Abstract
Homocysteine is an amino acid related to metabolism in human vivo, which is closely related to cardiovascular disease, senile dementia, bone fracture, et al. Currently, the usual medical test methods for homocysteine include high performance liquid chromatography (HPLC), fluorescence polarization immunoassay (FPIA) and enzyme-linked immunosorbent assay (ELISA), which are time-consuming or expensive. In this paper, we first analyze the vibration and rotation of homocysteine molecules by using density functional theory, and then we ensure that the theoretical absorption peaks are located in the range of the terahertz spectrum. Then, based on the terahertz time-domain spectroscopy system, we measured the absorption spectrum of homocysteine under different concentrations. It is found that as the detection of the concentration, the terahertz results present higher accuracy than that of the laser Raman spectrum, which can be used as the reference for the evaluation of pathological stage. These results are of great significance for the exact and quick diagnosis of homocysteine-related diseases in clinical medicine.
Introduction
Homocysteine (C4H9NO2S [1]) is a sulphydryl-containing amino acid derived from the metabolic demethylation of dietary methionine, which is abundant in animal protein [2]. Normally, the concentration of homocysteine is 8.9 ± 2.42 μmol/L [1,3] in human plasma and 3.5-9.5 μmol/L [4] in urine. The concentration of homocysteine is affected by many factors, such as nutrition, heredity, environment, et al [1,2], where the environmental factors include body contained vitamins (such as folic acid, B12 and B6), medications, and lifestyle [2]. Different from the beneficial effects of cysteine [5–7], if the homocysteine metabolizing pathways are inhibited due to enzymatic defects or vitamin deficiencies, homocysteine will be accumulated and then leads to an increase of homocysteine levels in plasma/urine [8].
Once the concentration of homocysteine is larger than the normal value, it will be defined as hyperhomocyteinemia, and can be classified as moderate (15-30 μmol/L), intermediate (30-100 μmol/L), and severe (>100 μmol/L) [8]. Specifically, excess homocysteine has many bad effects to human health: (1) the chronic elevation of homocysteine concentration in plasma may induce cellular methylation reactions, bringing about a disturbance of body metabolism [9]. (2) High concentration homocysteine can lead to various forms of vascular disease, including the brain or coronary arteries, and the formation of clots [2,10]. In serious cases, it can even lead to a stroke [11]. (3) High level of homocysteine can lead to inhibition of bone collagen synthesis [12]. It is even associated with bone fracture [13,14]. (4) Abnormal elevation of homocysteine concentration can also be considered as a marker of Alzheimer’s disease (AD), leading to cognitive dysfunction [14,15].
Currently, there are many medical standard methods for homocysteine detection, such as high performance liquid chromatography (HPLC), fluorescence polarization immunoassay (FPIA) and enzyme-linked immunosorbent assay (ELISA). HPLC is based on the separation of material (rely on the differences in the adsorption characteristics, surface charge, ligand specificity, and molecular size of protein molecules), which usually needs dozens of minutes to hours and high cost [13,16]. While the principle of FPIA and ELISA is the specific combination of antigen and antibody. However, FPLA can only detect the antigen with a molecular weight less than 160 kilo Dalton (KD) and be time-consuming [17]. ELISA kit is expensive and can only be used for one time [18].
Terahertz (THz) spectroscopy is a useful method for substances identification and analysis [19–23]. As the vibration and rotation frequency of many organic molecules lie in this THz band, their identifications can be made by THz characteristic absorption spectra named fingerprint spectra [24,25].Therefore, using terahertz spectroscopy is expected to realize the fast, accurate and low cost identification of homocysteine. In this paper, the absorption spectrum of homocysteine was firstly calculated and analyzed by density functional theory, then we used the terahertz time-domain spectroscopy (THz-TDS) to detect the absorption spectrum of homocysteine as the change of concentration. This study provides a reference for the later rapid detection of homocysteine content in human plasma/urine, which is of great significance for clinical medicine.
