Abstract
Background:
Despite providing a comparable level of care, it is uncertain why non-mechanically ventilated hospitalized older adults are transferred to long-term acute care hospitals (LTACs) versus remaining in the hospital.
Design:
Observational cohort
Setting:
National Medicare data
Participants:
12,875 non-mechanically ventilated hospitalized adults ≥65 years with fee-for-service Medicare in 2012 who were transferred to an LTAC (n=1,831) or had a prolonged hospitalization without transfer (≥ average hospital length of stay among those transferred to an LTAC), and who had one of the top 50 most common hospital diagnoses leading to LTAC transfer.
Measurements:
We assessed predictors of transfer with a multilevel model adjusting for patient, hospital, and hospital referral region (HRR)-level factors. We estimated proportions of variance at each level and adjusted hospital- and HRR-specific LTAC transfer rates using sequential models.
Results:
The strongest predictor of transfer was being hospitalized near an LTAC (<1.4 vs >33.6 miles, aOR 6.2, 95% CI 4.2–9.1). After adjusting for case-mix, differences between hospitals and between regions explained 15.4% and 27.8% of the variation in LTAC use respectively. Case-mix adjusted LTAC use was very high in the South where many HRRs had rates between 20.3%−53.1%, compared to the Pacific Northwest, North, and New England where many HRRs were <5.4%. From our fully adjusted model, the median adjusted hospital LTAC transfer rate was 7.2% (IQR 2.8–17.5%), with substantial within-region variation (intraclass coefficient 0.25, 95% CI 0.21–0.30).
Conclusions:
Nearly half of the variation in LTAC use is independent of patients’ illness severity and is explained by where the patient was hospitalized and in what region. Because of the increased fragmentation of care and greater Medicare spending with LTAC transfers (since LTACs generate a separate bundled payment from the hospital), greater attention is needed to define the optimal role of LTACs in caring for older adults.
Keywords: Post-acute care, long-term acute care hospital, Medicare, variation, health policy
INTRODUCTION
One in seven hospitalized older adults are transferred to a post-acute care facility rather than going home after hospitalization.1 Long-term acute care hospitals (LTACs) have become a major part of post-acute care, and account for over 130,000 stays and $5.3 billion in Medicare spending annually, which is approximately one-fifth of the spending on skilled nursing facilities (SNFs).2 Although LTACs were initially designed to care for individuals requiring prolonged mechanical ventilation, over the past few decades LTACs have cared for an expanded population of non-ventilated older adults with other complex and prolonged illness and a range of ongoing long-term inpatient care needs, such as antibiotic infusions or complex wound care.3,4 Given the uncertainty of the clinical and cost effectiveness of LTACs versus acute care hospitals for caring for these patients, it is unclear what factors influence the decision to transfer older adults to LTACs.
While SNFs are the principal alternative post-acute care setting for many LTAC patients who require subacute care,3,5 on average, patients in LTACs are more similar to patients in hospital step-down units with respect to clinical severity, cognitive and functional status, and treatments received.3 However, despite the overlap in levels of care between LTACs and acute care hospitals, we know very little about why non-ventilated older adults are transferred to an LTAC versus remaining in the acute care hospital. This transfer decision has important implications not only for recovery and outcomes among older adults but also for Medicare spending since an LTAC transfer generates a separate bundled payment.
Therefore, we sought to examine the patient, hospital, and regional-level factors associated with LTAC transfer (versus remaining in the hospital) and quantify the amount of variation explained at each level. We hypothesize that many non-patient factors will be strongly predictive of LTAC transfer, and that a sizeable proportion of the variation will be explained by differences between hospitals and between regions, rather than solely differences between patients.
METHODS
Study Design and Cohort
We conducted a retrospective cohort study using national 5% Medicare data from 2010–2012. We included non-mechanically ventilated hospitalized fee-for-service Medicare beneficiaries 65 years of age or older who were transferred to an LTAC or remained in the acute care hospital (henceforth referred to as hospital). To ensure adequate claims history to characterize baseline health we excluded older adults without continuous Medicare Parts A and B or having any Part C coverage in the year prior to hospitalization. For the LTAC group, we included hospitalized older adults transferred to an LTAC on the same or next day using a temporally adjacent algorithm.6,7 LTACs were identified using the Centers of Medicare and Medicaid Services (CMS) provider number, and verified by reviewing the facility name and conducting an Internet search if the facility type was uncertain.
Since most hospitalized older adults are not transferred to an LTAC, we restricted the cohort to older adults with a greater likelihood of LTAC transfer (see Supplementary Figure S1 and Table S1 for details on cohort assembly). First, we only included patients who had one of the 50 most common hospital Diagnosis Related Groups (DRG) as observed in our data among patients transferred to LTACs. Second, we excluded patients in the hospital cohort with a short length of stay (LOS), defined as having a hospital LOS less than the DRG-specific mean LOS among patients transferred to an LTAC. Third, since our goal was to examine predictors and variation for LTAC transfer versus remaining in the hospital, we excluded patients transferred to alternative inpatient post-acute care facilities within a comparable amount of time that a patient would have otherwise spent in an LTAC, which we defined as the sum of the DRG-specific mean time to LTAC transfer and the LTAC DRG-specific short-stay outlier (SSO) threshold. The SSO threshold is used by CMS to adjust reimbursement for LTAC stays that are considerably shorter than the average LTAC LOS for that diagnosis.
