Abstract
The mainstay of malaria diagnosis relies on rapid diagnostic tests (RDTs) and microscopy, both of which lack analytical sensitivity. This leads to repeat testing to rule out malaria. A prospective diagnostic trial of the Meridian illumigene Malaria assay (loop-mediated isothermal amplification [LAMP]) was conducted comparing it with reference microscopy and RDTs (BinaxNOW Malaria) in returning travelers between June 2017 and January 2018. Returning travelers with signs and symptoms of malaria were enrolled in the study. RDTs, microscopy, and LAMP assays were performed simultaneously. A total of 298 patients (50.7% male; mean age, 32.5 years) were enrolled, most visiting friends and relatives (43.3%), presenting with fever (88.9%), not taking prophylaxis (82.9%), and treated as outpatients (84.1%). In the prospective arm (n = 348), LAMP had a sensitivity of 98.1% (95% confidence interval [CI], 90.0%–100%) and a specificity of 97.6% (95% CI, 95.2%–99.1%) vs microscopy. After discrepant resolution with real-time polymerase chain reaction, LAMP had a sensitivity of 100% (95% CI, 93.7%–100%) and a specificity of 100% (95% CI, 98.7%–100%) vs microscopy. After discrepant resolution, RDTs had a sensitivity of 83.3% (95% CI, 58.6%–96.4%) and a specificity of 96.2% (95% CI, 93.2%–98.1%) vs microscopy. When including retrospective specimens (n = 377), LAMP had a sensitivity of 98.8% (95% CI, 93.2%–100%) and a specificity of 97.6% (95% CI, 95.2%–99.1%) vs microscopy, and after discrepant resolution of this set, LAMP had a sensitivity of 100% (95% CI, 95.8%–100%) and a specificity of 100% (95% CI, 98.7%–100%). A cost-benefit analysis of reagents and labor suggests savings of up to USD$13 per specimen using a novel algorithm with LAMP screening.
Keywords: malaria, LAMP, prospective study
In 2016, there were approximately 216 million cases of malaria in 91 countries, resulting in 445 000 deaths [1]. The World Health Organization (WHO) African Region bears the largest burden, with around 90% of the malaria cases. The WHO guidelines recommend that all suspected cases of malaria be confirmed with microscopy and/or rapid diagnostic tests. In Canada, there are on average 488 malaria cases per year across the country [2], and approximately 1700 annually in the United States [3]. However, in nonendemic settings such as North America, the vast majority of tests performed are negative.
Globally, the diagnosis of malaria is achieved with microscopy and rapid diagnostic tests (RDTs), or a combination of these. The WHO estimates that around 204 million patients were tested by microscopy and 269 million rapid diagnostic tests were sold in 2016 [4]. Although the increasing use of diagnostic-based treatment rather than symptom-based treatment undoubtedly has significant benefits, it is not without limitations. Microscopy has been found to have a limit of detection (LOD) of 50–100 parasites/μL under field conditions [5, 6]. However, this can only be achieved when laboratory facilities are available, along with a specialized microscopist. Studies have found that the LOD varies depending on the training and experience of the microscopist [7]. The method is labor-intensive and requires constant quality assurance. Due to these limitations, and therefore the possibility of false negatives, the Centers for Disease Control and Prevention (CDC) in the United States and Public Health Canada both recommend that 3 malaria slides be taken [8, 9]. This is not just an inconvenience to the patient, who may have to return the emergency room several times; it results in increased health care costs due to re-attendance and increased laboratory time.
Although RDTs have demonstrated a sensitivity and specificity >90% for Plasmodium falciparum infections with >200 parasites/μL, they cannot reliably diagnose cases with parasitemia below this level [10]. Additionally, false-negative RDT results can occur from parasites that do not produce histidine-rich protein 2 (HRP2), a growing concern in malaria-endemic regions [11–13]. Molecular testing such as polymerase chain reaction (PCR) can achieve an LOD of 1 parasites/μL with improved sensitivity, but these tests are expensive and simply not feasible without a well-equipped laboratory and expertise [14].
Given the limitations with the current testing methods of microscopy, RDTs, and PCR, there has been interest in validating simpler nucleic acid tests (NATs) such as loop-mediated isothermal amplification (LAMP) [15–19]. LAMP is a technology that was developed in 2000 that uses unique DNA polymerases and specially designed primers to amplify nucleic acid without thermocyclers [20]. LAMP can differentiate species, and variations can even be used to detect the C580Y mutation in the propeller domain of the kelch 13 gene, the most prevalent marker of artemisinin resistance [21]. The LOD varies slightly depending on the exact protocol used, but it can achieve limits of detection of ≤2 parasites/μL, similar to those achieved by PCR and surpassing those of microscopy and RDTs [22–25]. The illumigene Malaria LAMP assay (illumigene M; Meridian Biosciences, Cincinatti, OH) has shown good sensitivity and specificity vs microscopy, the current gold standard [15]. It performs well in terms of technological simplicity and cost. Commercial LAMP technology has been assessed against PCR in a number of studies, with extremely encouraging results (Table 1) [15–18, 22, 25–28]. Given the excellent negative predictive value properties of LAMP in malaria, our group has proposed replacing the current testing algorithm, which relies on 3 microscopy tests to rule out infection, with a single LAMP test [15]. This would improve testing turnaround time, optimize use of the emergency room, and reduce cost to the health care system.
