Abstract
Fast and reliable incision closure is critical in any surgical intervention. Common solutions are sutures and clips or adhesives, but they all present difficulties. These difficulties are especially pronounced in classical and robot-assisted minimally-invasive interventions. Laser soldering methods present a promising alternative, but their reproducibility is limited. We present a system that combines a previously reported laser soldering system with a robotic system, and demonstrate its feasibility on the incision-closure of ex-vivo mice skins. In this demonstration, we measured tearing forces of ~2.5N, 73% of the tearing force of a mouse skin without an incision. This robot-assisted laser soldering technique has the potential to make laser tissue soldering more reproducible and revolutionize surgical tissue bonding.
1. Introduction
Almost every surgical intervention requires some type of joining of adjacent sections of tissue. Technologies such as sutures, staples, clips, and adhesives have been used for many years to close surgical incisions and wounds. In suturing, the surgeon passes the needle through the tissues close to their edge, pulling them together and relying on tissue and suture strength to hold them tight. However, suturing is a delicate, demanding, and time-consuming procedure, requiring technical skill [1–3]. Sutured sites are at an increased risk for leaks, infections, and scarring [4,5]. Stapling is appropriate in some cases, but it also relies on the original tissue strength, and may be limited by the shape and size of the specific stapler: a poorly adapted stapler may result in bonding failure. Staples that are too small can lead to excessive tissue compression, which exceeds the tensile strength of the tissue, leading to tearing and perforation, whereas staples that are too large can fail to achieve a good seal [6]. Adhesives also have their limitations – they may exhibit insufficient tensile strength [7], and may be toxic [8,9].
Today, a growing percentage of surgical interventions are performed using standard minimally invasive surgery (MIS), or robot-assisted minimally invasive surgery (RAMIS). In both techniques, connecting adjacent tissue intracorporeally (inside the body) is an important component of many procedures. A common task is the closure or anastomosis of hollow organs such as bowel or bladder requiring achievement of a reliable, strong, airtight and watertight seal without causing tissue ischemia (inadequate blood supply). In standard MIS the surgeon inserts long hand-held instruments into the body via small incisions making this task more complicated than in open surgery. The reduced degrees of freedom limit the ability to properly rotate the needle through its arc in the right direction and knot tying is challenging, requiring significant training [1]. In RAMIS, a surgeon manipulates a pair of joysticks that teleoperate instruments and a camera inside the body of the patient [10,11]. In this case, the problem of limited degrees-of-freedom is solved, but a new technical challenge is introduced by the absence of haptic (touch) feedback [12]. The lack of haptic feedback can result in tearing of the tissue or the suture when approximating the tissues with the suture and when tying the knots. Anastomotic complications in minimally invasive colorectal surgery for example, are currently in the range of 8-10% [13] using these techniques. These complications have a profound effect on the morbidity and mortality of patients, hospitalization times, infection, as well as long-term effects such as local recurrence of cancer [14]. Reducing this rate of complications can bring about an enormous effect on patients undergoing minimally invasive bowel surgery.
Laser technology provides a promising alternative for reliable closure of incisions. Heating an incision by a laser beam accelerates wound adhesion and reduces scarring. There are different methods for laser-bonding two edges of tissue [15–21]. In laser tissue welding, the edges of the incision are approximated and a spot on the incision is heated. The laser is then moved to a neighboring spot, and the process is repeated until the full length of the incision is covered. In laser tissue soldering (LTS), heating commences on approximated edges of an incision that are covered by a biological solder such as albumin, fibrin, or other materials [22], prior to heating. The heated solder biochemically links to the surrounding tissue and creates a clot, thereby sealing the incision [23]. In some variations of LTS, the solder is heated by direct absorption of the laser. In other cases, a coloring agent (Indocyanine Green) is mixed with the solder and the wavelength of the laser is chosen to be absorbed by the coloring agent [24]. A recent development involves incorporation of nano-particles within the solder allowing absorption at specific wavelengths [25,26]. The LTS technique has been attempted by several groups, mostly in ex-vivo experiments [27]. Few attempts carried out in vivo testing [28], and even fewer performed clinical experiments on humans [29,30]. LTS does not require complex dexterous manipulation like suturing, and does not involve contact with the tissue. Moreover, LTS allows operation at various working distances, depending on the output optics of the radiation delivery system. These advantages make LTS particularly promising for improving the quality of tissue bonding in MIS and RAMIS.
Most of the prior studies examining LTS did not control the temperature during the laser soldering process [31]. Controlling the temperature, however, is very important because tissue over-heating causes thermal damage, scarring and weak bonding, whereas under-heating of tissue results in insufficient closure effects and weak bonding [32]. A robotic LTS system with a temperature monitoring has been demonstrated in [22]. However, the temperature of the heated spot was measured indirectly, with a thermocouple between the chitosan film and the tissue, thus reporting inaccurate results. To address this problem we developed a fiber-optic system for LTS under temperature control [33]. The system incorporates a bundle of fibers including a silica fiber for delivering the laser beam to heat a spot on the incision, and six infrared fibers for determining the temperature of the spot. A control sub-system was used to heat the spot to a specific temperature. The spot heated by the laser emitted infrared radiation which was detected by a detection system which was used to accurately determine the temperature of the spot. This information was fed through a feedback loop to control the laser power so that the spot was heated to a specific set of temperature (T) and time (t), obtaining optimal conditions in experiments in vitro where T~60 °C to 65°C, and t~10 sec. The ambient room temperature was 22 ± 5°C, with 50 ± 30% changes in the ambient humidity. Under these conditions, the bonding was strong and no thermal damage was observed.