To illustrate how the hospital group was constructed, we will use a hypothetical patient with DRG 592 (skin ulcers). Among LTAC patients with DRG 592, the mean time to transfer was 6 days and the LTAC SSO threshold was 21 days. Thus, patients with DRG 592 who were not transferred to an LTAC were included in the hospital group if their hospital LOS was ≥6 days and were not transferred to an alternative post-acute care facility before day 27, which is the sum of the mean time to transfer and the SSO threshold for DRG 592.
Predictors
We obtained patient-level characteristics from the Medicare data. We used Durable Medical Equipment claims to identify incident wheelchair use as a proxy of advanced debility.8,9 We used DRGs and major diagnostic categories (MDC) to characterize the primary reason for hospitalization. DRG weights are assigned multipliers that reflect the average resources used to treat patients in that DRG. Hospital characteristics were obtained from CMS Provider of Services (POS) and Impact Files. Regions were defined at the hospital-referral region (HRR).10 Regional population and healthcare intensity were ascertained from the Dartmouth Atlas.10 Linear arc distance from the hospital to the nearest LTAC was calculated using addresses in the POS file. State Certificate of Need laws restricting the opening or expansion of hospitals were obtained from the National Conference of State Legislatures.11
Statistical Analyses
Predictors of LTAC Transfer
To ascertain independent predictors of LTAC transfer, we developed a multilevel mixed-effects logistic regression model. We chose candidate predictors based on prior literature and our expertise.5,12–15 Fixed effects included significant patient, hospital, and region-level predictors in univariate analyses with a p-value <0.05. We retained all predictors in our final model with a p-value <0.05 using backward stepwise selection. We specified random effects at the hospital and HRR level to account for clustering of patients within hospitals and hospitals within HRRs. We graphically evaluated functional forms of continuous variables with restricted cubic splines. We group-mean centered patient- and hospital-level continuous predictors. Model diagnostics suggested excellent fit (C-statistic, 0.91; <1.6% absolute difference between observed and predicted LTAC transfer rates for the lowest 8 deciles of predicted risk; Supplementary Table S2).
Variation of LTAC Transfer
To estimate the variation explained by each level, we used variance partition coefficients (VPCs) from sequential multilevel models.16,17 VPCs represent the residual variation in LTAC transfer explained by unobserved differences at each level that remain after adjustment.16 From the case-mix model, which included the patient-level predictors from our final model, we created a heat map to illustrate the variation in adjusted HRR LTAC transfer rates. From the full model, we created a variation profile graph showing adjusted hospital LTAC transfer rates and a scatterplot of hospital variation within HRRs. We restricted hospital variation analyses to hospitals with ≥5 patients to enable more stable estimates. Lastly, we estimated the intraclass correlation coefficient (ICC) to examine hospital variation in LTAC use within the same HRR.
Sensitivity Analyses
We conducted 3 sensitivity analyses to examine the policy relevance and robustness of our estimates (see Supplementary Table S3 for sub-cohort details). First, we restricted our cohort by excluding patients who had an ICU stay <3 days during the index hospitalization or if they had a psychiatric or rehabilitation diagnosis. Beginning in fiscal year 2020, the CMS site-neutral payment policy will substantially decrease LTAC reimbursement for these patients, making LTAC transfer less likely in the future given the lower financial incentives. Second, we restricted our original cohort to patients hospitalized in HRRs with ≥1 LTAC. Third, because the group of patients who remained in the hospital have shorter inpatient LOS for the entire episode of care than those transferred to an LTAC (median of 10 vs. 32 days), we restricted the hospital cohort to patients with an index hospital LOS greater than or equal to the LTAC DRG-specific SSO threshold.
The UT Southwestern institutional review board approved this study. We conducted analyses using Stata (version 14.2, StataCorp), SAS (version 9.4, SAS Institute Inc), and ArcGIS (version 10.3, Esri Inc).
RESULTS
We included 12,875 patients from 2,448 hospitals across 301 HRRs (Supplementary Table S1). A total of 1,831 hospitalized older adults (14.2%) were transferred to an LTAC. The most common 50 diagnoses leading to an LTAC transfer comprised approximately two-thirds of the eligible non-ventilated LTAC population (63.2%) and one-third (34.4%) of the non-ventilated hospital population. The most common diagnoses among patients included in our cohort included sepsis, heart failure, chronic obstructive pulmonary disease, and pneumonia (Supplementary Table S4). Overall, a greater proportion of patients transferred to an LTAC were younger, non-white, and sicker than those who remained in the hospital (Table 1). Notably, patients transferred to LTACs were more likely to have a surgical diagnosis, sepsis, soft skin tissue or joint infection, and chronic wounds.
