Abstract
Purpose
CLIC1, a member of the highly conserved class ion-channel protein family, is frequently upregulated in multiple human malignancies and has been demonstrated to play a critical role in cell proliferation, apoptosis, and invasion. However, limited is known about its expression, biological functions, and action mechanism in oral malignancies. We aimed to evaluate whether CLIC1 could be a biomarker for oral squamous cell carcinoma (OSCC).
Methods
Immunohistochemistry was used to analyze the expression of CLIC1 in tissue. CLIC1 protein and mRNA were measured through Western immunoblotting and quantitative real-time PCR. CLIC1 protein expression in plasma was detected via ELISA. A total of 72 OSCC specimens were recruited in this study for evaluation of correlations of CLIC1 with clinicopathological features and survival.
Results
CLIC1 was significantly overexpressed in tissue and plasma of OSCC patients. It was found that upregulated CLIC1 was distinctly correlated with histological grade, TNM stage, and tumor size. Meanwhile, Kaplan–Meier survival analysis showed that OSCC patients with high CLIC1 expression had remarkably poorer overall survival rate than those with low CLIC1 expression. Multivariate Cox regression analysis revealed that CLIC1 was the independent prognostic factor for overall survival rate of OSCC patients. In addition, Pearson correlation analysis showed that CLIC1 was associated with multiple tumor-associated genes.
Conclusion
These results indicated that CLIC1 acts as a molecular target in OSCC and may present a novel diagnostic marker and therapeutic target for OSCC.
Keywords: chloride intracellular channel 1, oral squamous cell carcinoma, expression, prognosis, biomarker
Introduction
It has been reported that oral squamous cell carcinoma (OSCC) is the commonest oral malignancy, with ~300,000 new cases worldwide annually.1 Although tremendous progress has been achieved in radical surgery combined with postoperative radiotherapy/chemoradiotherapy, patient 5-year survival rate has not improved essentially in recent decades. Therefore, early detection, early diagnosis, and early treatment are very important to increase the 5-year survival rate of patients with OSCC. Novel and specific biomarkers are required to solve this problem.
Molecular changes are regarded as potential biomarkers for cancers. In OSCC, some genes have been reported as potential biomarkers, such as KPNA2, HOXA13, HOXD13, EZH2, and LEF1.2–5 They had certain value in the diagnosis, treatment, and prognosis of OSCC, but it was not enough to obtain sensitivity and specificity for OSCC only by them. CLIC1 is a member of the highly conserved category of chloride-channel proteins that exists in both integral membrane and soluble forms.6 It is abundant in the apical domains of epithelia.7 Recently, CLIC1 has been reported to be responsible for regulating the migration, invasion, apoptosis, and drug sensitivity of cancer cells and is involved in several signaling pathways, such as MAPK–ERK, PI3K–Akt, and MAPK–p38 (Table 1).8–24 However, whether CLIC1 plays an important role in tumorigenesis and progression of OSCC remains elusive.
Table 1.
Function of CLIC1 in different cancers
| Cancer | Sample | Function | Pathway | Reference(s) |
|---|---|---|---|---|
| Gastric cancer | Tissue | Promotes invasion and migration and inhibits apoptosis | MAPK–Akt | 14 |
| Gallbladder cancer | Tissue | Increases drug sensitivity | – | 17, 12 |
| Glioblastoma | Tissue | Regulator of glioblastoma growth | – | 18 |
| Pancreatic cancer | Tissue | Putative oncogene | – | 9 |
| Sarcoma | Tissue | Receptor oncotargets | ERK | 23 |
| Colon cancer | Cells | Metastasis | – | 11 |
| Breast cancer | Cells | Migrates or invades | – | 15 |
| Ovarian cancer | Serum | Serological tumorigenesis biomarkers | – | 13, 19, 22 |
| Colorectal cancer | Cells | Migration and invasion | RVD | 24 |
| Endometrioid endometrial cancer | Tissue | Chemoresistance | – | 21 |
| Laryngeal cancer | Cells | Promotion of ROS production | ROS | 10 |
| Nasopharyngeal carcinoma | Tissue/plasma | Plasma tumor marker | – | 8 |
| Gallbladder cancer | Cells | Metastasis | – | 20 |
| Hepatocellular carcinoma | Cells | New targets for drug development | – | 16 |
Abbreviations: RVD, regulatory volume decrease; ROS, reactive oxygen species.
To determine the role of CLIC1 in OSCC, we examined CLIC1 mRNA and protein expression in OSCC tissues and plasma. Moreover, we analyzed the association between CLIC1 expression and clinicopathological characteristic in OSCC. In addition, we elucidated the prognostic role of CLIC1 in OSCC patients. Finally, we found that CLIC1 was associated with multiple tumor-associated genes.