THz spectra calculated by the density function theory
THz absorption peak is originated from the resonance absorption between the irradiated THz waves and vibration/rotation of atoms/functional groups in the molecule [26]. Based on the calculation of the density functional theory (DFT), we can know the exact corresponding relation between the vibration/rotation modes and each absorption peak. Therefore, a series of calculations were carried out with Density Function Theory (DFT) to study the vibration and rational modes of the homocysteine, where Gaussian-09 packets were used with B3LYP hybrid functional and 3-21G basis set. For the convenience of comparing with the later experimental results, we only present the calculated results in the range of 0.2-2.8 THz, which is the effective spectral range of the THz-TDS system in our lab.
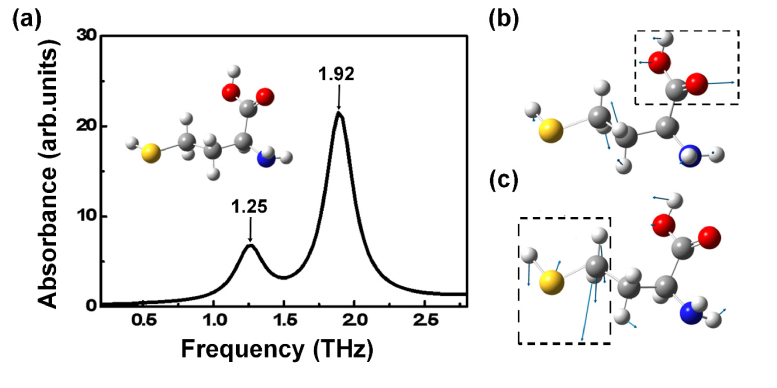
Figure 1 shows the calculated spectrum and the corresponding molecular vibration modes of homocysteine. We can see that homocysteine has two absorption peaks centered around 1.25 and 1.92 THz. Furthermore, the absorption peaks attribute to two different vibration modes of homocysteine: 1) the absorption peak at 1.25 THz is main contributed by the twisting vibration of O = C-OH group; 2) the absorption peak at 1.92 THz is mainly originated from the wagging vibration of H2C-S-H group.
Fig. 1.
(a) Spectrum of homocysteine from DFT calculation. (b, c) Typical vibration modes of homocysteine at 1.25 THz (b) and 1.92 THz (c). Gray, white, red, blue, and yellow atoms present C, H, O, N, S atoms, respectively. The blue arrows indicate the vibrational direction of the atoms, and the dominant functional groups in the modes of vibration are marked with black dotted boxes.
Experiment setup and sample preparation
After the theoretical calculation proves that the fingerprint spectrum of homocysteine is located in the detectable THz range, we used a standard THz time-domain spectroscopy setup (THz-TDS) to measure it. The experimental setup is a commercial Fast scanning system (EKSPLA), whose single scanning time is ~ 0.09s by using a voice coil stage. The details about setup and spectrum can be seen in Appendix 1. Here, we just emphasize that the effective bandwidth for the measured signals is from 0.2 to 2.8 THz, the spectrum resolution is better than 15 GHz, signal to noise radio (SNR) is larger than 1000:1. Besides, all the spectra were averaged 128 times to ensure a high SNR.
Pure homocysteine samples (≥95%, CAS: 454-29-5) were purchased from Sigma Aldrich. Before the sample pretreatment, the concentration ranges should be determined in advance. Considering the content of homocysteine in blood and urine per volume is extremely low, and the water has great absorption for terahertz wave. We propose this detection for homocysteine in solution can use the methods of filtration, purification and drying. Take the urine for instance, two liters of normal urine contains about 2.5 mg homocysteine after concentration and drying. If we mix it with 120 mg polyethylene (PE) powder (particle size is 40-48 µm, CAS: 9002-88-4) to get the standard sample, the concentration will be 0.14 mol/L. Therefore, 0.14 mol/L can be used as the baseline to determine whether human homocysteine content is higher than the normal concentration.
Basing on this concentration analysis, each sample was mixed with PE powder to obtain different concentrations and then pressed into a 1mm thick tablet with 3 tons of force [26,27]. The homocysteine concentration (C, mol/L) is calculated by using the following equations [20]:
| (1) |
| (2) |
where w, M, d, and r are the weight of the homocysteine powder, the molecular weight of homocysteine, and the thickness and radius of the pellet, respectively.
