Abstract
Objective
To develop an easy surgical approach to facilitate clinical management.
Study Design
A novel transnasal endoscopic 3-step surgical method for vidian neurectomy was designed and tried in 91 cases with a mild-to-severe degree of allergic and nonallergic rhinitis refractory to routine medical therapy.
Setting
Endoscopic vidian neurectomy requires accurate localization of the vidian canal. However, it is not easy to localize during surgery because of its deep location and the complex anatomy of the pterygopalatine fossa.
Subjects and Methods
This technique consists of 3 steps, including transnasal endoscopic perforation of the anterior wall of the sphenoidal sinus as the first step and removal of the anterior wall until the exposure of the vidian canal in the junction between the anterior wall and the floor of the sphenoid sinus as the second step. The last step is the accurate resection and cauterization of the vidian nerve. In some cases in which the sphenoid sinus developed well with a big lateral space, an extended procedure of posterior ethmoidectomy was included to allow good exposure of the vidian canal.
Results
Using this technique, successful endoscopic vidian neurectomy in this series of patients was confirmed by both histology and Schirmer test, showing its distinct advantages of easy localization of the vidian canal and less risk of injury to the nerve and vessel bundles within the pterygopalatine fossa.
Conclusion
Taken together, this novel 3-step procedure of endoscopic vidian neurectomy plus an extended procedure guarantees good exposure of the vidian canal and therefore accurate vidian neurectomy.
Keywords: vidian neurectomy, endoscope, transnasal, modification, allergic rhinitis, vasomotor rhinitis
Vidian neurectomy (VN) for airway hypersensitivity has been tried and reported since its first publication in 1961 by Golding-Wood.1 With the revolution of various surgical procedures and instruments, including the microscope and endoscope, its surgical approach changed greatly.2 Thus far, transnasal endoscopic VN has become the main surgical approach.3 Ideal surgical view and exact localization of the vidian canal are the advantages of endoscope-guided VN compared with the traditional method.4 VN via sphenoid sinus as well as via middle meatus along the middle turbinate were in most cases the choice of such surgery.3-6
So far, basically 2 different methods of surgical access have been used clinically—through the sphenoid sinus4,5 and through the pterygopalatine fossa.7,8 In the first case, the paper-thin vidian canal wall was often perforated with subsequent severing of the nerve. The key of this procedure is the anatomic protrusion of the vidian canal into the sphenoid sinus. If the canal is located deeply in the floor of the sinus, the procedure has to be modified accordingly.9 In the second situation, the vidian canal was exposed in the pterygopalatine fossa through the sphenopalatine foramen. The key to this procedure is the accurate location of the vidian canal on the posterior wall of the pterygopalatine fossa or between the sphenoid sinus and the pterygopalatine fossa. The advantages of the 2 techniques described above were obvious, but for a beginner, their difficulties were also evident in terms of their key techniques.
Based on the literature and our clinical practice, we modified the endoscopic VN and summarized the technique as a transnasal transsphenoidal endoscopic 3-step VN. The difference between our technique and that in the literature is its surgical exposure of the vidian canal and resection of its contents including the vidian nerve completely within the sphenoid sinus and absolutely under the anterior wall of the sinus. The advantage of our method is no worry about whether the protrusion of the vidian canal existed anatomically and no worry about possible severe bleeding resulting from the management in the pterygopalatine fossa.
The procedure is described in detail in this article, based on the clinical management of 91 Chinese cases.
Materials and Methods
Subjects and Data Collection
Ninety-one patients admitted to a tertiary hospital with a diagnosis of either nasal hypersensitivity, including nonallergic rhinitis such as vasomotor rhinitis and allergic rhinitis such as mite-allergic rhinitis, or nasal hypersensitivity complicated with asthma from 2012 to 2015 were enrolled; their ages varied from 16 to 64 years. All patients had unsuccessful prior treatment, including local (nasal) spray with corticosteroids and systemic antihistamine plus corticosteroids. Written informed consent was obtained from each patient. This study was approved by the Human Ethic Committee of the Second Hospital at Shanxi Medical University of China. The surgery was performed in accordance with approved guidelines. All patients who received the surgery were followed up after discharge from the hospital either by telephone or by outpatient interview.
