Abstract
Objective: To explore whether aspirin (ASA) enhances the sensitivity of hepatocellular carcinoma (HCC) side population (SP) cells to doxorubicin (Doxo) via miR-491/ATP-binding cassette sub-family G member 2 (ABCG2).
Methods: Non-SP and SP cells were isolated from MHCC-97L cell line using flow cytometry analysis and fluorescence-activated cell sorting. Colony formation assay was performed to determine the colony-formation ability of cells. Cell viability of SP cells was determined with the MTT assay. Luciferase reporter assay was applied in confirming the binding between miR-491 and ABCG2.
Results: Although the Doxo treatment lowered the colony-formation ability of both non-SP and SP cells, the colony-formation ability of SP cells was 2-fold higher than that of non-SP cells (P<0.05). Doxo slightly inhibited the cell viability of SP cells in a concentration-dependent manner; the addition of ASA dramatically enhanced the inhibitory effect of Doxo on SP cell viability in a concentration-dependent manner (P<0.05). Compared with non-SP cells, the miR-491 expression was significantly decreased in SP cells, which was significantly reversed by ASA (P<0.05). miR-491 directly controlled the ABCG2 expression. In the presence of Doxo, miR-491 inhibitor reduced the inhibitory effect of ASA on the cell viability of SP cells, which was significantly reversed by knockdown of ABCG2 (P<0.05).
Conclusion: ASA enhanced the sensitivity of SP cells to Doxo via regulating the miR-491/ABCG2 signaling pathway.
Keywords: Aspirin (ASA), hepatocellular carcinoma (HCC), side population (SP), doxorubicin (Doxo), miR-491, ATP-binding cassette sub-family G member 2 (ABCG2)
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths in the world [1]. Chemotherapeutic agents (including doxorubicin [Doxo]) are widely used in the clinical treatment of HCC [2]. However, drug resistance always results in the failure and thus limited use of chemotherapeutic drugs in treating HCC patients [3]. Therefore, enhancing the drug sensitivity of HCC cells is beneficial for the clinical treatment of patients with HCC.
Side population (SP) cell is a special type of tumor stem cell that exists in many solid tumor tissues, including human primary HCC [4–8]. In HCC cell lines, previous studies have also reported the existence of unique SP cells with cancer stem/stem cell properties [9–11]. Compared with non-SP cells, the SP cells showed much stronger anti-apoptotic and proliferative activities [12]. Besides, it was found that the resistance of SP cells to chemotherapy drugs was significantly higher than that of non-SP cells [13,14].
A common cause of drug resistance is that a large number of tumor cells express the ATP-binding cassette (ABC) pump, which causes tumors to have little response to conventional chemotherapy [15–18]. ATP-binding cassette sub-family G member 2 (ABCG2) is the main transport protein that mediates SP phenotype [19,20]. ABCG2 promotes drug resistance, and is a potential cancer stem cell (CSC) marker in HCC. The expression of ABCG2 is closely related to the occurrence, proliferation, drug resistance, and metastasis of tumor. As reported, the up-regulation of ABCG2 enhanced the proliferation, Doxo resistance, migration, and invasion of HCC, which were lowered by the down-regulation of ABCG2 [21]. Hu et al. [22] studied the expression pattern of ABCG2 in HCC, and proved that the expression of ABCG2 endowed HCC cells, especially SP cells, with the efflux capacity, which was modulated by Akt signaling.
It has been reported that aspirin (ASA), a cyclooxygenase inhibitor, promoted growth inhibition and apoptosis of HCC [23]. The ligation of ASA to cisplatin can lead to chemotherapy sensitization, thereby defeating resistance [24]. Recently, the scholars demonstrated that ASA inhibited the acquisition of chemoresistance in breast cancer by disrupting the NFkB–IL6 signaling pathway that was responsible for the generation of CSCs [25]. However, there have been few studies about the underlying mechanism of how ASA suppresses the drug resistance of HCC SP cells.
MiRNAs, a well-known class of small non-coding RNAs, participate in numerous pathophysiological processes [26]. Multiple miRNAs are related to HCC, including miR-491. miR-491 was reported to be related with the CSC-like properties of HCC [27]. Its expression was much lower in poorly differentiated HCC tissues compared with well-differentiated HCC tissues, and miR-491 was negatively associated with CSC-like properties in both cell line and tissue samples of HCC [27]. Bioinformatics analysis (microRNA.org) has shown the binding site of miR-491 in ABCG2. Therefore, in the present study, we investigated whether ASA enhances the sensitivity of HCC SP cells to Doxo via up-regulating miR-491 and down-regulating target gene ABCG2, providing theoretical basis for the clinical application of ASA.
