Abstract
Background
Neuroendocrine tumors (NETs) are a group of heterogeneous cancers arising from a variety of anatomic sites. Their incidence has increased in recent years. This study aimed to analyze the prognosis of NETs originating from different anatomic sites.
Methods
We identified 73,782 patients diagnosed with NETs from the Surveillance Epidemiology and Ends Results (SEER) database from 1973 to 2014. Clinical data were compared between patients with different primary tumor sites using the chi-squared test. Differences in survival among NET patients with different tumor sites were compared by Kaplan–Meier analysis. Cox proportional hazard models were performed to identify the prognostic factors of overall survival.
Results
In this cohort, the lung/bronchus was the most common site of NETs, accounting for 30.6%, followed by the small intestine (22.2%), rectum (16.2%), colon (13.4%), pancreas (10.8%), and stomach (6.8%). Totally, 73,782 patients were selected for this cohort from 1973 to 2014. The median survival duration was 41 months. The 1-, 3-, 5-, and 10-year overall survival rates for patients with NETs were 72.8%, 52.7%, 39.4%, and 18.1%, respectively. Patients with NETs located in the rectum had the best prognosis, followed by those with NETs in the small intestine (HR, 1.660, 95% CI, 1.579, 1.744), lung/bronchus (HR, 1.786, 95% CI, 1.703, 1.874), stomach (HR, 1.865, 95% CI, 1.755, 1.982), and colon (HR, 1.896, 95% CI, 1.799, 1.999). Patients with NETs in the pancreas had the highest risk of mortality (HR, 2.034, 95% CI, 1.925, 2.148).
Conclusion
Significant differences in survival were found among various primary tumor sites. NETs in the rectum had the best prognosis, while those in the pancreas had the worst. Primary tumor sites might be one of the most useful outcome predictors in patients with NETs.
Keywords: neuroendocrine tumor, SEER program, survival analysis, primary tumor sites
Introduction
Neuroendocrine tumors (NETs) are a group of rare cancers that arise from neuroendocrine cells throughout the body; they account for 0.46% of gastroenteropancreatic and bronchopulmonary malignancies.1 They are a heterogeneous group of malignancies that are most common in the lung/bronchus but also originate in the pancreas, small intestine, colon and rectum, ovaries, and thyroid and adrenal glands. Clinically, NETs are regarded as functional if they are associated with symptoms of hormonal hypersecretion or as non-functional if they are not associated with these symptoms.
Despite 100 years of clinical studies, NETs remain poorly understood because of their rarity, tumor heterogeneity, nonspecific presentation, unique indolent biology, and a lack of awareness. Recent studies have reported an increase in the incidence and prevalence of NETs in recent decades. According to a study by Hallet et al, from 1994 to 2009, the incidence increased almost 2.5-fold, reaching 5.86 per 100,000 per year in 2009.1 Although rare, NETs are more prevalent than gastric and pancreatic adenocarcinomas combined because of the indolent nature of the disease.2 These characteristics illustrate the increasing need for a better understanding of NETs.
Previously published articles have indicated the primary site as a prognostic factor for better survival in NETs.3 However, the association between race/ethnicity, gender and marital status, and various tumor sites is less well investigated. The present study used a population-based approach to examine the clinical characteristics and prognosis of NETs located at different sites.
Methods
Data
Our retrospective, population-based cohort was based on Incidence-SEER 18 Regs Research Data + Hurricane Katrina-Impacted Louisiana Cases, Nov 2016 Sub (1973– 2014 varying), a population-based registry that collects cancer information covering ~28.7% of the U.S. population.4 The SEER program routinely collects high-quality demographic, clinical, and follow-up information from 18 cancer registries.
Study population
Patients aged ≥18 years who were diagnosed with NETs between 1973 and 2014 were selected for the cohort (Figure 1). ICD-O-3 histology codes were used to identify NETs. These codes correspond to the following clinical/histologic diagnoses: islet cell carcinoma (8150), insulinoma (8151), glucagonoma (8152), gastrinoma (8153), mixed islet-cell/exocrine adenocarcinoma (8154), vipoma (8155), somatostatinoma (8156), enteroglucagonoma (8157), carcinoid (8240), enterochromaffin cell carcinoid (8241), enterochromaffin-like cell tumors (8242), goblet cell carcinoid (8243), composite carcinoid (8244), adenocarcinoid (8245), neuroendocrine carcinoma (8246), and atypical carcinoid (8249). Small-cell (8002 and 8040–8045) and large-cell neuroendocrine carcinoma (8013) of the lung, pheomochromocytoma (8700), paraganglioma (8680, 8693), and medullary carcinoma of the thyroid (8510) were excluded.5 Demographic and clinical information were extracted from the SEER database. In the SEER study, races were listed as white, black, and a grouping of other races represented as Asian, Pacific Islander, American Indian, and Alaska Native (A/PI/AI/AN). Tumor grade was assigned according to degree of differentiation, as defined in SEER using the following ICD-O-3 specifications: grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; and grade IV, undifferentiated (anaplastic). Survival time was calculated as the period from the date of diagnosis until death. Those alive as of the last follow-up date reported in SEER were treated as censored observations. Tumors were classified as localized, regional, or distant according to the SEER staging system for analysis. All the SEER data are freely available and do not require approval from an institutional review board or ethics committee. No personal identifying information was used in the study. Therefore, we did not require any informed consent.
