Abstract
Background:
Electroconvulsive therapy (ECT) is an important treatment option for patients with major depressive disorder (MDD). However, the mechanisms of ECT in MDD are still unclear.
Methods:
Twenty-four patients with severe MDD and 14 healthy controls were enrolled in this study. Eight ECT sessions were conducted for MDD patients using brief-pulse square-wave signal at bitemporal locations. To investigate the regional cerebral blood flow in MDD patients before and after ECT treatments by resting-state functional magnetic resonance imaging (rs-fMRI), the patients were scanned twice (before the first ECT and after the eighth ECT) for data acquisition. Afterward, we adopted fractional amplitude of low-frequency fluctuations (fALFF) to assess the alterations of regional brain activity.
Results:
Compared with healthy controls, the fALFF in the cerebellum lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus in MDD patients before ECT (pre-ECT) was significantly increased. In another comparison, the fALFF in the cerebellum anterior lobe, fusiform gyrus, insula, parahippocampal gyrus, middle frontal gyrus, and inferior frontal gyrus in pre-ECT patients was significantly greater than the post-ECT fALFF.
Limitations:
Only two rs-fMRI scans were conducted at predefined times: before the first and after the eighth ECT treatment. More scans during the ECT sessions would yield more information. In addition, the sample size in this study was limited. The number of control subjects was relatively small. A larger number of subjects would produce more robust findings.
Conclusions:
The fALFF of both healthy controls and post-ECT patients in cerebellum anterior lobe, fusiform gyrus, and parahippocampal gyrus is significantly lower than the fALFF of pre-ECT patients. This finding demonstrates that ECT treatment is effective on these brain areas in MDD patients.
Keywords: Electroconvulsive therapy, Resting-state functional magnetic resonance imaging (rs-fMRI), Major depressive disorder, Fractional Amplitude of low-frequency fluctuations
1. Introduction
Major depressive disorder (MDD) is an increasingly common mental disease and is of great concern. To some extent, several common treatments such as cognitive behavioral therapy (Ritchey et al., 2011, Siegle et al., 2006) and antidepressant medication (Sheline et al., 2012) are effective for depressive patients. However, electroconvulsive therapy (ECT) is regarded as the most effective and rapid treatment for depression (Bouckaert et al., 2016, Husain et al., 2004, Kellner et al., 2016, Kellner et al., 2016), and it is a vital antidepressant treatment selection for most patients with MDD. For patients with severe mental illness, ECT is considered to be psychiatry’s “good standard” treatment. Despite its effective outcome, the mechanisms of ECT on MDD are still unclear. It is important to understand the intrinsic mechanisms that may allow further improvements of ECT and reduce its side effects, such as nausea, headache, jaw pain, and muscle ache (Wei et al., 2014).
Previous studies on antidepressant treatment response have reported the effect of gray matter volumes and cortical thickness related to improvements in MDD patients (Ota et al., 2015, Sheline et al., 2012). For ECT treatment, certain changes were reported in the structure of brain gray matter in MDD patients. Bouckaert et al. (Bouckaert et al., 2016) drew the conclusion that ECT can significantly increase the volume of gray matter. Pirnia et al. (Pirnia et al., 2016) showed that several brain regions, including the bilateral anterior cingulate cortex (ACC); inferior and superior temporal, parahippocampal, entorhinal, and fusiform cortex; and distributed prefrontal areas became thicker after ECT treatment. Ota et al. (Ota et al., 2015) reported significant volume increases after ECT in the bilateral medial temporal cortices, inferior temporal cortices, and right anterior cingulate.
Besides assessing structural variations of the brain, functional alterations of brain activity have been measured in MDD patients using advanced imaging techniques. Functional magnetic resonance imaging (fMRI) was employed to explore functional alterations in MDD patients in comparison with healthy controls. Using this technique, Kaiser et al. (Kaiser et al., 2015) demonstrated large-scale network dysfunctions in MDD, including imbalanced connectivity among networks involved in regulating attention to the internal or external world and decreased connectivity among networks involved in regulating or responding to emotion or salience. fMRI has also been found to be a useful tool for measuring the response to antidepressant treatments (Liston et al., 2014, Posner et al., 2013). Beall et al. (Beall et al., 2012) adopted task fMRI and found that remission after ECT for MDD was characterized by decreased activation in emotional regulation but increased resting connectivity. Abbott et al. (Abbott et al., 2013) used resting-state fMRI (rs-fMRI) to investigate the response to ECT for MDD. It was found that successful ECT resulted in an increase in functional network connectivity between two pairs of the network: posterior default mode and the dorsomedial prefrontal cortex (MPFC) as well as posterior default mode and left dorsal lateral prefrontal cortex. Moreover, some data-driven approaches were used to find consistent changes after ECT treatment in MDD patients (Leaver et al., 2016, Perrin et al., 2012). However, there is still a lack of studies on the effectiveness of the ECT response in MDD patients using rs-fMRI (Dichter et al., 2015, Kong et al., 2017).
Because Biswal et al. (Biswal et al., 1995) adopted rs-fMRI to investigate human brain function, considerable studies have been conducted to reveal intrinsic spontaneous brain activity. Most researchers examined spontaneous low-frequency oscillations at the frequency band of 0.01 to 0.08 Hz. Meanwhile, many studies have presented different measures of the nature of rs-fMRI. Among them, the amplitude of low-frequency fluctuations (ALFF) is a reliable measure of whole-brain rs-fMRI signals (Kong et al., 2017, Zou et al., 2008). The ALFF has been widely adopted because it directly correlates to the intensity of spontaneous neural activity in the resting state with regard to energy metabolism (Qi et al., 2012, Yu-Feng et al., 2007). Moreover, Zou et al. (Zou et al., 2008) proposed that fractional ALFF (fALFF) reduces the effects of physiological noise. The fALFF represents the ratio of the power spectrum of low-frequency (0.01–0.08 Hz) to that of the entire frequency range. Using the fALFF, one can measure functional abnormalities in patients with affective disorders by rs-fMRI signals. As a whole-brain data-driven method with high test-retest reliability, the fALFF has been selected for carrying out many studies in patients with MDD (Guo et al., 2013, Lai and Wu, 2015, Liu et al., 2013, Wang et al., 2012). Some significant alterations have been reported in MDD patients in some brain regions, such as the limbic system and the cerebellum. Because ECT is an effective antidepressant treatment for MDD, there should be some significant alterations in specific brain regions in MDD patients before and after ECT. Thus, we adopted rs-fMRI and fALFF to assess brain function alterations and illustrate the effectiveness of ECT treatment.
