 Diaryl ketones are an important scaffold in drug discovery due to their prevalence in naturally occurring bioactive compounds. This review discusses molecules containing the benzophenone moiety that have potent biological activity.
Diaryl ketones are an important scaffold in drug discovery due to their prevalence in naturally occurring bioactive compounds. This review discusses molecules containing the benzophenone moiety that have potent biological activity.
Abstract
The benzophenone scaffold represents a ubiquitous structure in medicinal chemistry because it is found in several naturally occurring molecules which exhibit a variety of biological activities, such as anticancer, anti-inflammatory, antimicrobial, and antiviral. In addition, various synthetic benzophenone motifs are present in marketed drugs. They also represent important ingredients in perfumes and can act as photoinitiators. This review will provide an overview of benzophenone moieties with medicinal aspects synthesized in the last 15 years and will cover the most potent molecule in each report. In this review, only benzophenones with substitutions on their aryl rings, i.e. diphenyl ketone analogues, have been covered.
Introduction
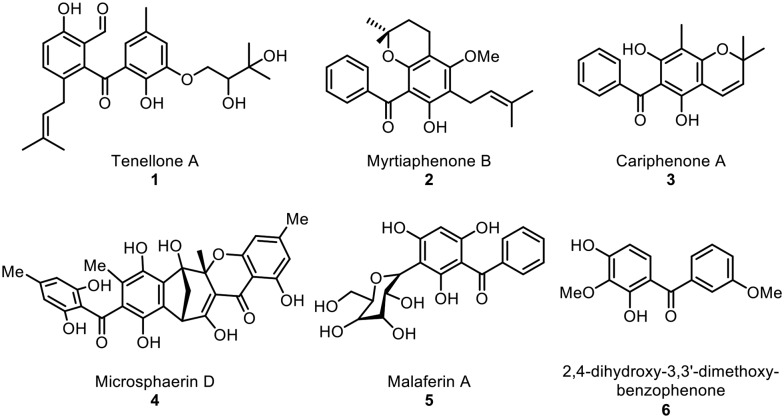
Benzophenone motifs are of considerable interest to researchers because they are found in pharmacologically relevant natural products and represent versatile synthetic building blocks. Natural benzophenones with structural diversity and bioactivity have been reported from higher plants and fungi. It has been found that about 77% of known natural benzophenones are found in the Clusiaceae (formerly Guttiferae) family.1,2 In addition, these natural benzophenones exhibit a range of biological activities, including antifungal, anti-HIV, antimicrobial, antiviral, and antioxidant (Fig. 1).
Fig. 1. Representative examples of naturally occurring benzophenones.
For example, prenylated benzophenone tenellone A (1),3 which is found in Diaporthe sp. fungi, showed ability to inhibit the cGMP dependent protein kinase activity of Eimeria tenella and also showed antiparasitic activity against the apicomplexan parasite Toxoplasma gondii (TgWC), with an IC50 of 1.8 mM. Cariphenone A (3), which was isolated from the leaves of Hypericum carinatum, showed antioxidant activity with IC50 = 3.2 μM and was also found to show similar inhibition of chemiluminescence to that of quercetin at the same concentration.4 Benzophenones containing hydroxy groups at C-2 and C-4 and methoxy groups at C-3 and C-3′ were isolated from the roots of the Popoluca Amerindian medicinal plant Securidaca diversifolia (L.) S. F. Blake and were found to show moderate inhibitory activity on herpes simplex virus type-1 (HSV-1).5
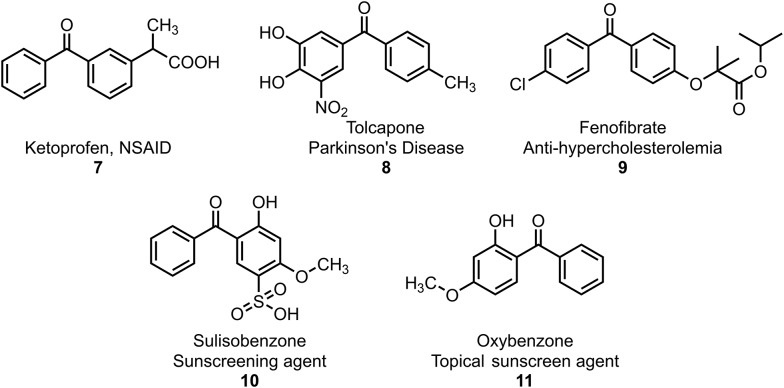
In addition, Fig. 2 depicts some of the benzophenone molecules available commercially as drugs and sun-protective materials. For example, ketoprofen (7) is a potent nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic effects.6–8 Meanwhile, tolcapone (8) (brand name Tasmar), a dihydroxy derivative of benzophenone, is used to treat Parkinson's disease.9 In addition, fenofibrate (8, Tricor) is mainly used to decrease cholesterol levels in patients at risk of cardiovascular disease.10 Moreover, benzophenones are one of the most important classes of substances in photochemistry and are used widely used as photosensitizers. In particular, UV-A/B filters, such as 2-hydroxybenzophenones 10 and 11, are known to be active constituents in sun protection products (e.g. sunscreens) that prevent ultraviolet light from passing through the skin.8,11–13
Fig. 2. Representative examples of benzophenone drugs.
Due to their importance as building blocks in the synthesis of natural products and pharmacological compounds, benzophenones have been synthesized in numerous ways. A few synthetic strategies to synthesize benzophenone and its derivatives are shown in Fig. 3. One of the most general methods for the preparation of these compounds is Friedel–Crafts acylation reactions.14–17 Alternative transformations used to construct these diaryl ketones involve metal-catalyzed carbonylative reactions. The carbonylative Suzuki–Miyaura reaction is one of the most important transition-metal-catalyzed carbonylations because it possesses unique features, such as readily accessible and stable substrates, high regioselectivity and stereoselectivity, broad substrate scope, and wide functional group compatibility.18–21 Although this reaction is a powerful method, gaseous CO is toxic, flammable, odorless, and difficult to handle (mainly at high pressure); hence, there are limitations to the application of this protocol.22 In addition, other approaches require a reaction between an aryl halide and an aldehyde or its derivative under metal catalysis. Because a range of aldehydes and aryl halides are readily available, a variety of aryl ketones can be synthesized using this reaction. For example, Cheng and co-workers reported the coupling of aryl iodides with aldehydes in the presence of Ni(dppe), Br2 and Zn to afford the corresponding biaryl ketones.23 Very recently, Kuninobu and Kanai reported the synthesis of diaryl ketones from aldehydes and aryl halides using a palladium/picolinamide catalyst.24 Various other catalysts have also been used with different coupling partners and aldehydes to afford various unsymmetrical diaryl ketones.25–30
Fig. 3. Various synthetic strategies for the synthesis of benzophenones.
In this area, transition-metal-catalyzed oxidative C–H acylation and decarboxylative acylation of unactivated sp2 arene C–H bonds using different acyl sources, such as aldehyde, benzyl alcohol, benzylamine, toluene, and α-keto acids, has been recently developed.31,32
Recently, a review on naturally occurring benzophenones, highlighting their biological activities, has been published;1 however, a review on their synthetic analogues is lacking. To the best of our knowledge, this review will be the first review on synthetic analogues of the benzophenone moiety in the field of medicinal chemistry. Due to the vast number of diaryl ketones known for their biological activities, we must limit ourselves; thus, this review will only discuss the benzophenone (diphenyl ketone) scaffold and its functionalized analogues reported in the last 15 years for various biological activities.
Anti-inflammatory activity
Inflammation is defined as an immediate response of the body to injuries of vascularized tissues; it delivers blood cells and fluid to the site of damage.33 Inflammation is biochemically induced by a group of physiologically active lipid compounds known as prostaglandins that induce hormone-like effects in animals.34 PGE2 is known to be the key mediator of inflammation, pain, and fever because it is the most abundant prostaglandin in humans.35,36 Further, all inflammatory diseases and various types of cancers are known to be associated with elevated levels of COX-2 and associated overproduction of PGE2.37 These observations led to the use of NSAIDs and specific COX-2 inhibitors as the first line drugs for the treatment of inflammation and pain.38
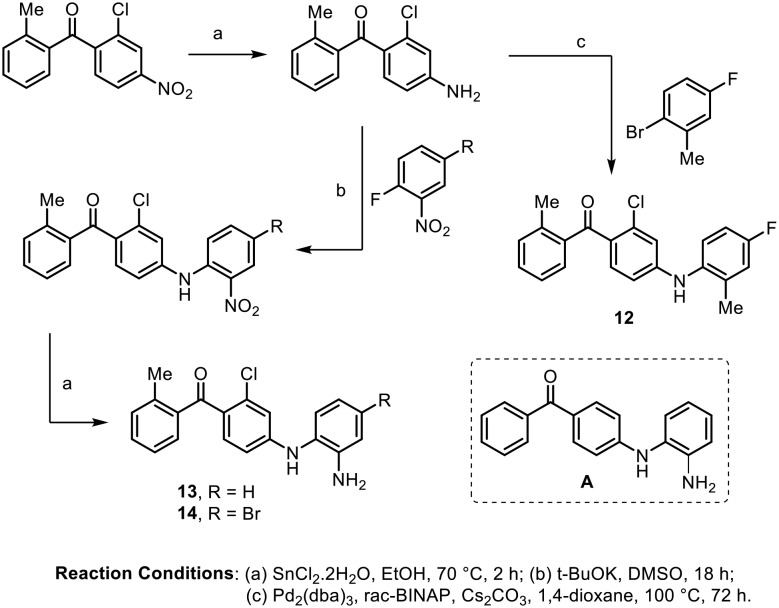
Natural and synthetic benzophenones have become known for their anti-inflammatory activity. In 2003, Ottosen and coworkers reported that the 4-aminobenzophenone class of compounds showed potent anti-inflammatory effects.39 It is known that tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) are among the principal pro-inflammatory cytokines responsible for many inflammatory diseases.40 Initial screening showed that the compound 4-aminobenzophenone derivative A displayed potent inhibition against TNF-α (IC50 = 159 nM) and IL-1β (IC50 = 226 nM) in LPS-stimulated human peripheral blood mononuclear cells (PBMC). Thus, the authors carried out the synthesis of various 4-aminobenzophenone analogues and performed structure activity relationship (SAR) studies. The synthesis of the three most active inhibitors (12, 13 and 14) is depicted in Scheme 1. Compounds 12, 13 and 14 inhibited TNF-α (IC50 = 4 to 6 nM) more potently than IL-1β (IC50 = 14 to 30 nM). Compounds 12, 13, and 14 were further evaluated for p38α MAP kinase activity; they showed good potency, with IC50 values of 4 nM, 10 nM and 39 nM, respectively.
Scheme 1.
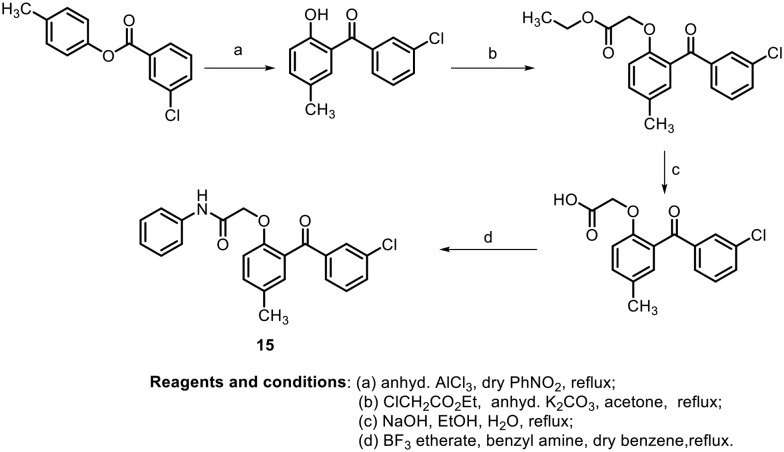
In this context, in 2004, S. A. Khanum et al. synthesized various hydroxybenzophenones, aroylaryloxyacetic acids and 2-(2-aroyl aryloxy)-N-phenyl acetamide derivatives and evaluated their anti-inflammatory activity (Scheme 2).41 The anti-inflammatory activity of the synthesized benzophenones was measured by carrageenan-induced foot pad edema (CPE) assay42 and by air pouch test.43 It was found that the synthesized benzophenones containing a chloro moiety at the meta position (15) showed the most effective anti-inflammatory activity and ulcerogenic activity compared with reference NSAIDs such as naproxen, indomethacin and phenylbutazone.
