Abstract
Peripheral artery disease (PAD) is characterized by functional and vascular impairments as well as elevated levels of inflammation which are associated with reduced nitric oxide (NO) bioavailability. Inorganic nitrate supplementation boosts NO bioavailability potentially improving functional and vasodilatory capacities and may reduce inflammation. Twenty-one patients with PAD were randomly assigned to sodium nitrate (NaNO3) or placebo supplementation groups for eight-weeks. Outcome measures included a six-minute walk test (6MWT), blood flow and vasodilator function in the forearm and calf, as well as plasma inflammatory and adhesion biomarker concentrations. NaNO3 elevated plasma nitrate (32.3±20.0 to 379.8±204.6μM) and nitrite (192.2±51.8 to 353.1±134.2nM), improved 6MWT performance (387±90 to 425±82m), peak calf blood flow (BFPeak; 11.6±4.9 to 14.1±5.1mL/dL tissue/min), and peak calf vascular conductance (VCPeak; 11.1±4.3 to 14.2±4.9mL/dL tissue/min/mmHg) (p<0.05 for all). Improvements in calf BFPeak (r=0.70, p<0.05) and VCPeak (r=0.61, p<0.05) correlated with changes in 6MWT distance. Placebo supplementation did not change plasma nitrate or nitrite, 6MWT, calf BFPeak, or calf VCPeak. Forearm vascular function nor inflammatory and adhesion biomarker concentrations changed in either group. Eight-weeks of NaNO3 supplementation improves vasodilatory capacity in the lower-limbs of patients with PAD, which correlated with improvement in functional capacity.
Keywords: peripheral artery disease, vascular function, exercise capacity, inorganic nitrate, nitric oxide
1. Introduction
Peripheral artery disease (PAD) is characterized by atherosclerotic stenosis of arteries supplying the extremities (32), associated with an increased risk of fatal cardiovascular events (10), and affects over eight million Americans (43). Phenotypically, PAD manifests in many ways (e.g., intermittent claudication, critical limb ischemia) and is associated with poor functional capacity (32). Functional limitations in patients with PAD are rooted in the mismatch of oxygen supply and demand induced by atherosclerosis; however, revascularization fails to restore functional capacity (41). Thus, it appears as if functional limitations in patients with PAD are likely more closely related to microvascular impairments than the degree of stenosis.
Regulation of microvascular blood flow involves complex interactions between vasodilatory and vasoconstrictor signaling (25). To this point, patients with PAD have impaired endothelium-dependent vasodilation (endothelial dysfunction) (2); due to, in part, reduced production or activity of nitric oxide (NO). Byproducts of NO metabolism (nitrate and nitrite) have been implicated as NO reservoirs through the nitrate-nitritenitric oxide pathway (26). This system operates in parallel to the classic L-arginine pathway but is differentially regulated as the nitrate-nitrite-nitric oxide pathway becomes more prolific during ischemia (36). Given the prevalence of ischemia in patients with PAD (32) and the low NO bioavailability in this patient population (33), supplementation of inorganic nitrate has significant potential to improve functional outcomes in patients with PAD.
Inorganic nitrate supplementation improves various health-related outcomes namely blood pressure and exercise capacity (36). Specifically, a single dose of inorganic nitrate acutely improves exercise tolerance in patients with PAD (20); however, longitudinal data supporting these findings are lacking. A recent study investigated the effects of sodium nitrite on brachial artery endothelial function and exercise capacity in patients with PAD (31). Albeit a novel study, vascular function was exclusively assessed in an upper-extremity conduit artery thus ignoring vascular function in the lower-limb. Thus, our primary aims were to investigate whether inorganic nitrate supplementation enhances limb blood flow, specifically in the lower-limbs, as well as vasodilatory and functional capacities in patients with PAD. We hypothesized that eight-weeks of sodium nitrate (NaNO3) supplementation would improve lower-limb blood flow and vasodilation, and increase total distance walked during a six-minute walk test (6MWT).
It is well known that patients with atherosclerotic disease express elevated levels of inflammation and adhesion biomarkers (22), which may in part explain the low bioavailability of NO in patients with PAD (8). Moreover, excessive inflammation and adhesion biomarkers are associated with poor functional capacity in patients with PAD (35). To this, NO has demonstrated an inhibitory-effect on several biomarkers of inflammation and adhesion (12, 18, 45). To our knowledge, translational work on this paradigm has yet been conducted. Therefore, a secondary aim of this study was to investigate if NaNO3 supplementation reduces circulating biomarkers of inflammation and adhesion in patients with PAD.
