Abstract
Background
Poststroke depression (PSD) is the most common and important neuropsychiatric consequences of stroke, which can result in longer hospital stay, compromise the effectiveness of rehabilitation, and reduce the patients’ quality of life. However, Iranian studies have reported different and conflicting prevalence rates for PSD.
Objective
The present systematic review and meta-analysis aimed to evaluate the overall prevalence of PSD in Iranian patients.
Patients and methods
A systematic search was conducted in 2018 for Persian or English articles on PSD, published in the Scientific Information Database (SID), MagIran, PubMed, Scopus, and Web of Science, using the following keywords: depression, depressive disorder, stroke, cerebrovascular disorder, and Iran and all of their possible combinations. Heterogeneity across the studies was evaluated using the Cochran’s Q test. According to the heterogeneity results, a random effects model was used to estimate the overall prevalence of PSD. The data were analyzed using the Stata 12 software.
Results
Overall, six articles with a total sample size of 641 patients were included in the analysis. The overall prevalence of PSD in Iran was 46.9% (95% CI: 30.1–63.7). In addition, the prevalence of PSD was higher in women (50.4%, 95% CI: 17.9–82.9) than in men (29.5%, 95% CI: 17.2–41.8). According to the results of univariate meta-regression, there was a significant relationship between PSD prevalence and sample size (P=0.010).
Conclusion
Around half of the stroke patients in Iran suffer from PSD. Given the overlap between neurological symptoms of stroke and depression, efforts should be made to quickly and accurately diagnose depression so that it can be effectively managed with minimum complications.
Keywords: depression, stroke, outbreak, systematic review, meta-analysis, Iran
Introduction
Stroke is a chronic and debilitating disease with an estimated socioeconomic burden far greater than that of cardiovascular disease and cancer.1 Stroke occurs due to an abnormality in the blood supply to the brain and is divided into two ischemic and hemorrhagic categories, according to the nature of the disorder.2 Annually, around 15 million people suffer from stroke worldwide, of whom about 5 million die and 5 million suffer from permanent disabilities.3 According to reports, 10% of patients with stroke and 25% of those suffering from minor complications are recovered. However, another 40% of stroke patients experience severe and serious complications and require special care, of whom 10% require permanent and lifelong care, whereas around 15% die shortly after stroke.4 Stroke is a very important incident in the patients’ lives and can lead to multiple psychosocial, social, and economic consequences, such as seizures, motor disorders, and psychiatric problems.5
Although stroke can have unpredictable effects on each person, poststroke depression (PSD) is the most common and major neuropsychiatric consequence of stroke that affects one-third of survivors.6 The results of various meta-analyses have shown a positive and significant relationship between stroke and depression.7,8 At least one-third of stroke survivors exhibit mood and depression symptoms following the stroke.9 Loss of energy, increase or decrease in the duration of sleep, restlessness, unconsciousness during activities, feeling useless, distraction, behavioral changes, and suicidal thoughts are among the common symptoms of PSD.10 Patients with PSD experience more functional disabilities, longer hospital stay, poorer rehabilitation results, lower quality of life, and high mortality in the first year after the stroke.10–12 The disease is often not diagnosed and left untreated.13,14 Several factors have been shown to predict PSD, including physical disability, severity of stroke, history of depression, and cognitive impairment.10,15,16
About 25%–79% of stroke survivors suffer from PSD. Different prevalence rates are partly due to the use of different diagnostic criteria and different assessment times.10 A meta-analysis evaluating the prevalence of PSD reported by observational studies found a prevalence of 33%, whereas another meta-analysis examining the prevalence of mood disorders after stroke reported a prevalence of 17.7% for major depressive disorder and a prevalence of 13.1% for minor depressive disorder.17,18 Similarly, Iranian studies have reported conflicting prevalence rates for PSD, ranging from 18% to 72.5%.19,20 Considering that any effort to prevent and treat depression in stroke patients requires accurate statistical data from the community, and given the lack of systematic studies focused on this issue in Iran, the present study is aimed at accurately estimating the prevalence of PSD in Iranian patients.
