Abstract
We report a cinchona alkaloid catalyzed addition of thiophenol into rapidly interconverting aryl-naphthoquinones, resulting in stable biaryl atropisomers upon reductive methylation. An array of thiophenols and naphthoquinone substrates were evaluated, and we observed selectivities up to 98.5:1.5 e.r. Control of the quinone redox properties allowed us to study the stereochemical stabilities of each oxidation state of the substrates. The resulting enantioenriched products can also be moved on via an SNAr-like reaction sequence to arrive at stable derivatives with excellent enantioretention.
Keywords: atroposelective, thiophenol, aryl-naphthoquinone, cinchona alkaloid, biaryl atropisomers
Graphical Abstract

Atropisomerism is a hallmark of many bioactive small molecules1,2 and privileged catalyst scaffolds.3 As such, there has been significant effort toward developing atroposelective methodology4–6 over the past decade. Seminal examples include strategies where the enantioselectivity is induced during formation of the chiral axis,7–9 via cyclization10,11 and point-toaxial chirality transfer.12 Recently, Miller13–15 and others16–18 have studied atropisomer selective dynamic kinetic resolutions (DKR), wherein a configurationally unstable atropisomer is rendered stereochemically stable through various means.
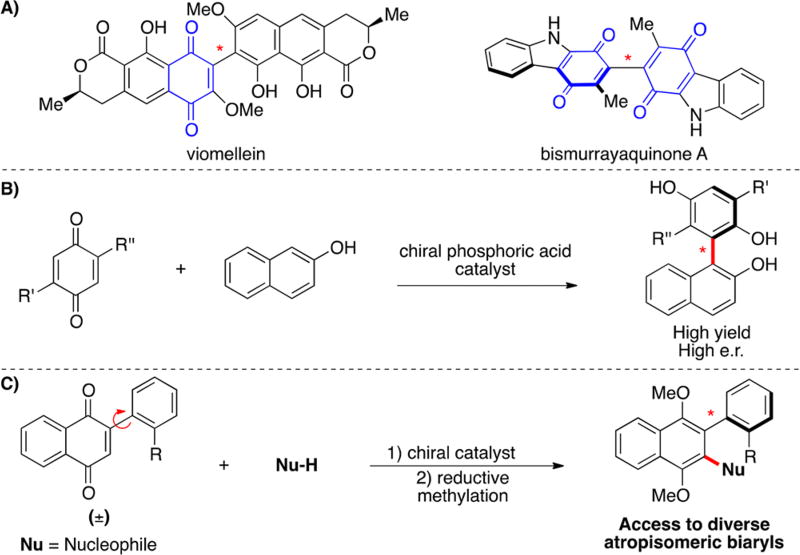
Quinones are common pharmacophores in small molecules and natural products, which can often covalently target nucleophilic protein residues.19 As redox-active moieties, quinones and hydroquinones can also play distinct roles in redox cycling and cytotoxicity.20,21 Many bioactive quinones and hydroquinones are atropisomeric (Figure 1A). Starting in 2015, Tan22 and others23–25 reported the atroposelective addition of 2-naphthols into quinones using a chiral catalyst (Figure 1B). Inspired by this seminal work, we posited that an enantioselective synthesis of atropisomers could be achieved by the addition of a nucleophile into a quinone, adjacent to a rapidly interconverting atropisomeric axis (Figure 1C). We felt this strategy, which is analogous to vicarious nucleophilic substitution, could potentially afford both biaryl and nonbiaryl atropisomers with unparalleled diversity because of the plethora of nucleophiles that are amenable to catalytic activation coupled with the myriad atropisomeric scaffolds that quinones can be embedded in.
Figure 1.
Quinone scaffold in atropisomerism. (A) Examples of axially chiral quinones in natural products. (B) Previous work: Chiral biaryldiols from 2-naphthols and quinones. (C) This work: Enantioselective synthesis of stable atropisomers from aryl-naphthoquinones.
To test this approach, we chose to study the addition of thiophenols into naphthoquinones (as in Figure 1C). The addition of thiophenols proximal to an “atropisomerically labile” aryl-naphthoquinone axis would be expected to yield stable biaryl atropisomers, and thus, this approach would be amenable to a catalytic atroposelective variant. Such an approach could hold utility for diverse applications. For example, atropisomers with sulfur functionality are known to be effective chiral ligands,26 and recent work has demonstrated that C–S bonds can be functionalized in a manner comparable to halogens, suggesting that the enantioenriched products from such a reaction could be further elaborated to diverse chiral scaffolds.27,28 In support of the feasibility of an atroposelective addition, the nucleophilic addition of thiophenols is a well-studied topic in enantioselective catalysis,29 exemplified by work by Wynberg.30,31 Furthermore, work from Smith32 and a recent kinetic resolution from our group33 have shown that chiral quaternary ammonium salts can effect atroposelective thiophenol addition via SNAr.