Schirmer I Test: Preoperative Computed Tomography Scan and Evaluation
The Schirmer I test was performed 1 day before the surgery and 1 week after the surgery to evaluate the secretion function of the lacrimal glands. A routine thin-layer coronal computed tomography (CT) scan was performed for each case to analyze the anatomic classification of the vidian nerve canal, as shown in Figure 1 . Such an image analysis helps surgeons to keep in mind the position and its relation of the vidian nerve canal to the adjacent structures in the sinus so as to orientate the surgery. In addition to the coronal CT images, both sagittal and axial positions were obtained to evaluate the pneumatization of the sinuses, especially the sphenoid sinus so as to design the surgical procedures as described below. Chronic rhinosinusitis, including pan-sinusitis and turbinate hypertrophy, as well as septum deviation are often complicated in these candidates.
Figure 1.
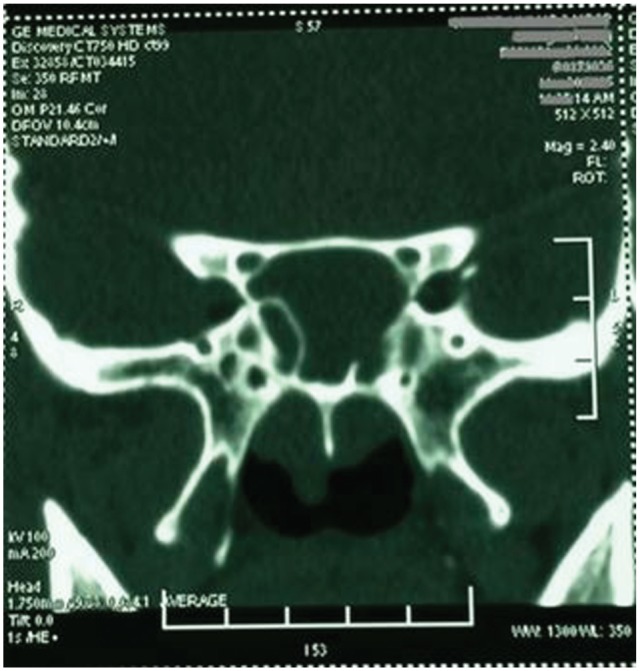
Computed tomography (CT) scan and location of the vidian canal. A coronal CT scan image for location of vidian nerve canal, showing the anatomy of the vidian nerve canal with a type III classification.
General Anesthesia
All patients underwent general anesthesia through oral endotracheal intubation.
Routine Surgical Procedures
The surgical procedures described here could be classified into routine 3-step and extended surgical approaches.
For the routine 3-step approach, after a topical preparation of the surgical field with cotton pledgets soaked with saline and 1:5000 epinephrine, an endoscopic 3-step procedure was performed. The first step was a transnasal endoscopic sphenoidectomy through sphenoethmoidal recess. A powered instrument, such as a hummer, facilitated the surgery. A hypertrophied middle turbinate was laterally displaced, and a deviated nasal septum—especially a posteriorly located deviation—was always treated so as to widen the surgical space and facilitate the exposure of the surgical field.
The second step was the removal of the anterior wall of the sinus at the junction of the anterior wall and the floor of the sinus. An electric burr was used to cut down the anterior wall in a medial to lateral order until the floor of the sinus could be seen clearly under endoscope. The lateral limit of such an electric burring was the lateral wall of the sinus. Bony structure, such as a septum, a protrusion, or even a mucous cyst in the sinus, should be removed so as to expose the vidian canal ideally.
The third step of the procedure is the exposure of the vidian canal followed by resection of the corresponding neurovascular bundle including the vidian nerve.
Here, 2 points should be emphasized. The first point is the burring level at the joining part of the anterior wall and the floor of the sinus, as this would be closely related to the final exposure of the vidian canal. Based on preoperative CT scan of the sinus, an anatomic classification of the vidian canal could be done, and this helps to decide the level of the electric burring on the joining part between the anterior wall and the floor of the sinus. If the vidian canal belongs to the type I and type II classification (indicating that the vidian canal either protruded into the sinus in type I or half-protruded into the sinus and half-embedded under the floor of the sinus in type II), then the electric burring level would be on the floor or a little below the floor depending on the degree of the protrusion. Direct visualization of the vidian canal in the sphenoid sinus under either a 0° endoscope or an angled endoscope (30° or 70°) helps to locate and expose the vidian canal at the joining part described above. If the vidian canal is located deeply under the floor of the sinus (anatomic classification type III), then the burring level has to be lower than that of the floor.
