Abstract
Background: Preclinical models have suggested a role for sex hormones in the development of glioblastoma multiforme (GBM). However, the impact of gender on the survival time of patients with GBM has not been fully understood. The objective of the present study was to clarify the association between gender and survival of patients with GBM by analyzing population-based data.
Methods: We searched the Surveillance, Epidemiology, and End-Results database who were diagnosed with GBM between 2000 and 2008 and were treated with surgery. Five-year cancer specific survival data were obtained. Kaplan–Meier methods and multivariable Cox regression models were used to analyze long-term survival outcomes and risk factors.
Results: A total of 6586 patients were identified; 61.5% were men and 38.5% were women. The 5-year cancer-specific survival (CSS) rates in the male and female groups were 6.8% and 8.3%, respectively (P=0.002 by univariate and P<0.001 by multivariate analysis). A stratified analysis showed that male patients always had the lowest CSS rate across localized cancer stage and different age subgroups.
Conclusions: Gender has prognostic value for determining GBM risk. The role of sex hormones in the development of GBM warrants further investigation.
Keywords: gender, glioblastoma, survival, SEER
Background
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most common primary brain tumor, with aggressive clinical manifestation [1]. The incidence rate of central nervous system tumors was reported to be 6.7 per 100,000 persons in 2000 [2]. Some researchers have demonstrated an increase in the incidence of brain tumors, which was partly result of the developments in diagnosis and changes in the classification system [3]. Despite radiotherapy plus temozolomide (TMZ) provided 2- and 5-year survival rates of 27 and 10%, median survival in GBM is generally less than 1 year, and even the patients with favorable situations, the survival month is still less than 2 years [4–7]. Except for Turcot’s syndrome and Li–Fraumeni syndrome, most GBM patients originate in a sporadic fashion without any known predisposing factors [8]. Therefore, little is known about the risk factors for brain tumors [9]. A better understanding of the distribution of GBM may provide indications of etiologic factors and contribute to the search for improved therapies.
Gender-related discrepancies in the incidence and survival of hepatocellular carcinoma [10], colorectal [11], and gastric cancers [12] have previously been reported. Above results support the protective role of estrogen in these malignancies. However, the protective role in GBM has not been investigated in a large population. To further clarify the issue of gender on GBM prognosis, Surveillance, Epidemiology, and End Results (SEER) population-based data were analyzed in our study.
Methods
Patients
The current SEER database consists of 17 population-based cancer registries representing approximately 26% of the U.S. population. The SEER Cancer Statistics Review (http://seer.cancer.gov/data/citation.html)—a report on the most recent cancer incidence, mortality, survival, prevalence, and lifetime risk statistics—is published annually by the Data Analysis and Interpretation Branch of the National Cancer Institute (Rockville, MD, U.S.A.). SEER data contain no identifiers and are publicly available for studies of cancer-based epidemiology and survival analysis. The National Cancer Institute’s SEER*Stat software, version 8.1.5 (Surveillance Research Program; www.seer.cancer.gov/seerstat) was used to identify patients whose pathological diagnosis as glioblastoma based on International Classification of Diseases for Oncology (ICD-O) topography codes (C71.0–C71.9) between 2000 and 2008. The definition of anatomical primary site of brain tumors was restricted to the following: C71.0-Cerebrum, C71.1-Frontal lobe, C71.2-Temporal lobe, C71.3-Parietal lobe, C71.4-Occipital lobe, C71.5-Ventricle, C71.6-Cerebellum, C71.7-Brain stem, C71.8-Overlapping lesion of brain, C71.9-Brain, and brain sites not otherwise specified (NOS). Morphology codes for glioblastoma were expanded to include the following histologies: 9440, 9441 and 9442 (i.e. glioblastoma, NOS, Giant cell glioblastoma, and Gliosarcoma). Only patients who underwent surgical treatment and who were between 18 and 70 years old at the time of diagnosis were included. Patients were excluded if they had incomplete staging, distant metastasis, or lacked an evaluation of histological type or follow-up. Age, sex, race, histological type, stage, tumor grade and size, and cancer-specific survival (CSS) were assessed. Adjuvant chemotherapy was not evaluated, since the SEER registry does not have this information. Tumor-node-metastasis classification was restaged according to criteria described in the American Joint Committee on Cancer Staging Manual (7th edition, 2010). The primary endpoint of the present study was CSS, which was calculated from the date of diagnosis to the date of cancer-related death. Deaths attributable to cancer were treated as events and deaths from other causes were treated as censored observations.