2. Materials and methods
2.1. Participants
Inpatients (14 women and 10 men, aged 31.33 ± 10.79, range 18–55 years) who had been diagnosed with major depression were recruited from the Mental Health Center, the First Affiliated Hospital of Chongqing Medical University. Healthy controls (10 women and 4 men, aged 33.29±10.36, range 20–56 years) were also selected to take part in this study. Ethical approval was acquired from the Local Medical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and this study was carried out in accordance with the Declaration of Helsinki. Two psychiatrists conducted the diagnoses and structured clinical interviews for all participant patients who had a unipolar depressive episode as determined by Diagnostic and Statistical Manual (DSM)-IV criteria for MDD (First et al., 2002) and the Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1967). All the patients were under severe depression and actively seeking effective treatment. Before ECT, they underwent physical examination, electroencephalogram, electrocardiogram, blood test, and X-ray. Moreover, they were excluded from the study if they had received ECT, mood stabilizers, antidepressants, or antipsychotics within the past 1 month.
Additional inclusion criteria for the MDD patients were (1) agreement to receive ECT (consent provided either by the patient or, in certain cases, a direct relative), (2) aged between 16 and 60 years, (3) HAMD scores greater than 21, and (4) no contraindication to ECT treatments. Exclusion criteria for all subjects included (1) contraindication to MRI scan, (2) neurological disorders, (3) severe somatic disease, (4) substance abuse, (5) pregnancy, (6) lactation, or (7) depression caused by or combined with somatic disease and other psychiatric disorders (Cao et al., 2018). Healthy controls had no personal or family history of any psychiatric disorder.
2.2. ECT procedure
ECT was performed using the Thymatron DGx system (Somatics LLC, Lake Bluff, IL, USA) at the Mental Health Center, the First Affiliated Hospital of Chongqing Medical University. Over the course of 3 weeks, all 24 patients underwent 8 sessions of ECT treatment, including 3 sessions per week (Monday, Wednesday, and Friday mornings) for the first 2 weeks and 2 sessions (Monday and Friday mornings) during the third week. All patients were restricted from water and food intake beginning at midnight before ECT. The time and frequency of ECT treatment were the same for all patients. They were administered the HAMD rating scale, MRI scan, and fMRI scan before the first ECT and after the eighth ECT. During this period, antipsychotics and antidepressants were not used.
The intensity of electric stimulation was individually adjusted based on seizure response and adverse effects experienced during the ECT process, if any. The energy setting percentage was adjusted by integral multiples of 5. During the first ECT session, the seizure threshold was measured by the smallest electrical dose that produced a seizure of at least 25 seconds on the electroencephalogram (Abrams, 2002). If the initial dosage failed to elicit a seizure, the age-adjusted titration was adopted to increase by 5% the output charge of the ECT device every time, and the patient was restimulated after 30 seconds. Electric stimulation was conducted at most 3 times during an ECT session. If the seizure threshold was not achieved in the first session, stimulation with twice the last dose in the next session was performed. The electrical dosage was set at 1.5 to 2 times the seizure threshold in consecutive ECT sessions according to the extent of the seizure (Kennedy et al., 2009). The patients were administered anesthesia with succinylcholine (0.5–1.0 mg/kg) and sodium thiopental (3.0–5.0 mg/kg) during the ECT procedure.
ECT was continued if clinical symptoms of depression had not sufficiently improved after 8 sessions, as determined by a clinician, for a maximum of 12 ECT sessions. However, for the sake of comparability, the MRI scans after treatments were performed after the fixed amount of 8 ECTs (Reininghaus et al., 2013). After the end of the ECT treatments, the doctor selected the antidepressant for the patient according to the patient’s age, course of disease, severity, physical condition, and previous medication.
2.3. Clinical measure of depression
The 24-item HAMD Rating Scale was adopted to measure depressive symptoms. The clinical assessments of depression were completed within 24 hours before the first ECT treatment (pre-ECT) and 24 hours after the eighth ECT treatment (post-ECT).
2.4. Image data acquisition
A 3.0-Tesla MRI system (GE Signa) using an echo planar imaging sequence was used to obtain the image data at the First Affiliated Hospital of Chongqing Medical University. During the scanning, all subjects relaxed, kept their eyes closed, and remained awake; meanwhile, they did not think anything specific. The parameters of resting-state functional images were recorded as follows: repetition time, 2000 milliseconds; echo time, 30 milliseconds; field of view, 240 × 240 mm2; data matrix, 64 × 64; flip angle, 90°; 30 slices; slice thickness, 5 mm; and scanner time, 6 minutes 50 seconds (200 volumes obtained).
2.5. Data preprocessing
Using the statistical parametric mapping (SPM) software platform, we adopted the Data Processing Assistant for rs-fMRI (DPABI, by YAN Chao-Gan et al (Yan et al., 2016), http://www.restfmri.net) to conduct functional image preprocessing. The first 10 volumes were abandoned to allow the subjects time to adapt to the scanning process. Thus, we analyzed the remaining 190 volumes. Head motion corrections were carried out primarily. We demanded that all subjects’ maximum displacements in the 3 coordinate axes to be no more than 1.5 mm and angular motions no more than 1.5°. In addition, the slice corrections were fulfilled for the acquisition delay. Then, fMRI images were registered to the standard Montreal Neurological Institute (MNI) space with normalization and resampling to 3 × 3 × 3 mm3. Moreover, a Gaussian kernel of 8-mm full-width at half-maximum was used to smooth the functional images. Finally, normalized image data were band-pass filtered (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency noise, such as cardiac and respiratory noise.