Table 1.
Commercial LAMP Performance Against PCR
| Article | Commercial Kit | Specimen Analysisb | Location | Sensitivity (95% CI), % |
Specificity (95% CI), % |
|---|---|---|---|---|---|
| Aydin-Schmidt 2014 | Eiken | Retrospective | Zanzibar – pre-elimination | 91.5 (84.8–95.8) | 100 (99.5–100) |
| Hopkins 2013 | Eiken | Prospective | Uganda – high endemicity | 90.0 (85.0–94.0) | 85.0 (75.0–92.0) |
| Lucchi 2016 | illumigene | Prospective and retrospective | Senegal – low endemicity | 97.2 (92.6–99.1) | 93.8 (84.2–98.0) |
| Marti 2015 | Eiken | Prospective | Switzerland – returning travelers | 100 (92.4–100) | 100 (97.7–100) |
| Polley 2013 | Eiken | Prospective | UK – returning travelers | 97.0 (89.6–99.6) | 99.2 (98.1–99.7) |
| Ponce 2017 | illumigene | Prospective | France – returning travelers | 100 (95.8–100) | 98.1 (95.3–99.5) |
| Rypien 2017 | illumigene | Retrospective | Canada – returning travelers | 97.3 (90.7–99.7) | 93.8 (84.8–98.3) |
| Sema 2015a | Eiken | Retrospective | Ethiopia - moderate endemicity | 96.8 (83.2–99.5) | 84.3 (71.4–92.9) |
| Tegegne 2017 | Eiken | Retrospective | Ethiopia - moderate endemicity | 100 (100–100) | 93.5 (86.5–100) |
Abbreviations: CI, confidence interval; LAMP, loop-mediated isothermal amplification; PCR, polymerase chain reaction.
aField values taken.
b"Prospective" defined as blood specimens tested within 72 hours without preservation; others considered "retrospective".
On a global note, if elimination is to be achieved, a diagnostic tool with a lower limit of detection is required. A recent study modeling eradication showed that reducing the limit of detection of a diagnostic test increased the detection of the asymptomatic infectious reservoir [29]. LAMP meets this criterion, and several studies have described the role it could play in malaria elimination [10, 25, 26, 30, 31].
Although LAMP has been shown in studies to surpass microscopy and RDTs, many studies to date have been retrospective in nature. In addition, studies have often focused on P. falciparum rather than all species of malaria. Our study was designed to closely reflect testing scenarios in North America. A single-center evaluation of LAMP performance against microscopy for all species of malaria in a major urban center (1.4 million people) was conducted. The primary objective of the study was to compare the clinical performance of a commercially available LAMP method (illumigene M) vs RDT (BinaxNOW) compared with microscopy in symptomatic patients presenting as potential cases of malaria in a nonendemic area. The secondary objective was to determine if certain clinical and epidemiological factors predicted malaria test results using regression analysis.
METHODS
Study Design, Patient Enrollment, and Ethics
The study design was pragmatic, containing both prospective and retrospective arms to provide an adequate distribution of all species of malaria. In the prospective arm, adult participants who underwent malaria testing were consecutively enrolled into the study from June 2017 until January 2018. Based on sample size calculation, a goal of 350 specimens was set with at least 10% overall positivity for all species of malaria. To achieve this, testing was performed on a continuous basis with interruptions only to ensure that enough positive samples were tested. Samples arriving at night were tested first thing in the morning by medical laboratory technologists using the LAMP technology. All prospectively enrolled participants had epidemiological data collected through a case history form that accompanied the specimens. Variables recorded included whether pretravel advice was received, reason for travel, country visited, symptoms, severity, whether the participant took antimalarial prophylaxis, and whether they were on treatment. In addition to the prospective arm of the study, 29 microscopy confirmed malaria specimens were retrospectively collected and included in the study. This design has been used by other studies, and it allows an adequate sample size for rare non–P. falciparum species [32]. Without this, due to the otherwise prospective nature of the study, only a small number of positive specimens for these species would have been included. Five Plasmodiumvivax and 5 Plasmodium ovale frozen whole-blood specimens (at –80°C) were obtained from a large teaching hospital in Edmonton, Canada. Thirteen Plasmodium malariae specimens were frozen whole-blood specimens obtained from Pará, Brazil, and 6 culture-derived Plasmodium knowlesi (A1-H.1 clone) specimens of varying parasitemia (range, 0.1–10 000 parasite per μL) were obtained from London, United Kingdom. Retrospective specimens were thawed and underwent the same LAMP testing protocol as the prospective arm. No epidemiological data were available on retrospective specimens. Ethical approval was obtained from the Conjoint Health Research Ethics Board (REB17-2220), and privacy review from Alberta Health Services.