Scheme 2.
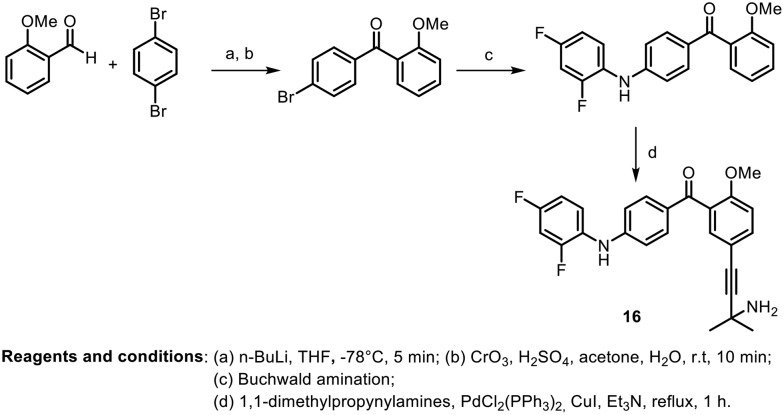
In the same year, another research group, L. Revesz et al., synthesized various benzoylpyridine and benzophenone derivatives; they evaluated them in vitro as p38α inhibitors and in vivo in several models of rheumatoid arthritis.44Scheme 3 depicts the synthesis of the most potent benzophenone derivative. The benzophenone was synthesized by cross coupling of aldehydes with 1,4-dibromobenzene to afford the corresponding alcohol; further Jones oxidation provided bromobenzophenone. Regioselective amination was performed on the formed benzophenone using Buchwald conditions. Further, Sonogashira coupling with 1,1-dimethylpropynylamine afforded the desired compound (16). Most of the compounds showed good inhibitory activity against p38α MAP kinase. The compound (16), shown in Scheme 3, was found to have an IC50 value of 14 nM and showed the highest efficacy and selectivity, with an ED50 value of 9.5 mg kg–1 po in CIA (collagen-induced arthritis in rats).
Scheme 3.
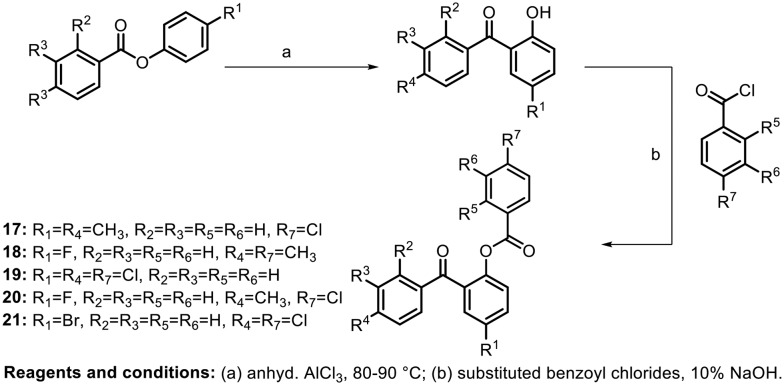
In 2007, Shashikanth et al. synthesized benzoate-substituted benzophenones and tested them for anti-inflammatory activity. The author utilized the Fries rearrangement reaction to synthesize the hydroxy benzophenones, followed by benzoylation to afford various benzoyloxy benzophenones, as shown in Scheme 4.45 The anti-inflammatory activity of the molecules was evaluated by the rat paw oedema inhibition test and cyclooxygenase assay. In addition, their ulcerogenic activity was evaluated. Based on the above tests, it was found that the compounds with chloro and methyl substituents at the para position in ring B and ring C (17 and 20) showed more potent anti-inflammatory activity than the standard. Compound 19, with a chloro group at the para position on all its phenyl rings, displayed lower ulcerogenic activity (10%).
Scheme 4.
Similar compounds were synthesized by Jun and coworkers in 2012 and their inhibitory effects on ICAM-1 expression, which is an important regulator of inflammatory immune response, were studied.46 They investigated the anti-inflammatory properties of the compounds on IFN-γ-induced ICAM-1 expression in HaCaT cells. IFN-γ-induced protein expression of ICAM-1 and IFN-γ-induced mRNA expression of ICAM-1 were analyzed by western blot and RT-PCR, respectively. It was found that the compound 19, containing chloro groups in all three aryl rings at the para position, showed the maximum inhibitory activity of ICAM-1 expression.
In continuation of their research on the anti-inflammatory evaluation of benzophenone analogues, in 2009, S. A. Khanum et al. synthesized a new sequence of benzophenone N-ethyl piperidine ether analogues, as shown in Scheme 5.47 The synthesized molecules were found to show interesting anti-inflammatory activities in CPE assays and in air-pouch tests; they decreased the total number of leukocytes, indicating inhibition of prostaglandin production. Benzophenone N-ethyl piperidine ether analogues act as orally active anti-inflammatory agents with decreased side effects. Halogen groups at the para position showed significant anti-inflammatory profiles, with low gastric ulceration incidence compared to the standards naproxen, indomethacin, and phenylbutazone. Further, they reported benzophenone N-ethyl morpholine ether analogues with good anti-inflammatory activity.48
Scheme 5.
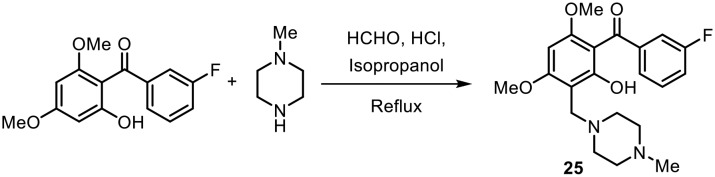
Bandgar and coworkers synthesized amine-containing benzophenone analogues and evaluated their inhibition of TNF-α and IL-6 as well as their antioxidant activity.49 The amine-containing benzophenone analogues were prepared by Mannich reactions, as shown in Scheme 6. Most of the synthesized molecules were found to inhibit TNF-α and IL-6. Furthermore, their antioxidant potentials were determined by DPPH radical scavenging activity studies, and kinetics studies were performed. The most potent molecule containing N-methylpiperidine (25) showed inhibition of TNF-α (54%) and IL-6 (97%) at a concentration of 10 μM, with lower cytotoxicity and considerable antioxidant activity.
Scheme 6.
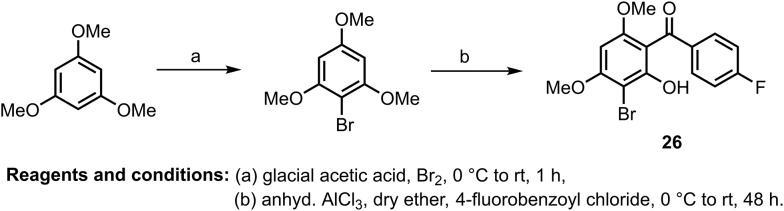
Later, in 2011, Sangamwar and co-workers synthesized a new series of benzophenone derivatives and studied their TNF-α and IL-6 inhibitory activities.50 These molecules were synthesized by bromination of 1,3,5-trimethoxy benzene using bromine water in glacial acetic acid. Further, via Friedel–Crafts acylation, the desired benzophenones were synthesized as shown in Scheme 7. The synthesized benzophenones were not found to have promising inhibitory activities against TNF-α. However, they showed promising inhibitory effects against IL-6; the para-fluoro-containing compound showed an IC50 value of 0.19 μM.
Scheme 7.
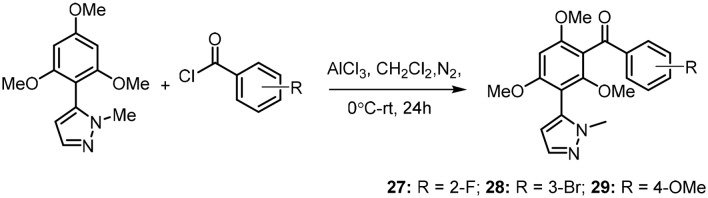
Further, the Bandgar research group synthesized a new series of benzophenone derivatives containing a biologically active pyrazole moiety, as shown in Scheme 8. The molecules were synthesized by carrying out acylation on 1-methyl-5-(2,4,6-trimethoxyphenyl)-1H-pyrazole with various benzoyl chlorides. All the pyrazole-linked benzophenones were screened for their in vitro inhibitory potentials against the COX-1 and COX-2 enzymes; they exhibited considerable inhibition of COX-2 (44% to 60%) at 100 μM. Further, their in vivo anti-inflammatory activity was studied by CPE assays in rats, and the antioxidant potentials of the compounds were evaluated against a variety of reactive oxygen and nitrogen species, such as hydroxyl (OH), superoxide (SOR) and 2,2-diphenyl-2-picrylhydrazyl (DPPH). The molecules (27, 28 and 29) shown in the scheme below were found to be anti-inflammatory agents with potent antioxidant activities.51
Scheme 8.
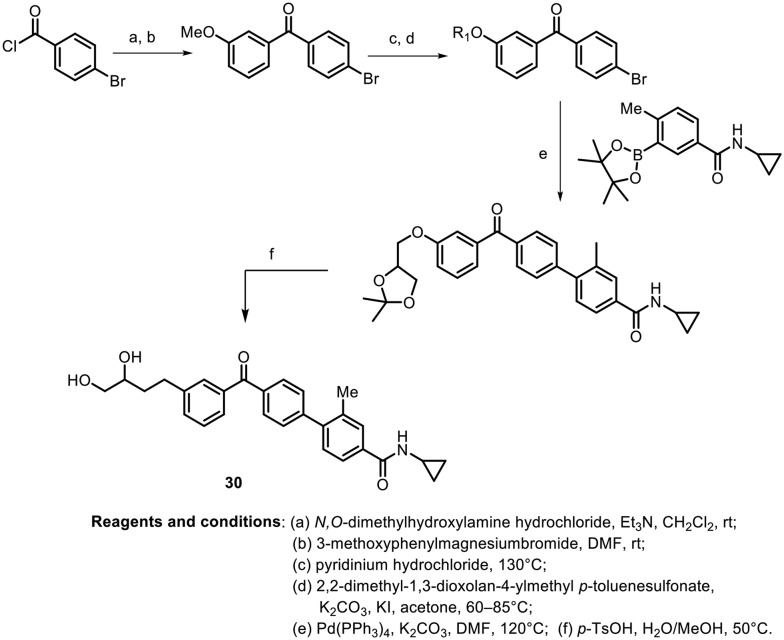
In 2015, Inn and Kim synthesized N-cyclopropylbenzamide-benzophenone hybrids and evaluated them as novel inhibitors of p38 mitogen-activated protein kinase (MAPK), which plays a crucial role in the production of pro-inflammatory cytokines.52 The synthesis of the most potent molecule is depicted in Scheme 9. Grignard addition with a Weinreb amide using methoxybenzene produces the benzophenone, which undergoes subsequent demethylation, O-alkylation, and arylation via a Suzuki–Miyaura coupling reaction followed by acetal deprotection to deliver the potent molecule. The N-cyclopropylbenzamide-benzophenone hybrid (30) shows potent p38α MAPK inhibitory activity, with an IC50 value of 0.027 μM, and high kinase selectivity. Further, the anti-inflammatory activity was evaluated on the THP-1 cell line, and decreases in the production of TNF-α and IL-6 were observed in a dose-dependent manner.
Scheme 9.
Anti-cancer activity
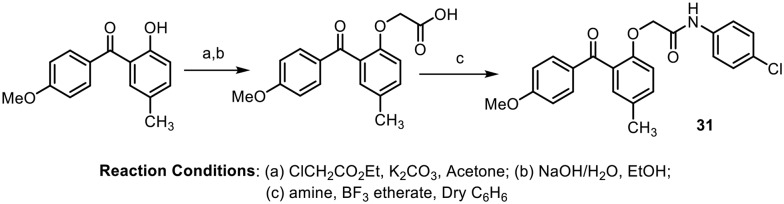
In 2006, Shashikanth et al. synthesized a series of substituted benzophenone analogues, (2-aroyl-4-methylphenoxy) acetamides, as shown in Scheme 10, and investigated their anti-tumor and pro-apoptotic effects in Ehrlich ascites tumor (EAT) cells.53 The in vitro results showed that compound 31, with a methoxy group at the para position in ring B, showed the highest anti-tumor activity of 61%; also, in in vivo experiments, 86% of a group animals treated with the same compound (31) remained alive for 23 days. Further, investigation suggested that these effects are likely to be induced by overexpression of p53, activation of caspase-3, and translocation of CAD into the nucleus, which is involved in apoptosis.