2. Materials and Methods
2.1. Subjects
Twenty-one patients (12 male/9 female, 72±10 years) with diagnosed PAD (classified as Fontaine Stage 1–2a, Rutherford 0–1) (34) were recruited from the University of Iowa’s Vascular Clinic into the study. This cohort of 21 patients were enrolled into a clinical trial (clinicaltrials.gov/ct2/show/NCT01983826) with several outcome measures; some of which, have previously been published by our group (23). Exclusion criteria were non-atherosclerotic vascular disease, critical limb ischemia, active ischemic ulceration, recent (six months) revascularization, symptomatic coronary artery disease, heart failure, renal disease, hypotension (systolic blood pressure < 90 mmHg), smoking or history of smoking within one-year, or the use of phosphodiesterase five inhibitor drugs. All women enrolled in the study were postmenopausal and not receiving hormone therapy. All subjects provided written informed consent approved by the University of Iowa’s Institutional Review Board.
2.2. Experimental design
A randomized, double-blind, placebo-controlled study design was employed using block randomization (block sizes of six with a 4:2 randomization). Subject randomization and supplement manufacturing were handled by a compounding pharmacy to ensure a true double-blinded study. Subjects were assigned to NaNO3 (1g/day, 8.5mM) or placebo (microcrystalline cellulose) groups for eight-weeks similar to previous works using sodium nitrite (15, 16, 31). Pre- and post-supplementation assessment protocols spanned two-days and were identical on both visits (described below). Subjects followed a low-nitrate diet 72 hours prior to all visits and abstained from exercise, alcohol use, as well as caffeine consumption 24 hours prior to visiting the laboratory. Subjects refrained from prescription medication use on the morning of all visits and arrived following an overnight fast. Furthermore, subjects did not take their respective supplement the morning of post-assessment to account for potential confounding influence of acute supplementation. Visit one consisted of venipuncture, ankle-brachial index assessment, and 6MWT. Vasodilator function was assessed on study day two as to avoid any confounding effects of acute exercise on blood flow.
2.3. Venous blood sampling
Venous blood samples were obtained from an antecubital vein for determination of plasma [nitrate], [nitrite], as well as inflammatory and adhesion biomarkers (listed below). Blood was collected in tubes containing ethylenediaminetetraacetic acid and immediately underwent centrifugation at 3,000 rpm for 15 minutes. Plasma was then aliquoted into Eppendorf tubes and frozen at −80°C for later analysis. Quantification of [nitrate] and [nitrite] were made by their addition to vanadium III chloride in hydrochloric acid at 90°C and to potassium iodide in acetic acid at room temperature, respectively, within 30 minutes of thawing using a Sievers chemiluminescence NO analyzer (NOA 280i; Sievers Instruments, Boulder, CO) as previously reported (6).
2.4. Inflammation and adhesion biomarkers
Inflammatory and adhesion biomarker concentrations were quantified using commercially available enzyme-linked immunosorbent assays (RnD Systems, Minneapolis, MN). Plasma samples were analyzed in triplicate for interleukin-six (IL-6), soluble vascular adhesion molecule-one (sICAM-1), soluble vascular adhesion molecule-one (sVCAM-1), and monocyte chemoattractant protein-one (MCP-1). Plate well optical densities were measured using a microplate reader and were wavelength-corrected per assay protocol. Values were compared to a standard curve created on the same plate. Intra- and inter-assay coefficients of variation were: 8.5 and 9.9% (IL-6), 2.1% and 5.5% (sICAM-1); 4.0% and 7.6% (sVCAM-1), 3.1% and 3.0% (MCP-1), respectively.
2.5. Functional capacity assessment
Functional capacity was assessed using a self-paced 6MWT per the American Thoracic Society (1) on a 30.5-meter long course with start and end points indicated by cones. Subjects walked from one cone to the other pivoting immediately after passing each one with total distance walked serving as the outcome measurement.
2.6. Venous occlusion plethysmography
Venous occlusion plethysmography (EC-6, D.E. Hokanson Inc. Indianapolis, IN) was used to determine endothelium-dependent reactivity in the left forearm and left calf (14). Baseline blood flow was measured by inflating the distal cuff (wrist or ankle) to a supra-systolic pressure (220 mmHg) then cyclically inflating the respective proximal cuff (upper arm or thigh) to 60 mmHg every 15 seconds for two minutes. Baseline blood flow was the average of all eight cycles. Following baseline, circulation to the experimental limb was arrested for five minutes by rapidly inflating the proximal cuffs to 240 mmHg inducing ischemia. Peak blood flow (BFPeak) was the highest measurement observed following deflation (reactive hyperemia). Total blood flow (BFTotal) for three minutes after cuff release was recorded and was defined as the area under the curve using the trapezium rule (28). Vascular conductance served as a surrogate of vasodilatory capacity and calculated as blood flow/mean arterial pressure (Nexfin, Edwards Lifesciences Irvine, CA). Peak and total vascular conductance (VCPeak and VCTotal, respectively) were determined in the same fashion as BFPeak and BFTotal, respectively. Blood pressure and plethysmography data were collected at 250 Hz and analyzed offline (WinDaq, DATAQ Instruments Akron, OH).