Following the results of these preliminary studies physicians in several medical centers carried experiments in vivo. These included incision soldering in various tissues, including skin [34], bowel [35], dura [36] and cornea [33,37,38]. In experiments for soldering of incisions on the skin of large farm pigs in-vivo, it was established that healing of the wounds was faster than those obtained after standard suturing and there were practically no scars [39]. These experiments culminated with a preliminary clinical experiment in which incisions in the abdominal skin of 10 patients undergoing laparoscopic cholecystectomy were successfully soldered, resulting in strong bonding with negligible scarring [40].
In all these previous experiments, the surgeon held the distal tip of the bundle and moved it along the incision. The main disadvantage of this method was that that the surgeon was unable to keep the distal tip at a constant height above the incision and was not able to move the tip at a constant speed with constant spacing between heating points or keep it at the same place for the specific predefined time. Although most of the bonding was performed successfully, some portions were over-heated or under-heated, leading to weak bonding in those regions. In all in-vitro, in-vivo experiments and in human trials it was difficult to manually obtain a consistent heating dose on the sample. If the bond-strength variations are the outcome of non-uniform heating, we submit that an improvement in bond-strength can be achieved if all the parameters: height, spacing, temperature, and heating time are optimized and made controllable and repeatable.
Combining laser technology with robotic precision and accuracy presents a promising opportunity for improving surgical interventions. This combination was previously used for performing laser-assisted cutting or ablation in different robot-assisted interventions [41–49]. Recently, two studies demonstrated a successful robot-assisted laser welding of a cornea [22,50]. However, this system did not include direct temperature control. The increasing popularity of RAMIS presents a promising opportunity to combine the advantages of LTS with the accuracy and controllability that robotic technology offers. In this paper, we present a novel concept of Robot-assisted Laser Tissue Soldering (RLTS) with built-in temperature control. Combining robotic precision with tight temperature control is expected to improve the repeatability of LTS and eventually improve clinical outcomes. We integrated our LTS system with a Robot-assisted Minimally-Invasive Surgery research platform – the Raven II [51,52]. As a proof of feasibility, we demonstrate RLTS soldering of incisions in mouse skin and mechanical testing of the strength of the resulting bonded tissue.
2. Materials and methods
Our novel system includes two parts: (a) an optical device, consisting of a diode-laser whose beam is transmitted through an optical fiber that heats the incision, point by point, under close temperature control; (b) a robotic system that accurately maneuvers the distal tip of the fiber to move at a predefined height and trajectory (path and speed) along the incision in open loop.
2.1 Laser soldering system
Optical fibers have been used for light delivery in confined spaces in many applications. Our studies concentrated on a system, conveniently packed in a single tube to be held by a surgeon or fixed on a robotic arm (i.e. applicator), based on two types of fibers: a transmitting visible radiation for heating the tissue, and a middle infrared (mid-IR) radiation fiber for monitoring the temperature, both shown in Fig. 1 [53]. In this system, we selected a diode laser emitting at λ = 1.9μm for heating the spot. The penetration depth of this wavelength is of the order of 1mm, and it ensures strong bonding, as was proved in our earlier experiments [33]. A visible fiber (silica, Thorlabs FT800EMT) was used to transmit this laser beam from the proximal end to the distal end and onto a spot on the tissue, thus heating it. The beam emitted from the distal end had a Gaussian profile and the width of the heated spot depended on the distance from the distal end to the tissue. The choice of working distance for this study was 1.5 and 3mm ± 0.5mm (tissue roughness), but other distances were also attempted such as 5mm ± 0.5mm [40].
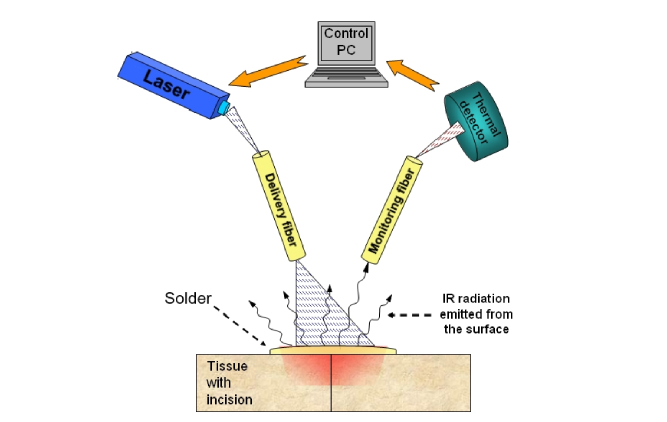
Fig. 1.
A schematic drawing of the cross section of an incised tissue with albumin layer spread over it. The cut is being laser-soldered using a system consisting of a heating laser, a delivery fiber, a mid-IR monitoring fiber and a mid-IR.