Participants and methods
This is a systematic review and meta-analysis of Iranian studies focused on the prevalence of PSD in Iran, published in the Iranian and international journals from inception until 2018. In this study, articles published in the Iranian and international databases, including Scientific Information Database (SID), MagIran, PubMed, Scopus, and Web of Science, were searched using the following keywords: depression, depressive disorder, stroke, cerebrovascular disorder, and Iran. For instance, the search strategy in the PubMed database was as follows: (“Depression”[All Fields] OR “depressive disorder”[All Fields]) AND ((“stroke”[MeSH Terms] OR “stroke”[All Fields]) OR “cerebrovascular disorder”[All Fields]) AND (“Iran”[MeSH Terms] OR “Iran”[All Fields]). The reference lists of the selected articles were also reviewed to find other related articles.
Study selection and data extraction
First, all the articles investigating the prevalence of depression in patients with stroke were chosen. The inclusion criteria were as follows: observational studies, published in Persian or English, and access to the full texts of articles. Nonrelated studies, case reports, letters to editors, interventional studies, and repeated studies were excluded. To reduce bias, the search process, study selection, evaluation of the studies’ quality, and data extraction were conducted by two researchers independently. Disagreements between the two researchers were resolved through discussions. The following information was extracted from each article and recorded in an appropriate form: name of the first author, year of publication, place of publication, total sample size, sample size by gender, language of the study, diagnostic criteria, and proportion of participants with depression and PSD by gender. Methodological quality was evaluated using a standard tool assessing the following five aspects of the studies: study plan, comparison groups, sample characteristics, sample size, and data collection tools; each item was rated on a scale from 0 to 3. Total scores ranged from 0 to 15, with higher scores indicating higher methodological quality. Accordingly, the articles were divided into the following three categories: poor quality (scored 0–5), moderate (scored 6–10), and strong (scored above 10).21–24
Statistical analysis
For each selected study, a point estimate and a 95% CI for PSD prevalence were calculated based on the binomial distribution. Due to heterogeneity among the selected studies, data analysis was performed using the random effects model (Dersimonian and Laird). A forest plot was used to display the prevalence of PSD in the selected articles, based on effect size and 95% CI. The I2 index was used to examine the percentage of overall change due to heterogeneity. Based on the I2 index, heterogeneity level was classified into low heterogeneity (<25%), moderate heterogeneity (25%–75%), and high heterogeneity (more than 75%).25 The univariate meta-regression was used to investigate the relationship of the prevalence of PSD with article’s year of publication and sample size. Using the subgroup analysis, the combined prevalence rate was estimated based on gender, screening criteria, and five regions of Iran. In addition, after assessing the publication bias and the effect of small sample sizes, the funnel plot was used based on the Egger’s regression test. Data analysis was performed using the Stata 12 software.
Results
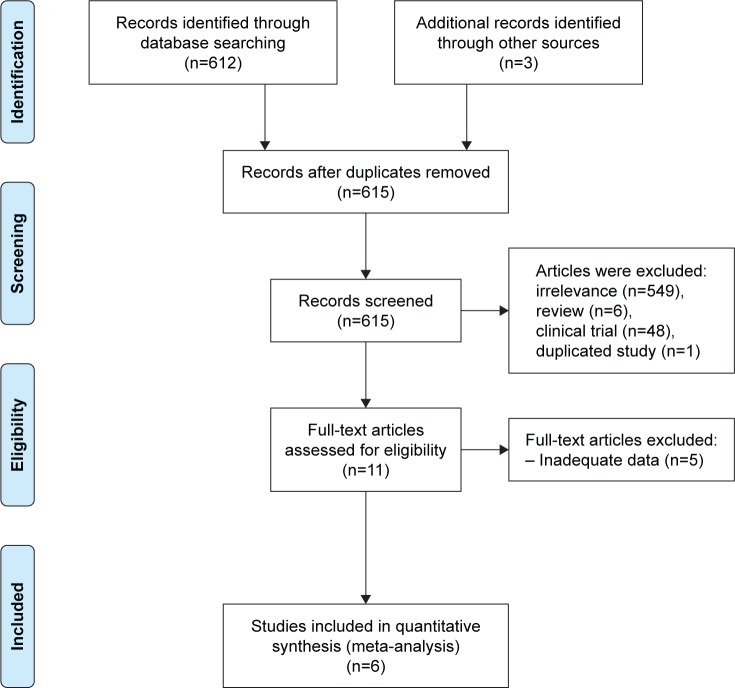
In this meta-analysis, the most recent articles published from 2007 to 2015 were reviewed. In the first step, a total of 615 articles were identified through the search process, of which a total of 604 articles were excluded based on the exclusion criteria. In the next step, the full-texts of the remaining 11 articles were analyzed. Among the 11 articles, five articles lacked adequate data for analysis and were discarded. Finally, a total of six articles examining the prevalence of PSD in Iran were analyzed based on the PRISMA statement (Figure 1).26
Figure 1.