The second point to be emphasized is the local anatomy of the sinus in relation to the reference marks. The location of the vidian canal in the CT scan is one thing, and exposure under endoscope is another. This means that in some cases in which the vidian canal was difficult to expose during the surgery, the local anatomy of the sinus, such as a bony protrusion, a septum, or even a mucous cyst, could be used as a landmark or a “road sign” to guide the surgery. For example, the vidian canal could be very close to a bony protrusion from the floor of the sinus. In such cases, the bony protrusion would be used as a landmark to help locate the vidian canal ( Figures 2 and 3 ).
Figure 2.
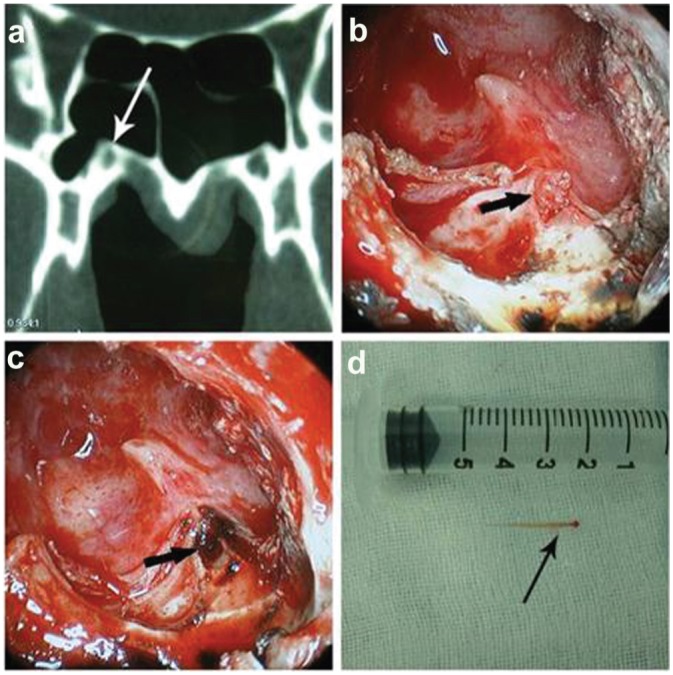
Computed tomography (CT) scan–guided surgery. Both CT scan of the vidian canal (a) and endoscopic view of the vidian nerve during surgery (b, nerve exposed; c, after cautery) with a section of resected vidian nerve (d, gross photograph).
Figure 3.
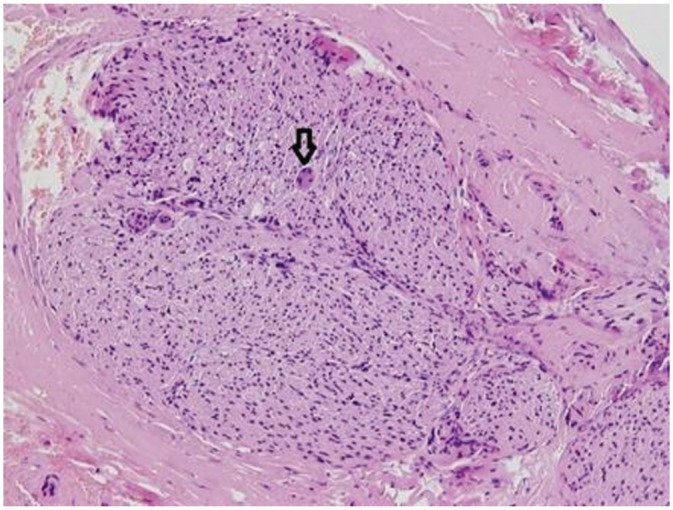
Histology of resected vidian nerve. Histology of the resected vidian nerve from Figure 2d , showing the nerve fibers and the soft tissue covering the nerve bundle. The arrow indicates a ganglion cell. Hematoxylin-eosin staining, 200×.