Scheme 10.
Pin1 is a protein that interacts with NIMA (never in mitosis gene a)-related kinase 1; it belongs to the parvulin family of peptidyl-prolyl cis/trans-isomerases (PPIase) and has distinctive substrate specificity.54 It is well known that Pin1 is frequently up-regulated in various tumors, and its overexpression is also associated with tumor grades and clinical outcomes in prostate cancer.55,56 In this context, in 2012, Xu and coworkers developed a series of diamino derivatives of benzophenone for hPin1 inhibitory activity, as shown in Scheme 11. The inhibitory activities on Pin1 of all the synthesized benzophenone compounds were evaluated by protease-coupled enzyme assays with Suc-Ala-Glu-Pro-Phe-pNA as the substrate. The most active molecule (32, shown in Scheme 11) was found to have an IC50 value of 5.99 μM.57
Scheme 11.
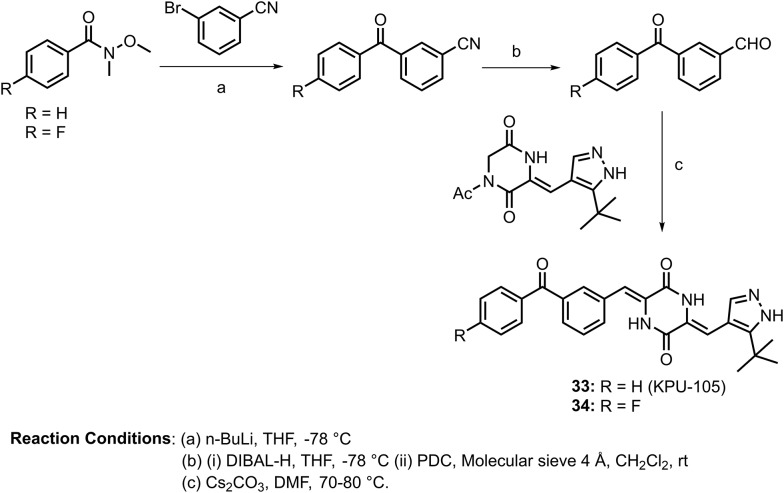
Plinabulin, which is a diketopiperazine (DKP) type vascular disrupting agent (VDA), demonstrated potent cytotoxicity, with an IC50 value of 10 nM against a variety of human cancer cell lines in addition to vascular disrupting activity.58 In 2012, Hayashi and coworkers developed more potent antimicrotubule and cytotoxic derivatives based on the didehydro-DKP skeleton plinabulin.59 After a structure–activity relationship (SAR) study, they identified a more potent derivative (33: KPU-105) containing a benzoyl group at the C-3 position of the phenyl ring. The synthesis of this molecule is shown in Scheme 12. KPU-105 was found to have cytotoxicity against HT-29 (human colon adenocarcinoma) cells, with an IC50 value of 1.4 nM, and the binding dissociation constant of this benzophenone derivative to porcine tubulin was 63 nM. In addition, the vascular disrupting activity of KUP-105 on human vascular endothelial cells (HuVECs) exhibited a lowest effective concentration of 1 nM for microtubule depolymerization. Furthermore, in the same year, they screened other benzoyl group derivatives and reported that a derivative bearing a fluorine atom substitution at the 4-position of the benzoyl group (34, shown in Scheme 12) had more potent cytotoxicity (IC50 = 0.5 nM) and a similar binding dissociation constant (Kd = 72 nM).60
Scheme 12.
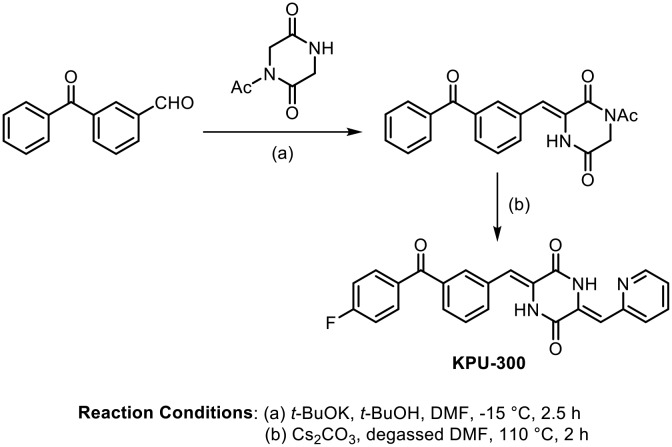
Continuing their work on benzophenone-DKP antimicrotubule derivatives, Hayashi and coworkers succeeded in replacing the imidazole moiety with a 2-pyridyl ring; this compound showed strong cytotoxicity, with IC50 values on the nanomolar level against several human cancer cells. They synthesized various benzophenone-DKP analogues, as shown in Scheme 13. The compound (KPU-300) exhibited potent cytotoxicity, with an IC50 value of 7.0 nM against HT-29 cells, by strongly binding to tubulin (Kd = 1.3 μM) and inhibiting tubulin depolymerization.61
Scheme 13.
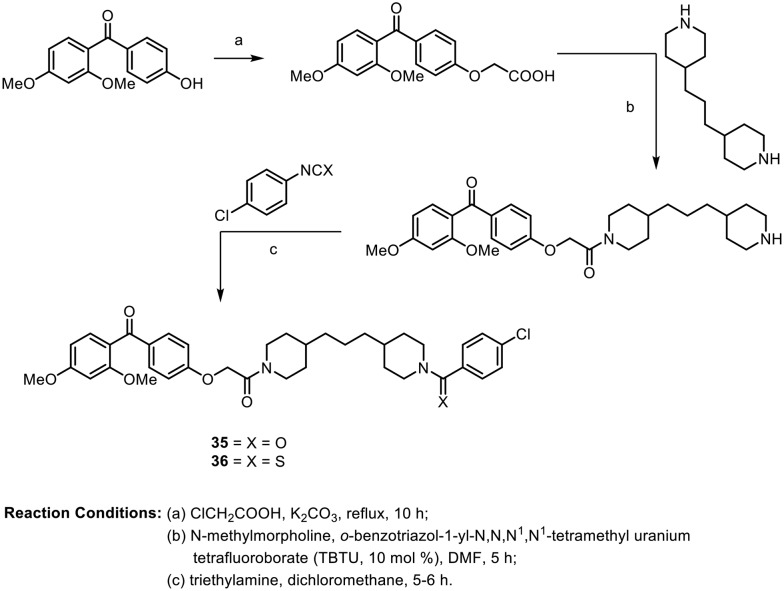
In 2012, Rangappa et al. synthesized novel molecules containing 2,4-dimethoxybenzophenone conjugated with piperidine analogues.62 These molecules were tested for their antileukemic activities against two human leukemic cell lines (K562 and CEM) using MTT assays. All the synthesized compounds were found to have good IC50 values, from 1.6 to 8.0 μM. The synthesis of two of the most potent compounds, 35 and 36, is depicted in Scheme 14.
Scheme 14.
In 2013, Khanum et al. carried out multistep synthesis of novel benzophenone–benzimidazole analogues synthesized for angioprotective activity and tumor inhibition activity. The synthesis of the desired molecules is depicted in Scheme 15; it involves benzoylation, Fries rearrangement, esterification, and an alkaline hydrolysis coupling reaction using TBTU/lutidine, followed by an acid-mediated cyclization step. These compounds were evaluated for their cytotoxic and antiproliferative effects against Ehrlich ascites carcinoma (EAC). Further, the most potent lead compound showed angioprevention of tumors by in vivo experiments in a chorioallantoic membrane (CAM) model and a tumor-induced vasculature in vivo model. The final compounds (37 and 38) shown in Scheme 15 were found to have strong cytotoxicity. In the SAR study, the authors found that the greater potency of these molecules is due to the increase in the number of methyl groups in addition to methoxy substitution at the para position of the benzoyl ring. Tumor inhibition was observed by CAM and a tumor-induced mice peritoneum model. Compound 38 showed potent activity by inducing minimum viability at an inhibitory concentration of 10 μM.63
Scheme 15.
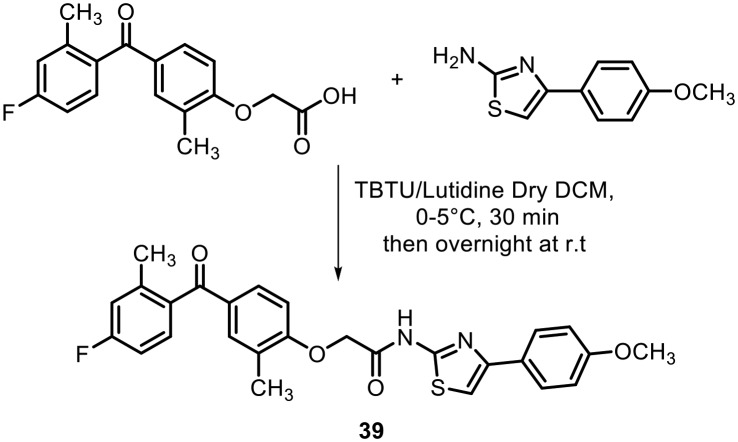
Vascular endothelial growth factor A (VEGF-A) is a potent secreted cytokine that plays a crucial role in angiogenesis under both physiological and pathological conditions. It is a key mediator of tumor angiogenesis by stimulating the growth of blood vessels from capillaries.64 To this end, in 2014, Khanum's research group synthesized a series of benzophenone-thiazole derivatives and screened them for VEGF-A inhibition. The molecules were synthesized by a similar strategy to that used earlier. The final molecules were synthesized by condensation of the acid analogue of benzophenone with 2-aminothiazole derivatives, as shown in Scheme 16. The synthesized benzophenones containing thiazole analogues were screened for their antiproliferative and cytotoxic effects in EAC and Dalton's lymphoma ascites (DLA) cell lines using the trypan blue dye exclusion method. After the SAR study, they found that compound 39, with methyl and fluoro groups on the benzophenone moiety and a methoxy group on the phenyl ring, was the most active; it exhibited antiproliferative effects through translational VEGF-A inhibition with IC50 values in the range of 5 μM in trypan blue, MTT and LDH release assays against EAC cells and DLA cells.
Scheme 16.
Clonis and coworkers targeted multiple drug resistance (MDR)-involved human glutathione S-transferase A1-1 (GSTA1-1), which is an important isoenzyme that is overexpressed in several tumors, leading to chemotherapeutic-resistant tumour cells.65
The group reported that 2,2′-hydroxybenzophenones and their N-acyl hydrazone derivatives, shown in Fig. 4, showed potent inhibitory activity toward MDR-involved human GST-isoenzyme A1-1; their IC50 values were in the range of 0.18 ± 0.02 to 1.77 ± 0.10 μM. Based on molecular modeling and docking studies, it was found that these molecules interact at the CDNB-binding site of the enzyme.
Fig. 4. 2,2′-Hydroxybenzophenones and their N-acyl hydrazone derivatives as anti-tumor agents.
Later, Khanum et al. synthesized a series of diamide-coupled benzophenone analogues as potential anticancer agents. The molecules were designed based on their earlier synthetic strategy using diamines.66 The cytotoxic and antiproliferative effects of the diamide-coupled benzophenone derivatives were investigated on multiple cancer cell lines with different origins: A549, MCF-7 and DLA. Compound 44, shown in Fig. 5, with three methyl groups at the ortho positions in rings A, B and D and a bromo group at the para position in ring E, was found to be a good lead for their studies. The IC50 values of the lead compound were 20 μM, 23 μM and 23 μM on A549, MCF-7 and DLA cells, respectively. In addition, the antitumour potency of the lead compound was verified against tumor regressing activity and against the murine ascites model DLA. The authors suggested that this potency is due to regressed neovessel formation both in tumorigenic and non-tumorigenic model systems.
Fig. 5. A diamide-coupled benzophenone derivative as a potent anti-cancer agent.
