Abstract
Background
Three recent reviews evaluated 19 studies testing the hemoglobin A1c (HbA1c) benefit of 16 diabetes apps, including 5 publicly available apps. Most studies relied on small samples and did not link app engagement with outcomes.
Objective
This study assessed both HbA1c change in a large sample of people using the One Drop | Mobile app and associations between app engagement and changes in HbA1c.
Methods
The One Drop | Mobile app for iOS and Android is designed to manually and passively (via Apple HealthKit, Google Fit, and the One Drop | Chrome blood glucose meter) store, track, and share data. Users can schedule medication reminders, view statistics, set goals, track health outcomes, and get data-driven insights. In June 2017, we queried data on people with diabetes using the app who had entered at least 2 HbA1c values in the app >60 and ≤365 days apart. Multiple imputation corrected for missing data. Unadjusted and adjusted mixed effects repeated measures models tested mean HbA1c change by time, diabetes type, and their interaction. Multiple regression models assessed relationships between using the app to track food, activity, blood glucose, and medications and HbA1c change.
Results
The sample (N=1288) included people with type 1 diabetes (T1D) (n=367) or type 2 diabetes (T2D) (n=921) who were 35% female, diagnosed with diabetes for a mean 9.4 (SD 9.9) years, and tracked an average 1646.1 (SD 3621.9) self-care activities in One Drop | Mobile between their first (mean 8.14% [SD 2.06%]) and second HbA1c entry (mean 6.98% [SD 1.1%]). HbA1c values were significantly associated with user-entered average blood glucose 90 days before the second HbA1c entry (rho=.73 to .75, P<.001). HbA1c decreased by an absolute 1.07% (unadjusted and adjusted F=292.03, P<.001) from first to second HbA1c entry. There was a significant interaction between diabetes type and HbA1c. Both groups significantly improved, but users with T2D had a greater HbA1c decrease over time than users with T1D (F=10.54, P<.001). For users with T2D (n=921), HbA1c decreased by an absolute 1.27% (F=364.50, P<.001) from first to second HbA1c entry. Finally, using One Drop | Mobile to record food was associated with greater HbA1c reductions even after adjusting for covariates and after also adjusting for insulin use for users with T2D (all P<.05).
Conclusions
People with T1D and T2D reported a 1.07% to 1.27% absolute reduction in HbA1c during a median 4 months of using the One Drop | Mobile app. Using the app to track self-care was associated with improved HbA1c. More research is needed on the health benefits of publicly available diabetes apps, particularly studies associating app engagement with short- and long-term effects.
Keywords: type 1 diabetes, type 2 diabetes, mobile app, tracking, self-care, glycemic control
Introduction
There are over 1500 mobile apps in the marketplace assisting with diabetes self-management but limited research on their clinical benefit. In the past year, a handful of systematic reviews and meta-analyses evaluated the impact of diabetes apps on glycemic control or hemoglobin A1c (HbA1c) [1-4]. Three reviews included a total of 19 studies evaluating 16 unique apps. Only 5 of those apps were publicly available (ie, dBees [5], Diabeo [6], Glucose Buddy [7], mDiab/Mobil Diab [8,9], and WellDoc [10,11]).
The trials evaluating publicly available apps offer insights into their clinical value. For example, people with type 1 diabetes (T1D) using the dBees self-care and glucose tracking app had no HbA1c improvement over time or compared to people tracking with a paper logbook [5]. Children and adolescents with T1D using mDiab/Mobil Diab [8] lowered their HbA1c, but not significantly more than a conventional care control group. In contrast, people with type 2 diabetes (T2D) using mDiab/Mobil Diab lowered their HbA1c significantly more than the usual care control group [9]. In 2 separate randomized controlled trials (RCTs), people with T1D using the Diabeo insulin dosing app [6] or the Glucose Buddy tracking app [7] lowered their HbA1c significantly more than controls did. Finally, people with T2D using the WellDoc tracking and coaching app substantially lowered their HbA1c relative to controls [10,11].
Limited clinical evidence supporting publicly available diabetes apps is promising, but there are still many unknowns. In the 7 trials reporting data, no studied sample was greater than 200 people, which has implications for generalizability. Moreover, effects on glycemic control were linked to being exposed to an entire intervention or app and not using the app or different aspects of it.