When a spot of a ~2-3 mm diameter was heated to a specific T, it emitted thermal middle infrared (mid-IR) radiation (i.e. blackbody radiation) of intensity (I). I was proportional to the value of T, and this was the non-contact method for accurately measuring T. A monitoring mid-IR fiber was used to transmit this radiation from the spot onto a suitable detector, that generated a voltage V, proportional to T. This voltage was then used in an automatic feedback loop that controlled the intensity emitted by the laser so that the T was kept constant. The temperature T was chosen to obtain strong bonding. This feedback loop minimized the temperature fluctuations due to changes in laser power or any other conditions. In this work, with the robot, the temperature fluctuations were reduced by ~50% compared to previous publications [32], assuring much smaller temperature variation on a heated spot.
With this system, the human operator held the applicator above the desired spot and made sure that the spot was heated for a chosen time t. The laser beam was then slowly shifted to a neighboring spot that was also heated to a reference temperature T for an approximate time t. In experiments on various tissues and using various lasers, we found that strong bonding was obtained if each spot was heated to a temperature T = 60 °C to 65°C for a time of approximately t = 8-12 seconds, which conforms to our previous result [33].
Calibration [32,54] of the temperature-readout system was performed using model thermal emitters with known emissivity, heated to temperatures 20°-60° and the results were validated by thermocouples. The voltage (V) generated by the IR detector at each temperature T was recorded, and a calibration curve was built. Using this approach, we could calibrate the temperature measurement for any mid-IR fiber of any numerical aperture and attenuation.
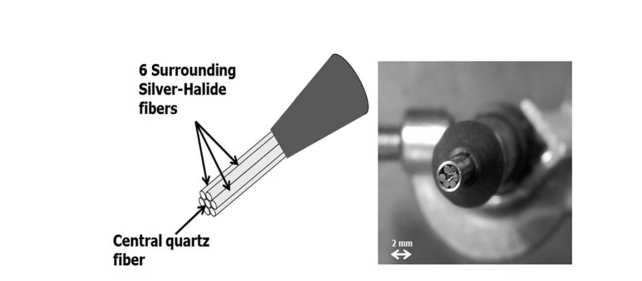
In the current experiment, the applicator included seven fibers, as shown in Fig. 2, all in the same bundle. The delivery fiber in the center of the bundle, composed of silica, transmitting near-IR radiation at a wavelength of λ = 1.9μm. The six monitoring fibers were made of silver halide (AgClBr) and were highly transparent in the mid-IR range. This configuration operates similarly to the two-fiber system, but it is more accurate and is suitable for integration with a RAMIS instrument.
Fig. 2.
The fiber bundle used in the laser soldering system. The radiation of a semiconductor diode laser is transmitted through a silica fiber and heats a spot on tissue. Six AgClBr mid-IR fibers are used to transmit the mid-IR emitted from the spot to a mid-IR detector for measuring the temperature of the spot.
We used a near-IR Semiconductor Disk Laser (SDL, Fraunhofer Institute, Freiburg, Germany) to generate the radiation [55]. The laser was operated in the CW mode with an output power in the range 0–0.5W.
2.2 Robotic hardware and software
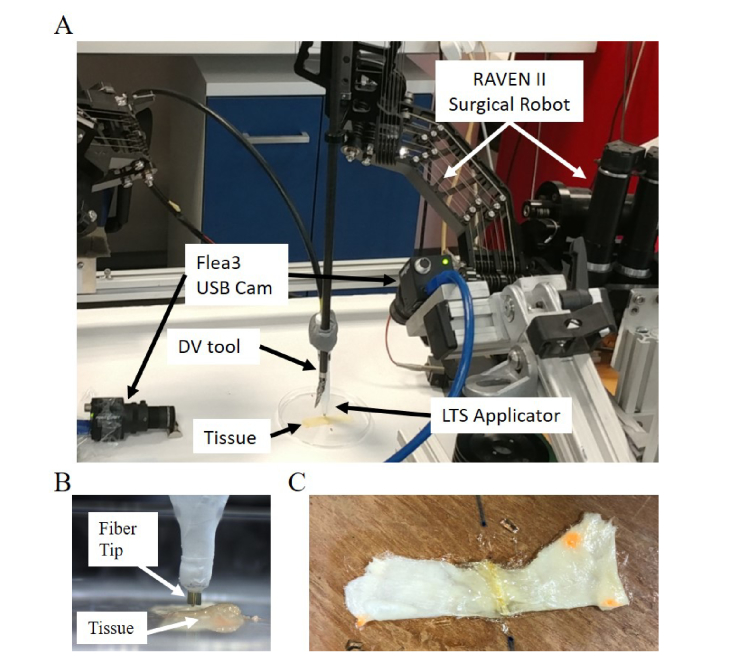
The current version of our RLTS system is depicted in Fig. 3(A-B). In this study, we used the Raven II RAMIS research platform (Applied Dexterity, Seattle, USA) [51] with the same control architecture as in [52]. We attached a da Vinci Surgical System Tool (Intuitive Surgical, Sunnyvale, USA) to the Raven II arm with a special adapter (Applied Dexterity, Seattle, USA). We also placed 2 Flea3 USB cameras (PointGrey, Richmond, Canada) in the surgical scene for visualization purposes during the manual stages of robot control. In this first demonstration we attached the LTS applicator tip to the Da Vinci Tool with a custom-made attachment (Fig. 3(A)).
Fig. 3.
Experimental setup. (A) The RAVEN II Surgical Robot connected to the LTS applicator and the experimental workspace. (B) The LTS applicator connected to the RAMIS tool. (C) The resulting soldered specimen.