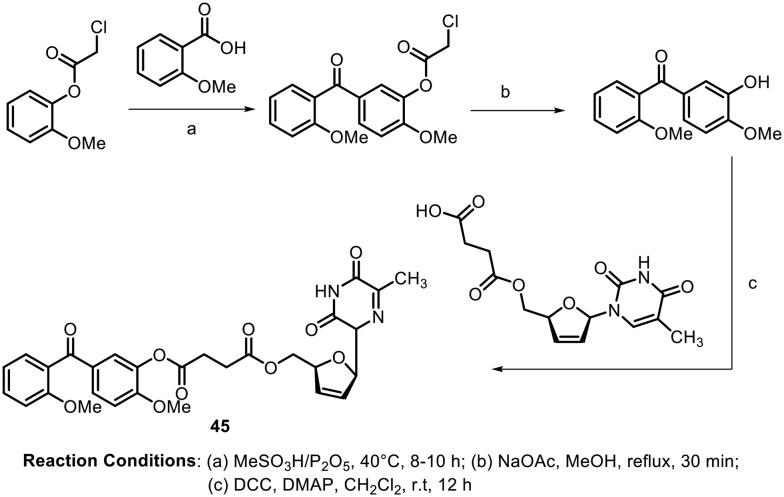
In 2016, Liu and Yao synthesized some benzophenone-nucleoside derivatives and tested them for telomerase inhibition activity.67 The author designed the molecules based on the mechanistic and structural similarities between telomerase reverse transcriptase (TERT) and HIV-1 reverse transcriptase; they anticipated that the presence of stavudine (a potent HIV-1 inhibitor) in the phenstatin moiety (a potent anticancer agent) would play an important role in the telomerase activity. Scheme 17 shows the synthesis of the potent molecule containing a benzophenone moiety linked with a stavudine derivative. The benzophenones were synthesized from carboxylic acids using Eaton's reagent (MeSO3H/P2O5) followed by deprotection of the chloroacetyl group and subsequent coupling with the stavudine derivative. Compound 45, shown in Scheme 15, showed potent activity against HeLa, SMMC-7721 and SGC-7901 cell lines with IC50 values of 1.58 ± 0.20, 0.82 ± 0.11 and 0.77 ± 0.33 μM, respectively. Further, in vivo studies revealed that the compound displayed inhibition of tumor growth of S180 and HepG2 tumor-bearing mice.
Scheme 17.
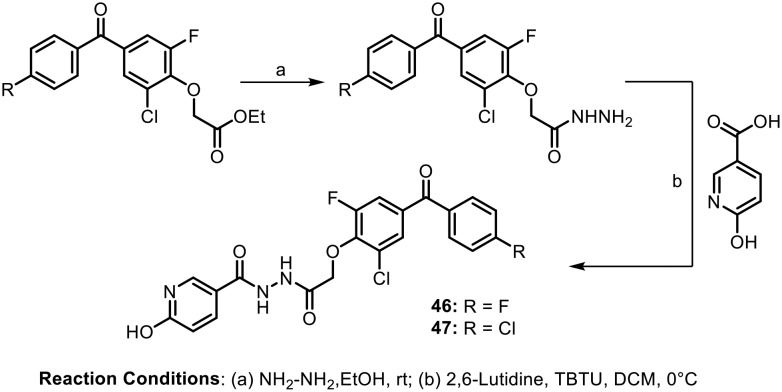
In continuation of their efforts towards the design of new anticancer agents, in 2016, Khanum et al. synthesized benzophenone-tagged pyridine analogues containing a hydrazide linker.68 The synthesized molecules were evaluated for their antiproliferative and cytotoxic properties against DLA cells by performing MTT, trypan blue and LDH release assays. The synthesis of these potent molecules is depicted in Scheme 18. After SAR studies, it was found that the compounds with fluoro (46) and chloro (47) groups at the para position of the benzoyl ring of the benzophenone scaffold and a hydroxyl group at the 2 position of the pyridine ring showed significant activity. Further, in vivo tests revealed that both these compounds (46 and 47) led to DNA fragmentation due to caspase-activated DNase (CAD) activation, resulting in apoptosis and thereby inhibiting the tumour growth of Dalton's lymphoma ascites.
Scheme 18.
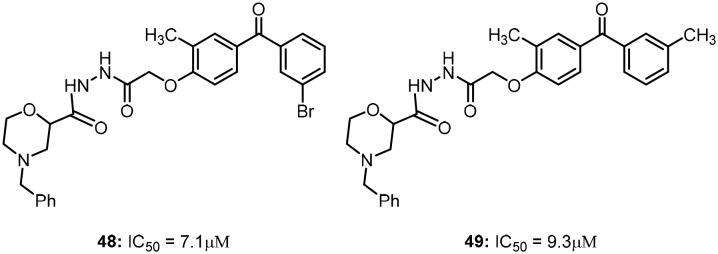
Recently, Khanum's research group reported novel morpholine-conjugated benzophenone analogues as an extension of their previous reports of benzophenones with hydrazide linkers. The synthesized molecules were tested for anti-proliferative activity against various neoplastic mouse and human cell lines, such as DLA, EAC, MCF-7 and A549 cells.69
Molecules 48 and 49, which are shown in Fig. 6, were found to exhibit IC50 values of 7.1 μM and 9.3 μM for antiproliferative activity, respectively, and showed sufficient potency to exhibit prolonged activity against cancer progression via cell cycle arrest in G2/M phase. In addition, these compounds (48 and 49) also inhibit murine ascites lymphoma through CAD-mediated apoptosis.
Fig. 6. Morpholine-conjugated benzophenones as potent anti-cancer agents.
Anti-HIV activity
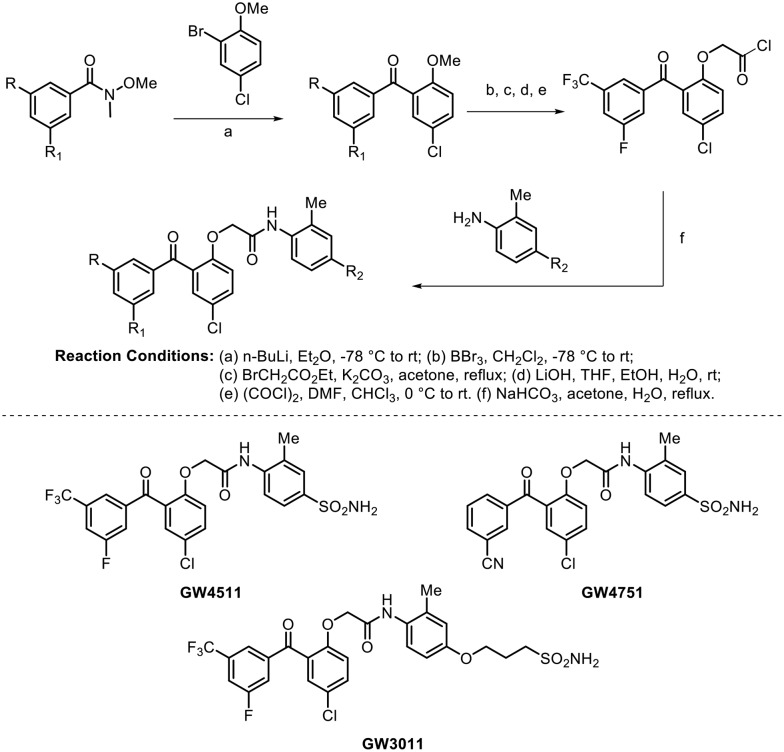
For the treatment of HIV infection and AIDS, non-nucleoside reverse transcriptase inhibitors (NNRTI) represent one of the most important classes of drugs and are key components of current antiretroviral therapy.70,71 In 1995, a series of benzophenone analogues with potent activity against wild-type HIV-1 were reported.72 To this end, in 2004, Chan et al. identified three compounds based on the benzophenone scaffold which showed potent broad spectrum antiviral activity against both wild-type and relevant NNRTI-resistant mutant viruses. The synthesis of these potent molecules (GW4751, GW4511, and GW3011) is shown in Scheme 19.73
Scheme 19.
These molecules were found to be extremely potent against the wild-type virus, with IC50 values <2 nM; they also showed potency against 16 of 20 resistant mutants, with IC50 values <10 nM. The authors were delighted to observe that all the three compounds showed significant potency against the Y181C-, 103N-, and K103N-containing double mutation NNRTI-resistant viruses, which were found to have resistance against the well-known marketed NNRTIs.
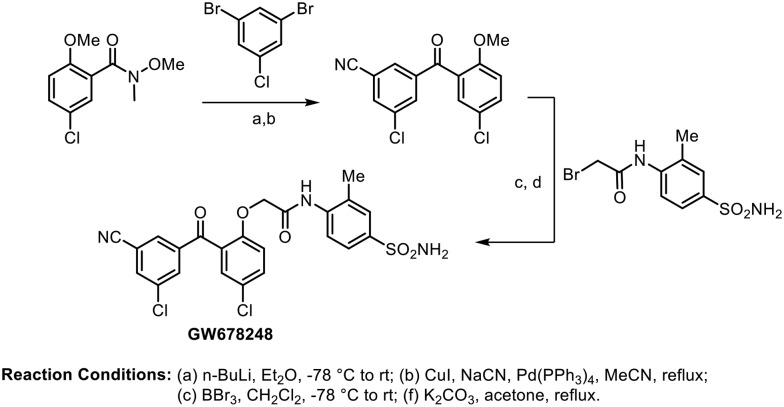
In 2006, Romines and coworkers performed an extensive investigation on the structure–activity relationships of benzophenone derivatives, which resulted in the identification of various compounds with very potent inhibition of both wild type and clinically important NNRTI-resistant mutant strains of HIV.74 After systematically exploring the A-ring, B-ring, linker region, and C-ring sections of this benzophenone template, they found an excellent molecule (GW678248) that possessed an attractive potency profile in their primary assays against both wild-type (IC50 = 0.5 nM) and mutant viruses, particularly those associated with clinical resistance to efavirenz and nevirapine. Further, the molecule showed a lower clearance rate and a longer elimination half-life in intravenous pharmacokinetic studies. The synthesis of GW678248 is shown in Scheme 20.
Scheme 20.
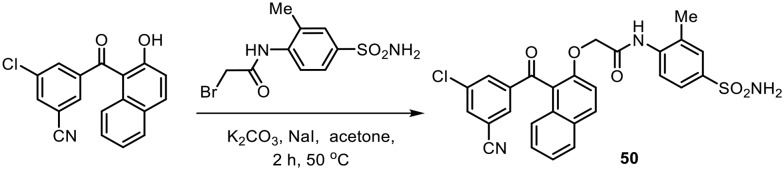
Further investigations on benzophenone scaffolds were carried out by Chen and coworkers to identify a new lead as an NNRTI.75 They synthesized a series of benzophenone derivatives with B-rings substituted by bulky naphthyl rings and evaluated them as non-nucleoside HIV-1 reverse transcriptase inhibitors (Scheme 21). Most of these compounds showed good to moderate activity against wild-type HIV-1 and mutated viruses. In particular, the analogue (50) without any substituent on the C6 position of the naphthyl ring was found to be the most potent against wild-type HIV-1 (EC50 = 4.8 nM); it was also proved to be active against the HIV-1 double mutant strain A17 (K103N + Y181C), with an EC50 value of 2.1 μM.
Scheme 21.
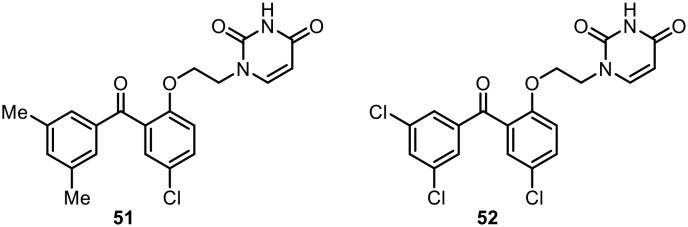
Seley-Radtke examined the anti-HIV activity of a series of hybrid molecules possessing benzophenone and uracil moieties tethered with an ether linkage.76 After SAR examination, two compounds (51 and 52), shown in Fig. 7, displayed the highest activity against the wild-type virus; however, different levels of activity were observed towards the K103N/Y181C double mutant HIV-1 strain. Also, both compounds (51 and 52) showed no activity against the V106A RT, while efavirenz retained its activity. The authors suggested that poor solubility may be the reason for these observations rather than a different mode of interaction with mutant HIV-RTs.