Qualitative studies indicate people with diabetes (PWD) want apps with automated self-care tracking [12], medication reminders [13,14], data sharing with peers and providers [15] including reports [16], and a Bluetooth-connected meter [17]. Publicly available apps offer these and other features (eg, One Drop | Mobile), but studies linking engagement with such features to health outcomes are limited.
Additional studies are needed to broaden generalizability by testing with larger samples and associating app engagement to health outcomes. To address these gaps, we assessed HbA1c changes among a large sample of people with T1D and T2D (N=1288) using the One Drop | Mobile app. We also assessed if using the app resulted in significant changes in glycemic control as measured by HbA1c values.
Methods
One Drop | Mobile
The One Drop | Mobile app was launched in April 2015. It is available for free on iOS, WatchOS, and Android operating systems.
One Drop | Mobile has a variety of features to support diabetes management (see Figure 1). Users can manually and passively (via HealthKit, Google Fit, and the Bluetooth-enabled One Drop | Chrome blood glucose meter) store and track blood glucose readings, medication doses, physical activity, and foods consumed. In addition, users can view daily, weekly, and monthly summary statistics regarding these data. A built-in food library facilitates tracking food. An optional medication scheduler reminds users when a dose is due and facilitates tracking medications. Users can also view the percentage of in-range blood glucose readings over time and store and track HbA1c values and body weight. Importantly, they can set daily goals (for time in range, medication adherence, carbohydrate intake, and physical activity) and monitor their progress toward these goals. Users can also access a wide array of diabetes-relevant information by using an in-app newsfeed that delivers health tips, articles, infographics, user polls, expert interviews, and scientific study results. A community section lets the user view and learn from other users’ data. A map displays dots representing other One Drop | Mobile users in a local area, anywhere, and provides an option to view another user’s data and give badges to offer support and encouragement. A notifications inbox delivers data-driven insights, achievements, reminders, and lists badges accumulated from other users.
Figure 1.
The One Drop | Mobile app.
Measures
User Characteristics
All One Drop | Mobile users complete a profile and can self-report gender, diabetes type, and year of diagnosis. We calculated years of diagnosed diabetes as the difference between a user’s year of diagnosis entered in the app and the year his or her One Drop | Mobile profile was created. We used passively collected time zone data to determine user location. Because few users outside the United States had entered 2 HbA1c values required for inclusion, we dichotomized location to United States versus outside the United States in analyses.
Self-Care
One Drop | Mobile users can track blood glucose, medication, and physical activity manually and passively (via HealthKit, Google Fit, and the Bluetooth-enabled One Drop | Chrome blood glucose meter) in the app. They can also track their food consumed (measured in grams of carbohydrate). We summed data tracked between HbA1c entries to obtain counts of blood glucose, medications, activity, and food tracked during that time.
Glycemic Control
Users can also self-report HbA1c test results and test dates. HbA1c values can be displayed in mmol/mol or percent but are stored as percent. Shortly after a One Drop | Mobile account is created, an in-app pop-up asks each user to enter his or her HbA1c information. This reminder appears again 3 months after the previously entered HbA1c test date. We used HbA1c test dates to calculate the number of days between HbA1c entries and converted days to months using the factor 30.42 (365 days/12 months.
Study Oversight
Solutions Institutional Review Board approved analyses and reporting of One Drop | Mobile’s data for research purposes.
Analyses
Summary statistics characterized the sample overall and stratified by diabetes type. Distributions of continuous variables were asymmetrical, so Mann Whitney U tests compared mean ranks of continuous user characteristics, app-tracked data, and HbA1c percent. Chi-square tests assessed differences in dichotomous variables by diabetes type. To examine and exclude invalid self-reported blood glucose and HbA1c data, we converted each user’s 90-day average blood glucose to an estimated HbA1c using the formula HbA1c=(90-day mean blood glucose+77.3)/35.6 [18]. We calculated the difference between the converted HbA1c and self-reported HbA1c and excluded users with more than a 2.0% difference. For the remaining users, Spearman’s rho correlations tested the relationship between self-reported HbA1c values and the prior 90-day average blood glucose to ensure consistency with the literature [19]. Because most users enter their first HbA1c when they initiate using the app, we were unable to assess the relationship between 90-day average blood glucose prior to the first self-reported HbA1c.