Flowchart of the screening and selection of qualified articles according to the PRISMA statement.
The total sample size was 641, ranging from 40 to 205 (M=106). Four articles were in Persian, and two articles were in English. In terms of methodological quality, all the studies were of moderate quality (Table 1).
Table 1.
Characteristics of the six selected articles
| Prevalence rate | Instrument | Region | Language | Place | Sample size (male/female) | Year of publication | First author |
|---|---|---|---|---|---|---|---|
| 45 | DSM-IV | 1 | Persian | Ramsar | 80 (Ns/Ns) | 2016 | Ebrahimi-Rad27 |
| 47.4 | HADS | 1 | Persian | Sari | 116 (57/59) | 2014 | Hassanzadeh28 |
| 72.5 | BDI | 1 | English | Tehran | 40 (21/19) | 2013 | Haghgoo20 |
| 59 | DSM-IV | 5 | Persian | Rafsanjan | 100 (35/65) | 2012 | Iranmanesh29 |
| 18 | DSM-IV | 1 | English | Babol | 205 (97/108) | 2009 | Ahangar19 |
| 42 | BDI | 5 | Persian | Zahedan | 100 (61/39) | 2008 | Lashkaripour30 |
Abbreviations: BDI, Beck Depression Inventory; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; HADS, Hospital Anxiety and Depression Scale; Ns, not stated.
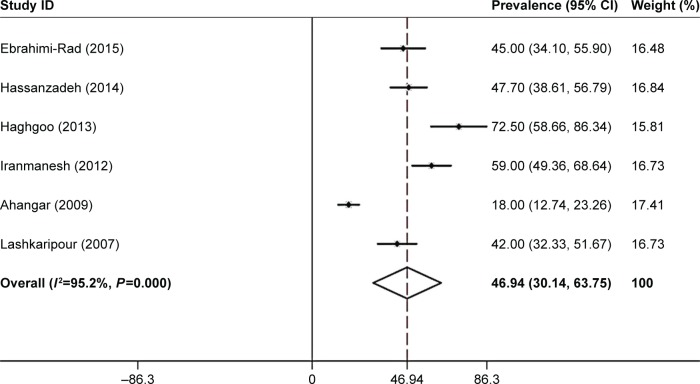
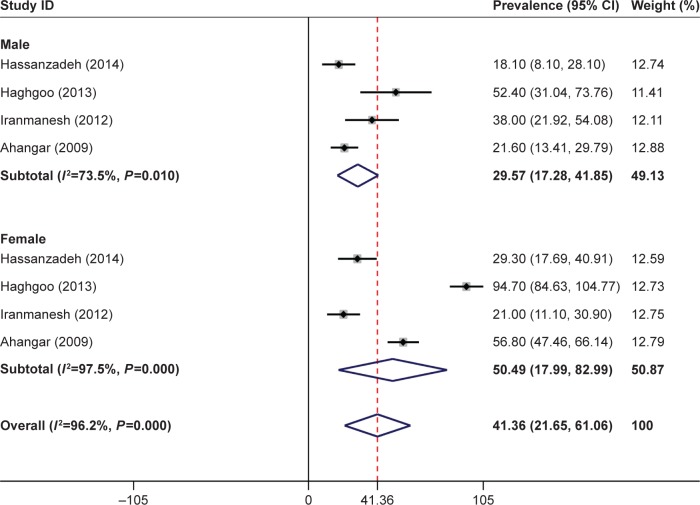
The overall prevalence of PSD in the Iranian patients was 46.9% (95% CI: 30.1–63.7). The overall prevalence of PSD was 29.5% (95% CI: 17.2–41.8) in men and 50.4% (95% CI: 17.9–82.9) in women. The findings based on screening tools showed that the prevalence of PSD was 53.2% (95% CI: 37.5–68.9) based on the Hospital Anxiety and Depression Scale (HADS) and Beck Depression Inventory (BDI) and 40.4% (95% CI: 13.3–67.4) based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). The prevalence of PSD reported by the studies conducted in region 5 (50.5%, 95% CI: 33.8–67.1) was higher than that reported by the studies conducted in region 1 (45.2%, 95% CI: 22.2–68.2) (Figures 2 and 3).