Routine Laboratory Testing for Malaria
Venous whole-blood samples were collected in EDTA vacutainers from medical centers in Calgary and the surrounding area and transported to Calgary Laboratory Services (CLS). CLS is a large centralized public microbiology laboratory service covering 1.4 million people. Samples from malaria-positive patients who were initially symptomatic, treated, and then retested were included in the study. Each specimen was tested according to standard operating procedure: Giemsa-stained thick and thin peripheral blood smears and RDTs (BinaxNOW Malaria, Alere, Waltham, MA) on study participants. For the purposes of analysis, microscopy repeated 3 times from the same individual to confirm negativity was classified once only. Microscopy, RDTs, and LAMP testing were conducted in real time by the same technologist who was trained in all diagnostic tests due to personnel availability.
Malaria Testing With illumigene M LAMP
illumigene Malaria (Meridian Biosciences, Cincinatti, OH) is a commercially available, loop-mediated amplification test that detects all species of human malaria. The assay provides a positive or negative result only and does not speciate. Medical laboratory technologists received training for the LAMP assay. Testing using LAMP occurred simultaneously with current standard operating procedure (SOP) for the duration of the study. Specimens received at night were tested by LAMP in the day shift due to technologist availability. LAMP was performed according to the manufacturer’s recommendations. Complete standard operating procedures for illumigene are available online (http://www.meridianbioscience.eu/media/pdf/Package%20Insert/280925_281125_MULTI_REV1215.pdf). Samples were frozen once at –80°C for subsequent real-time PCR (RT-PCR) analysis, which was performed off-site in a blinded manner.
Discrepancy Resolution
Discrepant results occurred when the results of microscopy and the LAMP assay results were different. All microscopy-positive and discrepant specimens underwent RT-PCR testing at an off-site location at the Provincial Reference Laboratory in Edmonton, Canada, in a blinded manner. The RT-PCR method was clinically validated and used routinely at the reference laboratory [33]. Concordant negative specimens were also tested by RT-PCR in Calgary, Canada.
Statistical Analysis
Data were tabulated in Microsoft Excel, version 16.11.1, and were analyzed using Stata, version 13.0. Crude statistical analysis of epidemiological dichotomous variables was determined using chi-square or Fisher exact statistical tests; t tests were used for comparison of means. Variables found to be statistically significant with initial analysis were then characterized through multivariate analysis with logistic regression. Retrospectively and prospectively collected specimens were pooled and analyzed together. Sensitivity, specificity, positive predictive rate, and negative predictive rate for LAMP were calculated against microscopy both before and after discrepant resolution with PCR. After being assessed for significance, multivariate logistic regression was utilized to calculate odds ratios. Both important variables and statistically significant ones were incorporated into the analysis. Admission status was excluded because it was a dependent variable, which was influenced by the malaria test result. Due to the small sample size of malaria-positive patients, variables were dichotomized to allow for reliable analysis. Continent visited was split into Africa and non-Africa, and reason for travel was split into those visiting friends and relatives (VFR) and non-VFR. The sample size for subanalyses was insufficient without this breakdown. As observed in the correlation matrix (Supplementary Table 1), there was reason to believe that there was a correlation between pretravel advice and prophylaxis taken, so only a single variable, “prophylaxis taken,” was utilized in the logistic analysis.
RESULTS
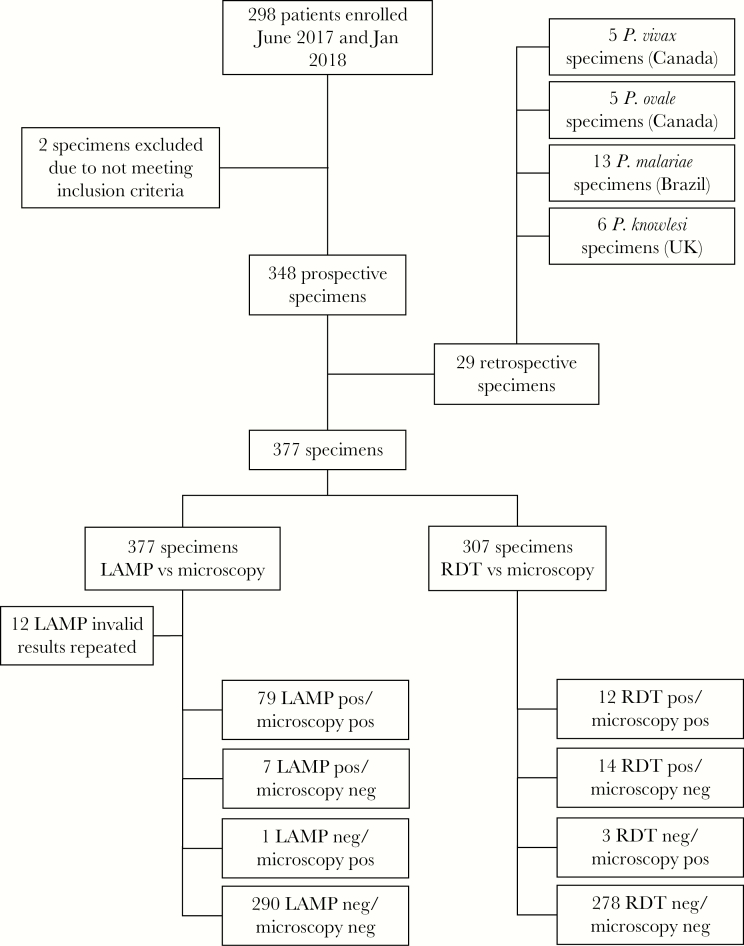
In the prospective arm, 350 specimens were collected from a total of 298 consecutive patients enrolled (Figure 1). Two specimens were excluded due to having insufficient samples for specimen resolution, leaving 348 specimens. Fifty specimens were repeats from some of the participants who initially tested positive, as it is routine practice to monitor parasitemia during treatment. In the retrospective arm, 29 additional frozen specimens were used to supplement the analysis. The prospective and retrospective specimens were combined, so there were 377 specimens in total. Microscopy and LAMP were available on all specimens, but RDT testing was only performed on participants in the prospective arm if deemed appropriate (n = 307). Frozen specimens had prior microscopy data at the time of diagnosis. There was an initial “invalid” test rate with LAMP illumigene M of 3.18%; however, all of these resolved with a single repeat test.