Table 1.
Characteristics of the Cohort
| Characteristic a | Transferred to LTAC (n=1,831) |
Remained in Hospital (n=11,044) |
|---|---|---|
| Patient Factors Prior to Hospitalization | ||
| Age, years | ||
| 65–69 | 265 (14.5) | 1653 (15.0) |
| 70–74 | 359 (19.6) | 1983 (18.0) |
| 75–79 | 356 (19.4) | 2159 (19.6) |
| 80–84 | 409 (22.3) | 2107 (19.1) |
| ≥ 85 | 442 (24.1) | 3142 (28.5) |
| Female | 1006 (54.9) | 6224 (56.4) |
| White | 1374 (75.0) | 8931 (80.9) |
| Prior LTAC use | 189 (10.3) | 218 (2.0) |
| Prior SNF use | 738 (40.3) | 2889 (26.2) |
| Wheelchair use | 357 (19.5) | 1564 (14.2) |
| Patient Factors of Index Hospitalization | ||
| Length of hospital stay, days | 8 (5–13) | 10 (8–14) |
| Prolonged ICU stay ≥ 3 days | 911 (49.8) | 4619 (41.8) |
| Primary diagnosis | ||
| DRG resource intensity weight | 1.91 (1.47–2.59) | 1.17 (1–1.84) |
| DRG with a MCC designation | 1378 (75.3) | 5614 (50.8) |
| Medical diagnosis type (vs surgical) | 1322 (72.2) | 10155 (92.0) |
| Respiratory MDC | 386 (21.1) | 3572 (32.3) |
| Circulatory MDC | 296 (16.2) | 2260 (20.5) |
| Urinary MDC | 148 (8.1) | 1309 (11.9) |
| Secondary diagnoses b | ||
| Respiratory failure | 612 (33.4) | 2792 (25.3) |
| Sepsis | 681 (37.2) | 2410 (21.8) |
| Skin, soft tissue, or joint infection | 322 (17.6) | 874 (7.9) |
| Chronic skin ulcer | 493 (26.9) | 1484 (13.4) |
| Delirium or dementia | 488 (26.7) | 2295 (20.8) |
| Select intensive treatments and procedures | ||
| Transient mechanical ventilation (<96 hours) | 127 (6.9) | 449 (4.1) |
| Central venous line | 498 (27.2) | 1614 (14.6) |
| Excisional debridement | 76 (4.2) | 89 (0.8) |
| Hospital Factors | ||
| For-profit ownership | 466 (25.5) | 1688 (15.3) |
| Urban | 1662 (90.8) | 9546 (86.4) |
| Region Factors | ||
| Linear arc distance to nearest LTAC, miles | ||
| >33.6 | 90 (4.9) | 2486 (22.5) |
| >11.4 – 33.6 | 204 (11.1) | 2399 (21.7) |
| >5.1– 11.4 | 400 (21.9) | 2161 (19.6) |
| >1.4 – 5.1 | 553 (30.2) | 1995 (18.1) |
| 0–1.4 | 584 (31.9) | 2003 (18.1) |
| State without Certificate of Need law | 685 (37.4) | 7380 (66.8) |
| HRR LTAC supply, beds per 100K persons | 11.1 (7.6–23) | 7 (2.7–11.5) |
| HRR Medicare spending per person, $ | 10,579 (10,003 – 11,646) | 10,091 (9,207 – 10,731) |
| HRR median household income,c $ | 51,761 (45,409 – 60,501) | 52,864 (45,948 – 61,250) |
Abbreviations: LTAC, long-term acute care hospital; SNF, skilled nursing facility; ICU, intensive care unit; DRG, Diagnosis-Related Group; MCC, major complication or comorbidity; MDC, Major Diagnostic Category; HRR, hospital referral region
Categorical variables shown as n (%) and continuous variables shown as median (interquartile range)
Categorized using the Agency for Healthcare Research and Quality Clinical Classification Software (CCS)
Median household income was obtained from the US Census Bureau and aggregated at the HRR level.35
Predictors of LTAC Transfer
Table 2 shows the independent predictors of LTAC transfer. Measures of greater illness severity and complexity were typically associated with greater odds of LTAC transfer, including previous post-acute care use and presence of delirium or dementia. However, patients hospitalized with respiratory or circulatory conditions were more likely to stay in the hospital. Receipt of a central venous line or excisional debridement were also strongly associated with LTAC transfer. We only identified two independent hospital-level predictors of transfer. For-profit hospitals had higher rates of LTAC transfer. Hospitals located in urban areas were less likely to send patients to an LTAC. Lastly, we identified many important region-level predictors of LTAC transfer. The strongest predictor of LTAC transfer was whether a patient was hospitalized in close proximity to the nearest LTAC (aOR 6.2, 95% CI 4.2–9.1, for distance ≤ 1.4 versus >33.6 miles).