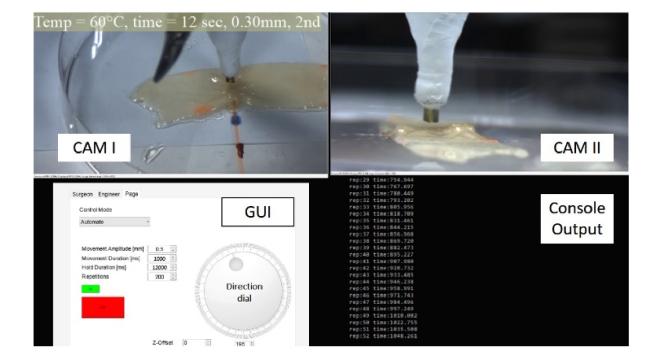
The surgeon’s operating console was a haptic manipulator that was connected to a personal computer via a custom-built graphic user interface. The surgeon viewed a graphical user interface (Fig. 4) and could control the surgical robot in three different modes: (1) manual control, (2) keyboard control, and (3) automatic control. The manual control allowed the use of a haptic manipulator (SIGMA 7, Force Dimension, Switzerland) to position the Raven II arm carrying the applicator to a desired location in the surgical field. Keyboard control allowed for fine adjustments of the position of the applicator, using keyboard keys which controlled the position and orientation of the distal tip of the fiber. Automatic control allowed for executed a 3D path (a straight line in the current demonstration) and a trajectory. In automatic control, the direction and amplitude of the motion, the increments of each segment of the motion, the time of each increment, and the hold time between increments, and the total motion time that was used for heating of each point were all controlled by the robotic system in open loop control.
Fig. 4.
The Graphical User Interface that was presented to the operator of our RLTS system.
2.3 Biological samples
Tissues: We used mouse skin tissues in the soldering experiment. They were stored in a freezer at −4°C and defrosted prior to the experiments. Immediately prior to the experiments, Samples of 1*5 cm in size and around ~1mm thick were cut into two segments, the segments were approximated, solder was spread on this area which was then placed under the distal tip of the power delivery fiber.
Solder: The solder was prepared from 67% w/v bovine albumin solution (Sigma-Aldich, LTD) in high purity water (Milli-Q, Millipore), homogenized and centrifuged, resulting in a gel-like solution. The solder was applied to the edges of each skin tissue as a thin layer.
2.4 Experimental protocol
The two cut segments of the skin were approximated on a Petri dish, solder was spread on this area and the coated, approximated sample was placed under the distal tip of the power delivery fiber. In each experiment run we initially used the manual and keyboard control of the surgical robot to aim the applicator to the beginning of the incision, and to set a height H between the tissue and the tip of the applicator. It is important to note, however, that the height was not feedback enforced because the control of the trajectory was open loop. We then applied the laser to heat the tissue to 60 °C, using the automatic open loop trajectory to control the movement along the incision. We used two experimental protocols for the automatic control: (1) discrete, where multiple spots were heated one-by-one along the incision line for a predefined dwell-time, and (2) continuous, where the heated spot was constantly moved along the incision at a predefined speed.
Immediately after the experiment we measured the bond strength using a Universal Testing Machine (UTS) (Lloyd LC2.5, Lloyd instruments, Bognor Regis, Sussex, UK). We placed the tissue sample in the machine and used a creeping protocol to measure the tearing strength [N] – the maximal force before the sample teared. We repeated this measurement for each of the samples, and for an additional control sample of tissue without an incision to determine an upper boundary for bond strength.
3. Results
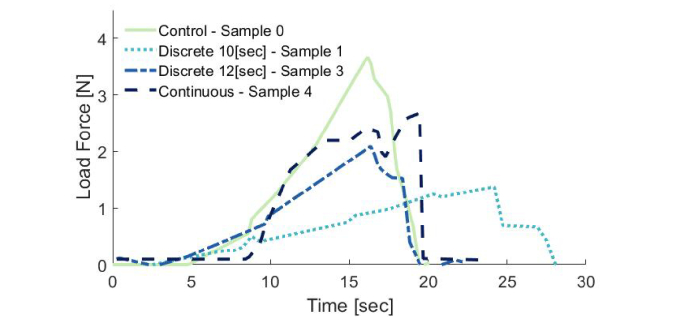
To demonstrate the feasibility of our novel RLTS system, we soldered several incisions using different versions of the discrete and the continuous protocols. The results of the tearing strength of 5 incisions are reported in Table 1 (samples 1-5): Three with discrete spot-by-spot and 2 with continuous irradiation along the incision line of the mouse skin samples after albumin application. An additional piece of skin without an incision (sample 0) was tested for strength as a control. The maximum strength measured in the discrete trials, was 2.1 N for 12 sec dwell time, and in the continuous trials 2.7 N. The maximum strength of a control sample was 3.8 N, and hence, our best samples yielded 55% and 70% of the tearing force of an intact tissue, respectively. An example of a soldered tissue sample can be seen in Fig. 3(C). Figure 5 demonstrates the detailed results of the creeping test using the UTS machine for the control tissue and the best sample from each of the discrete and continuous protocols.