2.7. Statistical analyses
Data are expressed as mean±standard deviation, unless otherwise noted. Subject characteristics were assessed between groups via independent samples t-tests or a chi-squared test where appropriate. A two-way repeated measures analysis of variance was used to compare outcome variables between groups pre- and post-supplementation. When significant F-ratios were detected, pairwise comparisons were made using Tukey’s post hoc analysis with effect sizes calculated using Cohen’s D (d). Additionally, Pearson correlations were used to quantify the relationship between improvements in 6MWT distance and calf vascular function (BFPeak and VCPeak). All statistical analyses were deemed significant a priori at α<0.05 and completed using SigmaPlot version 11.0 (Systat Software Inc., San Jose, CA).
3. Results
3.1. Subjects’ Demographical Data
Subjects’ demographical data are displayed in Table 1 and may also be referenced in our previous works (23). No differences were observed between groups for any data. All subjects whom started the study completed pre- and post-supplementation visits consuming 98.7% of their respective supplement based upon pill bottles collected on the final study visit. None of the subjects reported experiencing adverse effects from supplementation, medication changes, or surgical procedures during post-supplementation study visits. Systolic blood pressure was reduced following NaNO3 (136±15 to 129±17 mmHg, p<0.05, d=0.21) but not placebo (132±13 to 132±12 mmHg, p=0.97) supplementation. Diastolic blood pressure was unchanged in NaNO3 (72±9 to 70±10 mmHg) and placebo (77±10 to 75±9 mmHg) groups (group-by-time interaction p=0.71). Additionally, ankle-brachial index was unchanged following supplementation of NaNO3 (0.76±0.21 to 0.86±0.21) and placebo (0.81±0.14 to 0.85±0.15, group-by-time interaction p=0.24)
Table 1.
Demographic and clinical characteristics of the PAD cohort.
| NaNO3 (n=13) | Placebo (n=8) | p-value | |
|---|---|---|---|
| Age (years) | 73±9 | 69±10 | 0.32 |
| Sex (Male/Female) | 6/7 | 6/2 | 0.20 |
| Body mass index (kg/m2) | 29.2±5.9 | 28.1±3.6 | 0.63 |
| Systolic blood pressure (mmHg) | 136±15 | 132±13 | 0.46 |
| Diastolic blood pressure (mmHg) | 72±9 | 77±10 | 0.30 |
| Ankle-brachial index | 0.76±0.21 | 0.81±0.14 | 0.57 |
| Previous revascularization | 12 (92) | 6 (75) | 0.34 |
| Coronary artery disease | 3 (23) | 0 (0) | 0.65 |
| Type II diabetes mellitus | 4 (31) | 2 (25) | 0.89 |
| Use of a statin | 12 (92) | 7 (88) | 0.78 |
| Use of ACE inhibitors or ARBs | 6 (46) | 2 (25) | 0.62 |
| Use of beta-blocker | 6 (46) | 3 (38) | 0.83 |
| Use of calcium channel blocker | 5 (38) | 2 (25) | 0.76 |
| Use of anticoagulants | 6 (46) | 2 (25) | 0.62 |
| Use of insulin | 2 (15) | 1 (13) | 0.97 |
Data are mean±standard deviation or n (%). NaNO3=sodium nitrate group; ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker. Groups were compared using an independent samples t-test or chi-squared analysis when appropriate.
3.2. Plasma nitrate and nitrite
NaNO3 supplementation increased plasma [nitrate] (32.3±20.0 to 379.8±204.6 μmol/L, p<0.05, d=2.36) and [nitrite] (192.2±51.8 to 353.1±134.2 nmol/L, p<0.05, d=1.52). Placebo supplementation did not change plasma [nitrate] (34.3±18.7 to 77.5±69.9 μmol/L, p=0.23) or [nitrite] (249.7±33.2 to 230.3±76.5 nmol/L, p=0.47). Subsequently, plasma [nitrate] and [nitrite] were higher following eight-weeks of NaNO3 than placebo (p<0.05 for both).