Figure 1.
Flow chart of study design and initial results of malaria testing by each method. Abbreviations: LAMP, loop-mediated isothermal amplification; RDT, rapid diagnostic test.
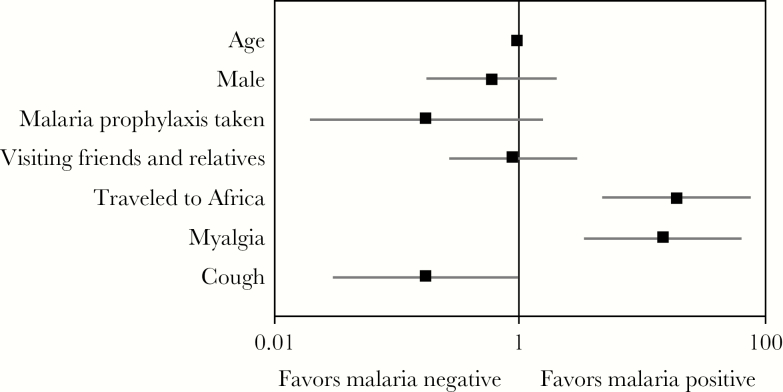
In the prospective arm, clinical and epidemiological data were analyzed based on the participant’s final malaria result after discrepant resolution. Twenty-five participants out of 298 tested positive for malaria after discrepant resolution. Twenty-two out of the 25 participants who tested positive were positive by microscopy; the remaining 3 were confirmed by PCR. The median parasitemia (interquartile range) was 0.2 (1.275). The mean age in the cohort was 32.5 (95% confidence interval [CI], 29.9–35.0) years, and 50.7% of participants were male (Table 2). Fever was by far the most common symptom (265/298; 88.9%), followed by headache (124/298; 41.6%), cough (102/298; 34.3%), night sweats (96/298; 32.2%), and myalgia (92/298; 30.9%). Myalgia was statistically more common in the malaria-positive group (16/25; 64.0%) compared with the malaria-negative group (76/273; 27.8%; P < .001) (Figure 2). Cough was statistically less common in the malaria-positive group (4/25; 16.0%) compared with the malaria-negative group (98/273; 35.9%; P = .047). Malaria-positive patients were more likely to be admitted than those who tested negative (P < .001). For every year increase in age, the odds of testing positive for malaria decreased by 6% (odds ratio [OR], 0.94), which was statistically significant (95% CI, 0.91–0.98). The odds of testing positive for males was slightly lower than females (OR, 0.60; 95% CI, 0.18–1.98), but this was not statistically significant. Participants traveling to Africa were significantly more likely to test positive for malaria (OR, 18.90; 95% CI, 4.81–74.48) compared with those visiting another location. There was no difference in the odds for those visiting friends and relatives compared with those traveling for other reasons (OR, 0.88; 95% CI, 0.27–2.89). Antimalarial prophylaxis was protective, with the odds ratio for those taking it being 0.17 (95% CI, 0.02–1.48), but this was not statistically significant. Evidence of effect measure modification (EMM) was looked for between those taking prophylaxis and also visiting Africa, but none was observed. In addition, EMM was assessed for those taking prophylaxis and also in the VFR group, but the sample size was insufficient to perform the analysis.
Table 2.