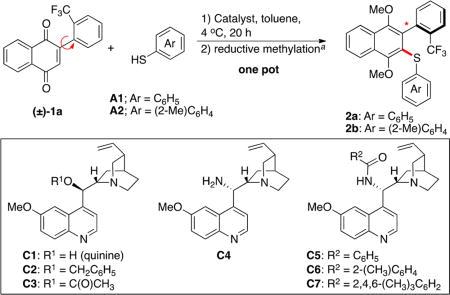
To begin our studies, we evaluated different quinine-derived catalysts for the addition of thiophenol into quinone biaryl 1a. To remove any complexities caused by the quinone oxidation state (vida infra), we quenched each reaction by reductive methylation using Na2S2O4 and dimethyl sulfate. The reaction of 1a with thiophenol in the absence of catalyst proceeded with minimal conversion (Table 1, entry 1). Quinine (C1) did catalyze the addition of thiophenol, yielding 49% 2a with preliminary levels of enantioselectivity (57:43 e.r.; Table 1, entry 2). Benzyl quinine (C2) and acetylated quinine (C3) proved to be ineffective catalysts (Table 1, entries 3 and 4); however, 9-amino-epi-cinchona alkaloid analogues proved to be promising, with primary amine C4 yielding 79% of 2a in 75:25 e.r. and benzoylated analogue C5 yielding similar results (Table 1, entries 5 and 6).
Table 1.
Reaction Optimization
| ||||
|---|---|---|---|---|
| Entry | Cat. (mol%) | Conc. [M] | Yieldb | e.r.b |
| 1 | None | .025 | <5% | n/a |
| 2 | C1 (10) | .025 | 49% | 57:43 |
| 3 | C2 (10) | .025 | 48% | 50:50 |
| 4 | C3 (10) | .025 | 17% | 46:54 |
| 5 | C4 (10) | .025 | 79% | 75:25 |
| 6 | C5 (10) | .025 | 70% | 78:22 |
| 7 | C6 (10) | .025 | 65% | 87:13 |
| 8 | C7 (10) | .025 | 69% | 93:7 |
| 9 | C7 (10) | .05 | 84% | 93:7 |
| 10c | C7 (10) | .05 | 75% | 87:13 |
| 11d | C7 (10) | .05 | 67% | 86:14 |
| 12 | C7 (2.5) | .1 | 80% | 88:12 |
| 13 | C7 (2.5) | .05 | 73% | 93:7 |
| 14 | C7 (5) | .05 | 89% | 93:7 |
| 15e | C7 (5) | .05 | 68% | 96:4 |
| 16e,f | C7 (5) | .05 | 82% | 96:4 |
Na2S2O4(aq), THF, MeOH, TBAB at 0 °C then KOH(aq), Me2SO4.
Isolated yields and enantiomeric ratios (e.r.) are reported as an average of two trials with A1 used as the nucleophile.
Reaction was run at room temperature.
Reaction was placed in −18 °C freezer for 20 h without stirring.
A2 was used as the nucleophile.
Reaction was allowed to stir for 44 h before reductive quench.
