Abstract
Test-to-test consistency was evaluated for a single binary combination of organic chemicals using an assay that examined toxicity over multiple exposure times. Six experiments were conducted. The toxicity of 3-chloro-2-butanone (3C2B), methyl crotonate (MC) and the mixture (MX) was evaluated in each experiment at 15, 30 and 45-min of exposure using the Microtox® system. Concentration-response (x/y) curves were generated via the five-parameter logistic minus one-parameter (5PL-1P) curve-fitting function and used to develop predicted x/y curves for the dose-addition (DA) and independence (I) models of combined effect. A variety of toxicity (e.g., EC50) and time-dependent toxicity (TDT) endpoints, 5PL-1P parameters, and various combined effects metrics (e.g., MX/DA) were calculated. Test-to-test consistency was evaluated via the coefficient of variation (CV) or, for TDT, the standard deviation of mean values. In the study, CVs obtained for single-chemical and mixture toxicity endpoints (EC25, EC50, and EC75) at each exposure time were <20, as were those for the predicted DA and I curves. For the MX/DA metric, mixture toxicity was consistent with that predicted for DA at each exposure time in each experiment with CVs <6, despite some substantial variation in TDT for MC-alone at the EC25 and for the 30 to 45-min time-interval. There was lower variation in TDT for 3C2B and for MX. Mean and CV values for 5PL-1P-derived slope and asymmetry parameters were also assessed to provide bases for comparisons in future reports.
Keywords: Microtox®, methodology, dose-addition, bioluminescence, time-dependent toxicity
Introduction
Research on chemical mixture toxicity is an area of considerable activity. Recent studies have included research in the areas of environmental (Altenburger et al. 2015) and human health (Toccalino et al. 2012, Muthusamy et al. 2016), ecotoxicology (Kim et al. 2013, Białk-Bielińska et al. 2017), and aquatic toxicology (Dietrich et al. 2010, Tipping and Lofts 2013). Commonly examined chemicals included pesticides (Belden et al. 2007, Phillips et al. 2010, Nowell et al. 2014), polycyclic aromatic hydrocarbons (Landrum et al. 2012), pharmaceuticals (Houtman et al. 2014, Watanabe et al. 2016), nanoparticles (Lopes et al. 2016) endocrine disruptors (Webster 2013), and metals (Balistrieri et al. 2015). Such studies have been conducted on simple and complex environmental mixtures. The variety of these studies and the types of organisms, assays, and predictive models used have been based on the goals of the studies and derived from the complexities of chemical fate and distribution, exposure and uptake processes, chemical reactivity, toxicokinetics and metabolism, and abiotic factors.
Recent work in this lab has utilized the Microtox® system to evaluate time-dependence in mixture toxicity. These studies have examined toxicity of selected mixtures of industrial organic chemicals (e.g., Gagan et al. 2007, Dawson et al. 2008), developed and evaluated the 5PL-1P function for concentration-response curve-fitting (Dawson et al. 2012, 2016) and examined the viability of using time-dependent toxicity (TDT) of the individual chemicals for predicting TDT of the mixtures (Dawson et al. 2014a). The design of these studies has included multiple tests of individual chemicals (Dawson et al. 2010, 2011, 2014b, 2016) thereby allowing for assessment of test-to-test consistency of those data, but none of these studies had evaluated such consistency over several tests of the same mixture. For this system to be a reliable approach for evaluating mixture toxicity of commonly used industrial organics such an assessment was needed.
To conduct the study it was determined that both of the individual chemicals selected for mixture testing should have increasing toxicity over exposure time (i.e., TDT >0). Chemicals lacking TDT tend to be unreactive (Gagan et al. 2007), thereby exerting reversible toxicity primarily via effects on the cell membrane (e.g., non-polar narcosis). Additionally, it was determined that neither chemical should have fully time-dependent toxicity (i.e., TDT ≥100%) as those agents appear to exert only irreversible toxic effects. Previous testing had suggested that there would be higher test-to-test variability in TDT for chemicals with mid-range TDT (i.e., TDT between about 20 and 80%), as such chemicals appear to have two modes of toxic action –reversible toxicity, especially at lower concentrations during earlier exposure times, and some form of irreversible action, most notably at higher concentrations and later in exposure (Gagan et al. 2007). For these reasons 3C2B and MC were selected as the single-chemicals to be used in this study as they have mid-range TDT. Six experiments of the chemical combination 3C2B-MC were conducted to evaluate test-to-test consistency of the model system. Data from each experiment were recorded and analyzed to evaluate consistency of the results for this model system.
Materials and Methods
Chemicals and reagents
Chemicals used in toxicity testing were purchased (Sigma-Aldrich, Milwaukee, WI) at ≥97% purity and used as received. Stock solutions for 3C2B, MC and the 3C2B-MC mixture (MX) were freshly prepared just prior to testing. Microtox® reagent, reconstitution solution, and diluent were obtained from Modern Water, Inc. (New Castle, DE).
Toxicity testing
Utilizing the quorum-sensing activity of the marine bacterium Aliivibrio fischeri (Urbanczyk et al. 2007), inhibition of bioluminescence was assessed using a calibrated Microtox® 500 analyzer (Dawson et al. 2014a, b). Six experiments were conducted. Each consisted of three toxicity tests: 3C2B-alone, MC-alone and MX within a 4.5 – 5 hr period. For all experiments the tested concentrations (nominal) were prepared via serial dilution (dilution factor = 1.75) and corrected for density since both chemicals were liquids at room temperature.
In each test, seven concentrations were evaluated in duplicate along with a duplicated control treatment. The 3C2B concentrations tested were the same in experiments 1–3 and were changed slightly for experiments 4–6. Concentrations of MC were also the same for experiments 1–3, but varied in each of experiments 4–6. For a given experiment, the MX concentration series always used the second highest concentration of 3C2B-alone and of MC-alone to form its highest concentration, with the other MX concentrations resulting from the 1.75 dilution factor used (see Supplementary Tables S1–S6). Initial bioluminescent readings were taken before chemical exposure; following exposure light readings were made at 15, 30 and 45-min.
Curve-fitting
Concentration-response (x/y) data were input to SigmaPlot® (v. 11.0; Systat Software, Chicago, IL) and fitted to sigmoid curves using the 5PL-1P function (Dawson et al. 2012). This method utilized four parameters: EC50, slope, maximum effect and asymmetry.
Curve fitting was performed using:
with y = % effect, max = maximum effect, x = concentration, s = asymmetry. The variable xb was determined using:
Initial parameters for the regressions were automatically estimated while employing three constraints: a) EC50 > 0; b) 0.1< s <10; and c) max = 100. Maximum effect values were constrained to 100% to provide consistency in calculating TDT values (Dawson et al. 2016). For each exposure time, single-chemical x/y data were used to calculate EC25, EC50, and EC75 values. The EC50 was defined as the half-maximal effective concentration, while the EC25 and EC75 were the one-quarter and three-quarters-maximal effective concentrations, respectively. Percent effect values for each replicate were determined using Microtox® Omni software. Slope and asymmetry values were also calculated.
For analyzing MX data, concentrations of MC were converted to concentration-equivalents of 3C2B using a conversion factor obtained by dividing the concentration of 3C2B by the concentration of MC (Dawson et al. 2010). This conversion allowed the total chemical concentration of the mixture to be made relative to those for 3C2B-alone, so that toxicity plots of the mixture at any exposure duration could be visualized alongside the plots of 3C2B and MC. The same curve-fitting methods used for the single-chemical data were used for MX data.