Figure 1.
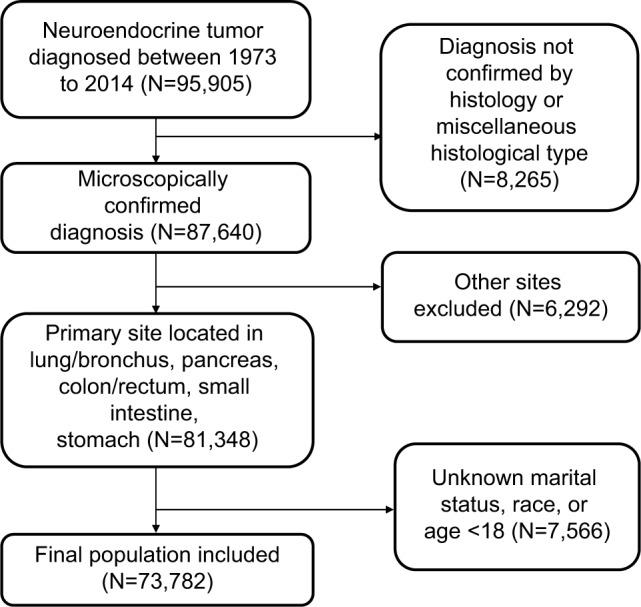
Inclusion of patients in the study.
Statistical analysis
We used the chi-squared test to compare the clinicopathological characteristics among different NET sites. One-way ANOVA was used to compare continuous variables between groups. We used Kaplan–Meier analysis and log-rank tests for survival comparisons. We employed the Cox proportional model for multivariate analyses of the patient population; accordingly, HRs with corresponding 95% CIs were generated. Statistical significance was considered if a two-tailed P-value<0.05 was achieved. All the statistical analyses were performed using SPSS Statistics 20.0 (IBM Corporation, Armonk, NY, USA). Figures were created using GraphPad Prism software (Graph-Pad Software, Inc., La Jolla, CA, USA).
Results
In total, 73,782 patients were selected for this cohort from 1973 to 2014. Table 1 summarizes the characteristics of the identified patients. Briefly, their ages ranged from 18 to 100 years, with a median age of 61.6 years. Patients who were younger than 50 years were more likely to be in the group of NETs happened in colon (25.0%). Yet NETs of lung/bronchus had the highest proportion of patients older than 65 years (53.4%). In white, the most common sites were lung/bronchus (33.8%), followed by small intestine (22.7%), colon (13.6%), rectum (12.1%), pancreas (10.9%), and stomach (6.8%). However, in other races, rectum was the most common sites of NETs. For 15,522 cases (21%), the disease stage was unreported; of the remaining 58,260 cases, 29,367 (39.8%) were localized, 12,659 (17.2%) were regional, and 16,234 (22%) were distant. NETs of the pancreas had the highest proportion of distant stage at diagnosis (40.3%). NETs in the lung/bronchus tended to be poorly differentiated or undifferentiated (18.9%).
Table 1.