Comparison of Clinical and Epidemiological Characteristics of Study Participants According to their Malaria Test Outcome (n = 298)
| Total | Malaria Positive | Malaria Negative | P Valuea | |
|---|---|---|---|---|
| No. of patients | 298 | 25 | 273 | |
| Age, mean (95% CI), y | 32.5 (29.9–35.0) | 25.2 (18.3–32.1) | 33.1 (30.5–35.8) | .089b |
| Male, No. (%) | 151 (50.7) | 13 (52.0) | 138 (50.5) | .890e |
| Parasitemia, median (IQR) | 0.2 (1.275) | |||
| Pretravel advice (n = 208),c No. (%) | 69 (33.2) | 2 (11.8) | 67 (35.1) | .061f |
| Malaria prophylaxis taken (n = 234),c No. (%) | 40 (17.1) | 2 (9.1) | 38 (17.9) | .385f |
| Reason for travel,d No. (%) | ||||
| Business | 15 (5.0) | 1 (4.0) | 14 (5.1) | 1.000f |
| Not recorded | 26 (8.7) | 2 (8.0) | 24 (8.8) | 1.000f |
| Visiting friends/relatives | 129 (43.3) | 10 (40.0) | 119 (43.6) | .692e |
| Volunteer | 1 (0.3) | 1 (4.0) | 0 | .085f |
| New immigrant | 32 (10.7) | 6 (24.0) | 26 (9.5) | .038f |
| Tourism | 89 (29.9) | 4 (16.0) | 85 (31.1) | .111f |
| Visitor to Canada | 6 (2.0) | 1 (4.0) | 5 (1.8) | .414f |
| Continent visited (n = 287),c No. (%) | ||||
| Non-Africa | 186 (64.8) | 4 (16.0) | 182 (69.5) | |
| Africa | 101 (35.2) | 21 (84.0) | 80 (30.5) | <.001f |
| Symptoms,d No. (%) | ||||
| Fever | 265 (88.9) | 23 (92.0) | 242 (88.7) | .704f |
| Night sweats | 96 (32.2) | 8 (32.0) | 88 (32.2) | .862e |
| Myalgia | 92 (30.9) | 16 (64.0) | 76 (27.8) | <.001e |
| Headache | 124 (41.6) | 13 (52.0) | 111 (37.2) | .361e |
| Cough | 102 (34.3) | 4 (16.0) | 98 (35.9) | .047f |
| Sore throat | 66 (22.1) | 2 (8.0) | 64 (23.4) | .080f |
| Diarrhea | 66 (22.1) | 5 (20.0) | 61 (22.3) | .808f |
| Other | 8 (2.7) | 2 (8.0) | 6 (2.2) | .148f |
| Pre-employment | 4 (1.3) | 0 | 4 (1.5) | 1.000f |
| Not recorded | 12 (4.0) | 0 | 12 (4.4) | .608f |
| New immigrant screen | 4 (1.3) | 0 | 4 (1.5) | 1.000f |
| Admitted (n = 295),c No. (%) | 47 (15.9) | 12 (50.0) | 35 (12.9) | <.001e |
Abbreviations: CI, confidence interval; IQR, interquartile range.
a P value compares malaria-positive with malaria-negative group, missing values excluded.
b T test of means.
cNo. is less than the grand total due to missing data; percentages were calculated excluding missing data and statistical tests excluding missing data.
dTotal does not add up to 100 as participants reported more than 1 symptom.
eChi-square test.
fFisher exact test.
Figure 2.
Odds ratio (OR) and 95% confidence interval (CI) for clinical and epidemiological characteristics among study participants for malaria-positive versus malaria-negative patients. ORs and 95% CIs enumerated on the x-axis were calculated using multivariate logistic regression.
Data were analyzed both including and excluding retrospective specimens. For the prospective arm, when repeat testing on the same participant was included, 57 specimens tested positive by LAMP, whereas 51 tested positive by microscopy (Table 3). For microscopy-positive specimens, 38 tested positive for P. falciparum, 7 for P. vivax, 6 for P. ovale, and none for P. malariae or P. knowlesi; the remainder tested negative. Due to repeat testing of malaria-positive patients, a test positivity rate of 14.7% was obtained in this study. Surveillance data in Calgary from 2013–2018 and excluding repeat testing within 2 months of an index revealed a test positivity rate of 4.4% (unpublished data) used to calculate the PPV and NPV. In the prospective arm, LAMP had a sensitivity of 98.1% (95% CI, 90.0%–100%) and a specificity of 97.6% (95% CI, 95.2%–99.1%) vs microscopy before discrepant resolution for all samples. Based on a prevalence of 4.4%, the positive predictive value (PPV) was 65.3%, and the negative predictive value (NPV) was 99.9%. Seven specimens were negative with microscopy but tested positive with LAMP. One specimen was positive with microscopy but negative with LAMP. All specimens, both discordant and concordant, were tested by RT-PCR for confirmation. Discrepant resolution confirmed that the 7 false-positive specimens by LAMP tested positive by RT-PCR (Table 4). One false-negative specimen by LAMP tested negative by RT-PCR. After discrepant resolution, LAMP had a sensitivity of 100% (95% CI, 93.9%–100%) and a specificity of 100% (95% CI, 98.7%–100%) vs microscopy. The PPV and NPV were both 100%. For 1 negative specimen by LAMP and RT-PCR, only P. ovale gametocytes were found.
Table 3.
Performance of illumigene M Assay vs Microscopy on Returning Travelers in the Prospective Arm Before and After Discrepant Resolution by RT-PCR (n = 348)
| Before Discrepant Resolution | Microscopy | After Discrepant Resolution | PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | ||||
| LAMP | Positive | 50 | 7 | 57 | LAMP | Positive | 57 | 0 | 57 |
| Negative | 1 | 290 | 291 | Negative | 0 | 291 | 291 | ||
| Total | 51 | 297 | 348 | Total | 57 | 291 | 348 | ||
| Percentage | 95% CI, % | Percentage | 95% CI, % | ||||||
| Sensitivity | 98.1 | 90.0–100 | Sensitivity | 100 | 93.7–100 | ||||
| Specificity | 97.6 | 95.2–99.1 | Specificity | 100 | 98.7–100 | ||||
| Positive predictive valuea | 65.3 | Positive predictive valuea | 100 | ||||||
| Negative predictive valuea | 99.9 | Negative predictive valuea | 100 | ||||||
Abbreviations: CI, confidence interval; LAMP, loop-mediated isothermal amplification; PCR, polymerase chain reaction; RT-PCR, real-time polymerase chain reaction.
aBased on a prevalence of 4.4%.