Ethics statement
The present study was based on public data from the SEER database, and permission was obtained to access the files (reference no. 12578-Nov2013). The analysis did not involve interaction with human subjects or use personal identifying information. The study did not require informed consent and was approved by the Review Board of Nanjing Medical University (Nanjing, China). Patient records/information was anonymized and de-identified prior to analysis, and the methods were carried out in accordance with the approved guidelines.
Statistical analysis
The association between gender (male or female) and clinicopathological parameters was analyzed by the χ2 test. Continuous variables were analyzed using the Student’s t test. Survival curves were generated based on Kaplan–Meier estimates, and differences between the curves were analyzed by the log-rank test. Multivariate Cox regression models were generated with hazard ratio (HR) and 95% confidence interval (CI) to analyze risk factors for survival. Statistical analyses were performed using SPSS version 17 for Windows (SPSS Inc., Chicago, IL, U.S.A.). Results were considered statistically significant for a two-tailed P value < 0.05.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available in the SEER dataset repository. https://seer.cancer.gov/.
Results
Patient characteristics
We identified 6586 eligible patients with GBM in the SEER database during the 8-year study period (between 2000 and 2008). A total of 4049 (61.5%) were men, and 2537 (38.5%) were women. The median follow-up period was 17 months. The median follow-up period was 17 months in the male group and 19 months in the female group. Patient demographics and pathologic features are summarized in Table 1.
Table 1. Characteristics of patients from SEER Database by gender.
| Number of patients (%) | ||||
|---|---|---|---|---|
| Characteristic | Total | Male | Female | |
| n=6586 | n=4049 | n=2537 | P value | |
| Media follow up (mo) | 17(5–20) | 17(5–19) | 19(5–22) | |
| (IQR) | ||||
| Years of diagnosis | 0.533 | |||
| 2000–2004 | 3469(52.7) | 2145(53.0) | 1324(52.2) | |
| 2005–2008 | 3117(47.3) | 1904(47.0) | 1213(47.8) | |
| Age | 0.296 | |||
| <40 | 581(8.8) | 358(8.8) | 223(8.8) | |
| 41–60 | 3635(55.2) | 2263 (55.9) | 1372(54.1) | |
| >60 | 2370(36.0) | 1428(35.3) | 942(37.1) | |
| Race | P<0.001 | |||
| Caucasian | 5425(82.4) | 3361(83.0) | 878(34.6) | |
| African American | 914(13.9) | 384(9.5) | 312(12.3) | |
| Others* | 2282(34.6) | 1712(42.3) | 209(8.2) | |
| Primary site | 0.870 | |||
| Cerebrum | 235(3.6) | 144(3.6) | 91(3.6) | |
| Frontal lobe | 1663(25.3) | 1028(25.4) | 635(25.0) | |
| Temporal lobe | 1556(23.6) | 957(23.6) | 599(23.6) | |
| Parietal lobe | 1097(16.7) | 661(16.3) | 436(17.2) | |
| Occipital lobe | 274(4.2) | 158(3.9) | 116(4.6) | |
| Ventricle, NOS | 27(0.4) | 15(0.4) | 12(0.5) | |
| Cerebellum, NOS | 36(0.5) | 22(0.5) | 14(0.6) | |
| Brain stem | 28(0.5) | 19(0.5) | 9(0.4) | |
| Overlapping lesion of brain | 1158(17.6) | 719(17.8) | 439(17.3) | |
| Brain, NOS | 512(7.8) | 326(8.1) | 186(7.3) | |
| Pathological grading | 0.144 | |||
| High/Moderate | 25(0.4) | 20 (0.5) | 5 (0.2) | |
| Poor/UD | 2655(40.3) | 1640(40.5) | 1015(40.0) | |
| Unknown | 3906(59.3) | 2389(59.0) | 1517(59.8) | |
| Stage | 0.127 | |||
| Localized | 5067(76.9) | 3152 (77.8) | 1915(75.5) | |
| Regional | 1172(17.8) | 697(17.2) | 475 (18.7) | |
| Distant | 70(1.1) | 38(0.9) | 32(1.3) | |
| Unstaged | 277(4.2) | 162(4.0) | 115(4.5) | |
| Tumor size | 0.015 | |||
| <3 cm | 797 (12.1) | 463 (11.4) | 334 (13.2) | |
| 3–5 cm | 2498(37.9) | 1511(37.3) | 987 (38.9) | |
| >5 cm | 1666(25.3) | 1071(26.5) | 595(23.5) | |
| Not stated | 1625(24.7) | 1004(24.8) | 621(24.5) | |
Abbreviation: NOS, not otherwise specified.