All tablets were controlled at 150 mg, and the mass loss was controlled less than 1%. The mass mixing ratio (mg:mg) between homocysteine and PE powder was 1:48, 1:24, 1:16, 1:12, 1:10, 1:8, 1:7, 1:6, 1:5 and 1:4, which corresponding to the concentration of 0.14 mol/L, 0.28 mol/L, 0.42 mol/L, 0.56 mol/L, 0.67 mol/L, 0.84 mol/L, 0.96 mol/L, 1.12 mol/L, 1.34 mol/L and 1.68 mol/L . The entire spectrum system was placed in a sealed box filled with dry air (humidity less than 3%) to reduce the impact of water vapor [25]. Here, a tablet of 100% PE was tested and used as the reference [Appendix 2], and the same reference spectrum was used for each absorbance spectrum of samples.
Results and discussion
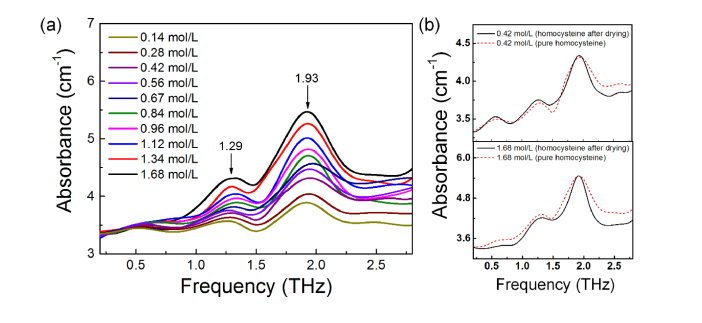
Figure 2(a) presents the experimental absorption spectra of homocysteine samples under ten different concentrations. It is obvious that, all of the spectra has the same absorption peaks at 1.29 and 1.93 THz, which agrees well with the theoretical calculation results.
Fig. 2.
(a) THz absorption spectra of homocysteine samples under different concentrations. The corresponding concentrations are labeled with different colors. (b) Comparison of the absorption spectra of homocysteine solution after drying and that of pure homocysteine, 0.42 mol/L(upper) and 1.68mol/L (down).
Here, we must notice that in real cases such as blood or urine, homocysteine molecules may bond with other molecules such as H2O in the solvent. Even the sample is purified and dried, the final THz absorption may be different. In order to verify this possible problem, we tested this process in experiments: pure homocysteine powder was dissolved in water and made into homocysteine solution. It was then placed in a drying oven (BG2-30), heated at 45°C for 90 mins, which will not affect the property of homocysteine. To ensure the degree of drying, we place the sample in a dry gas environment for another 60 mins. According to the method mentioned above, two samples with different concentrations (0.42 mol/L and 1.68 mol/L) was made and measured. As compared with the absorption spectra of pure homocysteine [see Fig. 2(b)], the dried homocysteine has the same absorption peaks. This shows that the related methods and results are feasible and effective in actual detection.
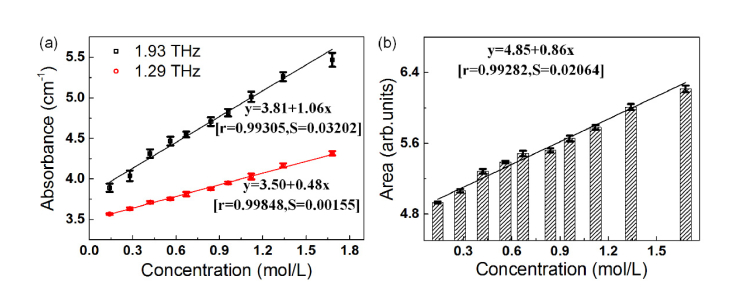
To further quantify the change of absorption peaks with the increase of the homocysteine concentration, we respectively extracted the amplitude and area of the two absorption peaks of all spectra as a function of concentration. Then, according to the Beer-Lambert Law, we performed a linear fitting for the data (see Fig. 3), and the corresponding linear fitting function expression are:
| (3) |
| (4) |
| (5) |
x is the sample concentration, and y is the amplitude or area in the expression, r is the correlation coefficient, S is the standard deviations. Equations (3) and (4) correspond to the amplitude at 1.93 and 1.29 THz, Eq. (5) corresponds to the area during the spectral range of 0.84-2.22 THz.