After the complete exposure of the vidian canal and the resection of the corresponding neurovascular bundle including the vidian nerve, a piece of resected nerve was sent for histology in some cases before bipolar electric cauterization on the proximal end of the vidian nerve instead of that on the distal part, as a previous study showed that electric cauterization on only the proximal part of the vidian nerve helps to prevent the complication of eye dryness.10
Extended Approach
On the other hand, if the sphenoid sinus developed very well with a highly pneumatized sinus cavity, then it would be difficult for a routine surgical approach to expose the vidian canal, even if the angled endoscope was used, as in these cases, the location of the vidian canal on the floor of the sinus could be positioned more laterally toward the lateral wall of the sphenoid sinus. In such cases, an extended approach or a combination technique of both intrasphenoid sinus visualization and transposterior ethmoid sinus procedure would be selected. Such an extended procedure could be described as the routine 3-step method described above plus a posterior ethmoidectomy. The posterior ethmoidectomy has been well described in the literature.11 In brief, the ethmoid bulla was opened and removed followed by dissection across the basal lamella of the middle turbinate into the posterior ethmoid with the identification and sharp resection of the inferior third of the superior turbinate as the last step in some cases. The ostium was usually medial to it. For maximum unilateral sphenoid exposure, such a combined technique would guarantee ideal exposure of the vidian canal under direct visualization. To make sure that the laterally located vidian canal could be localized under direct visualization, a 2-way endoscopic procedure was suggested. That is an intrasphenoid sinus endoscopic visualization plus an electric burring or bipolar cauterization through the posterior ethmoid.
Such a 3-step surgical procedure, as in the routine approach or an extended approach with a transposterior ethmoidectomy through the basal lamella of the middle turbinate, had its obvious advantages of easy location and exposure of the vidian canal with the anterior wall of the sinus as a stable landmark. Avoidance of management in the pterygopalatine fossa provided a clear subsequent surgical field.
To make sure that the vidian canal instead of the palatovaginal canal (PVC) was located and exposed, these 2 different canals had to be identified both preoperatively in the CT scan and during the surgery ( Figure 4 ).
Figure 4.
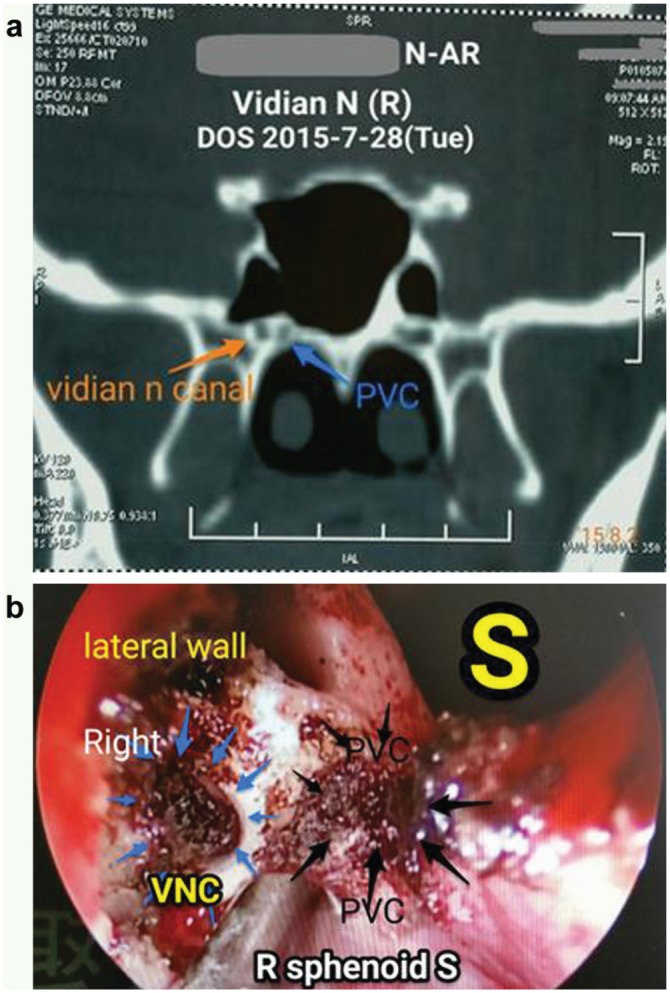
Identification and differentiation of the vidian canal and palatovaginal canal (PVC). Note the lateral position and relation to the PVC on the median side in (a) and the corresponding positions under endoscope in (b).
Concomitant surgery of either a deviated septum or hyperplasia of inferior turbinate would help to widen access, therefore facilitating the exposure of the vidian canal.