Calculation of time-dependent toxicity (TDT) values
To quantify changes in toxicity over exposure time, TDT values were calculated at the 25, 50 and 75% effect-levels (Gagan et al. 2007) and for the 15 to 30-min, 30 to 45-min and 15 to 45-min time-intervals using the appropriate time-factors for each. Time-factors (0.5 for 15 to 30-min, 0.333 for 30 to 45-min and 0.667 for 15 to 45-min) were calculated via the following equation:
with t2 being the later time of the exposure interval and t1 the earlier time of that interval. Then the equations below were used to calculate TDT:
with ECx being the effect-level (i.e. 25, 50, or 75%) and being the appropriate factor for the time-interval. As an example, for a hypothetical chemical with a 15-min EC50 of 90 mg/L and a 45-min EC50 of 30 mg/L, the TDT at 50% effect for the 15 to 45-min time-interval was 100%, as shown below:
Values for the TDT25 or TDT75 were calculated in the same manner by incorporating the appropriate time-factor and EC25 or EC75 data, respectively.
In addition to effect-level and time-interval TDT values for each individual experiment, mean TDT values for effect-level (i.e., mTDT25, mTDT50, and mTDT75) and time-interval (i.e., mTDT15:30, mTDT30:45, and mTDT15:45) were also calculated for each experiment. Mean TDT values for a given effect-level were determined by:
in which x represents the selected effect-level (i.e., 25, 50, or 75%). Mean TDT values for a given time-interval were calculated as follows:
in which t1:t2 represents the selected time-interval (i.e., 15 to 30, 30 to 45 or 15 to 45-min). It was previously shown that mTDT15:45 values for single-chemicals were of value in predicting mTDT15:45 for binary mixtures (Dawson et al. 2014a).
Calculation of predicted dose-addition and independence curves
Predicted x/y curves for dose-addition (DA) were calculated as described previously (Dawson et al. 2011). When chemicals A and B (i.e., 3C2B and MC respectively, in this study) are dose-additive the EC50 for the A+B mixture is graphically left-shifted by a dose-ratio (DR) factor of 2 when the chemicals are equieffective. This is calculated using the equation:
with Add50 being the EC50 for dose-addition, a50 the EC50 for the more potent chemical and b50 (see below) the EC50 for the less potent agent. The DR50 was determined by:
Calculations of EC25 and EC75 values for the predicted DA curve were also made. Taken together these predicted values (EC25, EC50, and EC75) permitted calculation of the predicted dose-addition curve via the curve-fitting procedures described above.
Predicted curves for the independence (I) model (Bliss 1939) were calculated using a user-generated transform within SigmaPlot® as:
with yA and yB being the percent effect values for chemicals A and B, respectively.
Combined effects determination
Dose-additivity quotient (AQ) and independence quotient (IQ) values were calculated as metrics of combined effect for the mixtures. These values were determined by dividing the respective EC50 value for the mixture at a given exposure time by the predicted EC50 value for DA or I, respectively (Dawson et al. 2014b) at that exposure time. For example, a hypothetical AQ50 calculation for a MX with a 45-min EC50 of 22.1 mg/L and a predicted 45-min EC50 value of 23.4 mg/L for DA is 0.94. Previously this lab has considered AQ and IQ values from 0.90 to 1.10 as being consistent with DA and I, respectively (Dawson et al. 2016). The AQ and IQ values were also determined for the 25 and 75% effect-levels at each exposure time.
Since the slope of a MX curve can be different than that for chemical A, chemical B or both a second combined effects metric was developed for both DA (i.e., MX/DA) and I (i.e., MX/I) (Dawson et al. 2016). To calculate MX/DA and MX/I the concentrations of 3C2B and concentration equivalents of MC (calculated as noted above) at the EC25, EC50, and EC75 of the mixture were summed (i.e., EC25+50+75) and divided by the sum of those same effect-level concentrations for the predicted DA curve and for the predicted I curve, respectively. These metrics allowed for a more complete assessment of combined effect from the 25 – 75% effect-levels than did examining mixture toxicity at only the 50% effect-level (Dawson et al. 2016).
Slope quotients
A variety of slope quotient values were also calculated for the toxicity data. Slopes of the x/y curves were determined at each exposure time for 3C2B-alone, MC-alone, and MX and for the predicted DA and I curves. Slope quotient values for each single-chemical and mixture versus DA and I were determined simply by dividing the observed slope by the predicted DA or I slope at the exposure time of interest.
Calculation of observed minus predicted TDT
By calculating TDT for each single-chemical and mixture at each effect-level and time-interval it was possible to compare observed mixture TDT (MX TDTobs) with predicted mixture TDT (MX TDTpred) from the single-chemical data (Dawson et al. 2014a). The MX TDTobs value for a given experiment was its mTDT15:45 value. The MX TDTpred value for that experiment was calculated using the single-chemical mTDT15:45 values for that experiment; calculated as follows:
Then observed minus predicted TDT for the mixture (MX TDTobs – pred) was calculated as:
Because MX TDTpred can be either greater-than or less-than the MX TDTobs, to accurately calculate the mean for MX TDTobs – pred values, the absolute values were used.
Data Quality Assessments and Statistical Procedures
The quality of x/y data was evaluated in two ways. Fitting of x/y data using the 5PL-1P function was assessed by calculating coefficient of determination (r2) values. Test-to-test consistency for ECx, EC25+50+75, slope, slope quotient, s, and the combined effects metrics (i.e., MX/DA, MX/I, AQ50, and IQ50) was evaluated by calculating the coefficient of variation (CV) using:
This approach has been noted to be preferable to using the standard deviation (s.d.) when data are collected by multiple operators (Steel and Torrie 1980). For TDT data, since some effect-level and time-interval TDT values for MC were negative, s.d. of mean values were directly used to evaluate test-to-test consistency.
For assessing statistical significance, data means were evaluated differently depending on whether the mean values of interest were paired or consisted of more than two groups. For pairwise comparisons the t-test was used unless the Shapiro-Wilk Normality Test failed; in the latter instances the Mann-Whitney Rank Sum Test was used to evaluate median values. For comparison of means across three groups, One-Way Analysis of Variance (ANOVA) was used. When there were significant differences among groups and the data were normally distributed, the Holm-Sidak multiple-comparison test was used. When the data were not normally distributed, the Kruskal-Wallis One-Way ANOVA on Ranks test was used to evaluate median values. All statistical procedures were conducted within SigmaPlot. Statistical significance was set at p < 0.05.
Results
Concentration-response (x/y) data
Six experiments were conducted in the study with each experiment including a test of 3C2B-alone, MC-alone and MX. The x/y data for each experiment are provided in the supplementary data tables (S1–S6).
Single-Chemical Toxicity Data
For each experiment, the EC25, EC50, and EC75 values were calculated for 3C2B-alone and MC-alone after 15, 30 and 45-min of exposure (Table 1). At each effect-level, toxicity increased upon longer exposure for both chemicals, as noted by the lower ECx values at longer exposure times. For each chemical, effect-level, and exposure time the coefficient of variation (CV) for mean values was <20.0 (Table 1), ranging from a low of 5.6 (3C2B 15-min EC50) to a high of 17.7 (MC 45-min EC25).
Table 1.
