Abstract
Purpose
We evaluated prevention of transforming growth factor β (TGFβ)–induced transdifferentiation of cultured scleral fibroblasts to myofibroblasts by rho-associated protein kinase (ROCK) inhibitors. Additionally, we tested whether local delivery of ROCK inhibitors reduced scleral fibroblast proliferation in response to chronic intraocular pressure (IOP) elevation.
Methods
Primary human peripapillary sclera (PPS) fibroblasts were cultured and treated with TGFβ to induce myofibroblast transdifferentiation, as determined by immunoblot assessment of α smooth muscle actin (SMA) levels and collagen gel contraction. Cells were treated with the ROCK inhibitors Y27632, fasudil, and H1152 before TGFβ treatment. ROCK activity in TGFβ-treated fibroblasts and sclera from ocular hypertensive mice was assessed by measuring phosphorylation of the ROCK substrate MYPT1 at Thr696. Fibroblast proliferation following IOP elevation and ROCK inhibitor treatment was assessed by an enzyme-linked immunosorbent (ELISA) assay.
Results
ROCK inhibitors H1152 (10μM), Y27632 (10 μM), and fasudil (5μM) reduced SMA expression 72%, 85%, and 68%, respectively. Collagen gel contraction was reduced by 36% (P < 0.001), 27% (P = 0.0003), and 33% (P = 0.0019) following treatment with fasudil (25 μM), Y27632 (10 μM), and H1152 (10μM). ROCK activity induced by TGFβ rose 4.74 ± 1.9 times over control at 4 hours (P = 0.0004) and 2.4 ± 0.47-fold (P = 0.0016) in sclera after IOP elevation. Proliferation of scleral fibroblasts after chronic IOP elevation was reduced 77% by Y27632 (P = 0.001) and 84% by fasudil (P = 0.0049).
Conclusions
ROCK inhibitors reduce TGFβ-induced myofibroblast transdifferentiation and glaucoma-induced scleral cell proliferation.
Translational Relevance
These findings suggest altered fibroblast activity promoted by ROCK inhibitors could modify scleral biomechanics and be relevant to glaucoma treatment.
Keywords: intraocular pressure, peripapillary sclera, Y27632, fasudil, H1152, ROCK
Introduction
Glaucoma is a potentially blinding disease in which retinal ganglion cell (RGC) death is caused by axonal injury at the optic nerve head (ONH). The ONH of glaucomatous eyes exhibits several characteristic features, including excavation of the lamina cribrosa (LC), the portion of the ONH at which blockade of anterograde and retrograde axonal transport occurs, as well as changes in ONH astrocyte reactivity.1 Several lines of evidence implicate a role for the biomechanical behavior of the sclera in causing axonal injury at the ONH. The peripapillary sclera (PPS), the sclera immediately surrounding the ONH, translates intraocular pressure (IOP)–induced stress to the ONH and RGC axons in ex vivo studies2 and finite element modeling.3,4 Changes in the biomechanical properties of the PPS can alter the strain experienced by the LC5 and the degree of axonal damage in animal models.6 Lastly, treatment with the angiotensin II receptor type 1 blocker, losartan, reduced axonal damage in a mouse model of glaucoma by altering the scleral response to IOP elevation.7 Therefore, factors that regulate the material properties of the PPS likely influence the ONH response to IOP-generated stress and affect the extent of axonal damage that occurs at a given IOP.
Alterations in scleral material properties are observed in glaucomatous eyes. Scleral stiffening occurs following bead-induced murine glaucoma8 and chronic IOP elevation in monkeys.9 Postmortem human glaucomatous eyes are stiffer than nonglaucoma controls.10 Stiffening of the sclera in monkey eyes is preceded by alterations in the viscoelastic properties of the peripapillary sclera that occur before axonal damage.11 Changes in the mechanical behavior of sclera in animal models and glaucomatous human eyes are associated with alterations in collagen microarchitecture and extracellular matrix organization.2,10,12 The response of the main cellular component of the sclera—the fibroblast—to glaucoma is not as well defined. Transmission electron microscopy imaging of mouse PPS showed that one-fourth of the alternating scleral layers consist of fibroblasts, and in experimental glaucomatous eyes the cellular lamellae occupied a greater proportion of the scleral wall.13 In experimental glaucoma eyes, scleral fibroblasts proliferate and increase their expression of α smooth muscle actin (α-SMA) and contractile proteins, such as myosin II, that signify an increase in scleral myofibroblasts.14 These previous results suggest strongly that the cellular layers of the sclera are not static, but adapt and respond to IOP elevation and glaucoma. Modification of these adaptations could be the basis of new glaucoma therapy.
Fibroblasts are a diverse set of mesenchymal cells that are found in every tissue of the body; depending on their location, they can display multiple morphologies.15 The transition from fibroblast to myofibroblast is characterized by proliferation, altered expression of proteins that modify the extracellular matrix (ECM), expression of α-SMA, cellular migration, and adoption of a contractile phenotype.16 The transition from a fibroblast to myofibroblast phenotype can be induced by cytokine signaling, mechanical strain, or altered tissue stiffness. When regulated appropriately, this transition aids in ECM deposition and wound contraction that leads to proper wound healing. Unchecked myofibroblast activity leads to tissue fibrosis, tissue stiffening, and diminished organ function.16,17
Inhibition of rho-associated protein kinase (ROCK) prevents myofibroblast differentiation and reduces organ fibrosis. Pharmacologic ROCK inhibition reduces myofibroblast differentiation induced by cytokine signaling, stretch,18 and stiffness.19 In addition, ROCK inhibitor treatment prevents fibrosis in ocular tissues. ROCK inhibition reduced myofibroblast differentiation of conjunctival fibroblasts,20 improved surgical outcomes following glaucoma filtering surgery in rabbits by reducing myofibroblasts,21 reduced myofibroblast differentiation of keratinocytes following corneal injury,22 and showed promise in treatment of vitreoretinal fibrosis.23,24 The antifibrotic activity of ROCK inhibitors is becoming more relevant since several are available for clinical use. Systemic delivery of the inhibitor, fasudil, is approved in China and Japan for treatment of cerebral vasospasm.25 Local topical delivery of the ROCK inhibitor, ripasudil, and the ROCK/norepinephrine transporter inhibitor (ROCK/Net), netarsudil, is approved in Japan26 and the United States,27 respectively, to treat ocular hypertension and glaucoma. We examined whether three ROCK inhibitors prevent transdifferentiation of cultured scleral fibroblasts and whether local delivery of these inhibitors can affect scleral fibroblast proliferation in a preclinical glaucoma model.