Clinical characteristics of NETs at different primary sites based on the SEER database
| Variables | Total (%) | Colon (%) | Lung/bronchus (%) | Small intestine (%) | Pancreas (%) | Stomach (%) | Rectum (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sex | |||||||
| Male | 34,876 | 4,616 | 9,200 | 8,644 | 4,415 | 2028 | 5,973 |
| (47.3) | (46.6) | (40.8) | (52.7) | (55.6) | (40.4) | (50.0) | |
| Female | 38,906 | 5,284 | 13,369 | 7,744 | 3,531 | 2,994 | 5,984 |
| (52.7) | (53.4) | (59.2) | (47.3) | (44.4) | (59.6) | (50.0) | |
| Year | |||||||
| 1973–1986 | 3,599 | 687 | 1,088 | 1,058 | 425 | 106 | 235 |
| (4.9) | (6.9) | (4.8) | (6.5) | (5.3) | (2.1) | (2.0) | |
| 1987–2000 | 13,558 | 1,823 | 4,791 | 3,078 | 1,098 | 779 | 1992 |
| (18.4) | (18.4) | (21.2) | (18.8) | (13.8) | (15.5) | (16.7) | |
| 2001–2014 | 56,625 | 7,390 | 16,690 | 12,252 | 6,423 | 4,137 | 9,730 |
| (76.7) | (74.6) | (74.0) | (74.8) | (80.8) | (82.4) | (81.4) | |
| Age, years | |||||||
| <50 | 13,558 | 2,474 | 3,373 | 2,178 | 1,776 | 929 | 2,831 |
| (18.4) | (25.0) | (14.9) | (13.3) | (22.4) | (18.5) | (23.7) | |
| 50–65 | 27,409 | 3,677 | 7,154 | 5,838 | 2,939 | 1,677 | 6,122 |
| (37.1) | (37.1) | (31.7) | (35.6) | (37.0) | (33.4) | (51.2) | |
| >65 | 32,815 | 3,749 | 12,042 | 8,372 | 3,231 | 2,416 | 3,004 |
| (44.5) | (37.9) | (53.4) | (51.1) | (40.7) | (48.1) | (25.1) | |
| Race | |||||||
| White | 58,984 | 8,039 | 19,928 | 13,408 | 6,437 | 4,018 | 7,154 |
| (79.9) | (81.2) | (88.3) | (81.8) | (81.0) | (80.0) | (59.8) | |
| Black | 10,296 | 1,368 | 1,853 | 2,540 | 902 | 705 | 2,928 |
| (14.0) | (13.8) | (8.2) | (15.5) | (11.4) | (14.0) | (24.5) | |
| Other | 4,502 | 493 | 788 | 440 | 607 | 299 | 1,875 |
| (6.1) | (5.0) | (3.5) | (2.7) | (7.6) | (6.0) | (15.7) | |
| Marital status | |||||||
| Married | 45,434 | 5,903 | 13,036 | 10,363 | 5,252 | 2,997 | 7,886 |
| (61.6) | (59.6) | (57.8) | (63.2) | (66.1) | (59.7) | (66.0) | |
| Unmarried | 28,348 | 399 | 9,533 | 6,025 | 2,694 | 2025 | 4,071 |
| (38.4) | (40.4) | (42.2) | (36.8) | (33.9) | (40.3) | (34.0) | |
| Grade | |||||||
| Good | 15,683 | 1,762 | 3,211 | 4,717 | 2,379 | 1,244 | 2,373 |
| (21.3) | (17.8) | (14.2) | (28.8) | (29.9) | (24.8) | (19.8) | |
| Moderate | 4,855 | 738 | 1,348 | 1,169 | 819 | 286 | 493 |
| (6.6) | (7.5) | (6.0) | (7.1) | (10.3) | (5.7) | (4.1) | |
| Poor | 5,531 | 1,170 | 2,990 | 209 | 523 | 369 | 272 |
| (7.5) | (11.8) | (13.2) | (1.3) | (6.6) | (7.3) | (2.3) | |
| Undifferentiated | 2,108 | 354 | 1,294 | 69 | 182 | 98 | 107 |
| (2.9) | (3.6) | (5.7) | (0.4) | (2.3) | (2.0) | (0.9) | |
| Unknown | 45,605 | 5,876 | 13,726 | 10,224 | 4,043 | 3,025 | 8,712 |
| (61.8) | (59.4) | (60.8) | (62.4) | (50.9) | (60.2) | (72.9) | |
| Stage | |||||||
| Localized | 29,367 | 3,420 | 7,373 | 4,697 | 1,987 | 2,970 | 8,911 |
| (39.8) | (34.5) | (32.7) | (28.7) | (25.0) | (59.1) | (74.5) | |
| Regional | 12,659 | 2,367 | 3,354 | 4,987 | 1,389 | 335 | 231 |
| (17.2) | (23.9) | (14.9) | (30.4) | (17.5) | (6.7) | (1.9) | |
| Distant | 16,234 | 1,891 | 7,305 | 2,959 | 3,206 | 474 | 401 |
| (22.0) | (19.1) | (32.4) | (18.1) | (40.3) | (9.4) | (3.4) | |
| Unknown | 15,522 | 2,222 | 4,537 | 3,745 | 1,364 | 1,243 | 2,414 |
| (21.0) | (22.4) | (20.1) | (22.9) | (17.2) | (24.8) | (20.2) | |
| Surgery | |||||||
| Yes | 42,229 | 7,001 | 9,156 | 11,298 | 3,351 | 2,660 | 8,754 |
| (57.2) | (70.7) | (40.6) | (68.9) | (42.2) | (53.0) | (73.2) | |
| No | 19,382 | 1,030 | 9,452 | 1,963 | 3,437 | 1,762 | 1,744 |
| (26.3) | (10.4) | (41.9) | (12.0) | (43.3) | (35.1) | (14.6) | |
| Unknown | 12,171 | 1,869 | 3,961 | 3,127 | 1,158 | 600 | 1,459 |
| (16.5) | (18.9) | (17.6) | (19.1) | (14.6) | (11.9) | (12.2) | |
| Radiation | |||||||
| Yes | 5,517 | 138 | 4,314 | 176 | 501 | 125 | 263 |
| (7.5) | (1.4) | (19.1) | (1.1) | (6.3) | (2.5) | (2.2) | |
| No | 68,265 | 9,762 | 18,255 | 16,212 | 7,445 | 4,897 | 11,694 |
| (92.5) | (98.6) | (80.9) | (98.9) | (93.7) | (97.5) | (97.8) | |
| Chemotherapy | |||||||
| Yes | 11,078 | 1,376 | 5,798 | 925 | 2,178 | 390 | 415 |
| (15.0) | (13.9) | (25.7) | (5.6) | (27.4) | (7.8) | (3.5) | |
| No | 62,704 | 8,524 | 16,771 | 15,463 | 5,768 | 4,632 | 11,542 |
| (85.0) | (86.1) | (74.3) | (94.4) | (72.6) | (92.2) | (96.5) | |
Abbreviations: NETs, neuroendocrine tumors; SEER, Surveillance, Epidemiology and End Results.