including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Clinicopathological differences between the groups
As illustrated in Table 1, there were significant differences observed between the two groups, including race (more frequent in Caucasian, 82.4%; P<0.001) and tumor size (more 3–5 cm, 37.9%; P=0.015). Whereas, no differences were observed in years of diagnosis, age, primary site, pathological grading, and stage between the two groups.
Impact of gender on survival outcomes
The univariate log-rank test showed that the 1-, 3- and 5-year CSS were 45.9%, 11.4% and 6.8% in male group, 47.9%, 14.3% and 8.3% in female group (P=0.002) (Figure 1). Moreover, an early year of diagnosis (2000–2004), age more than 60 years, African American race, brain stem tumor, poor/undifferentiated tumor grade (P=0.014), higher stage, and larger tumor size (P<0.001) were regarded as significant risk factors by univariate analysis (Table 2). Multivariate analysis with Cox regression was performed, and the following seven factors were found to be independent prognostic factors (Table 3), including year of diagnosis (2005–2008: HR, 0.783; 95% CI, 0.743–0.826), gender (female: HR, 0.906; 95% CI, 0.859–0.954), age (41–60 years: HR, 2.036; 95% CI, 1.840–2.254; >60 years: HR, 3.033; 95% CI, 2.729–3.371), race (African American: HR, 1.025; 95% CI, 0.908–1.158), primary site (frontal lobe: HR, 0.996; 95% CI, 0.861–1.151; temporal lobe: HR, 1.018; 95% CI, 0.880–1.178; parietal lobe: HR, 0.953; 95% CI, 0.821–1.107; occipital lobe: HR, 1.025; 95% CI, 0.853–1.232; ventricle, NOS: HR, 1.268; 95% CI, 0.848–1.898; cerebellum, NOS: HR, 1.044; 95% CI, 0.719–1.516; brain stem: HR, 1.518; 95% CI, 1.023–2.254; overlapping lesion of brain: HR, 0.983; 95% CI, 0.847–1.141; brain, NOS: HR, 0.787; 95% CI, 0.667–0.929); pathological grading (poor/undifferentiated: HR, 1.418; 95% CI, 0.912–2.205), stage (regional: HR, 1.568; 95% CI, 1.465–1.678; distant: HR, 1.580; 95% CI, 1.238–2.017), tumor size (3–5 cm: HR, 1.029; 95% CI, 0.946–1.119; >5 cm: HR 1.145; 95% CI, 1.046–1.253).