Materials and Methods
Fibroblast Isolation and Culture
Methods for isolation and culture of peripapillary fibroblasts were described previously in detail28 and summarized briefly here. Donor eyes were obtained from the National Disease Research Interchange within 24 hours postmortem. Fibroblasts were isolated from both eyes of two European-derived persons, an 80-year old woman (lines 1 and 2) and a 79-year old man (lines 3 and 4); the woman was bilaterally phakic and the man bilaterally pseudophakic. Eyes were nonglaucomatous without a history of ocular disease besides cataract as defined by review of medical records. A 2-mm wide, circular scleral band surrounding the ONH was isolated and cut into 1 × 1 mm sections. Sclera pieces then were placed on 35 mm collagen-coated, tissue culture dishes containing Roswell Park Memorial Institute (RPMI)–1640 medium, 20% fetal bovine serum (FBS), nonessential amino acids, 1% penicillin/streptomycin, and sodium pyruvate. After 14 days, cells were passaged and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 1% penicillin/streptomycin, and sodium pyruvate. Experiments were either conducted medium containing 2% FBS for immunoblots or 0.2% FBS for cell contraction assays. All experiments were conducted on cells between passages 3 and 10. Recombinant human TGFβ1 was obtained from R&D Systems (Minneapolis, MN). Y27632, fasudil, and H1152 were obtained from TOCRIS (Minneapolis, MN).
Immunoblot and Densitometry Analyses
Cell lysates containing 6 μg total protein were resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels under reducing conditions. Proteins then were transferred to nitrocellulose membranes that were blocked with tris-buffered saline (TBS) containing 5% nonfat milk with 0.1% Tween 20, and incubated with antibody (Supplementary Table S1). Antigen–antibody reactions were detected using chemiluminescence and bands were quantified by Image Lab Software (Biorad, Hercules, CA).
Collagen Gel Contraction Assay
Cell contraction was determined using a kit from Cell Biolabs (San Diego, CA). Briefly, cells were seeded in collagen solution according to the manufacturer's instructions and treated with media containing inhibitor and transforming growth factor β (TGFβ) for 12 hours before release from the plates with a sterile spatula. Gel images were taken after release and the surface area of each gel was measured using ImageJ.29
Animal Studies
Animal experimentation was conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee of Johns Hopkins University and according to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Mice were kept in a 12-hour (7 AM–7 PM) light-dark cycle. Following sedation with a mixture of ketamine (Fort Dodge Animal Health, Fort Dodge, IA), xylazine (VedCo, Inc., Saint Joseph, MO), and acepromazine (Phoenix Pharmaceuticals, Burlingame, CA), 8- to 12-week old female CD1 mice (Charles River, Inc., Wilmington, MA) underwent unilateral intracameral bead injection as described previously.7 Briefly, a glass cannula with a 50 μM tip diameter connected to a Hamilton syringe (Hamilton, Inc., Reno, NV) was used to inject the anterior chamber of the mouse with Polybead Microspheres® (Polysciences, Inc., Warrington, PA) of 6 and 1 μM diameter followed by 10 mg/mL sodium hyaluronate (Healon; Advanced Medical Optics, Inc., Santa Ana, CA). Experimental and control animals were treated in a masked fashion. Five mice were used per experimental group (105 total).
IOPs were measured using the TonoLab rebound tonometer (iCare, Vantaa, Finland) according to the manufacturer's instructions with the magnetic probe in the horizontal position. IOPs were taken under anesthesia through inhalation of isoflurane administered by a RC2-Rodent Circuit Controller (VetEquip, Inc., Pleasanton, CA). Mice spent two minutes in an induction chamber and 1 minute on a nose cone before measurements were taken. The mean of two to three recordings per eye, with each recording from the tonometer resulting from six readings, was used as the final IOP measurement. Eyes did not receive topical anesthesia.
Subconjunctival injections were performed under inhalational anesthesia. After application of a drop of 1% proparacaine (Alcaine®, Alcon Laboratories, Inc., Fort Worth, TX) conjunctival tissue was grasped with forceps and elevated. Then, 50 μL solution was injected into the subconjunctival space using a 27-gauge needle with an intradermal bevel. After injection, the needle was kept in the subconjunctival space for at least 10 seconds to prevent leakage.
Scleral Isolation, ROCK-Activity Assay, and PCNA Immunoassay
After animals were euthanized, eyes were enucleated and the posterior pole was isolated under a dissecting microscope. Choroidal tissue was removed using a blunt spatula and sclera were incubated in PBS at 4°C for 10 minutes followed by vigorous agitation for 15 seconds. This wash was repeated three times before use of sclera in downstream assays. Commercially available kits were used for ROCK activity assay (Cell Biolabs, San Diego, CA) and PCNA immunoassay (Cell Biolabs). For the ROCK activity assay, five sclera were pooled per experimental group (n = 5). For the proliferating cell nuclear antigen (PCNA) enzyme-linked immunosorbent assay (ELISA) values were obtained for each individual sclera.