3.3. Blood flow and conductance
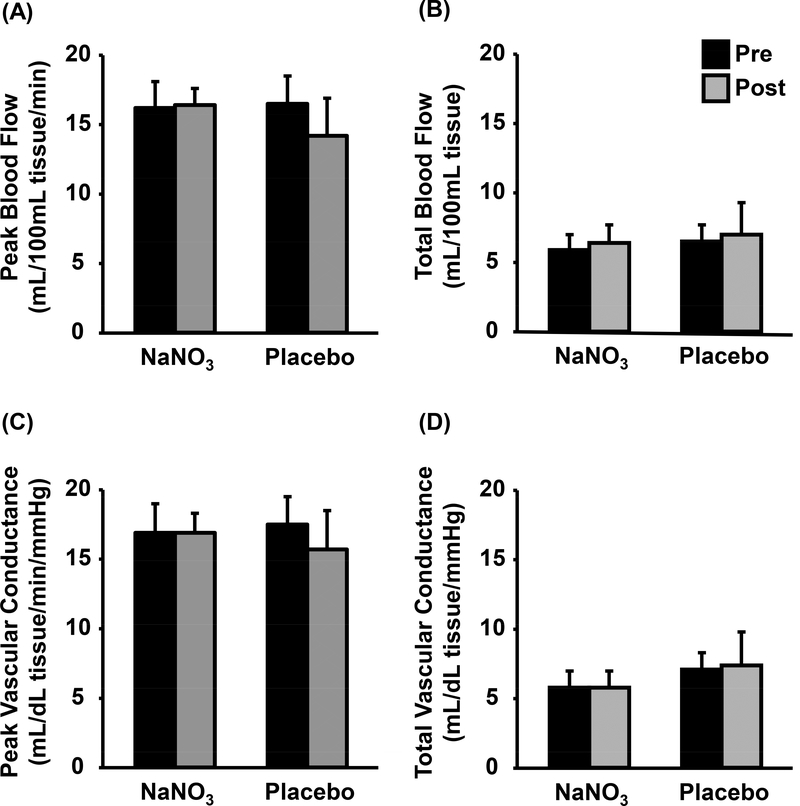
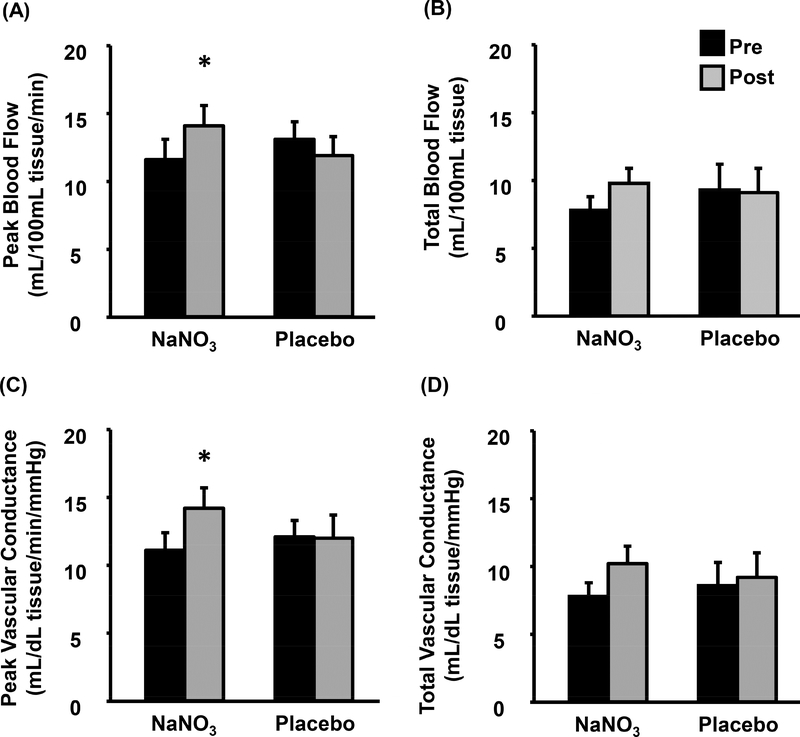
Forearm blood flow and vascular conductance results are displayed in Figure 1. No group-by-time interactions were observed in forearm BFPeak (p=0.22) or BFTotal (p=0.99). Likewise, forearm VCPeak and VCTotal were also unchanged following NaNO3 and placebo supplementation (group-by-time interactions p=0.45 and 0.90, respectively). Calf blood flow and vascular conductance are illustrated in Figure 2. Due to discomfort during thigh cuff inflation, testing was prematurely stopped for three subjects. Subsequently, data collected on the calf reflects 11 and seven subjects from the NaNO3 and placebo groups, respectively. Calf BFPeak increased following NaNO3 supplementation (11.6±4.9 to 14.1±5.1 mL/dL tissue/min, p<0.05, d=0.50) but was unchanged following placebo (13.1±3.5 to 11.9±3.7 mL/dL tissue/min, p=0.32). However, calf BFTotal was unchanged after NaNO3 and placebo supplementation (group-by-time interaction p=0.11). Similarly, calf VCPeak was increased following NaNO3 (11.1±4.3 to 14.2±4.9 mL/dL tissue/min/mmHg, p<0.05, d=2.21) but not placebo (12.1±3.2 to 12.0±4.6 mL/dL tissue/min/mmHg, p=0.92) supplementation whereas calf VCTotal was unchanged in both groups (group-by-time interaction p=0.18).
Figure 1.
Forearm blood flow (Peak [A] and Total [B]) and conductance (Peak [C] and Total [D]) obtained by venous occlusion plethysmography pre- (black) and postsupplementation (grey) of sodium nitrate (NaNO3) or placebo. Data presented as mean±standard error.