Methods
Sample preparation and clinicopathological data
An immunohistochemistry analysis was conducted on OSCC specimens collected from 72 patients who had undergone radical surgical resection (without prior radiotherapy or chemotherapy) from January 2012 to February 2013 at the Research Center of Pathology, Chongqing Medical University. A total of 26 pairs freshly frozen OSCC and corresponding noncancerous tissue samples were used for quantitative real-time (qRT) PCR and Western blot; 76 plasma samples included 54 OSCC plasma samples collected from OSCC patients and 22 healthy controls. All tissue and blood samples were collected at the Stomatological Hospital of Chongqing Medical University, China from 2017 to 2018 and used for qRT-PCR, Western blotting, and ELISA analysis. This study was approved by the Ethics Committee of the Stomatological Hospital of Chongqing Medical University, and all patients provided informed written consent before enrollment. Collection and processing of tissue and blood samples were conducted in accordance with the Declaration of Helsinki.
All tissue samples and blood samples were collected from patients and stored using the standard procedure.25 These samples had undergone only one freeze–thaw cycle before the study was carried out. Telephone interviews were conducted routinely for at least 6 months during follow-up. Time from diagnosis to death or last follow-up visit was regarded as survival time and was checked at the last telephone interview in February of 2018, regardless of whether the patient was alive or not.
Immunohistochemistry
Standard immunoperoxidase staining was used for immunohistochemical staining and CLIC1 protein expression in normal and malignant samples evaluated. Each section was semiquantitatively scored based on the extent and intensity of immunoreactivity: 0, 10% immunoreactive cells; 1, ≤25% immunoreactive cells; 2, 25%–75% immunoreactive cells; and 3, >75% immunoreactive cells. Additionally, staining intensity was graded on a 0–3 scale: 0, negative; 1, weak; 2, intermediate; and 3, strong. The final score was designated as the sum of extent and intensity and samples categorized as negative (0), weak (1–2), moderate (3), and strong staining (4–6). For statistical calculations, moderate and strong staining scores were considered positive and other final scores considered negative.
Western immunoblotting
Total protein extracts from tissue were separated by 10% SDS-PAGE (20 mg per lane), then transferred onto a polyvinylidene fluoride membrane. Membranes were blocked and then probed with primary antibodies against CLIC1 (1:800 dilution; Abcam, Cambridge, UK) and GAPDH (1:500 dilution; Sino Biological, Beijing, China) at 4°C overnight. The secondary antibody (1:500 dilution; SAB, China) was placed under infrared light at room temperature for 1 hour and visualized using an enhanced chemiluminescence-detection reagent.
Real-time quantitative PCR
Total RNA was isolated from tissue using Trizol reagent (Takara, Kusatsu, Japan). The primers used in this study were shown in Table 2. RNA was reverse-transcribed into cDNA with a ReverTra Ace qRT-PCR kit (Takara, Japan). qRT PCR was performed on a StepOnePlus RT-PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The reaction comprised an initial denaturation step of 95°C for 30 minutes, followed by 39 cycles of 95°C for 5 seconds, 60°C for 34 seconds, and a final extension step of 95°C for 10 minutes. The mRNA-expression level of the target gene in each sample was normalized to the GADPH control.
Table 2.
Quantitative real-time PCR primers used in this study
| Gene | Primer sequence | |
|---|---|---|
| CLIC1 | Forward: AATCAAACCCAGCACTCAATG | Reverse: CAGCACTGGTTTCATCCACTT |
| GAPDH | Forward: CAACGACCCCTTCATTGACC | Reverse: CGCCAGTAGACTCCACGACAT |
| PA28B | Forward: CTTTTCCAGGAGGCTGAGGAA | Reverse: GGGAAGTCAAGTCAGCCACA |
| MMP2 | Forward: GCGCCGTCGCCCATCATCAA | Reverse: AGCTCTCCTTGGGGCAGCCA |
| MMP9 | Forward: GGGACGCAGACATCGTCATC | Reverse: TCGTCATCGTCGAAATGGGC |
| ANXA7 | Forward: GCTATCCCCCAACAGGCTAC | Reverse: CACGTTCCTGAGTTCCTGCT |
| GSN | Forward: CCGCTGTCGCCACCAT | Reverse: GAACTTCTCCACACGCCAGA |
| MMP12 | Forward: CCAACGCTTGCCAAATCCT | Reverse: CCACGGTAGTGACAGCATCAA |
| MMP13 | Forward: ACCCTGGAGCACTCATGTTTCCTA | Reverse: TGGCATCAAGGGATAAGGAAGGGT |
| SERPINB5 | Forward: CACGAGTTGTGCTCCTCGC | Reverse: CATACAGAACGTGGCCTCCA |
ELISA
Blood samples in heparin tubes were centrifuged (2,000 g) at 4°C for 10 minutes to obtain plasma and frozen at −80°C for further analysis. CLIC1 protein levels in plasma were measured with human ELISA kits according to the manufacturer’s instructions. The results were processed with an automated analyzer (PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
Data were analyzed using SPSS 20.0. All data are expressed as means ± SD. Independent Student’s t-tests and χ2 tests were used to assess the effects of CLIC1 on tissue and plasma. The Kaplan–Meier test was used for univariate survival analysis. The Cox proportional-hazard model was used for multivariate analysis and for determining 95% CIs. Pearson correlation coefficients were used to assess correlations among tumor-associated genes. Results were regarded as significant at P<0.05.