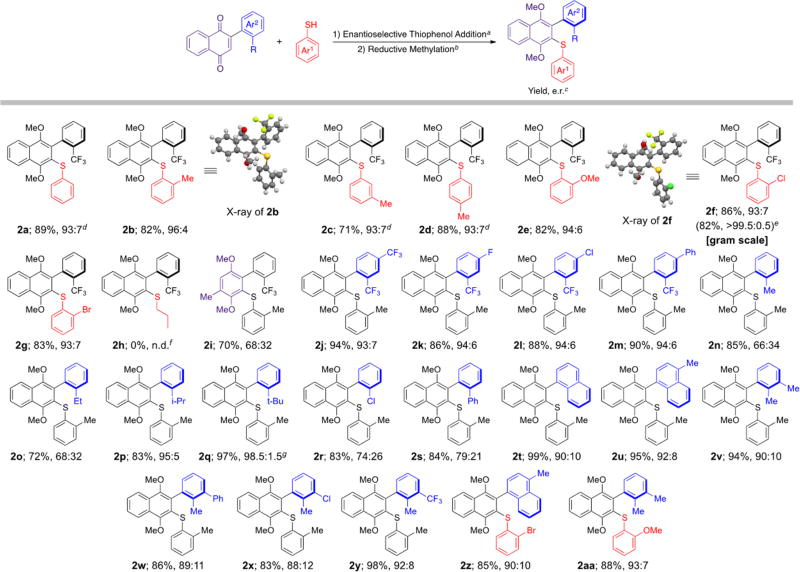
Methodical elaboration of C4 to diverse ureas, carbamates, and amides were met with little improvement in enantioselectivity. These results, however, proved to be informative and suggested a reliance on steric bulk distal to the “active site” of the catalyst (see Supporting Information). On the basis of these results, we hypothesized an ortho-substituted benzamide might improve selectivity because of catalyst conformational changes related to the “magic methyl effect.”34 For example, adding a methyl group to the 2-position of the benzamide, as in C6, would be expected to bias the arene-carbonyl bond toward a pseudoperpendicular conformation, thereby increasing the effective radius of the benzamide. This hypothesis proved to be fruitful, as C6 yielded a notable increase in enantioselectivity (87:13 e.r., Table 1, entry 7). The addition of a second orthomethyl group led to our best catalyst, C7, which yielded 69% of 2a in 93:7 e.r. (Table 1, entry 8). Further modification of reaction conditions led to 89% yield of 2a with retained 93:7 e.r. (Table 1, entries 9–14). Finally, using o-toluenethiol as the nucleophile afforded 2b with an improved e.r. of 96:4 (Table 1, entries 15 and 16).
With an optimized atroposelective synthesis in hand, we set out to gain a better understanding of the effect of the thiophenol structure (Scheme 1). Moving the methyl group on the thiophenol away from the ortho- position (2c and 2d) resulted in decreased e.r. values. Replacing the o-methyl group with methoxy, chlorine, or bromine also led to slight decreases in e.r. (94:6, 93:7, 93:7; respectively). We were able to recrystallize 2f on the gram scale in 82% overall yield as a near enantiopure atropisomer (>99.5:0.5 e.r.). We obtained the crystal structures of 2b and 2f, revealing the major enantiomer from this reaction to be the (Ra) atropisomer. Because of structural similarity, the stereochemistry of all other substrates were assigned by analogy. Reacting 1a with 1-propanethiol, rather than thiophenol, resulted in no observable reaction. Lastly, performing this DKR on a quinone-based scaffold gave 2i in 70% yield and 68:32 e.r., suggesting this chemistry could be applicable to quinone scaffolds upon further optimization.
Scheme 1. Substrate Scope.
aAryl-naphthoquinone (0.1 mmol), toluene (2 mL), C7 (0.005 mmol), thiophenol (0.2 mmol) was stirred at 4 °C for 44 h. bNa2S2O4(aq), toluene, THF, MeOH, TBAB at 0 °C, then KOH(aq), Me2SO4. cIsolated yields are reported, and e.r. was determined by HPLC using a chiral stationary phase (average of three trials). dEnantioselective thiophenol addition ran for 20 h. eAfter recrystallization. fNo reaction was observed by TLC after 20 h. gEnantioselective thiophenol addition ran for 68 h.
Continuing with the naphthoquinone scaffold, we sought out to explore a range of substitutions on the nonquinone aryl ring (Scheme 1). In general, adding substitution at the para- position had little effect on enantioselectivity, giving 2j–2m in high yields with 93:7 e.r. or better. However, replacing the –CF3 group with methyl (2n) resulted in a drop in e.r. to 66:34. Enantioselectivity was improved as we increased the steric bulk of the alkyl substituent, with –i-Pr (2p) resulting in 95:5 e.r. and –t-Bu (2q) resulting in 98.5:1.5 e.r. Moreover, replacing –CF3 with –Cl (2r) resulted in a drop in e.r. to 74:26, and a similar result was observed with a phenyl group ortho- to the atropisomeric axis (2s, 79:21 e.r.). The [1,2′-binaphthalene] atropisomer (2t) was isolated in 99% yield and 90:10 e.r., and the structurally analogous 4-methyl-[1,2′-binaphthalene], 2u, was isolated in 95% yield and 92:8 e.r.. These results encouraged us to probe the effect of meta- substitution on the nonquinone aryl, finding that adding adjacent meta-substituents to poor substrates such as 2n, (2v–2y), afforded significantly improved enantioselectivities ranging from 88:12 to 92:8 e.r., all in high yields. Notably, these selectivities were conserved when different thiophenols were used, as exemplified by 2z and 2aa. Overall, this methodology proved amenable to a diversity of substitution patterns within the confines of atropisomer stability.