Statistical Analysis
All values are ± standard deviation (SD). Fold change was calculated relative to untreated or fellow eye, control values. Unless otherwise noted, 1-way analysis of variance test (ANOVA) was used for means and Dunnett's test.
Results
Isolation and Characterization of Primary PPS Fibroblasts
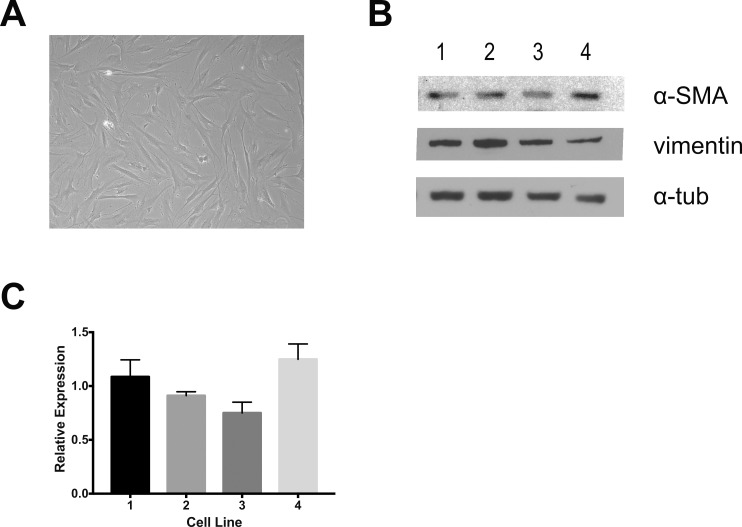
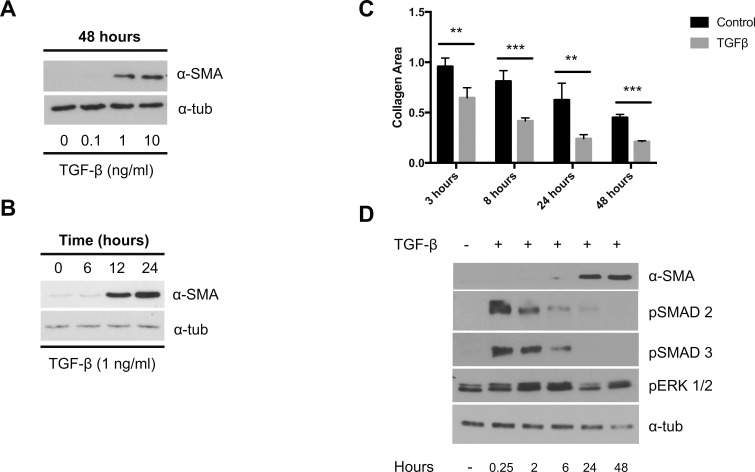
Primary PPS fibroblasts were isolated from four nonglaucomatous eyes of two donors. Cells demonstrated the characteristic spindle shape of fibroblasts and expressed vimentin and αSMA (Fig. 1). Baseline αSMA expression was shown previously to vary as much as 10-fold between PPS cell lines28; however, αSMA expression between PPS cell lines 1 to 4 varied <25%. Treatment with ng/mL dosages of TGFβ increased αSMA expression in a dosage- and time-dependent manner (Figs. 2a, 2b). TGFβ treatment increased cell contractility (Fig. 2c) as early as 3 hours after release of the collagen plug. Collagen area was 64% ± 10% of the original area in gels with TGFβ-treated cells versus 95% ± 8% in nontreated gels at 3 hours (P = 0.003). A significant increase in contraction over prerelease with TGFβ was present at 8, 24, and 48 hours (P = 0.0008, 0.008, and 0.00002, respectively). Taken together, these results point to promotion of a myofibroblast phenotype following TGFβ treatment. TGFβ is known to use signaling pathways that include activation of SMAD-dependent (canonical) pathways and through activation of noncanonical pathways, including phosphorylation of extracellular signal-related kinase (ERK). Treatment of PPS fibroblasts with TGFβ rapidly induced SMAD2/3 phosphorylation that subsided to baseline levels by 48 hours after treatment. A more subtle and delayed induction of ERK phosphorylation (Fig. 2d) peaked 2 hours after TGFβ treatment.
Figure 1.
Isolation of primary peripapillary fibroblasts. (a) Light microscopy of primary cells. (b) Immunoblots for α-SMA, vimentin, and α-tubulin (α-tub) for four primary cell lines. (c) Densitometric analysis of α-SMA expression normalized to α-tub levels.
Figure 2.
TGFβ induces α-SMA expression and a contractile phenotype. (a, b) Immunoblots for α-SMA following TGFβ treatment. (c) Collagen contraction following TGFβ treatment (2 ng/mL; n = 3). (d) Immunoblots for pSMAD2/3 and pERK1/2 following TGFβ treatment. **P < 0.01, ****P < 0.0001.