Consistencya of ECx toxicity data (mg/L) for 3C2B and MC tested alone
| 15-min |
30-min |
45-min |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment # | EC25 | EC50 | EC75 | EC25 | EC50 | EC75 | EC25 | EC50 | EC75 |
| 3C2B – 1 | 7.9 | 21.5 | 56.2 | 5.3 | 14.1 | 35.4 | 4.2 | 10.9 | 26.6 |
| 3C2B – 2 | 6.9 | 19.7 | 52.0 | 4.8 | 13.5 | 34.3 | 3.7 | 10.4 | 25.9 |
| 3C2B – 3 | 6.4 | 18.1 | 47.8 | 4.6 | 13.0 | 32.8 | 3.6 | 10.2 | 25.4 |
| 3C2B – 4 | 7.1 | 19.9 | 54.1 | 5.4 | 15.1 | 39.1 | 4.7 | 12.6 | 31.1 |
| 3C2B – 5 | 6.7 | 19.7 | 54.3 | 5.5 | 15.4 | 39.0 | 4.6 | 12.8 | 31.2 |
| 3C2B – 6 | 7.0 | 20.2 | 55.4 | 5.8 | 15.6 | 40.5 | 5.2 | 13.0 | 32.1 |
| mean | 7.0 | 19.8 | 53.3 | 5.2 | 14.4 | 36.8 | 4.4 | 11.6 | 28.7 |
| CV | 7.1 | 5.6 | 5.7 | 8.2 | 7.6 | 8.5 | 14.1 | 10.9 | 10.6 |
| MC – 1 | 83.2 | 245.1 | 610.4 | 77.5 | 204.4 | 456.6 | 74.2 | 193.8 | 407.2 |
| MC – 2 | 70.0 | 211.6 | 541.9 | 68.7 | 184.5 | 421.0 | 70.3 | 183.5 | 385.9 |
| MC – 3 | 70.8 | 221.1 | 554.5 | 67.9 | 193.7 | 436.7 | 67.4 | 187.2 | 395.8 |
| MC – 4 | 54.7 | 175.1 | 461.5 | 49.5 | 146.6 | 342.9 | 50.2 | 140.5 | 302.8 |
| MC – 5 | 59.8 | 182.4 | 469.7 | 59.0 | 155.1 | 348.4 | 51.2 | 138.3 | 297.0 |
| MC – 6 | 63.3 | 202.7 | 502.6 | 53.1 | 156.9 | 360.5 | 52.6 | 141.9 | 304.2 |
| mean | 66.9 | 206.3 | 523.4 | 62.6 | 173.5 | 394.4 | 61.0 | 164.2 | 348.8 |
| CV | 15.0 | 12.5 | 10.8 | 16.9 | 13.7 | 12.6 | 17.7 | 16.1 | 15.1 |
mean and coefficient of variation (CV) values obtained from data calculated to four decimal places
Mixture and predicted dose-addition and independence curve data
As with the single-chemical tests, effect-level toxicity values were calculated at each exposure time for MX data. Toxicity increased with increased exposure time at each effect-level (Table 2: columns 3, 6, and 9). As with the single-chemical data, CV values for MX data were always <12.0 (Table 2), ranging from a low of 10.2 (15-min EC25) to a high of 11.8 (15-min EC50).
Table 2.
Consistencya of experimental (mg/L) mixture (MX) toxicity and predicted (mg/L) mixture toxicity for the dose-addition (DA) and independence (I) models
| EC25 |
EC50 |
EC75 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Experiment # | MX | DA | I | MX | DA | I | MX | DA | I |
| 15-min | MX – 1 | 3.9 | 4.5 | 4.1 | 11.5 | 12.6 | 11.0 | 31.5 | 32.4 | 25.4 |
| MX – 2 | 3.6 | 3.9 | 3.4 | 11.0 | 11.3 | 9.6 | 29.8 | 29.4 | 22.7 | |
| MX – 3 | 3.4 | 3.7 | 3.2 | 10.0 | 10.9 | 9.2 | 27.6 | 28.3 | 22.0 | |
| MX – 4 | 3.5 | 3.8 | 3.3 | 10.4 | 11.4 | 9.5 | 28.9 | 30.6 | 22.9 | |
| MX – 5 | 3.0 | 3.3 | 2.9 | 8.9 | 9.9 | 8.3 | 24.9 | 26.3 | 19.7 | |
| MX – 6 | 3.0 | 3.1 | 2.6 | 8.5 | 9.4 | 7.8 | 24.1 | 24.4 | 18.8 | |
| mean | 3.4b | 3.7b | 3.2b | 10.1c | 10.9c | 9.3c | 27.8d | 28.6d | 21.9e | |
| CV | 10.2 | 13.3 | 15.0 | 11.8 | 10.7 | 12.2 | 10.3 | 10.2 | 10.8 | |
| 30-min | MX – 1 | 3.3 | 3.4 | 3.2 | 9.2 | 9.1 | 8.4 | 22.8 | 21.9 | 18.6 |
| MX – 2 | 2.7 | 3.1 | 2.8 | 8.5 | 8.5 | 7.7 | 21.8 | 20.8 | 17.4 | |
| MX – 3 | 2.7 | 3.0 | 2.6 | 8.1 | 8.4 | 7.4 | 20.7 | 20.5 | 17.2 | |
| MX – 4 | 2.9 | 3.1 | 2.7 | 8.4 | 9.0 | 7.8 | 21.5 | 22.4 | 18.2 | |
| MX – 5 | 2.5 | 2.9 | 2.7 | 7.1 | 8.0 | 7.3 | 18.3 | 19.2 | 16.1 | |
| MX – 6 | 2.6 | 2.6 | 2.3 | 7.0 | 7.3 | 6.4 | 17.6 | 17.6 | 14.5 | |
| mean | 2.8f | 3.0f | 2.7f | 8.1g | 8.4g | 7.5g | 20.5h | 20.4h | 17.0i | |
| CV | 10.5 | 9.5 | 11.2 | 10.6 | 8.1 | 8.7 | 10.1 | 8.6 | 8.7 | |
| 45-min | MX – 1 | 2.9 | 2.9 | 2.7 | 7.9 | 7.5 | 7.0 | 18.9 | 17.5 | 15.5 |
| MX – 2 | 2.3 | 2.6 | 2.4 | 7.3 | 7.2 | 6.6 | 18.3 | 16.8 | 14.9 | |
| MX – 3 | 2.2 | 2.5 | 2.2 | 6.9 | 7.1 | 6.3 | 17.2 | 16.8 | 14.7 | |
| MX – 4 | 2.6 | 2.9 | 2.6 | 7.5 | 7.9 | 7.1 | 18.4 | 18.6 | 16.1 | |
| MX – 5 | 2.2 | 2.5 | 2.2 | 6.2 | 6.9 | 6.2 | 15.2 | 15.9 | 13.8 | |
| MX – 6 | 2.2 | 2.4 | 2.4 | 5.9 | 6.3 | 6.0 | 14.0 | 14.5 | 12.6 | |
| mean | 2.4j | 2.7j | 2.4j | 6.9k | 7.2k | 6.5k | 17.0l | 16.7l | 14.6l | |
| CV | 11.0 | 7.8 | 8.6 | 11.2 | 7.7 | 7.0 | 11.6 | 8.5 | 8.4 | |
mean and coefficient of variation (CV) values were obtained from data to four decimal places
grouped means with the same designation were not significantly different (one-way ANOVA, p > 0.05)
means at these exposure times were significantly different (Holm-Sidak test, p < 0.05)
To assess mixture toxicity against the DA and I models, predicted DA and I curves were calculated from the single-chemical data. Predicted toxicity values (presented as 3C2B-equivalent concentrations in mg/L) also showed increased toxicity with increased exposure time (Table 2: columns 4, 7, and 10 for DA; columns 5, 8 and 11 for I). For both DA and I, CV values at each effect-level and exposure time were ≤15.0.