Figure 2.
Calf blood flow (Peak [A] and Total [B]) and conductance (Peak [C] and [D]) obtained by venous occlusion plethysmography pre- (black) and post-supplementation (grey) of sodium nitrate (NaNO3) or placebo. Data presented as mean±standard error. *p<0.05 vs. pre-supplementation.
3.4. Functional capacity
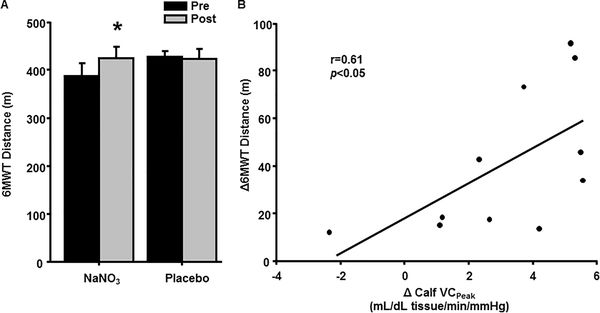
As shown in Figure 3A, patients in the NaNO3 group increased their 6MWT distance (p<0.01) whereas patients in the placebo group demonstrated no change (p=0.74). Moreover, improvements in 6MWT performance following NaNO3 supplementation correlated with calf BFPeak (n=11, r=0.70, p<0.05) and calf VCPeak (Figure 3B, r=0.61, p<0.05).
Figure 3.
Six-minute walk test (6MWT) distance before (Pre) and after (Post) eight weeks of NaNO3 and placebo supplementation (A). The relationship between individual changes (Δ) in 6MWT distance and peak calf vascular conductance (VCPeak) from the NaNO3 group (B). Data in (A) are presented as mean±standard error. Data in (B) reflect the 11 subjects with calf data from the NaNO3 group. * p<0.05 compared to Pre.
3.5. Inflammatory and adhesion biomarkers
Table 2 presents plasma inflammatory and adhesion biomarker data prior to, and following supplementation in both groups. No group-by-time interactions were found for any investigated biomarkers.
Table 2.
Plasma concentrations of inflammatory and adhesion biomarkers pre- and postsupplementation.
| NaNO3 | Placebo | ||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Interaction | |
| IL-6 (pg/ml) | 4.0±1.8 | 3.4±1.4 | 2.9±0.8 | 3.1±1.2 | p = 0.11 |
| sICAM-1 (ng/ml) | 338±116 | 325±99 | 265±39 | 263±35 | p = 0.72 |
| sVCAM-1 (ng/ml) | 908±302 | 820±197 | 703±208 | 687±185 | p = 0.47 |
| MCP-1 (pg/ml) | 212±56 | 220±61 | 203±42 | 198±34 | p = 0.71 |
Data are mean±standard deviation. NaNO3=sodium nitrate; IL-6=interleukin-six; sICAM1=soluble vascular adhesion molecule-one; sVCAM-1=soluble vascular adhesion molecule-one; MCP-1=monocyte chemoattractant protein-one. Data were compared using a two-way repeated measures analysis of variance.
4. Discussion
This is the first study investigating the effects of long-term inorganic nitrate supplementation on functional and vasodilatory capacities in patients with PAD. We demonstrate that inorganic nitrate elevates circulating [nitrate] and [nitrite] while improving functional and vasodilator capacities of patients with PAD. Specifically, eight-weeks of daily NaNO3 supplementation increases 6MWT distance as well as calf blood flow and vasodilation in response to reactive hyperemia. Additionally, patients who demonstrated the greatest improvement in calf BFPeak and VCPeak had the largest increases in 6MWT distance. Importantly, none of the patients enrolled in this study experienced adverse responses to sodium nitrate supplementation contrasting similar works using sodium nitrite (31). Collectively, these findings provide novel insight into the safe therapeutic potential of inorganic nitrate in patients with PAD.
Recently, a single dose of inorganic nitrate was shown to enhance functional capacity in patients with PAD (20). While this study provided proof-of-concept, longitudinal investigations were lacking. Data from the present study extend the findings of Kenjale et al. (20) as we demonstrated prolonged inorganic nitrate supplementation increases functional capacity (6MWT, Figure 3A). Although criteria for a clinically-meaningful change in the 6MWT has yet been established for patients with PAD, several studies in aged populations with and without chronic diseases suggest a change of 20 meters is small yet meaningful, whereas large meaningful change is defined as 50 meters (39). In the present study, 6MWT distance increased nearly 40 meters which is comparable to improvements following exercise training in patients with PAD (38). This finding yields strong clinical significance as 6MWT performance (total distance walked) is predictive of all-cause as well as cardiovascular mortality (29). The authors do not propose inorganic nitrate supplementation is an alternative to exercise in patients with PAD as there are immense benefits associated with regular physical activity (24); rather, we suggest the benefits of inorganic nitrate supplementation and exercise training may be synergistic. Indeed, this concept was investigated by Woessner et al. (48) who demonstrated that exercise combined with beetroot juice (source of inorganic nitrate) improved exercise tolerance and hyperemic blood flow in patients with PAD compared to exercise alone. Nonetheless, our data suggest that daily inorganic nitrate supplementation over eight-weeks is sufficient to improve functional capacity in a population with well documented exercise intolerance.