Materials and methods
Isolation of non-SP and SP cells from the HCC cell line MHCC-97L
The isolation of non-SP and SP cells from MHCC-97L cell line was conducted as previously reported [22]. Briefly, the adherent cells were dissolved by trypsin and suspended in DMEM medium containing 2% FBS and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (1 × 106 cells/ml). The cells were then stained by 5 μg/ml Hoechst 33342 (Invitrogen) at 37°C for 1.5 h in the absence or presence of 10 μM fumitremorgin C (FTC). Afterward, the cells were centrifuged at 4°C and resuspended in ice-cold PBS with 2 μg/ml propidium iodide (PI). Then flow cytometry analysis and fluorescence-activated cell sorting were performed to isolate non-SP and SP cells.
Colony formation assay
To determine the colony-formation ability of MHCC-97L cells with drug treatment, the SP and non-SP cells were sorted in the presence of Doxo (500 ng/ml). The sorted cells were planted in 96-well microplates in triplicate (20 cells/well) and cultured in DMEM medium with 10% FBS. After 2 weeks, the cells were stained with 0.01% crystal violet and the number of colonies was counted under a microscope.
MTT assay
The cell viability of SP cells was determined with the MTT assay. Briefly, SP cells were planted into 96-well microplates and cultured for 24 h. Then the cells were treated with different concentrations of ASA (0, 1.25, 2.5, and 5 μmol/ml) for 48 h in the presence of Doxo (500 ng/ml) [28]. Afterward, 10 μl of MTT was added to every well and cultured at 37 °C for 2 h in darkness. After the medium was changed to DMSO, and the absorbance at 570 nm was determined.
Quantitative real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. After the purity and concentration were determined, the purified RNA was used to synthesize the first‐strand cDNA using Reverse Transcription Kit (Qiangen, Germany) under the guidance of the manufacturer. The SYBR Green Master Mix (Applied Biosystems, U.S.A.) was used for quantitative real-time PCR (qPCR), which was analyzed on an ABI 7900HT Fast Real-Time PCR System. The relative expression levels of miR-130b-3p, miR-7-5p, miR-491, miR-612, and miR-3650 were normalized to those of U6, and the relative expression levels of ABCG2 were normalized to those of GAPDH. The gene expression was quantified using the comparative Ct (ΔΔCt) approach.
Western blotting
The radioimmunoprecipitation assay (RIPA) lysis buffer (containing protease and phosphatase inhibitors) was used to extract total proteins from cells. After measuring the protein concentration, we loaded the equal amounts of protein samples to SDS-PAGE. The proteins were then transferred to a PVDF membrane. After being incubated with 5% non-fat milk for 30 min at room temperature, the PVDF membrane was probed with the primary antibodies, anti-ABCG2 (1:20, Abcam), and anti‐β-actin (1:5000, Abcam), at 4°C overnight. After being washed three times, the membrane was incubated with secondary antibody containing horse radish peroxidase (HRP) at room temperature for 2 h. Bands were visualized with ECL (GE Healthcare).
Cell transfection
The SP cells were seeded in six-well plates. After 24 h, the SP cells were transfected with miR-491 inhibitor, ABCG2 siRNA (siRNA-ABCG2) or negative control using Lipofectamine 2000 (Invitrogen, U.S.A.) according to the protocol of manufacturer. Forty-eight hours after transfection, the cells were collected for determining gene expression and cell viability.
Luciferase reporter assay
Genomic DNA was isolated from SP cells and used as a template. The wild-type 3′UTR of ABCG2 was inserted into the pGL3-Basic Luciferase Reporter Vector (Promega) to construct the ABCG2-WT plasmid. Then the mutant ABCG2 3′UTR (ABCG2-Mut) plasmid was constructed based on the ABCG2-WT plasmid. The SP cells were cultured in 12-well plates for 24 h, and then transfected with one of the pGL3-based 3′UTR-reporter plasmids together with miR-491 mimic, miR-491 inhibitor, or negative control using Lipofectamine 2000. The cells were collected after 48 h of transfection, and the luciferase activity was measured using Dual-Luciferase Reporter Assay (Promega, U.S.A.) under the guidance of the manufacturer.