Mean toxicity data were evaluated for statistical differences (Table 2). Mean values for MX and DA showed no significant differences at any exposure time. Mean I values were not significantly different from those for MX and DA at the EC25 and EC50 at any exposure time. However, mean I values at the EC75 were significantly different than the respective EC75 means for MX and DA at 15 and 30-min of exposure.
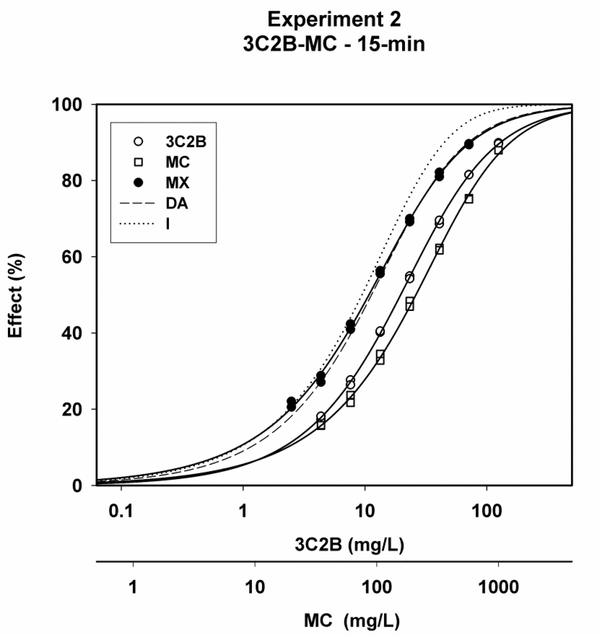
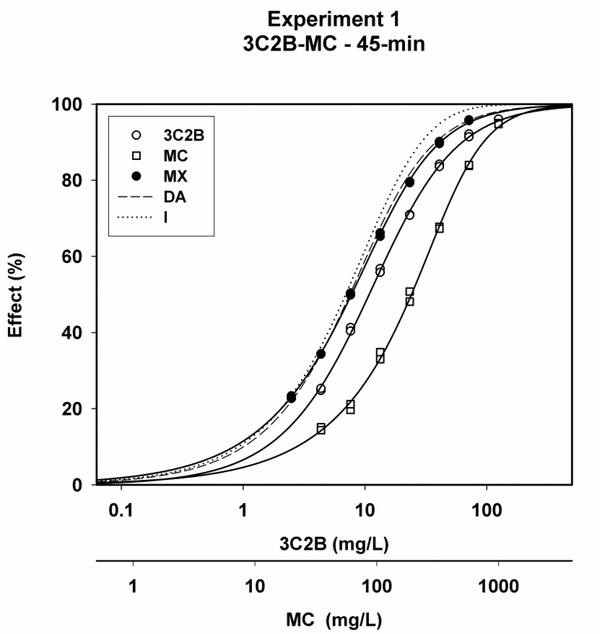
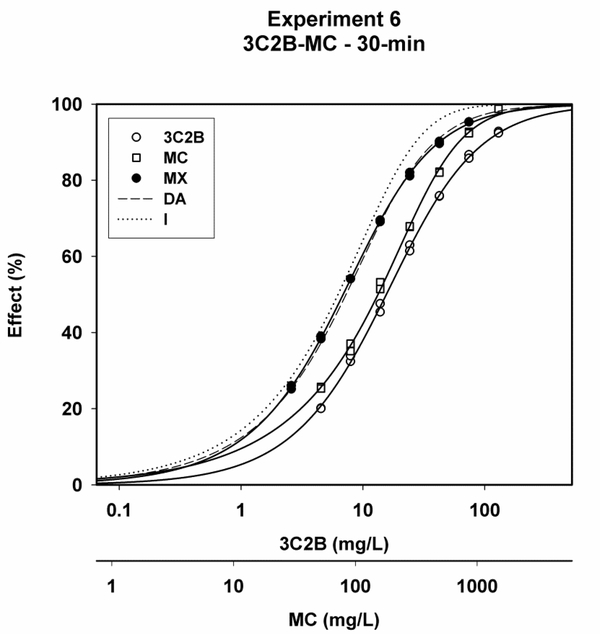
Representative plots of x/y data for 3C2B, MC, MX, and the predicted DA and I curves showed that MX curves plotted more closely to the predicted DA curves than to the predicted I curves (Figs. 1–3).
Figure 1.
Concentration-response curves for 3-chloro-2-butanone (3C2B), methyl crotonate (MC) and the 3C2B-MC mixture (MX) after 15-min of exposure in experiment #2, along with predicted curves for the dose-addition (DA) and independence (I) combined effects models. Experimental MX toxicity was better aligned visually with the predicted DA curve than the predicted I curve.
Figure 3.
Concentration-response curves for 3-chloro-2-butanone (3C2B), methyl crotonate (MC) and the 3C2B-MC mixture (MX) after 45-min of exposure for experiment #1, along with predicted curves for the dose-addition (DA) and independence (I) combined effects models.
Slope, asymmetry (s) and coefficient of determination (r2) values
As an important element of 5PL-1P curve-fitting, slope values were recorded for 3C2B, MC, and MX at each effect-level and exposure time (Supplementary Table S7: columns 2–4). Individual slope values tended to increase upon increased exposure time for the single-chemicals and mixtures; as did the mean values. Standard deviations for mean slope at 15, 30 and 45-min were ±0.05, ±0.05, and ±0.08, respectively for 3C2B, ±0.11, ±0.10, and ±0.09, respectively for MC and ±0.08, ±0.08, and ±0.07, respectively for MX. Resulting CV values for slope means were <10.0, ranging from 3.7–7.2. Slopes for MC were significantly different from those for 3C2B and MX at each exposure time, but slopes were only significantly different between 3C2B and MX at 45-min (Holm-Sidak test, p <0.05).
Asymmetry (s) values were not equal to 1.0, thereby indicating that plotted x/y data were not symmetrical (Supplementary Table S7: columns 5 – 7). Mean and CV values for s were calculated for each chemical and mixture at each effect-level and exposure time. All mean s values were <1.0 and CV values ranged from 7.7 to 24.1. Median or mean s values were significantly different between MC and both 3C2B and MX at 15-min (Tukey test, p <0.05) and 30-min (Holm-Sidak test, p <0.05), respectively. At 45-min, the mean s values were significantly different between 3C2B and MC (Holm-Sidak test, p <0.05) but not between MC and MX or 3C2B and MX (Holm-Sidak test, p ≥0.05).
To assess the quality of x/y data curve-fitting with the 5PL-1P function, r2 values were determined for all tests at each exposure time (Supplementary Table S7: columns 8–10). Individual test r2 values ranged from 0.9971 to 0.9998. Mean r2 values were ≥0.9990 for the 3C2B and MX tests and ≥0.9982 for MC tests. In all cases CV values for the means were ≤0.07.
Time-dependent toxicity (TDT) data for individual tests
Individual TDT values were calculated for each test of 3C2B and MC at the 25, 50 and 75% effect-levels and each of the three exposure time-intervals: 15 to 30-min, 30 to 45-min and 15 to 45-min (Table 3). For 3C2B, individual TDT values were generally lower at 25% effect than at the 50 and 75% effect-levels for each time-interval. For MC, TDT also increased with increasing effect-level, but the increase was more marked than for 3C2B. By calculating the averages for TDT values across all six experiments, s.d. values at each effect level and time-interval were determined. These showed higher test-to-test variability for MC than for 3C2B. This was especially noted for the 30 to 45-min interval at TDT25 (7.7%±17.1). For 3C2B, the highest s.d. value for TDT data was also for the 30 to 45-min interval at TDT25 (50.7%±16.2).