Results
CLIC1 significantly upregulated in OSCC tissue
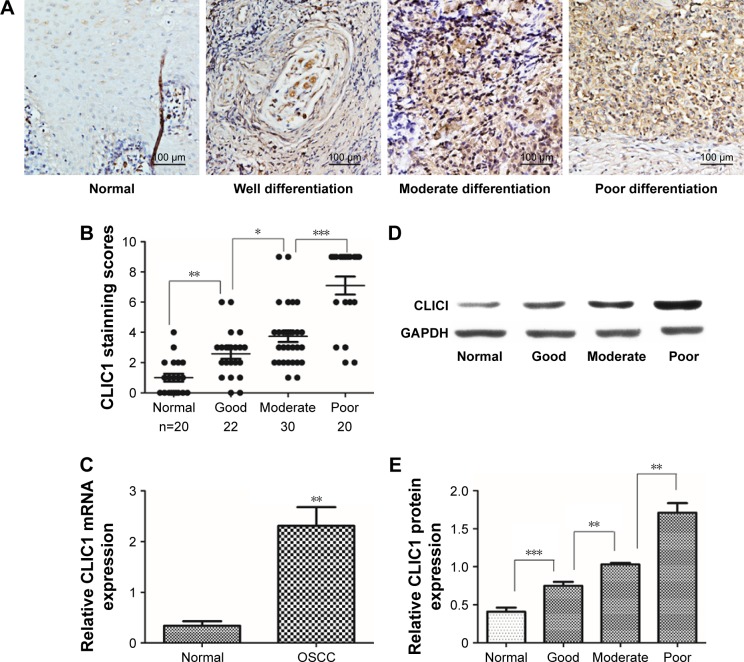
To evaluate CLIC1-expression status in human OSCC tissue, 26 pairs of OSCC and noncancerous oral tissue were collected to examine CLIC1 mRNA expression by qRT-PCR. Results showed that CLIC1 mRNA expression was upregulated in OSCC tissue (2.31±1.27) compared with noncancerous oral tissue (0.34±0.32, P=0.003; Figure 1C). CLIC1 protein expression was higher in OSCC tissue (1.16±0.44) than paired noncancerous oral tissue (0.41±0.93) via Western blot, and this was consistent with qRT-PCR results. Furthermore, CLIC1 protein expression in different histological grades showed significant differences (P<0.001; Figure 1D and E).
Figure 1.
CLIC1 expression was significantly increased in oral squamous cell carcinoma (OSCC) tissue.
Notes: (A) CLIC1 expression in normal, well differentiated, moderately differentiated, and poorly differentiated OSCC tissue via IHC. (B) Average staining scores of CLIC1 expression in normal, well differentiated, moderately differentiated, and poorly differentiated OSCC tissue. (C) Quantitative real-time PCR showed CLIC1-expression levels in 26 tissue pairs (P=0.002). (D) Western blotting of CLIC1 protein expression. (E) CLIC1 protein expression in normal tissue, well differentiated OSCC tissue, moderately differentiated OSCC tissue, and poorly differentiated OSCC tissue via Western blot. P-values calculated by Student’s t-test. Data presented as mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
CLIC1 expression associated with clinicopathological features in OSCC patients
To elucidate the biological functions of CLIC1 in OSCC development, the expression of this protein in OSCC was compared with normal control tissue via IHC. Our results showed that CLIC1 is localized mainly in the nucleus and can also be observed in membranes and cytoplasm of cancer cells. Its protein expression differed in tumors of different differentiation (Figure 1A). Approximately 70.83% (51 of 72) of the OSCC patients displayed positive CLIC1-immunoreactive cells, while only 10.00% (two of 20) of the patients had positive immunoreactive cells in the normal control group (P<0.001; Figure 1B). In addition, there were significant CLIC1 proteins of histological grade. Then, we evaluated correlations between CLIC1 expression and histopathological parameters in the OSCC patients. Table 3 showed the CLIC1 overexpression remarkably related to histological grade (P=0.049), TNM stage (P=0.025), and tumor size (P=0.021), but not with age, sex, or lymph-node metastasis. These results indicated a potential role of CLIC1 expression in promoting incursionary OSCC phenotypes.