Statistical analysis
Statistical analysis was conducted using SPSS 18.0 software. Student’s t-tests or ANOVA (used in cases where there are more than two groups) were performed to compare the mean of groups. All data were presented as mean ± S.D. P<0.05 was considered as statistically significant.
Results
ASA enhances the sensitivity of SP cells to Doxo
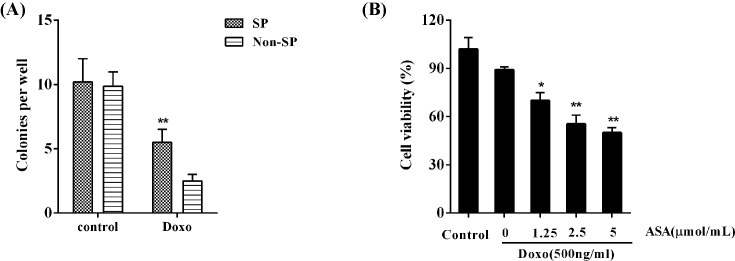
Without drug treatment, non-SP and SP cells showed the equivalent colony-formation ability. Although the Doxo treatment lowered the colony-formation ability of both non-SP and SP cells, the colony-formation ability of SP cells was much higher than that of non-SP cells (P<0.01; Figure 1A). We then focussed on studying the SP cells. Doxo slightly inhibited the cell viability of SP cells; the addition of ASA dramatically enhanced the inhibitory effect of Doxo on SP cell viability in a concentration-dependent manner (P<0.05; Figure 1B).
Figure 1. Effect of ASA on drug resistance of HCC SP cells.
Flow cytometry analysis and fluorescence-activated cell sorting were performed to isolate non-SP and SP cells from the HCC cell line MHCC-97L. (A) The colony-formation ability of non-SP and SP cells was determined in the absence or presence of Doxo (500 ng/ml); **P<0.01, compared with Non-SP cells. (B) The cell viability of SP cells treated with different concentrations of ASA (0, 1.25, 2.5, and 5 μmol/ml) for 48 h in the presence of Doxo (500 ng/ml) was analyzed by MTT assay; *P<0.05, **P<0.01, compared with control.
Decreased miR-491 expression in SP cells is reversed by ASA
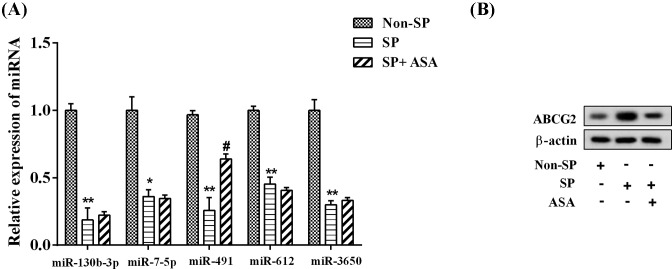
Compared with non-SP cells, the expression of miR-130b-3p, miR-7-5p, miR-491, miR-612, and miR-3650 was significantly decreased in SP cells; however, ASA can only reverse the miR-491 expression (P<0.05; Figure 2A). Consistent with this finding, the ABCG2 protein expression in SP cells was much higher than that in non-SP cells, which was reversed by ASA treatment (Figure 2B).
Figure 2. Decreased miR-491 expression in SP cells was reversed by ASA.
ASA (2.5 μmol/ml) was used to treat MHCC-97L SP cells. There were three groups as follows: non-SP group, SP group, and SP + ASA group. (A) The expression of multiple miRNAs (miR-130b-3p, miR-7-5p, miR-491, miR-612, and miR-3650), confirmed by qPCR; *P<0.05, **P<0.01, compared with non-SP cells; #P<0.05, compared with SP. (B) The expression of ABCG2, determined by Western blotting. β-Actin was used as the loading control.