Table 3.
Consistencya of individual test time-dependent toxicity (TDTx)b values for 3C2B, MC and their binary mixtures (MX)
| TDT25 |
TDT50 |
TDT75 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment # | 15–30 min | 30–45 min | 15–45 min | 15–30 min | 30–45 min | 15–45 min | 15–30 min | 30–45 min | 15–45 min |
| 3C2B – 1 | 64.9 | 61.0 | 69.3 | 69.1 | 68.6 | 74.3 | 74.0 | 74.8 | 79.1 |
| 3C2B – 2 | 61.2 | 66.3 | 68.9 | 63.5 | 67.6 | 70.7 | 68.2 | 73.3 | 75.3 |
| 3C2B – 3 | 55.3 | 64.8 | 64.9 | 56.5 | 64.0 | 65.4 | 62.9 | 67.2 | 70.2 |
| 3C2B – 4 | 48.4 | 36.9 | 50.3 | 47.6 | 50.3 | 54.8 | 55.6 | 61.1 | 63.7 |
| 3C2B – 5 | 37.7 | 48.6 | 48.0 | 43.4 | 51.2 | 52.6 | 56.2 | 59.9 | 63.7 |
| 3C2B – 6 | 34.9 | 26.9 | 37.3 | 45.0 | 51.1 | 53.6 | 53.9 | 62.5 | 63.3 |
| average | 50.4 | 50.7 | 56.4 | 54.2 | 58.8 | 61.9 | 61.8 | 66.5 | 69.2 |
| ± s.d. | 12.3 | 16.2 | 13.2 | 10.6 | 8.8 | 9.5 | 8.0 | 6.4 | 6.8 |
| MC – 1 | 13.7 | 12.6 | 16.2 | 33.2 | 15.5 | 31.4 | 50.4 | 32.4 | 49.9 |
| MC – 2 | 3.7 | -7.1 | -0.7 | 25.7 | 1.6 | 19.9 | 44.6 | 25.0 | 43.2 |
| MC – 3 | 8.1 | 2.2 | 7.1 | 24.8 | 10.1 | 23.0 | 42.5 | 28.1 | 42.9 |
| MC – 4 | 18.7 | -3.7 | 12.3 | 32.5 | 12.5 | 29.6 | 51.4 | 35.1 | 51.6 |
| MC – 5 | 2.7 | 39.8 | 21.7 | 30.0 | 32.6 | 36.3 | 51.6 | 44.3 | 55.2 |
| MC – 6 | 32.3 | 2.4 | 25.2 | 45.2 | 28.7 | 45.0 | 56.5 | 46.9 | 59.2 |
| average | 13.2 | 7.7 | 13.6 | 31.9 | 16.8 | 30.9 | 49.5 | 35.3 | 50.3 |
| ± s.d. | 11.1 | 17.1 | 9.5 | 7.4 | 11.7 | 9.1 | 5.1 | 8.7 | 6.5 |
| MX – 1 | 30.7 | 40.9 | 40.4 | 40.8 | 42.8 | 47.7 | 54.8 | 51.5 | 59.8 |
| MX – 2 | 48.5 | 49.3 | 55.1 | 45.7 | 44.9 | 51.6 | 54.0 | 48.4 | 58.1 |
| MX – 3 | 41.3 | 51.6 | 51.5 | 39.6 | 45.0 | 47.7 | 50.4 | 50.8 | 56.8 |
| MX – 4 | 31.7 | 41.0 | 41.0 | 38.4 | 34.8 | 42.9 | 50.9 | 44.6 | 54.8 |
| MX – 5 | 30.8 | 39.9 | 40.0 | 39.7 | 39.6 | 45.6 | 53.0 | 51.0 | 58.5 |
| MX – 6 | 31.7 | 40.4 | 40.8 | 35.9 | 48.1 | 46.7 | 53.7 | 62.0 | 62.9 |
| average | 35.8 | 43.9 | 44.8 | 40.0 | 42.5 | 47.0 | 52.8 | 51.4 | 58.5 |
| ± s.d. | 7.4 | 5.2 | 6.7 | 3.3 | 4.7 | 2.9 | 1.7 | 5.8 | 2.7 |
average and standard deviation (s.d.) values were obtained from data calculated to four decimal places
percentage-based TDTx values were calculated at the 25 (TDT25), 50 (TDT50), and 75% (TDT75) effect levels for each time-interval in each experiment
Mixture TDT was also determined at each effect-level and time-interval (Table 3). As with the single-chemicals, TDT increased with increased effect-level and was always highest for the 15 to 45-min interval. The highest s.d. value for MX was for the 15 to 30-min interval at TDT25 (35.8%±7.4). Variability in TDT was lower for MX than for either 3C2B or MC.
Mean effect-level and time-interval TDT values for individual tests
Individual TDT values for each of the six experiments were used to generate mean TDT (mTDT) values for each experiment – at both effect-level and time-interval (Supplementary Table S8). The trend for mTDT values showed increased mTDT with increased effect-level for 3C2B, MC and MX. The time-interval with the highest mTDT was 15 to 45-min for each. In addition for MC, mTDT for the 30 to 45-min interval (i.e., mTDT30:45) was lower than for mTDT15:30 and mTDT15:45.
Combined effects determination vs. the dose-addition and independence models
For each of the six MX tests, the sum of the 3C2B-equivalent concentrations at the EC25, EC50, and EC75 (i.e., EC25+50+75) were calculated at each exposure time (Table 4: column 3). These sums were also calculated for the predicted DA curve and the predicted I curve at each exposure time (Table 4: columns 4, 5). One-way analysis of variance and the Holm-Sidak multiple comparison test indicated that the mean EC25+50+75 values for I at 15-min and 30-min were significantly different than those for MX and DA, whereas there was no statistical difference in mean EC25+50+75 values between MX, DA and I at 45-min.
Table 4.