Fig. 3.
(a) The absorbance of absorption peaks at 1.29 (circles) and 1.93 THz (squares) for different concentrations of homocysteine samples, corresponding to the red and black curve, respectively. (b) The area under the absorption peaks of all homocysteine samples in the range of 0.84-2.22 THz. Each value was the average result of six measurements, and the corresponding error bars were labeled to show the standard deviations. The calculated functions are shown in the figure with the correlation coefficients (r) and the standard deviations (S). Each value was the average result of six measurements, and the corresponding error bars were labeled to demonstrate the analytical utility.
By comparison, it is found that the change rule presented in the black square curve (1.93 THz) in Fig. 3 (a) and 3(b) are approximately consistent. This change trend will help us to detect different concentration levels of homocysteine in human plasma/urine with high sensitivity and to realize quick quantitative analysis based on the linear fitting equation. This is useful for the later clinical test with high speed and sensitivity.
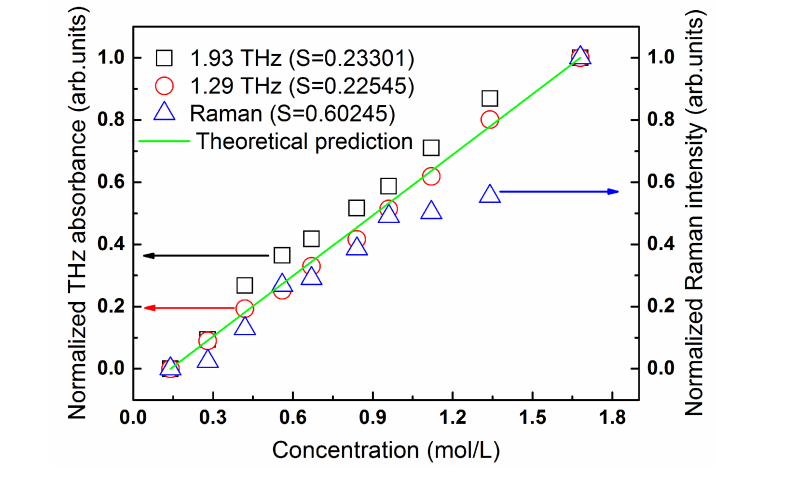
After the quantitative analysis of homocysteine samples, we also want to compare our detection methods with other measurement techniques. Here, we mainly evaluate the accuracy of the test results. We used Raman spectroscopy [Appendix 3] to repeat the above experiments, and then compared the results of the two detection methods. Considering the difference in unit of measurement, we normalize the terahertz absorbance and Raman intensity, and compare their linear deviation in the same figure. The theoretical prediction is the fitting line basing Beer-Lambert Law. As shown in Fig. 4, the standard deviation (S) of Raman spectrum to theoretical prediction line is much larger than that of terahertz spectra (SRaman = 0.60245, S1.29THz = 0.22545, S1.93THz = 0.23301). Therefore, we can deduce that in the detection of samples with different concentrations, the terahertz method has higher accuracy than that of the Raman method.
Fig. 4.
Comparison of homocysteine measured by terahertz spectroscopy vs Raman spectroscopy. The green solid curve represents standard values of concentration and the corresponding methods are labeled with different symbols. S is the standard deviations.
Conclusion
In this paper, we provide a new detection method to realize the fast, accurate and low cost identification of homocysteine. Based on the DFT function in Gaussian software, we got the calculated spectrum and corresponding relationship between absorption peak and molecular vibration mode. After proving the effective fingerprint spectrum in terahertz frequency range, we have done a series of tests. Combining with the fitting equations, as long as we can obtain the amplitude or areas at the two absorption peaks, we can accurately detect the concentrations of homocysteine samples.