Missing data were handled using multiple imputation [20]. We used predictive mean matching (PMM) [21,22] to impute 100 datasets. PMM is a multiple-imputation method robust to violations of distributional assumptions (eg, normality) [23,24]. Multiple imputation was carried out in SPSS 23.0 (IBM Corp).
Next, 3 mixed effects repeated measures models tested mean HbA1c change by time (pre- to posttest), diabetes type (T1D vs T2D), and their interaction. Only these effects were in the first model (ie, the unadjusted model). The second model adjusted for a priori covariates: gender, location (US vs non-US), years since a diagnosis of diabetes, and the number of months between the first and second HbA1c entries. We restricted the third model to users with T2D, excluded the time by diabetes type interaction term, and adjusted for gender, location, years since diagnosis, number of months between HbA1c entries, and insulin use.
Finally, 4 multiple regression models assessed the relationships between change in HbA1c and using the app to track blood glucose, activity, medications, and food. The first, unadjusted model assessed the relationships between HbA1c change and the 4 types of self-care tracking. The second model included diabetes type (T1D vs T2D), and the third model included gender, location, years since diagnosis, and number of months between the first and second HbA1c entries. We restricted the fourth model to users with T2D and included insulin use as well as the a priori covariates. Given the skewness of self-care data and assumption violations for statistical testing, we dichotomized each variable to indicated tracking or nontracking of blood glucose, medications, activity, and food.
Results
As of June 6, 2017, 2365 One Drop | Mobile users had entered 2 HbA1c values into the app at least 60 days but no more than 1 year apart. They reported a diagnosis of T2D (1526/2365, 64.5%), T1D (591/2365, 25%), prediabetes (122/2365, 5.2%), latent autoimmune diabetes in adults (LADA) (72/2365, 3.0%), gestational diabetes (9/2365, 0.4%), other types of diabetes (eg, surgically or chemically induced diabetes; 29/2365, 1.2%), or did not enter a diabetes type (16/2365, 0.7%).
We restricted analyses to users reporting a diagnosis of T1D or T2D and confirmed the diagnosis through examination of the names of diabetes medications logged or scheduled in One Drop | Mobile. A total of 408 T1D or T2D users were excluded from the sample because they had either no medication data or because the medications logged or scheduled were inconsistent with their stated diabetes type (eg, T1D on metformin or sulfonylurea, T2D setting an auto basal insulin).
We excluded an additional 288 users with >2.0% HbA1c difference between their second self-reported HbA1c and the HbA1c calculated from their 90-day mean blood glucose. This criterion resulted in correlations of rho=.75 and rho=.73 between the 90-day mean blood glucose and second self-reported HbA1c for subjects with T1D (n=367) and T2D (n=921), respectively (both P<.001). This is consistent with previous cohort studies reporting correlations between average blood glucose and HbA1c varying from 0.71 to 0.86 [19].
Three of the up to 14 variables included in analyses had missing data: gender (242/1288, 18.8%), location (14/1288, 1.1%), and duration of diagnosed diabetes (325/1288, 25.5%). Multiple imputation was used to make corrections for missing data on these variables.
Analyses included N=1288 users (see Table 1) who were 35% (454/1288) female, diagnosed with diabetes for a mean 9.4 (SD 9.9) years, and tracked an average 1646.1 (SD 3621.9) self-care activities in One Drop | Mobile between their first (mean 8.14% [SD 2.06%]) and second (mean 6.98% [SD 1.1%]) HbA1c (calculations prior to multiple imputation).
Table 1.
Sample characteristics with tests of difference by diabetes type.