Consistencya of actual mixture (MX) vs. predicted dose-addition (DA) and independence (I) concentrations (mg/L) and combined effects values for the mixtures
| EC25+50+70b |
||||||||
|---|---|---|---|---|---|---|---|---|
| Time | Experiment # | MX | DA | I | MX/DAc | MX/Id | AQ50e | IQ50f |
| 15-min | MX – 1 | 46.95 | 49.50 | 40.52 | 0.95 | 1.16 | 0.91 | 1.05 |
| MX – 2 | 44.37 | 44.57 | 35.66 | 1.00 | 1.24 | 0.97 | 1.15 | |
| MX – 3 | 40.99 | 42.92 | 34.40 | 0.96 | 1.19 | 0.92 | 1.09 | |
| MX – 4 | 42.82 | 45.87 | 35.70 | 0.93 | 1.20 | 0.91 | 1.09 | |
| MX – 5 | 36.80 | 39.42 | 30.90 | 0.93 | 1.19 | 0.90 | 1.07 | |
| MX – 6 | 35.62 | 36.85 | 29.27 | 0.97 | 1.22 | 0.90 | 1.08 | |
| mean | 41.26g | 43.19g | 34.41h | 0.96i | 1.20j | 0.92k | 1.09l | |
| CV | 10.6 | 10.5 | 11.6 | 2.5 | 2.4 | 3.0 | 3.1 | |
| 30-min | MX – 1 | 35.35 | 34.38 | 30.22 | 1.03 | 1.17 | 1.01 | 1.09 |
| MX – 2 | 33.03 | 32.32 | 27.82 | 1.02 | 1.19 | 1.00 | 1.11 | |
| MX – 3 | 31.42 | 31.91 | 27.28 | 0.98 | 1.15 | 0.96 | 1.08 | |
| MX – 4 | 32.90 | 34.57 | 28.70 | 0.95 | 1.15 | 0.93 | 1.08 | |
| MX – 5 | 27.97 | 30.17 | 26.09 | 0.93 | 1.07 | 0.89 | 0.98 | |
| MX – 6 | 27.17 | 27.47 | 23.27 | 0.99 | 1.17 | 0.96 | 1.09 | |
| mean | 31.31m | 31.80m | 27.23n | 0.98i | 1.15j | 0.96k | 1.07l | |
| CV | 10.1 | 8.4 | 8.8 | 4.0 | 3.5 | 4.8 | 4.4 | |
| 45-min | MX – 1 | 29.68 | 27.88 | 25.26 | 1.06 | 1.18 | 1.06 | 1.12 |
| MX – 2 | 27.85 | 26.63 | 23.85 | 1.05 | 1.17 | 1.05 | 1.11 | |
| MX – 3 | 26.30 | 26.43 | 23.24 | 1.00 | 1.13 | 0.97 | 1.09 | |
| MX – 4 | 28.37 | 29.47 | 25.81 | 0.96 | 1.10 | 0.94 | 1.05 | |
| MX – 5 | 23.61 | 25.25 | 22.29 | 0.94 | 1.06 | 0.90 | 1.00 | |
| MX – 6 | 22.07 | 23.22 | 20.99 | 0.95 | 1.05 | 0.93 | 0.98 | |
| mean | 26.32o | 26.48o | 23.57o | 0.99i | 1.11j | 0.97k | 1.06l | |
| CV | 11.1 | 8.1 | 7.7 | 5.3 | 4.7 | 5.8 | 5.5 | |
mean and coefficient of variation (CV) values obtained from data to four decimal places
sum of the EC25, EC50, and EC75 values expressed as 3C2B-equivalent concentrations
MX/DA = MX EC25+50+75 / DA EC25+50+75 at a given exposure time
MX/I = MX EC25+50+75 /I EC25+50+75 at a given exposure time
dose-additivity quotient value at the EC50 only
independence quotient value at the EC50 only
grouped means with different letters were statistically different (Holm-Sidak test, p < 0.05)
mean MX/DA and MX/I values at that exposure time were statistically different (t-test, p ≤ 0.003)
mean AQ50 and IQ50 values at that exposure time were statistically different (t-test, p ≤ 0.02 or lower)
grouped EC25+50+75 means with different letters were statistically different (Holm-Sidak test, p < 0.05)
grouped EC25+50+75 means were not significantly different in one-way ANOVA
Calculating EC25+50+75 for MX, DA and I facilitated comparison of MX toxicity vs. the DA and I combined effects models using the MX/DA and MX/I metrics, respectively (Table 4: columns 6, 7). In each individual test, mixture toxicity was consistent with dose-addition at each exposure time, with MX/DA values being from 0.93–1.00 at 15-min, 0.93–1.03 at 30-min and 0.94–1.06 at 45-min. Mean MX/DA values were 0.96, 0.98 and 0.99 at those respective times with the CV for each being <6.0 (Table 4: column 6). However, individual MX/I values were consistent with independence (i.e., 0.90 ≤ MX/I ≤ 1.10) in only 4 of 18 cases over the three exposure times (Table 4: column 7). Mean MX/I values decreased across increased exposure time, from 1.20 at 15-min to 1.11 at 45-min, with CV values for MX/I means being <5.0 at each exposure time. Statistically at each exposure time, MX/DA mean values were significantly different from those for MX/I (t-test, p ≤0.003).
Determinations of mixture toxicity using AQ and IQ values at the 50% effect-level for the individual mixtures were made (Table 4: columns 8–9) for comparison with the MX/DA and MX/I metrics. At a given exposure time, both the individual and mean AQ50 and IQ50 values were most often lower than the respective MX/DA and MX/I values. Additionally, the AQ50 values were consistent with DA, with one exception at 30-min (MX–#5), and the IQ values were more frequently consistent with I than were the MX/I values (i.e., 14/18 for the former, 4/18 for the latter). At each exposure time, mean AQ50 and IQ50 values were significantly different from each other (t-test, p ≤0.02).
Predicted dose-addition and independence slope and slope quotient values
Slopes of predicted x/y curves for DA and I were determined at each exposure time (Supplementary Table S9: columns 3, 4). The mean DA slope increased from 1.32 to 1.47 to 1.57 as exposure time increased from 15 to 30 to 45-min, respectively. The mean I slope also increased with increased exposure time from 1.73 to 1.88 to 1.95. At each exposure time the difference in mean slope values for DA and I curves was statistically significant (p <0.001).
Slope quotient (SQ) values for 3C2B, MC and MX versus DA and I were determined at each exposure time (Supplementary Table S9: columns 5–10). The respective mean SQ values at 15, 30 and 45-min for 3C2B with DA or I changed little over exposure time. In contrast, the respective mean SQ values for both MC and MX with DA or I increased with increased exposure time. In all instances the differences in mean SQ values between DA and I at a given exposure time were statistically significant at p ≤0.002.
Observed vs. predicted TDT for the mixtures
Mixture TDT was determined for each experiment. A previous study demonstrated that, for binary combinations, averaging the 25, 50 and 75% effect-level TDT values for the individual chemicals over the 15 to 45-min interval could be used to predict MX TDT over the same interval (Dawson et al. 2014a). This was done in this study to determine test-to-test consistency of the prediction (Supplementary Table S10). Observed MX TDT15:45 varied from 46.2–54.9% (mean = 50.1±3.1), while predicted MX TDT15:45 was from 43.7–53.4% (mean 47.1±3.3). Values for observed minus predicted TDT15:45 (TDTobs – pred) were from −4.1% to +8.7% (mean = 4.4%±2.7).
Mean and s.d. values for TDTobs – pred values obtained for the six MX tests in this study were compared with those for chemicals with irreversible toxicity (i.e., TDT ≥ 100%; Supplementary Table S10). The data for six combinations of agents having irreversible toxicity were taken from a previous study (Dawson et al., 2014a). In the current study, as noted above, TDTobs – pred ranged from −4.1 to +8.7% while a tighter range (−1.4% to +4.5%) and a smaller mean value (1.7%±2.0) for TDTobs – pred were observed for the selected combinations in which both chemicals had irreversible toxicity.
Discussion
Consistency of single-chemical toxicity data
Test-to-test consistency of single-chemical toxicity data was assessed by CV values at each effect-level for each exposure time (Table 1). Calculating the CV allows one to relate the variation observed for one chemical to that of another. In this study, these values were always <20 for both 3C2B and MC, well within the acceptable range for aquatic toxicity tests as CV values were below the upper limit of 40 for intra-laboratory variability (Moore et al. 2000; Hagen et al. 2009, Piazza et al. 2012). Previous studies with this system have reported CVs for the EC50 similar to those obtained herein (Dawson et al. 2014b, 2016). While CVs tended to be slightly higher for the EC25 than for the EC50 or EC75, they were similar.