Of course, in human blood and urine, there are many other amino acids, which also have their own THz fingerprint spectra and may superpose with the homocysteine spectrum. For this problem, it can be solved from four aspects: (1) using high speed centrifuge to separate different substances with different molecular weight; (2) using chromatographic instrument; (3) using filter paper with different pore size to separate different substances with different molecular size; (4) combing with the purified spectra obtained in this study and the mixture algorithm [26,28] to extra the absorption peaks of homocysteine. These measurements are also our next work plan.
Overall, the result of this study not only provide reference value for the detection of homocysteine in human plasma/urine, but also realize early monitoring and post-treatment of the diseases like cardiovascular disease, senile dementia, bone fracture, et al. These results are of great significance to the diagnosis of clinical medicine.
Appendix 1: Experimental setup, standard waveform and Fourier transform of THz radiation
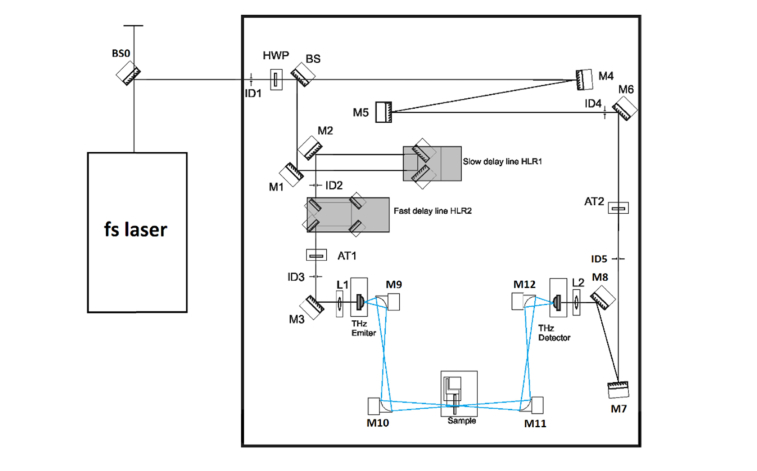
The experimental setup we used is a commercial Fast scanning system (EKSPLA) [see Fig. 5
Fig. 5.
Optical layout of THz spectroscopy kit in transmission configuration.
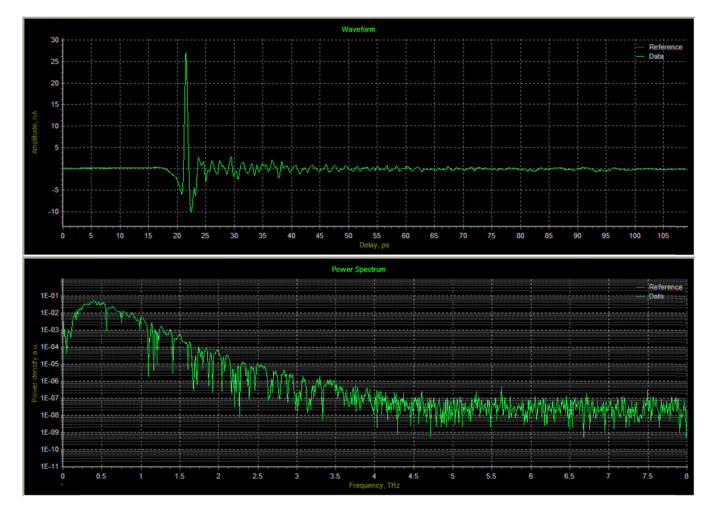
], whose time-domain waveform and frequency spectrum are shown in Fig. 6
Fig. 6.
Waveform and Fourier transform of THz radiation (room temperature and undried).
. The single scanning time is ~0.09 s by using a voice coil stage, and the time required to collect each set of 128 spectra is about 12 s.