Table 2.
Predictors of LTAC Transfer versus Staying in the Hospital
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Patient Factors Prior to Hospitalization | ||
| Age, years | ||
| 65–69 | REF | REF |
| 70–74 | 1.11 (0.91,1.35) | 1.11 (0.89,1.39) |
| 75–79 | 0.99 (0.81,1.21) | 1.08 (0.87,1.35) |
| 80–84 | 1.25 (1.03,1.53) | 1.37 (1.10,1.70) |
| ≥ 85 | 0.83 (0.69,1.01) | 0.94 (0.76,1.16) |
| Prior LTAC use | 3.34 (2.62,4.26) | 1.77 (1.36,2.31) |
| Prior SNF use | 2.17 (1.92,2.46) | 1.73 (1.50,1.99) |
| Wheelchair | 1.49 (1.28,1.73) | 1.30 (1.10,1.53) |
| Patient Factors of Index Hospitalization | ||
| Primary diagnosis | ||
| DRG resource intensity weight, per unit | 1.82 (1.73,1.91) | 1.16 (1.04,1.29) |
| DRG with a MCC designation | 3.52 (3.08,4.02) | 2.16 (1.82,2.57) |
| Medical diagnosis type (vs surgical) | 0.16 (0.14, 0.19) | 0.41 (0.29,0.58) |
| Respiratory MDC | 0.50 (0.44,0.58) | 0.71 (0.60,0.85) |
| Circulatory MDC | 0.77 (0.66,0.90) | 0.65 (0.54,0.78) |
| Urinary MDC | 0.61 (0.50,0.75) | 0.71 (0.56,0.90) |
| Secondary diagnoses | ||
| Respiratory failure | 1.67 (1.47,1.90) | 1.37 (1.17,1.60) |
| Skin, soft tissue, or joint infection | 3.18 (2.68,3.78) | 2.02 (1.65,2.46) |
| Chronic skin ulcer | 3.18 (2.75,3.68) | 1.73 (1.46,2.04) |
| Delirium or dementia | 1.36 (1.19,1.56) | 1.19 (1.01,1.39) |
| Select intensive treatments and procedures | ||
| Transient mechanical ventilation (vs none) | 2.06 (1.61,2.62) | 0.66 (0.50,0.88) |
| Central venous line | 2.61 (2.27,3.01) | 1.57 (1.34,1.83) |
| Excisional debridement | 10.4 (6.95,15.5) | 2.05 (1.31,3.22) |
| Hospital Factors | ||
| For-profit ownership | 1.39 (1.14,1.71) | 1.58 (1.27,1.95) |
| Urban | 1.77 (1.37, 2.27) | 0.54 (0.39,0.73) |
| Region Factors | ||
| Linear arc distance to nearest LTAC, miles | ||
| >33.6 | REF | REF |
| >11.4 – 33.6 | 2.04 (1.47,2.82) | 2.24 (1.56,3.21) |
| >5.1– 11.4 | 4.41 (3.18,6.10) | 4.83 (3.27,7.14) |
| >1.4 – 5.1 | 5.69 (4.13,7.84) | 6.14 (4.18,9.04) |
| 0–1.4 | 6.49 (4.71,8.93) | 6.18 (4.21,9.09) |
| State without Certificate of Need law | 2.63 (1.98,3.49) | 2.31 (1.81,2.94) |
| LTAC supply, per 5 beds/100K persons | 1.47 (1.37,1.58) | 1.23 (1.16,1.32) |
| Median household income, per $10,000 | 0.81 (0.71,0.93) | 0.88 (0.79,0.97) |
| Medicare spending per person, per $3000 | 4.97 (3.37,7.33) | 1.48 (1.06,2.06) |
Regional Variation
After adjusting for case-mix, over one-quarter of the variation (27.8%) was explained by unmeasured differences between regions (Table 3), with an 11-fold difference in LTAC transfer rates between HRRs with the lowest 10th and the highest 90th percentile transfer rate (Figure 1). There was far greater LTAC use in the South, particularly in Texas, Oklahoma, and Louisiana, with transfer rates between 20.3%−53.1% for several HRRs, compared to New England, the North, and the Pacific Northwest, where many HRRs had transfer rates between 1.06%−5.37%. Three-quarters of the regional variation identified in the case-mix model was explained by the five region-level predictors included in our full model (proportion of variation explained: [27.8%−7.1%]/27.8%=74.5%).
Table 3.