Rho-kinase Inhibition Reduces Myofibroblast Phenotype Following TGFβ Treatment
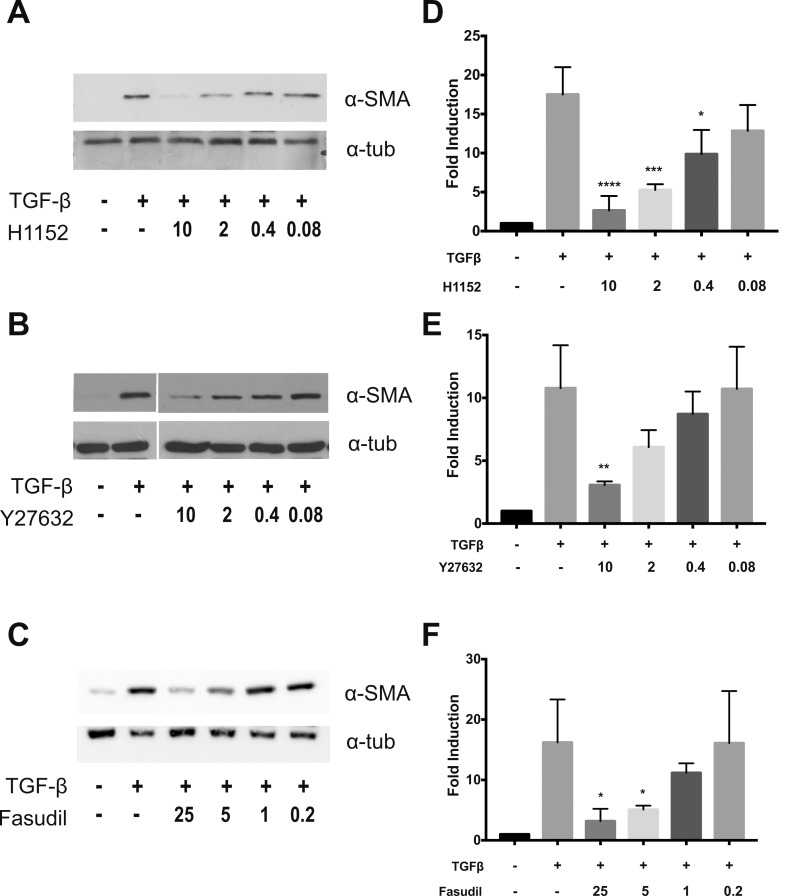
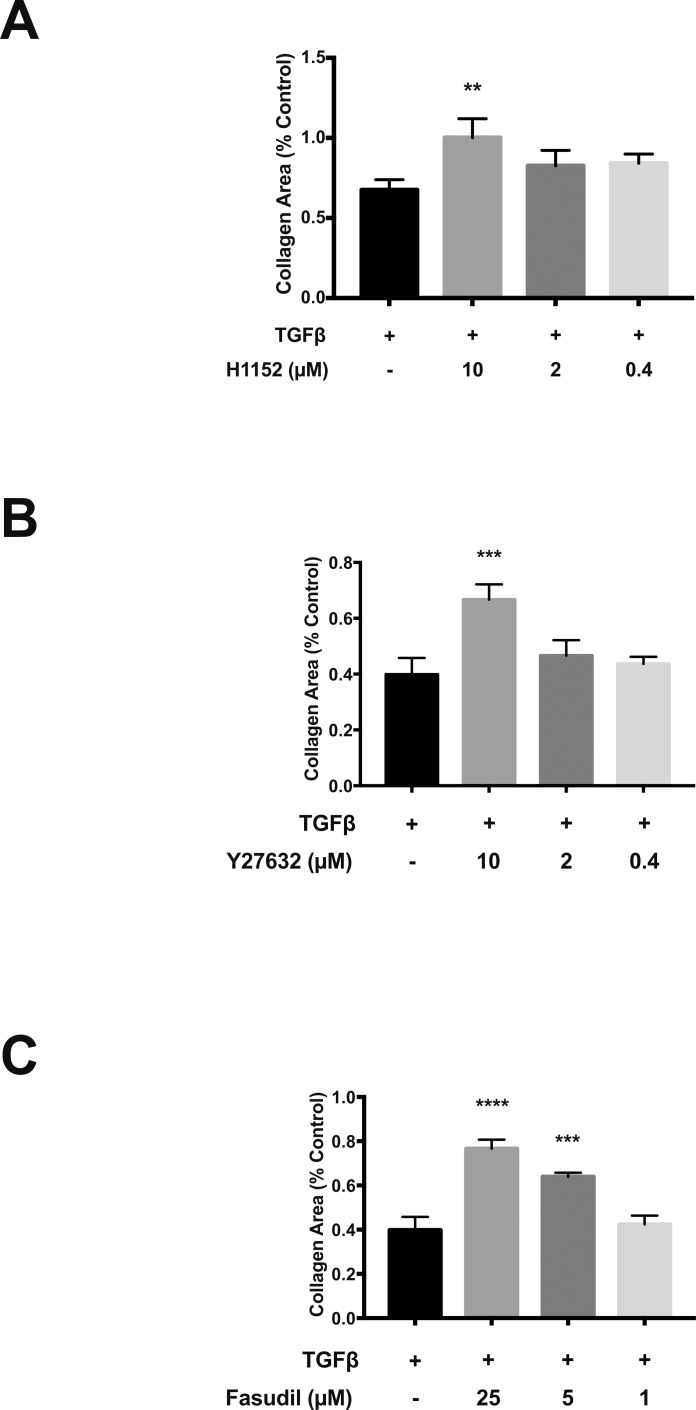
To determine the role of ROCK signaling in TGFβ-dependent promotion of the myofibroblast phenotype, we used three ROCK inhibitors: H1152, Y27632, and fausidil. Pretreatment with ROCK inhibitors reduced TGFβ-dependent αSMA induction in a dosage-dependent manner (Fig. 3). Each compound inhibited αSMA at micromolar concentrations. Treatment with 10 μM H1152 and Y-27632 αSMA levels by 85% and 72% from baseline, respectively. Treatment with 25 or 5 μM fasudil reduced TGFβ-induced αSMA levels 80% and 68% from baseline. ROCK inhibition also reduced TGFβ-induced cell contractility at micromolar dosages (Fig. 4), as assessed by collagen gel contraction assay. When compared to TGFβ-treated controls, collagen gel area was increased 33% (P = 0.0019), 27% (P = 0.0003), and 36% (P < 0.001) following treatment with H1152 (10 μM), Y27632 (10 μM), and fasudil (25 μM), respectively. Taken together, these results demonstrated that ROCK inhibition prevented TGFβ-induced myofibroblast transdifferentiation of PPS fibroblasts.