Fig. 7. Benzophenone and uracil hybrids as potent anti-HIV-1 agents.
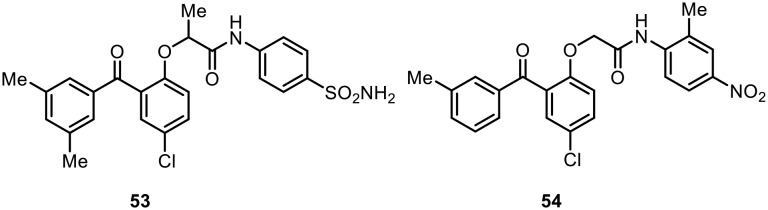
Recently, Zheng and Ma evaluated a series of novel benzophenone derivatives (BPs) and identified molecules with nanomolar anti-HIV-1 activity.77 Two of the most potent compounds (53 and 54) identified by them are shown in Fig. 8. They found that compound 53, which contains a methyl group at the linker position, was the most active inhibitor; it had an EC50 value of 2.9 nM, comparable to the lead compound GW678248, in inhibiting wild-type HIV-1 virus. Additionally, the benzophenone analogue 54, with nitro functionality at the para position of the C ring, showed strong inhibitory activity against the HIV-1 virus (EC50 = 4.2 nM) and possessed very low cytotoxicity.
Fig. 8. Benzophenone derivatives with amide linkers as potent anti-HIV-1 agents.
Anti-Alzheimer's activity
Acetylcholinesterase (AChE) is a major drug target, and its inhibitors have demonstrated functionality in the symptomatic treatment of Alzheimer's disease (AD).78 In 2009, Belluti and coworkers carried out the synthesis of various benzophenones inspired by donepezil, which is an FDA-approved AChE inhibitor. The synthesis of these molecules is shown in Scheme 22.79
Scheme 22.
Their synthetic approach was based on a Friedel–Crafts acylation reaction followed by bromination and subsequent nucleophilic substitution with different amines. The compounds were tested for anti-Alzheimer's activity on the enzymes human AChE and butyrylcholinesterase (BChE) by Ellman's assay. The compounds (55 and 56) shown in Scheme 22 were found to have lower μM ranges of IC50 values. Compound 55 showed IC50 values of AChE = 0.46 ± 0.04 μM and BChE = 60.7 ± 1.3 μM; compound 56 showed IC50 values of AChE = 0.57 ± 0.04 μM and BChE = 71.5 ± 3.7 μM.
Further, in 2011, Belluti and coworkers extended their investigation of their previous lead molecule for AChEI based on the benzophenone nucleus.80 They developed a new series of acetylcholinesterase inhibitors (AChEIs) endowed with anti-Aβ aggregating capability. Their approach was to convert the lead molecule into a chemical entity involving insertion, through spacers, of peripheral-site interacting functions into the benzophenone scaffold; thereby, they were able to modulate AChE proaggregating activity. Based on the diethylmethylammonium alkyl group of propidium, which is a potent peripheral site ligand, various amino moieties were inserted in an attempt to mimic its function. The synthesis is shown in Scheme 23.
Scheme 23.
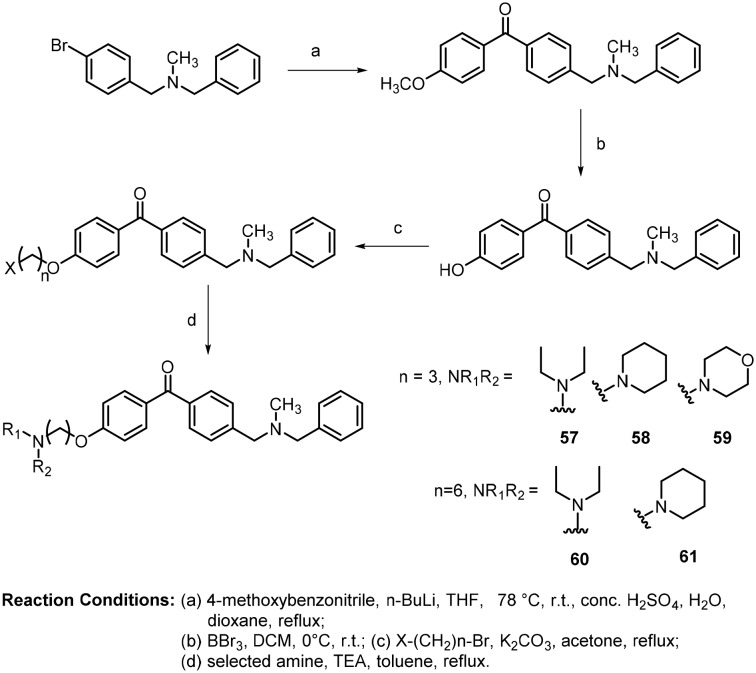
These molecules were intended for selective dual inhibition of acetylcholinesterase and acetylcholinesterase-induced beta amyloid aggregation. The synthesized compounds with propyloxy (57, 58 and 59) and hexyloxy (60 and 61) ethers showed good activity against AChE-induced Aβ aggregation. In addition, molecular modeling studies also confirmed that the diethylaminopropyloxy (57) and diethylaminohexyloxy (60) side chains interact at the peripheral anionic side (PAS) pockets of the enzyme.
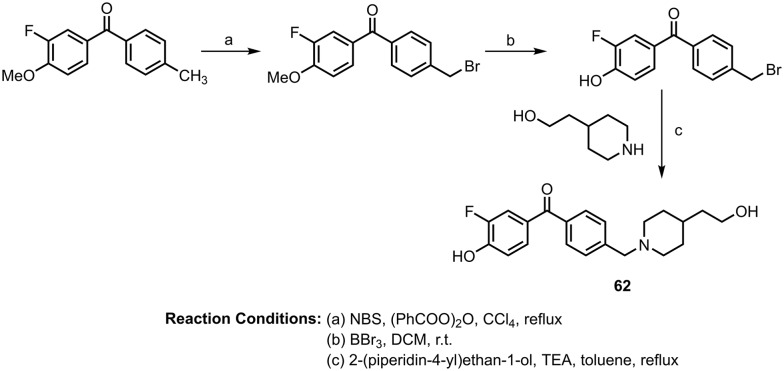
Later, the same author synthesized fluorinated benzophenone derivatives as multipotent agents for Alzheimer's disease against β-secretase (BACE-1) and acetylcholinesterase (AChE).81 These benzophenone-based analogues were investigated for their ability to inhibit BACE-1 enzyme activity using the fluorescence resonance energy transfer (FRET) method. The most potent molecule (62) is shown in Scheme 24; it showed good potency to inhibit BACE1 (IC50 value: 2.32 μM). These molecules also showed antioxidant activity by inhibiting ROS formation in human neuronal SH-SY5Y cells. They also found that the most potent inhibitor of BACE1 is a weak inhibitor of AChE, with an IC50 value of 2.52 μM.
Scheme 24.
Antiparasitic activity
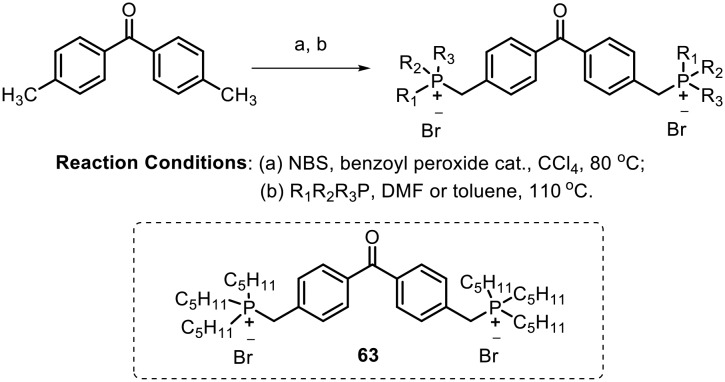
Leishmaniasis is a parasitosis transmitted by several species of the protozoan genus Leishmania.82,83 The phosphonium derivatives act as lipophilic ions with delocalized charges and enhanced hydrophobicity; considering the strong anionic character of the parasite plasma membrane84 and the key role of mitochondria in the bioenergetics of the parasite (Leishmania), Rivas and Dardonville synthesized benzophenone-derived bisphosphonium salts and assayed them for lethal activity against the human protozoan parasite Leishmania.85 They synthesized the desired molecules by reaction of 4,4′-bisbromomethylbenzophenone with an excess of the corresponding phosphine in either anhydrous DMF or anhydrous toluene, as shown in Scheme 25. The most potent compound (63) bears an n-pentyl group on the phosphine; it showed negligible toxicity on mammalian cells. The LC50 values of the most potent compound on L. donovani promastigotes and L. pifanoi axenic amastigotes were found to be 1.09 ± 0.14 μM and 0.97 ± 0.01 μM, respectively. Based on various parameters evaluated in in vitro assays, the authors suggested that compound 63 targets complex II of the respiratory chain of the parasite.
Scheme 25.
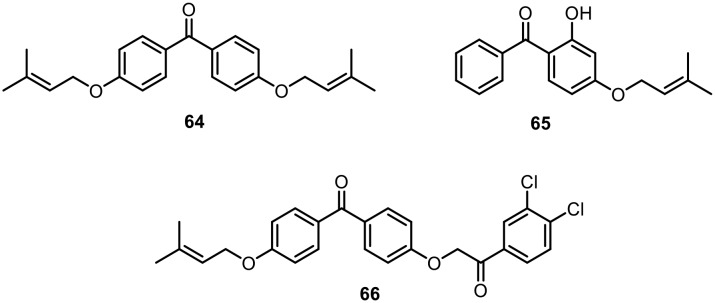
In 2010, dos Santos and coworkers found that natural polyprenylated benzophenones isolated from the fruits of Garcinia brasiliensis, such as guttiferone-A, 7-epi-clusianone and garciniaphenone, exhibited leishmanicidal activity against amastigote and promastigote forms of L. amazonensis.86 In this regard, in 2013, they synthesized O-alkyl and O-prenyl derivatives of benzophenone at the para position of the aryl ring and evaluated their leishmanicidal activity against the promastigote form of Leishmania (L.) amazonesis; they also evaluated their cytotoxicity in murine macrophages. Fig. 9 shows the compound (64) that showed the best relationship between anti-leishmanial and cytotoxic properties against macrophages. After performing in vitro assays, the authors suggested that variation in the lipophilicity of the target molecules was essential to the modulation of anti-leishmanial activity and toxic effects in macrophages.87
Fig. 9. O-Prenyl derivatives of benzophenone with leishmanicidal activity.
Later, in 2015, L. de Almeida et al. synthesized a similar analogue of O-prenylated benzophenone and studied its leishmanicidal activity against intramacrophage amastigote forms of Leishmania (L.) amazonesis as well as its cytotoxic effects against murine peritoneal macrophages. The compound (65), shown in Fig. 9, was found to have good inhibitory activity against papain, which is one of the most exploited cysteine protease representatives. The compound (65) also showed potent inhibition of trypanosomatid cysteine proteases, with IC50 values in the low micromolar range.88
Very recently, Khan and Choudhary reported the anti-leishmanial activities of a series of 4-substituted benzophenone ethers.89 The synthesized compounds were tested in vitro for anti-leishmanial activity on Leishmania major promastigotes. The compound 2-(4-benzoylphenoxy)-1-(3,4-dichlorophenyl)ethan-one (66), shown in Fig. 9, showed the highest inhibitory effect, with an IC50 value of 1.19 μg ml–1.