Proportion of Variation in LTAC Transfer Explained by Patients, Hospitals, and Regions a
| VPC (95% CI) | Case-mix only | Case-mix + hospital | Case-mix + hospital + region (full model) |
|---|---|---|---|
| Between patients | 56.9% (51.7%−62.1%) | 57.8% (52.4%−62.8%) | 74.5% (70.0%−79.0%) |
| Between hospitals | 15.4% (11.7%−19.0%) | 15.4% (11.7%−19.1%) | 18.4% (14.1%−22.7%) |
| Between regions | 27.8% (22.1%−33.5%) | 27.0% (21.3%−32.7%) | 7.1% (3.9%−10.2%) |
Abbreviations: LTAC, long-term acute care hospital; VPC, variance partition coefficient; CI, confidence interval
Variance partition coefficients (VPC) describe the proportion of variation explained by unobserved differences between patients, hospitals, and hospital referral regions. We conducted sequential multilevel mixed-effects logistic regression models to estimate the impact of adjusting for each successive level of predictors shown in Table 2.
Figure 1. Adjusted LTAC Transfer Rate by Region.
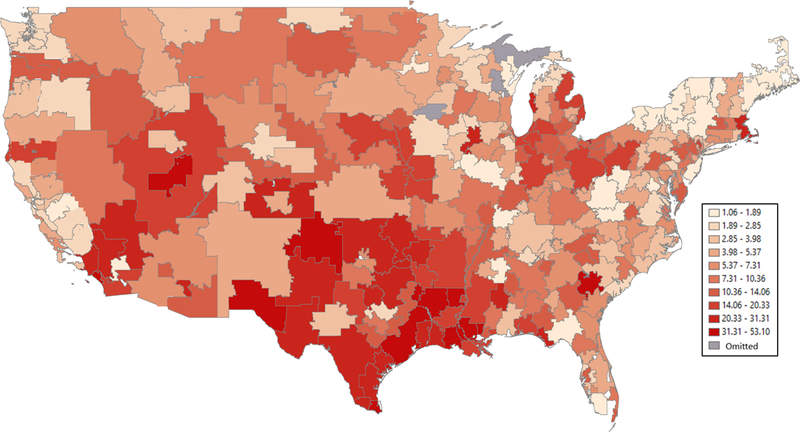
The mean adjusted LTAC transfer rate (versus remaining in the hospital) by hospital referral region among non-mechanically ventilated hospitalized older adults was estimated from the case-mix only multilevel model adjusted for all patient-level predictors shown in Table 2. Hospital referral regions (n=304) are defined as regional healthcare markets for tertiary medical care.10
Hospital Variation
From our fully adjusted model, 18.4% of the variation in LTAC use was explained by unobserved differences between hospitals. The average adjusted transfer rate for individual hospitals varied widely (Figure 2A). The median adjusted hospital LTAC transfer rate was 7.2% (interquartile range, 2.8%−17.5%). Even within a specific region, the adjusted hospital LTAC transfer rates varied substantially (ICC, 0.25, 95% CI, 0.21–0.30; Figure 2B). In low-use regions, there were hospitals with relatively high adjusted transfer rates, and in high-use regions there were many hospitals with very low LTAC use.
Figure 2. Hospital Variation in LTAC Use : A. Distribution of adjusted hospital LTAC transfer rates : B. Variation in adjusted hospital LTAC transfer rate within regions.
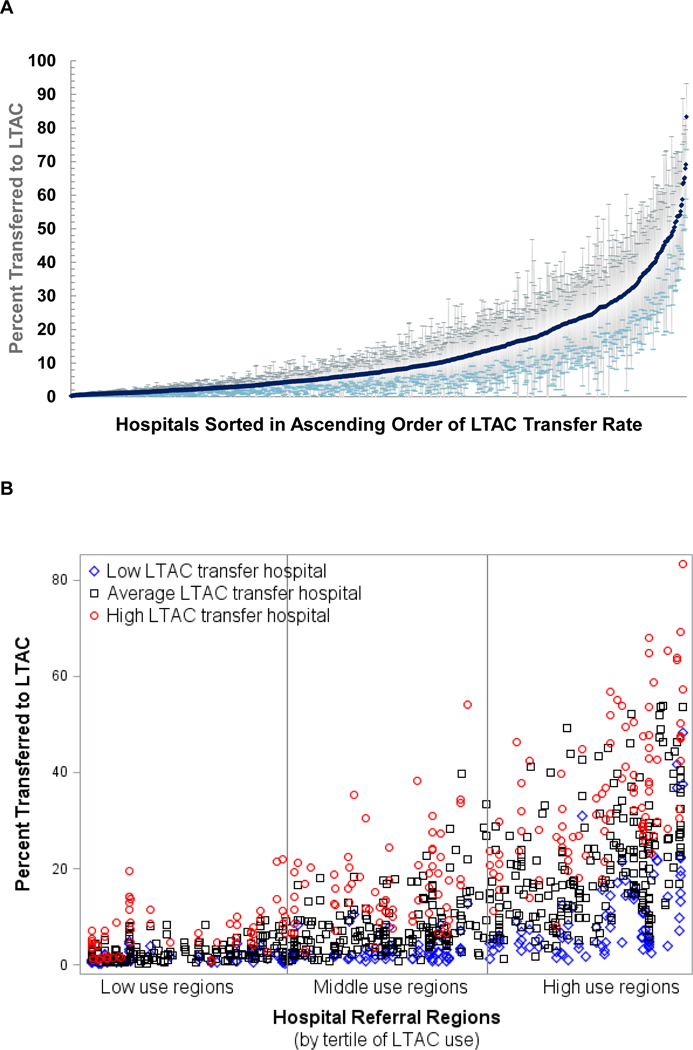
(A) Adjusted hospital LTAC transfer rates were estimated from the full multilevel model and shown for hospitals with ≥5 patients (n=1,038). (B) Hospitals are shown as individual markers within hospital referral regions (HRRs). HRRs were sorted in ascending order by their case-mix adjusted LTAC transfer rates (as per Figure 1) and further categorized by tertiles of use. For each of the HRRs, we estimated the HRR-specific 25th and 75th percentile values for adjusted hospital LTAC transfer rates. A low LTAC transfer hospital (blue diamond) was defined as having an adjusted transfer rate less than their HRR-specific 25th percentile hospital transfer rate. An average LTAC transfer hospital (black square) was defined as having between the 25th-75th percentile transfer rate. A high LTAC transfer hospital (red circle) was defined as greater than the 75th percentile rate. All hospitals in HRRs with fewer than 4 hospitals were defined as average. This approach compares a hospital’s adjusted LTAC transfer rate to their peers within the same HRR.
Sensitivity Analyses
Findings were similar among the sicker patients who would be exempt from reduced site-neutral payment (Supplementary Tables S5 and S6). Among patients hospitalized in HRRs with ≥1 LTAC, the magnitude of the effect size for distance to the nearest LTAC was attenuated, and differences between regions explained less of the variation in LTAC transfer (18.6% vs 27.8% for the full cohort). Otherwise findings were comparable. Lastly, when restricting the cohort of patients who remained in the hospital to those with a longer length of stay, the effect sizes for patient illness severity measures were either greatly attenuated or no longer associated with LTAC transfer, and the region-level predictors were more strongly associated with transfer. Furthermore, unobserved differences between regions (VPC, 41.0%) and between hospitals (VPC, 20.0%) explained much more of the variation in LTAC transfer after adjusting for differences in case-mix than in our original cohort.
DISCUSSION