Results
All 91 cases in which patients received the surgery were successful in term of the localization, exposure, and resection of the vidian canal under endoscopes according to the method described here. The vidian canal shown in a preoperative CT scan image was 100% in accordance with endoscopic findings during the surgery confirmed by CT scan after surgery ( Figure 5 ).
Figure 5.
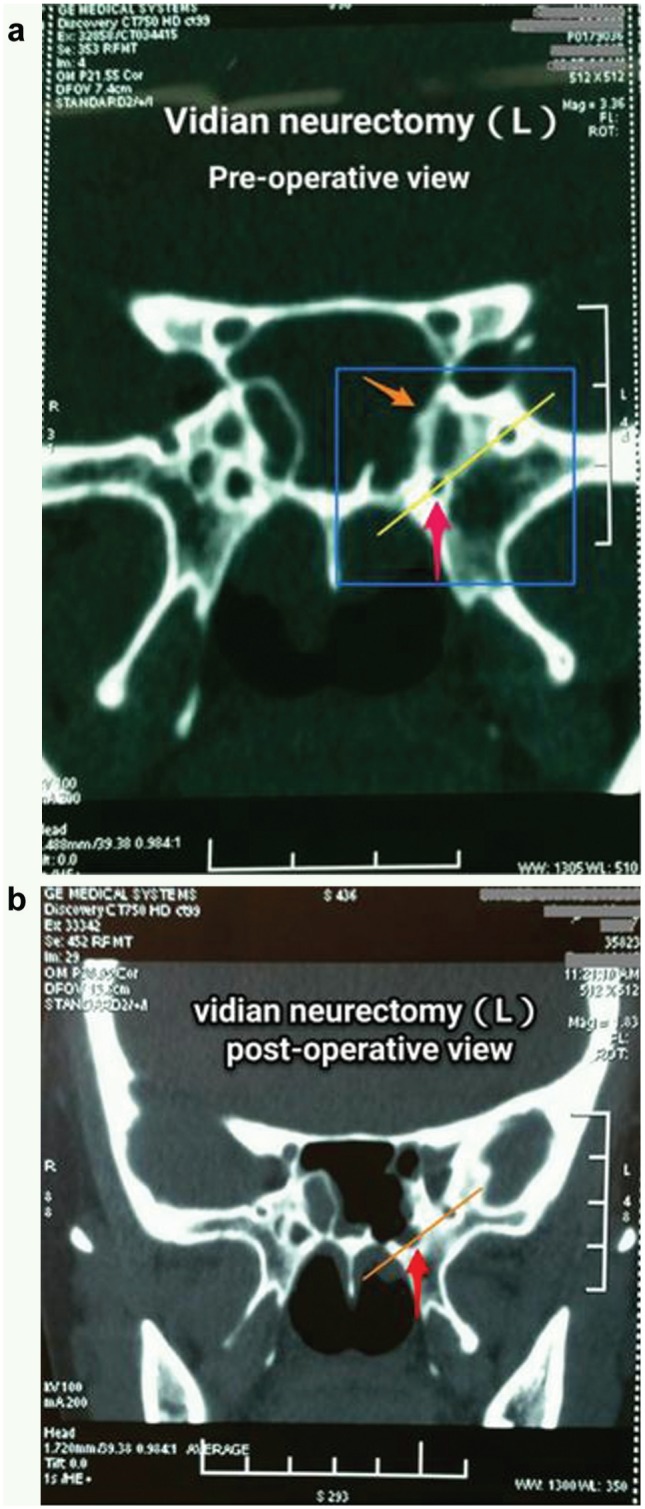
Computed tomography (CT) scan of vidian canal with pre- (a) and postoperative (b) comparison. Bony part of vidian canal indicated by red arrows in Figure 5a has been removed, as shown in Figure 5b . Bony septum referred to by orange arrow has been removed to expose the vidian nerve canal, as shown in Figure 5b .
In most cases, the surgery was performed according to routine procedures, while in some of the patients, surgery was performed by using an extended surgical approach depending on the degree of pneumatization of the sphenoid sinus.
Complications include mild dry eye in some cases and cheek numbness on the surgical side in a few patients (6/91), with delayed bleeding at day 16 after surgery in 1 patient. The eye dryness recovered in most cases within a short period of time after surgery (<3 months), with no persistent cheek numbness. The bleeding from a branch of the terminal vessel of the internal maxillary artery was confirmed and coagulated by bipolar electric cautery.