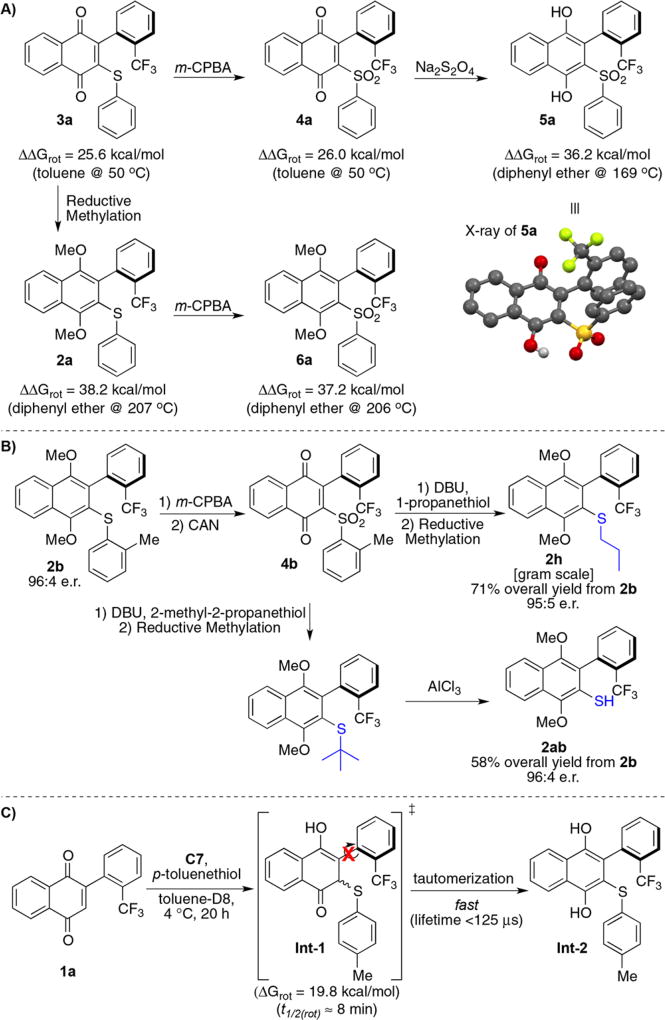
When we did not quench the reaction via reductive methylation, we observed spontaneous oxidation of the dihydroquinone to the naphthoquinone product (i.e., 3a), resulting in lower yields and enantioselectivities. The lower selectivity in the absence of reductant is likely due to racemization of the quinone as the reaction warms to room temperature. We experimentally determined the barrier to rotation of 3a to be 25.6 kcal/mol in toluene at 50 °C, which translates to an undesirable stereochemical stability at room temperature (Figure 2A). Upon oxidizing 3a to sulfone 4a, we observed a small increase in stereochemical stability to 26.0 kcal/mol, also in toluene at 50 °C. Moreover, this quinone could now be reduced to 5a, which does not spontaneously oxidize because of the electron-withdrawing sulfone. This allowed us to obtain a crystal structure (enantiomer was Ra) and determine its stereochemical stability, observing a barrier to rotation of 36.2 kcal/mol for 5a in diphenyl ether at 169 °C. Thus, the observed barrier to rotation should preclude any observable racemization at room temperature. This drastic increase in stereochemical stability compared with 4a is likely due to the longer C–O bond length in hydroquinones. Furthermore, analysis of aryl-quinone crystal structures reveal an out-of-plane distortion across the atropisomeric axis, which could also account for the lower observed barriers to rotation in arylquinones. 35 The methylated dihydroquinones proved even more stable, as 2a exhibits an experimentally determined barrier to rotation of 38.2 kcal/mol in diphenyl ether at 207 °C. Next, we performed an SNAr-like reaction sequence on 2b to arrive at 2h in 71% overall yield on the gram scale with nearly complete enantioretention (Figure 2B), despite proceeding through the low-barrier quinone oxidation state. Moreover, when 2-methyl-2-propanethiol is used instead of 1-propanethiol, the tert-butyl group could be removed from the resulting tert-butyl sulfane with AlCl3 to furnish the corresponding atropisomeric biaryl thiol with complete enantioretention. This result suggests that enantioretentive substitutions with other nucleophiles are possible; however, that is perhaps beyond the scope of this work. Furthermore, having alkyl-sulfide substitution also provides a starting point for atropisomer diversification as there are several recent methodologies concerning C–S bond activation.36–39
Figure 2.