| Characteristics | Total N=1288 |
Type 1 diabetes n = 367 |
Type 2 diabetes n=921 |
P value | |
| Gender, n (%) | |||||
| Male | 592 (46.0) | 154 (42.0) | 438 (47.6) | .61 | |
| Female | 454 (35.2) | 152 (41.4) | 302 (32.8) | ||
| Other | 2 (0.2) | 1 (0.3) | 1 (0.1) | ||
| Location, n (%) | |||||
| America/United States | 1077 (83.6) | 292 (80.7) | 785 (86.1) | .001 | |
| Europe | 111 (8.6) | 51 (14.1) | 60 (6.6) | ||
| Asia | 44 (3.4) | 7 (1.9) | 37 (4.1) | ||
| Pacific | 16 (1.2) | 4 (1.1) | 1.3 (14) | ||
| Australia | 19 (1.5) | 5 (1.4) | 1.5 (3) | ||
| Africa | 6 (0.5) | 3 (0.8) | 3 (0.3) | ||
| Atlantic | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||
| Insulin, n (%) | |||||
| Yes | 717 (55.7) | 367 (100) | 350 (38) | .001 | |
| Diabetes duration in years, median (IQR) | 6 (15) | 10 (19) | 5 (12) | .001 | |
| Food entries, n (%) | 4 (88) | 10 (99) | 3 (82) | .04 | |
| Activity entries, n (%) | 271.5 (809) | 182 (786) | 294 (814) | .09 | |
| Blood glucose entries, n (%) | 72 (200) | 102 (356) | 67 (165) | .001 | |
| Medication entries, n (%) | 118.5 (366) | 121 (609) | 117 (331) | .02 | |
| Months between HbA1c entries, median (IQR) | 4.0 (3.1) | 4.6 (1.5) | 3.9 (2.7) | .001 | |
| First HbA1c %, median (IQR) | 7.6 (2.4) | 7.65 (2.1) | 7.6 (2.5) | .43 | |
| Second HbA1c %, median (IQR) | 6.9 (1.4) | 7.30 (1.5) | 6.7 (1.3) | .001 | |
Table 1 presents preimputed median and interquartile range (IQR) or n (%) with P values for differences between diabetes type on app-entered user characteristics, app-tracked data, and HbA1c entries. Chi-square tests compared dichotomous variables. Mann Whitney U tests compared mean ranks of continuous variables in Table 1.
Compared to users with T2D (367/1288), users with T1D (921/1288) were diagnosed with diabetes for more years (U958= 71,571, z=–7.07, P<.001), had more months between their first and second HbA1c (for both U1286=140,143.5, z=–4.79, P<.001), and tracked more food (U1286=156,703.5, z=–2.09, P=.04), blood glucose (U1286=147,630, z=–3.56, P<.001), and medications (U1286=155,500, z=–2.26, P=.02). They were also more likely than users with T2D to log or schedule insulin in the app (χ21,N=1288=408.7, P<.001), use the app in Europe (χ21,N=1274=24.1, P<.001), and report a higher second HbA1c (U1286=125,966.5, z=–7.14, P<.001).
In the unadjusted model (Multimedia Appendix 1), HbA1c decreased by an absolute 1.07% (F=292.03, P<.001) in the median 4.0 (IQR 3.1) months from first (mean HbA1c 8.15%) to second entry (mean 7.08%). Users with T1D (mean 7.74%) had an absolute .25% (F=9.52, P=.002) higher HbA1c than users with T2D (mean 7.49%). There was a significant interaction between diabetes type and HbA1c entry (Figure 2). Both groups improved over time, but users with T2D had a greater HbA1c decrease over time than users with T1D (F=10.54, P<.001).
Figure 2.
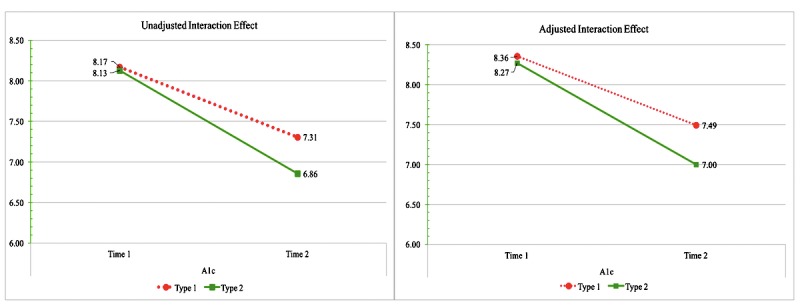
The unadjusted and adjusted interaction between diabetes type and hemoglobin A1c over time.