Concomitant septoplasty and inferior turbinectomy as well as sinus surgery were done in those patients with these complications.
The effect of VN on the secretion function of lacrimal glands within a short period of time was confirmed by Schirmer I test (Student t test, P ≤ .05), indicating that VN was successful.
The treatment efficacy was statistically significant based on the clinical follow-up results, and it will be discussed elsewhere in detail.
Discussion
Transnasal endoscopic VN with different surgical approaches has been reported.1-6 Currently, 2 basic surgical approaches are used, with each having advantages and disadvantages.4-6 An alternative surgical method was introduced and clinically tried in 91 cases with a 100% success rate in this series of patients. The unique benefit of this method was its easy localization and exposure of the vidian canal with the anterior wall of the sphenoid sinus as the only stable landmark. A routine and an extended approach would always guarantee the exposure of the vidian canal. Anatomically, the vidian canal between the foramen lacerum and its exit on the posterior wall of the pterygopalatine fossa went above, under, or parallel to the floor of the sphenoid sinus; therefore, the vidian canal was classified into 3 types.4 The technique described in this article is different from what was reported previously. Although direct exposure of the vidian canal through the anterior wall of the sphenoid sinus for a beginner is not so difficult in type I and type II vidian canal, indicating a positive protrusion of the vidian canal into the sinus, it is a big challenge if the vidian canal belongs to type III, suggestive of a negative protrusion. In this case, an antigrade tracing into the exit of the vidian canal on the posterior wall of the pterygopalatine fossa had to be done.9 But such a surgical procedure in the pterygopalatine fossa would increase not only the difficulty but also the bleeding.7,8
Some special considerations for ideal exposure of vidian canal according to the method described in this series of patients should be kept in mind. This includes the imaginary line through the foramen rotundum and the vidian canal (the so-called VR line; Figure 5 ). The VR line was used to evaluate the degree of the pneumatization of the sinus as well as the space lateral to the line. If a big space were present, then an extended procedure would be added so as to expose the vidian canal easily.
Another consideration was the distinguishing of the vidian canal from the PVC, as different neurovascular bundles were included in these 2 canals, with the vidian nerve and vessels passing through the vidian canal and the pharyngeal nerve (Bock’s nerve) through the PVC. If a PVC were mistaken as a vidian canal during the surgery, then the surgical effect would be different. One of the distinct differences between these 2 canals is their location, with vidian canal located more laterally and PVC located closer to the midline. In this series of patients, the VN in each case was accurate and successful, confirmed by Schirmer I test.
Special attention should also be paid to the management strategy for those complicated with asthma. In this group of patients, 7 cases of nasal hypersensitivity were complicated with asthma (7.7%, 7/91). In this series of candidates, a pulmonary function test was assigned for each of these 7 patients as a screening step in addition to the routine allergy test. If abnormal results were reported, an asthma specialist was consulted, with possible examinations as well as medication following. In this series of candidates, a patient with asthma as a complication was transferred to the intensive care unit and was treated there overnight following surgery, although preoperative medication was administered.
In addition to the surgical technique described above, the injury of the second branch of the trigeminal nerve (V2) and bleeding should always be avoided. In this series of candidates, 5 patients had numbness after surgery, although they recovered within 6 months. A retrospective analysis showed that the injury occurred in the first 30 patients because of the relative unfamiliarity of the local anatomy. The delayed bleeding from the surgical field in 1 patient was due to an incomplete bipolar electric cautery of the artery from the branch of the internal maxillary artery and strong physical exercise after surgery.
Concomitant septoplasty and inferior turbinectomy as well as sinus surgery helped to expose the surgical field and, therefore, the vidian canal.
In conclusion, based on the literature and our experience, a routine transnasal endoscopic intrasphenoid 3-step surgical procedure in most cases and extended procedures in some cases were suggested and tried in 91 cases in a Chinese population with a 100% success rate confirmed under the endoscope during the surgery by histology, Schirmer I test, and imaging (pre- and postoperative CT scan). The key step was the localization and exposure of the vidian canal in the junction between the anterior wall and the floor of the sinus, no matter which anatomic type the canal belonged to according to its anatomic classification. The unique advantage of this surgical approach was its ideal exposure of the vidian canal by referring to the anterior wall of the sphenoid sinus as the only stable landmark and the benefit of less bleeding via avoidance of exploring the pterygopalatine fossa and, therefore, having no trouble with bleeding caused by injury to the branches from sphenopalatine artery.