Figure 2.
Forest plot of the prevalence of poststroke depression in Iranian patients.
Notes: The 95% CI for each study is shown in the form of horizontal lines around the central mean, the midpoint of the dotted line represents the mean of the overall score, and the lozenge shape shows the CI of the prevalence of this disorder. Weights are from random-effects analysis.
Figure 3.
Forest plot of the prevalence of poststroke depression based on gender.
Note: Weights are from random-effects analysis.
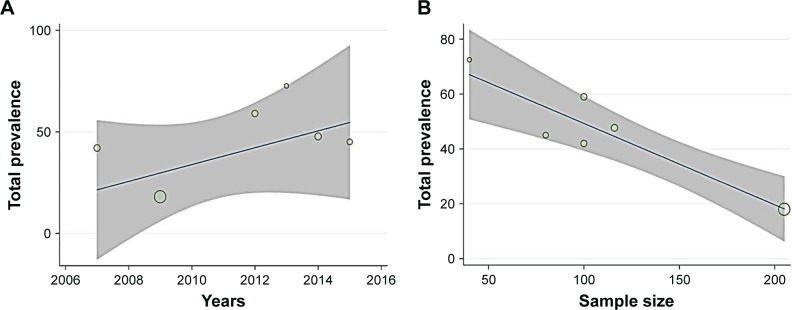
According to the meta-regression results, there was no significant relationship between PSD prevalence and article’s year of publication, but PSD prevalence significantly reduced with an increase in sample size (Figure 4).
Figure 4.
Meta-regression results for the prevalence of poststroke depression in the Iranian patients.
Notes: The prevalence of poststroke depression by the year of publication (A) and sample size (B). Circles indicate the weight of the studies.
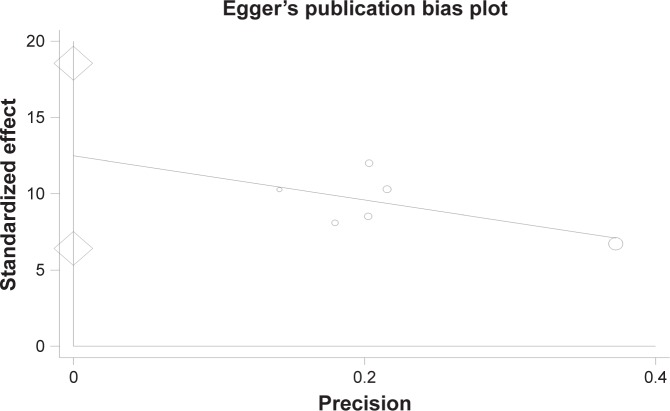
The findings indicated that the publication bias in the present study was significant (P=0.005) (Figure 5).
Figure 5.
Publication bias.
Discussion
The aim of this study was to estimate the overall prevalence of PSD in Iranian patients. A total of six qualified articles were examined. Among these, four articles had reported similar prevalence rates. The lowest (18%) and the highest (72%) prevalence rates were in Ahangar and Hosseini19 and Haghgoo et al,20 respectively. The highest prevalence of PSD had been reported by Haghgoo et al. In their study, 65.5% of the patients were completely or partially dependent on others in performing the activity of daily living (ADL) and did not have independence in everyday tasks. It seems logical that losing independence can lead to depression. In the study by Ahangar and Hosseini also, no clear reason had been reported for the low prevalence of depression.