In this national Medicare study, we found marked variation in LTAC transfer among non-mechanically ventilated hospitalized older adults versus remaining in the acute care hospital for the duration of their inpatient care. Nearly half of the variation is explained by differences between hospitals and between regions, independent of patients’ illness complexity and severity. By far, the strongest predictor of LTAC transfer was how close patients were hospitalized to the nearest LTAC facility. Our analyses were robust when we focused on the population of patients who will be exempt from reduced LTAC reimbursement beginning in 2020, making these findings relevant to the current and future post-acute care policy environment. Furthermore, when we limited our comparison group to older adults with considerably longer hospital lengths of stay who may have a greater likelihood of LTAC transfer, we found that patient-level predictors were much less important and unmeasured differences between hospitals and regions were much stronger drivers of LTAC use. Taken together, the large amount of variation in LTAC transfer that is unrelated to differences between patients suggests great uncertainty about the optimal role of LTACs for non-mechanically ventilated individuals.
Transferring non-ventilated hospitalized older Medicare beneficiaries to LTACs compared to continued care in a traditional acute care hospital has several important clinical and economic implications. LTACs may facilitate quicker recovery given their greater focus on interdisciplinary rehabilitation.18 Furthermore, given their focus on the sickest patients, LTACs have unique expertise in caring for patients with complex care needs (i.e., wound care), which may lead to better outcomes and lower costs.18 Conversely, LTAC transfer could potentially worsen recovery through fragmentation of the initial episode of acute care, which has been shown to lead to unfavorable outcomes in other clinical scenarios.19–21 Problems during the hospital-to-LTAC care transition may arise from non-interoperable information technology systems, as well as from personnel changes with a new team of nurses, ancillary staff, and physicians. Additionally, LTACs are financially incentivized to delay discharges until after patients reach their diagnosis-specific short-stay outlier threshold in order to qualify for full reimbursement, potentially leading to unnecessarily long LTAC stays.22 Unnecessary LTAC days expose frail, vulnerable older adults to hazards of hospitalization, including hospital-acquired infections from multidrug resistant organisms which are more prevalent in LTACs than acute care hospitals.23–26 Furthermore, despite having a high burden of palliative care needs,27,28 older adults in LTACs may have less access to geriatrician and palliative care clinicians than the hospital, which may worsen quality of life.29 With respect to financial implications for CMS, an LTAC transfer generates a separate payment from the bundled payment hospitals receive under the CMS’ Inpatient Prospective Payment System (PPS). While costs of care may be lower by transferring certain patients to an LTAC than receiving continued care in a traditional hospital, Medicare spending is greater due to the dual PPS reimbursement structure.30 This would also have adverse implications for Medicare Accountable Care Organizations, since participants are benchmarked to spending targets and not costs of care. Conversely, from a hospital’s perspective, transferring patients to an LTAC is a financially sensible decision since LTACs can substitute for a prolonged hospitalization, and thus decreases the length of stay and costs of care.31,32
Additional comparative effectiveness research is needed to provide greater clarity as to which older adults would benefit from LTAC transfer. LTACs are thought to be most effective for caring for chronically critically ill patients who require prolonged mechanical ventilation.12,30 For non-ventilated patients, existing evidence suggests lower mortality among those transferred to an LTAC after surviving a critical illness or having multi-organ failure, but this comparison was limited by differences in severity of illness among patients in the comparison group since LTAC transfer was compared to all other alternative disposition options combined, including discharge to home and to a SNF.33 Furthermore, these studies only examined mortality, and did not examine other patient-centered outcomes, including cognitive and functional recovery, important outcomes relevant to older adults with advanced illnesses.