Figure 3.
ROCK inhibitors reduce TGFβ-induced α-SMA expression. (a–c) Immunoblots for α-SMA and α-tubulin following treatment with TGFβ (2 ng/mL) and rho-kinase inhibitors (μM; n = 3). (d–f) Densitometric analysis of immunoblots normalized to α-tubulin expression. *P < 0.05, **P < 0.01, ****P < 0.0001.
Figure 4.
ROCK inhibitors reduce TGFβ-induced collagen contraction. (a–c) Collagen contraction assays following treatment with TGFβ alone (control) and TGFβ with ROCK inhibitors (n = 3). Collagen area was measured 8 hours after release of collagen from the plate. *P < 0.05, **P < 0.01, ****P < 0.0001.
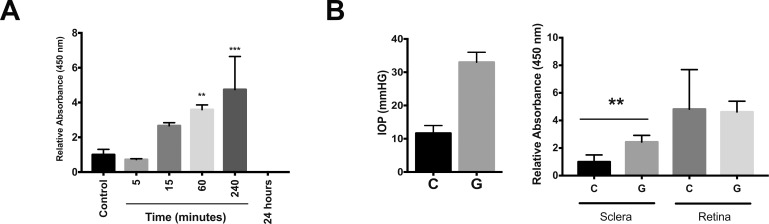
Rho-Kinase Activation Occurs following TGFβ Exposure and IOP Elevation
ROCK activity was assessed by incubating cell and scleral lysates with the ROCK substrate, MYPT1, and assessing phosphorylation at Thr696 by colorimetric immunoassay. Compared to untreated control cell lysates, lysates from TGFβ-treated cells showed increased MYPT1 phosphorylation that was present 60 minutes after treatment (3.59 ± 0.3 vs. 1.00 ± 0.3, P = 0.008), peaked 4 hours after treatment (4.74 ± 1.9, P = 0.0004), and was absent 24 hours after treatment (−0.35 ± 0.22, P = 0.20; Fig. 5a). Sclera and retina then were isolated from mice 12 hours following induction of ocular hypertension by anterior chamber bead injection. In nondrug-treated animals, a significant increase in IOP was observed 12 hours after bead injection compared to fellow eye controls (33.0 ± 3.0 vs. 11.67 ± 2.3 mm Hg, P = 0.0006, t-test; Fig. 5b). This elevation was associated with increased ROCK activity in sclera of bead-injected eyes compared to fellow eye controls (2.44 ± 0.5- vs. 1.0 ± 0.5-fold, P = 0.0016; Fig. 5b). Retinal ROCK activity was elevated compared to control sclera; however, bead injection did not significantly alter retinal ROCK activity (4.82 ± 2.9 vs. 4.62 ± 0.8, P = 0.9).
Figure 5.
Scleral ROCK activation following TGFβ-treatment and ocular hypertension. (a) ROCK activation following TGFβ treatment of cultured PPS scleral fibroblasts (n = 5). (b) Scleral and retinal ROCK activation (right) 12 hours after bead-induced ocular hypertension (left; C = control, G = glaucoma; n = 5). *P < 0.05, **P < 0.01, ****P < 0.0001.
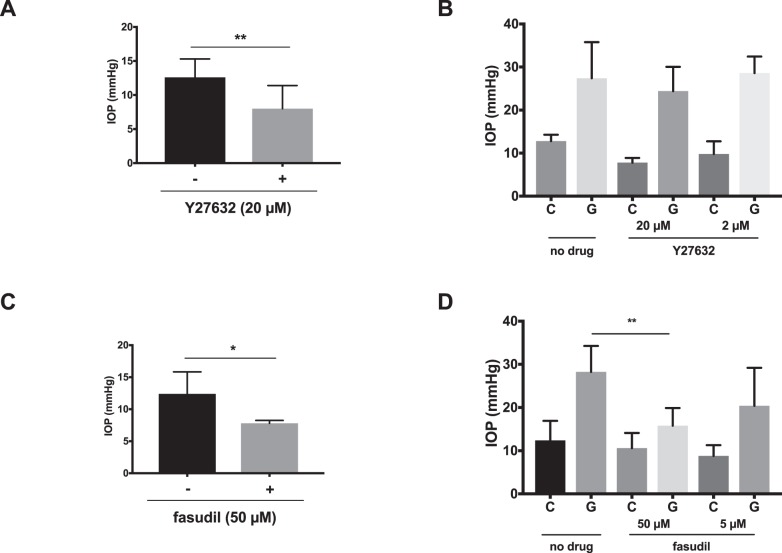
Rho-Kinase Inhibition Reduces Glaucoma-Induced Fibroblast Proliferation
We found that subconjunctival injection of Y27632 and fasudil significantly lowered IOP from baseline in normotensive mice and to a lesser extent in a mouse model of ocular hypertension. Injection of Y27632 did significantly reduce IOP when injected in nonhypertensive eyes from a baseline IOP of 12.6 ± 2.7 to 6.5 ± 0.6 mm Hg (P = 0.003; Fig. 6a). When injected in the subconjunctival space of eyes with ocular hypertension induced by anterior chamber microparticle injection, a significant IOP reduction was not seen IOP (P = 0.1 for 20 μM and P = 0.45 for 2 μM; Fig. 6b). Subconjunctival injection of fasudil significantly reduced IOP in nonhypertensive eyes from 12.4 ± 3.4 mm Hg in control, fellow eyes to 7.8 ± 0.4 mm Hg (P = 0.02; Fig. 6c). Interestingly, following bead-induced ocular hypertension mean IOP was reduced from 28.3 ± 6.0 to 15.8 ± 4.1 mm Hg (P = 0.0076) following injection of 50 μM fasudil; however, significant reduction was not seen following injection of 5 μM fasudil (P = 0.17; Fig. 6d).