ASA enhances the sensitivity of SP cells to Doxo via miR-491
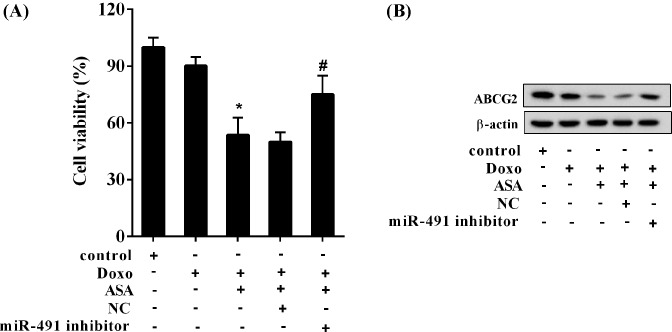
In the presence of Doxo, the cell viability of SP cells was 90%, which was dramatically decreased by ASA with a result of 55%, and further significantly reversed by the miR-491 inhibitor with a result of 72% (P<0.05; Figure 3A). In line with this finding, the ABCG2 expression was reduced by ASA, which was reversed by the miR-491 inhibitor (Figure 3B).
Figure 3. ASA enhanced the sensitivity of SP cells to Doxo via miR-491.
The SP cells were divided into five groups (Control, Doxo, Doxo + ASA, Doxo + ASA + NC, and Doxo + ASA + miR-491 inhibitor). The concentration of Doxo was 500 ng/ml, and ASA was 2.5 μmol/ml. (A) The cell viability of SP cells; *P<0.05, compared with Doxo; #P<0.05, compared with Doxo + ASA + NC. (B) The expression of ABCG2.
miR-491 directly controls the ABCG2 expression
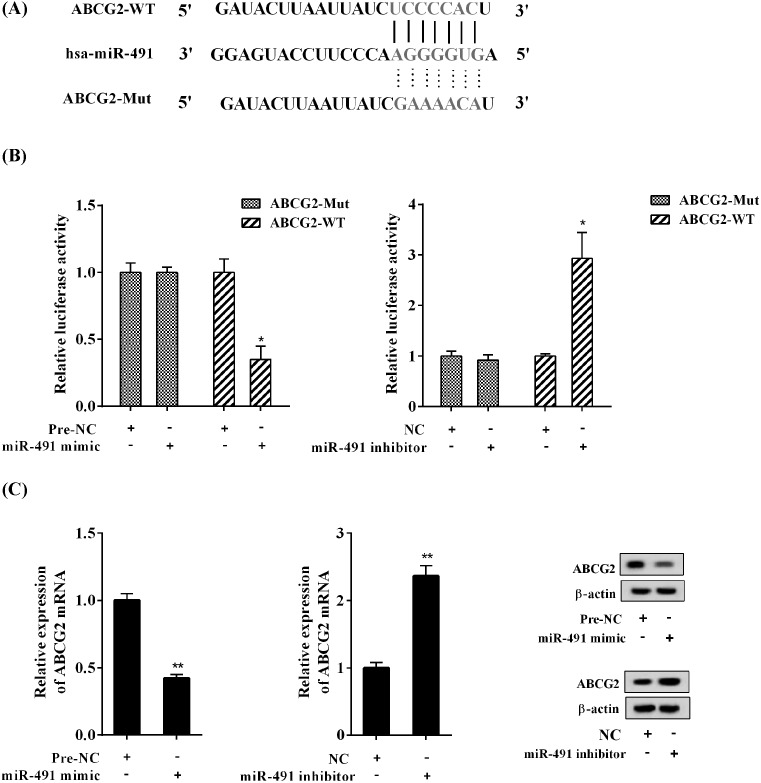
The bioinformatics analysis showed the miR-491-binding site in the 3′UTR of ABCG2 mRNA (Figure 4A). In SP cells, the luciferase reporter assay showed that miR-491 mimic inhibited the luciferase activity of wild-type ABCG2 3′UTR (P<0.05), without affecting the mutant ABCG2 3′UTR; in contrast, miR-491 inhibitor enhanced the luciferase activity of wild-type ABCG2 3′UTR (P<0.05), without impacting the mutant ABCG2 3′UTR (Figure 4B). Further, miR-491 mimic reduced the mRNA and protein expression of ABCG2, while miR-491 inhibitor increased the mRNA and protein expression of ABCG2 (Figure 4C).
Figure 4. miR-491 directly controlled the ABCG2 expression in SP cells.
(A) The miR-491-binding site existed in the 3′UTR of ABCG2 mRNA. (B) The effect of miR-491 mimic or inhibitor on the luciferase activity of wild-type ABCG2 3′UTR and mutant ABCG2 3′UTR. (C) The effect of miR-491 mimic or inhibitor on the mRNA and protein expression of ABCG2. *P<0.05 and **P<0.01, compared with pre-NC or NC.