Stereochemical stability and discussion of stereoinduction. (A) Effect of quinone oxidation state on conformational stability. (B) Enantioretention in gram-scale nucleophilic substitution. (C) Tautomerism is likely much faster than atropisomer bond rotation.
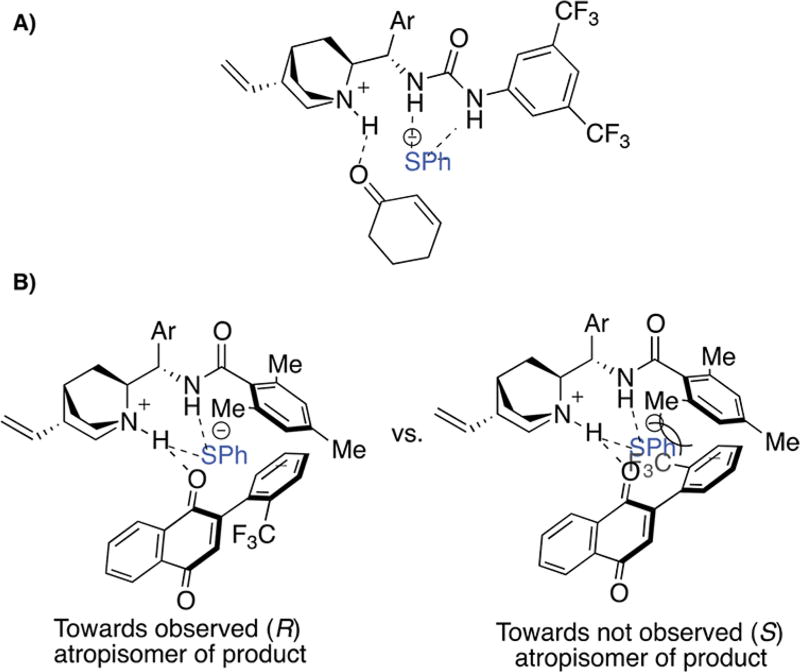
Houk has recently reported a Brønsted acid-hydrogen bonding model (Figure 3A)40,41 for cinchona alkaloid-catalyzed sulfa-michael reactions, based on Wynberg’s work.30,31 We postulate this transformation likely parallels Houk’s model, as we found that C7 can effect the addition of thiophenol into cyclohexanone with moderate levels of enantioselectivity (see Supporting Information). This result opens the possibility that the stereoselectivity of this transformation is induced via a “point-to-axial” chirality transfer mechanism in which the thiophenol adds enantioselectively into the quinone to give diastereomeric Int-1, followed by the equilibration of the atropisomeric axis to the more stable diastereomer and tautomerization of Int-1 to the corresponding hydroquinone Int-2. To probe this, we monitored the reaction of 1a with p-toluenethiol in the presence of C7 by 1H NMR, observing no evidence of the point chiral addition adduct Int-1, meaning tautomerization of Int-1 to the corresponding hydroquinone (Int-2) likely occurs on the hundred-millisecond time scale or faster (Figure 2C, refer to discussion of NMR kinetics study in Supporting Information for more details). We next computationally determined the barrier to rotation of Int-1 to be approximately 19.8 kcal/mol in the gas phase, which roughly translates to a t1/2 to atropisomer bond rotation of about 8 min at 4 °C. While the error to the calculated barriers to rotation may not be accurate enough to draw an absolute conclusion, these experiments do suggest that tautomerization of Int-1 to Int-2 likely occurs faster than rotation of the atropisomeric bond, offering evidence against point-to-axial chirality, and perhaps in favor of a mechanism in which the axis is set prior to thiophenol addition.
Figure 3.
Mechanistic model. (A) Houk’s Brønsted acid-hydrogen bonding model. (B) Plausible model for stereochemical induction.