After adjusting for gender, location, duration of diabetes, and months between HbA1c entries, HbA1c continued to decrease by an absolute 1.07% (F=292.03, P<.001; Multimedia Appendix 1) from first (mean HbA1c 8.31%) to second entry (mean HbA1c 7.24%). Regardless of time, users with T1D (mean 7.92%) continued to have a higher HbA1c (.29% HbA1c difference; F=11.66, P<.001) than users with T2D (mean 7.63%). In the adjusted model, the interaction between diabetes type and HbA1c entry persisted (Figure 2). Users with T2D continued to have a greater HbA1c decrease over time than users with T1D (F=10.54, P<.001). After adjusting for gender, location, duration of diabetes, months between HbA1c entries, and insulin use, users with T2D reported a 1.27% absolute HbA1c reduction (F=364.43, P<.001) from first (mean HbA1c 8.16%) to second entry (mean HbA1c 6.89%).
Finally, using the app to record food was associated with greater HbA1c reductions even after adjusting for covariates and after further adjusting for insulin use for users with T2D (Multimedia Appendix 2, P<.05).
Discussion
Principal Findings
We assessed changes in HbA1c for people with T1D or T2D who used the One Drop | Mobile app over a period of 1 year. We also evaluated relationships between tracking self-care with the app and HbA1c change during that time. App users reported up to a 1.27% absolute decrease in HbA1c depending on their diabetes type. Using the app to track food intake was associated with greater HbA1c reductions.
Landmark studies, including the Diabetes Control and Complications Trial [25] and United Kingdom Prospective Diabetes Study [26], found lowering HbA1c closer to normal levels reduced the risk of diabetes complications. According to recent reviews, diabetes apps are associated with reduction of HbA1c [1-3], but their effectiveness varies widely across studies and by diabetes type.
One review reported people with T1D who used diabetes apps had a 0.36% HbA1c reduction in 3 to 9 months [1]. For people with T1D using the dBees self-care and blood glucose tracking app, there was no HbA1c reduction over time or relative to controls using a paper logbook [5]. In another trial, 34 children and teenagers with T1D using the mDiab/Mobil Diab tracking and self-care support app reduced their HbA1c by 0.72%, but HbA1c also fell by 0.98% in the control group [8]. In a nonrandomized controlled trial, 90 adults with T1D and HbA1c≥8% using the Diabeo digital diary and insulin calculator lowered their HbA1c by 0.91% relative to controls [6]. Among 36 people with T1D in Australia using the Glucose Buddy tracking app, HbA1c was reduced by 1.10% [7].
Based on 367 people with T1D using the One Drop | Mobile app, we found HbA1c declined by 0.86%—an amount consistent with other studies evaluating publicly available apps but more than two-fold larger than the overall effect of diabetes apps tested among people with T1D [1]. Moreover, unlike the previous trials described above, we related HbA1c change to tracking self-care with an app, and found, regardless of diabetes type, using the app to track food consumption was associated with a greater HbA1c reduction.
For people with T2D, an evaluation of 10 studies testing diabetes apps found an average HbA1c reduction of 0.49% [3]. One of those studies was an RCT evaluating the publicly available WellDoc app (now available as Bluestar) that reported a 2.03% drop in HbA1c among 15 people with T2D in one urban area. Our observational study with no control group or randomization included a sample of 921 people with T2D, and found HbA1c decreased by 1.27%. This HbA1c improvement is comparable to the difference in HbA1c improvement between the WellDoc intervention and control groups and more than double the effect of diabetes apps used by people with T2D in a recent meta-analysis by Hou et al [3]. In that meta-analysis, one other trial evaluated a publicly available app [9]. The trial evaluated the mDiab/Mobil Diab app as used by 40 people with T2D in Butembo, Democratic Republic of Congo [9]. HbA1c improved by 1.78% [9]. The baseline HbA1c was 0.54% higher than in our study.