Table 1. Tearing strength results.
| Sample | Protocol | Dwell time ( ± 0.5 sec) |
Laser–Tissue distance H ( ± 0.5 mm) |
Tissue sample size (width × length [mm] ( ± 0.1 mm) |
Trial time, sec | Tearing Strength, N ( ± 0.01N) |
|---|---|---|---|---|---|---|
| 0 | Control | 11.9 × 0.3 | 3.66 | |||
| 1 | Discrete | 10 sec | 1.5 mm | 13 × 0.3 | 1.37 | |
| 2 | Discrete | 12 sec | 3 mm | 10.1 × 0.3 | 853 | 1.02 |
| 3 | Discrete | 12 sec | 3 mm | 11.4 × 0.3 | 940 | 2.09 |
| 4 | Continuous | 1.12 mm/sec | 3 mm | 9.2 × 0.5 | 718 | 2.67 |
| 5 | Continuous | 1.12 mm/sec | 3 mm | 10.3 × 0.27 | 669 | 2.08 |
Fig. 5.
Representative test results on the quality of bonding in a Universal Testing Machine. The line numbers in the legend refer to the line number in Table 1.
4. Discussion and conclusions
In this study, we developed a novel Robot-assisted Laser Tissue Soldering system by integrating a fiber-optic LTS device with a RAMIS research platform. Our system allows for an open loop continuous or discrete movement of the distal tip of the fiber bundle along the incision. This made it possible to keep a fairly consistent height between the fiber tip and the tissue, and the temperature T and heating time at the desired values. Our results demonstrate that Robotic Laser Tissue Soldering can achieve a strong bonding in discrete and continuous movements, yielding approximately 55% and 70% of the tearing force of intact tissue respectively. We noted an increase in tearing strength upon implementation of longer heating times, or a reduction of speed, but future studies are needed to determine these relationships and optimize the parameters of the RLTS. Based on our experience these parameters are likely to vary between different tissues, e.g. between bowel, skin, and cornea samples. Taken together, these results suggest that RLTS is a very promising technique that can reduce the completion time of many procedures. For example, novice surgeons may take up to 25 minutes to incise and close a 2.5 cm incision in the bladder in the common task of cystotomy and repair [56]. It may also be a promising alternative to automatic suturing that is very difficult to implement with RAMIS systems [57].
In the current demonstration, our focus was on integrating the two systems and showing the potential advantage of accurate control of the soldering path and timing on soldering ex- vivo tissue. Therefore, we implemented the simplest open loop controller for the automatic control mode and used the manual and keyboard control for adjustments.
We demonstrated the feasibility of our RLTS system by showing good results in soldering mouse skins. In future studies, we intend to continue optimization towards an eventual integration with clinical RAMIS systems and other robotic platforms. The soldering of incisions may be used both on the surface of tissues or intracorporeally in MIS or RAMIS.
We plan to develop efficient automatic and teleoperated controllers for our RLTS system. We believe that these improvements will also allow for achieving consistent results in terms of tearing strength. In future developments, the teleoperated nature of RAMIS will allow for the choice between surgeon teleoperated, shared, and autonomous modes of soldering.
We aim to achieve (1) faster and more precise tissue bonding (2) high and uniform bond-strength throughout the incision, with improved bonding strength, and (3) fully automated or shared control soldering with a rapid learning curve of operating the device. This technology has the potential to revolutionize tissue bonding in many surgical disciplines such as colorectal surgery, bariatric surgery, urology, gynecology, thoracic, ophthalmic and many other types of robot-assisted minimally-invasive surgical procedures.
Funding
ABC Robotics Initiative at BGU; ISF (grant number 823/15); Alon Fellowship; Ministry of Science and Technology, Israel; BGU Kreitman Fellowship
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Allen J. W., Rivas H., Cocchione R. N., Ferzli G. S., “Intracorporeal suturing and knot tying broadens the clinical applicability of laparoscopy,” JSLS 7(2), 137–140 (2003). [PMC free article] [PubMed] [Google Scholar]
- 2.Sharon Y., Nisky I., “Expertise, teleoperation, and task constraints affect the speed–curvature–torsion power law in RAMIS,” J. Med. Robot. Res. 2018, 1841008 (2018). 10.1142/S2424905X18410088 [DOI] [Google Scholar]
- 3.Nisky I., Che Y., Quek Z. F., Weber M., Hsieh M. H., Okamura A. M., “Teleoperated versus open needle driving: Kinematic analysis of experienced surgeons and novice users,” in 2015 IEEE International Conference on Robotics and Automation (ICRA) (IEEE, 2015), pp. 5371–5377. 10.1109/ICRA.2015.7139949 [DOI] [Google Scholar]