The median survival duration was 41 months. The 1-, 3-, 5-, and 10 year overall survival (OS) rates for patients with NETs were 72.8%, 52.7%, 39.4%, and 18.1%, respectively (Table 2). The survival rates differed significantly according to the primary tumor site (P<0.001); for example, 55.7% of patients with NETs originating in the rectum were still alive after 5 years, while those with NETs in the pancreas had a 5-year survival rate of 22.7%, the shortest of all sites (P<0.001).
Table 2.
Survival analysis for patients with NETs diagnosed from 1973 to 2014
| Variables | Median survival (months) | Survival rate (%)
|
|||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | 10 years | ||
|
| |||||
| All patients | 41 | 72.8 | 52.7 | 39.4 | 18.1 |
| Sex | |||||
| Male | 36 | 70.7 | 50.4 | 37.3 | 16.9 |
| Female | 45 | 75.8 | 55.5 | 41.9 | 19.5 |
| Year | |||||
| 1973–1986 | 98 | 80.1 | 67.2 | 59.6 | 46.0 |
| 1987–2000 | 91 | 78.2 | 65.6 | 57.8 | 44.5 |
| 2001–2014 | 34 | 71.8 | 49.3 | 34.2 | 10.4 |
| Age, years | |||||
| <50 | 72 | 84.6 | 67.9 | 55.3 | 32.1 |
| 50–65 | 49 | 77.9 | 58.1 | 44.1 | 20.4 |
| >65 | 26 | 64.9 | 42.8 | 29.5 | 10.7 |
| Race | |||||
| White | 40 | 73.0 | 52.8 | 39.6 | 18.3 |
| Black | 41 | 74.7 | 53.6 | 39.1 | 17.2 |
| Other | 44.5 | 75.1 | 55.7 | 42.0 | 20.2 |
| Marital status | |||||
| Married | 46 | 76.3 | 56.3 | 42.9 | 20.5 |
| Unmarried | 33 | 68.7 | 47.9 | 34.6 | 14.7 |
| Grade | |||||
| Good | 32 | 75.8 | 45.8 | 26.1 | 6.7 |
| Moderate | 29 | 72.6 | 43.3 | 26.2 | 9.2 |
| Poor | 8 | 40.8 | 16.9 | 10.7 | 3.8 |
| Undifferentiated | 8 | 36.9 | 13.5 | 8.7 | 3.5 |
| Unknown | 61 | 78.3 | 63.0 | 50.9 | 25.7 |
| Stage | |||||
| Localized | 54 | 83.8 | 63.0 | 46.7 | 18.1 |
| Regional | 40 | 77.5 | 53.7 | 36.8 | 12.5 |
| Distant | 10 | 46.0 | 23.5 | 14.2 | 3.8 |
| Unknown | 77 | 79.0 | 65.2 | 55.8 | 39.0 |
| Surgery | |||||
| Yes | 52 | 82.7 | 61.4 | 44.7 | 16.9 |
| No | 11 | 49.0 | 26.4 | 16.8 | 5.0 |
| Unknown | 93 | 79.9 | 66.9 | 59.0 | 44.6 |
| Radiation | |||||
| Yes | 11 | 47.9 | 18.9 | 10.8 | 3.8 |
| No | 45 | 75.5 | 55.9 | 42.1 | 19.5 |
| Chemotherapy | |||||
| Yes | 12 | 52.0 | 22.4 | 12.8 | 3.9 |
| No | 50 | 77.2 | 58.6 | 44.5 | 20.8 |
| Primary site | |||||
| Colon | 41 | 72.3 | 53.3 | 40.9 | 20.5 |
| Lung and bronchus | 25 | 63.9 | 44.1 | 33.7 | 17.3 |
| Small intestine | 51 | 81.3 | 60.6 | 44.2 | 17.9 |
| Pancreas | 22 | 65.1 | 36.9 | 22.7 | 7.3 |
| Stomach | 41 | 74.7 | 53.7 | 38.3 | 15.8 |
| Rectum | 70 | 86.5 | 70.3 | 55.7 | 27.1 |
Notes: Bold indicates level of statistical significance at a=0.05.