Limitations
This study has limitations. There was no control group or randomization. Multiple potential confounding factors may have contributed to the observed results, making it impossible to ascribe causal relationships between using the One Drop | Mobile app and HbA1c change. The significant relationship between using the app to track self-care and HbA1c benefit enhances confidence of a direct link. Users were also self-selected in terms of their using the app and self-reporting 2 or more HbA1c values, introducing external validity and generalizability concerns. This possibility, however, is also a concern with any RCT in which participants self-select to participate. Our sample also reflects people willing to use a diabetes app. It is plausible to assume these people are younger, have a higher socioeconomic status (ie, a higher income, education) and are more comfortable using technology. To protect privacy, One Drop | Mobile does not collect user age, precluding the ability to describe this and other characteristics (eg, education, income, insurance status) of the sample or adjust for them in analyses. One Drop | Mobile also has other features we did not relate to HbA1c change or adjust for in our analyses. HbA1c was self-reported rather than assessed with a laboratory assay. Because the app is a tool for the user and not subject to review by others, it is unlikely users altered their HbA1c values in response to social desirability bias. Consistent with prior studies that used laboratory HbA1c values, we found a greater HbA1c improvement among people with T2D than people with T1D [1]. Also, self-reported HbA1c was highly correlated with average blood glucose 90 days before the HbA1c, increasing confidence in its utility as an indicator of glycemic control in this study. Finally, our sample included over 1200 PWD from both within and outside the United States, differentiating it from other studies that included people from only one country or region.
Conclusion
There are currently no best practices for evaluating mobile health apps [27], and clearly more research is needed. This study adds to that body of work. Diabetes app developers collect data that can both improve product offerings and user experience and evaluate how users may be benefiting.
We believe people want and deserve mobile health apps that address their self-care needs and enhance their ability to improve the management of their chronic health condition [17,28]. Selecting an app is challenging. There are over 1500 diabetes apps to choose from with more being developed. A review of 65 publicly available diabetes apps concluded 86% were unfit for promoting self-management [29]. Ratings by consumers can be a poor indication of an app’s clinical efficacy [30]. The results of carefully developed clinical evaluations will help consumers select better apps and assist providers in recommending efficacious apps to patients.
Abbreviations
- HbA 1c
hemoglobin A1c
- IQR
interquartile range
- LADA
latent autoimmune diabetes in adults
- PMM
predictive mean matching
- PWD
people with diabetes
- RCT
randomized controlled trial
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Tests of mean hemoglobin A1c change by time, diabetes type, and their interaction.
Tests of the relationships between tracking food, activity, blood glucose, and medications in One Drop | Mobile and hemoglobin A1c change.
Footnotes
Conflicts of Interest: Chandra Y Osborn, Mark Heyman, Brian Huddleston, and Jeff Dachis are full-time employees and have equity in Informed Data Systems Inc, manufacturer of the One Drop | Mobile app. Joost van Ginkel received a consulting fee to assist with analyses but otherwise has no conflict of interest. Informed Data Systems Inc has paid David Rodbard of Biomedical Informatics Consultants LLC for services unrelated to this study. David G Marrero serves as a clinical advisory board member for the One Drop | Experts program on which this study does not report.
References
- 1.Wu Y, Yao X, Vespasiani G, Nicolucci A, Dong Y, Kwong J, Li L, Sun X, Tian H, Li S. Mobile app-based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR mHealth uHealth. 2017;5(3):e35. doi: 10.2196/mhealth.6522. http://mhealth.jmir.org/2017/3/e35/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonoto B, de Araujo VE, Godói IP, de Lemos LL, Godman B, Bennie M, Diniz L. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR mHealth uHealth. 2017;5(3):e4. doi: 10.2196/mhealth.6309. http://mhealth.jmir.org/2017/3/e4/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39(11):2089–2095. doi: 10.2337/dc16-0346. [DOI] [PubMed] [Google Scholar]