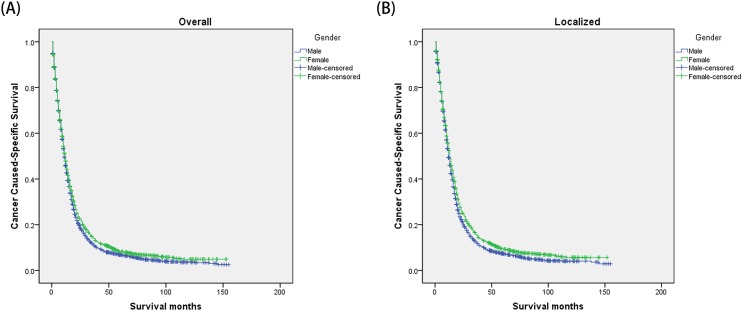
Figure 1. Kaplan–Meier estimates of glioblastoma cancer caused-specific survival in different gender groups.
(A) The overall group; male versus female: χ2 = 9.616, P=0.002. (B) The localized stage group; male versus female: χ2 = 12.959, P<0.001.
Table 2. Univariate survival analyses of GBM patients according to various clinicopathological variables.
| Variable | N | 1-year CSS (%) | 3-year CSS (%) | 5-year CSS (%) | Log rank χ2 test | P |
|---|---|---|---|---|---|---|
| Years of diagnosis | 61.795 | P<0.001 | ||||
| 2000–2004 | 3469 | 41.7% | 10.2% | 6.5% | ||
| 2005–2008 | 3117 | 52.1% | 15.0% | 8.3% | ||
| Gender | 9.616 | 0.002 | ||||
| Male | 4049 | 45.9% | 11.4% | 6.8% | ||
| Female | 2537 | 47.9% | 14.3% | 8.3% | ||
| Age | 477.901 | P<0.001 | ||||
| <40 | 581 | 74.3% | 35.0% | 26.1% | ||
| 41–60 | 3635 | 50.5% | 12.3% | 6.9% | ||
| >60 | 2370 | 33.7% | 7.2% | 3.5% | ||
| Race | 29.078 | P<0.001 | ||||
| Caucasian | 5425 | 46.2% | 11.6% | 7.0% | ||
| African American | 914 | 45.8% | 16.8% | 9.9% | ||
| Others* | 2282 | 55.8% | 24.3% | 13.9% | ||
| Primary site | 51.553 | P<0.001 | ||||
| Cerebrum | 235 | 49.5% | 11.2% | 6.2% | ||
| Frontal lobe | 1663 | 47.6% | 11.1% | 7.1% | ||
| Temporal lobe | 1556 | 44.2% | 10.8 | 5.5 | ||
| Parietal lobe | 1097 | 45.3% | 14.3% | 7.9% | ||
| Occipital lobe | 274 | 42.3% | 11.8% | 4.7% | ||
| Ventricle, NOS | 27 | 29.6% | 3.7% | NI | ||
| Cerebellum, NOS | 36 | 53.5% | 11.9 | 5.9 | ||
| Brain stem | 28 | 28.6% | 3.6% | NI | ||
| Overlapping lesion of brain | 1158 | 46.3% | 13.3% | 7.9% | ||
| Brain, NOS | 512 | 56.9% | 18.3% | 13.9% | ||
| Pathological grading | 8.529 | 0.014 | ||||
| High/Moderate | 25 | 68.0% | 32.0% | 22.4% | ||
| Poor/UD | 2655 | 46.8% | 12.0% | 7.2% | ||
| Unknown | 3906 | 46.4% | 12.7% | 7.4% | ||
| Stage | 159.412 | P<0.001 | ||||
| Localized | 5067 | 50.5% | 13.7% | 8.1% | ||
| Regional | 1172 | 32.1% | 7.3% | 4.8 | ||
| Distant | 70 | 30.3% | 4.5% | NI | ||
| Unstaged | 277 | 41.4% | 13.7% | 7.9 | ||
| Tumor size | 29.108 | P<0.001 | ||||
| <3 cm | 797 | 53.2 | 14.0 | 7.5 | ||
| 3–5 cm | 2498 | 49.7 | 13.1 | 7.6 | ||
| >5 cm | 1666 | 43.4 | 12.4 | 7.5 | ||
| Not stated | 1625 | 42.0 | 10.8 | 7.1 |
Abbreviation: NI, not included.
including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Table 3. Multivariate Cox model analyses of prognostic factors of GBM.