Table 4.
Results of Discrepant Resolution by Sample Number (n = 8)
| Sample Number | Microscopy | illumigene M | Alternate PCR | Final |
|---|---|---|---|---|
| 018 | Neg | Pos | Pos | True positive – P. falciparum |
| 035 | Neg | Pos | Pos | True positive – P. falciparum |
| 068 | Neg | Pos | Pos | True positive – P. falciparum |
| 095 | Neg | Pos | Pos | True positive – P. vivax |
| 112 | Neg | Pos | Pos | True positive – P. falciparum |
| 122 | Po gametocytes | Neg | Neg | True negative |
| 253 | Neg | Pos | Pos | True positive – P. falciparum |
| 265 | Neg | Pos | Pos | True positive – P. falciparum |
Abbreviation: PCR, polymerase chain reaction; Po, plasmodium ovale.
When including retrospective specimens, an additional 5 P. vivax, 5 P. ovale, 13 P. malariae, and 6 P. knowlesi specimens were included, so that in total by microscopy there were 38 P. falciparum specimens, 12 P. vivax, 11 P. ovale, 13 P. malariae, and 6 P. knowlesi specimens, with the remainder being negative (Table 5). With retrospective specimens included, LAMP had a sensitivity of 98.8% (95% CI, 93.2%–100%) and a specificity of 97.6% (95% CI, 95.2%–99.1%) vs microscopy. After discrepant resolution, LAMP had a sensitivity of 100% (95% CI, 95.8%–100%) and a specificity of 100% (95% CI, 98.7%–100%). There were no additional discrepancies following inclusion of retrospective specimens.
Table 5.
Performance of illumigene M Assay vs Microscopy on Returning Travelers in the Prospective and Retrospective Arms Combined (n = 377) Before and After Discrepant Resolution
| Before Discrepant Resolution | Microscopy | After Discrepant Resolution | PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | ||||
| LAMP | Positive | 79 | 7 | 86 | LAMP | Positive | 86 | 0 | 86 |
| Negative | 1 | 290 | 291 | Negative | 0 | 291 | 291 | ||
| Total | 80 | 297 | 377 | Total | 86 | 291 | 377 | ||
| Percentage | 95% CI, % | Percentage | 95% CI, % | ||||||
| Sensitivity | 98.8 | 93.2–100 | Sensitivity | 100 | 95.8–100 | ||||
| Specificity | 97.6 | 95.2–99.1 | Specificity | 100 | 98.7–100 | ||||
| Positive predictive valuea | 65.5 | Positive predictive valuea | 100 | ||||||
| Negative predictive valuea | 100 | Negative predictive valuea | 100 | ||||||
Abbreviations: CI, confidence interval; LAMP, loop-mediated isothermal amplification; PCR, polymerase chain reaction.
aBased on a prevalence of 4.4%.
The performance of RDT compared with microscopy was evaluated (n = 307) (Supplementary Table 2). After discrepant resolution using RT-PCR, the sensitivity was 83.3% (95% CI, 58.6%–96.4%) and specificity was 96.2% (95% CI, 93.2%–98.1%). These results correlated with a PPV of 50.2% and an NPV of 99.2%. The species-specific performance of LAMP after discrepant resolution is shown in Supplementary Table 3.
DISCUSSION
The commonest reason for travel was recent immigrants traveling to visit friends and relatives abroad. These individuals have been found in other studies to stay in endemic areas for longer, are more likely to stay in local houses, so they are more exposed to mosquitos, and are less likely to seek pretravel advice and antimalarial prophylaxis [34]. The patient cohort here represents a typical population presenting with the symptoms of malaria. As a result, this study is generalizable to urban centers with a large immigrant population across North America and Europe. Although numerous studies have examined the role of LAMP for the diagnosis of malaria in both the nonendemic and endemic settings, this is the first evaluation of LAMP in a “real-time” prospective trial in returning travelers to North America. A recent meta-analysis found that LAMP appears to have excellent sensitivity against PCR [35]. Due to its limit of detection, LAMP is thought to be particularly useful in diagnosing patients with a low parasitemia below the LOD of microscopy [26]. It has several advantages over other molecular tests: simple to use, no major capital equipment needs, and limited training needed [16, 20, 36]. Laboratory technologists were trained in illumigene M, and they were able to perform it without difficulty in under an hour once they received the specimen.
Currently, the CDC recommendation for malaria testing is to release an RDT result as a preliminary result and then repeat thick and thin films every 6 to 8 hours on average 3 times to ensure that no parasites are present [9]. This is accompanied by a significant labor cost in both the laboratory and in repeated emergency room use. A previous study from our group proposed a new testing algorithm for malaria with LAMP as the firstline diagnostic test [15]. In the nonendemic setting, the proportion of malaria tests that are positive is relatively small. Repeated testing of patients without malaria based on existing algorithms results in additional costs to the laboratory and hospital. We estimated from this study that a single LAMP test with a high NPV would lead to a laboratory cost savings of up to USD$13 per malaria test in a nonendemic setting. Further savings for the hospital may be possible resulting from the earlier discharge of the patient from the emergency room or doctor's office. Multiple studies have found that LAMP had an excellent NPV compared with PCR. However, they are often retrospective, do not run continuously, and/or commonly have a small number of non–P. falciparum species included.