Table 3.
Association of CLIC1 expression with the clinicopathological characteristics of oral squamous cell carcinoma
| Category | Cases | CLIC1
|
|||
|---|---|---|---|---|---|
| Positive cases, n (%) | X2 | P-value | |||
|
| |||||
| Age, years | <60 | 28 | 18 (64.29) | 0.019 | 0.888 |
| ≥60 | 44 | 29 (65.91) | |||
| Sex | Male | 47 | 30 (63.83) | 0.125 | 0.723 |
| Female | 25 | 17 (68.00) | |||
| Location | Tongue | 18 | 12 (66.67) | 1.558 | 0.669 |
| Buccal mucosa | 17 | 13 (76.47) | |||
| Gingiva | 8 | 6 (75.00) | |||
| Others* | 29 | 16 (55.17) | |||
| Histological grade | Well differentiated | 32 | 16 (50.00) | 6.042 | 0.049# |
| Moderately differentiated | 20 | 15 (75.00) | |||
| Poorly differentiated | 20 | 16 (80.00) | |||
| TNM stage | 0–I | 20 | 9 (45.00) | 5.023 | 0.025# |
| II–IV | 52 | 38 (69.23) | |||
| Lymph-node metastasis | Negative | 58 | 35 (60.34) | 2.18 | 0.139 |
| Positive | 14 | 12 (85.71) | |||
| Tumor size (cm) | <3 | 30 | 15 (50.00) | 5.3 | 0.021* |
| ≥3 | 42 | 32 (76.19) | |||
Notes:
Included floor of mouth, lips and palate;
statistically significant. P-values determined using Kruskal–Wallis and χ2 tests.
Evaluation of CLIC1 expression predicts poor prognosis in patients
Among the 72 OSCC patients, only 64 patients’ survival information was collected through phone-call follow-up. Histopathological subtype (P=0.001), TNM stage (P=0.046), and lymph-node metastasis (P<0.001) were significantly relevant to survival time by Kaplan–Meier survival analysis. Survival figures showed that the survival time of the CLIC1-positive patients was obviously lower than that of patients with CLIC1-negative expression (P=0.025; Table 4, Figure 2). To identify risk factors of prognosis precisely, univariate Cox regression analysis was used. These data revealed that CLIC1 expression, histological grade, and lymph-node metastasis were adversely correlated with postoperative survival time, suggesting that overexpression of CLIC1 is a risk factor for OSCC (Table 5).
Table 4.
Univariate analysis of overall survival of oral squamous cell carcinoma patients
| Category | Cases (n) | Mean survival time, months (95% CI) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age, years | <60 | 22 | 56.818 (46.840–66.796) | 0.38 |
| ≥60 | 42 | 55.490 (48.087–62.892) | ||
| Sex | Male | 43 | 54.680 (47.618–61.742) | 0.746 |
| Female | 21 | 54.828 (44.793–64.863) | ||
| Location | Tongue | 17 | 62.035 (53.044–71.026) | 0.231 |
| Buccal mucosa | 15 | 46.558 (31.198–61.917) | ||
| Gingiva | 7 | 70.167 (68.676–71.685) | ||
| Others* | 25 | 53.520 (43.876–43.963) | ||
| Histological grade | Well differentiated | 30 | 62.182 (55.205–69.158) | 0.001# |
| Moderately differentiated | 16 | 63.063 (56.012–70.113) | ||
| Poorly differentiated | 18 | 38.333 (24.606–52.060) | ||
| TNM stage | 0–I | 19 | 66.819 (60.844–72.793) | 0.046# |
| II–IV | 45 | 51.386 (43.666–59.105) | ||
| Lymph-node metastasis | Negative | 51 | 61.833 (55.942–67.725) | <0.001# |
| Positive | 13 | 33.359 (21.257–45.461) | ||
| Tumor size (cm) | <3 | 27 | 58.213 (50.116–66.310) | 0.833 |
| ≥3 | 37 | 53.304 (45.157–61.451) | ||
| CLIC1 | Negative | 26 | 63.917 (56.705–71.129) | 0.025# |
| Positive | 38 | 50.431 (42.098–58.765) | ||
Notes:
Floor of mouth, lips, and palate;
statistically significant. P-valued determined using log-rank test.