Figure 6.
ROCK inhibitors lower baseline IOP. (a, c) IOP measurements in nonglaucomatous eyes treated with subconjunctival ROCK inhibitors (+) or fellow eye control (-) and (b, d) glaucomatous eyes treated with local injection of Y27632 or fasudil (G) and fellow eye controls (C). *P < 0.05, **P < 0.01, ****P < 0.0001.
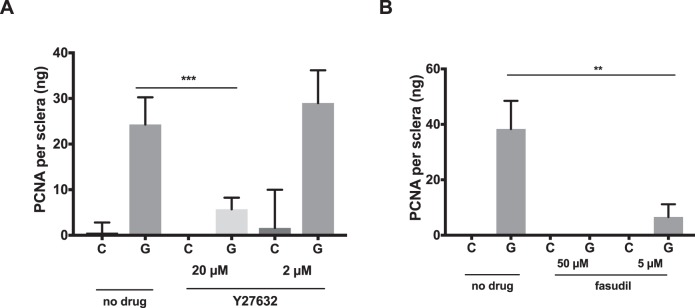
We showed previously that bead-induced glaucoma causes a scleral proliferative response as evidenced by increased expression of the proliferative marker Ki67.14 This response was present as early as 7 days after induction of ocular hypertension and remained significant for at least 6 weeks. Here, we showed that lysates from the sclera of bead-injected mice had increased expression of the proliferation marker PCNA by colorimetric immunoassay as soon as 48 hours after ocular hypertension induction (Fig. 7). Subconjunctival injection of Y27632 and fasudil reduced cellular proliferation following 48 hours of ocular hypertension (Figs. 7a, 7b). Injection of 20 μM Y27632 reduced glaucoma-induced scleral PNCA levels from 24.3 ± 5.9 to 5.7 ± 2.5 pg/sclera (P = 0.0002). A significant reduction of PCNA levels was not seen following injection of 2 μM Y27632 (P = 0.82). Injection of 5 and 50 μM fasudil reduced glaucoma-induced scleral PNCA levels from 38.3 ± 10.2 to undetectable levels and 6.6 ± 4.9 pg/sclera (P = 0.0002 and 0.0049), respectively (Fig. 7c).
Figure 7.
ROCK inhibitors reduce glaucoma-induced cell proliferation. (a, b) PCNA levels are reduced in glaucomatous (G) eyes treated with local injection of Y27632 or fasudil as compared to fellow eye controls (C; n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We demonstrated that ROCK inhibition reduced development of myofibroblast phenotype in scleral fibroblasts following TGFβ exposure. Treatment with the ROCK inhibitors, Y27632, H1152, or fasudil, reduced TGFβ-induced αSMA expression and cell contractility of cultured primary human PPS fibroblasts. TGFβ treatment transiently induced ROCK activation in vitro and in the sclera of ocular hypertensive mice. We showed previously that mouse sclera contains few proliferating cells at baseline with approximately 1% to 2% of cells labeling with the proliferative marker Ki67. Ocular hypertension increases this fraction to 10% following 1 week of ocular hypertension and this increase remains statistically significant at 6 weeks following induction of ocular hypertension.14 Here, we showed that local injection of the ROCK inhibitors, Y27632 and fasudil, inhibited this proliferative response by measuring levels of another proliferative marker, PCNA. Y27632 and fasudil lowered IOP from baseline in nonhypertensive eyes; however, treatment with 50 μM, but not 5 μM fasudil lowered IOP in ocular hypertensive eyes. The reduction in PPS fibroblast proliferation conferred by fasudil at the 50 μM treatment could be due to pressure reduction as well as a direct effect on scleral fibroblasts.
Identification of the proliferative response of scleral cells as a pharmacologically modifiable marker of scleral response to ocular hypertension is a potentially useful tool to quantify scleral behavior in experimental glaucoma. Altered scleral biomechanics and microarchitecture was demonstrated previously in glaucomatous eyes and in animal models of glaucoma following exposure to elevated IOP for prolonged periods. To understand scleral remodeling better, it is essential to understand the early changes that drive this process; scleral fibroblast proliferation is a rapidly induced and marked response that follows ocular hypertension. Ongoing work in our laboratory aims to better define the initial response of fibroblasts to IOP elevation and determine if this early response leads to altered scleral biomechanics and microarchitecture. Lastly, assessing fibroblast proliferation following IOP elevation could serve as a useful early marker for measuring the scleral response to IOP elevation following medication treatment or in genetically modified mouse strains. Understanding how to modify this response could, in turn, help identify therapeutic interventions that could prevent glaucomatous scleral remodeling and possibly prevent vision loss.
Multiple stimuli that trigger myofibroblast differentiation are present in glaucomatous eyes, including mechanical stretch,28 cytokine signaling, and alterations in tissue stiffness.19,30 In this study, we focused on TGFβ signaling. Evidence supports increased TGFβ-signaling in the ONH of glaucomatous eyes. TGFβ1 and TGFβ2 expression was shown previously by immunohistochemistry in glaucomatous human optic nerves, and TGFβ2 release from glaucomatous optic nerves was 70 to 100 times that of control nerves.31 Additionally, Fukuchi et al.32 showed monkey eyes with established glaucomatous ONH damage had increased TGFβ1 and TGFβ2 expression at the level of the lamina cribrosa.32 Lastly, we found previously that the sclera of glaucomatous mice had elevated levels of the TGFβ-activating protein thrombospondin compared to control sclera.14 This finding suggested that TGFβ present in glaucomatous tissue has a higher likelihood of being activated.