ASA enhances the sensitivity of SP cells to Doxo via miR-491/ABCG2
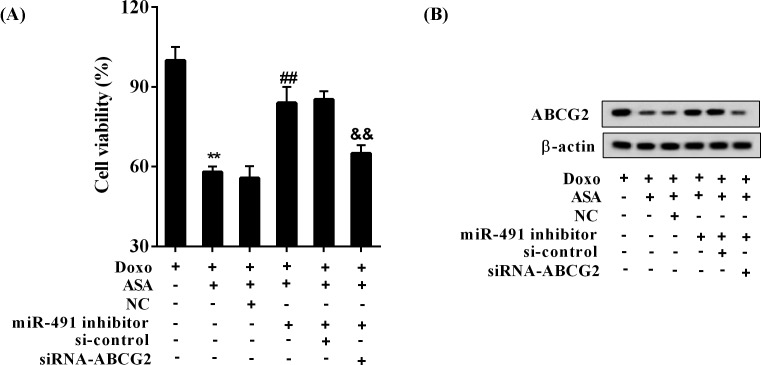
In the presence of Doxo, miR-491 inhibitor reduced the inhibitory effect of ASA on the cell viability of SP cells, which was significantly reversed by the knockdown of ABCG2 (P<0.05; Figure 5A). Consistent with this finding, the promoting effect of ASA on ABCG2 expression was decreased by miR-491 inhibitor, which was reversed by the knockdown of ABCG2 (Figure 5B).
Figure 5. ASA enhanced the sensitivity of SP cells to Doxo via miR-491/ABCG2.
The SP cells were allocated to five groups (Doxo, Doxo + ASA, Doxo + ASA + NC, Doxo + ASA + miR-491 inhibitor, Doxo + ASA + miR-491 inhibitor + si-control, and Doxo + ASA + miR-491 inhibitor + siRNA-ABCG2). The concentration of Doxo was 500 ng/ml, and ASA was 2.5 μmol/ml. (A) The cell viability of SP cells; **P<0.01, compared with Doxo; ##P<0.01, compared with Doxo + ASA + NC; &&P<0.01, compared with Doxo + ASA + miR-491 inhibitor + si-control. (B) The protein expression of ABCG2 in SP cells.
Discussion
In the present study, we found that the colony-formation ability of SP cells were much higher than that of non-SP cells, and that ASA enhanced the sensitivity of SP cells to Doxo via up-regulating miR-491 and down-regulating target gene ABCG2.
Chemotherapy is one of the main methods for clinical treatment of HCC patients [1]. However, the use of chemotherapeutic drugs, including Doxo and cisplatin, was limited due to the drug toxicity, low drug efficacy, and acquired drug resistance of cancer cells [29]. Due to the high incidence of developing resistance to drug in HCC [3], it is important to enhance the drug sensitivity of HCC cells in clinical treatment.
SP cell is a special type of tumor stem cell that has been isolated from multiple solid tumor tissues, such as small-cell lung cancer (SCLC), cervical cancer, osteosarcoma, and HCC [4–7,30,31]. In human primary HCC, the cell viability, colony forming ability, anti-apoptosis, self-renewal, invasion, and tumorigenicity of SP cells were much higher than those of non-SP cells [32]. Moreover, it has also been reported that the resistance of SP cells to chemotherapy drugs was significantly higher than that of non-SP cells [9,10,13,14]. Consistent with this, in the present study, we found that the colony-formation ability of SP cells were much higher than that of non-SP cells, although the Doxo treatment lowered the colony-formation ability of both non-SP and SP cells. Besides, Doxo can only slightly suppress the cell viability of SP cells. As reported, ABCG2 promoted the drug resistance, and was a potential CSC marker in HCC [21]. Moreover, the expression of ABCG2 endowed HCC cells, especially SP cells, with the efflux capacity. In our study, we also found that the ABCG2 expression in SP cells was much higher than that in non-SP cells.