The results showed that nearly half of the patients with stroke suffered from depression. This rate is higher than those reported in USA (31%) and New Zealand (23%).31,32 A meta-analysis study by Hackett et al17 showed that the prevalence of PSD (at any time after stroke) was 33%. In the study by Ariful Islam et al,33 70% of the patients in Bangladesh had depression symptoms. Various studies have led to different results. This variation could be attributed to the demographic characteristics of the countries in which the studies were conducted and the use of different measurement instruments. Wolfe believes that measuring depression in patients with stroke is a unique challenge, since many stroke-related neurological symptoms, such as energy loss, fatigue, restlessness, sleep disturbances, and concentration problems, can have symptoms similar to those of depression. The patients may also have multiple neuropsychiatric disorders, making neuropsychiatric assessments more challenging.1 PSD is a stressful response to experiencing stroke, especially when the patient is faced with the negative outcomes of the illness; some also attribute it to early organic dysthymia, which is due to neurobiological changes during stroke.34 Heterogeneity across the studies can be due to different populations and difference in the study design, using different assessment methods, small sample sizes, and definitions for the variables. The articles reviewed in our meta-analysis had different sample sizes and assessment tools. In our meta-analysis, there was a high level of heterogeneity across the studies that could be due to clinical or methodological differences. This heterogeneity could influence the estimation of the combined effect size for stroke; therefore, the findings should be interpreted with caution.
In this study, the prevalence of PSD in women was higher than that in men (50.4 vs 29.5%), which was consistent with the results of the studies conducted in Bangladesh and China.33,35 A systematic review study with 47 selected studies aimed at assessing the prevalence of PSD in men and women showed that, in 35 studies, there was a higher prevalence of PSD in women than in men.36 According to the WHO, following stroke, women tend to be hospitalized longer than men and are less likely to use rehabilitation programs. As a result, they become more disabled than men, which could be a potential reason for the higher prevalence of PSD in women. Other causes, such as genetic and social factors, age, cognitive impairment, and access to rehabilitation programs, may also play a role in the higher prevalence of PSD in women.37 It should also be noted that the prevalence of PSD in many studies is based on a single type of stroke; therefore, more precise studies are needed on the relationship between gender and PSD prevalence in different types of stroke.
Considering that, so far, few studies have been conducted on the prevalence of PSD in Iran and that the earliest meta-analysis on this topic in Iran dates back to 2007, we can argue that this group of patients in Iran has not received enough attention regarding their psychological needs after treatment. Despite the high level of heterogeneity in the present meta-analysis, the high prevalence of PSD in Iran was expected, considering the limited studies on this topic and not paying enough attention to depressive symptoms in this group of patients.
The meta-regression findings showed an increase in the prevalence of PSD at this time but were not statistically significant. Other studies have examined changes in PSD over a short period of time and reported different findings. In the study by Verdelho et al,38 the prevalence of PSD in the first 6 months after stroke was 43%; this was reduced to 18% in the third year after the disease. However, in another study, in the first year after stroke, the prevalence of PSD was 16%, but in the third year after stroke, it was increased to 29%.39 In our meta-analysis, meta-regression was used to identify the source of heterogeneity among the following two predetermined covariates: article’s year of publication and sample size; the results indicated that different sample sizes were the source of high heterogeneity.
The prevalence rates of PSD in the studies conducted in the region 1 were lower than those in the studies conducted in region 5. The reason for the difference is that region 1 includes Tehran (the capital of Iran) and neighboring cities. Therefore, the patients in this region have better access to health care facilities; therefore, they are more easily and quickly identified and treated. PSD is a multifactorial phenomenon that is influenced by many factors. Among the six reviewed articles, four articles had been conducted in region 1 of Iran. Iran’s region 1 includes Tehran (Iran’s capital) and the adjacent cities, which have almost similar populations in terms of cultural, ethnic, and religious characteristics. Therefore, we can be pretty much sure that the cultural, ethnic, and religious effects were adjusted. Publication bias was significant in our meta-analysis, perhaps because articles with negative or insignificant results were not published, or even because the sponsors of the studies did not approve their results. Articles written in English are usually of better methodological quality (blinding, random allocation, and adequate sample sizes) compared to the studies written in other languages. In addition, studies with significant results are more likely to be published in the top-ranked journals.