One possible model, for stereochemical induction, largely inspired by Houk’s model, is proposed in Figure 3B. Thiophenol is deprotonated by the quinuclidine base and oriented via H-bonding with the C-9 benzamide. The quinone is activated by an H-bond with the quinuclidinium ion, orienting the atropisomer axis in a way such that the ortho- substitution of the aryl group avoids steric interaction with the catalyst. More detailed studies are underway, including those aimed to better understand the atropisomer differentiation by C7 to further probe the likelihood of potential modes of stereoinduction.
In conclusion, we have developed a novel atroposelective addition of thiophenol into rapidly interconverting aryl-naphthoquinones to afford stereochemically stable enantioenriched biaryl sulfides. This report lends itself as a proof-of-concept toward a general strategy toward the enantioselective synthesis of diverse biaryl and nonbiaryl atropisomers. With proper control of the redox properties of the quinone moiety, we showed that we can modulate the atropisomer’s stereochemical stability, as well as its reactivity. Furthermore, the resulting biaryl sulfide was moved on in an SNAr-like reaction sequence with near complete enantioretention. Finally, an argument was made for the mode of asymmetric induction, as point-to-axial chirality transfer may not be the likely driving force for stereoinduction.
Supplementary Material
Acknowledgments
We thank Dr. Bennet Addison and Professor Joann Um for technical assistance. This work was funded by the NIH/ NIGMS (R35GM124637). G.A.D. and A.B.A. are grateful for support from the NIH funded Initiative for Maximizing Student Development (IMSD) (5R25GMO58906).
Footnotes
ASSOCIATED CONTENT
The authors declare no competing financial interest.
References
- 1.Glunz PW. Recent Encounters with Atropisomerism in Drug Discovery. Bioorg. Med. Chem. Lett. 2018;28:53–60. doi: 10.1016/j.bmcl.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Zask A, Murphy J, Ellestad GA. Biological Stereoselectivity of Atropisomeric Natural Products and Drugs. Chirality. 2013;25:265–274. doi: 10.1002/chir.22145. [DOI] [PubMed] [Google Scholar]
- 3.Yoon TP, Jacobsen EN. Privileged Chiral Catalysts. Science. 2003;299:1691–1693. doi: 10.1126/science.1083622. [DOI] [PubMed] [Google Scholar]
- 4.Bencivenni G. Organocatalytic Strategies for the Synthesis of Axially Chiral Compounds. Synlett. 2015;26:1915–1922. [Google Scholar]
- 5.Bringmann G, Menche D. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Acc. Chem. Res. 2001;34:615–624. doi: 10.1021/ar000106z. [DOI] [PubMed] [Google Scholar]
- 6.Bringmann G, Price Mortimer AJ, Keller PA, Gresser MJ, Garner J, Breuning M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem., Int. Ed. 2005;44:5384–5427. doi: 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Jones GO, Watson Da, Bhayana B, Buchwald SL. Enantioselective Synthesis of Axially Chiral Biaryls by the Pd-Catalyzed Suzuki-Miyaura Reaction: Substrate Scope and Quantum Mechanical Investigations. J. Am. Chem. Soc. 2010;132:11278–11287. doi: 10.1021/ja104297g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, Hayashizaki K, Kiyoi T, Ito Y. Asymmetric Synthesis Catalyzed by Chiral Ferrocenylphosphine-Transition-Metal Complexes. 6. Practical Asymmetric Synthesis of 1,1′-Binaphthyls via Asymmetric Cross-Coupling with a Chiral [(Alkoxyalkyl)ferrocenyl]-monophosphine/Nickel Catalyst. J. Am. Chem. Soc. 1988;110:8153–8156. [Google Scholar]
- 9.Nicolaou KC, Li H, Boddy CNC, Ramanjulu JM, Yue T-Y, Natarajan S, Chu X-J, Bräse S, Rübsam F. Total Synthesis of Vancomycin—Part 1: Design and Development of Methodology. Chem. - Eur. J. 1999;5:2584–2601. [Google Scholar]