Weinreb et al.33 showed that prostaglandin treatment of scleral explants increased scleral permeability; thus, proving that the “sclera is metabolically active and pharmacologically responsive.” Experimental alterations in scleral behavior affect glaucoma severity. Stiffening of the sclera by subconjunctival glyceraldehyde injection increased axonal damage and RGC death in a mouse glaucoma model.6 Stiffening of explanted human peripapillary sclera decreased laminar stress with IOP elevation, which would be anticipated to reduce axonal damage.34 Previously, we showed that systemic losartan treatment reduced RGC loss, axonal damage, and axonal transport blockade in mouse IOP glaucoma. These effects occurred in the setting of altered biomechanical behavior of the sclera of losartan-treated mice. Importantly, no RGC protection was observed after losartan treatment following optic nerve crush, indicating its effect was not a direct rescue of ganglion cells independent of biomechanical alterations. This set of findings further supported the hypothesis that sclera is a therapeutic target in glaucoma treatment. We showed evidence that, in addition to their other activities (IOP reduction), ROCK inhibitors may have potential as therapeutic tools to target the sclera in glaucoma treatment.
ROCK inhibition holds therapeutic promise in glaucoma and the potential to affect multiple disease processes. Current glaucoma therapy relies on IOP reduction to slow or stop progressive vision loss. Physicians can medically reduce IOP by using established drugs that reduce aqueous production (carbonic anhydrase inhibitors, β-blockers, α2 agonists) or facilitate improved aqueous outflow from the eye (prostaglandin analogues, ROCK inhibition). Several novel approaches to IOP reduction are in clinical development or have been introduced recently to clinical use as well (ROCK/Net inhibition, nitric oxide signaling, among others).35 It is the potent activity of ROCK inhibitors on trabecular meshwork cells and the resulting IOP reduction that has opened the door to clinical use of ROCK inhibitors in glaucoma treatment.36 In addition to IOP reduction, ROCK inhibition has promise in neuroprotection in glaucoma. Systemic treatment with the ROCK inhibitors fasudil and K115 prevented RGC loss and axonal damage following optic nerve crush injury.37 This protection was associated with reduction in reactive oxygen species and oxidized lipids, although alternative targets of neuroprotection have been proposed.38 Interestingly, topical application of the ROCK/Net inhibitor netarsudil reduced RGC loss following rat optic nerve crush.39 Topical application of netarsudil inhibited phosphorylation of the downstream ROCK targets cofillin and LIM kinase in isolated retina—suggesting ROCK inhibition in the posterior segment of the eye.
Approaches to ROCK-inhibitor delivery could affect the site and mechanism of action. Topically applied ROCK inhibitors achieve IOP reduction by affecting trabecular meshwork cells and downstream outflow pathways. Thus, for IOP reduction to be maximized, drug delivery should be optimized for delivery to the anterior segment of the eye. Topically applied ripasudil and netarsudil do reach therapeutic levels in the aqueous humor and cornea.40,41 Interestingly, drug also was detected in the iris-ciliary body and retina-choroid of pigmented rabbits, demonstrating that ocular pharmacokinetics of ROCK inhibitors could be affected by drug-pigment binding. After repeated instillation, ripasudil was present in the posterior sclera, suggesting that even topically applied ROCK inhibitors could be affecting scleral fibroblast behavior. If alternate cell types, such as RGCs, optic nerve head astrocytes, scleral fibroblasts, or even optic nerve axons, are the desired target, alternate approaches to drug delivery (intravitreal injection, subconjunctival injection, or suprachoroidal injection) could be required.
Our study has some recognized limitations. We do not yet know whether ROCK-inhibition of scleral fibroblast transdifferentiation alters glaucoma progression. Given the multiple potential effects of ROCK inhibition, isolating the specific role of ROCK inhibition on scleral fibroblasts could be challenging. In addition, the ROCK inhibitors used in our study have off target activity.42 Using these inhibitors, it is not possible to attribute these effects to ROCK inhibition. We did not examine the potential for ROCK inhibition to alter the scleral fibroblast response to stretch and increased substrate stiffness—two stimuli that could affect the sclera with IOP elevation and glaucoma. Future studies aim to define further the response of scleral fibroblasts to the diverse stimuli present in glaucomatous eyes and uncover the role of scleral fibroblasts in glaucoma progression. Lastly, we focused these studies on PPS fibroblasts; however, the influence of ROCK inhibitors on anterior scleral fibroblasts could have implications for glaucoma. It would be of interest to examine how clinically available ROCK inhibitors affect limbal scleral fibroblast behavior and scleral biomechanics. In addition, the potential role of limbal scleral fibroblasts on aqueous humor outflow dynamics and IOP regulation warrants further investigation.
Supplementary Material
Acknowledgments
Supported by a Research to Prevent Blindness Career Development Award and K08EY024952 (IP), and EY02120 (HQ).
Disclosure: I. Pitha, None; E. Oglesby, None; A. Chow, None; E. Kimball, None; M. E. Pease, None; J. Schaub, None; H. Quigley, None
References
- 1.Quigley HA. The contribution of the sclera and lamina cribrosa to the pathogenesis of glaucoma: diagnostic and treatment implications. Prog Brain Res. 2015;220;:59–86. doi: 10.1016/bs.pbr.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53:1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–4199. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- 4.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2004;45:4378–4387. doi: 10.1167/iovs.04-0133. [DOI] [PubMed] [Google Scholar]
- 5.Thornton IL, Dupps WJ, Sinha Roy A, Krueger RR. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapillary scleral collagen cross-linking. Invest Ophthalmol Vis Sci. 2009;50:1227–1233. doi: 10.1167/iovs.08-1960. [DOI] [PubMed] [Google Scholar]
- 6.Kimball EC, Nguyen C, Steinhart MR, et al. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp Eye Res. 2014;128:129–140. doi: 10.1016/j.exer.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, Pitha IF, Welsbie DS, et al. Losartan treatment protects retinal ganglion cells and alters scleral remodeling in experimental glaucoma. PLoS One. 2015;10:e0141137. doi: 10.1371/journal.pone.0141137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen C, Cone FE, Nguyen TD, et al. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2013;54:1767–1780. doi: 10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52:5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coudrillier B, Pijanka JK, Jefferys JL, et al. Glaucoma-related changes in the mechanical properties and collagen micro-architecture of the human sclera. PLoS One. 2015;10:e0131396. doi: 10.1371/journal.pone.0131396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46:540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- 12.Pijanka JK, Coudrillier B, Ziegler K, et al. Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human sclerae. Invest Ophthalmol Vis Sci. 2012;53:5258–5270. doi: 10.1167/iovs.12-9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cone-Kimball E, Nguyen C, Oglesby EN, Pease ME, Steinhart MR, Quigley HA. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol Vis. 2013;19:2023–2039. [PMC free article] [PubMed] [Google Scholar]
- 14.Oglesby EN, Tezel G, Cone-Kimball E, et al. Scleral fibroblast response to experimental glaucoma in mice. Mol Vis. 2016;22:82–99. [PMC free article] [PubMed] [Google Scholar]
- 15.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 16.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy HS. Fibroblasts, myofibroblasts, and fibrosis: fact, fiction, and the future. J Cardiovasc Pharmacol. 2011;57:373–375. doi: 10.1097/FJC.0b013e3182155a38. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Futakuchi A, Inoue T, Fujimoto T, Inoue-Mochita M, Kawai M, Tanihara H. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp Eye Res. 2016;149:107–115. doi: 10.1016/j.exer.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Van de Velde S, Van Bergen T, Vandewalle E, et al. Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. Prog Brain Res. 2015;220:283–297. doi: 10.1016/bs.pbr.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Quantock AJ, Young RD, et al. A selective inhibitor of the Rho kinase pathway, Y-27632, and its influence on wound healing in the corneal stroma. Mol Vis. 2012;18:1727–1739. [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi M, Nakao S, Arima M, et al. Rho-kinase/ROCK as a potential drug target for vitreoretinal diseases. J Ophthalmol. 2017;2017:8543592. doi: 10.1155/2017/8543592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollanders K, Van Bergen T, Kindt N, et al. The effect of AMA0428, a novel and potent ROCK inhibitor, in a model of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:1335–1348. doi: 10.1167/iovs.14-15681. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Zhou D, Guo J, et al. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2006;46:421–428. doi: 10.2176/nmc.46.421. [DOI] [PubMed] [Google Scholar]
- 26.Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94:e26–34. doi: 10.1111/aos.12829. [DOI] [PubMed] [Google Scholar]
- 27.Lewis RA, Levy B, Ramirez N, et al. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100:339–344. doi: 10.1136/bjophthalmol-2015-306778. [DOI] [PubMed] [Google Scholar]
- 28.Qu J, Chen H, Zhu L, et al. High-magnitude and/or high-frequency mechanical strain promotes peripapillary scleral myofibroblast differentiation. Invest Ophthalmol Vis Sci. 2015;56:7821–7830. doi: 10.1167/iovs.15-17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Htwe SS, Cha BH, Yue K, Khademhosseini A, Knox AJ, Ghaemmaghami AM. Role of rho-associated coiled-coil forming kinase isoforms in regulation of stiffness-induced myofibroblast differentiation in lung fibrosis. Am J Respir Cell Mol Biol. 2017;56:772–783. doi: 10.1165/rcmb.2016-0306OC. [DOI] [PubMed] [Google Scholar]
- 31.Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. Br J Ophthalmol. 1999;83:209–218. doi: 10.1136/bjo.83.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuchi T, Ueda J, Hanyu T, Abe H, Sawaguchi S. Distribution and expression of transforming growth factor-beta and platelet-derived growth factor in the normal and glaucomatous monkey optic nerve heads. Jpn J Ophthalmol. 2001;45:592–599. doi: 10.1016/s0021-5155(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 33.Weinreb RN. Enhancement of scleral macromolecular permeability with prostaglandins. Trans Am Ophthalmol Soc. 2001;99:319–343. [PMC free article] [PubMed] [Google Scholar]
- 34.Coudrillier B, Campbell IC, Read AT, et al. Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa. Invest Ophthalmol Vis Sci. 2016;57:2666–2677. doi: 10.1167/iovs.15-18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bucolo C, Platania CBM, Drago F, et al. Novel therapeutics in glaucoma management. Curr Neuropharmacol. 2018;16:978–992. doi: 10.2174/1570159X15666170915142727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SK, Chang RT. An emerging treatment option for glaucoma: rho kinase inhibitors. Clin Ophthalmol. 2014;8:883–890. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K, Maruyama K, Himori N, et al. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:7126–7136. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]
- 38.Van de Velde S, De Groef L, Stalmans I, Moons L, Van Hove I. Towards axonal regeneration and neuroprotection in glaucoma: rho kinase inhibitors as promising therapeutics. Prog Neurobiol. 2015;131:105–119. doi: 10.1016/j.pneurobio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Shaw PX, Sang A, Wang Y, et al. Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp Eye Res. 2017;158:33–42. doi: 10.1016/j.exer.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34:40–51. doi: 10.1089/jop.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isobe T, Kasai T, Kawai H. Ocular penetration and pharmacokinetics of ripasudil following topical administration to rabbits. J Ocul Pharmacol Ther. 2016;3:405–414. doi: 10.1089/jop.2016.0028. [DOI] [PubMed] [Google Scholar]
- 42.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.