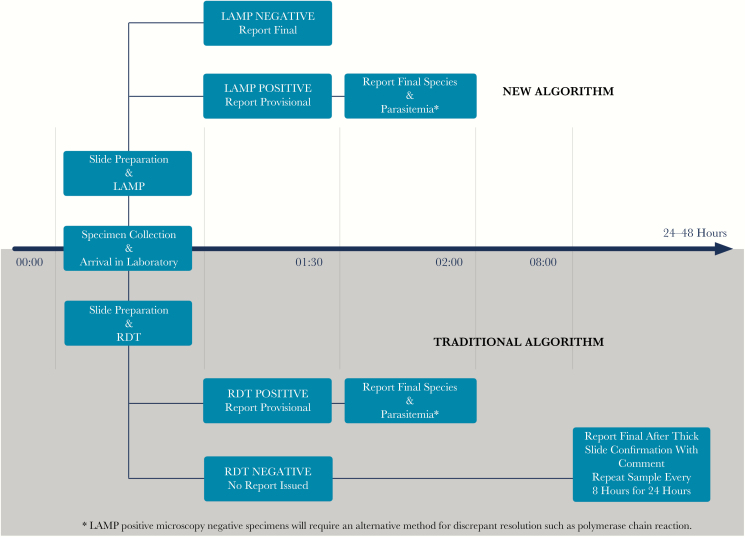
A strength of the study is that all species of malaria were tested and correctly identified, which is essential if this test is to be used routinely. The study was prospective in nature and ran continuously in real time for 8 months to reflect current practice, albeit supplemented with retrospective specimens. Technologists were easily trained and were able to complete testing with illumigene M without supervision. This has potential advantages after-hours as technologists may not be experienced with reading the thick films required to ultimately rule out infection. Based on the LAMP sensitivity and NPV (100%) found in this study, patients presenting after-hours who test negative can safely be discharged home within 1 hour of specimen receipt at the laboratory (Figure 3). The lower sensitivity of RDT (83.3%) in this study does not permit this confidence, especially for non–P. falciparum species, for which it fails more routinely to detect infection. Previously, the time to final reading of the thick film (where sensitivity is achieved) was 9 to 24 hours, depending on specialist technologist availability, because of the lack of confidence in the RDT result. The ability to discharge patients leads to a cost savings for the health facility because patients do not have to re-attend for repeat microscopy smears.
Figure 3.
Comparison of a traditional malaria testing algorithm with the novel algorithm proposed in this study. Abbreviations: LAMP, loop-mediated isothermal amplification; RDT, rapid diagnostic test.
A limitation of this study is that the same technologist often performed LAMP, microscopy, and RDT testing for each specimen. It was not feasible to have multiple technologists available who could perform testing, so consequently operators were not blinded to other results. This may have introduced some misclassification bias. For discordant results, PCR testing occurred off-site and operators were blinded, thereby reducing misclassification bias for the most important results. Also, although P. knowlesi samples were contrived from a lab strain, LAMP has been able to detect patient samples on dried blood spots by our group (our unpublished observations) and patient whole-blood samples by others (Martin et al., unpublished data, 2018).
A limitation of the illumigene M platform is that it does not identify the species of malaria. This is not essential as a rapid screening test. Positive LAMP tests can be followed up with microscopy to determine species and parasitemia. An “invalid” rate of 3.18% was observed. The reasons for this are unclear (possibly due to high parasitemia, DNA amplification inhibitor, delay in the extraction process), but this study did have a smaller proportion of invalid results compared with other relevant studies [15, 30].
In this study, invalid results resolved with single repeated testing; however, they caused a delay in diagnosis. Specimens that did not resolve with repeated testing would require an alternative method to be used.
In a nonendemic setting where the prevalence of malaria in returning travelers is relatively low, LAMP is able to rule out malaria with a faster turnaround time without the need for repeat testing. A novel, highly sensitive testing algorithm for malaria screening with associated cost savings in the nonendemic setting is proposed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all the medical laboratory staff at Calgary Laboratory Services who contributed to this study. We also thank Dr. Janet Cox-Singh for kindly providing P. knowlesi dried blood spots for evaluation.
Financial support. This work was supported by Calgary Laboratory Services.
Potential conflicts of interest. Meridian Biosciences Inc. provided the equipment and reagents for the illumigene M LAMP assay. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Malaria. 2017. Available at: http://www.who.int/mediacentre/factsheets/fs094/en/. Accessed 29 November 2017. [Google Scholar]
- 2. Public Health Agency of Canada. Surveillance of malaria. 2018. Available at: https://www.canada.ca/en/public-health/services/diseases/malaria/surveillance-malaria.html. Accessed 18 March 2018.
- 3. Centers for Disease Control and Prevention. Facts about malaria. 2017. Available at: https://www.cdc.gov/malaria/about/facts.html. Accessed 13 March 2018.