- 10.Link A, Sparr C. Organocatalytic Atroposelective Aldol Condensation: Synthesis of Axially Chiral Biaryls by Arene Formation. Angew. Chem., Int. Ed. 2014;53:5458–5461. doi: 10.1002/anie.201402441. [DOI] [PubMed] [Google Scholar]
- 11.Fäseke VC, Sparr C. Stereoselective Arene-Forming Aldol Condensation: Synthesis of Axially Chiral Aromatic Amides. Angew. Chem., Int. Ed. 2016;55:7261–7264. doi: 10.1002/anie.201602689. [DOI] [PubMed] [Google Scholar]
- 12.Jolliffe JD, Armstrong RJ, Smith MD. Catalytic Enantioselective Synthesis of Atropisomeric Biaryls by a Cation-Directed O-Alkylation. Nat. Chem. 2017;9:558–562. doi: 10.1038/nchem.2710. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson JL, Lim D, Miller SJ. Dynamic Kinetic Resolution of Biaryl Atropisomers via Peptide-Catalyzed Asymmetric Bromination. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett KT, Miller SJ. Enantioselective Synthesis of Atropisomeric Benzamides through Peptide-Catalyzed Bromination. J. Am. Chem. Soc. 2013;135:2963–2966. doi: 10.1021/ja400082x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diener ME, Metrano AJ, Kusano S, Miller SJ. Enantioselective Synthesis of 3-Arylquinazolin-4(3H)-Ones via Peptide-Catalyzed Atroposelective Bromination. J. Am. Chem. Soc. 2015;137:12369–12377. doi: 10.1021/jacs.5b07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann G, Hartung T. First Atropo-Enantioselective Ring Opening of Achiral Biaryls Containing Lactone Bridges with Chiral Hydride-Transfer Reagents Derived from Borane. Angew. Chem., Int. Ed. Engl. 1992;31:761–762. [Google Scholar]
- 17.Mori K, Itakura T, Akiyama T. Enantiodivergent Atroposelective Synthesis of Chiral Biaryls by Asymmetric Transfer Hydrogenation: Chiral Phosphoric Acid Catalyzed Dynamic Kinetic Resolution. Angew. Chem., Int. Ed. 2016;55:11642–11646. doi: 10.1002/anie.201606063. [DOI] [PubMed] [Google Scholar]
- 18.Bhat V, Wang S, Stoltz BM, Virgil SC. Asymmetric Synthesis of QUINAP via Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2013;135:16829–16832. doi: 10.1021/ja409383f. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS. Developing Irreversible Inhibitors of the Protein Kinase Cysteinome. Chem. Biol. 2013;20:146–159. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrax J, Delvaux M, Beghein N, Taper H, Gallez B, Calderon PB. Enhancement of Quinone Redox Cycling by Ascorbate Induces a Caspase-3 Independent Cell Death in Human Leukemia Cells. An in Vitro Comparative Study. Free Radical Res. 2005;39:649–657. doi: 10.1080/10715760500097906. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Distinct Roles of Cytochrome P450 Reductase in Mitomycin C Redox Cycling and Cytotoxicity. Mol. Cancer Ther. 2010;9:1852–1863. doi: 10.1158/1535-7163.MCT-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YH, Cheng DJ, Zhang J, Wang Y, Liu XY, Tan B. Atroposelective Synthesis of Axially Chiral Biaryldiols via Organocatalytic Arylation of 2-Naphthols. J. Am. Chem. Soc. 2015;137:15062–15065. doi: 10.1021/jacs.5b10152. [DOI] [PubMed] [Google Scholar]
- 23.Moliterno M, Cari R, Puglisi A, Antenucci A, Sperandio C, Moretti E, Di Sabato A, Salvio R, Bella M. Quinine-Catalyzed Asymmetric Synthesis of 2,2′-Binaphthol-Type Biaryls Under Mild Reaction Conditions. Angew. Chem., Int. Ed. 2016;55:6525–6529. doi: 10.1002/anie.201601660. [DOI] [PubMed] [Google Scholar]
- 24.Wang J-Z, Zhou J, Xu C, Sun H, Kürti L, Xu Q-L. Symmetry in Cascade Chirality-Transfer Processes: A Catalytic Atroposelective Direct Arylation Approach to BINOL Derivatives. J. Am. Chem. Soc. 2016;138:5202–5205. doi: 10.1021/jacs.6b01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YH, Qi LW, Fang F, Tan B. Organocatalytic Atroposelective Arylation of 2-Naphthylamines as a Practical Approach to Axially Chiral Biaryl Amino Alcohols. Angew. Chem., Int. Ed. 2017;56:16308–16312. doi: 10.1002/anie.201710537. [DOI] [PubMed] [Google Scholar]