- 4.Severin M., Bartz-Schmidt K.-U., Penetrating Keratoplasty: Diagnosis and Treatment of Postoperative Complications (Springer Berlin Heidelberg, 2000). [Google Scholar]
- 5.Hjortdal J., Søndergaard A., Fledelius W., Ehlers N., “Influence of suture regularity on corneal astigmatism after penetrating keratoplasty,” Acta Ophthalmol. 89(5), 412–416 (2011). 10.1111/j.1755-3768.2009.01729.x [DOI] [PubMed] [Google Scholar]
- 6.Baker R. S., Foote J., Kemmeter P., Brady R., Vroegop T., Serveld M., “The Science of Stapling and Leaks,” Obes. Surg. 14(10), 1290–1298 (2004). 10.1381/0960892042583888 [DOI] [PubMed] [Google Scholar]
- 7.Chow A., Marshall H., Zacharakis E., Paraskeva P., Purkayastha S., “Use of tissue glue for surgical incision closure: a systematic review and meta-analysis of randomized controlled trials,” J. Am. Coll. Surg. 211(1), 114–125 (2010). 10.1016/j.jamcollsurg.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 8.Oelker A. M., Grinstaff M. W., “Ophthalmic adhesives: a materials chemistry perspective,” J. Mater. Chem. 18(22), 2521–2536 (2008). 10.1039/b719791h [DOI] [Google Scholar]
- 9.Chen W.-L., Lin C.-T., Hsieh C.-Y., Tu I.-H., Chen W. Y. W., Hu F.-R., “Comparison of the bacteriostatic effects, corneal cytotoxicity, and the ability to seal corneal incisions among three different tissue adhesives,” Cornea 26(10), 1228–1234 (2007). 10.1097/ICO.0b013e3181506129 [DOI] [PubMed] [Google Scholar]
- 10.Jarc A. M., Nisky I., “Robot-assisted surgery: an emerging platform for human neuroscience research,” Front. Hum. Neurosci. 9, 315 (2015). 10.3389/fnhum.2015.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMaio S., Hanuschik M., Kreaden U., “The da Vinci Surgical System,” in Surgical Robotics (Springer US, 2011), pp. 199–217. [Google Scholar]
- 12.Milstein A., Mintz Y., Nisky I., “The Effect of Gripper Scaling on Grip Force Adjustment in Robot - Assisted Surgery – a Preliminary Study,” in 2016 Hamlyn Symposium on Medical Robotics, Yang G. Z., Dazri A A., eds. (Royal Geographical Society, 2016), pp. 94–95. [Google Scholar]
- 13.Halabi W. J., Kang C. Y., Jafari M. D., Nguyen V. Q., Carmichael J. C., Mills S., Stamos M. J., Pigazzi A., “Robotic-assisted colorectal surgery in the United States: A nationwide analysis of trends and outcomes,” World J. Surg. 37(12), 2782–2790 (2013). 10.1007/s00268-013-2024-7 [DOI] [PubMed] [Google Scholar]
- 14.Mirnezami A., Mirnezami R., Chandrakumaran K., Sasapu K., Sagar P., Finan P., “Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis,” Ann. Surg. 253(5), 890–899 (2011). 10.1097/SLA.0b013e3182128929 [DOI] [PubMed] [Google Scholar]
- 15.Bass L. S., Treat M. R., “Laser tissue welding: A comprehensive review of current and future clinical applications,” Lasers Surg. Med. 17(4), 315–349 (1995). 10.1002/lsm.1900170402 [DOI] [PubMed] [Google Scholar]
- 16.McNally K. M., Sorg B. S., Chan E. K., Welch A. J., Dawes J. M., Owen E. R., “Optimal parameters for laser tissue soldering. Part I: Tensile strength and scanning electron microscopy analysis,” Lasers Surg. Med. 24(5), 319–331 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Talmor M., Bleustein C. B., Poppas D. P., “Laser Tissue Welding: a Biotechnological Advance for the Future,” Arch. Facial Plast. Surg. 3(3), 207–213 (2001). 10.1001/archfaci.3.3.207 [DOI] [PubMed] [Google Scholar]
- 18.Rasier R., Ozeren M., Artunay O., Bahçecioğlu H., Seçkin I., Kalaycoğlu H., Kurt A., Sennaroğlu A., Gülsoy M., “Corneal tissue welding with infrared laser irradiation after clear corneal incision,” Cornea 29(9), 985–990 (2010). 10.1097/ICO.0b013e3181cc7a3e [DOI] [PubMed] [Google Scholar]
- 19.Vaddavalli P. K., Yoo S. H., “Technology needs for corneal transplant surgery,” Proc. SPIE 7885, 788502 (2011). 10.1117/12.881526 [DOI] [Google Scholar]
- 20.Al-Mubarak L., Al-Haddab M., “Cutaneous wound closure materials: an overview and update,” J. Cutan. Aesthet. Surg. 6(4), 178–188 (2013). 10.4103/0974-2077.123395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ark M., Cosman P. H., Boughton P., Dunstan C. R., “Review: photochemical tissue bonding (PTB) methods for sutureless tissue adhesion,” Int. J. Adhes. Adhes. 71, 87–98 (2016). 10.1016/j.ijadhadh.2016.08.006 [DOI] [Google Scholar]
- 22.Garcia P., Mines M. J., Bower K. S., Hill J., Menon J., Tremblay E., Smith B., “Robotic laser tissue welding of sclera using chitosan films,” Lasers Surg. Med. 41(1), 59–67 (2009). 10.1002/lsm.20727 [DOI] [PubMed] [Google Scholar]