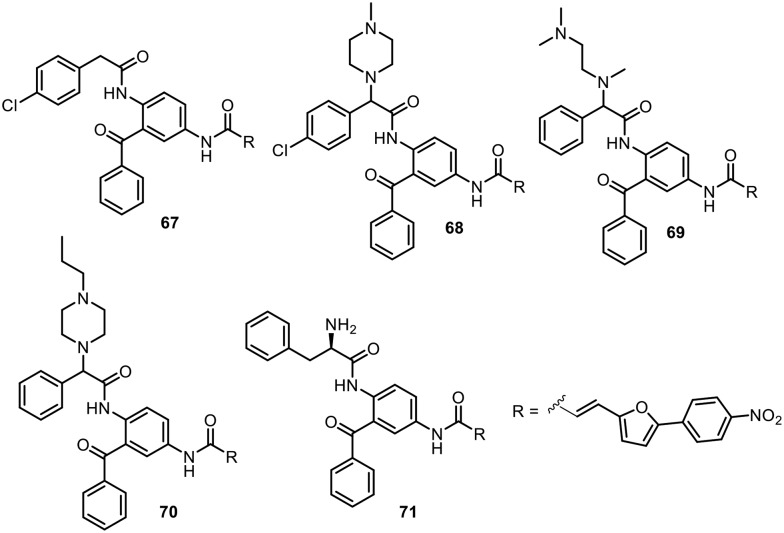
Farnesyltransferase (FTase) is known as a potential target for the development of anticancer drugs. Thus, various farnesyltransferase inhibitors have been subjected to clinical trials.90 In addition, it was found that other eukaryotic organisms, such as the pathogenic protozoa Plasmodium falciparum,91,92 Trypanosoma brucei,93–95 Trypanosoma cruzi (T. cruzi),96Leishmania major,97Toxoplasma gondii,97 and Entamoeba histolytica,98 also contain farnesyltransferase. Hence, FTase has also been suggested as a new target for the treatment of parasitic infections.99 In this context, Schlitzer's research group focused on the development of FTase inhibitors based on the 2,4-diaminobenzophenone scaffold as antimalarial drugs (Fig. 10). In 2003, benzophenones with farnesyltransferase inhibitory effects were tested by in vitro assays against the multi-drug resistant Plasmodium falciparum strain Dd2.100 Compound 67 was found to have an IC50 value of 64 nM. In 2004, Schlitzer and coworkers found that piperazinyl analogue 2 displayed low IC50 values compared to compound 67 in in vitro assays; however, compound 68 showed more potent in vivo activity against plasmodium infections.101 In this report, the authors established that the antimalarial activity of the compounds relies on FTase inhibition. Later, in 2008, they found that replacement of the piperazinyl moiety with N,N,N′-trimethylethylenediamine significantly enhanced the in vitro and in vivo activities of the compound.102 Compound 69 showed IC50 values of 21 nM against FTase and 32 nM against P. falciparum. The ED50 of compound 3 in a mouse model was found to be 16 mg kg–1. In parallel to their search for novel anti-malarial compounds, in 2005, Schlitzer and coworkers tested FTase inhibitors with the 2,4-daminobenzophenone scaffold against the human parasite T. cruzi, which can cause Chagas's disease.103 Compounds 70 and 71 showed encouraging in vitro activities against T. cruzi, with LC50 values of 1 nM and 10 nM, respectively. Further, 115 days postinfection with T. cruzi, 60% of the mice survived when treated with compound 70 at 7 mg kg–1 per day, and 80% survived when treated with compound 71 at 6.4 mg kg–1 per day. Schlizer's research group is actively developing novel FTase inhibitors containing 2,4-diaminobenophenone as antimalarial and antitrypanosomal agents.104,105
Fig. 10. 2,4-Diaminobenzophenone scaffolds as FTase inhibitors.
Antimicrobial activity
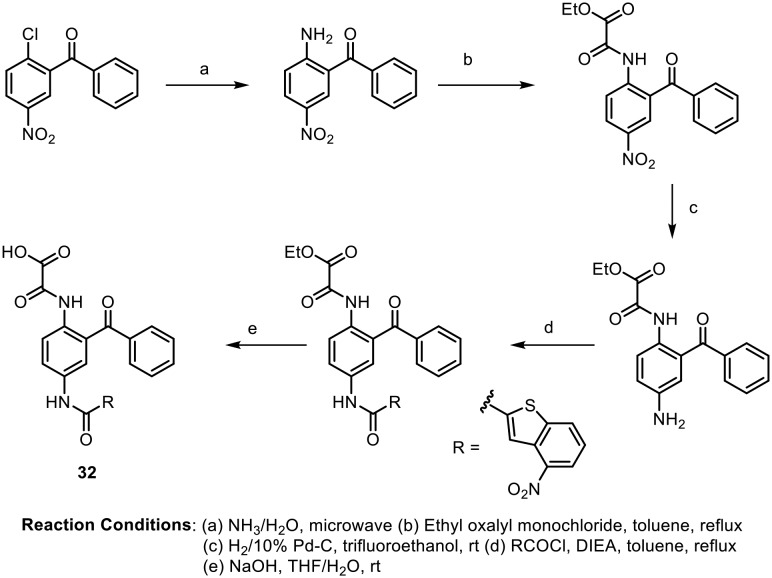
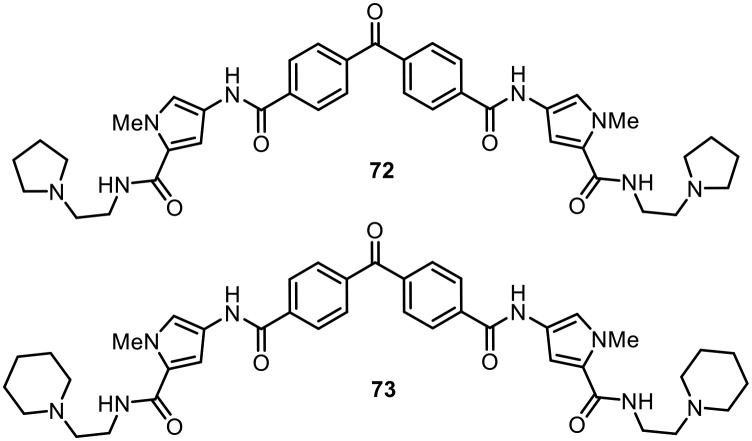
It is known that many antibiotics show activity due to disruption of critical biochemical processes involving DNA.106 Firestine and coworkers developed a unique set of benzophenones containing tetraamides which showed good DNA binding affinity, although mechanistic studies revealed that the antibacterial activity of these molecules is due to membrane depolarization of bacterial cells.107,108 The synthesized compounds were screened against a wide range of antibiotic-resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-resistant Staphylococcus aureus (VRSA) and vancomycin-resistant Enterococci (VRE). Most of the benzophenone tetraamides displayed good activities, with low MIC values. Two of the most potent molecules (72 and 73) are shown in Fig. 11.
Fig. 11. Benzophenone-containing tetraamides as potent antimicrobial agents.
Later, they reported the synthesis of these benzophenone-tetraamides through solution-phase parallel synthesis and evaluated their activities against various antibiotic-resistant bacteria.109 They synthesized about 218 compounds and found that 6 molecules displayed MIC values of 2.0 mg L–1 against Staphylococcus aureus. It was found that cyclic amines at the tails of the compounds were required to show good potency. Further, both unsymmetrical and symmetrical compounds displayed good activities, and the tail region is very sensitive to modifications.
Antihyperlipidemic activity
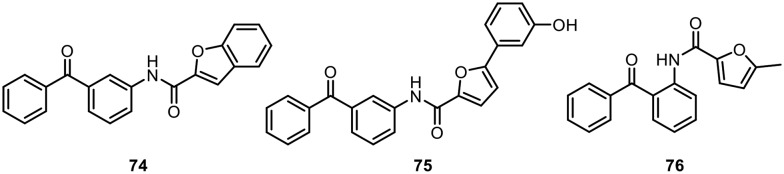
One of the major risk factors for cardiovascular disease is hyperlipidemia, which is an increase in the lipids in plasma. In 2012, Al-Qirim and co-workers synthesized hybrids of benzofuran and benzophenone molecules tethered with an amide group and studied their antihyperlipidemic activities in male Wistar rats.110 Hyperlipidemia in rats was induced using Triton WR-1339. The rat plasma lipid profiles of the molecules were checked, i.e., total cholesterol (TC), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) levels. Compound 74, shown in Fig. 12, showed promising results, with low TG levels and increased HDL-C levels. Further, in 2014, the same research group found that 2-aryl-substituted furan–benzophenone conjugates with carboxamide linkers showed similar antihyperlipidemic activities.111 Compound 75, which contains a meta-hydroxyphenyl group, was found to decrease the TC and TG levels in triton-induced rats by 88% and 84%, respectively, after 18 h at a dose of 15 mg kg–1. Recently, in 2017, they further studied the antihyperlipidemic activities of a few more furamide derivatives containing benzophenone.112 Compound 76 was found to be the most active. The observations of the authors suggested that the presence of a lipophilic moiety (benzophenone), a carboxamide linkage and a furan ring (which can form hydrogen bonds) are three important requirements for the activity of the synthesized molecules.
Fig. 12. Benzophenones with a furamide linkage as antihyperlipidemic agents.
Conclusions
The plethora of biological and pharmaceutical applications of naturally occurring benzophenones have prompted researchers to explore methodologies for the construction of this class of compounds and to evaluate their biological activities. In this review, benzophenone has been highlighted as a unique scaffold in medicinal chemistry. In particular, the review covers only synthetic derivatives of benzophenone (diphenyl ketone); no other diaryl ketones, heteroaryl–aryl ketones, heteroaryl–heteroaryl ketones or naturally occurring benzophenones have been discussed. Based on the substitution pattern on the aryl ring of benzophenone, the biological properties of these molecules vary. Although benzophenone showed potential in various biological activities, such as anti-inflammatory, anticancer, anti-HIV, etc., most work has been performed on the derivatization of hydroxyl groups on benzophenone. This may be due to mimicking of the natural analogues and multiple facets of the phenolic moiety. Very few examples have been reported with biological activity other than derivatives of hydroxyl-substituted benzophenone. It should be noted that various strategies have been reported for the synthesis and functionalization of benzophenones with diverse substitutions; however, their biological activity, which we believe can lead to various new drug candidates, is lacking. This review may stimulate researchers to use benzophenone scaffolds and further carry out diverse functionalizations to develop new structural motifs in drug design and medicinal chemistry.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The authors thank the Department of Science and Technology (DST) for their financial support through an INSPIRE faculty award [DST/INSPIRE/04/2016/000414]. The authors would also like to thank the Department of Pharmaceutical, Ministry of Chemicals and Fertilizers and NIPER-Ahmedabad for providing necessary support.
Biographies

Khemchand Surana
Khemchand Surana obtained his bachelor's degree (B. Pharm) at Savitribai Phule Pune University in 2016. He obtained his master's degree (M. S. Pharm) in medicinal chemistry in 2018 from NIPER-A. He has worked on the synthesis of novel anti-inflammatory agents via C–H acylation in the research group of Dr. Satyasheel Sharma.

Bharatkumar Chaudhary
Bharatkumar Chaudhary obtained his B. Pharm degree from Gujarat Technological University (GTU), India in 2012 and completed a master's degree in medicinal chemistry in 2014 at NIPER-A, India. Then, he joined Piramal Discovery Solution (PDS), Ahmedabad, India and worked there for about one year as a research associate. He began his PhD studies in 2015 at NIPER-A in the Medicinal Chemistry department and is currently working in the research group of Dr. Satyasheel Sharma. His research is focused on the synthesis of fluorinated molecules of medicinal importance via C–H bond activation using transition metal catalysts.

Monika Diwaker
Monika Diwaker obtained her Bachelor of Pharmacy degree at the Jai Narayan Vyas University, India in 2016. She received her master's in Medicinal Chemistry at NIPER-A in 2018 under the guidance of Dr. Satyasheel Sharma on the synthesis of novel medicinally active compounds by C–H activation. Currently, she is working as an R&D officer at Sahajanand Medical Technologies Pvt. Ltd., Surat, Gujarat.

Satyasheel Sharma
Satyasheel Sharma received his PhD in synthetic organic chemistry from the Department of Chemistry, University of Delhi, India in 2011 under the supervision of Prof. Mahendra Nath. After his doctoral studies, he performed postdoctoral research in South Korea under the supervision of Prof. In Su Kim at Sungkyunkawn University. He worked on transition metal-catalyzed C–H activation for the synthesis and functionalization of organic molecules of medicinal importance. He received the DST INSPIRE faculty award in 2016; then, he joined NIPER-A as an Assistant Professor (DST INSPIRE faculty) in 2017. Currently, his research focuses on the synthesis of fluorine-containing molecules of biological importance via a C–H activation approach and late stage functionalization of pharmaceutically important molecules.