Appendix 2: Reference spectrum
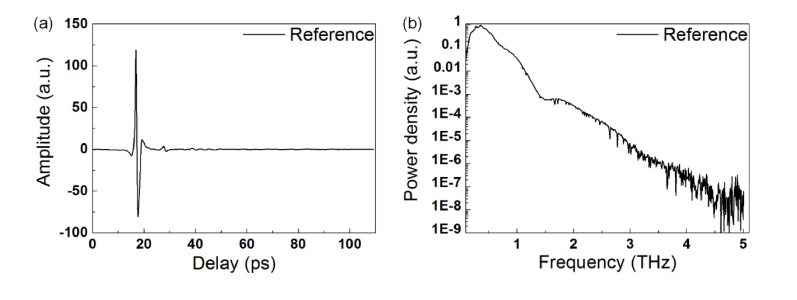
In our experiments, we used the 120 mg 100% PE tablet as the reference, whose time domain waveform is shown in Fig. 7(a)
Fig. 7.
Time domain waveform and frequency spectrum of 100% PE tablet (room temperature and 3% humidity).
. Before the FFT, the reflection peak was removed and 1578 points was zero-padding to keep the total points is 2048. The frequency spectrum is shown in Fig. 7(b).
Appendix 3: Raman spectroscopy and spectrum
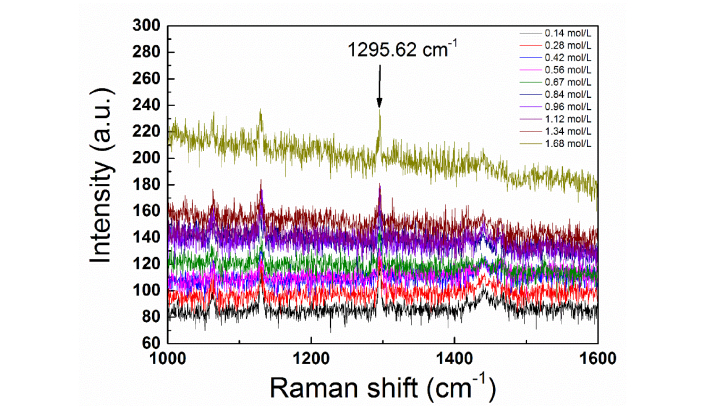
The Spectroscopy we used is Raman station 400F, excitation wavelength is 532 nm, optional power is 250 mW. Here, the characteristic peak we selected is located at 1295.62 cm−1, which is originate from the vibration of ωCH2 + τNH2 + βCCH [29]. The Raman spectrum of homocysteine with different concentrations was shown in Fig. 8
Fig. 8.
Raman spectrum of homocysteine samples under different concentrations. The corresponding concentrations are labeled with different colors.
. We extracted the Raman intensity at 1295.62 cm−1 under different concentrations. Considering the signal intensity stands for the amounts of radicals and molecules, thereby, we obtained the corresponding relationship between Raman intensity and homocysteine concentration [see Fig. 4].
Typographical corrections were made to the author affiliations.
Funding
National Development Project of Scientific Instrument and Equipment (2017YFF0106300); National Natural Science Foundation of China (61722111, 61771314); Shanghai Rising-Star Program (17QA1402500); Shuguang Program (17SG45); Shanghai Pujiang Program (16PJD033); Shanghai Youth Talent Support Program; Young Yangtse Rive Scholar (Q2016212).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Mudd S. H., Finkelstein J. D., Refsum H., Ueland P. M., Malinow M. R., Lentz S. R., Jacobsen D. W., Brattström L., Wilcken B., Wilcken D. E. L., Blom H. J., Stabler S. P., Allen R. H., Selhub J., Rosenberg I. H., “Homocysteine and its disulfide derivatives: a suggested consensus terminology,” Arterioscler. Thromb. Vasc. Biol. 20(7), 1704–1706 (2000). 10.1161/01.ATV.20.7.1704 [DOI] [PubMed] [Google Scholar]
- 2.Hankey G. J., Eikelboom J. W., “Homocysteine and vascular disease,” Lancet 354(9176), 407–413 (1999). 10.1016/S0140-6736(98)11058-9 [DOI] [PubMed] [Google Scholar]
- 3.Jacques P. F., Selhub J., Bostom A. G., Wilson P. W. F., Rosenberg I. H., “The effect of folic acid fortification on plasma folate and total homocysteine concentrations,” N. Engl. J. Med. 340(19), 1449–1454 (1999). 10.1056/NEJM199905133401901 [DOI] [PubMed] [Google Scholar]
- 4.Refsum H., Helland S., Ueland P. M., “Radioenzymic determination of homocysteine in plasma and urine,” Clin. Chem. 31(4), 624–628 (1985). [PubMed] [Google Scholar]
- 5.David K. R., “Source, metabolism, and function of cysteine and glutathione in the central nervous system,” Methods in neurosciences 30, 167– 177 (1996). [Google Scholar]