In the present meta-analysis, for the first time, the Iranian studies on the prevalence of PSD were systematically reviewed and analyzed; this can be regarded as strength of it. However, lack of access to sufficient information, such as type of stroke (hemorrhagic or thromboembolic), location of the lesion (right or left), and the overall score on the disability index were some of the limitations of the study that prevented us from conducting a more comprehensive analysis.
Conclusion
The study findings showed that nearly half of the Iranian stroke patients suffered from PSD. Depression leads to reduced treatment adherence and ultimately worsens the patient’s condition. Given the overlap between neurological and depressive symptoms and the presence of several neurological disorders in the stroke patients, developing a good understanding of PSD is important in controlling the illness and providing the required interventions for the patients.
Acknowledgments
This work was done without any external funding.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wolfe CD. The impact of stroke. Br Med Bull. 2000;56(2):275–286. doi: 10.1258/0007142001903120. [DOI] [PubMed] [Google Scholar]
- 2.Iranmanesh F. Post-stroke depression and hospital admission. A need for nursing care partition according to the clinical condition. Neurosciences. 2010;15(1):33–36. [PubMed] [Google Scholar]
- 3.de Ryck A, Brouns R, Geurden M, Elseviers M, de Deyn PP, Engelborghs S. Risk factors for poststroke depression: identification of inconsistencies based on a systematic review. J Geriatr Psychiatry Neurol. 2014;27(3):147–158. doi: 10.1177/0891988714527514. [DOI] [PubMed] [Google Scholar]
- 4.Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, Todorovic L, Tiric-Campara M. Post stroke depression. Med Arch. 2014;68(1):47–50. doi: 10.5455/medarh.2014.68.47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel K, Wolfe CD, Busch MA, Mckevitt C. What are the social consequences of stroke for working-aged adults? A systematic review. Stroke. 2009;40(6):e431–e440. doi: 10.1161/STROKEAHA.108.534487. [DOI] [PubMed] [Google Scholar]
- 6.Gainotti G, Marra C. Determinants and consequences of post-stroke depression. Curr Opin Neurol. 2002;15(1):85–89. doi: 10.1097/00019052-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(1):32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- 8.van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 9.Loubinoux I, Kronenberg G, Endres M, et al. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med. 2012;16(9):1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Benedetto P. Post-stroke depression. European Journal of Physical and Rehabilitation Medicine. 2000;36(2):53. [Google Scholar]
- 11.Wei N, Yong W, Li X, et al. Post-stroke depression and lesion location: a systematic review. J Neurol. 2015;262(1):81–90. doi: 10.1007/s00415-014-7534-1. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki S, Hashimoto M, Yuki S, Koyama A, Hirata Y, Ikeda M. The relationship between post-stroke depression and physical recovery. J Affect Disord. 2015;176:56–60. doi: 10.1016/j.jad.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Sarfo FS, Jenkins C, Singh A, et al. Post-stroke depression in Ghana: Characteristics and correlates. J Neurol Sci. 2017;379:261–265. doi: 10.1016/j.jns.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paolucci S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr Dis Treat. 2008;4(1):145–154. doi: 10.2147/ndt.s2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 16.Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(10):2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 17.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AJ, Sheth B, Gill J, et al. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry. 2017;47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Ahangar AA, Hosseini S. Epidemiological evaluation of post stroke depression in Babol, Northern Iran. Neurosciences. 2009;14(1):102–103. [PubMed] [Google Scholar]