Figure 2.
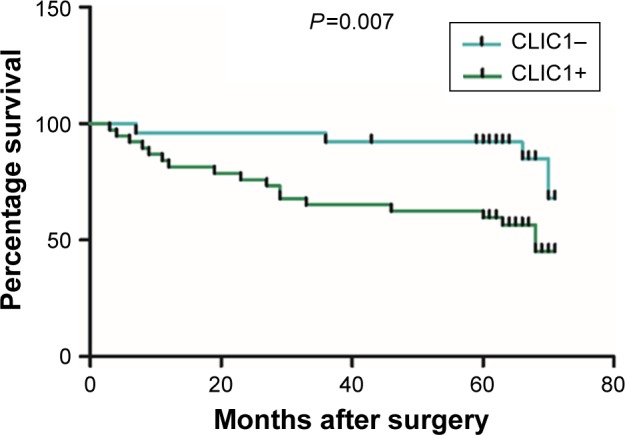
CLIC1 was correlated with overall survival rate in oral squamous cell carcinoma patients.
Notes: Kaplan–Meier plots of overall survival of patients with positive and negative CLIC1-expression scores. P-values calculated by log-rank test.
Table 5.
Multivariate analysis of overall survival of oral squamous cell carcinoma patients
| Category | HR (95% CI) | P-value | |
|---|---|---|---|
|
| |||
| CLIC1 expression | Negative | 5.354 (1.463–19.586) | 0.011a |
| Positive | |||
| Histological grade | Well differentiated | 0.180 (0.038–0.859) | 0.032a |
| Moderately differentiated | |||
| Poorly differentiated | |||
| TNM stage | 0–I | 1.680 (0.413–6.836) | 0.469 |
| II–IV | |||
| Lymph-node metastasis | Negative | 4.743 (1.607–14.001) | 0.005a |
| Positive | |||
| Tumor size (cm) | <3 | 1.197 (0.244–5.871) | 0.825 |
| ≥3 | |||
Note:
Statistically significant.
Determination of CLIC1 as a plasma OSCC biomarker
As CLIC1 was overexpressed in OSCC tissues, we speculated that CLIC1 could be detected in blood samples of OSCC patients. To determine this possibility, a sensitive ELISA was conducted to detect the expression of CLIC1 protein in plasma. Plasma samples of 30 OSCC patients without any treatment, 14 who had undergone resection only, ten who had undergone resection and chemotherapy, and 22 healthy controls were examined. CLIC1 protein expression was significantly higher in OSCC plasma than that of healthy controls (P=0.023), CLIC1 protein expression obviously lower in resection patients than those of OSCC patients (P=0.039), and CLIC1-expression levels in resection- + chemotherapy-treated patients aberrantly lower than in resection-only patients and healthy controls (P=0.002 and P=0.000, respectively; Figure 3A). Moreover, CLIC1-expression level in OSCC patients’ plasma was also obviously higher in stage I–II than III–IV (P=0.002; Figure 3B). However, there was no statistical difference between moderate differentiation and good differentiation (P=0.459; Figure 3C).
Figure 3.
Elevated CLIC1 protein levels in oral squamous cell carcinoma (OSCC) plasma samples via ELISA.
Notes: (A) Plasma CLIC1 protein levels of healthy controls (n=22), OSCC patients (n=30), resection operation (RO) patients (n=14), and RO + chemotherapy (CT)-treated patients (n=10). (B) Plasma CLIC1 protein levels of TNM stage (P=0.009). (C) Plasma CLIC1 protein levels of histological grade (P=0.459). *P<0.05; **P<0.01; ***P<0.001; P-values calculated by Student’s t-test.