- 6. Burns A., Olszowy P., Ciborowski P., “Biomolecules,” in Proteomic Profiling and Analytical Chemistry (Elsevier, 2016), pp. 7–14. 10.1016/B978-0-444-63688-1.00002-1 [DOI] [Google Scholar]
- 7.Kuśmierek K., Głowacki R., Bald E., “Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography,” Anal. Bioanal. Chem. 385(5), 855–860 (2006). 10.1007/s00216-006-0454-x [DOI] [PubMed] [Google Scholar]
- 8.Refsum H., Ueland P. M., Nygård O., Vollset S. E., “Homocysteine and cardiovascular disease,” Annu. Rev. Med. 49, 31–62 (1998). 10.1146/annurev.med.49.1.31 [DOI] [PubMed] [Google Scholar]
- 9.Yi P., Melnyk S., Pogribna M., Pogribny I. P., Hine R. J., James S. J., “Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation,” J. Biol. Chem. 275(38), 29318–29323 (2000). 10.1074/jbc.M002725200 [DOI] [PubMed] [Google Scholar]
- 10.Nygård O., Nordrehaug J. E., Refsum H., Ueland P. M., Farstad M., Vollset S. E., “Plasma homocysteine levels and mortality in patients with coronary artery disease,” N. Engl. J. Med. 337(4), 230–237 (1997). 10.1056/NEJM199707243370403 [DOI] [PubMed] [Google Scholar]
- 11.Perry I. J., Refsum H., Morris R. W., Ebrahim S. B., Ueland P. M., Shaper A. G., “Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men,” Lancet 346(8987), 1395–1398 (1995). 10.1016/S0140-6736(95)92407-8 [DOI] [PubMed] [Google Scholar]
- 12.Enneman A. W., Swart K. M. A., Zillikens M. C., van Dijk S. C., van Wijngaarden J. P., Brouwer-Brolsma E. M., Dhonukshe-Rutten R. A. M., Hofman A., Rivadeneira F., van der Cammen T. J. M., Lips P., de Groot C. P. G. M., Uitterlinden A. G., van Meurs J. B. J., van Schoor N. M., van der Velde N., “The association between plasma homocysteine levels and bone quality and bone mineral density parameters in older persons,” Bone 63, 141–146 (2014). 10.1016/j.bone.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Frantzen F., Faaren A. L., Alfheim I., Nordhei A. K., “Enzyme conversion immunoassay for determining total homocysteine in plasma or serum,” Clin. Chem. 44(2), 311–316 (1998). [PubMed] [Google Scholar]
- 14.Miller J. W., “Homocysteine, Alzheimer’s disease, and Cognitive Function,” Nutrition 16(7-8), 675–677 (2000). 10.1016/S0899-9007(00)00307-5 [DOI] [PubMed] [Google Scholar]
- 15.Seshadri S., Beiser A., Selhub J., Jacques P. F., Rosenberg I. H., D’Agostino R. B., Wilson P. W. F., Wolf P. A., “Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease,” N. Engl. J. Med. 346(7), 476–483 (2002). 10.1056/NEJMoa011613 [DOI] [PubMed] [Google Scholar]
- 16.Vervoort N., Daemen D., Török G., “Performance evaluation of evaporative light scattering detection and charged aerosol detection in reversed phase liquid chromatography,” J. Chromatogr. A 1189(1-2), 92–100 (2008). 10.1016/j.chroma.2007.10.111 [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer C. M., Twite D., Shih J., Holets-McCormack S. R., Gunter E. W., “Method comparison for total plasma homocysteine between the Abbott IMx analyzer and an HPLC assay with internal standardization,” Clin. Chem. 45(1), 152–153 (1999). [PubMed] [Google Scholar]