- 23.Duarte A. P., Coelho J. F., Bordado J. C., Cidade M. T., Gil M. H., “Surgical adhesives: Systematic review of the main types and development forecast,” Prog. Polym. Sci. 37(8), 1031–1050 (2012). 10.1016/j.progpolymsci.2011.12.003 [DOI] [Google Scholar]
- 24.Rossi F., Matteini P., Pini R., Esposito G., Puca A., Albanese A., Maira G., “Laser soldering improves microsuturing procedures in neurosurgery,” SPIE Newsroom (2013). 10.1117/2.1201312.005242 [DOI]
- 25.Matteini P., Ratto F., Rossi F., de Angelis M., Cavigli L., Pini R., “Hybrid nanocomposite films for laser-activated tissue bonding,” J. Biophotonics 5(11-12), 868–877 (2012). 10.1002/jbio.201200115 [DOI] [PubMed] [Google Scholar]
- 26.Mushaben M., Urie R., Flake T., Jaffe M., Rege K., Heys J., “Spatiotemporal modeling of laser tissue soldering using photothermal nanocomposites,” Lasers Surg. Med. 50(2), 143–152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo S., Petroni G., Quaglia C., Niccolini M., Rossi F., Menabuoni L., Pini R., Fortuna D., Dario P., Menciassi A., “ESPRESSO: A novel device for laser-assisted surgery of the anterior eye segment,” Minim. Invasive Ther. Allied Technol. 25(2), 70–78 (2016). 10.3109/13645706.2015.1092450 [DOI] [PubMed] [Google Scholar]
- 28.Esposito G., Rossi F., Matteini P., Scerrati A., Puca A., Albanese A., Rossi G., Ratto F., Maira G., Pini R., “In vivo laser assisted microvascular repair and end-to-end anastomosis by means of indocyanine green-infused chitosan patches: A pilot study,” Lasers Surg. Med. 45(5), 318–325 (2013). 10.1002/lsm.22145 [DOI] [PubMed] [Google Scholar]
- 29.Pini R., Menabuoni L., Starnotti L., “First application of laser welding in clinical transplantation of the cornea,” Proc. SPIE 4244, 266–271 (2001). 10.1117/12.427800 [DOI] [Google Scholar]
- 30.Buzzonetti L., Capozzi P., Petrocelli G., Valente P., Petroni S., Menabuoni L., Rossi F., Pini R., “Laser welding in penetrating keratoplasty and cataract surgery in pediatric patients: Early results,” J. Cataract Refract. Surg. 39(12), 1829–1834 (2013). 10.1016/j.jcrs.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 31.Matossian C., Makari S., Potvin R., “Cataract surgery and methods of wound closure: a review,” Clin. Ophthalmol. 9, 921–928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabay I., Abergel A., Vasilyev T., Rabi Y., Fliss D. M., Katzir A., “Temperature-controlled two-wavelength laser soldering of tissues,” Lasers Surg. Med. 43(9), 907–913 (2011). 10.1002/lsm.21123 [DOI] [PubMed] [Google Scholar]
- 33.Gabay I., Basov S., Varssano D., Barequet I., Rosner M., Rattunde M., Wagner J., Platkov M., Harlev M., Rossman U., Katzir A., “Closure of incision in cataract surgery in-vivo using a temperature controlled laser soldering system based on a 1.9μm semiconductor laser,” Proc. SPIE 9702, 97020B (2016). [Google Scholar]
- 34.Levanon D., Katzir A., Ravid A., “A scanning electron microscopy study of CO2 laser-albumin soldering in the rabbit model,” Photomed. Laser Surg. 22(6), 461–469 (2004). 10.1089/pho.2004.22.461 [DOI] [PubMed] [Google Scholar]
- 35.Spector D., Rabi Y., Vasserman I., Hardy A., Klausner J., Rabau M., Katzir A., “In vitro large diameter bowel anastomosis using a temperature controlled laser tissue soldering system and albumin stent,” Lasers Surg. Med. 41(7), 504–508 (2009). 10.1002/lsm.20799 [DOI] [PubMed] [Google Scholar]
- 36.Forer B., Vasileyev T., Gil Z., Brosh T., Kariv N., Katzir A., Fliss D. M., “CO2 laser fascia to dura soldering for pig dural defect reconstruction,” Skull Base 17(1), 17–23 (2007). 10.1055/s-2006-959332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barak A., Ma-Naim T., Belkin M., Katzir A., “Temperature-Controlled CO2 laser tissue welding of ocular tissues,” Proc. SPIE 2971, 103–105 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Norman G., Rabi Y., Assia E., Katzir A., “In vitro conjunctival incision repair by temperature-controlled laser soldering,” J. Biomed. Opt. 14(6), 064016 (2009). 10.1117/1.3262610 [DOI] [PubMed] [Google Scholar]
- 39.Simhon D., Halpern M., Brosh T., Vasilyev T., Ravid A., Tennenbaum T., Nevo Z., Katzir A., “Immediate tight sealing of skin incisions using an innovative temperature-controlled laser soldering device: in vivo study in porcine skin,” Ann. Surg. 245(2), 206–213 (2007). 10.1097/01.sla.0000232554.13719.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simhon D., Gabay I., Shpolyansky G., Vasilyev T., Nur I., Meidler R., Hatoum O. A., Katzir A., Hashmonai M., Kopelman D., “Temperature-controlled laser-soldering system and its clinical application for bonding skin incisions,” J. Biomed. Opt. 20(12), 128002 (2015). 10.1117/1.JBO.20.12.128002 [DOI] [PubMed] [Google Scholar]