- 4.Cui M, Wu X, Mao J, Wang X, Nie Min. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166718. doi: 10.1371/journal.pone.0166718. http://dx.plos.org/10.1371/journal.pone.0166718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drion I, Pameijer L, van Dijk P, Groenier K, Kleefstra N, Bilo HJG. The effects of a mobile phone application on quality of life in patients with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Sci Technol. 2015;9(5):1086–1091. doi: 10.1177/1932296815585871. http://europepmc.org/abstract/MED/25963412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charpentier G, Benhamou P, Dardari D, Clergeot A, Franc S, Schaepelynck-Belicar P, Catargi B, Melki V, Chaillous L, Farret A, Bosson J, Penfornis A, TeleDiab Study Group The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study) Diabetes Care. 2011;34(3):533–539. doi: 10.2337/dc10-1259. http://europepmc.org/abstract/MED/21266648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15(11):e235. doi: 10.2196/jmir.2588. http://www.jmir.org/2013/11/e235/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndt R, Takenga C, Preik P, Kuehn S, Berndt L, Mayer H, Kaps A. Impact of information technology on the therapy of type-1 diabetes: a case study of children and adolescents in Germany. J Pers Med. 2014;4(2):200–217. doi: 10.3390/jpm4020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takenga C, Berndt R, Musongya O, Kitero J, Katoke R, Molo K, Kazingufu B, Meni M, Vikandy M, Takenga H. An ICT-based diabetes management system tested for health care delivery in the African context. Int J Telemed Appl. 2014:1–10. doi: 10.1155/2014/437307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 11.Quinn C, Shardell M, Terrin M, Barr E, Ballew S, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934–1942. doi: 10.2337/dc11-0366. http://europepmc.org/abstract/MED/21788632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabin C, Bock B. Desired features of smartphone applications promoting physical activity. Telemed J E Health. 2011;17(10):801–803. doi: 10.1089/tmj.2011.0055. [DOI] [PubMed] [Google Scholar]
- 13.Nelson L, Mulvaney S, Johnson K, Osborn CY. mHealth intervention elements and user characteristics determine utility: a mixed-methods analysis. Diabetes Technol Ther. 2017 Jan;19(1):9–17. doi: 10.1089/dia.2016.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheibe M, Reichelt J, Bellmann M, Kirch W. Acceptance factors of mobile apps for diabetes by patients aged 50 or older: a qualitative study. Med 2 0. 2015;4(1):e1. doi: 10.2196/med20.3912. http://www.medicine20.com/2015/1/e1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafazzo J, Casselman M, Hamming N, Katzman D, Palmert MR. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;14(3):e70. doi: 10.2196/jmir.2058. http://www.jmir.org/2012/3/e70/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight BA, McIntyre HD, Hickman IJ. Qualitative assessment of user experiences of a novel smart phone application designed to support flexible intensive insulin therapy in type 1 diabetes. BMC Med Inform Decis Mak. 2016;16(19) doi: 10.1186/s12911-016-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lithgow K, Edwards A, Rabi D. Smartphone app use for diabetes management: evaluating patient perspectives. JMIR Diabetes. 2017;2(1):e2. doi: 10.2196/diabetes.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohlfing C, Wiedmeyer H, Little R, England J, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the diabetes control and complications trial. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 19.Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572–1583. doi: 10.1177/193229681100500634. http://europepmc.org/abstract/MED/22226280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 21.Little RJA. Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6(3):287–296. [Google Scholar]
- 22.Rubin DB. Statistical matching using file concatenation with adjusted weights and multiple imputations. J Bus Econ Stat. 1986;4(1):87–94. [Google Scholar]
- 23.Marshall A, Altman DG, Royston P, Holder RL. Comparison of techniques for handling missing covariate data within prognostic modelling studies: a simulation study. BMC Med Red Methodol. 2010;10(1):7. doi: 10.1186/1471-2288-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Burren S. Flexible Imputation of Missing Data. Boca Raton: Chapman & Hall/CRC Press; 2012. [Google Scholar]
- 25.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 26.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 27.McKay F, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare. 2016 doi: 10.1177/1357633X16673538. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez V, Johnson E, Gonzalez C, Ramirez V, Rubino B, Rossetti G. Assessing the use of mobile health technology by patients: an observational study in primary care clinics. JMIR mHealth uHealth. 2016 Apr 19;4(2):e41. doi: 10.2196/mhealth.4928. http://mhealth.jmir.org/2016/2/e41/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brzan P, Rotman E, Pajnkihar M, Klanjsek P. Mobile applications for control and self management of diabetes: a systematic review. J Med Syst. 2016;40(9):210. doi: 10.1007/s10916-016-0564-8. [DOI] [PubMed] [Google Scholar]
- 30.Singh K, Drouin K, Newmark LP, Lee J, Faxvaag A, Rozenblum R, Pabo EA, Landman A, Klinger E, Bates DW. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood) 2016;35(12):2310–2318. doi: 10.1377/hlthaff.2016.0578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tests of mean hemoglobin A1c change by time, diabetes type, and their interaction.
Tests of the relationships between tracking food, activity, blood glucose, and medications in One Drop | Mobile and hemoglobin A1c change.