- 18.Rosén J., “Efficient and sensitive screening and confirmation of residues of selected polyether ionophore antibiotics in liver and eggs by liquid chromatography-electrospray tandem mass spectrometry,” Analyst (Lond.) 126(11), 1990–1995 (2001). 10.1039/b105133b [DOI] [PubMed] [Google Scholar]
- 19.Zhong H., Redo-Sanchez A., Zhang X. C., “Identification and classification of chemicals using terahertz reflective spectroscopic focal-plane imaging system,” Opt. Express 14(20), 9130–9141 (2006). 10.1364/OE.14.009130 [DOI] [PubMed] [Google Scholar]
- 20.Ueno Y., Rungsawang R., Tomita I., Ajito K., “Quantitative measurements of amino acids by terahertz time-domain transmission spectroscopy,” Anal. Chem. 78(15), 5424–5428 (2006). 10.1021/ac060520y [DOI] [PubMed] [Google Scholar]
- 21.Zeitler J. A., Newnham D. A., Taday P. F., Strachan C. J., Pepper M., Gordon K. C., Rades T., “Temperature dependent terahertz pulsed spectroscopy of carbamazepine,” Thermochemical ACTA 436(1–2), 71–77 (2005). 10.1016/j.tca.2005.07.006 [DOI] [Google Scholar]
- 22.Ajito K., Ueno Y., “THz chemical imaging for biological applications,” IEEE Trans. Terahertz Sci. Technol. 1(1), 293–300 (2011). 10.1109/TTHZ.2011.2159562 [DOI] [Google Scholar]
- 23.Nishizawa J., Sasaki T., Suto K., Tanabe T., Saito K., Yamada T., Kimura T., “THz transmittance measurements of nucleobases and related molecules in the 0.4-to 5.8-THz region using a GaP THz wave generator,” Opt. Commun. 246(1–3), 229–239 (2005). 10.1016/j.optcom.2004.10.076 [DOI] [Google Scholar]
- 24.Sun J., Shen J., Liang L., Xu X., Liu H., Zhang C., “Experimental investigation on terahertz spectra of amphetamine Type Stimulants,” Chin. Phys. Lett. 22(12), 3176–3178 (2005). 10.1088/0256-307X/22/12/054 [DOI] [Google Scholar]
- 25.Zheng X. M., McLaughlin C. V., Cunningham P., Hayden L. M., “Origin broadband terahertz sources and sensors,” Journal of Nanoelectronics and Optoelectronics 2(1), 58–76 (2007). 10.1166/jno.2007.005 [DOI] [Google Scholar]
- 26.Peng Y., Yuan X., Zou X., Chen W., Huang H., Zhao H., Song B., Chen L., Zhu Y., “Terahertz identification and quantification of neurotransmitter and neurotrophy mixture,” Biomed. Opt. Express 7(11), 4472–4479 (2016). 10.1364/BOE.7.004472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W., Peng Y., Jiang X., Zhao J., Zhao H., Zhu Y., “Isomers Identification of 2-hydroxyglutarate acid disodium salt (2HG) by Terahertz Time-domain Spectroscopy,” Sci. Rep. 7(1), 12166 (2017). 10.1038/s41598-017-11527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y., Shi C., Xu M., Kou T., Wu X., Song B., Ma H., Guo S., Liu L., Zhu Y., “Qualitative and quantitative identification of components in mixture by Terahertz spectroscopy,” IEEE Trans. Terahertz Sci. Technol. 2018, 1 (2018). 10.1109/TTHZ.2018.2867816 [DOI] [Google Scholar]
- 29.Gunasekarana S., Brightb A., Renuga Devib T. S., Arunbalajic R., Anandd G., Dhanalakshmie J., Kumaresanf S., “Experimental and semi-empirical computations of the vibrational spectra of methionine, homocysteine and cysteine,” Archives of Physics Research 1(1), 12– 26 (2010). [Google Scholar]