Vascular function was assessed in the present study using reactive hyperemia allowing us to index microvascular function in the forearm and calf. Given that our intervention was effective in boosting NO bioavailability, our study provides insight into the relationship between NO and microvascular function in patients with PAD. Although factors such as endothelium-derived hyperpolarizing factor (37) and potassium channels (9) contribute to reactive hyperemia, NO is also considered influential (30, 44, 47). We have previously shown the reliance on NO for vasodilation during exercise becomes greater in experimentally hypoperfused contracting skeletal muscle (7). In addition to chronic hypoperfusion, a hallmark of PAD is endothelial dysfunction characterized by abnormal vascular reactivity, mediated in part by reduced levels of endothelium-derived NO (2). Interestingly, the degree of endothelial dysfunction in patients with PAD is indicative of exercise capacity (2). Specifically, peak hyperemic blood flow in the calf of patients with PAD is associated with functional impairments (42). To this, pathological reductions of blood flow and vasodilation during exercise impair functional capacity as patients with PAD have a well-documented attenuation in functional capacity compared to healthy adults (32). Within the current study, inorganic nitrate supplementation improved both calf blood flow and vasodilation which were associated with greater functional capacity (Figure 3B). Collectively, our results demonstrate a beneficial effect of longer-term inorganic nitrate supplementation on microvascular health in the lower-leg and functional capacity of patients with PAD.
Despite PAD being viewed as exclusive to the lower extremities, endothelial dysfunction has also been found in the upper limbs of patients with PAD (31). Subjects in our study corroborate this notion as their forearm BFPeak and VCPeak appear to be lower than previously reported (46). Despite lower forearm blood flow responses in patients with PAD, there were no improvements in forearm vascular function following inorganic nitrate supplementation (Figure 1). These results support the recent findings of Mohler et al. (31) who also did not observe improvements in brachial artery reactivity following 10 weeks of sodium nitrite supplementation. Collectively, the limb-specific benefits of NO-boosting therapies indicate that vascular dysfunction may be more pronounced and more amendable to interventions in lower- compared to upper-extremities of patients with PAD.
Patients with PAD exhibit elevated levels of inflammatory and adhesion biomarkers (22); some of which have been directly associated with limitations in functional capacity (35). Subjects from the present study align with these notions as their plasma levels of inflammation and cellular adhesion exceeded those reported in healthy subjects as well as patients with claudication and critical limb ischemia (11). In the present study, prolonged inorganic nitrate supplementation did not affect circulating biomarkers of inflammation or adhesion and is the first study to examine the anti-inflammatory properties of inorganic nitrate in humans. Our findings support recent preclinical data suggesting prolonged inorganic nitrate supplementation does not influence systemic inflammation (27), yet contradict other preclinical studies demonstrating anti-inflammatory effects of inorganic nitrate (21). Despite demonstrating no statistical improvements in inflammation, subjects in the present study increased their functional capacity with concomitant improvement in vascular reactivity following inorganic nitrate supplementation. Thus, functional and vasodilator capacities can be enhanced without change in inflammation or adhesion biomarkers; however, these results should be interpreted with caution as our investigation of inflammation was not comprehensive.
While novel, our study contains a few limitations that should be considered. First, we only examined a single dose of inorganic nitrate with outcome assessments occurring exclusively before and after supplementation. While a study containing incremental doses and multiple measurement points is indeed more comprehensive, it would reduce feasibility and complicate statistical analysis. However, it should be noted that the increases in plasma [nitrate] and [nitrite] observed in the current study (eight weeks, 8.5mM) are similar to other studies utilizing shorter supplementation periods (314 days) and similar doses (8.0–8.4mM) of inorganic nitrate in various populations (3, 4, 13, 19). Second, the smaller sample size of our study is also worth noting given our a priori power analysis proposing 15 subjects per group. While our findings were adequately powered to detect differences in functional and vasodilatory capacities, a sample of 21 subjects is not sufficient to make conclusive statements on the efficacy of inorganic nitrate in this population. Along these lines, a larger sample size may have powered our study enough to observe a significant group-by-time interaction for the total calf blood flow and vascular conductance. Despite the relatively small sample size included in the NaNO3 group, none of the subjects reported adverse events on study visit two and thus it appears that inorganic nitrate supplementation can be considered safe in patients with PAD. It was recently shown that females have greater nitrate-reducing bacteria in their saliva (17); thus, the potential for sex-related differences in the present study is also worth noting where 54% of the NaNO3 group and 25% of the placebo group were female. Additionally, differences in sex breakdown between groups, in concert with our smaller sample size, may have contributed to the differences in baseline 6MWT distances. Lastly, a collective four biomarkers of inflammation and adhesion were investigated and that a more comprehensive analysis may yield different results as an array of circulating inflammation and adhesion markers are implicated in this population; specifically, high-sensitivity C-reactive protein (22). However, biomarkers assessed in the present study are known to be inhibited by NO (12, 18, 45). Despite these considerations, we demonstrate that vasodilatory and functional capacities can be improved in patients with PAD following eight-weeks of a nutraceutical intervention aimed at boosting NO levels.