Abbreviation: NETs, neuroendocrine tumors.
We then evaluated survival patterns according to site and stage (Figure 2). We excluded 15,522 cases for which stage information was not shown in SEER. Among the cases of localized NETs, the median OS ranged from 27 months for NETs in the pancreas to 66 months for NETs in the rectum. Among the cases of regional NETs, NETs in the stomach had the worst median OS (22 months), while the small intestine had the best median OS (50 months). Among patients in the distant stage, those with NETs in the small intestine had the best median OS of 40 months; those with NETs in the lung/bronchus had the worst OS of 5 months.
Figure 2.
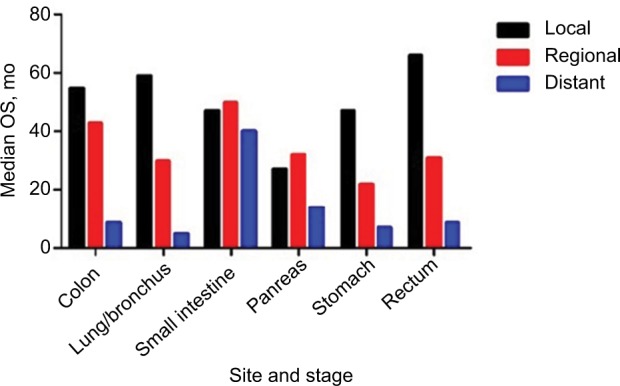
Median OS of NETs by stage.
Abbreviations: NETs, neuroendocrine tumors; OS, overall survival.
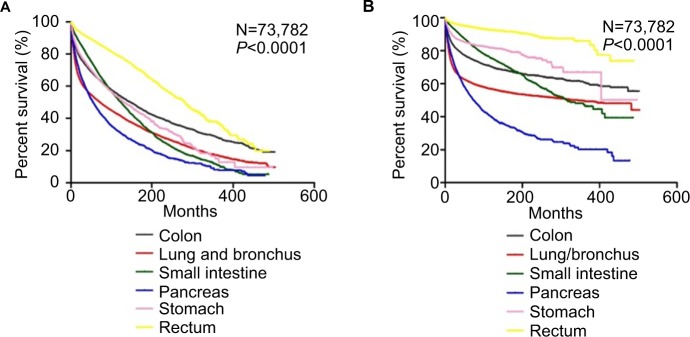
Furthermore, we performed univariate Cox regression analysis to identify variables that might influence the survival. We found that gender, year of diagnosis, age, race, marital status, grade, stage, therapies, and primary sites influence overall survival and cancer-specific survival among patients with NETs (Tables 3 and 4). We then performed a multivariate analysis of the impact of the primary site while adjusting for prognostic factors influencing the survival of patients with NECs using the Cox proportional hazards model (Tables 3 and 4). Significant differences in overall and cancer-specific mortality were observed among the six sites (both log-rank test P<0.0001, Figure 3). NETs in rectum had the best prognosis, while NETs in pancreas had the highest risk of mortality (using rectum as a reference: colon, HR, 1.896, 95% CI, 1.799, 1.999; lung/bronchus, HR, 1.786, 95% CI, 1.703,1.874; pancreas, HR, 2.034, 95% CI, 1.925, 2.148; small intestine, HR, 1.660, 95% CI, 1.579, 1.744; stomach, HR, 1.865, 95% CI, 1.755, 1.982).
Table 3.