- 26.Mellah M, Voituriez A, Schulz E. Chiral Sulfur Ligands for Asymmetric Catalysis. Chem. Rev. 2007;107:5133–5209. doi: 10.1021/cr068440h. [DOI] [PubMed] [Google Scholar]
- 27.Pan F, Shi ZJ. Recent Advances in Transition-Metal-Catalyzed C-S Activation: From Thioester to (Hetero)aryl Thioether. ACS Catal. 2014;4:280–288. [Google Scholar]
- 28.Liu J, Robins MJ. SNAr Displacements with 6-(Fluoro, Chloro, Bromo, Iodo, and Alkylsulfonyl)purine Nucleosides: Synthesis, Kinetics, and Mechanism. J. Am. Chem. Soc. 2007;129:5962–5968. doi: 10.1021/ja070021u. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan P, Mahajan S, Enders D. Organocatalytic Carbon-Sulfur Bond-Forming Reactions. Chem. Rev. 2014;114:8807–8864. doi: 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]
- 30.Helder R, Arends R, Bolt W, Hiemstra H, Wynberg H. Alkaloid Catalyzed Asymmetric Synthesis III the Addition of Mercaptans to 2-Cyclohexene-1-One; Determination of Enantiomeric Excess Using 13C NMR. Tetrahedron Lett. 1977;18:2181–2182. [Google Scholar]
- 31.Hiemstra H, Wynberg H. Addition of Aromatic Thiols to Conjugated Cycloalkenones, Catalyzed by Chiral β-Hydroxy Amines. A Mechanistic Study on Homogeneous Catalytic Asymmetric Synthesis. J. Am. Chem. Soc. 1981;103:417–430. [Google Scholar]
- 32.Armstrong RJ, Smith MD. Catalytic Enantioselective Synthesis of Atropisomeric Biaryls: A Cation-Directed Nucleophilic Aromatic Substitution Reaction. Angew. Chem., Int. Ed. 2014;53:12822–12826. doi: 10.1002/anie.201408205. [DOI] [PubMed] [Google Scholar]
- 33.Cardenas MM, Toenjes ST, Nalbandian CJ, Gustafson JL. Enantioselective Synthesis of Pyrrolopyrimidine Scaffolds through Cation-Directed Nucleophilic Aromatic Substitution. Org. Lett. 2018;20:2037. doi: 10.1021/acs.orglett.8b00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönherr H, Cernak T. Profound Methyl Effects in Drug Discovery and a Call for New C-H Methylation Reactions. Angew. Chem., Int. Ed. 2013;52:12256–12267. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- 35.Konkol LC, Guo F, Sarjeant AA, Thomson RJ. Enantioselective Total Synthesis and Studies into the Configurational Stability of Bismurrayaquinone A. Angew. Chem., Int. Ed. 2011;50:9931–9934. doi: 10.1002/anie.201104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugahara T, Murakami K, Yorimitsu H, Osuka A. Palladium-Catalyzed Amination of Aryl Sulfides with Anilines. Angew. Chem., Int. Ed. 2014;53:9329–9333. doi: 10.1002/anie.201404355. [DOI] [PubMed] [Google Scholar]
- 37.Dubbaka SR, Vogel P. Organosulfur Compounds: Electrophilic Reagents in Transition-Metal-Catalyzed Carbon-Carbon Bond-Forming Reactions. Angew. Chem., Int. Ed. 2005;44:7674–7684. doi: 10.1002/anie.200463007. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Xiao J, Chen T, Yin SF, Han LB. Efficient Nickel-Catalyzed Phosphinylation of C-S Bonds Forming C-P Bonds. Chem. Commun. 2016;52:12233–12236. doi: 10.1039/c6cc06048j. [DOI] [PubMed] [Google Scholar]
- 39.Bhanuchandra M, Baralle A, Otsuka S, Nogi K, Yorimitsu H, Osuka A. Palladium-Catalyzed Ipso-Borylation of Aryl Sulfides with Diborons. Org. Lett. 2016;18:2966–2969. doi: 10.1021/acs.orglett.6b01305. [DOI] [PubMed] [Google Scholar]
- 40.Grayson MN, Houk KN. Cinchona Alkaloid-Catalyzed Asymmetric Conjugate Additions: The Bifunctional Brønsted Acid-Hydrogen Bonding Model. J. Am. Chem. Soc. 2016;138:1170–1173. doi: 10.1021/jacs.5b13275. [DOI] [PubMed] [Google Scholar]
- 41.Grayson MN, Houk KN. Cinchona Urea-Catalyzed Asymmetric Sulfa-Michael Reactions: The Brønsted Acid-Hydrogen Bonding Model. J. Am. Chem. Soc. 2016;138:9041–9044. doi: 10.1021/jacs.6b05074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.