Consistency of mixture and predicted dose-addition and independence curve data
In addition to that assessed for single-chemical toxicity, test-to-test consistency was evaluated for MX and the predicted DA and I data. In all cases, CV values were ≤15.0 (Table 2) and those at the EC25 tended, again, to be slightly higher than those for the higher effect-levels. As with the single-chemical data, the mixture data were considered to have acceptable test-to-test consistency, since all CVs were <40.
Consistency of slope, asymmetry (s) and coefficient of determination (r2) values
Slope CV values were <8 for 3C2B, MC, MX and the predicted DA and I curves at each effect-level and exposure time (Supplementary Tables S7, S9). It is not clear from the literature what CV values for slope are considered to be acceptable in examining test-to-test consistency of a toxicity assay, as these are not often reported. However, by examining the standard deviations for mean values, they were less-than ±10% of the slope value. Calculating CVs is a simple way to put standard deviation values on a percentage basis to facilitate comparisons from lab-to-lab and study-to-study.
For asymmetry data, CVs were as high as 24.1 (Supplementary Table S7) suggesting more variability than that observed for ECx and slope. This is not surprising as s values below 1.0 indicate that x/y data at lower effect-levels are less symmetrical than at higher effect-levels. In this system, generally, for a given test there tended to be greater variability in replicate responses at lower concentrations than at higher concentrations.
Quality of fitting x/y data using the 5PL-1P function was assessed by r2 values. The x/y data for 3C2B, MC and MX at each effect-level and exposure time were well-fitted using 5PL-1P with mean r2 values being ≥0.9982. The s.d. values for mean r2 data were from ±0.0002 to ±0.0007 and CVs were ≤0.07, both indicating minimal variation in quality of fitting the x/y data.
Consistency of time-dependent toxicity (TDT) data
As noted above (Methods), calculating CV values for TDT data would be misleading in instances where toxicity actually decreased (i.e., TDT <0%) in a given time-interval. Therefore, since MC had some instances of negative TDT, s.d. values were directly used to assess test-to-test consistency of TDT data – for both the individual tests (Table 3) and for mean effect-level and time-interval values within each individual test (Supplementary Table S8). These s.d. values tended to be higher at the EC25 than at the higher effect-levels. Overall, they were lowest for MX and highest for MC. If it is the case that chemicals having mid-range TDT exert toxicity that is partly due to irreversible toxicity and partly due to reversible effects, it is likely that variability in the irreversible toxic effects associated with a chemical or mixture would be observed most prominently at lower effect-levels where those effects may just be beginning to be manifested. For MC, especially from 30 to 45-min, the variation was sizable but worst at the EC25, for which actual TDT was <10% in four tests and negative (i.e., decreased toxicity) for two of those four. It is also likely that from one test to the next, there is variability in the time it takes for the bacteria to become fully metabolically active, thereby affecting TDT.
By examining s.d. values for mean TDT of each individual test across the six experiments, test-to-test consistency was deemed acceptable even though the variability in individual tests of MC was rather high in the instances noted above. It was noted that the MX generally had lower s.d. for TDT than did either 3C2B or MC alone. The reason for this result is unclear.
Consistency of combined effects vs. the dose-addition and independence models
By summing the 3C2B and the 3C2B-equivalent concentrations for MC, it was possible to compare the sum of the chemical concentrations at the 25, 50 and 75% effect-levels (i.e., EC25+50+75) in MX testing with the predicted values for DA and I (Table 4). Across the six experiments CVs for mean EC25+50+75 were <12, so test-to-test consistency was deemed likely to be acceptable; although this is the first time this calculation has been made.
The EC25+50+75 values served as the basis for calculating two combined effects metrics for mixture toxicity versus the DA (i.e., MX/DA) and I (i.e., MX/I) models. For these metrics, at each exposure time CVs were <6.0 suggesting acceptable test-to-test variability for mixture toxicity. For DA, MX/DA values were consistent with DA (i.e., 0.90 ≤ MX/DA ≤ 1.10) in each test at each exposure time. Based on the MX/I metric, mixture toxicity was generally not consistent with I.
Statistical analyses supported the combined effects findings. First, the mean EC25+50+75 values were not significantly different between MX and DA at any of the exposure times, but those values for MX and DA were significantly different from those for I at 15 and 30 minutes. Also, MX/DA values were significantly different from MX/I values at each respective exposure time, as were AQ50 vs. IQ50 values.
As noted in previous studies with this system (Dawson et al. 2014b, 2016), the results obtained herein support the use of the MX/DA and MX/I metrics in preference to AQ50 and IQ50 values. The former provide relevant information on closeness of mixture toxicity to the chosen model within the range from 25 to 75% effect, rather than just at 50% effect. This conclusion was drawn because the actual MX curve can be close to that for a given model at 50% effect but not necessarily at lower or higher effect-levels (e.g., Dawson et al. 2016, Figs. 3–4).
Consistency of slope quotient data
Although x/y curve slope and AQ and IQ slope quotient (SQ) values have been noted in previous studies (Dawson et al. 2010, 2011), they have not yet received scrutiny as to their value in mixture toxicity assessment. They are presented here to provide a baseline for forthcoming reports, as slope CVs for 3C2B, MC, MX, DA and I were always <8.0 and across the six experiments, CVs for SQ values were always ≤8.5 (Supplementary Tables S7, S9).
Consistency of observed vs. predicted TDT for the mixtures
Time-dependent toxicity (TDT) of the individual chemicals has been shown to be useful for predicting TDT of the mixtures (Dawson et al. 2014a). For the six 3C2B-MC mixture experiments in this study, values for MX TDTobs were always within 10% of the predicted values (i.e., MX TDTpred; see Supplementary Table S10) with the standard deviation being ±2.7%. The range was only slightly larger than that for a series of six selected combinations in which both chemicals had irreversible toxicity. Likewise the mean for MX TDTobs – pred values for 3C2B-MC mixtures was only slightly higher than that for chemical combinations that induced only irreversible toxic effects. Since chemicals having both reversible and irreversible toxicity were expected to have greater variability than those having only irreversible toxicity, the results suggest that this time-dependence mixture toxicity assay is repeatable and can be useful as part of risk assessment.
Conclusions
Consistency of the toxicity results across the six experiments was within the acceptable range, based on CV values for ECx, EC25+50+75, slope, s, and the four combined effects metrics. For TDT, higher levels of variation in testing were observed for MC at the EC25 and the 30 to 45-min time-interval. For 3C2B and MX, TDT values showed less variability across the effect-levels and time-intervals evaluated. Despite the higher TDT variability for MC, the combined effects metric MX/DA was consistent with predicted effects for the DA model for all 18 assessments (i.e., at each of the three exposure times for each of the six experiments). The results of this study suggest that this simple assay is a useful approach for examining mixture toxicity on a time-dependent basis. Additionally, the substantial amount of data generated in testing could prove useful in risk assessment.
Supplementary Material
Figure 2.
Concentration-response curves for 3-chloro-2-butanone (3C2B), methyl crotonate (MC) and the 3C2B-MC mixture (MX) after 30-min of exposure for experiment #6, along with predicted curves for the dose-addition (DA) and independence (I) combined effects models.
Acknowledgments:
This study was made possible in part by grants 2 R15 ES08019 – 03/04 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). Its content is solely the responsibility of the investigators and does not represent the official views of NIEHS, NIH.
Footnotes
Disclosure Statement: No potential conflict of interest was reported by the authors.