- 20.Haghgoo HA, Pazuki ES, Hosseini AS, Rassafiani M. Depression, activities of daily living and quality of life in patients with stroke. J Neurol Sci. 2013;328(1–2):87–91. doi: 10.1016/j.jns.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Ghanei Gheshlagh R, Ebadi A, Dalvandi A, Rezaei M, Nourozi Tabrizi K. A systematic study of resilience in patients with chronic physical diseases. Nurs Midwifery Stud. 2017;6(2):e36401. [Google Scholar]
- 22.Hoodin F, Weber S. A systematic review of psychosocial factors affecting survival after bone marrow transplantation. Psychosomatics. 2003;44(3):181–195. doi: 10.1176/appi.psy.44.3.181. [DOI] [PubMed] [Google Scholar]
- 23.Tsimicalis A, Stinson J, Stevens B. Quality of life of children following bone marrow transplantation: critical review of the research literature. Eur J Oncol Nurs. 2005;9(3):218–238. doi: 10.1016/j.ejon.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Ebtekar F, Dalvand S, Gheshlagh RG. The prevalence of metabolic syndrome in postmenopausal women: a systematic review and meta-analysis in Iran. Diabetes Metab Syndr. 2018;12(6):955–960. doi: 10.1016/j.dsx.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimi-Rad R, Nasiri M, Gholizadeh B, Arabpuor M, Fotokian Z, Jannat Alipoor Z. Prevalence and Risk Factors of Early Post-Stroke Depression. J Zanj Univ Med Sci. 2016;24(103):115–124. [Google Scholar]
- 28.Hassanzadeh R, Hosseini H, Abedini M, Enayati H. The relationship of possible risk factors in prevalence of post stroke depression. J Mazand Univ Med Sci. 2014;24(1):60–66. [Google Scholar]
- 29.Iranmanesh F, Vazirinejad R, Gaderi F, Rajabpour N. Study of relationship between prevalence of post-stroke depression and stroke risk factors. J Fas Univ Med Sci. 2012;2(2):66–70. [Google Scholar]
- 30.Lashkaripour K. Prevalence of post stroke depression and its relationship with disability and lesion location. Journal of Fundamentals of Mental Health. 2008;10(39):191–199. [Google Scholar]
- 31.Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Arch Clin Neuropsychol. 2007;22(4):519–531. doi: 10.1016/j.acn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Fei K, Benn EK, Negron R, Arniella G, Tuhrim S, Horowitz CR. Prevalence of depression among stroke survivors: racial-ethnic differences. Stroke. 2016;47(2):512–515. doi: 10.1161/STROKEAHA.115.010292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ariful Islam M, Rahman A, Aleem MA, Islam SMS. Prevalence and associated factors of depression among post-stroke patients in Bangladesh. Int J Ment Health Addict. 2016;14(2):154–166. [Google Scholar]
- 34.Lewin-Richter A, Volz M, Jöbges M, Werheid K. Predictivity of early depressive symptoms for post-stroke depression. J Nutr Health Aging. 2015;19(7):754–758. doi: 10.1007/s12603-015-0540-x. [DOI] [PubMed] [Google Scholar]
- 35.Sun N, Li QJ, Lv DM, Man J, Liu XS, Sun ML. A survey on 465 patients with post-stroke depression in China. Arch Psychiatr Nurs. 2014;28(6):368–371. doi: 10.1016/j.apnu.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics. 2009;50(6):563–569. doi: 10.1176/appi.psy.50.6.563. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Zhu M, Su Z, et al. Post-stroke depression: different characteristics based on follow-up stage and gender-a cohort perspective study from Mainland China. Neurol Res. 2017;39(11):996–1005. doi: 10.1080/01616412.2017.1364514. [DOI] [PubMed] [Google Scholar]
- 38.Verdelho A, Hénon H, Lebert F, Pasquier F, Leys D. Depressive symptoms after stroke and relationship with dementia: A three-year follow-up study. Neurology. 2004;62(6):905–911. doi: 10.1212/01.wnl.0000115107.66957.8c. [DOI] [PubMed] [Google Scholar]
- 39.Aström M, Adolfsson R, Asplund K. Major depression in stroke patients. A 3-year longitudinal study. Stroke. 1993;24(7):976–982. doi: 10.1161/01.str.24.7.976. [DOI] [PubMed] [Google Scholar]