To date, the chemotherapy of HCC is widely accepted in the world. For example, Doxo is widely used in Asia and the North-African region; Sorafenib is the most popular drug for the HCC treatment worldwide, new PD1-inhibitors and regorafenib are also recently approved. ASA is widely applied in the chemotherapy of HCC owing to its anti-platelet effect [33]. ASA was found to enhance IFN-α-induced growth inhibition and apoptosis of HCC via controlling the Janus kinase 1 (JAK1)/signal transducer and activator of transcription 1 (STAT1) signaling pathway [23]. Subsequently, researchers demonstrated that the ligation of ASA to cisplatin could take significant synergistic effects on tumor cells [24]. Recently, it has been reported that ASA inhibited the acquisition of chemoresistance in breast cancer by disrupting the NFkB–IL6 regulatory axis that contributed to the generation of CSCs [25]. In the present study, we explored the underlying mechanism of how ASA suppressed the drug resistance of HCC SP cells. miR-491 is widely involved in the pathogenesis of multiple tumors, including glioma, osteosarcoma, cervical cancer, esophageal cancer, and liver cancer by regulating cell proliferation, apoptosis, migration, invasion, etc [34–38]. In HCC, miR‐491 was shown to be involved in metastasis by blocking epithelial-to-mesenchymal transition (EMT) and reducing matrix metalloproteinase (MMP)‐9 expression [39]. Besides, miR-491 was shown to decrease CSC-like properties of HCC by inhibition of GIT-1/NFκB-mediated EMT [27]. In the present study, we found that the miR-491 expression was significantly decreased in SP cells compared with non-SP cells, which was significantly reversed by ASA. Moreover, miR-491 directly controlled the ABCG2 expression. In the presence of Doxo, miR-491 inhibitor reduced the inhibitory effect of ASA on the cell viability of SP cells, which was significantly reversed by knockdown of ABCG2. Therefore, ASA enhanced the sensitivity of HCC SP cells to Doxo via up-regulating miR-491 and down-regulating target gene ABCG2. However, the ASA/miR-491/ABCG2 regulatory axis was not confirmed in vivo, requiring further investigations.
In conclusion, ASA enhanced the sensitivity of SP cells to Doxo via regulating the miR-491/ABCG2 signaling pathway, providing theoretical basis for the clinical application of ASA.
Abbreviations
- ABC
ATP-binding cassette
- ABCG2
ATP-binding cassette sub-family G member 2
- ASA
aspirin
- CSC
cancer stem cell
- DMEM
Dulbecco’s modified Eagle medium
- Doxo
doxorubicin
- EMT
epithelial-to-mesenchymal transition
- FTC
fumitremorgin C
- HCC
hepatocellular carcinoma
- JAK1
Janus kinase 1
- NC
negative control
- PI
propidium iodide
- RIPA
radioimmunoprecipitation assay
- SCLC
small-cell lung cancer
- SP
side population
- STAT1
signal transducer and activator of transcription 1
- WT
wild type
Funding
The study was supported by Key Research and Development Program of Jiangxi Provincial Department of Science and Technology [grant number 20071BBG70085]; Science and Technology Project Foundation of Education Department of Jiangxi Province, China [grant number 160162]; Foundation of The Second Affiliated Hospital of Nanchang University [grant number 2016YNZJ12008]; Science and Technology Planning Project of Health and Family Planning Commission of Jiangxi Province [grant number 20181062]; Science and Technology Planning Project of Administration of Traditional Chinese Medicine of Health and Family Planning Commission of Jiangxi Province [grant number 2017A263]; and the National Natural Science Foundation of China [grant number 81860119].