Correlation of CLIC1 and tumor-associated gene expression in OSCC tissue
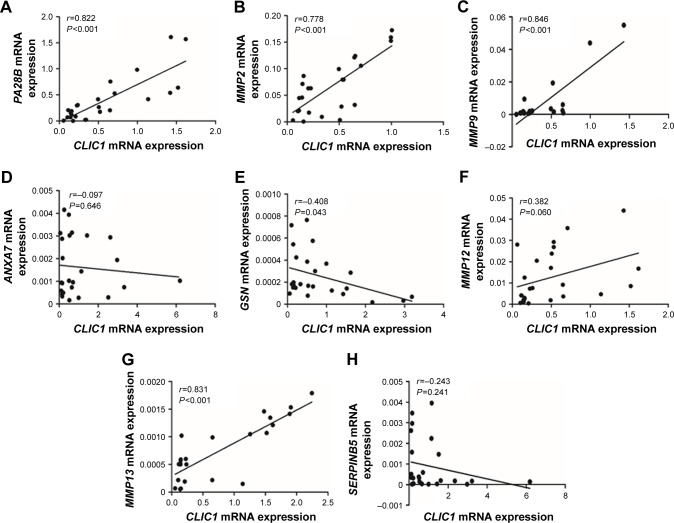
In order for profound understanding of CLIC1, tumor-associated genes were detected. In OSCC tissue, CLIC1 mRNA-expression level of was positively correlated with PA28B, MMP2, MMP9, MMP13, and MMP12 mRNA-expression levels, whereas CLIC1 mRNA expression level was negatively correlated with ANXA7, GSN, and SERPINB5 mRNA-expression levels (Figure 4). Correlations between mRNA expression of GSN, PA28B, MMP2, MMP9, and MMP13 were significantly different from those of CLIC1 (P=0.043 and P<0.001).
Figure 4.
Correlations between CLIC1 and PA28B, MMP2, MMP9, ANXA7, GSN, MMP12, MMP13, and SERPINB5 mRNA in OSCC according to qRT-PCR data.
Notes: (A) Correlation between CLIC1 and PA28B. (B) Correlation between CLIC1 and MMP2. (C) Correlation between CLIC1 and MMP9. (D) Correlation between CLIC1 and ANXA7. (E) Correlation between CLIC1 and GSN. (F) Correlation between CLIC1 and MMP12. (G) Correlation between CLIC1 and MMP13. (H) Correlation between CLIC1 and SERPINB5. r-values calculated by Pearson correlation analysis. P-values calculated by Student’s t-test.
Abbreviations: OSCC, oral squamous cell carcinoma; qRT-PCR, quantitative real-time PCR.
Discussion
In this research, our results suggested that CLIC1 plays an important role in the tumorigenic potential of human OSCC. CLIC1 mRNA and protein were overexpressed in OSCC tissue and CLIC1 protein closely associated with patients’ overall survival. Investigation indicated that CLIC1 is an independent metastasis and prognostic factor in OSCC. Moreover, CLIC1 protein was highly expressed in OSCC plasma and associated with therapy. Furthermore, our data demonstrated that CLIC1 may be involved in the pathway of development and metastasis of OSCC. Therefore, we consider CLIC1 has potential as a novel molecular therapeutic target and a possible outcome predictor.
CLIC1 has been reported to be upregulated in human malignant tumors of the colon, brain, liver, stomach, and lung.14,22,24,26,27 It was evaluated to be the most penetrating receptor among commonly dysregulated proteins within three sarcomas.23 This protein plays an important role in the metastasis of colonic cancer.24 In gliomas, CLIC1 expression is inversely related to patient survival.27 Moreover, CLIC1 participates in regulation of aggression in human lung adenocarcinoma.26 Consistently with upregulated CLIC1 expression in a large number of tumors, our data suggested that CLIC1 mRNA and protein were highly expressed in OSCC tissue. Firstly, our study discovered that CLIC1 exists mainly in the nucleus, cytoplasm, and cell membranes of OSCC patients. Next, this research found that increased CLIC1 protein expression was relevant not only to tumor progression but also to poor patient survival, indicating its materiality in OSCC advancement. Notably, Cox multivariate analysis suggested that CLIC1-overexpression level was an independent prognostic factor for OSCC patients. Therefore, CLIC1 is a potential molecular therapeutic target for OSCC.
Increasing attention has been paid to blood research. CLIC1 has also been detected in the blood of patients, suggesting that CLIC1 could be useful as a blood marker. CLIC1 proteins secrete from tumor cells and can enter the circulation.28 Chang et al first reported that there exists the possibility of CLIC1 as a plasma marker for nasopharyngeal carcinoma.8 CLIC1 has been confirmed to be overexpressed in ovarian cancer patient serum.22 It seems to have recently become a popular view that CLIC1 may be applied as general cancer screening markers. We detected CLIC1 protein in plasma samples of OSCC patients, and found that its expression level was significantly higher than in normal controls. CLIC1 protein expression in plasma was also correlated with advanced tumor status. Furthermore, it was closely associated with treatment. These results indicated that CLIC1 expression could be regarded as a biomarker and in indices for tumor metastasis and prognosis in OSCC. However, CLIC1 needs to be evaluated with further testing of larger patient cohorts to avoid false positives or false negatives before clinical application. In addition, because CLIC1 is associated with histological grade, TNM, and tumor size, but not with lymph-node metastasis, it may require coordination with other biomarkers for diagnosis and treatment of OSCC. CLIC1 could be included in a group of select biomarkers for early detection.
Although the potential role of CLIC1 in cancer is not clear, there are have been many advances. Some studies have reported that CLIC1 is linked to many tumor-associated proteins. Wei et al found that downregulation of CLIC1 increased maspin expression and reduced MMP2, MMP9, MMP12, and VEGF expression. Conversely, upregulation of CLIC1 decreased maspin expression and increased MMP2, MMP12, VEGF, and MMP13 expression.29 Zhang et al provided the novel insight that CLIC1 has a vital role in hepatocarcinoma metastasis, perhaps via regulation of gelsolin and annexin A7 expression.30 Other research reported that knockdown of PA28B increased tumor invasion and migration through upregulation of CLIC1.31 What is more, CLIC1 may be correlated with the regulation of integrin-family proteins, which leads to subsequent regulation of the PI3K–AKT, MAPK–p38, and MAPK–ERK pathways.14 To evaluate the biological function of CLIC1 in OSCC pathogenesis further, we compared CLIC1 with tumor-associated genes using PCR. We found that CLIC1 was relevant to a variety of tumor genes, especially PA28B, MMP2, and MMP13, whereas PA28B and matrix metalloproteinases (MMPs) played an important role in tumor metastasis and invasiveness.31,32 These data indicated that CLIC1 may be involved in the pathway of development and metastasis of OSCC. In vitro experiments and experiments on animals are required to elucidate the mechanism of CLIC1 acting in OSCC.
Conclusion
In summary, this study detected the expression of CLIC1 in OSCC tissue and plasma. The results pointed to CLIC1 perhaps being a novel biomarker for OSCC diagnosis, prognosis, and even early screening. The potential use of CLIC1 as an OSCC biomarker needs to be evaluated with further testing of larger patient cohorts and in-depth mechanical experiments.
Acknowledgments
This work was supported by funds provided by the Program for Innovation Team Building at the Institutions of Higher Education in Chongqing in 2016, Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing Research Program of Basic Research and Frontier Technology (PYZD201601), the Scientific and Technological Research Program of the Health and Family Planning Commission of Chongqing Province (2017ZDXM019 and 2016MSXM046), and Science and Technology Projects Foundation of Chongqing Yubei District Agricultural Bureau (2017-37).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31(6):744–751. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CI, Yu CJ, Huang Y, et al. Association of overexpressed karyopherin alpha 2 with poor survival and its contribution to interleukin-1β-induced matrix metalloproteinase expression in oral cancer. Head Neck. 2018;40(8):1719–1733. doi: 10.1002/hed.25145. [DOI] [PubMed] [Google Scholar]
- 3.Aquino G, Franco R, Sabatino R, et al. Deregulation of paralogous 13 HOX genes in oral squamous cell carcinoma. Am J Cancer Res. 2015;5(10):3042–3055. [PMC free article] [PubMed] [Google Scholar]
- 4.Su KJ, Lin CW, Chen MK, Yang SF, Yu YL. Effects of EZH2 promoter polymorphisms and methylation status on oral squamous cell carcinoma susceptibility and pathology. Am J Cancer Res. 2015;5(11):3475–3484. [PMC free article] [PubMed] [Google Scholar]
- 5.Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7(6):1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 6.Harrop SJ, DeMaere MZ, Fairlie WD, et al. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolution. J Biol Chem. 2001;276(48):44993–45000. doi: 10.1074/jbc.M107804200. [DOI] [PubMed] [Google Scholar]
- 7.Ulmasov B, Bruno J, Woost PG, Edwards JC. Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 2007;8:8. doi: 10.1186/1471-2121-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang YH, Wu CC, Chang KP, Yu JS, Chang YC, Liao PC. Cell secretome analysis using hollow fiber culture system leads to the discovery of CLIC1 protein as a novel plasma marker for nasopharyngeal carcinoma. J Proteome Res. 2009;8(12):5465–5474. doi: 10.1021/pr900454e. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Dong Q, Zhang B, et al. Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol. 2015;32(6):616. doi: 10.1007/s12032-015-0616-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Chang JW, Yun HS, et al. Chloride intracellular channel 1 identified using proteomic analysis plays an important role in the radiosensitivity of HEp-2 cells via reactive oxygen species production. Proteomics. 2010;10(14):2589–2604. doi: 10.1002/pmic.200900523. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Zeng Y, Liu T, et al. Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. 2014;20(8):2071–2078. doi: 10.3748/wjg.v20.i8.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wang Z, Li M, et al. Chloride intracellular channel 1 regulates the antineoplastic effects of metformin in gallbladder cancer cells. Cancer Sci. 2017;108(6):1240–1252. doi: 10.1111/cas.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y, Yin M, Huang B, Wang Y, Li X, Lou G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015;36(6):4175–4179. doi: 10.1007/s13277-015-3052-8. [DOI] [PubMed] [Google Scholar]