Our findings demonstrate novel insight into the relationship between inorganic nitrate supplementation, functional capacity, and vasodilatory capacity in patients with PAD. Additionally, the present study supports the notion that diets rich in nitrate are strongly associated with attenuation in adjusted all-cause mortality in patients with atherosclerosis (5). Moreover, we suggest that therapies aimed at increasing NO bioavailability may exert beneficial effects in other patient populations as evidenced by ongoing clinical trials (40).
5. Conclusion
The present study demonstrates that eight-weeks of daily NaNO3 supplementation improved exercise tolerance and vasodilator capacity in patients with PAD. Our findings are of clinical relevance as these patients are known to have exercise intolerance (32) which is partially attributable to poor vascular health (2). Moreover, improvements in exercise tolerance exceeded the minimal important difference (39) and are similar to habitual exercise in this patient population (29). Collectively, our results carry implications for NO-boosting therapies in patients with systemic atherosclerosis.
Highlights to “Inorganic nitrate supplementation enhances functional capacity and lowerlimb microvascular reactivity in patients with peripheral artery disease”
Sodium nitrate increases functional capacity in patients with PAD
Improvements in functional capacity were related to greater calf vasodilation
Biomarkers of inflammation and adhesion were unchanged with sodium nitrate use
No adverse events were reported with 8-weeks of sodium nitrate supplementation
Acknowledgments
Funding
This study was supported by the American Heart Association 13GRNT16490002 (D.P.C.) and National Institutes of Health Clinical and Translational Science Award U54TR001356. S.L.N. was supported by an Undergraduate Summer Research Fellowship program from the American Physiological Society.
Footnotes
Disclosures
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine 166: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, and Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric oxide : biology and chemistry 20: 231–237, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano T, Okushima D, Breese BC, Bailey SJ, Koga S, and Kondo N. Influence of dietary nitrate supplementation on local sweating and cutaneous vascular responses during exercise in a hot environment. European journal of applied physiology 2018. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, and Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, O(2) uptake kinetics, and exercise tolerance at high but not low pedal rates. Journal of applied physiology (Bethesda, Md : 1985) 118: 1396–1405, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Woodman RJ, Croft KD, Lim WH, Wong G, Beilin LJ, Prince RL, and Hodgson JM. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. The American journal of clinical nutrition 106: 207–216, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Bock JM, Ueda K, Schneider AC, Hughes WE, Limberg JK, Bryan NS, and Casey DP. Inorganic nitrate supplementation attenuates peripheral chemoreflex sensitivity but does not improve cardiovagal baroreflex sensitivity in older adults. American journal of physiology Heart and circulatory physiology 314: H45–h51, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DP, and Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. Journal of applied physiology (Bethesda, Md : 1985) 107: 1685–1692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, and MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovascular research 64: 172–178, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, and Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circulation research 113: 1023–1032, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, and Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. The New England journal of medicine 326: 381–386, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Danielsson P, Truedsson L, Eriksson KF, and Norgren L. Inflammatory markers and IL-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vascular medicine (London, England) 10: 191–198, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Desai A, Miller MJ, Huang X, and Warren JS. Nitric oxide modulates MCP-1 expression in endothelial cells: implications for the pathogenesis of pulmonary granulomatous vasculitis. Inflammation 27: 213–223, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, and Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric oxide : biology and chemistry 40: 67–74, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Joyner MJ, Dietz NM, and Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. Journal of applied physiology (Bethesda, Md : 1985) 91: 2431–2441, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Justice JN, Gioscia-Ryan RA, Johnson LC, Battson ML, de Picciotto NE, Beck HJ, Jiang H, Sindler AL, Bryan NS, Enoka RM, and Seals DR. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. Journal of applied physiology (Bethesda, Md : 1985) 118: 163–169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice JN, Johnson LC, DeVan AE, Cruickshank-Quinn C, Reisdorph N, Bassett CJ, Evans TD, Brooks FA, Bryan NS, Chonchol MB, Giordano T, McQueen MB, and Seals DR. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: a pilot trial in middle-aged and older adults. Aging (Albany NY) 7: 1004–1021, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapil V, Rathod KS, Khambata RS, Bahra M, Velmurugan S, Purba A, Watson D, Barnes MR, Wade WG, and Ahluwalia A. Sex differences in the nitratenitrite-NO() pathway: role of oral nitrate-reducing bacteria. Free radical biology & medicine 2018. [DOI] [PubMed] [Google Scholar]
- 18.Kawachi S, Cockrell A, Laroux FS, Gray L, Granger DN, van der Heyde HC, and Grisham MB. Role of inducible nitric oxide synthase in the regulation of VCAM-1 expression in gut inflammation. The American journal of physiology 277: G572–576, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kelly J, Vanhatalo A, Bailey SJ, Wylie LJ, Tucker C, List S, Winyard PG, and Jones AM. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. American journal of physiology Regulatory, integrative and comparative physiology 307: R920–930, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, and Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. Journal of applied physiology (Bethesda, Md : 1985) 110: 1582–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khambata RS, Ghosh SM, Rathod KS, Thevathasan T, Filomena F, Xiao Q, and Ahluwalia A. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proceedings of the National Academy of Sciences of the United States of America 114: E550–e559, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khawaja FJ, and Kullo IJ. Novel markers of peripheral arterial disease. Vascular medicine (London, England) 14: 381–392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse NT, Ueda K, Hughes WE, and Casey DP. Eight weeks of nitrate supplementation improves blood flow and reduces the exaggerated pressor response during forearm exercise in peripheral arterial disease. American journal of physiology Heart and circulatory physiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O'Keefe JH, Milani RV, and Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circulation research 117: 207–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, and Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circulation research 68: 1027–1034, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg JO, Carlstrom M, Larsen FJ, and Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovascular research 89: 525–532, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Marsch E, Theelen TL, Janssen BJ, Briede JJ, Haenen GR, Senden JM, van Loon LJ, Poeze M, Bierau J, Gijbels MJ, Daemen MJ, and Sluimer JC. The effect of prolonged dietary nitrate supplementation on atherosclerosis development. Atherosclerosis 245: 212–221, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Matthews JN, Altman DG, Campbell MJ, and Royston P. Analysis of serial measurements in medical research. BMJ 300: 230–235, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, and Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 130: 61–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, and Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. The American journal of physiology 270: H1435–1440, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Mohler ER 3rd, Hiatt WR, Gornik HL, Kevil CG, Quyyumi A, Haynes WG, and Annex BH Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: safety, walking distance and endothelial function. Vascular medicine (London, England) 19: 9–17, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Muller MD, Reed AB, Leuenberger UA, and Sinoway LI. Physiology in medicine: peripheral arterial disease. Journal of applied physiology (Bethesda, Md : 1985) 115: 1219–1226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabel EG, Selwyn AP, and Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium-dependent mechanism that fails in patients with atherosclerosis. Journal of the American College of Cardiology 16: 349–356, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, and Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of vascular surgery 45 Suppl S: S5–67, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Nylaende M, Kroese A, Stranden E, Morken B, Sandbaek G, Lindahl AK, Arnesen H, and Seljeflot I. Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vascular medicine (London, England) 11: 21–28, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Omar SA, Webb AJ, Lundberg JO, and Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. Journal of internal medicine 279: 315–336, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, and Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 123: 2244–2253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parmenter BJ, Dieberg G, and Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports medicine (Auckland, NZ) 45: 231–244, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Perera S, Mody SH, Woodman RC, and Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society 54: 743–749, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Reddy YNV, Lewis GD, Shah SJ, LeWinter M, Semigran M, Davila-Roman VG, Anstrom K, Hernandez A, Braunwald E, Redfield MM, and Borlaug BA. INDIEHFpEF (Inorganic Nitrite Delivery to Improve Exercise Capacity in Heart Failure With Preserved Ejection Fraction): Rationale and Design. Circ Heart Fail 10: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regensteiner JG, Hargarten ME, Rutherford RB, and Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology 44: 1–10, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, and Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. Journal of applied physiology (Bethesda, Md : 1985) 111: 81–86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, and Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, and Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi M, Ikeda U, Masuyama J, Funayama H, Kano S, and Shimada K. Nitric oxide attenuates adhesion molecule expression in human endothelial cells. Cytokine 8: 817–821, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Taylor JL, Hines CN, Nicholson WT, Joyner MJ, and Barnes JN. The effect of ageing and indomethacin on forearm reactive hyperaemia in healthy adults. Experimental physiology 99: 859–867, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O'Keefe Z, Ives SJ, Conklin JD, Reese V, and Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. American journal of physiology Regulatory, integrative and comparative physiology 305: R893–899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woessner MN, VanBruggen MD, Pieper CF, Sloane R, Kraus WE, Gow AJ, and Allen JD. Beet the Best? Dietary Inorganic Nitrate to Augment Exercise Training in Lower Extremity Peripheral Artery Disease with Intermittent Claudication. Circulation research 2018. [Google Scholar]