Author contribution
Z.X. and M.L. conceived and designed the study and drafted the manuscript. C.Z. and P.C. collected the data. Z.X. contributed to the statistical analysis and interpreted the data. F.W. put forward the concept of the study and reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Cox J. and Weinman S. (2016) Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat. Oncol. 3, 57–59 10.2217/hep.15.41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeda M., Mitsunaga S., Ohno I., Hashimoto Y., Takahashi H., Watanabe K.. et al. (2015) Systemic Chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Diseases 3, 360–381 10.3390/diseases3040360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Grazie M., Biagini M.R., Tarocchi M., Polvani S. and Galli A. (2017) Chemotherapy for hepatocellular carcinoma: the present and the future. World J. Hepatol. 9, 907–920 10.4254/wjh.v9.i21.907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taga T. (2006) Identification of cancer stem cells in the “side population”. Gan To Kagaku Ryoho 33, 295–299 . [PubMed] [Google Scholar]
- 5.Yang X., Wang J., Qu S., Zhang H., Ruan B., Gao Y.. et al. (2015) MicroRNA-200a suppresses metastatic potential of side population cells in human hepatocellular carcinoma by decreasing ZEB2. Oncotarget 6, 7918–7929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai J.M., Yin X.Y., Hou X., Hao X.Y., Cai J.P., Liang L.J.. et al. (2013) Analysis of the genome-wide DNA methylation profile of side population cells in hepatocellular carcinoma. Dig. Dis. Sci. 58, 1934–1947 10.1007/s10620-013-2663-4 . [DOI] [PubMed] [Google Scholar]
- 7.Nakayama M., Ogasawara S., Akiba J., Ueda K., Koura K., Todoroki K.. et al. (2014) Side population cell fractions from hepatocellular carcinoma cell lines increased with tumor dedifferentiation, but lack characteristic features of cancer stem cells. J. Gastroenterol. Hepatol. 29, 1092–1101 10.1111/jgh.12484 . [DOI] [PubMed] [Google Scholar]
- 8.Guo Z., Jiang J.H., Zhang J., Yang H.J., Zhong Y.P., Su J.. et al. (2016) Side population in hepatocellular carcinoma HCCLM3 cells is enriched with stem-like cancer cells. Oncol. Lett. 11, 3145–3151 10.3892/ol.2016.4343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraguchi N., Utsunomiya T., Inoue H., Tanaka F., Mimori K., Barnard G.F.. et al. (2006) Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 24, 506–513 10.1634/stemcells.2005-0282 . [DOI] [PubMed] [Google Scholar]
- 10.Chiba T., Kita K., Zheng Y.W., Yokosuka O., Saisho H., Iwama A.. et al. (2006) Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology 44, 240–251 10.1002/hep.21227 . [DOI] [PubMed] [Google Scholar]
- 11.Qiu L., Li H., Fu S., Chen X. and Lu L. (2018) Surface markers of liver cancer stem cells and innovative targeted-therapy strategies for HCC. Oncol. Lett. 15, 2039–2048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Y., Lin M., Jiang X., Ye J., Guo T., Shi Y.. et al. (2017) The recent advances on liver cancer stem cells: biomarkers, separation, and therapy. Anal. Cell. Pathol. (Amst.) 2017, 5108653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou G.X., Liu P.P., Zhang S., Yang M., Liao J., Yang J.. et al. (2018) Elimination of stem-like cancer cell side-population by auranofin through modulation of ROS and glycolysis. Cell Death Dis. 9, 89 10.1038/s41419-017-0159-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phi L.T.H., Sari I.N., Yang Y.G., Lee S.H., Jun N., Kim K.S.. et al. (2018) Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 5416923 10.1155/2018/5416923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu A.X. (2006) Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist 11, 790–800 10.1634/theoncologist.11-7-790 . [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Rahman O. and Fouad M. (2015) Second line systemic therapy options for advanced hepatocellular carcinoma; a systematic review. Expert Rev. Anticancer Ther. 15, 165–182 10.1586/14737140.2015.978295 . [DOI] [PubMed] [Google Scholar]
- 17.Heery R., Finn S.P., Cuffe S. and Gray S.G. (2017) Long non-coding RNAs: key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel) 9, 38, 10.3390/cancers9040038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean M., Fojo T. and Bates S. (2005) Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284 10.1038/nrc1590 . [DOI] [PubMed] [Google Scholar]
- 19.Zhou S., Schuetz J.D., Bunting K.D., Colapietro A.M., Sampath J., Morris J.J.. et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7, 1028–1034 10.1038/nm0901-1028 . [DOI] [PubMed] [Google Scholar]