Univariate and multivariate analyses of OS in patients with NETs
| Variables | Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| OS HR (95% CI) | P-value | OS HR (95% CI) | P-value | |
|
| ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.796 (0.779, 0.813) | <0.001 | 0.746 (0.729, 0.763) | <0.001 |
| Year | ||||
| 1973–1994 | Reference | Reference | ||
| 1995–2004 | 1.041 (0.996, 1.087) | 0.074 | 0.937 (0.895, 0.982) | <0.001 |
| 2005–2014 | 0.880 (0.844, 0.919) | <0.001 | 0.802 (0.756, 0.851) | <0.001 |
| Age, years | ||||
| <50 | Reference | Reference | ||
| 50–65 | 2.058 (1.975, 2.144) | <0.001 | 2.072 (1.988, 2.159) | <0.001 |
| >65 | 4.890 (4.703, 5.085) | <0.001 | 4.215 (4.050, 4.387) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.896 (0.868, 0.926) | <0.001 | 1.107 (1.070, 1.144) | <0.001 |
| Other | 0.686 (0.651, 0.723) | <0.001 | 0.914 (0.866, 0.963) | 0.001 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Unmarried | 1.415 (1.384, 1.447) | <0.001 | 1.358 (1.327, 1.389) | <0.001 |
| Grade | ||||
| Good | Reference | Reference | ||
| Moderate | 1.890 (1.777, 2.010) | <0.001 | 1.437 (1.351, 1.529) | <0.001 |
| Poor | 7.503 (7.154, 7.869) | <0.001 | 3.140 (2.983, 3.306) | <0.001 |
| Undifferentiated | 8.399 (7.912, 8.917) | <0.001 | 3.044 (2.857, 3.245) | <0.001 |
| Unknown | 1.964 (1.887, 2.044) | <0.001 | 1.540 (1.478, 1.605) | <0.001 |
| Stage | ||||
| Localized | Reference | Reference | ||
| Regional | 2.067 (1.989, 2.148) | <0.001 | 1.533 (1.472, 1.597) | <0.001 |
| Distant | 7.050 (6.831, 7.276) | <0.001 | 3.206 (3.088, 3.329) | <0.001 |
| Unknown | 2.337 (2.260, 2.416) | <0.001 | 1.180 (1.110, 1.254) | <0.001 |
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 4.624 (4.506, 4.745) | <0.001 | 2.188 (2.116, 2.262) | <0.001 |
| Unknown | 1.876 (1.820, 1.933) | <0.001 | 1.711 (1.597, 1.834) | <0.001 |
| Radiation | ||||
| Yes | Reference | Reference | ||
| No | 0.275 (0.266, 0.284) | <0.001 | 0.829 (0.798, 0.860) | <0.001 |
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| No | 0.266 (0.259, 0.274) | <0.001 | 0.778 (0.753, 0.803) | <0.001 |
| Primary site | ||||
| Rectum | Reference | Reference | ||
| Colon | 2.613 (2.484, 2.748) | <0.001 | 1.896 (1.799, 1.999) | <0.001 |
| Lung and bronchus | 3.948 (3.778, 4.127) | <0.001 | 1.786 (1.703, 1.874) | <0.001 |
| Small intestine | 2.566 (2.449, 2.690) | <0.001 | 1.660 (1.579, 1.744) | <0.001 |
| Pancreas | 4.364 (4.147, 4.592) | <0.001 | 2.034 (1.925, 2.148) | <0.001 |
| Stomach | 2.606 (2.455, 2.767) | <0.001 | 1.865 (1.755, 1.982) | <0.001 |
Abbreviations: NETs, neuroendocrine tumors; OS, overall survival.
Table 4.
Univariate and multivariate analyses of CSS in patients with NETs
| Variables | Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| CSS HR (95% CI) | P-value | CSS HR (95% CI) | P-value | |
|
| ||||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.769 (0.748, 0.791) | <0.001 | 0.770 (0.748, 0.793) | <0.001 |
| Year | ||||
| 1973–1994 | Reference | Reference | ||
| 1995–2004 | 1.051 (0.988, 1.118) | 0.115 | 0.946 (0.885, 1.010) | 0.096 |
| 2005–2014 | 0.875 (0.825, 0.927) | <0.001 | 0.796 (0.734, 0.863) | <0.001 |
| Age, years | ||||
| <50 | Reference | Reference | ||
| 50–65 | 1.656 (1.577, 1.739) | <0.001 | 1.599 (1.522, 1.681) | <0.001 |
| >65 | 3.087 (2.947, 3.234) | <0.001 | 2.471 (2.355, 2.592) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.789 (0.755, 0.825) | <0.001 | 1.033 (0.987, 1.080) | 0.163 |
| Other | 0.740 (0.693, 0.790) | 0.002 | 0.995 (0.931, 1.063) | 0.877 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Unmarried | 1.328 (1.291, 1.367) | <0.001 | 1.274 (1.237, 1.313) | <0.001 |
| Grade | ||||
| Good | Reference | Reference | ||
| Moderate | 2.724 (2.510, 2.957) | <0.001 | 1.808 (1.665, 1.963) | <0.001 |
| Poor | 13.128 (12.314, 13.995) | <0.001 | 4.133 (3.860, 4.425) | <0.001 |
| Undifferentiated | 14.789 (13.720, 15.941) | <0.001 | 3.958 (3.655, 4.287) | <0.001 |
| Unknown | 2.638 (2.489, 2.797) | <0.001 | 1.822 (1.716, 1.935) | <0.001 |
| Stage | ||||
| Localized | Reference | Reference | ||
| Regional | 4.306 (4.055, 4.573) | <0.001 | 2.913 (2.736, 3.102) | <0.001 |
| Distant | 18.827 (17.878, 19.826) | <0.001 | 7.062 (6.659, 7.489) | <0.001 |
| Unknown | 4.730 (4.473, 5.002) | <0.001 | 1.792 (1.631, 1.970) | <0.001 |
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 6.869 (6.639, 7.107) | <0.001 | 2.345 (2.243, 2.452) | <0.001 |
| Unknown | 2.433 (2.334, 2.536) | <0.001 | 2.223 (2.015, 2.454) | <0.001 |
| Radiation | ||||
| Yes | Reference | Reference | ||
| No | 0.202 (0.195, 0.209) | <0.001 | 0.804 (0.771, 0.838) | <0.001 |
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| No | 0.177 (0.172, 0.183) | <0.001 | 0.749 (0.722, 0.777) | <0.001 |
| Primary site | ||||
| Rectum | Reference | Reference | ||
| Colon | 2.613 (2.484, 2.748) | <0.001 | 2.744 (2.523, 2.983) | <0.001 |
| Lung and bronchus | 3.948 (3.778, 4.127) | <0.001 | 2.565 (2.371, 2.775) | <0.001 |
| Small intestine | 2.566 (2.449, 2.690) | <0.001 | 1.756 (1.616, 1.909) | <0.001 |
| Pancreas | 4.364 (4.147, 4.592) | <0.001 | 3.022 (2.780, 3.285) | <0.001 |
| Stomach | 2.606 (2.455, 2.767) | <0.001 | 1.941 (1.754, 2.148) | <0.001 |
Abbreviations: CSS, cancer-specific survival; NETs, neuroendocrine tumors.