| Variable | Hazard ratio | 95%CI | P |
|---|---|---|---|
| Years of diagnosis | P<0.001 | ||
| 2000–2004 | 1 | Reference | |
| 2005–2008 | 0.783 | 0.743–0.826 | |
| Gender | |||
| Male | 1 | Reference | P<0.001 |
| Female | 0.906 | 0.859–0.954 | |
| Age | P<0.001 | ||
| <40 | 1 | Reference | |
| 41–60 | 2.036 | 1.840–2.254 | |
| >60 | 3.033 | 2.729–3.371 | |
| Race | P<0.001 | ||
| Caucasian | 1 | Reference | |
| African American | 1.025 | 0.908–1.158 | |
| Others* | 0.750 | 0.663–0.848 | |
| Primary site | P<0.001 | ||
| Cerebrum | 1 | Reference | |
| Frontal lobe | 0.996 | 0.861–1.151 | |
| Temporal lobe | 1.018 | 0.880–1.178 | |
| Parietal lobe | 0.953 | 0.821–1.107 | |
| Occipital lobe | 1.025 | 0.853–1.232 | |
| Ventricle, NOS | 1.268 | 0.848–1.898 | |
| Cerebellum, NOS | 1.044 | 0.719–1.516 | |
| Brain stem | 1.518 | 1.023–2.254 | |
| Overlapping lesion of brain | 0.983 | 0.847–1.141 | |
| Brain, NOS | 0.787 | 0.667–0.929 | |
| Pathological grading | 0.178 | ||
| High/Moderate | 1 | Reference | |
| Poor/UD | 1.418 | 0.912–2.205 | |
| Unknown | 1.454 | 0.935–2.259 | |
| Stage | P<0.001 | ||
| Localized | 1 | Reference | |
| Regional | 1.568 | 1.465–1.678 | |
| Distant | 1.580 | 1.238–2.017 | |
| Unstaged | 0.988 | 0.865–1.129 | |
| Tumor size | P<0.001 | ||
| <3 cm | 1 | Reference | |
| 3–5 cm | 1.029 | 0.946–1.119 | |
| >5 cm | 1.145 | 1.046–1.253 | |
| Not stated | 1.158 | 1.056–1.269 |
including other (American Indian/AK Native, Asian/Pacific Islander) and unknowns.
Stratified analysis of gender effect on CSS rates
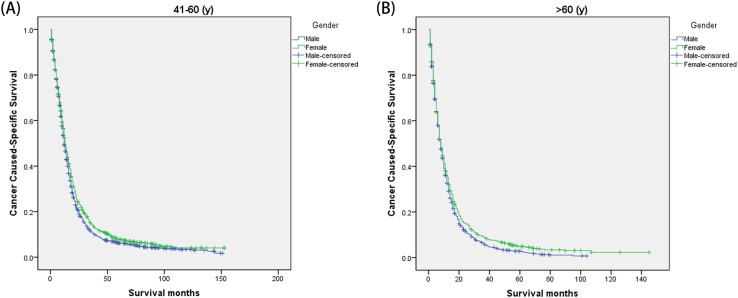
We then further analyzed the effect of gender on CSS rates in each stage (Figure 1). The univariate analysis of gender on CSS showed that female had increased 1-, 3-, and 5-year CSS in localized stage (P<0.001), but not in regional (P=0.619) and distant stage (P=0.259). And gender was validated as an independent predictor of survival in multivariate Cox regression in the localized stages (P<0.001) (Figure 1) (Table 4). Furthermore, we made further stratified analysis of survival rates and hazard by age (Figure 2). Male always had the lowest CSS rate in 41–60 years and >60 years group, which were consistent with above results (Table 5).
Table 4. Univariate and multivariate analyses for evaluating gender influencing CSS in GBM based on different cancer stage.
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | 1-year CSS (%) | 3-year CSS (%) | 5-year CSS (%) | Log rank χ2 test | P | HR (95%CI) | P |
| Localized | |||||||
| Gender | 12.959 | P<0.001 | P<0.001 | ||||
| Male | 49.3 | 12.4 | 7.2 | Reference | |||
| Female | 52.4 | 15.8 | 9.3 | 0.898(0.845–0.954) | |||
| Regional | |||||||
| Gender | 0.247 | 0.619 | |||||
| Male | 31.7 | 6.3 | 5.1% | ||||
| Female | 32.7 | 8.6 | 4.4% | ||||
| Distant | |||||||
| Gender | 1.273 | 0.259 | |||||
| Male | 39.4 | 5.6 | NI | ||||
| Female | 16.4 | 3.3 | NI | ||||
Figure 2. Subgroup analysis for evaluating the effect of gender for glioblastoma patients according to different age.
(A) 41–60 years: χ2 = 8.389, P=0.004; (B) >60 years: χ2 = 6.233, P=0.013.
Table 5. Univariate and multivariate analyses for evaluating gender influencing CSS in GBM based on different age.
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | 1-year CSS (%) | 3-year CSS (%) | 5-year CSS (%) | Log rank χ2 test | P | HR (95%CI) | P |
| <40 | |||||||
| Gender | 0.072 | 0.788 | NI | ||||
| Male | 75.9 | 34.6 | 26.1% | ||||
| Female | 71.9 | 35.6 | 24.9% | ||||
| 41–60 | |||||||
| Gender | 8.389 | 0.004 | 0.003 | ||||
| Male | 49.3 | 11.0 | 6.1% | Reference | |||
| Female | 52.5 | 14.4 | 7.8% | 0.899(0.837–0.965) | P<0.001 | ||
| >60 | |||||||
| Gender | 6.233 | 0.013 | 0.029 | ||||
| Male | 32.6 | 6.0 | 2.4% | Reference | |||
| Female | 35.2 | 8.9 | 4.9% | 0.908(0.832–0.990) | |||
NI: not included in multivariate survival analysis.
P values were adjusted for years of diagnosis, age, race, pathological grading, stage and tumor size as covariates between the two groups.
Discussion
GBM accounts for 17% of intracranial tumors and be considered as the most common brain tumor in adults [13]. Despite surgical resection followed by adjuvant radiotherapy and chemotherapy has been applied, prognosis remains poor and long-term survival is rare [14]. Thus, further understanding and improvements in GBM prognosis may affect the choice of salvage therapy and follow-up strategies.
The higher percentage of GBM in men compared with women has been reported in some literature, with a mean male/female ratio ranging from 1.0 to 1.9 [15–17]. However, to the best of our knowledge, there is limited information regarding the impact of sex on survival in patients with GBM. Our study revealed a correlation between female sex and improved CSS and OS in patients with GBM. This survival discrepancy still existed after stratified analysis. Interestingly, female patients have an equivalent percentage in poor/undifferentiation grade (40.0% versus 40.5%) and more than 3 cm tumor size (63.8% versus 62.4%) when compared with male patients. In addition, even after adjusting confounding factors, gender remained to serve as an independent prognostic predictor.
Sex disparities in cancer mortality arise from the sex differences have been analyzed widely. However, the evidence regarding the influence of reproductive factors and hormones on GBM has not been well verified. Epidemiological studies provided very limited evidence regarding the impact of sex on survival in patients with GBM [18–21]. Some studies have reported that female have longer survival than male [22,23]. Barone et al. [24] demonstrated that estrogen increased survival in an orthotopic model of glioblastoma, and estradiol-based study may be beneficial in treating GBM. Li et al. [25]observed high frequency of estrogen receptor methylation GBMs, indicating that estrogen protect patients from GBM. Moreover, Yu et al. [26] found that androgen receptor signaling could promote tumorigenesis of GBM in adult men by inhibiting TGF-β (transforming growth factor β) receptor signaling. The findings of our study suggest that estrogen may protect against GBM genesis and promote a more favorable biology once GBM develops.
Univariate analysis showed that female had a better 1-, 3-, and 5-year CSS compared with male patients, but this failed to reach statistical significance in multivariable Cox regression models of regional and distant stages. A total of 4049 male GBM patients and 2537 females were included in our study, the largest sample size up to now. Due to the protective role of estrogen in the female groups, these patients exhibited better survival. The survival disadvantage in women aged more than 60 years may reflect the lasting effect of estrogen on the biology of GBM. In addition to the impact of sex on survival, we explored potential interactions between sex and age. Male patients were at an increased risk of cancer mortality in contrast with female patients with different age subgroups after adjusted for confounding factors. When comparing with male patients, female patients always had the worse CSS in regional and distant subgroups.
Although the present study is based on a large population, there are still limitations. First, its retrospective nature may affect the analysis. Second, several important pieces of information regarding GBM predisposing factors were not included in the SEER database. Moreover, current classification of tumors of the CNS does not include the term glioblastoma multiforme, thus we cannot adjust the nomenclature according to the newest criteria. Besides, information on menopausal status or use of hormone therapy was not included in the SEER database, thus limit our ability to reach definitive conclusions in this regard. Despite these limitations, our large population-based study may render our conclusions more convincing.
Conclusions
The results of the present study demonstrate that sex influences survival among patients with GBM. Compared with male patients, female patients with GBM have a higher CSS after surgery. Future studies are warranted to validate these confounding factors and present unique opportunities for novel therapeutics.
Acknowledgments
Not applicable
Abbreviations
- CSS
cancer-specific survival
- GBM
glioblastoma multiforme
- HR
hazard ratio
- SEER
Surveillance, Epidemiology, and End-Results
Author Contribution
M.J.T., J.P.S., and Y.D.Z. designed the study. W.Y.M., Y.Q.C., and Y.Y. provided the databases. M.J.T., D.L.Z., and J.P.S. assembled and analyzed the data. M.J.T. and Y.D.Z. wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was partially supported by Nanjing Medical Science and Technology Development Project [YKK15113].
References
- 1.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.. et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Hess K.R., Broglio K.R. and Bondy M.L. (2004) Adult glioma incidence trends in the United States, 1977-2000. Cancer 101, 2293–2299 10.1002/cncr.20621 [DOI] [PubMed] [Google Scholar]
- 3.Jukich P.J., McCarthy B.J., Surawicz T.S., Freels S. and Davis F.G. (2001) Trends in incidence of primary brain tumors in the United States, 1985-1994. Neuro. Oncol. 3, 141–151 10.1093/neuonc/3.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner J.C. (2003) Factors influencing survival in high-grade gliomas. Semin. Oncol. 30, 10–14 10.1053/j.seminoncol.2003.11.031 [DOI] [PubMed] [Google Scholar]
- 5.DeAngelis L.M. (2001) Brain tumors. N. Engl. J. Med. 344, 114–123 10.1056/NEJM200101113440207 [DOI] [PubMed] [Google Scholar]
- 6.Saito T., Muragaki Y., Shioyama T., Komori T., Maruyama T., Nitta M.. et al. (2018) Malignancy index using intraoperative flow cytometry is a valuable prognostic factor for glioblastoma treated with radiotherapy and concomitant temozolomide. Neurosurgery 10.1093/neuros/nyy089 [DOI] [PubMed] [Google Scholar]
- 7.Rusthoven C.G., Koshy M., Sher D.J., Ney D.E., Gaspar L.E., Jones B.L.. et al. (2016) Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era: a national cancer database analysis. JAMA Neurol. 73, 821–828 10.1001/jamaneurol.2016.0839 [DOI] [PubMed] [Google Scholar]