While this study compared patients transferred to LTACs versus remaining in the hospital, SNFs are also a major alternative to LTACs for post-acute care. Though hospitals and SNFs care for patients with quite different illness severity and care needs, we found remarkable similarity in the variation of LTAC use with our previous study comparing LTACs to SNFs.5 Regions with high case-mix adjusted LTAC use in this study were also identified as high LTAC use regions when compared to SNFs. Additionally, hospitals’ adjusted LTAC transfer rates are highly correlated, such that the same hospitals that preferably transfer patients to LTACs instead of keeping them hospitalized also send more patients to LTACs rather than SNFs (Pearson Correlation Coefficient=0.74, p<0.01; Supplementary Figure S2). The consistency of our findings further suggests that regional and hospital use of LTACs is more related to availability and practice culture, rather than patients’ illness severity.
Our study has certain limitations. First, our findings may not be generalizable to privately insured, Medicare Advantage, or young populations. However, fee-for-service Medicare beneficiaries account for the majority of national LTAC use.2 Second, we likely have omitted important patient and hospital-level predictors owing to data limitations. However, the VPCs we estimated for our variation analyses capture unobserved differences at each level beyond what was included in our models.16 For example, patient-level VPCs include unmeasured differences in cognition, functional status, frailty, patient preference, among other domains of illness severity and complexity. Third, our findings may not generalize to patients with a diagnosis not among the most common 50 DRGs leading to LTAC transfer.
In conclusion, nearly half of the variation in transferring non-ventilated hospitalized older adults is explained by differences between hospitals and between regions, independent of patients’ illness severity and preferences for care. Regional differences account for over a quarter of the variation, with far greater use in the South. However, ‘geography is not entirely destiny’ given the considerable hospital variation in LTAC use, even among hospitals with the same potential LTAC access from the same region. Variation in LTAC use may in part be driven by a scarcity of evidence of which model of care is most effective in improving outcomes for hospitalized older adults with prolonged illness. The decision for LTAC transfer has important clinical and economic consequences that will need to be explored further in future comparative effectiveness research, especially given that the burden of prolonged acute or chronic critical illness is likely to expand with an aging population and advances in medical care and technologies.34
Supplementary Material
ACKNOWLEDGMENTS
Funding: This work was funded by the National Institute on Aging (R03AG053291, K23AG052603), with additional support by the National Center for Advancing Translational Sciences (UL1TR001105) and the Agency for Healthcare Research and Quality (R24HS022418). Dr. Nguyen received funding from the National Heart, Lung, and Blood Institute (K23HL133441).
Sponsor’s Role: The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Meeting Presentations: This paper was presented at the 2018 AGS Annual Meeting during the Presidential Poster Session on May 3rd, 2018. This paper will also be presented at the 2018 AcademyHealth Annual Research Meeting as an oral presentation.
Footnotes
Impact Statement: We certify that this work is novel. This study identifies many patient-related and non-patient-related reasons why non-mechanically ventilated older adults are transferred to LTACs versus remaining in the acute care hospital, and finds that nearly half of the variation in LTAC transfer is explained by what hospital and in what region of the country a patient is hospitalized in.
Co-author Twitter handles: @OanhKieuNguyen
Conflict of Interest: The authors have no conflicts of interest to disclose, financial or otherwise.