- 4. World Health Organization. Overview of diagnostic testing. 2018. Available at: http://www.who.int/malaria/areas/diagnosis/overview/en/. Accessed 13 March 2018.
- 5. Joanny F, Löhr SJ, Engleitner T, et al. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar J 2014; 13:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milne LM, Kyi MS, Chiodini PL, Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol 1994; 47:740–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimmerman PA, Howes RE. Malaria diagnosis for malaria elimination. Curr Opin Infect Dis 2015; 28:446–54. [DOI] [PubMed] [Google Scholar]
- 8. Public Health Agency of Canada. Canadian Recommendations for the Prevention and Treatment of Malaria Among International Travellers. 2009. Available at: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2009-35/canadian-recommendations-prevention-treatment-malaria-among-international-travellers/malaria-diagnosis.html. Accessed 13 March 2018. [Google Scholar]
- 9. Centers for Disease Control and Prevention. Malaria Diagnosis & Treatment - Guidelines for Clinicians. 2009. Available at: https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.html. Accessed 13 March 2018.
- 10. McMorrow ML, Aidoo M, Kachur SP. Malaria rapid diagnostic tests in elimination settings—can they find the last parasite?Clin Microbiol Infect 2011; 17:1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamboa D, Ho MF, Bendezu J, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 2010; 5:e8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koita OA, Doumbo OK, Ouattara A, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 2012; 86:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Araia Berhane KA, Mihreteab S, Gresty K, et al. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2, Eritrea. Emerg Infect Dis 2018; 24:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofmann N, Mwingira F, Shekalaghe S, et al. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015; 12:e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rypien C, Chow B, Chan WW, et al. Detection of plasmodium infection by the illumigene Malaria assay compared to reference microscopy and real-time PCR. J Clin Microbiol 2017; 55:3037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polley SD, González IJ, Mohamed D, et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis 2013; 208:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sema M, Alemu A, Bayih AG, et al. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malar J 2015; 14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tegegne B, Getie S, Lemma W, et al. Performance of loop-mediated isothermal amplification (LAMP) for the diagnosis of malaria among malaria suspected pregnant women in Northwest Ethiopia. Malar J 2017; 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frickmann H, Hinz R, Rojak S, et al. Evaluation of automated loop-mediated amplification (LAMP) for routine malaria detection in blood samples of German travelers - a cross-sectional study. Travel Med Infect Dis 2018; 24:25–30. [DOI] [PubMed] [Google Scholar]
- 20. Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000; 28:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohon AN, Menard D, Alam MS, et al. A novel SNP-LAMP assay for detection of artemisinin-resistant Plasmodium falciparum malaria. Open Forum Infect Dis 2018;XXX(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucchi NW, Gaye M, Diallo MA, et al. Evaluation of the illumigene Malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep 2016; 6:36808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lucchi NW, Demas A, Narayanan J, et al. Real-time fluorescence loop mediated isothermal amplification for the diagnosis of malaria. PLoS One 2010; 5:e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polley SD, Mori Y, Watson J, et al. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J Clin Microbiol 2010; 48:2866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins H, González IJ, Polley SD, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 2013; 208:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aydin-Schmidt B, Xu W, González IJ, et al. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS One 2014; 9:e103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marti H, Stalder C, González IJ. Diagnostic accuracy of a LAMP kit for diagnosis of imported malaria in Switzerland. Travel Med Infect Dis 2015; 13:167–71. [DOI] [PubMed] [Google Scholar]
- 28. Pöschl B, Waneesorn J, Thekisoe O, et al. Comparative diagnosis of malaria infections by microscopy, nested PCR, and LAMP in Northern Thailand. Am J Trop Med Hyg 2010; 83:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slater H, Ross A, Ouédraogo AL, et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 2015; 528:S94–101. [DOI] [PubMed] [Google Scholar]
- 30. Aydin-Schmidt B, Morris U, Ding XC, et al. Field evaluation of a high throughput loop mediated isothermal amplification test for the detection of asymptomatic plasmodium infections in Zanzibar. PLoS One 2017; 12:e0169037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perera RS, Ding XC, Tully F, et al. Development and clinical performance of high throughput loop-mediated isothermal amplification for detection of malaria. PLoS One 2017; 12:e0171126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrington SM, Buchan BW, Doern C, et al. Multicenter evaluation of the BD max enteric bacterial panel PCR assay for rapid detection of Salmonella spp., Shigella spp., Campylobacter spp. (C. jejuni and C. coli), and Shiga toxin 1 and 2 genes. J Clin Microbiol 2015; 53:1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 2009; 47:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. CDC - malaria - travelers - recommendations for immigrants from malaria-endemic countries planning to return 2017. Available at: https://www.cdc.gov/malaria/travelers/vfr.html. Accessed 02 May 2018.
- 35. Roth JM, Korevaar DA, Leeflang MM, Mens PF. Molecular malaria diagnostics: a systematic review and meta-analysis. Crit Rev Clin Lab Sci 2016; 53:87–105. [DOI] [PubMed] [Google Scholar]
- 36. Sattabongkot J, Tsuboi T, Han ET, et al. Loop-mediated isothermal amplification assay for rapid diagnosis of malaria infections in an area of endemicity in Thailand. J Clin Microbiol 2014; 52:1471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.