2.6. fALFF analyses
The filtered time series of a given voxel was converted to a frequency domain through the fast Fourier transform process. Then, the ALFF value for the voxel was calculated as the average square root of the power spectrum. To enhance the specificity and sensitivity, the fALFF value was computed as the ratio of the power in the specific frequency band to that of the whole detected frequency range for suppressing nonspecific signals in the rs-fMRI data. In our study, we computed the fALFF values before and after ECT in MDD patients.
2.7. Statistical analyses
To investigate the fALFF distinctions between the pre-ECT and the post-ECT, the influence of the ECT treatment for MDD was analyzed by the Resting-State fMRI Data Analysis (REST) Toolkit (Song et al., 2011). We applied a 2-sample, two-sided t-tests to measure whether the fALFF value of one brain area is significantly different from the other one. Paired t-tests were used to assess the fALFF difference between pre-ECT and post-ECT. We corrected statistical maps for multiple comparisons to a significance level of ?? < 0.05 (bilateral) by AlphaSim combining the height threshold P < 0.01 and minimum cluster size = 85. The regions of interesting (ROIs) in the brain regions described the significantly different fALFF values before and after ECT. The coordinates of the ROIs were indicated at the peak density region of the paired t-test fALFF map.
3. Results
3.1. Clinical results
Twenty-four patients (14 women, 10 men, right-handed, 31.33 ± 10.79 years old) with severe MDD were enrolled in the study from the Inpatient Department of Psychiatry at the First Affiliated Hospital of Chongqing Medical University. The patients were examined using the 24-item HAMD, and the mean pre-ECT score was 31.33 ± 4.55 . Details are provided in Table 1. Their depression symptoms were significantly improved after the ECT sessions ( t21 = 15.52, p < 0.0001; paired t-test). From the clinical data, 22 patients experienced at least a 50% reduction in post-ECT score compared with pre-ECT score. Moreover, 12 patients were in remission (their HAMD scores were lower than 7) after ECT treatment.
Table 1.
Demographic data of the MDD patients and healthy control groups
| Characteristic | MDD group | HC group | P | |
|---|---|---|---|---|
| 24 | 14 | |||
| Sex (Male/Female) | 10/14 | 4/10 | ||
| Age (mean (SD)) | 31.33±10.79 | 33.29±10.36 | 0.520* | |
| Education year (mean ±SD) |
11.04±2.76 | 15.07±3.47 | 0.087* | |
| HAMD | Pre-ECT | Post-ECT | 2.21±1.25 | <0.001** |
| 31.33±4.55 | 8.58±5.62 | |||
MDD=major depressive disorder; HC=healthy control; ECT=electroconvulsive therapy; HAMD=Hamilton Rating Scale for Depressive.
Mann-whitney U nonparametric tests (criteria alpha=0.05).
Paired t tests between pre-ECT and post-ECT MDD group.
3.2. fALFF results
In this study, alterations in fALFF were observed in patients with MDD before and after ECT. We compared the alterations between the pre-ECT patients and healthy controls. A two-sample t-test was performed. The results are shown in Figure 1. It can be observed that fALFF in some brain regions increased significantly. Table 2 shows the group differences in fALFF between the healthy controls and pre-ECT patients with MDD. Compared with healthy controls, fALFF in the cerebellum lobe, limbic lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus increased significantly in MDD patients pre-ECT.
Fig. 1.
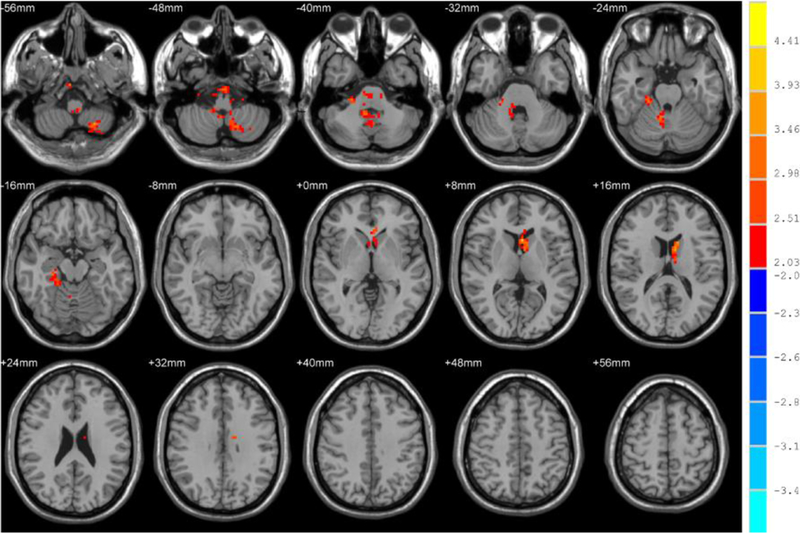
Brain regions that showed significant alterations in fALFF in the typical band (0.01–0.08 Hz) between healthy controls and patients before ECT. For display purposes only, all statistical maps are overlaid on a T1-weighted MNI template. The hot color denotes pre-ECT > healthy controls in the fALFF values, and T-score bars are shown on the right side. The brain regions with significant increases in fALFF include the cerebellum lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus.
Table 2.
fALFF differences in brain regions between pre-ECT patients and HC
| Brain region | Side | MNI coordinates |
Voxels | t values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Pre-ECT > HC | ||||||
| Cerebellum Lobe | R&L | −21 | −63 | −51 | 273 | 4.0060 |
| Parahippocampal | ||||||
| Gyrus, Fusiform | R | 21 | −36 | −18 | 91 | 3.3836 |
| Gyrus | ||||||
| Anterior Cingulate gyrus, Thalamus |
L | −3 | 15 | 9 | 155 | 4.0818 |
The fALFF alterations for the post-ECT patients and healthy controls were then assessed by two-sample t-tests. The results are shown in Figure 2 and Table 3. The cerebellum, left middle frontal gyrus, and caudate increased significantly in MDD patients after ECT as compared with healthy controls. However, the temporal lobe, parahippocampal gyrus, right middle frontal gyrus, and cingulate gyrus decreased significantly.
Fig. 2.
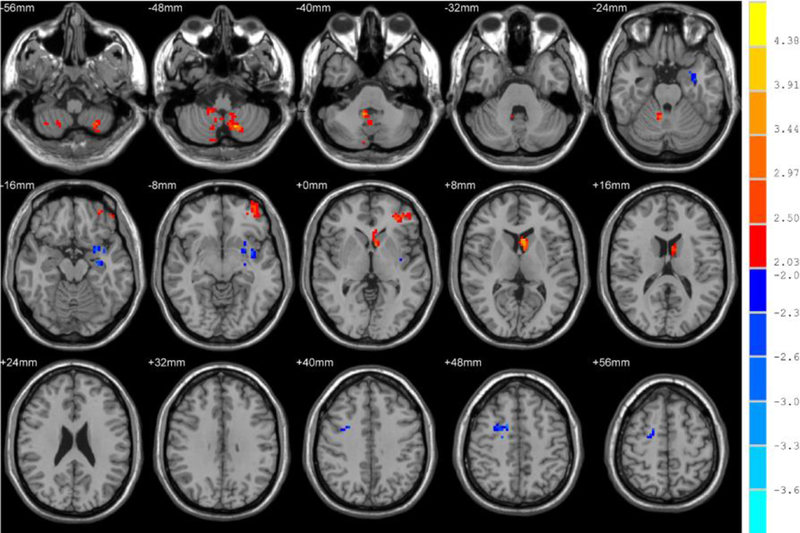
Brain regions that showed significant alterations in fALFF in the typical band (0.01–0.08 Hz) between healthy controls and patients after ECT. For display purposes only, all statistical maps are overlaid on a T1-weighted MNI template. The hot color denotes post-ECT > healthy controls in the fALFF values and the blue cold color denotes post-ECT < healthy controls. The T-score bars are shown on the right side. The brain regions with significant increases in fALFF include the cerebellum, left middle frontal gyrus, and caudate. However, temporal lobe, parahippocampal gyrus, right middle frontal gyrus, and cingulate gyrus decreased significantly.
Table 3.
fALFF differences in brain regions between post-ECT patients and HC
| Brain region | Side | MNI coordinates |
Voxels | t values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Post-ECT > HC | ||||||
| Left Cerebellum | L | −21 | −63 | −51 | 214 | 4.0134 |
| Middle Frontal Gyrus |
L | −36 | 45 | −3 | 111 | 3.9041 |
| Caudate | L | −3 | 12 | 6 | 93 | 4.1648 |
| Right Cerebellum | R | 21 | −60 | −54 | 88 | 3.1829 |
| Post-ECT < HC | ||||||
| Temporal Lobe, Parahippocampal |
L | −33 | −6 | −18 | 104 | −3.0025 |
| Gyrus | ||||||
| Middle Frontal | ||||||
| Gyrus, Cingulate Gyrus |
R | 15 | 6 | 48 | 101 | −3.1408 |
Finally, we used paired t-tests to display the differences shown in Figures 3 and 4. There were some significant fALFF differences between MDD patients after ECT and before ECT. As shown in Table 4, the cerebellum anterior lobe, parahippocampal gyrus, fusiform gyrus, middle frontal lobe, and inferior frontal gyrus decreased significantly in MDD patients after ECT compared with before ECT.
Fig. 3.
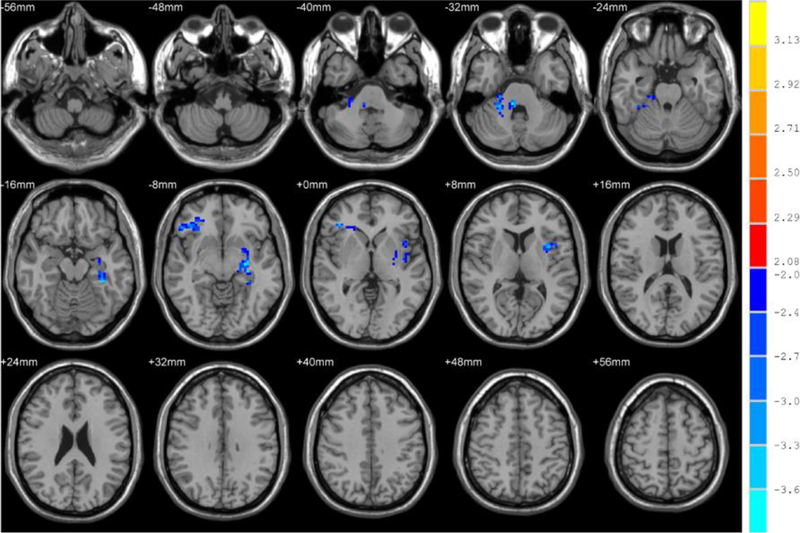
Brain regions that showed significant alterations in fALFF in the typical band (0.01–0.08 Hz) for patients before and after ECT. For display purposes only, all statistical maps are overlaid on a T1-weighted MNI template. The blue cold color denotes post-ECT < pre-ECT and T-score bars are shown on the right side. The brain regions with significant decreases in fALFF include the cerebellum lobe, parahippocampal gyrus, fusiform gyrus, middle frontal lobe, and inferior frontal gyrus.
Fig. 4.
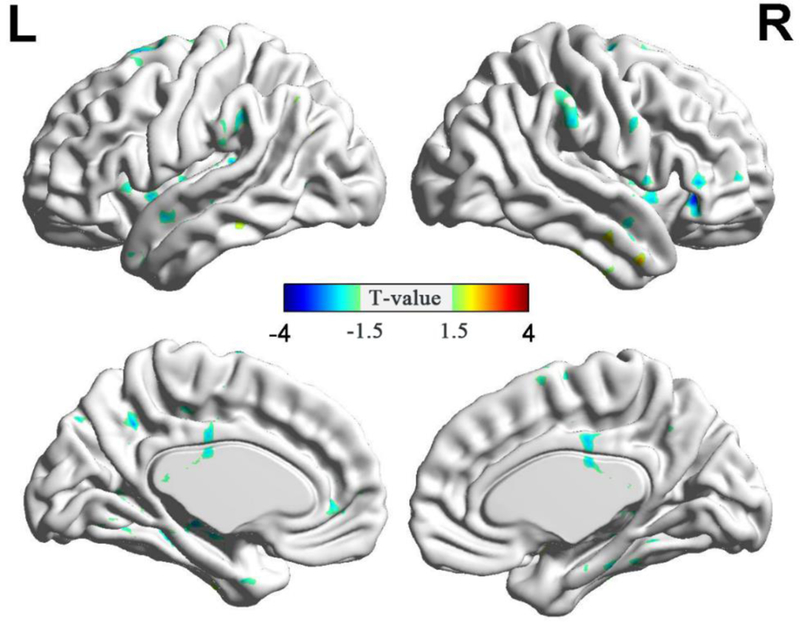
Significant alterations (P<0.05, AlphaSim-corrected; combined height threshold P<0.01 and minimum cluster size=85 voxels) in fALFF in the typical band (0.01–0.08 Hz) for patients before and after ECT. They are presented as inflated surface maps created using BrainNet Viewer (www.nitrc.org/projects/bnv). The blue cold color denotes post-ECT < pre-ECT.
Table 4.
fALFF differences in brain regions between post-ECT patients and pre-ECT
| Brain region | Side | MNI coordinates |
Voxels | t values | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Post-ECT<Pre-ECT | ||||||
| Cerebellum Anterior Lobe; Fusiform Gyrus. |
R | 9 | −36 | −33 | 89 | −3.7495 |
| Insula; Parahippocampal Gyrus. |
L | −30 | −24 | −9 | 186 | −3.9051 |
| Middle Frontal Gyrus; Inferior Frontal Gyrus |
R | 39 | 36 | −6 | 85 | −3.9811 |
4. Discussion
In this study, spontaneous neural activity changes in fALFF were observed in patients with MDD before and after ECT. Twenty-four patients with severe MDD were enrolled. Meanwhile, 14 healthy controls were also selected to take part in this study. The clinical assessments of depression were completed using the 24-item HAMD rating scale. All patients’ depressive symptoms were alleviated after ECT according to the evaluation. To illustrate the improvement between post-ECT and pre-ECT patients, we analyzed the changes in the different groups. First, we employed two-sample t-tests to compare the fALFF between the pre-ECT patients and the healthy controls. Cerebellum lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus increased significantly in MDD patients before ECT. Second, we assessed the differences in fALFF between the post-ECT patients and healthy controls using the two-sample t-tests. The brain regions including the cerebellum, middle frontal gyrus, and caudate demonstrated significant increases. However, the middle frontal gyrus, temporal lobe, parahippocampal gyrus, and cingulate gyrus decreased significantly. Finally, to compare the effectiveness of the ECT treatment, we compared the fALFF in the post-ECT patients with that of the pre-ECT patients using paired t-tests. Cerebellum lobe, parahippocampal gyrus, fusiform gyrus, middle frontal lobe, and inferior frontal gyrus decreased significantly in MDD patients after ECT.
Amplitude abnormalities were found in a wide variety of regions, including the cerebellum, limbic system, and frontal, temporal, parietal, and occipital regions (Dutta et al., 2014, Wang et al., 2012). Several previous rs-fMRI studies (Guo et al., 2013, Guo et al., 2013, Lai and Wu, 2015, Liu et al., 2013, Liu et al., 2013, Wang et al., 2012) also reported significantly different alterations in fALFF values in the specific brain regions in MDD patients. We conducted a comparison between pre-ECT patients and healthy controls. In our findings, the cerebellum lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus were increased. The fALFF values in the cerebellum lobe and fusiform gyrus were increased, which was consistent with the results reported by Wang et al. (Wang et al., 2012). On the other hand, Liu et al. (Liu et al., 2013) found a decrease in the cerebellum lobe and parahippocampal gyrus, which was opposite to our findings. In the present study, the fALFF of the parahippocampal gyrus and cingulate gyrus in pre-ECT patients increased significantly compared with the healthy controls. However, they decreased significantly in post-ECT patients after 8 ECT treatment sessions. The fALFF was even lower than those in the healthy controls. Moreover, the fALFF in the temporal lobe and middle frontal gyrus decreased significantly in post-ECT patients. These findings may further suggest that ECT has a therapeutic effect by reducing the increased local brain activity in MDD patients.
In our study, we observed a decreased fALFF in the medial frontal gyrus in post-ECT compared with pre-ECT patients. The MPFC is one of the two brain network hubs in the default mode network (DMN) (Bullmore and Sporns, 2009). The increased fALFF of the MPFC may be suggested as a biomarker for MDD (Liu et al., 2013). The finding of decreased fALFF in MPFC in post-ECT patients is in line with previous antidepressant treatments. In addition, we showed that the fALFF in the subgenual anterior cingulate cortex (sgACC) was significantly increased in pretreatment MDD patients compared with healthy controls. Although it is still unclear whether the ALFF in the ACC is increased or decreased between MDD patients and healthy controls (Liu et al., 2014, Liu et al., 2015), the sgACC has been demonstrated to be a critical region related to depression (Drevets et al., 2008). The DMN, which contains some important brain regions such as the MPFC, the posterior cingulate, and the precuneus, has been reported to be hyperactive in depressive patients (Nejad et al., 2013, Sheline et al., 2010). Abnormal hyperactivity and hyperconnectivity between the sgACC and other DMN areas are also reported in MDD (Liston et al., 2014). In this study, the alterations in the MPFC and the sgACC showed that DMN might be associated with MDD and the efficacy of the ECT treatment.
What’s more, we determined that the pre-ECT patients had a higher fALFF in the parahippocampal gyrus than the healthy controls. Accordingly, the post-ECT patients had a reduced fALFF as compared with the pre-ECT patients. The parahippocampal gyrus, located in the inferior medial temporal lobe, is one of the key parts of the memory loop. The parahippocampal region plays a major role in detailed memory retrieval of both the spatial and temporal context (Eichenbaum and Lipton, 2008). Because it is associated with contextual associations or episodic memory, the parahippocampal gyrus is found as a center of reliable overactivity during affective processing tasks (Miller et al., 2015). Previous studies (Miller et al., 2015, Sankar et al., 2015, Wang et al., 2017, Young et al., 2012) also reported a hyperactive response in the parahippocampus in depressive patients as compared with healthy controls. Thus, our findings of decreased fALFF values in post-ECT patients compared with pre-ECT patients provide further evidence that the parahippocampus is associated with the efficacy of ECT treatment in patients with MDD.
There was also an increase in the cerebellum in pre-ECT MDD patients compared with the healthy subjects in our study. For further comparison, we observed that the post-ECT patients had a lower fALFF in the cerebellum than pre-ECT patients. As a result, both healthy controls and post-ECT MDD patients have decreased fALFF in the cerebellum as compared with the pre-ECT patients. Many frontal and limbic regions are connected by the cerebellum. These areas are important for emotional and cognitive processing (Dutta et al., 2014, Schmahmann and Caplan, 2006). Fitzgerald et al. identified that the cerebellum is relevant to depression (Fitzgerald et al., 2008). Furthermore, the increased brain activity of pre-ECT patients in the cerebellum has been reported by previous studies (Guo et al., 2012, Guo et al., 2013, Wang et al., 2012). In particular, Wang et al. (Wang et al., 2012) used the fALFF to observe the increase in the cerebellum in MDD patients. Considering these reports and the effectiveness of the ECT treatment on the MDD patients in our study, the depressive symptoms of post-ECT patients may be improved by the changes in fALFF in the cerebellum.
The fusiform gyrus is within the visual recognition network and is considered to be involved in facial stimuli (Tao et al., 2013). The abnormal activity of the fusiform gyrus has been related to some symptoms of depression (Demenescu et al., 2011). Moreover, the altered responsiveness of impaired facial emotion processing has been employed as a biomarker of the early diagnosis of MDD (Hahn et al., 2011). Wang et al. (Wang et al., 2012) reported that the bilateral fusiform gyrus of MDD patients was increased in fALFF. Similarly, we observed that the fALFF in the fusiform gyrus of the pre-ECT patients increased significantly in our study. For the post-ECT patients, their fALFF was significantly lower than pre-ECT.
In previous rs-fMRI studies, abnormal activity in the thalamus was found in depressed subjects (Miller et al., 2015, Peng et al., 2011, Zhao et al., 2014). We also observed a higher fALFF in pre-ECT patients as compared with healthy controls in our study. However, we did not find significant alterations in the thalamus between post-ECT and pre-ECT patients. The insula is another brain region associated with depressive symptoms in MDD (Avery et al., 2014, Hamilton et al., 2015, Pigoni et al., 2017). Although there are no significant changes between pre-ECT patients and healthy controls, we observed a decreased fALFF in post-ECT patients compared with pre-ECT patients.
The voxel-based morphology method was adopted to study the effect of ECT treatment on the brain structure of patients with depression in our previous work (Qiu et al., 2016). We found that the volumes of the bilateral amygdala and hippocampus were increased. Some studies (Bouckaert et al., 2016, Ota et al., 2015, Pirnia et al., 2016) reported that the brain structure in MDD patients had changed through ECT treatment. After ECT, brain regions including the bilateral medial temporal, parahippocampal, entorhinal, and fusiform cortex became thicker. In this study, we used the fALFF to measure the brain’s functional activity and found alterations in related brain regions. The findings may be helpful in understanding the mechanisms of ECT’s antidepressant effects.
Several limitations in our study should be considered. First, we scanned the patients only twice at predefined times (before and after ECT) separately. More scans during the ECT sessions could be performed to detect more alterations of brain activity in MDD patients. In addition, the number of ECT treatments usually depends on the speed of recovery in clinical practice, so unresponsive patients tend to receive more ECT sessions on average (Oltedal et al. 2018). However, for the sake of comparability, we decided to conduct MRI scans after a fixed amount of 8 ECT treatments. This might have obscured the real connection between fALFF and clinical symptoms. In this study, we concluded that the fALFF was changed in some brain regions in MDD after 8 ECT sessions. A more detailed longitudinal study should be conducted to determine whether the fALFF and clinical symptoms are correlated before and after ECT in MDD. In addition, the sample size was quite small. The number of control subjects was relatively small. A greater number of subjects would help us observe more robust findings. Future studies may consider enrolling more subjects in our experiments. Finally, although ECT has been demonstrated to be an effective treatment of MDD, side effects may sometimes still emerge due to ECT.
5. Conclusion
In the present study, we used the fALFF to measure abnormalities in MDD patients and the changes between pre-ECT and post-ECT. Compared with healthy controls, pre-ECT patients showed a significant increase in fALFF in the cerebellum lobe, parahippocampal gyrus, fusiform gyrus, anterior cingulate gyrus, and thalamus. Moreover, the cerebellum anterior lobe, temporal lobe, middle frontal lobe, and inferior frontal gyrus decreased significantly in post-ECT patients who underwent the ECT treatment sessions. In comparison, ECT has been shown to have an effect on the decrease in cerebellum anterior lobe, fusiform gyrus, and parahippocampal gyrus in post-ECT patients, similar to that of healthy controls. These findings may partly demonstrate the mechanism of ECT’s antidepressant effects.
Highlights.
fALFF was used to study ECT response in MDD patients.
The pairwise comparisons were made between healthy controls, pre-and post-ECT MDD.
The antidepressant and side effects were related to the changes in specific regions.
Acknowledgements
We gratefully acknowledge and thank all the subjects for their time and effort participating in the study.
Funding
This work was financially supported in part by the Natural Science Foundation of China (Grant nos. 61501069, 51377179), the Basic and Advanced Research Project in Chongqing (Grant no. cstc2018jcyjAX0440), the Fundamental Research Funds for the Central Universities of China (Grant nos. 106112015CDJXY160003, 10611CDJXZ238826), and the National Institutes of Health (Grant no. R01EB013174) in the United States. The fundings listed here were used to design the study and collect the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
Reference
- Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA, 2013. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front Psychiatry 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams R, 2002. Electroconvulsive therapy (4th ed.).
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK, 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76 (3), 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EB, Malone DA, Dale RM, Muzina DJ, Koenig KA, Bhattacharrya PK, Jones SE, Phillips MD, Lowe MJ, 2012. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. J ECT 28 (4), 234–241. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magnetic resonance in medicine 34 (4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bouckaert F, De Winter FL, Emsell L, Dols A, Rhebergen D, Wampers M, Sunaert S, Stek M, Sienaert P, Vandenbulcke M, 2016. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci 41 (2), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Sporns O, 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience 10 (3), 186–198. [DOI] [PubMed] [Google Scholar]
- Cao B, Luo QH, Fu YX, Du L, Qiu T, Yang XY, Chen XL, Chen QB, Soares JC, Cho RY, Zhang XY, Qiu HT, 2018. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Scientific Reports 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu LR, Renken R, Kortekaas R, van Tol MJ, Marsman JBC, van Buchem MA, van der Wee NJA, Veltman DJ, den Boer A, Aleman A, 2011. Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study. Psychological Medicine 41 (11), 2253–2264. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ, 2015. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord 172, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M, 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13 (8), 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, McKie S, Deakin JF, 2014. Resting state networks in major depressive disorder. Psychiatry Res 224 (3), 139–151. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA, 2008. Towards a Functional Organization of the Medial Temporal Lobe Memory System: Role of the Parahippocampal and Medial Entorhinal Cortical Areas. Hippocampus 18 (12), 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P) New York: Biometrics Research. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ, 2008. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping 29 (6), 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. b., Liu F, Xue Z. m., Xu X. j., Wu R. r., Ma C. q., Wooderson SC, Tan C. l., Sun X. l., Chen J. d., Liu Z. n., Xiao C. q., Chen H. f., Zhao J. p., 2012. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: A resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 37 (1), 153–160. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Liu J, Yu L, Zhang Z, Zhang J, Chen H, Xiao C, 2013. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry 46, 13–18. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Zhang J, Zhang Z, Yu L, Liu J, Chen H, Xiao C, 2013. Dissociation of regional activity in the default mode network in first-episode, drug-naive major depressive disorder at rest. J Affect Disord 151 (3), 1097–1101. [DOI] [PubMed] [Google Scholar]
- Hahn T, Marquand AF, Ehlis A-C, Dresler T, Kittel-Schneider S, Jarczok TA, Lesch K-P, Jakob PM, Mourao-Miranda J, Brammer MJ, Fallgatter AJ, 2011. Integrating Neurobiological Markers of Depression. Archives of General Psychiatry 68 (4), 361–368. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry 78 (4), 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1967. Development of a rating scale for primary depressive illness. British Journal of Clinical Psychology 6 (4), 278–296. [DOI] [PubMed] [Google Scholar]
- Husain SS, Kevan IM, Linnell R, Scott AI, 2004. Electroconvulsive therapy in depressive illness that has not responded to drug treatment. Journal of affective disorders 83 (2), 121–126. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72 (6), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM, Mueller M, Prudic J, Greenberg RM, Weiner RD, Bailine SH, Rosenquist PB, Raza A, Kaliora S, Latoussakis V, Tobias KG, Briggs MC, Liebman LS, Geduldig ET, Teklehaimanot AA, Dooley M, Lisanby SH, Group CPW, 2016. A Novel Strategy for Continuation ECT in Geriatric Depression: Phase 2 of the PRIDE Study. Am J Psychiatry 173 (11), 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM, Mueller M, Prudic J, Greenberg RM, Weiner RD, Bailine SH, Rosenquist PB, Raza A, Kaliora S, Latoussakis V, Tobias KG, Briggs MC, Liebman LS, Geduldig ET, Teklehaimanot AA, Lisanby SH, Group CPW, 2016. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Psychiatry 173 (11), 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, Parikh SV, Patten SB, Ravindran AV, 2009. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. Journal of Affective Disorders 117, S1–S2. [DOI] [PubMed] [Google Scholar]
- Kong XM, Xu SX, Sun Y, Wang KY, Wang C, Zhang J, Xia JX, Zhang L, Tan BJ, Xie XH, 2017. Electroconvulsive therapy changes the regional resting state function measured by regional homogeneity (ReHo) and amplitude of low frequency fluctuations (ALFF) in elderly major depressive disorder patients: An exploratory study. Psychiatry Res 264, 13–21. [DOI] [PubMed] [Google Scholar]
- Lai CH, Wu YT, 2015. The patterns of fractional amplitude of low-frequency fluctuations in depression patients: the dissociation between temporal regions and fronto-parietal regions. J Affect Disord 175, 441–445. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Espinoza R, Pirnia T, Joshi SH, Woods RP, Narr KL, 2016. Modulation of intrinsic brain activity by electroconvulsive therapy in major depression. Biological psychiatry: cognitive neuroscience and neuroimaging 1 (1), 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ, 2014. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76 (7), 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Ma X, Wu X, Fan TT, Zhang Y, Zhou FC, Li LJ, Li F, Tie CL, Li SF, Zhang D, Zhou Z, Dong J, Wang YJ, Yao L, Wang CY, 2013. Resting-state brain activity in major depressive disorder patients and their siblings. J Affect Disord 149 (1–3), 299–306. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z, Wang Y, Li J, Hu M, Zhang J, Du H, Zeng L, Liu Z, Wooderson SC, Tan C, Zhao J, Chen H, 2013. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 146 (3), 401–406. [DOI] [PubMed] [Google Scholar]
- Liu J, Ren L, Womer FY, Wang J, Fan G, Jiang W, Blumberg HP, Tang Y, Xu K, Wang F, 2014. Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Hum Brain Mapp 35 (10), 4979–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Du L, Li Y, Liu H, Zhao W, Liu D, Zeng J, Li X, Fu Y, Qiu H, Li X, Qiu T, Hu H, Meng H, Luo Q, 2015. Antidepressant Effects of Electroconvulsive Therapy Correlate With Subgenual Anterior Cingulate Activity and Connectivity in Depression. Medicine (Baltimore) 94 (45), e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, Gotlib IH, 2015. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry 72 (10), 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C, 2013. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci 7, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Narr KL, Abbott C, Anand A, et al. , 2018. Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy. Biol Psychiat [Epub ahead of print] https://doi.org/10.1016/j.biopsych.2018.05.017. [DOI] [PMC free article] [PubMed]
- Ota M, Noda T, Sato N, Okazaki M, Ishikawa M, Hattori K, Hori H, Sasayama D, Teraishi T, Sone D, 2015. Effect of electroconvulsive therapy on gray matter volume in major depressive disorder. Journal of affective disorders 186, 186–191. [DOI] [PubMed] [Google Scholar]
- Peng DH, Jiang KD, Fang YR, Xu YF, Shen T, Long XY, Liu J, Zang YF, 2011. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl) 124 (3), 369–373. [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C, 2012. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proceedings of the National Academy of Sciences 109 (14), 5464–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigoni A, Delvecchio G, Altamura AC, Soares JC, Fagnani C, Brambilla P, 2017. The role of genes and environment on brain alterations in Major Depressive Disorder: A review of twin studies. Journal of Affective Disorders [DOI] [PubMed]
- Pirnia T, Joshi S, Leaver A, Vasavada M, Njau S, Woods R, Espinoza R, Narr K, 2016. Electroconvulsive therapy and structural neuroplasticity in neocortical, limbic and paralimbic cortex. Translational psychiatry 6 (6), e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS, 2013. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry 70 (4), 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, Zhang L, Wu S, Zhong J, Zhang Z, Zhong Y, Ni L, Zhang Z, Li K, Jiao Q, 2012. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology 264 (1), 187–195. [DOI] [PubMed] [Google Scholar]
- Qiu HT, Li XR, Zhao WJ, Du L, Huang PY, Fu YX, Qiu T, Xie P, Meng HQ, Luo QH, 2016. Electroconvulsive Therapy-Induced Brain Structural and Functional Changes in Major Depressive Disorders: A Longitudinal Study. Medical Science Monitor 22, 4577–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus EZ, Reininghaus B, Ille R, Fitz W, Lassnig R-M, Ebner C, Annamaria P, Hofmann P, Kapfhammer H-P, Reingard A, Fazekas F, Ropele S, Enzinger C, 2013. Clinical effects of electroconvulsive therapy in severe depression and concomitant changes in cerebral glucose metabolism—An exploratory study. Journal of Affective Disorders 146 (2), 290–294. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R, 2011. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res 45 (5), 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar A, Scott J, Paszkiewicz A, Giampietro VP, Steiner H, Fu CH, 2015. Neural effects of cognitive-behavioural therapy on dysfunctional attitudes in depression. Psychol Med 45 (7), 1425–1433. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D, 2006. Cognition, emotion and the cerebellum. Brain 129, 290–292. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Disabato BM, Hranilovich J, Morris C, D’Angelo G, Pieper C, Toffanin T, Taylor WD, MacFall JR, Wilkins C, Barch DM, Welsh-Bohmer KA, Steffens DC, Krishnan RR, Doraiswamy PM, 2012. Treatment course with antidepressant therapy in late-life depression. Am J Psychiatry 169 (11), 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107 (24), 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME, 2006. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry 163 (4), 735–738. [DOI] [PubMed] [Google Scholar]
- Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, He Y, Yan C-G, Zang Y-F, 2011. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one 6 (9), e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, Feng J, 2013. Depression uncouples brain hate circuit. Molecular Psychiatry 18 (1), 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai W, Su Y, Wang G, Tan Y, Jin Z, Zeng Y, Yu X, Chen W, Wang X, Si T, 2012. Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS One 7 (10), e48658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WN, Zhao YJ, Hu XY, Huang XQ, Kuang WH, Lui S, Kemp GJ, Gong QY, 2017. Conjoint and dissociated structural and functional abnormalities in first-episode drug-naive patients with major depressive disorder: a multimodal meta-analysis. Scientific Reports 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Tian Y, Yu Y, Zhang F, Hu X, Dong Y, Chen Y, Hu P, Hu X, Wang K, 2014. Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Translational Psychiatry 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14 (3), 339–351. [DOI] [PubMed] [Google Scholar]
- Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey ML, Drevets WC, 2012. Functional anatomy of autobiographical memory recall deficits in depression. Psychol Med 42 (2), 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, Li-Xia T, Tian-Zi J, Yu-Feng W, 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development 29 (2), 83–91. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Du MY, Huang XQ, Lui S, Chen ZQ, Liu J, Luo Y, Wang XL, Kemp GJ, Gong QY, 2014. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med 44 (14), 2927–2937. [DOI] [PubMed] [Google Scholar]
- Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, Wang Y-F, Zang Y-F, 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods 172 (1), 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