REFERENCES
- 1.Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295–296. [DOI] [PubMed] [Google Scholar]
- 2.Medicare Payment Advisory Commision. Report of the Congress: Medicare payment policy. Washington D.C. 2017. [Google Scholar]
- 3.Dalton K, Kandilov AM, Kennell DK, Wright A. Determining medical necessity and appropriateness of care for Medicare long-term care hospitals. Falls Church, VA: Kennell and Associates, Inc. and RTI International; 2012. [Google Scholar]
- 4.Eskildsen MA. Long-term acute care: a review of the literature. J Am Geriatr Soc. 2007;55(5):775–779. [DOI] [PubMed] [Google Scholar]
- 5.Makam AN, Nguyen OK, Xuan L, Miller ME, Goodwin JS, Halm EA. Factors Associated With Variation in Long-term Acute Care Hospital vs Skilled Nursing Facility Use Among Hospitalized Older Adults. JAMA Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Dartmouth Atlas of Health Care. The Dartmouth Atlas of Health Care. 2016; http://www.dartmouthatlas.org/. Accessed October 18, 2016.
- 11.Certificate of Need State Laws. http://www.ncsl.org/research/health/con-certificate-of-need-state-laws.aspx. Accessed October 17, 2016.
- 12.Medicare Payment Advisory Commision. Defining Long-Term Acute Care Hospitals. Washington D.C.2004. [Google Scholar]
- 13.Gage B, Morley M, Smith L, et al. Post-Acute Care Payment Reform Demonstration: Final report Volume 3 of 4 Research Triangle Park, N.C.: RTI International; 2012. [Google Scholar]
- 14.Gage B, Morley M, Spain P, Ingber MJ. Examing Post Acute Care Relationships in an Integrated Hospital System. Waltham, MA: RTI International; 2009. [Google Scholar]
- 15.Kahn JM, Werner RM, Carson SS, Iwashyna TJ. Variation in long-term acute care hospital use after intensive care. Med Care Res Rev. 2012;69(3):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gage B, Pilkauskas N, Dalton K, et al. Long-Term Care Hospital Payment System Monitoring and Evaluation. Phase II Report. Waltham, MA: RTI International; 2007. [Google Scholar]
- 19.Hua M, Gong MN, Miltiades A, Wunsch H. Outcomes after Rehospitalization at the Same Hospital or a Different Hospital Following Critical Illness. Am J Respir Crit Care Med. 2017;195(11):1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai TC, Orav EJ, Jha AK. Care fragmentation in the postdischarge period: surgical readmissions, distance of travel, and postoperative mortality. JAMA Surg. 2015;150(1):59–64. [DOI] [PubMed] [Google Scholar]
- 21.van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398–405. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Kleerup EC, Ganz PA, Ponce NA, Lorenz KA, Needleman J. Medicare Payment Policy Creates Incentives For Long-Term Care Hospitals To Time Discharges For Maximum Reimbursement. Health Aff (Millwood). 2015;34(6):907–915. [DOI] [PubMed] [Google Scholar]
- 23.Chitnis AS, Edwards JR, Ricks PM, Sievert DM, Fridkin SK, Gould CV. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect Control Hosp Epidemiol. 2012;33(10):993–1000. [DOI] [PubMed] [Google Scholar]
- 24.de Lissovoy G, Pronovost PJ, Faden R. Long-term acute care hospitals: a clinical, economic, and ethical dilemma. Med Care. 2013;51(1):1–3. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Price LS. Long-term acute care hospitals. Clin Infect Dis. 2009;49(3):438–443. [DOI] [PubMed] [Google Scholar]
- 26.Wolfenden LL, Anderson G, Veledar E, Srinivasan A. Catheter-associated bloodstream infections in 2 long-term acute care hospitals. Infect Control Hosp Epidemiol. 2007;28(1):105–106. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin MR, Wunsch H, Reyfman PA, et al. High burden of palliative needs among older intensive care unit survivors transferred to post-acute care facilities. a single-center study. Ann Am Thorac Soc. 2013;10(5):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamas DJ, Owens RL, Nace RN, et al. Opening the Door: The Experience of Chronic Critical Illness in a Long-Term Acute Care Hospital. Crit Care Med. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn JM, Werner RM, David G, Ten Have TR, Benson NM, Asch DA. Effectiveness of long-term acute care hospitalization in elderly patients with chronic critical illness. Med Care. 2013;51(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall WB, Willis LE, Medvedev S, Carson SS. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185(1):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seneff MG, Wagner D, Thompson D, Honeycutt C, Silver MR. The impact of long-term acute-care facilities on the outcome and cost of care for patients undergoing prolonged mechanical ventilation. Crit Care Med. 2000;28(2):342–350. [DOI] [PubMed] [Google Scholar]
- 33.Koenig L, Demiralp B, Saavoss J, Zhang Q. The Role of Long-term Acute Care Hospitals in Treating the Critically Ill and Medically Complex: An Analysis of Nonventilator Patients. Med Care. 2015;53(7):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn JM, Angus DC. Health policy and future planning for survivors of critical illness. Curr Opin Crit Care. 2007;13(5):514–518. [DOI] [PubMed] [Google Scholar]
- 35.2011 American Community Survey 5-year Estimates, Table S1901: Income in the Past 12 Months (in 2011 Inflation–adjusted Dollars). http://www.ncsl.org/research/health/con-certificate-of-need-state-laws.aspx. Accessed December 15, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