Figure 3.
Kaplan–Meier survival curves according to different sites in patients with NETs.
Notes: (A) Overall survival. (B) Cancer-specific survival.
Subsequently, we performed a stratified analysis of overall mortality by race, gender, and marital status, which were considered prognostic factors for cancer in previous studies. The magnitudes of the associations are presented in Table 5. Regarding marital status, married patients with NETs had a worse prognosis than unmarried NET patients. Regarding gender, females had a higher risk of mortality in all sites except the lung/bronchus. Regarding race, American Indians/Alaskan Natives and Asian/Pacific Islanders had a significantly worse prognosis than other racial groups for all sites except the colon.
Table 5.
Adjusted HRs for OS associated with different primary sites (colon as reference) by marital status, gender, and race
| Variables | Colon HR (95% CI) | Lung/bronchus HR (95% CI) | Small intestine HR (95% CI) | Pancreas HR (95% CI) | Stomach HR (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Marital status | |||||
| Married | 1.976 (1.842, 2.118) | 1.913 (1.795, 2.038) | 1.756 (1.644, 1.875) | 2.178 (2.028, 2.339) | 1.907 (1.757, 2.070) |
| Unmarried | 1.767 (1.632, 1.914) | 1.608 (1.496, 1.729) | 1.523 (1.412, 1.643) | 1.808 (1.659, 1.969) | 1.778 (1.623, 1.947) |
| Gender | |||||
| Male | 1.728 (1.608, 1.856) | 1.962 (1.838, 2.094) | 1.512 (1.414, 1.616) | 1.916 (1.781, 2.061) | 1.841 (1.690, 2.005) |
| Female | 2.109 (1.951, 2.279) | 1.699 (1.582, 1.824) | 1.857 (1.724, 2.001) | 2.270 (2.090, 2.466) | 1.947 (1.783, 2.125) |
| Race | |||||
| White | 1.975 (1.854, 2.104) | 1.774 (1.674, 1.879) | 1.672 (1.574, 1.776) | 2.025 (1.897, 2.161) | 1.772 (1.649, 1.906) |
| Black | 1.493 (1.326, 1.681) | 1.750 (1.561, 1.962) | 1.507 (1.357, 1.673) | 1.887 (1.650, 2.158) | 2.036 (1.772, 2.340) |
| Other | 1.899 (1.556, 2.318) | 2.141 (1.786, 2.565) | 1.946 (1.587, 2.386) | 2.470 (2.032, 3.001) | 2.663 (2.136, 3.319) |
Notes: Bold indicates level of statistical significance achieved at a=0.05. Adjusted for grade, therapy, stage, age, and year of diagnosis.
Abbreviation: OS, overall survival.
Discussion
NETs are biologically and clinically heterogeneous, which poses significant challenge in the management of this disease. NETs are more indolent than other epithelial malignancies; however, they can be aggressive and resistant to therapy.6 Most likely because of the widespread use of endoscopy and computed tomography for cancer screening, the incidence of NETs has increased yearly.5,7 Attribute to the increased awareness and new treatment modalities, a trend toward improved OS had been seen in 1995–2004 (HR, 0.937, 95% CI, 0.895, 0.982, P<0.001) and 2005–2014 (HR, 0.802, 95% CI, 0.756, 0.851, P<0.001) when compared to 1973–1994.