- 20.Scharenberg C.W., Harkey M.A. and Torok-Storb B. (2002) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99, 507–512 10.1182/blood.V99.2.507 . [DOI] [PubMed] [Google Scholar]
- 21.Zhang G., Wang Z., Luo W., Jiao H., Wu J. and Jiang C. (2013) Expression of potential cancer stem cell marker ABCG2 is associated with malignant behaviors of hepatocellular carcinoma. Gastroenterol. Res. Pract. 2013, 782581 10.1155/2013/782581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C., Li H., Li J., Zhu Z., Yin S., Hao X.. et al. (2008) Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis 29, 2289–2297 10.1093/carcin/bgn223 . [DOI] [PubMed] [Google Scholar]
- 23.Li T., Dong Z.R., Guo Z.Y., Wang C.H., Tang Z.Y., Qu S.F.. et al. (2013) Aspirin enhances IFN-alpha-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 20, 366–374 10.1038/cgt.2013.29 . [DOI] [PubMed] [Google Scholar]
- 24.Cheng Q., Shi H., Wang H., Min Y., Wang J. and Liu Y. (2014) The ligation of aspirin to cisplatin demonstrates significant synergistic effects on tumor cells. Chem. Commun. (Camb.) 50, 7427–7430 10.1039/C4CC00419A . [DOI] [PubMed] [Google Scholar]
- 25.Saha S., Mukherjee S., Khan P., Kajal K., Mazumdar M., Manna A.. et al. (2016) Aspirin suppresses the acquisition of chemoresistance in breast cancer by disrupting an NFkappaB-IL6 signaling axis responsible for the generation of cancer stem cells. Cancer Res. 76, 2000–2012 10.1158/0008-5472.CAN-15-1360 . [DOI] [PubMed] [Google Scholar]
- 26.Schueller F., Roy S., Vucur M., Trautwein C., Luedde T. and Roderburg C. (2018) The role of miRNAs in the pathophysiology of liver diseases and toxicity. Int. J. Mol. Sci. 19, 10.3390/ijms19010261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Ye J., Yan H., Tang Z., Shen J., Zhang J.. et al. (2016) MiR-491 attenuates cancer stem cells-like properties of hepatocellular carcinoma by inhibition of GIT-1/NF-kappaB-mediated EMT. Tumour Biol. 37, 201–209 10.1007/s13277-015-3687-5 . [DOI] [PubMed] [Google Scholar]
- 28.Hossain M.A., Kim D.H., Jang J.Y., Kang Y.J., Yoon J.H., Moon J.O.. et al. (2012) Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo. Int. J. Oncol. 40, 1636–1642 10.3892/ijo.2011.1304 . [DOI] [PubMed] [Google Scholar]
- 29.Sun W. and Cabrera R. (2018) Systemic treatment of patients with advanced, unresectable hepatocellular carcinoma: emergence of therapies. J. Gastrointest. Cancer, 49, 107–115 10.1007/s12029-018-0065-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Z.T., Yu X.W., He J.X., Liu Y. and Zhang S.L. (2017) Characteristics of primary side population cervical cancer cells. Oncol. Lett. 14, 3536–3544 10.3892/ol.2017.6606 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M., Yan M., Zhang R., Li J. and Luo Z. (2011) Side population cells isolated from human osteosarcoma are enriched with tumor-initiating cells. Cancer Sci. 102, 1774–1781 10.1111/j.1349-7006.2011.02028.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y., Gao H., Liu M. and Mao Q. (2016) Sorting and biological characteristics analysis for side population cells in human primary hepatocellular carcinoma. Am. J. Cancer Res. 6, 1890–1905 . [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang I.C., Chang J., Kim K. and Park S.M. (2018) Aspirin use and risk of hepatocellular carcinoma in a national cohort study of korean adults. Sci. Rep. 8, 4968 10.1038/s41598-018-23343-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Z., Ding H., He E., Chen J. and Li M. (2017) Up-regulation of microRNA-491-5p suppresses cell proliferation and promotes apoptosis by targeting FOXP4 in human osteosarcoma. Cell Prolif. 50, e12308, 10.1111/cpr.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi Z., Cai S., Cai J., Chen L., Yao Y., Chen L.. et al. (2016) miR-491 regulates glioma cells proliferation by targeting TRIM28 in vitro. BMC Neurol. 16, 248 10.1186/s12883-016-0769-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Q., Zhai Y.X., Liu H.Q., Shi Y.A. and Li X.B. (2015) MicroRNA-491-5p suppresses cervical cancer cell growth by targeting hTERT. Oncol. Rep. 34, 979–986 10.3892/or.2015.4013 . [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Jiang F., Mu J., Ye X., Si L., Ning S.. et al. (2014) Arsenic trioxide attenuates the invasion potential of human liver cancer cells through the demethylation-activated microRNA-491. Toxicol. Lett. 227, 75–83 10.1016/j.toxlet.2014.03.016 . [DOI] [PubMed] [Google Scholar]
- 38.Niu H., Gong L., Tian X., Fang L., Wang C. and Zhu Y. (2015) Low expression of miR-491 Promotes Esophageal Cancer Cell Invasion by targeting TPX2. