Abstract
Purpose:
We highlight evidence for sexual dimorphism in preclinical and clinical studies investigating the etiology and treatment of cancer cachexia.
Recent Findings:
Cancer cachexia is unintended bodyweight loss occurring with cancer, and skeletal muscle wasting is a critical predictor of negative outcomes in the cancer patient. Skeletal muscle exhibits sexual dimorphism in fiber type, function, and regeneration capacity. Sex differences have been implicated in skeletal muscle metabolism, mitochondrial function, immune response to injury, and myogenic stem cell regulation. All of these processes have the potential to be involved in cancer-induced muscle wasting. Unfortunately, the vast majority of published studies examining cancer cachexia in preclinical models or cancer patients either have not accounted for sex in their design or have exclusively studied males. Preclinical studies have established that ovarian function and estradiol can affect skeletal muscle function, metabolism and mass; ovarian function has also been implicated in the sensitivity of circulating inflammatory cytokines and the progression of cachexia.
Summary:
Females and males have unique characteristics that effect skeletal muscle’s microenvironment and intrinsic signaling. These differences provide a strong rationale for distinct etiologies for cancer cachexia development and treatment in males and females.
Keywords: Sex hormones, Skeletal Muscle, Cachexia, Cancer, Wasting
1. INTRODUCTION
Cancer-induced cachexia is a complex and progressive wasting condition that is clinically identified by >5% bodyweight loss over a 6-month period (1, 2). Cancer-induced cachexia commonly occurs in 40–60% of male and 40–50% of female patients > 60 years of age (3, 4). While bodyweight loss is an indicator of cachexia, skeletal muscle wasting is a critical predictor of negative outcomes (5–7). Muscle mass loss is also related to reduced physical function and fatigue (1, 2, 8, 9), which can further aggravate cachexia through sedentary behavior. Systemic inflammation and hypogonadism are cancer-induced disruptions to the systemic environment that are directly linked to muscle wasting (1, 2, 8, 9).
Outcomes following cancer diagnosis and treatment are influenced by many factors, including age, body composition, and activity level (8, 10, 11). However, sex-based differences in cancer outcomes and treatment success only recently have garnered significant interest (3, 12–14). Although males and females both experience cachexia-induced functional and metabolic decrements (15–17), the vast majority of published studies examining cancer cachexia in preclinical models and cancer patients either have not accounted for sex in their design or have exclusively studied males. Despite the high prevalence of females presenting as cachectic, little is known about the biological differences between sexes during wasting, despite estrogens and androgens being acknowledged as important regulators of muscle mass and function (18–20). The role of sex in the etiology and treatment of cancer cachexia certainly warrants further study. The National Institutes of Health (NIH) is requiring experimental designs that account for sex as a biological variable (12, 14, 21). Through this effort the NIH intends to increase the reproducibility and translational value of biomedical research (13). Therefore, we highlight the rationale for investigating sex differences in the development and treatment of cancer-induced muscle wasting. The review emphasizes several well-described sex differences in skeletal muscle physiology and function that have clear implications for advancing the understanding of mechanistic drivers of cancer-induced muscle wasting.
2. CANCER-INDUCED DISRUPTION to SKELETAL MUSCLE & SEXUAL DIMORPHISM
Skeletal Muscle Fiber Type and Function
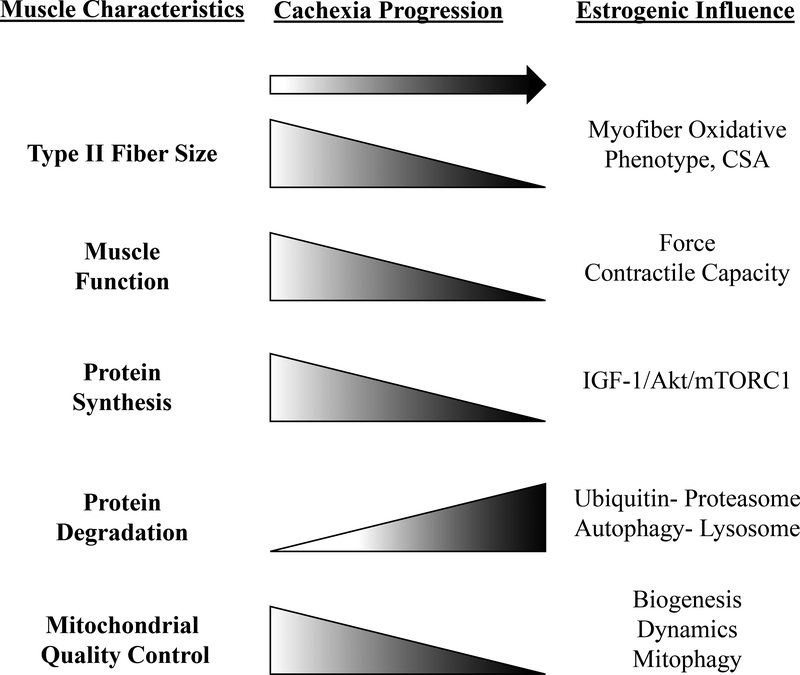
Skeletal muscle has been classically characterized by contractile, fatigue, and metabolic properties (22–24). This characterization has led to broad fiber type classifications: glycolytic (II(B,X)), oxidative-glycolytic (IIA), and oxidative (I) (23). Type II glycolytic myofibers have been reported to be more sensitive to cancer-induced muscle wasting than type I myofibers in cancer patients and mouse models (25). Cancer-induced muscle wasting is also associated with reduced myofiber oxidative metabolism (26, 27). Age-related wasting, sarcopenia, also causes pronounced type II fiber atrophy (28–30). However, both elderly people and cancer patients tend to engage in high levels of sedentary behavior, which can exacerbate atrophy (8, 31). Interestingly, muscle disuse regulates muscle mass and metabolism (31), and preferentially atrophies oxidative myofibers (25). Shifts in muscle fiber distribution with cancer cachexia have not been observed clinically (32), but altered contractile function of type II fibers has been found (32). Only recently have studies acknowledged a role for muscle disuse and sedentary behavior as drivers of cancer-induced muscle wasting (31). While several studies have demonstrated reduced physical function with cancer cachexia (33–36), the role of skeletal muscle fiber type in these changes warrants further investigation.
While skeletal muscle sexual dimorphisms have been well-documented and reviewed (24, 37, 38), the mechanisms responsible for these differences remain to be elucidated. For example, skeletal muscle gene expression profiling has revealed thousands of genes that exhibit sexually dimorphic expression (37), including evidence that muscle fiber type distribution is impacted by sex (24, 38). However, differences are not easy to quantify and interpret. Muscle fiber type is very heterogenous, varying by muscle type and species (23). Males have been reported to have greater muscle mass and ratio of type II:I fibers than females, demonstrating a more glycolytic phenotype (23, 24, 37). Type I fibers have been reported to account for a greater percentage of muscle area in females (23, 24, 38). Interestingly, estrogen status has been reported to not affect muscle fiber type distribution in humans (39) and mice (24). Since glycolytic and oxidative fibers can differentially respond to cancer and disuse, further research is warranted to determine if inherent fiber type differences contribute to differential responses to cancer-induced muscle wasting between men and women.
Sex-based differences have been widely reported for muscle fatigue susceptibility (24, 40), including evidence that male muscles are more fatigable than female muscles (16, 41). While the source of female fatigue resistance has not yet been established, it does coincide with increased oxidative fiber incidence. Interestingly, estrogen signaling can regulate fatigue and muscle contractile response in males and females; estrogen receptor (ER) beta loss decreases female endurance (42). Further work is needed to establish regulators of fatigue resistance in females (43). While estrogen status does not play a role in fiber type distribution, it can impact myofiber morphology, contraction, and function (24). Strength losses occur earlier in the aging process for postmenopausal females than males of the same age, but can be restored with hormone replacement therapy (44). Furthermore, cachectic males have greater deficits in handgrip strength when compared to cachectic female (15), and male cancer patients have increased muscle fatigue when compared to female patients (16). While whole body (45) and muscle (46) fatigue have been reported in preclinical cachexia models, sex regulation of cachexia-induced fatigue requires further study. Nevertheless, tumor-bearing female mice with decrements in estrogenic signaling have demonstrated increased tumor growth (47–49) and exacerbated cachectic pathology (33) that coincided with diminished muscle function (48, 49). While the cancer can increase muscle fatigue and decrease contractile function in preclinical cancer cachexia models (46), the sex-specific mechanisms that regulate these critical outcomes have not been established. A better understanding of sexual dimorphism’s role in cachexia-induced decrements to muscle function is needed and should benefit the development of therapeutics to improve cancer patient quality of life and survival.
Skeletal Muscle Protein Turnover Regulation
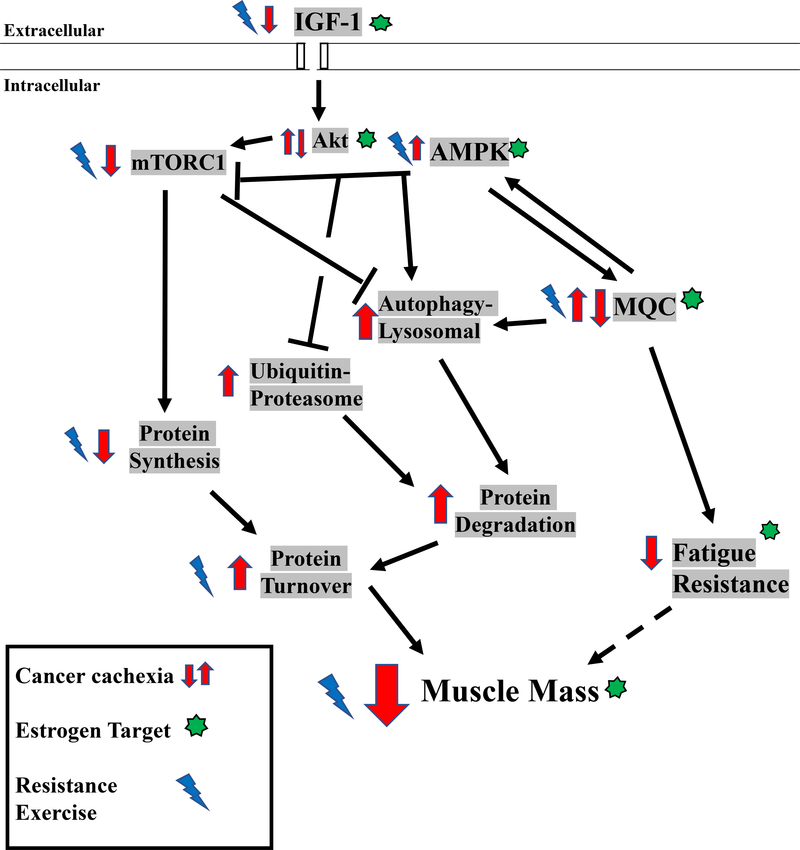
Muscle mass regulation comprises the constant flux of protein turnover related to protein degradation and synthesis (22). Endocrine, immune, physical activity, and nutritional signaling are integrated to provide this vital and dynamic regulation (22). When protein synthesis consistently exceeds degradation, net protein accretion results in hypertrophy (22). Insulin-like growth factor-1 (IGF-1) induces muscle protein synthesis and hypertrophy (22), and conversely the suppression of this pathway occurs with atrophic conditions (50, 51). The protein kinase B (Akt)/ mammalian target of rapamycin complex 1 (mTORC1) axis is a critical downstream effector of IGF-1 that induces muscle protein synthesis (22, 52, 53). mTORC1 has also emerged as a critical integration point for resistance exercise and amino acid induction of protein synthesis (54–58), which is independent of Akt signaling (22). Furthermore, protein synthesis regulation by nutrition and exercise is a critical indicator of skeletal muscle metabolic health (22, 59, 60). Anabolic resistance, the inability for anabolic stimuli to induce muscle protein synthesis, has been investigated as a driver of age-related sarcopenia (61, 62) and disuse atrophy (31). While anabolic resistance has been reported in cancer patients (56, 63), further investigation is needed to determine the mechanistic underpinnings of this resistance, and to identify the upstream mediators responsible for suppressed anabolic signaling in cachectic muscle (64). Protein degradation is also a tightly regulated process mediated by ubiquitin-proteasomal activation and proteasomal-autophagy-lysosomal pathways (65). Akt/mTORC1 signaling also regulates these degradation pathways (65, 66). Muscle ubiquitin-proteasomal degradation and autophagy pathways are induced during cancer cachexia (65, 67), and can be regulated by systemic inflammation and inflammatory cytokines (8, 52). Furthermore, 5’-adenosine monophosphate-activated protein kinase (AMPK) can promote autophagy through UNC-51 like kinase (ULK1) (68, 69). Additional research is warranted to determine if muscle AMPK and mTORC1 signaling can serve as therapeutic targets for cancer-induced autophagy (67, 70, 71).
The male and female response to anabolic stimuli has been well documented (24, 43, 72). Protein turnover regulation in young adult muscles does not exhibit overt sexual dimorphism (44, 72). However, differential protein turnover regulation in post-menopausal females has been reported (20, 72). Mediators of muscle anabolism decrease with advancing age and exhibit estrogen sensitivity (73, 74). Estrogen regulation has been implicated in AMPK signaling (75, 76) and upregulation of the IGF-1/mTORC1/Akt axis (73). Estradiol also promotes anabolism in preclinical models (18, 77), and disrupts muscle protein turnover through autophagy regulation by the Akt/forkhead-BoxO (FoxO) signaling pathway (78). Estrogen can also regulate inflammatory signaling (33, 79) and apoptosis (80). Therefore, decreased circulating estrogen has the potential to alter cachectic pathology through dysregulated protein turnover driven by increased muscle inflammation, attenuated anabolic metabolism, and increased autophagy (3, 77) (Figure 1). While the importance of sex on the ubiquitin-proteasome and autophagy-lysosomal pathways has only begun to be investigated (81), it remains an exploitable target for treating cachexia. Since muscle inflammatory signaling mediated through STAT3 and NF-κB can induce ubiquitin proteasome degradation during cachexia (27, 82), altered inflammatory signaling has the potential to suppress or activate these pathways. In general, a greater understanding of the sex-specific regulation of protein turnover should provide valuable insight for the successful development of therapies to prevent cancer-induced muscle wasting in both males and females.
Figure 1:
Potential Targets of Estrogen in Muscle in the Systemic Cachectic Environment
Skeletal Muscle Mitochondrial Quality Control and Oxidative Metabolism
The impact of mitochondrial function on skeletal muscle homeostasis and function has been widely acknowledged and recently and thoroughly reviewed (83–85). Mitochondrial quality control is a highly coordinated process that facilitates mitochondrial homeostasis (84, 85). Mitochondrial quality control encompasses the regulation of mitochondrial biogenesis, dynamics, and mitophagy processes (84). These pathways are integrated with muscle anabolic and catabolic signaling (83) that impacts muscle metabolic health (9, 63, 85). Cancer cachexia is associated with mitochondrial dysfunction and loss of muscle oxidative capacity and recent research has identified these changes as critical drivers of cancer cachexia progression (26, 27, 46, 86–88). Indeed, mitochondrial dysfunction and degeneration can precede muscle wasting in tumor-bearing (86) and sarcopenic (28) mice. Disrupted mitophagy regulation has also been investigated as a central mechanism for muscle wasting (67, 70, 88–91). Additionally, reactive oxygen species (ROS) and oxidative stress can accompany mitochondrial dysfunction and have also been widely investigated in muscle wasting (92, 93). While cachexia research has largely focused on defining the role of mitochondrial dysfunction as a driver of wasting, there is strong rationale for investigating the function of sex hormones in the cancer-induced disruption to muscle oxidative metabolism.
Mitochondrial homeostasis can be regulated by sex and has been comprehensively reviewed (77, 80, 94, 95). Basal mitochondria respiratory function and content is higher in females, recapitulating the oxidative phenotype (94). Sexually dimorphic properties of mitochondria quality control and oxidative metabolism can also be observed. Integration of cellular signaling pathways involving the mitochondria regulation, such as AMPK activity (18, 75, 76), are estrogen sensitive (96, 97). These are well-defined sex-mediated regulators of oxidative metabolism (77, 98). Mitochondrial gene expression is influenced by estrogen (80, 96), and has demonstrated sexual dimorphisms in genomic (37) and proteomic (38) analyses. Recently it has been shown that mitochondrial membrane viscosity can be regulated by estrogenic signaling and that it promotes electron transport chain activity (98). Research examining drivers of cancer cachexia in males and females provides a strong premise to investigate the role of sex in mitochondrial processes that regulate cancer cachexia (94).
3. POTENTIAL THERAPEUTIC INTERVENTIONS
Resistance Exercise
Resistance exercise is a non-pharmacological intervention that can modulate muscle fiber composition, protein turnover, oxidative phosphorylation, and hormones, and it has been extensively reviewed (64, 99, 100). Resistance exercise can benefit the cancer patient’s quality of life and physical function (11, 99–102). Regular physical activity during chemotherapy treatment (103) and prior to cancer can have positive effects on skeletal muscle metabolism and attenuate comorbidities (104–106). A multimodal approach to cachexia treatment could impact patient survival, and a recent clinical trial validated the feasibility of an exercise intervention in cachectic patients (107). A clinical trial is being conducted to investigate the effect of resistance exercise on skeletal muscle during chemotherapy treatment (NIH#:NCT02330926). While resistance exercise has the ability to attenuate wasting (56, 108, 109), mimicked resistance exercise in preclinical cancer models has demonstrated a blunted anabolic response in cachectic muscle (110). Exercise benefits have been as observed in male cancer patients (105, 111) and female cancer patients (104, 108), cancer patients of both sexes (99, 100, 107, 112, 113) and those with sarcopenia (56, 62, 114). However, there is a strong rationale for resistance exercise to impact cancer-induced muscle wasting (64) in a sexually dimorphic manner (99). Resistance exercise regulates many sexually dimorphic cellular signaling pathways in skeletal muscle (18, 24, 38, 72, 115, 116). Furthermore, resistance exercise can modulate sex hormone levels in men and women (99, 104, 117, 118). Given that exercise is a modifiable risk factor for chronic disease, resistance exercise adaptation and responses in both male and female cancer patients suffering from cachexia warrants further investigation.
Hormone Therapy
Hypogonadism is associated with decreased circulating sex hormones that can result from aging, gonad dysfunction, and chronic disease (9, 20). Testosterone impacts skeletal muscle hypertrophy and anabolic signaling (119, 120). Estrogen has genomic and nongenomic properties (18, 19, 116), and skeletal muscle fibers are highly sensitive to changes in circulating estrogen (18). While the prevalence of hypogonadism increases with age (20), it is more common among cancer patients (3, 121) and is related to cachexia development (122, 123). While sex hormones play established roles in many aspects of skeletal muscle function, their role in cancer-induced muscle wasting is not fully understood (19). Testosterone treatment in male and female cancer patients has positive effects on skeletal muscle function and health (124) (NIH#:NCT00878995), but may exacerbate comorbidities (119, 125). Similarly, the positive effects of estrogen-related therapy (18, 126) have the potential to be offset by adverse consequences (127, 128) and risk of cancer recurrence (129, 130). Therefore, hormone therapy alternatives have generated interest and ongoing study. Selective androgen receptor modulators (SARMs) are a class of tissue-selective androgen receptor ligands that target selected androgen effects and avoid comorbidities observed with traditional hormone therapy (3, 131–133). SARMs have been shown to benefit muscle mass, inflammation, and catabolism in hypogonadal states of both sexes (134, 135). The complex interaction of sex hormones with cancer-induced muscle metabolic and contractile dysfunction warrants further investigation.
4. CONCLUSION
Males and females have demonstrated sex-specific regulation related to aging and several disease states (12, 20, 136). This understanding extends to well-described sexual dimorphism in aspects of skeletal muscle function and metabolism. Sex differences also extend to essential muscle properties involving muscle regeneration after injury (3). Despite a strong premise for sexual dimorphism in mechanisms that drive muscle wasting, we have a very limited understanding of how sex can influence cancer cachexia progression. We highlight several mechanistic regulators of skeletal muscle wasting that have potential for sexually dimorphic responses that could impact the prevention and treatment of cancer cachexia in both males and females (Figure 2).
Figure 2:
Cancer Environment’s Effect on Dysregulated Protein Turnover in Skeletal Muscle
Key Points:
Skeletal muscle has sexual dimorphic properties that have strong potential to influence the mechanistic underpinnings of cancer-induced muscle wasting.
Cancer-induced systemic inflammation can disrupt the IGF-1/Akt/mTORC1 axis and mitochondria function, which have been widely investigated as regulators of cachexia progression and also have strong potential to be differentially regulated in males and females.
The role of hypogonadism in cancer-induced muscle wasting has significant implications for the identification of sexually dimorphic mechanisms of cachexia regulation.
Resistance exercise has strong therapeutic potential for the maintenance and recovery of muscle mass in both male and female cancer patients.
Sex is an important biological variable that needs to be accounted for in the design of human and preclinical cancer cachexia research.
ACKNOWLEDGEMENTS
The authors thank Gaye Christmus for the editorial review of the manuscript. This work was supported by National Institutes of Health Grants R01 CA-121249 (National Cancer Institute) to James A. Carson.
Funding: This work was supported by National Institutes of Health Grant R01 CA-121249 (National Cancer Institute) to James A. Carson
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 2. *.Mattox TW. Cancer Cachexia: Cause, Diagnosis, and Treatment. Nutr Clin Pract. 2017;32(5):599–606. Treatment agents need to be personalized to each patient’s appetite, weight gain, quality of life, exposure to adverse effects, and the cost/ availability of the agent. [DOI] [PubMed] [Google Scholar]
- 3. **.Anderson LJ, Liu H, Garcia JM. Sex Differences in Muscle Wasting. Adv Exp Med Biol. 2017;1043:153–97. This book chapter reviews estrogen’s mechanistic regulation on cachexia associated wasting, sarcopenia, and disuse; and its therapeutic role on reducing inflammation. [DOI] [PubMed] [Google Scholar]
- 4. *.Vagnildhaug OM, Blum D, Wilcock A, Fayers P, Strasser F, Baracos VE, et al. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J Cachexia Sarcopenia Muscle. 2017;8(5):789–97. A large study of men and women with weight loss helps observe rates of cachexia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. *.Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer. 2018;7(1):90–103. Skeletal muscle mass is inversely associated to all-cause mortality in patients with hepatocellular carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. *.Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han SW, et al. Skeletal muscle depletion predicts survival of patients with advanced biliary tract cancer undergoing palliative chemotherapy. Oncotarget. 2017;8(45):79441–52. Skeletal muscle mass is rarely evaluated in biliary tract cancer, despite the positive association to prognosis, therefore rendering a substantial component clinicals should evaluate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. *.Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9(2):315–25. Skeletal muscle loss during foregut cancer development and concomitant chemotherapy reduces cancer patient survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–71. [DOI] [PubMed] [Google Scholar]
- 9.Carson JA, Hardee JP, VanderVeen BN. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 2018;26(3):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. *.Cormie P, Zopf EM, Zhang X, Schmitz KH. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017;39(1):71–92. Exercise concomitant with cancer treatment decreases risk of recurrence and mortality. [DOI] [PubMed] [Google Scholar]
- 12. **.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31(1):29–34. Methods and techniques for inclusion of both sexes in research in an effort to guide preclinical investigators in NIH guide notice NOT-OD-15–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. *.Kim HI, Lim H, Moon A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol Ther (Seoul). 2018;26(4):335–42. Sex plays a key role in cancer prognosis, chemotherapy toxicity and efficacy, and cancer progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. **.Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018;187:2–5. Biomedical research has not accurately studied or addressed clinical or research-based sexual dimorphism. The NIH’s SABV methodologies should guide clinical and preclinical studies. [DOI] [PubMed] [Google Scholar]
- 15.Norman K, Stobaus N, Reiss J, Schulzke J, Valentini L, Pirlich M. Effect of sexual dimorphism on muscle strength in cachexia. J Cachexia Sarcopenia Muscle. 2012;3(2):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJ, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin Nutr. 2012;31(4):499–505. [DOI] [PubMed] [Google Scholar]
- 17. *.Yoon SL, Grundmann O, Williams JJ, Gordan L, George TJ, Jr. Body composition changes differ by gender in stomach, colorectal, and biliary cancer patients with cachexia: Results from a pilot study. Cancer Med. 2018. Sex has specific influence in outcomes of patients with biliary tract cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spangenburg EE, Geiger PC, Leinwand LA, Lowe DA. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc. 2012;44(9):1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen’s beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38(2):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. *.Lee H, Pak YK, Yeo EJ, Kim YS, Paik HY, Lee SK. It is time to integrate sex as a variable in preclinical and clinical studies. Exp Mol Med. 2018;50(7):82 This letter is one of the first international pleas of efficacious sex-based research that recapitulates the NIH-based initiatives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–314. [DOI] [PubMed] [Google Scholar]
- 23.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531. [DOI] [PubMed] [Google Scholar]
- 24.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45(10):2191–9. [DOI] [PubMed] [Google Scholar]
- 26. *.Hardee JP, Montalvo RN, Carson JA. Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal Muscle Oxidative Metabolism. Oxidative Medicine and Cellular Longevity. 2017;2017:14 Decrements in oxidative metabolism driving anabolic resistance is central to cachectic pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. *.VanderVeen BN, Fix DK, Carson JA. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxid Med Cell Longev. 2017;2017:3292087 Disruptions to mitochondrial homeostasis through inflammation is central to cancer-induced wasting pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. *.Del Campo A, Contreras-Hernandez I, Castro-Sepulveda M, Campos CA, Figueroa R, Tevy MF, et al. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging (Albany NY). 2018;10(1):34–55. This study demonstrated that decrements to mitochondrial quality control are evident prior to wasting, as observed in pre-clinical cachexia, and shows the vital role of mitochondria in wasting progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. *.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. Sarcopenia is driven by loss of exercise capacity with age, compounded by disease states, such as cachexia, and clinical treatment lacking a multifactorial approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, et al. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017;19(11):2396–409. [DOI] [PubMed] [Google Scholar]
- 31.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985). 2009;107(3):645–54. [DOI] [PubMed] [Google Scholar]
- 32.Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, et al. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin-actin cross-bridge formation and kinetics. J Appl Physiol (1985). 2013;114(7):858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. **.Hetzler KL, Hardee JP, LaVoie HA, Murphy EA, Carson JA. Ovarian function’s role during cancer cachexia progression in the female mouse. Am J Physiol Endocrinol Metab. 2017;312(5):E447–E59. This novel investigation demonstrated interaction of estrogenic signaling and inflammation causing cachexia. Functioning ovary production demonstrated protective effects in the prevention of wasting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. **.Gale N, Wasley D, Roberts S, Backx K, Nelson A, van Deursen R, et al. A longitudinal study of muscle strength and function in patients with cancer cachexia. Support Care Cancer. 2018. Investigators evaluated severely cachectic patients, with high attrition, but demonstrated stability and the little change in performance over 8 weeks. [DOI] [PubMed] [Google Scholar]
- 35. *.Schwarz S, Prokopchuk O, Esefeld K, Groschel S, Bachmann J, Lorenzen S, et al. The clinical picture of cachexia: a mosaic of different parameters (experience of 503 patients). BMC Cancer. 2017;17(1):130 Decrements in physical activity of cachectic and non-cachectic patients is based in anemia and impaired kidney and liver function, which are central to quality of life in cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. *.Bye A, Sjoblom B, Wentzel-Larsen T, Gronberg BH, Baracos VE, Hjermstad MJ, et al. Muscle mass and association to quality of life in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle. 2017;8(5):759–67. Quality of life of NSCLC patients is primarily dictated by muscle mass, early detection of muscle mass loss can benefit patient outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3(1):e1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maher AC, Fu MH, Isfort RJ, Varbanov AR, Qu XA, Tarnopolsky MA. Sex differences in global mRNA content of human skeletal muscle. PLoS One. 2009;4(7):e6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widrick JJ, Maddalozzo GF, Lewis D, Valentine BA, Garner DP, Stelzer JE, et al. Morphological and functional characteristics of skeletal muscle fibers from hormone-replaced and nonreplaced postmenopausal women. J Gerontol A Biol Sci Med Sci. 2003;58(1):3–10. [DOI] [PubMed] [Google Scholar]
- 40. *.Bouffard J, Yang C, Begon M, Cote J. Sex differences in kinematic adaptations to muscle fatigue induced by repetitive upper limb movements. Biol Sex Differ. 2018;9(1):17 Shoulder muscles of males are more fatigued then women despite similar exertion, recapitulating sexual dimorphism in skeletal muscle fatigue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29(3):109–12. [DOI] [PubMed] [Google Scholar]
- 42.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2004;287(6):E1125–31. [DOI] [PubMed] [Google Scholar]
- 43. **.Montero D, Madsen K, Meinild-Lundby AK, Edin F, Lundby C. Sexual dimorphism of substrate utilization: Differences in skeletal muscle mitochondrial volume density and function. Exp Physiol. 2018;103(6):851–9. No sex differences observed in respiratory exchange ratio and skeletal muscle variables. Females oxidize fats for fuels more efficiently, correlated to mitochondrial function and oxidative phenotype. [DOI] [PubMed] [Google Scholar]
- 44.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84(1):95–8. [DOI] [PubMed] [Google Scholar]
- 45. *.Ishida J, Saitoh M, Doehner W, von Haehling S, Anker M, Anker SD, et al. Animal models of cachexia and sarcopenia in chronic illness: Cardiac function, body composition changes and therapeutic results. Int J Cardiol. 2017;238:12–8. Cachexia is often concomitant with sarcopenia resulting in increased whole-body fatigue, likely related to cardiac function. [DOI] [PubMed] [Google Scholar]
- 46. *.VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal Muscle Function During the Progression of Cancer Cachexia in the Male Apc(Min/+) Mouse. J Appl Physiol (1985). 2017:jap008972017. Inflammatory signaling drives the fatigued skeletal muscle phenotype observed in tumor-bearing mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. *.Dou M, Zhu K, Fan Z, Zhang Y, Chen X, Zhou X, et al. Reproductive Hormones and Their Receptors May Affect Lung Cancer. Cell Physiol Biochem. 2017;44(4):1425–34. Increased AR and ER in female mice injected with LLC tumor cells, during which estrogen plays a protective role in reducing tumor pathogenesis in an OVX model. [DOI] [PubMed] [Google Scholar]
- 48. **.Wright LE, Harhash AA, Kozlow WM, Waning DL, Regan JN, She Y, et al. Aromatase inhibitor-induced bone loss increases the progression of estrogen receptor-negative breast cancer in bone and exacerbates muscle weakness in vivo. Oncotarget. 2017;8(5):8406–19. Observed in an OVX tumor-bearing model estrogen-ER signaling regulate contraction and plays a protective role in skeletal muscle homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. **.Bohlen J, McLaughlin SL, Hazard-Jenkins H, Infante AM, Montgomery C, Davis M, et al. Dysregulation of metabolic-associated pathways in muscle of breast cancer patients: preclinical evaluation of interleukin-15 targeting fatigue. J Cachexia Sarcopenia Muscle. 2018. Disruptions in oxidative metabolism associated with preclinical and clinical breast cancer is characterized by increased fatigue related to the mitochondria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Y, Arfat Y, Wang H, Goswami N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front Physiol. 2018;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. *.Carr RM, Enriquez-Hesles E, Olson RL, Jatoi A, Doles J, Fernandez-Zapico ME. Epigenetics of cancer-associated muscle catabolism. Epigenomics. 2017. Muscle wasting is propogated by reductions in anabolic pathways. [DOI] [PubMed] [Google Scholar]
- 52.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–9. [DOI] [PubMed] [Google Scholar]
- 53.Yoon MS. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front Physiol. 2017;8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep. 2017;7(1):5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. *.Dutchak PA, Estill-Terpack SJ, Plec AA, Zhao X, Yang C, Chen J, et al. Loss of a Negative Regulator of mTORC1 Induces Aerobic Glycolysis and Altered Fiber Composition in Skeletal Muscle. Cell Rep. 2018;23(7):1907–14. Loss of negative regulation on mTORC1 increases aerobic glycolysis, carbohydrate utilization, and fiber type shift. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. **.Antoun S, Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Ann Oncol. 2018;29(suppl_2):ii10–ii7. Cancer patients experiencing anabolic resistance can be treated with multimodal interventions involving elevated protein intake and regular physical activity to stimulate mTOR. [DOI] [PubMed] [Google Scholar]
- 57.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169(2):361–71. [DOI] [PubMed] [Google Scholar]
- 58.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Ruegg MA, et al. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol. 2017;313(6):C604–C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients. 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackman SR, Witard OC, Philp A, Wallis GA, Baar K, Tipton KD. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front Physiol. 2017;8:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. *.Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care. 2018;24(2):124–30. Sarcopenic-based skeletal muscle atrophy is largely driven by anabolic wasting. [DOI] [PubMed] [Google Scholar]
- 62. *.Strasser B, Volaklis K, Fuchs D, Burtscher M. Role of Dietary Protein and Muscular Fitness on Longevity and Aging. Aging Dis. 2018;9(1):119–32. Anabolic resistance is observed in elderly patients with sarcopenia, culminating in decreased physical activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. *.Schcolnik-Cabrera A, Chavez-Blanco A, Dominguez-Gomez G, Duenas-Gonzalez A. Understanding tumor anabolism and patient catabolism in cancer-associated cachexia. Am J Cancer Res. 2017;7(5):1107–35. Cancer patients demonstrate difficulty gaining muscle mass due to anabolic resistance, which is related to the systemic catabolic environment. [PMC free article] [PubMed] [Google Scholar]
- 64. *.Montalvo RN, Hardee JP, VanderVeen BN, Carson JA. Resistance Exercise’s Ability to Reverse Cancer-Induced Anabolic Resistance. Exerc Sport Sci Rev. 2018. Anabolic resistance can be ameliorated with resistance exercise, benefiting the anabolic pathways that contribute to skeletal muscle homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandri M Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45(10):2121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. *.Conciatori F, Ciuffreda L, Bazzichetto C, Falcone I, Pilotto S, Bria E, et al. mTOR Cross-Talk in Cancer and Potential for Combination Therapy. Cancers (Basel). 2018;10(1). Cancer cachexia demonstrates decrements to Akt/mTORC1 pathways and mediates muscle atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. **.Pettersen K, Andersen S, Degen S, Tadini V, Grosjean J, Hatakeyama S, et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep. 2017;7(1):2046 Novel study of cancer patients and preclinical models show the role of IL-6 and autophagy in the development and progression of cachexia, demonstrating a sexually dimorphic basis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nwadike C, Williamson LE, Gallagher LE, Guan JL, Chan EYW. AMPK Inhibits ULK1-Dependent Autophagosome Formation and Lysosomal Acidification via Distinct Mechanisms. Mol Cell Biol. 2018;38(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. *.Monkkonen T, Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018;14(2):190–8. Macroautophagy and autophagy are the key signaling cascades associated with tumor-induced inflammation, involving regulation with mTOR and more largely AMPK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. *.Paquette M, El-Houjeiri L, Pause A. mTOR Pathways in Cancer and Autophagy. Cancers (Basel). 2018;10(1). In cancer pathology mTOR is a key mediator of autophagy, demonstrating significant interaction with AMPK and potential mechanisms to be targeted with therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith GI, Mittendorfer B. Sexual dimorphism in skeletal muscle protein turnover. J Appl Physiol (1985). 2016;120(6):674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollanen E, Ronkainen PH, Horttanainen M, Takala T, Puolakka J, Suominen H, et al. Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Horm IGF Res. 2010;20(5):372–9. [DOI] [PubMed] [Google Scholar]
- 74. *.Hansen M Female hormones: do they influence muscle and tendon protein metabolism? Proc Nutr Soc. 2018;77(1):32–41. Postmenopausal females demonstrate decreased circulating estrogen, which negatively impacts skeletal muscle protein synthesis. [DOI] [PubMed] [Google Scholar]
- 75. **.Park YM, Pereira RI, Erickson CB, Swibas TA, Kang C, Van Pelt RE. Time since menopause and skeletal muscle estrogen receptors, PGC-1alpha, and AMPK. Menopause. 2017;24(7):815–23. Study of pre- and early and late postmenopausal women demonstrate decreased mitochondrial quality control with time since menopause, specifically in PGC-1alpha and AMPK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kjobsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmoller C, et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018;32(4):1741–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, et al. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8(334):334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4(4):524–6. [DOI] [PubMed] [Google Scholar]
- 79. *.Rothenberger NJ, Somasundaram A, Stabile LP. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci. 2018;19(2). Tumor-derived inflammation is highly sensitive to estrogen and can be positively regulated by estrogenic influence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. *.Yasar P, Ayaz G, User SD, Gupur G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol. 2017;16(1):4–20. Mitochondria-derived apoptosis can be positively regulated by estrogen in various tissue types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. *.Rossetti ML, Steiner JL, Gordon BS. Increased mitochondrial turnover in the skeletal muscle of fasted, castrated mice is related to the magnitude of autophagy activation and muscle atrophy. Mol Cell Endocrinol. 2018. Androgen-mediated regulation of protein balance is central to efficacious treatment during wasting and should be addressed in future studies. [DOI] [PubMed] [Google Scholar]
- 82. *.Sakuma K, Aoi W, Yamaguchi A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. 2017;469(5–6):573–91. Inflammatory cytokine-mediated ubiquitin proteasome degradation plays a key role in cachectic pathology and provides potential mechanisms for therapeutic intervention. [DOI] [PubMed] [Google Scholar]
- 83.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiriyama Y, Nochi H. Intra- and Intercellular Quality Control Mechanisms of Mitochondria. Cells. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pickles S, Vigie P, Youle RJ. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol. 2018;28(4):R170–R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. *.Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017. Novel investigation into the potential of muscle wasting being preceded by mitochondrial aberrations is vital to understanding the onset and progression of cachexia and prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. *.Penna F, Ballaro R, Beltra M, De Lucia S, Costelli P. Modulating Metabolism to Improve Cancer-Induced Muscle Wasting. Oxid Med Cell Longev. 2018;2018:7153610 Improvements to cachectic pathology are primarily mediated through metabolic pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. **.Marzetti E, Lorenzi M, Landi F, Picca A, Rosa F, Tanganelli F, et al. Altered mitochondrial quality control signaling in muscle of old gastric cancer patients with cachexia. Exp Gerontol. 2017;87(Pt A):92–9. Biopsies from gastric cancer patients with and without cachexia showed aberration of mitochondrial dynamics and mitophagy, providing mechanistic targets for therapies. [DOI] [PubMed] [Google Scholar]
- 89.Shi R, Guberman M, Kirshenbaum LA. Mitochondrial quality control: The role of mitophagy in aging. Trends Cardiovasc Med. 2018;28(4):246–60. [DOI] [PubMed] [Google Scholar]
- 90. *.Bordi M, Nazio F, Campello S. The Close Interconnection between Mitochondrial Dynamics and Mitophagy in Cancer. Front Oncol. 2017;7:81 Disrupted mitophagy drives cancer pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pare MF, Baechler BL, Fajardo VA, Earl E, Wong E, Campbell TL, et al. Effect of acute and chronic autophagy deficiency on skeletal muscle apoptotic signaling, morphology, and function. Biochim Biophys Acta. 2017;1864(4):708–18. [DOI] [PubMed] [Google Scholar]
- 92. *.Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8(3):349–69. Various wasting modalities highlight ROS as a causative factor in pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim Y, Triolo M, Hood DA. Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxid Med Cell Longev. 2017;2017:3165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. **.Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, et al. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond). 2017;131(9):803–22. Novel review of the specific regulation of mitochondrial processes by sex hormones and the roles in various disease states identifies opportunities for treatment intervention. [DOI] [PubMed] [Google Scholar]
- 95. **.Nye GA, Sakellariou GK, Degens H, Lightfoot AP. Muscling in on mitochondrial sexual dimorphism; role of mitochondrial dimorphism in skeletal muscle health and disease. Clin Sci (Lond). 2017;131(15):1919–22. Mitochondria can be regulated by sex hormones, which has unique implications into the treatment and study of various disease modalities. [DOI] [PubMed] [Google Scholar]
- 96. *.Hevener AL, Zhou Z, Drew BG, Ribas V. The Role of Skeletal Muscle Estrogen Receptors in Metabolic Homeostasis and Insulin Sensitivity. Adv Exp Med Biol. 2017;1043:257–84. Estrogenic influence in mitochondrial quality control and is central to metabolic homeostasis and anabolic input. [DOI] [PubMed] [Google Scholar]
- 97. **.Klinge CM. Estrogens regulate life and death in mitochondria. J Bioenerg Biomembr. 2017;49(4):307–24. Mitochondria are highly regulated by estrogen, which can have clinical implications in the continued regulation of metabolic homeostasis. [DOI] [PubMed] [Google Scholar]
- 98. **.Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, et al. 17beta-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metab. 2018;27(1):167–79e7. Estrogen receptor signaling at the mitochondria demonstrates benefits to mitochondrial function and homeostasis but may not be primarily mediated by estrogen receptor alpha. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. *.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018;27(1):10–21. Exercise has a myriad of beneficial effects to whole-body homeostasis and can have a beneficial molecular influence in cancer pathology. [DOI] [PubMed] [Google Scholar]
- 100. *.Heywood R, McCarthy AL, Skinner TL. Efficacy of exercise interventions in patients with advanced cancer: A systematic review. Arch Phys Med Rehabil. 2018. Exercise benefits physical function and general quality of life scores in cancer patients but has not bene observed to increase survivability. [DOI] [PubMed] [Google Scholar]
- 101. *.Schulz SVW, Laszlo R, Otto S, Prokopchuk D, Schumann U, Ebner F, et al. Feasibility and effects of a combined adjuvant high-intensity interval/strength training in breast cancer patients: a single-center pilot study. Disabil Rehabil. 2018;40(13):1501–8. High intensity interval training and strength training can be safely used in breast cancer patients and has beneficial effects to quality of life outcomes. [DOI] [PubMed] [Google Scholar]
- 102. *.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: A systematic review. Prev Med. 2017;104:124–32. Exercise functions to benefit quality of life even in later stage cancers and may prevent further complications. [DOI] [PubMed] [Google Scholar]
- 103. *.Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2017;8(2):305–16. Breast cancer patients receiving chemotherapy demonstrate increased fatigue and decrease muscle strength, which can be ameliorated with early exercise intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. *.Dethlefsen C, Pedersen KS, Hojman P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat. 2017;162(3):399–408. Physical activity can prevent breast cancer incidence and progression. [DOI] [PubMed] [Google Scholar]
- 105. *.Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8(5):e022899 Current ongoing trial will demonstrate if resistance exercise can benefit prostate cancer patients with the main outcome being survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. *.Owen PJ, Daly RM, Livingston PM, Mundell NL, Dalla Via J, Millar JL, et al. Efficacy of a multi-component exercise programme and nutritional supplementation on musculoskeletal health in men treated with androgen deprivation therapy for prostate cancer (IMPACT): study protocol of a randomised controlled trial. Trials. 2017;18(1):451 Multimodal approaches to involving exercise and nutraceuticals can benefit cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. *.Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(5):778–88. Cancer patients with lung and pancreatic cancer-induced cachexia are capable of performing exercise, phase II trials of exercise efficacy to follow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. *.Adraskela K, Veisaki E, Koutsilieris M, Philippou A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin Breast Cancer. 2017;17(6):408–17. Exercise benefits skeletal muscle quality and function in patients with breast cancer [DOI] [PubMed] [Google Scholar]
- 109. *.Padilha CS, Borges FH, Costa Mendes da Silva LE, Frajacomo FTT, Jordao AA, Duarte JA, et al. Resistance exercise attenuates skeletal muscle oxidative stress, systemic pro-inflammatory state, and cachexia in Walker-256 tumor-bearing rats. Appl Physiol Nutr Metab 2017;42(9):916–23. Resistance exercise ameliorates muscle wasting by reducing the inflammatory mechanisms that damage skeletal muscle homeostasis. [DOI] [PubMed] [Google Scholar]
- 110. *.Hardee JP, Counts BR, Gao S, VanderVeen BN, Fix DK, Koh HJ, et al. Inflammatory signalling regulates eccentric contraction-induced protein synthesis in cachectic skeletal muscle. J Cachexia Sarcopenia Muscle. 2017. Skeletal muscle demonstrates anabolic resistance mediated by inflammation in a tumor-bearing mouse despite eccentric contractions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. *.Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients. 2017;9(9). Prostate cancer patients can withstand and benefit from moderately intense guided resistance and aerobic exercise, which functions to improve overall fatigue and quality of life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. *.Pyszora A, Budzynski J, Wojcik A, Prokop A, Krajnik M. Physiotherapy programme reduces fatigue in patients with advanced cancer receiving palliative care: randomized controlled trial. Support Care Cancer. 2017;25(9):2899–908. Patients with advanced cancers receiving end-of-life treatment can decrease fatigue with physical activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. *.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017;3(7):961–8. Exercise as a non-pharmaceutical approach is an effective method to attenuate cancer-related fatigue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. *.Hardee JP, Carson JA. Understanding Sarcopenia Development: A Role for Healthy Behaviors. Am J Lifestyle Med. 2017;11(1):17–20. The effects of sarcopenia and demonstrates a role for physical activity to combat age-related muscle wasting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. **.Farhat F, Amerand A, Simon B, Guegueniat N, Moisan C. Gender-dependent differences of mitochondrial function and oxidative stress in rat skeletal muscle at rest and after exercise training. Redox Rep. 2017;22(6):508–14. Exercise benefits both male and female rats: females benefit from the protective role of estrogen in inflammation and males demonstrate increased mitochondrial capacity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. *.Haines M, McKinley-Barnard SK, Andre TL, Gann JJ, Hwang PS, Willoughby DS. Skeletal Muscle Estrogen Receptor Activation in Response to Eccentric Exercise Up-Regulates Myogenic-Related Gene Expression Independent of Differing Serum Estradiol Levels Occurring during the Human Menstrual Cycle. J Sports Sci Med. 2018;17(1):31–9. Exercise is capable of increasing ER-alpha, which has significant impacts into gene expression regulated by estrogen. [PMC free article] [PubMed] [Google Scholar]
- 117.Sellami M, Dhahbi W, Hayes LD, Kuvacic G, Milic M, Padulo J. The effect of acute and chronic exercise on steroid hormone fluctuations in young and middle-aged men. Steroids. 2018;132:18–24. [DOI] [PubMed] [Google Scholar]
- 118.Hayes LD, Herbert P, Sculthorpe NF, Grace FM. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr Connect. 2017;6(5):306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. *.Rossetti ML, Steiner JL, Gordon BS. Androgen-mediated regulation of skeletal muscle protein balance. Mol Cell Endocrinol. 2017;447:35–44. The effects of castration in autophagy in this novel mechanism are mediated by anabolic signaling (Akt, ERK) and the activity of AMPK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. *.Fink J, Schoenfeld BJ, Nakazato K. The role of hormones in muscle hypertrophy. Phys Sportsmed. 2018;46(1):129–34. Circulating testosterone interacts with anabolic factors (IGF-1) to increase skeletal muscle hypertrophy. [DOI] [PubMed] [Google Scholar]
- 121. *.Isaksson S, Bogefors K, Stahl O, Eberhard J, Giwercman YL, Leijonhufvud I, et al. High risk of hypogonadism in young male cancer survivors. Clin Endocrinol (Oxf). 2018;88(3):432–41. Hypogonadism is highly correlated to cancer in males even following survival. [DOI] [PubMed] [Google Scholar]
- 122. **.Slowikowski BK, Lianeri M, Jagodzinski PP. Exploring estrogenic activity in lung cancer. Mol Biol Rep. 2017;44(1):35–50. Decreased circulating estrogen levels associated with age and cancer status influence lung cancer pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, et al. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39(6):1016–24. [DOI] [PubMed] [Google Scholar]
- 124. *.Wright TJ, Dillon EL, Durham WJ, Chamberlain A, Randolph KM, Danesi C, et al. A randomized trial of adjunct testosterone for cancer-related muscle loss in men and women. J Cachexia Sarcopenia Muscle. 2018;9(3):482–96. Testosterone therapy benefits quality of life and lean body mass in men and women with cachexia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clavell-Hernandez J, Wang R. Emerging Evidences in the Long Standing Controversy Regarding Testosterone Replacement Therapy and Cardiovascular Events. World J Mens Health. 2018;36(2):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lagranha CJ, Silva TLA, Silva SCA, Braz GRF, da Silva AI, Fernandes MP, et al. Protective effects of estrogen against cardiovascular disease mediated via oxidative stress in the brain. Life Sci. 2018;192:190–8. [DOI] [PubMed] [Google Scholar]
- 127. *.Patel S, Homaei A, Raju AB, Meher BR. Estrogen: The necessary evil for human health, and ways to tame it. Biomed Pharmacother. 2018;102:403–11. The many positive effects of estrogen hormone replacement therapy are confounded by increased incidences of disease. [DOI] [PubMed] [Google Scholar]
- 128. *.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33 Estrogen-based hormone replacement therapy can benefit the heart and cardiovascular processes, but long-term use of exogenous estrogen may increase cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. *.Wang K, Li F, Chen L, Lai YM, Zhang X, Li HY. Change in risk of breast cancer after receiving hormone replacement therapy by considering effect-modifiers: a systematic review and dose-response meta-analysis of prospective studies. Oncotarget. 2017;8(46):81109–24. The risk of long-term hormone replacement therapy peaks at approximately 8–10 years of consistent use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. *.Wang Y, Lewin N, Qaoud Y, Rajaee AN, Scheer AS. The oncologic impact of hormone replacement therapy in premenopausal breast cancer survivors: A systematic review. Breast. 2018;40:123–30. Extended hormone replacement therapy for pre-menopausal breast cancer survivors increases risk of recurrence post-menopause. [DOI] [PubMed] [Google Scholar]
- 131. *.Argiles JM, Lopez-Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J. 2017;474(16):2663–78. Clinical studies involving SARMs show promise to reduce cancer-cachexia associated pathology. [DOI] [PubMed] [Google Scholar]
- 132. *.Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9(5):369–82. The tissue-specific non-steroidal nature of SARMs provides a unique therapeutic alternative to traditional hormone replacement therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. *.Anderson LJ, Albrecht ED, Garcia JM. Update on Management of Cancer-Related Cachexia. Curr Oncol Rep. 2017;19(1):3 Pre-clinical evaluation of SARMs demonstrates improved muscle mass without damaging reproductive organs in male rats. [DOI] [PubMed] [Google Scholar]
- 134. *.Morimoto M, Aikawa K, Hara T, Yamaoka M. Prevention of body weight loss and sarcopenia by a novel selective androgen receptor modulator in cancer cachexia models. Oncol Lett. 2017;14(6):8066–71. The novel role of SARMs to attenuate cachectic pathology using tumor-bearing and castrated mice demonstrates that SARMs are capable of attenuating wasting and reducing inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. *.Ponnusamy S, Sullivan RD, Thiyagarajan T, Tillmann H, Getzenberg RH, Narayanan R. Tissue Selective Androgen Receptor Modulators (SARMs) Increase Pelvic Floor Muscle Mass in Ovariectomized Mice. J Cell Biochem. 2017;118(3):640–6. This study provided new information in the novel study of SARMs to increase skeletal muscle homeostasis and prevent catabolism and an OVX model. [DOI] [PubMed] [Google Scholar]
- 136. *.Pinto JA, Vallejos CS, Raez LE, Mas LA, Ruiz R, Torres-Roman JS, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open. 2018;3(3):e000344 Lung cancer has historically demonstrated sexual dimorphism, effecting males more often than females. [DOI] [PMC free article] [PubMed] [Google Scholar]