NETs usually originate in the gastrointestinal tract, lung, and pancreas, according to previous studies.8 Survival outcomes vary significantly among tumors in different primary sites. In the present study, we compared the clinical characteristics, OS, and mortality among different anatomic sites of NETs and found that NETs originating in the pancreas had the worst prognosis (HR, 2.034, 95% CI, 1.925, 2.148, P<0.001). Primary tumor site could be one of the most useful outcome predictors in patients with NETs. We observed variations in the association between primary site and prognosis across race, gender, and marital status. Regarding marital status, we found that married patients had a higher risk of mortality than unmarried patients if their tumors originated in the colon, lung/bronchus, small intestine, pancreas, and stomach. Males also had a better prognosis for tumors originating in the colon, small intestine, pancreas, and stomach, but not in the lung/bronchus. Compared to white or black people, American Indians/Alaskan Natives and Asian/Pacific Islander had a significantly worse prognosis for tumors at all sites except the colon. It should be noted that the magnitude of the association between primary sites and survival benefits varied across races, genders, and marital status. Thus, gender, race, and marital status might partly explain the influence of primary tumor site on OS.
In both the univariable and multivariable analyses, survival was best for patients with localized disease and worst for patients with distant disease. For patients with localized disease, surgical resection should always be considered as surgery is the only curative treatment for localized NETs.2 However, according to the study by Pavel et al, 40%–50% of NET patients present with distant metastases at initial diagnosis.9 In our study, NETs originating from pancreas (40.3%) had a highest proportion of distant stage disease and rectum (3.4%) had the least. In the meanwhile, NETs of rectum (73.2%) had a highest proportion of patients who accepted surgery. That might partly explain the highest mortality in the patients with NETs in the pancreas and lowest in the rectum. Patients with advanced disease may suffer from fatal complications caused by secreted hormones or tumor progression. For advanced NETs, systematic agents, bio-therapy, and targeted radionuclide therapy are used to delay tumor growth.10 The most promising systematic agents are somatostatin analogs, temozolomide, vascular endothelial growth factor pathway inhibitors, and mammalian target of rapamycin inhibitor.10–13
This study used a large, nationally representative sample from the United States collected using the SEER program. Taking advantage of this program, we minimized discrepancies and biases to present results that can be generalized to the US adult population. Moreover, the sample size was particularly helpful because, to date, most studies have been performed using small case series, single centers, and limited data sets. However, some limitations remain. First, SEER does not classify patients according to the World Health Organization 2010 classification, nor does it record the Ki-67 proliferation index, which are important data for evaluating the aggressiveness of NETs.14 The stratification is therefore based on the morphological characteristics reported in the pathology report and known disease behavior of the actual neoplasm. This may slightly underestimate the survival in the well differentiated group and overestimate the survival in the undifferentiated group. Besides, high percentage of unknown information about tumor grade has been found in our studies which is regarded as an important prognostic factor. Such drawbacks are inherent to most retrospective, population-based study and may raise concerns about the generalizability of the findings. As we aimed to emphasize the prognostic value of the primary tumor sites, we still include these patients in our cohort. Second, the SEER registries likely underestimate the total number of patients with NETs. Only patients with malignant NETs are included in this database.
Conclusion
Our study showed significant differences of survival outcome of patients with NETs according to various primary sites. NETs happened in rectum had best prognosis however pancreas had the worst. Primary tumor sites might be one of the most useful predictors of outcome in patients with NETs.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 2.Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol. 2015;33(16):1855–1863. doi: 10.1200/JCO.2014.60.2532. [DOI] [PubMed] [Google Scholar]
- 3.Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer. 2018;124(4):807–815. doi: 10.1002/cncr.31124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute Surveillance, Epidemiology, and End Results Program [webpage on the Internet] Overview of the SEER program. [Accessed October 17, 2018]. Available from: https://seer.cancer.gov/about/overview.html.
- 5.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 6.Boyar Cetinkaya R, Aagnes B, Myklebust TÅ, Thiis-Evensen E. Survival in neuroendocrine neoplasms; A report from a large Norwegian population-based study. Int J Cancer. 2018;142(6):1139–1147. doi: 10.1002/ijc.31137. [DOI] [PubMed] [Google Scholar]
- 7.Shen C, Dasari A, Chu Y, et al. Clinical, pathological, and demographic factors associated with development of recurrences after surgical resection in elderly patients with neuroendocrine tumors. Ann Oncol. 2017;28(7):1582–1589. doi: 10.1093/annonc/mdx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavel M, O’Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103(2):172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 10.Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs. Gut. 2012;61(1):6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29(7):934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamberti G, Brighi N, Maggio I, et al. The role of mTOR in neuroendocrine tumors: future cornerstone of a winning strategy? Int J Mol Sci. 2018;19(3):747. doi: 10.3390/ijms19030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical biomarkers of gastrointestinal, pancreatic, pulmonary, and thymic neuroendocrine neoplasms. Endocr Pathol. 2018;29(2):150–168. doi: 10.1007/s12022-018-9522-y. [DOI] [PubMed] [Google Scholar]