References
- Altenburger R, Ait-Aissa S, Antczak P, et al. (2015). Future water quality monitoring — Adapting tools to deal with mixtures of pollutants in water resource management. Sci Total Environ 512–513:540–551 [DOI] [PubMed] [Google Scholar]
- Balistrieri LS, Mebane CA, Schmidt TS, Keller W (2015) Expanding metal mixture toxicity models to natural stream and lake invertebrate communities. Environ Toxicol Chem 34:761–776 [DOI] [PubMed] [Google Scholar]
- Belden JB, Gilliom RJ, Lydy MJ (2007) How well can we predict the toxicity of pesticide mixtures to aquatic life? Integ Environ Assess Manag 3:364–372 [PubMed] [Google Scholar]
- Białk-Bielińska A, Caban M, Pieczyńska A, Stepnowski P (2017) Mixture toxicity of six sulfonamides and their two transformation products to green algae Scenedesmus vacuolatus and duckweed Lemna minor. Chemosphere 173:542–550 [DOI] [PubMed] [Google Scholar]
- Bliss CI (1939) The toxicity of poisons applied jointly. Ann Appl Biol 26:585–615 [Google Scholar]
- Dawson DA, Allen EMG, Allen JL, Baumann HJ, Bensinger HM, Genco N, Guinn D, Hull MW, Il’Giovine ZJ, Kaminski CM, Peyton JR, Schultz TW, Pöch G (2014a) Time-dependence in mixture toxicity prediction. Toxicology 326:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Allen JL, Schultz TW, Pöch G (2008) Time-dependence in mixture toxicity with soft electrophiles: 2. Effects of relative reactivity level on time-dependent toxicity and combined effects for selected Michael acceptors. J Environ Sci Health Part A 43:43–52. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Genco N, Bensinger H, Guinn D, Il’Giovine Z, Schultz TW, Pöch G (2012) Evaluation of an asymmetry parameter for single-chemical and mixture toxicity assessment. Toxicology 292:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Guinn D, Pöch G (2016) Evaluation of time-dependent toxicity and combined effects for a series of mono-halogenated acetonitrile-containing binary mixtures. Toxicol Rep 3:572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Pöch G, Schultz TW (2014b) Mixture toxicity of SN2-reactive soft electrophiles: 3. Evaluation of ethyl α-halogenated acetates with α-halogenated acetonitriles. Arch Environ Contam Toxicol 66:248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Jeyaratnam J, Mooneyham T, Pöch G, Schultz TW (2010) Mixture toxicity of SN2-reactive soft electrophiles: 1. Evaluation of mixtures containing α-halogenated acetonitriles. Arch Environ Contam Toxicol 59:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Mooneyham T, Jeyaratnam J, Schultz TW, Pöch G (2011) Mixture toxicity of SN2-reactive soft electrophiles: 2-Evaluation of mixtures containing ethyl α-halogenated acetates. Arch Environ Contam Toxicol 61:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Ploessl F, Bracher F, Laforsch C (2010) Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in Daphnia magna – A multigenerational study. Chemosphere 79:60–66 [DOI] [PubMed] [Google Scholar]
- Gagan EM, Hull MW, Schultz TW, Pöch G, Dawson DA (2007) Time dependence in mixture toxicity with soft electrophiles: 1. Combined effects of selected SN2 and SNAr-reactive agents with a nonpolar narcotic. Arch Environ Contam Toxicol 52:283–293 [DOI] [PubMed] [Google Scholar]
- Hagen T, Allinson G, Wightwick A, Nugegoda D, (2000) Assessing the performance of a bdelloid rotifer Philodina acuticornisodiosa—acute toxicity assay. Bull Env Contam Tox 82:285–289 [DOI] [PubMed] [Google Scholar]
- Houtman CJ, Kroesbergen J, Lekkerkerker-Teunissen K, van der Hoek JP (2014) Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data. Sci Total Environ 496:54–62 [DOI] [PubMed] [Google Scholar]
- Kim J, Kim S, Schaumann GE (2013) Reliable predictive computational toxicology methods for mixture toxicity: toward the development of innovative integrated models for environmental risk assessment. Rev Environ Sci BioTech 12:235–256 [Google Scholar]
- Landrum PF, Chapman PM, Neff J, Page DS (2012) Evaluating aquatic toxicity of complex organic chemical mixtures: Lessons learned from polycyclic aromatic hydrocarbons and petroleum case studies. Integr Environ Assess Manage 8:217–230 [DOI] [PubMed] [Google Scholar]
- Lopes S, Pinheiro C, Soares AMVM, Louriero S (2016) Joint toxicity prediction of nanoparticles and ionic counterparts: Simulating toxicity under a fate scenario. J Hazard Mat 320:1–9 [DOI] [PubMed] [Google Scholar]
- Moore DRJ, Warren-Hicks W, Parkhurst BR, Teed RS, Baird RB, Berger R, Denton DL, Pletl JJ (2000) Intra- and inter-treatment variability in reference toxicant tests: implications for whole effluent toxicity testing programs. Env Tox Chem 19:105–112 [Google Scholar]
- Muthusamy S, Peng C, Ng JC (2016) Effects of binary mixtures of benzo[ a ]pyrene, arsenic, cadmium, and lead on oxidative stress and toxicity in HepG2 cells. Chemosphere 165:41–51 [DOI] [PubMed] [Google Scholar]
- Nowell LH, Norman JE, Moran PW, Martin JD, Stone WW (2014) Pesticide Toxicity Index – A tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. Sci Total Environ 476–477:144–157 [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Nowell LH, Gilliom RJ, Nakagaki N, Murray K, VanAlstyne C (2010) Composition, distribution, and potential toxicity of organochlorine mixtures in bed sediments of streams. Sci Total Environ 408:594–606 [DOI] [PubMed] [Google Scholar]
- Piazza V, Ferioli A, Giacco E, Melchiorre N, Valenti A, DelPrete F, Biandolino F, Dentone L, Frisenda P, Faimali M (2012) A standardization of Amphibalanus (Balanus) amphitrite (Crustacea, Cirripedia) larval bioassay for ecotoxicological studies. Ecotox Environ Saf 79:134–138 [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH (1980) Principles and procedures of statistics: A biometrical approach, 2nd ed. McGraw-Hill, New York [Google Scholar]
- Tipping E, Lofts S (2013) Metal mixture toxicity to aquatic biota in laboratory experiments: Application of the WHAM-FTOX model. Aquat Toxicol 142–143:114–122 [DOI] [PubMed] [Google Scholar]
- Toccalino PL, Norman JE, Scott JC. (2012) Chemical mixtures in untreated water from public-supply wells in the U.S. - Occurrence, composition, and potential toxicity. Sci Total Environ 431:262–270 [DOI] [PubMed] [Google Scholar]
- Urbanczyk H, Ast JC, Higgins MJ, Carson J, Dunlap PV (2007) Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida, and Vibrio wodanis as Aliivibrio fischeri gen. nov. comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov., and Aliivibrio wodanis comb. nov. Int J Syst Evol Microbiol 57:2823–2829 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Tamura I, Abe R, Takanobu H, Nakamura A, Suzuki T, Hirose A, Nishimura T, Tatarazako N (2016) Chronic toxicity of an environmentally relevant mixture of pharmaceuticals to three aquatic organisms (alga, daphnid, and fish). Environ Toxicol Chem 35:996–1006 [DOI] [PubMed] [Google Scholar]
- Webster TF (2013) Mixtures of endocrine disruptors: How similar must mechanisms be for concentration addition to apply? Toxicology 313:94–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.