- 14.Li BP, Mao YT, Wang Z, et al. CLIC1 Promotes the Progression of Gastric Cancer by Regulating the MAPK/AKT Pathways. Cell Physiol Biochem. 2018;46(3):907–924. doi: 10.1159/000488822. [DOI] [PubMed] [Google Scholar]
- 15.Nanaware PP, Ramteke MP, Somavarapu AK, Venkatraman P. Discovery of multiple interacting partners of gankyrin, a proteasomal chaperone and an oncoprotein – evidence for a common hot spot site at the interface and its functional relevance. Proteins. 2014;82(7):1283–1300. doi: 10.1002/prot.24494. [DOI] [PubMed] [Google Scholar]
- 16.Huang JS, Chao CC, Su TL, et al. Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2004;315(4):950–958. doi: 10.1016/j.bbrc.2004.01.151. [DOI] [PubMed] [Google Scholar]
- 17.He YM, Zhang ZL, Liu QY, et al. Effect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cells. J Cell Mol Med. 2018;22(5):2569–2579. doi: 10.1111/jcmm.13499. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Setti M, Osti D, Richichi C, et al. Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget. 2015;6(31):31413–31427. doi: 10.18632/oncotarget.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu H, Chen Y, Cao G, et al. Identification and validation of differentially expressed proteins in epithelial ovarian cancers using quantitative proteomics. Oncotarget. 2016;7(50):83187–83199. doi: 10.18632/oncotarget.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JW, Peng SY, Li JT, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281(1):71–81. doi: 10.1016/j.canlet.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Lomnytska MI, Becker S, Gemoll T, et al. Impact of genomic stability on protein expression in endometrioid endometrial cancer. Br J Cancer. 2012;106(7):1297–1305. doi: 10.1038/bjc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang HY, Beer LA, Tanyi JL, Zhang R, Liu Q, Speicher DW. Protein isoform-specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancer. J Proteomics. 2013;89:165–178. doi: 10.1016/j.jprot.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray E, Hernychová L, Scigelova M, et al. Quantitative proteomic profiling of pleomorphic human sarcoma identifies CLIC1 as a dominant pro-oncogenic receptor expressed in diverse sarcoma types. J Proteome Res. 2014;13(5):2543–2559. doi: 10.1021/pr4010713. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Zhang C, Yu P, et al. Regulation of colon cancer cell migration and invasion by CLIC1-mediated RVD. Mol Cell Biochem. 2012;365(1–2):313–321. doi: 10.1007/s11010-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 25.Jewell SD, Srinivasan M, McCart LM, et al. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002;118(5):733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Xu X, Wang W, et al. The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol. 2011;32(6):1199–1208. doi: 10.1007/s13277-011-0223-0. [DOI] [PubMed] [Google Scholar]
- 27.Setti M, Savalli N, Osti D, et al. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst. 2013;105(21):1644–1655. doi: 10.1093/jnci/djt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littler DR, Harrop SJ, Goodchild SC, et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584(10):2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Li J, Xie H, et al. Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J Gastroenterol Hepatol. 2015;30(1):208–216. doi: 10.1111/jgh.12668. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Li M, Song M, et al. Clic1 plays a role in mouse hepatocarcinoma via modulating Annexin A7 and Gelsolin in vitro and in vivo. Biomed Pharmacother. 2015;69:416–419. doi: 10.1016/j.biopha.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Zheng DL, Huang QL, Zhou F, Huang QJ, Lin JY, Lin X. PA28β regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem. 2012;113(5):1537–1546. doi: 10.1002/jcb.24022. [DOI] [PubMed] [Google Scholar]
- 32.Vijaya Kumar A, Salem Gassar E, Spillmann D, et al. HS3ST2 modulates breast cancer cell invasiveness via MAP kinase- and Tcf4 (Tcf7l2)-dependent regulation of protease and cadherin expression. Int J Cancer. 2014;135(11):2579–2592. doi: 10.1002/ijc.28921. [DOI] [PubMed] [Google Scholar]