- 8.Hamilton S.R., Liu B., Parsons R.E., Papadopoulos N., Jen J., Powell S.M.. et al. (1995) The molecular basis of Turcot’s syndrome. N. Engl. J. Med. 332, 839–847 10.1056/NEJM199503303321302 [DOI] [PubMed] [Google Scholar]
- 9.Soni S., Ruhela R.K. and Medhi B. (2016) Nanomedicine in central nervous system (CNS) disorders: a present and future prospective. Adv. Pharm. Bull. 6, 319–335 10.15171/apb.2016.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D., Hanna D.L., Usher J., LoCoco J., Chaudhari P., Lenz H.J.. et al. (2014) Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer 120, 3707–3716 10.1002/cncr.28912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendifar A., Yang D., Lenz F., Lurje G., Pohl A., Lenz C.. et al. (2009) Gender disparities in metastatic colorectal cancer survival. Clin. Cancer Res. 15, 6391–6397 10.1158/1078-0432.CCR-09-0877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D., Hendifar A., Lenz C., Togawa K., Lenz F., Lurje G.. et al. (2011) Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J. Gastrointest Oncol. 2, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A.. et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamson C., Kanu O.O., Mehta A.I., Di C., Lin N., Mattox A.K.. et al. (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin. Investig. Drugs 18, 1061–1083 10.1517/13543780903052764 [DOI] [PubMed] [Google Scholar]
- 15.Grossman S.A., O’Neill A., Grunnet M., Mehta M., Pearlman J.L., Wagner H.. et al. (2003) Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J. Clin. Oncol. 21, 1485–1491 10.1200/JCO.2003.10.035 [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Lv Y., Liu J., Wang D., Huang Q., Wang X.. et al. (2009) Survival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in China. J. Clin. Neurosci. 16, 1595–1598 10.1016/j.jocn.2009.02.036 [DOI] [PubMed] [Google Scholar]
- 17.McGirt M.J., Mukherjee D., Chaichana K.L., Than K.D., Weingart J.D. and Quinones-Hinojosa A. (2009) Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65, 463–469, discussion 9-70 10.1227/01.NEU.0000349763.42238.E9 [DOI] [PubMed] [Google Scholar]
- 18.Huang K., Whelan E.A., Ruder A.M., Ward E.M., Deddens J.A., Davis-King K.E.. et al. (2004) Reproductive factors and risk of glioma in women. Cancer Epidemiol. Biomarkers Prev. 13, 1583–1588 [PubMed] [Google Scholar]
- 19.Hatch E.E., Linet M.S., Zhang J., Fine H.A., Shapiro W.R., Selker R.G.. et al. (2005) Reproductive and hormonal factors and risk of brain tumors in adult females. Int. J. Cancer 114, 797–805 10.1002/ijc.20776 [DOI] [PubMed] [Google Scholar]
- 20.Benson V.S., Pirie K., Green J., Casabonne D. and Beral V. (2008) Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br. J. Cancer 99, 185–190 10.1038/sj.bjc.6604445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nizamutdinov D., Stock E.M., Dandashi J.A., Vasquez E.A., Mao Y., Dayawansa S.. et al. (2018) Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurgery 109, e67–e74 10.1016/j.wneu.2017.09.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng M.Y. and Tseng J.H. (2005) Survival analysis for adult glioma in England and Wales. J. Formos. Med. Assoc. 104, 341–348 [PubMed] [Google Scholar]
- 23.Claus E.B. and Black P.M. (2006) Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program Cancer 106, 1973–2001 10.1002/cncr.21733 [DOI] [PubMed] [Google Scholar]
- 24.Barone T.A., Gorski J.W., Greenberg S.J. and Plunkett R.J. (2009) Estrogen increases survival in an orthotopic model of glioblastoma. J. Neurooncol. 95, 37–48 10.1007/s11060-009-9904-6 [DOI] [PubMed] [Google Scholar]
- 25.Li Q., Jedlicka A., Ahuja N., Gibbons M.C., Baylin S.B., Burger P.C.. et al. (1998) Concordant methylation of the ER and N33 genes in glioblastoma multiforme. Oncogene 16, 3197–3202 10.1038/sj.onc.1201831 [DOI] [PubMed] [Google Scholar]
- 26.Yu X., Jiang Y., Wei W., Cong P., Ding Y., Xiang L.. et al. (2015) Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol. 36, 967–972 10.1007/s13277-014-2709-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available in the SEER dataset repository. https://seer.cancer.gov/.