- 41.Fichera L., Pardo D., Illiano P., Ortiz J., Caldwell D. G., Mattos L. S., “Online estimation of laser incision depth for transoral microsurgery: approach and preliminary evaluation,” Int. J. Med. Robot. 12(1), 53–61 (2016). 10.1002/rcs.1656 [DOI] [PubMed] [Google Scholar]
- 42.Remacle M., Prasad V. M. N., “Preliminary experience in transoral laryngeal surgery with a flexible robotic system for benign lesions of the vocal folds,” Eur. Arch. Otorhinolaryngol. 275(3), 761–765 (2018). 10.1007/s00405-018-4900-0 [DOI] [PubMed] [Google Scholar]
- 43.Russo S., Dario P., Menciassi A., “A novel robotic platform for laser-assisted transurethral surgery of the prostate,” IEEE Trans. Biomed. Eng. 62(2), 489–500 (2015). 10.1109/TBME.2014.2358711 [DOI] [PubMed] [Google Scholar]
- 44.Solares C. A., Strome M., “Transoral robot-assisted CO2 laser supraglottic laryngectomy: Experimental and Clinical Data,” Laryngoscope 117(5), 817–820 (2007). 10.1097/MLG.0b013e31803330b7 [DOI] [PubMed] [Google Scholar]
- 45.Acemoglu A., Fichera L., Kepiro I. E., Caldwell D. G., Mattos L. S., “Laser incision depth control in robot-assisted soft tissue microsurgery,” J. Med. Robot. Res. 02(03), 1740006 (2017). 10.1142/S2424905X17400062 [DOI] [Google Scholar]
- 46.Mattos L. S., Deshpande N., Barresi G., Guastini L., Peretti G., “A novel computerized surgeon-machine interface for robot-assisted laser phonomicrosurgery,” Laryngoscope 124(8), 1887–1894 (2014). 10.1002/lary.24566 [DOI] [PubMed] [Google Scholar]
- 47.Chauhan M., Deshpande N., Barresi G., Pacchierotti C., Prattichizzo D., Caldwell D. G., Mattos L. S., “Design and control of a novel robotic microsurgical forceps for transoral laser microsurgery,” in 2017 IEEE International Conference on Advanced Intelligent Mechatronics (AIM) (IEEE, 2017), pp. 737–742. 10.1109/AIM.2017.8014105 [DOI] [Google Scholar]
- 48.Gonzalez-Martinez J., Vadera S., Mullin J., Enatsu R., Alexopoulos A. V., Patwardhan R., Bingaman W., Najm I., “Robot-assisted stereotactic laser ablation in medically intractable epilepsy: operative technique,” Neurosurgery 10(2), 167–172 (2014). 10.1227/NEU.0000000000000286 [DOI] [PubMed] [Google Scholar]
- 49.Olivieri E., Barresi G., Caldwell D. G., Mattos L. S., Olivieri E., Barresi G., Caldwell D. G., Mattos L. S., Olivieri E., Caldwell D. G., Barresi G., Mattos L. S., “Haptic feedback for control and active constraints in contactless laser surgery: concept, implementation, and evaluation,” IEEE Trans. Haptics 11(2), 241–254 (2018). 10.1109/TOH.2017.2786243 [DOI] [PubMed] [Google Scholar]
- 50.Rossi F., Micheletti F., Magni G., Pini R., Menabuoni L., Leoni F., Magnani B., “Laser assisted robotic surgery in cornea transplantation,” Proc. SPIE 10056, 100560T (2017). [Google Scholar]
- 51.Hannaford B., Rosen J., Friedman D. W., King H., Roan P., Cheng L., Glozman D., Ma J., Kosari S. N., White L., “Raven-II: an open platform for surgical robotics research,” IEEE Trans. Biomed. Eng. 60(4), 954–959 (2013). 10.1109/TBME.2012.2228858 [DOI] [PubMed] [Google Scholar]
- 52.Milstein A., Ganel T., Berman S., Nisky I., “Human-centered transparency of grasping via a robot-assisted minimally invasive surgery system,” IEEE Trans. Human-Machine Syst. 48(4), 349–358 (2018). 10.1109/THMS.2018.2846033 [DOI] [Google Scholar]
- 53.Strassmann E., Loya N., Gaton D. D., Ravid A., Kariv N., Weinberger D., Katzir A., “Temperature controlled CO2 laser soldering of pig cornea,” Proc. SPIE 4609, 222–228 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Gabay I., Barequet I., Varssano D., Rosner M., Katzir A., “Bonding surgical incisions using a temperature-controlled laser system based on a single infrared fiber,” J. Biomed. Opt. 18(11), 111416 (2013). 10.1117/1.JBO.18.11.111416 [DOI] [PubMed] [Google Scholar]
- 55.Holl P., Rattunde M., Adler S., Kaspar S., Bronner W., Bachle A., Aidam R., Wagner J., “Recent advances in power scaling of GaSb-based semiconductor disk lasers,” IEEE J. Sel. Top. Quantum Electron. 21(6), 324–335 (2015). 10.1109/JSTQE.2015.2414919 [DOI] [Google Scholar]
- 56.Hung A. J., Patil M. B., Zehnder P., Cai J., Ng C. K., Aron M., Gill I. S., Desai M. M., “Concurrent and predictive validation of a novel robotic surgery simulator: a prospective, randomized study,” J. Urol. 187(2), 630–637 (2012). 10.1016/j.juro.2011.09.154 [DOI] [PubMed] [Google Scholar]
- 57.Shademan A., Decker R. S., Opfermann J. D., Leonard S., Krieger A., Kim P. C. W., “Supervised autonomous robotic soft tissue surgery,” Sci. Transl. Med. 8(337), 337ra64 (2016). 10.1126/scitranslmed.aad9398 [DOI] [PubMed] [Google Scholar]