Author Contributions
Changqing Zhao, substantial contributions to the conception and design of the work; Yongjin Ji, performed the surgical approach for VN and joined writing; Yunfang An, one of the operators who collected patients’ history information; Jinmei Xue, one of the operators who collected patients’ history information; Qingfeng Li, organized follow-up and managed original data; Limin Suo, organized follow-up and managed original data; Ran Hou, involved in the follow-up and health care after surgery; Yanting Zhang, took part in most of the surgeries and recorded patients’ basic information; Zhigang Geng, responsible for choosing suitable patients and preparing for some videos during the surgery; Huimei Shen, helped to complete some medical checks for patients before and after surgery; Jianjun Ren, helped to analyze the data and called patients back in time; Pingchang Yang, major contributions to revising the manuscript critically for important intellectual content.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: National Natural Science Foundation of China (No. 81271059, 81670914), Shanxi Scholarship Council of China (No. 2014, key project 3), and Shanxi Science and Technology Key Project (20130313022-1).
Acknowledgments
The authors thank the following colleagues for their contribution to this article either in reviewing the manuscript or proposing good suggestions. Dr Hwang, from the Division of Rhinology & Endoscopic Skull Base Surgery, Department of Otorhinolaryngology–Head & Neck Surgery, Stanford University School of Medicine, Stanford, California, USA; Professor Zhaori, from the Chinese Medical Association; Dr Li, from the Department of Otorhinolaryngology, Affiliated Hospital, Nanjing University of Traditional Chinese Medicine, China; Dr Gao, from Pathology, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, Massachusetts, USA.
Footnotes
This article was presented at the National Academic Conference of Otolaryngology–Head and Neck Surgery; October 17, 2015; Hangzhou, Zhejiang, People’s Republic of China.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1. Golding-Wood PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. J Laryngol Otol. 1961;75:232-247. [DOI] [PubMed] [Google Scholar]
- 2. Lee JC, Lin YS. Endoscopic vidian neurectomy: update on techniques and evidence. Curr Opin Otolaryngol Head Neck Surg. 2012;20:66-72. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Micomonaco DC, Dziegielewski PT, et al. Endoscopic vidian neurectomy: a prospective case series. Int Forum Allergy Rhinol. 2015;5:423-430. [DOI] [PubMed] [Google Scholar]
- 4. Lee JC, Kao CH, Hsu CH, et al. Endoscopic transsphenoidal vidian neurectomy. Eur Arch Otorhinolaryngol. 2011;268:851-856. [DOI] [PubMed] [Google Scholar]
- 5. Lee JC, Kao CH, Hsu CH. Endoscopic vidian neurectomy. An on-line video tutorial: how to do it. Clin Otolaryngol. 2010;35:496-499. [DOI] [PubMed] [Google Scholar]
- 6. Tan G, Ma Y, Li H, et al. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138:492-497. [DOI] [PubMed] [Google Scholar]
- 7. Hwang SH, Joo YH, Seo JH, et al. Three-dimensional computed tomography analysis to help define an endoscopic endonasal approach of the pterygopalatine fossa. Am J Rhinol Allergy. 2011;25:346-350. [DOI] [PubMed] [Google Scholar]
- 8. Isaacs SJ, Goyal P. Endoscopic anatomy of the pterygopalatine fossa. Am J Rhinol. 2007;21:644-647. [DOI] [PubMed] [Google Scholar]
- 9. Su WF, Liu SC, Chiu FS, et al. Antegrade transsphenoidal vidian neurectomy: short-term surgical outcome analysis. Am J Rhinol Allergy. 2011;25;e217-e220. [DOI] [PubMed] [Google Scholar]
- 10. Su WF, Liu SC, Hsu WC, et al. Randomized, double-blind, controlled study to evaluate the effect of vidian nerve cauterization on lacrimation. Am J Rhinol Allergy. 2014;28:255-259. [DOI] [PubMed] [Google Scholar]
- 11. Kennedy DW, Ramakrishnan VR. Functional endoscopic sinus surgery: concepts, surgical indications, and techniques. In: Kennedy DW, Hwang PH. eds. Rhinology: Diseases of the Nose, Sinuses, and Skull Base. New York, NY: Thieme; 2011:306-335. [Google Scholar]