References
- Wu S. B., Long C., Kennelly E. J. Nat. Prod. Rep. 2014;31:1158–1174. doi: 10.1039/c4np00027g. [DOI] [PubMed] [Google Scholar]
- Acuna U. M., Jancovski N., Kennelly E. J. Curr. Top. Med. Chem. 2009;9:1560–1580. doi: 10.2174/156802609789909830. [DOI] [PubMed] [Google Scholar]
- Zhang C., Ondeyka J. G., Herath K. B., Guan Z., Collado J., Platas G., Pelaez F., Leavitt P. S., Gurnett A., Nare B., Liberator P., Singh S. B. J. Nat. Prod. 2005;68:611–613. doi: 10.1021/np049591n. [DOI] [PubMed] [Google Scholar]
- Bernardi A. P., Ferraz A. B., Albring D. V., Bordignon S. A., Schripsema J., Bridi R., Dutra-Filho C. S., Henriques A. T., von Poser G. L. J. Nat. Prod. 2005;68:784–786. doi: 10.1021/np040149e. [DOI] [PubMed] [Google Scholar]
- Casu L., Solinas M. N., Saba A. R., Cottiglia F., Caboni P., Floris C., Laconi S., Pompei R., Leonti M. Phytochem. Lett. 2010;3:226–229. [Google Scholar]
- Bosca F., Miranda M. A., Carganico G., Mauleon D. Photochem. Photobiol. 1994;60:96–101. doi: 10.1111/j.1751-1097.1994.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Bosca F., Miranda M. A. J. Photochem. Photobiol., B. 1998;43:1–26. doi: 10.1016/s1011-1344(98)00062-1. [DOI] [PubMed] [Google Scholar]
- Cuquerella M. C., Lhiaubet-Vallet V., Cadet J., Miranda M. A. Acc. Chem. Res. 2012;45:1558–1570. doi: 10.1021/ar300054e. [DOI] [PubMed] [Google Scholar]
- Leegwater-Kim J., Waters C. Expert Rev. Neurother. 2007;7:1649–1657. doi: 10.1586/14737175.7.12.1649. [DOI] [PubMed] [Google Scholar]
- Rosenson R. S. Expert Rev. Cardiovasc. Ther. 2008;6:1319–1330. doi: 10.1586/14779072.6.10.1319. [DOI] [PubMed] [Google Scholar]
- Forbes, Jr. M. A., Brannen M., King W. C. South. Med. J. 1966;59:321–324. doi: 10.1097/00007611-196603000-00020. [DOI] [PubMed] [Google Scholar]
- Heurung A. R., Raju S. I., Warshaw E. M. Dermatitis. 2014;25:3–10. doi: 10.1097/DER.0000000000000025. [DOI] [PubMed] [Google Scholar]
- Gibson J. R. Cutis. 1993;51:406. [PubMed] [Google Scholar]
- Bernardo P. H., Chai C. L. J. Org. Chem. 2003;68:8906–8909. doi: 10.1021/jo035049c. [DOI] [PubMed] [Google Scholar]
- Nishimoto Y., Babu S. A., Yasuda M., Baba A. J. Org. Chem. 2008;73:9465–9468. doi: 10.1021/jo801914x. [DOI] [PubMed] [Google Scholar]
- Sartori G., Maggi R. Chem. Rev. 2011;111:PR181–PR214. doi: 10.1021/cr100375z. [DOI] [PubMed] [Google Scholar]
- Sartori G., Maggi R. Chem. Rev. 2006;106:1077–1104. doi: 10.1021/cr040695c. [DOI] [PubMed] [Google Scholar]
- Bjerglund K. M., Skrydstrup T., Molander G. A. Org. Lett. 2014;16:1888–1891. doi: 10.1021/ol5003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.-F., Neumann H., Beller M. Chem. Soc. Rev. 2011;40:4986–5009. doi: 10.1039/c1cs15109f. [DOI] [PubMed] [Google Scholar]
- Wu X.-F. RSC Adv. 2016;6:83831–83837. [Google Scholar]
- Gadge S. T., Bhanage B. M. RSC Adv. 2014;4:10367–10389. [Google Scholar]
- Friis S. D., Lindhardt A. T., Skrydstrup T. Acc. Chem. Res. 2016;49:594–605. doi: 10.1021/acs.accounts.5b00471. [DOI] [PubMed] [Google Scholar]
- Huang Y.-C., Majumdar K. K., Cheng C. H. J. Org. Chem. 2002;67:1682–1684. doi: 10.1021/jo010289i. [DOI] [PubMed] [Google Scholar]
- Wakaki T., Togo T., Yoshidome D., Kuninobu Y., Kanai M. ACS Catal. 2018;8:3123–3128. [Google Scholar]
- Rao M. L. N., Ramakrishna B. S. Eur. J. Org. Chem. 2017;2017:5080–5093. [Google Scholar]
- Ko S., Kang B., Chang S. Angew. Chem., Int. Ed. 2005;44:455–457. doi: 10.1002/anie.200462006. [DOI] [PubMed] [Google Scholar]
- Tetsuya S., Tomoaki I., Masahiro M., Masakatsu N. Chem. Lett. 1996;25:823–824. [Google Scholar]
- Karthikeyan J., Parthasarathy K., Cheng C. H. Chem. Commun. 2011;47:10461–10463. doi: 10.1039/c1cc13771a. [DOI] [PubMed] [Google Scholar]
- Pucheault M., Darses S., Genet J. P. J. Am. Chem. Soc. 2004;126:15356–15357. doi: 10.1021/ja044749b. [DOI] [PubMed] [Google Scholar]
- Qin C., Chen J., Wu H., Cheng J., Zhang Q., Zuo B., Su W., Ding J. Tetrahedron Lett. 2008;49:1884–1888. [Google Scholar]
- Xiao-Feng W. Chem. – Eur. J. 2015;21:12252–12265. doi: 10.1002/chem.201501548. [DOI] [PubMed] [Google Scholar]
- Sharma S., Mishra N. K., Shin Y., Kim I. S. Curr. Org. Chem. 2016;20:471–511. [Google Scholar]
- Medzhitov R. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Smith W. L. Biochem. J. 1989;259:315. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K., Hohjoh H., Inazumi T., Tsuchiya S., Sugimoto Y. Biochim. Biophys. Acta. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Park J. Y., Pillinger M. H., Abramson S. B. Clin. Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Pugh S., Thomas G. A. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consalvi S., Biava M., Poce G. Expert Opin. Ther. Pat. 2015;25:1357–1371. doi: 10.1517/13543776.2015.1090973. [DOI] [PubMed] [Google Scholar]
- Ottosen E. R., Sørensen M. D., Björkling F., Skak-Nielsen T., Fjording M. S., Aaes H., Binderup Lise. J. Med. Chem. 2003;46:5651–5662. doi: 10.1021/jm030851s. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Curr. Opin. Immunol. 1991;3:941–948. doi: 10.1016/s0952-7915(05)80018-4. [DOI] [PubMed] [Google Scholar]
- Khanum S. A., Shashikanth S., Deepak A. V. Bioorg. Chem. 2004;32:211–222. doi: 10.1016/j.bioorg.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Whiteley P. E., Dalrymple S. A. Curr. Protoc. Pharmacol. 1998:5.4.1–5.4.3. [Google Scholar]
- Sin Y. M., Sedgwick A. D., Chea E. P., Willoughby D. A. Ann. Rheum. Dis. 1986;45:873–877. doi: 10.1136/ard.45.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz L., Blum E., Di Padova F. E., Buhl T., Feifel R., Gram H., Hiestand P., Manning U., Rucklin G. Bioorg. Med. Chem. Lett. 2004;14:3601–3605. doi: 10.1016/j.bmcl.2004.03.111. [DOI] [PubMed] [Google Scholar]
- Venu T. D., Shashikanth S., Khanum S. A., Naveen S., Firdouse A., Sridhar M. A., Shashidhara Prasad J. Bioorg. Med. Chem. 2007;15:3505–3514. doi: 10.1016/j.bmc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- Kwon E.-M. K., Gi C., Goh A.-R., Park J.-S., Jun J.-G. Bull. Korean Chem. Soc. 2012;33:1939–1944. [Google Scholar]
- Khanum S. A., Girish V., Suparshwa S. S., Khanum N. F. Bioorg. Med. Chem. Lett. 2009;19:1887–1891. doi: 10.1016/j.bmcl.2009.02.070. [DOI] [PubMed] [Google Scholar]
- Khanum S. A., Begum B. A., Girish V., Khanum N. F. Int. J. Biomed. Sci. 2010;6:60–65. [PMC free article] [PubMed] [Google Scholar]
- Bandgar B. P., Patil S. A., Totre J. V., Korbad B. L., Gacche R. N., Hote B. S., Jalde S. S., Chavan H. V. Bioorg. Med. Chem. Lett. 2010;20:2292–2296. doi: 10.1016/j.bmcl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Bandgar B., Hote B., Gangwal R., Sangamwar A. Med. Chem. Res. 2012;21:3177–3181. [Google Scholar]
- Bandgar B. P., Chavan H. V., Adsul L. K., Thakare V. N., Shringare S. N., Shaikh R., Gacche R. N. Bioorg. Med. Chem. Lett. 2013;23:912–916. doi: 10.1016/j.bmcl.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Heo J., Shin H., Lee J., Kim T., Inn K. S., Kim N. J. Bioorg. Med. Chem. Lett. 2015;25:3694–3698. doi: 10.1016/j.bmcl.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Prabhakar B. T., Khanum S. A., Jayashree K., Salimath B. P., Shashikanth S. Bioorg. Med. Chem. 2006;14:435–446. doi: 10.1016/j.bmc.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Lu K. P., Hanes S. D., Hunter T. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- Bao L., Kimzey A., Sauter G., Sowadski J. M., Lu K. P., Wang D. G. Am. J. Pathol. 2004;164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala G., Wang D., Wulf G., Frolov A., Li R., Sowadski J., Wheeler T. M., Lu K. P., Bao L. Cancer Res. 2003;63:6244–6251. [PubMed] [Google Scholar]
- Liu C., Jin J., Chen L., Zhou J., Chen X., Fu D., Song H., Xu B. Bioorg. Med. Chem. 2012;20:2992–2999. doi: 10.1016/j.bmc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Nicholson B., Lloyd G. K., Miller B. R., Palladino M. A., Kiso Y., Hayashi Y., Neuteboom S. T. Anti-Cancer Drugs. 2006;17:25–31. doi: 10.1097/01.cad.0000182745.01612.8a. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Tanaka K., Nicholson B., Deyanat-Yazdi G., Potts B., Yoshida T., Oda A., Kitagawa T., Orikasa S., Kiso Y., Yasui H., Akamatsu M., Chinen T., Usui T., Shinozaki Y., Yakushiji F., Miller B. R., Neuteboom S., Palladino M., Kanoh K., Lloyd G. K., Hayashi Y. J. Med. Chem. 2012;55:1056–1071. doi: 10.1021/jm2009088. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Sumikura M., Masuda Y., Hayashi Y., Yasui H., Kiso Y., Chinen T., Usui T., Yakushiji F., Potts B., Neuteboom S., Palladino M., Lloyd G. K. Bioorg. Med. Chem. 2012;20:4279–4289. doi: 10.1016/j.bmc.2012.05.059. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Takeno H., Chinen T., Muguruma K., Okuyama K., Taguchi A., Takayama K., Yakushiji F., Miura M., Usui T. ACS Med. Chem. Lett. 2014;5:1094–1098. doi: 10.1021/ml5001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinaya K., Kavitha C. V., Prasanna D. S., Chandrappa S., Ranganatha S. R., Raghavan S. C., Rangappa K. S. Chem. Biol. Drug Des. 2012;79:360–367. doi: 10.1111/j.1747-0285.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- Ranganatha V. L., Vijay Avin B. R., Thirusangu P., Prashanth T., Prabhakar B. T., Khanum S. A. Life Sci. 2013;93:904–911. doi: 10.1016/j.lfs.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Banks R. E., Forbes M. A., Kinsey S. E., Stanley A., Ingham E., Walters C., Selby P. J. Br. J. Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perperopoulou F. D., Tsoungas P. G., Thireou T. N., Rinotas V. E., Douni E. K., Eliopoulos E. E., Labrou N. E., Clonis Y. D. Bioorg. Med. Chem. 2014;22:3957–3970. doi: 10.1016/j.bmc.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Zabiulla, Shamanth Neralagundi H. G., Bushra Begum A., Prabhakar B. T., Khanum S. A. Eur. J. Med. Chem. 2016;115:342–351. doi: 10.1016/j.ejmech.2016.03.040. [DOI] [PubMed] [Google Scholar]
- Shi J. B., Chen L. Z., Wang Y., Xiou C., Tang W. J., Zhou H. P., Liu X. H., Yao Q. Z. Eur. J. Med. Chem. 2016;124:729–739. doi: 10.1016/j.ejmech.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Al-Ghorbani M., Thirusangu P., Gurupadaswamy H. D., Girish V., Shamanth Neralagundi H. G., Prabhakar B. T., Khanum S. A. Bioorg. Chem. 2016;65:73–81. doi: 10.1016/j.bioorg.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Al-Ghorbani M., Thirusangu P., Gurupadaswamy H. D., Vigneshwaran V., Mohammed Y. H., Prabhakar B. T., Khanum S. A. Bioorg. Chem. 2017;71:55–66. doi: 10.1016/j.bioorg.2017.01.011. [DOI] [PubMed] [Google Scholar]
- de Bethune M. P. Antiviral Res. 2010;85:75–90. doi: 10.1016/j.antiviral.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Usach I., Melis V., Peris J. E. J. Int. AIDS Soc. 2013;16:1–14. doi: 10.7448/IAS.16.1.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt P. G., Bethell R. C., Cammack N., Charon D., Dodic N., Dumaitre B., Evans D. N., Green D. V., Hopewell P. L., Humber D. C. J. Med. Chem. 1995;38:1657–1665. doi: 10.1021/jm00010a010. [DOI] [PubMed] [Google Scholar]
- Chan J. H., Freeman G. A., Tidwell J. H., Romines K. R., Schaller L. T., Cowan J. R., Gonzales S. S., Lowell G. S., Andrews, 3rd C. W., Reynolds D. J., Clair M. St, Hazen R. J., Ferris R. G., Creech K. L., Roberts G. B., Short S. A., Weaver K., Koszalka G. W., Boone L. R. J. Med. Chem. 2004;47:1175–1182. doi: 10.1021/jm030255y. [DOI] [PubMed] [Google Scholar]
- Romines K. R., Freeman G. A., Schaller L. T., Cowan J. R., Gonzales S. S., Tidwell J. H., Andrews, 3rd C. W., Stammers D. K., Hazen R. J., Ferris R. G., Short S. A., Chan J. H., Boone L. R. J. Med. Chem. 2006;49:727–739. doi: 10.1021/jm050670l. [DOI] [PubMed] [Google Scholar]
- Ma X. D., Zhang X., Dai H. F., Yang S. Q., Yang L. M., Gu S. X., Zheng Y. T., He Q. Q., Chen F. E. Bioorg. Med. Chem. 2011;19:4601–4607. doi: 10.1016/j.bmc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Novikov M. S., Ivanova O. N., Ivanov A. V., Ozerov A. A., Valuev-Elliston V. T., Temburnikar K., Gurskaya G. V., Kochetkov S. N., Pannecouque C., Balzarini J., Seley-Radtke K. L. Bioorg. Med. Chem. 2011;19:5794–5802. doi: 10.1016/j.bmc.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Wang P., Huang S., Wang C., Wang R.-R., Yang L.-M., Zhen Y., Liu K., Zheng Y.-T., Ma X. Med. Chem. 2017;13:398–405. [Google Scholar]
- Colovic M. B., Krstic D. Z., Lazarevic-Pasti T. D., Bondzic A. M., Vasic V. M. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluti F., Piazzi L., Bisi A., Gobbi S., Bartolini M., Cavalli A., Valenti P., Rampa A. Eur. J. Med. Chem. 2009;44:1341–1348. doi: 10.1016/j.ejmech.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Belluti F., Bartolini M., Bottegoni G., Bisi A., Cavalli A., Andrisano V., Rampa A. Eur. J. Med. Chem. 2011;46:1682–1693. doi: 10.1016/j.ejmech.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Belluti F., De Simone A., Tarozzi A., Bartolini M., Djemil A., Bisi A., Gobbi S., Montanari S., Cavalli A., Andrisano V., Bottegoni G., Rampa A. Eur. J. Med. Chem. 2014;78:157–166. doi: 10.1016/j.ejmech.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Zulfiqar B., Shelper T. B., Avery V. M. Drug Discovery Today. 2017;22:1516–1531. doi: 10.1016/j.drudis.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Ghorbani M., Farhoudi R. Drug Des., Dev. Ther. 2018;12:25–40. doi: 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva E. M., Vannier-Santos M. A., Silva-Filho F. C., de Souza W. J. Cell Sci. 1989;93:481–489. doi: 10.1242/jcs.93.3.481. [DOI] [PubMed] [Google Scholar]
- Luque-Ortega J. R., Reuther P., Rivas L., Dardonville C. J. Med. Chem. 2010;53:1788–1798. doi: 10.1021/jm901677h. [DOI] [PubMed] [Google Scholar]
- Pereira I. O., Marques M. J., Pavan A. L., Codonho B. S., Barbieri C. L., Beijo L. A., Doriguetto A. C., D'Martin E. C., dos Santos M. H. Phytomedicine. 2010;17:339–345. doi: 10.1016/j.phymed.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Maciel-Rezende C. M., de Almeida L., Costa E. D., Pires F. R., Alves K. F., Viegas Jr. C., Dias D. F., Doriguetto A. C., Marques M. J., dos Santos M. H. Bioorg. Med. Chem. 2013;21:3114–3119. doi: 10.1016/j.bmc.2013.03.045. [DOI] [PubMed] [Google Scholar]
- de Almeida L., Alves K. F., Maciel-Rezende C. M., Jesus Lde O., Pires F. R., Junior C. V., Izidoro M. A., Judice W. A., dos Santos M. H., Marques M. J. Biomed. Pharmacother. 2015;75:93–99. doi: 10.1016/j.biopha.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Arshia, Ahad F., Ghouri N., Kanwal, Khan K. M., Perveen S., Choudhary M. I. R. Soc. Open Sci. 2018;5:171771. doi: 10.1098/rsos.171771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner T. B., Hahn S. M., Gupta A. K., Muschel R. J., McKenna W. G., Bernhard E. Cancer Res. 2003;63:5656–5668. [PubMed] [Google Scholar]
- Chakrabarti D., Azam T., DelVecchio C., Qiu L., Park Y., Allen C. M. Mol. Biochem. Parasitol. 1998;94:175–184. doi: 10.1016/s0166-6851(98)00065-6. [DOI] [PubMed] [Google Scholar]
- Chakrabarti D., Da Silva T., Barger J., Paquette S., Patel H., Patterson S., Allen C. M. J. Biol. Chem. 2002;277:42066–42073. doi: 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Trobridge P., Buckner F. S., Van Voorhis W. C., Stuart K. D., Gelb M. H. J. Biol. Chem. 1998;273:26497–26505. doi: 10.1074/jbc.273.41.26497. [DOI] [PubMed] [Google Scholar]
- Buckner F. S., Yokoyama K., Nguyen L., Grewal A., Erdjument-Bromage H., Tempst P., Strickland C. L., Xiao L., Van Voorhis W. C., Gelb M. H. J. Biol. Chem. 2000;275:21870–21876. doi: 10.1074/jbc.M000975200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Trobridge P., Buckner F. S., Scholten J., Stuart K. D., Van Voorhis W. C., Gelb M. H. Mol. Biochem. Parasitol. 1998;94:87–97. doi: 10.1016/s0166-6851(98)00053-x. [DOI] [PubMed] [Google Scholar]
- Buckner F. S., Eastman R. T., Nepumuceno-Silva J. L., Speelmon E. C., Myler P. J., Van Voorhis W. C., Yokoyama K. Mol. Biochem. Parasitol. 2002;122:181–188. doi: 10.1016/s0166-6851(02)00099-3. [DOI] [PubMed] [Google Scholar]
- Ibrahim M., Azzouz N., Gerold P., Schwarz R. T. Int. J. Parasitol. 2001;31:1489–1497. doi: 10.1016/s0020-7519(01)00268-5. [DOI] [PubMed] [Google Scholar]
- Kumagai M., Makioka A., Takeuchi T., Nozaki T. J. Biol. Chem. 2003;279:2316–2323. doi: 10.1074/jbc.M311478200. [DOI] [PubMed] [Google Scholar]
- Cox A. D., Der C. J. Curr. Opin. Pharmacol. 2002;2:388–393. doi: 10.1016/s1471-4892(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Schlitzer M. Curr. Pharm. Des. 2002;8:1713–1722. doi: 10.2174/1381612023394061. [DOI] [PubMed] [Google Scholar]
- Jochen W., Kettler K., Sakowski J., Ortmann R., Katzin A. M., Kimura E. A., Silber K., Klebe G., Jomaa H., Schlitzer M. Angew. Chem., Int. Ed. 2004;43:251–254. doi: 10.1002/anie.200351169. [DOI] [PubMed] [Google Scholar]
- Kohring K., Wiesner J., Altenkamper M., Sakowski J., Silber K., Hillebrecht A., Haebel P., Dahse H. M., Ortmann R., Jomaa H., Klebe G., Schlitzer M. ChemMedChem. 2008;3:1217–1231. doi: 10.1002/cmdc.200800043. [DOI] [PubMed] [Google Scholar]
- Esteva M. I., Kettler K., Maidana C., Fichera L., Ruiz A. M., Bontempi E. J., Andersson B., Dahse H. M., Haebel P., Ortmann R., Klebe G., Schlitzer M. J. Med. Chem. 2005;48:7186–7191. doi: 10.1021/jm050456x. [DOI] [PubMed] [Google Scholar]
- Altenkämper M., Bechem B., Perruchon J., Heinrich S., Mädel A., Ortmann R., Dahse H. M., Freunscht E., Wang Y., Rath J., Stich A., Hitzler M., Chiba P., Lanzer M., Schlitzer M. Bioorg. Med. Chem. 2009;17:7690–7697. doi: 10.1016/j.bmc.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Heinrich S., Altenkämper M., Bechem B., Perruchon J., Ortmann R., Dahse H. M., Wang Y., Lanzer M., Schlitzer M. Eur. J. Med. Chem. 2011;46:1331–1342. doi: 10.1016/j.ejmech.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Hu W., Burli R. W., Kaizerman J. A., Johnson K. W., Gross M. I., Iwamoto M., Jones P., Lofland D., Difuntorum S., Chen H., Bozdogan B., Appelbaum P. C., Moser H. E. J. Med. Chem. 2004;47:4352–4355. doi: 10.1021/jm049712g. [DOI] [PubMed] [Google Scholar]
- Vooturi S. K., Cheung C. M., Rybak M. J., Firestine S. M. J. Med. Chem. 2009;52:5020–5031. doi: 10.1021/jm900519b. [DOI] [PubMed] [Google Scholar]
- Vooturi S. K., Dewal M. B., Firestine S. M. Org. Biomol. Chem. 2011;9:6367–6372. doi: 10.1039/c1ob05643c. [DOI] [PubMed] [Google Scholar]
- Vooturi S. K., Firestine S. M. J. Comb. Chem. 2010;12:151–160. doi: 10.1021/cc900138h. [DOI] [PubMed] [Google Scholar]
- Al-Qirim T., Shattat G., Sweidan K., El-Huneidi W., Sheikha G. A., Khalaf R. A., Hikmat S. Arch. Pharm. Chem. Life Sci. 2012;345:401–406. doi: 10.1002/ardp.201100225. [DOI] [PubMed] [Google Scholar]
- Al-Qirim T., Shattat G., Sheikha G. A., Sweidan K., Al-Hiari Y., Jarab A. Drug Res. 2015;65:158–163. doi: 10.1055/s-0034-1375646. [DOI] [PubMed] [Google Scholar]
- Hikmat S., Al-Qirim T., Alkabbani D., Shattat G., Sheikha G. A., Sabbah D., Khalaf R. A., Al-Hiari Y. Trop. J. Pharm. Res. 2017;16:193–201. [Google Scholar]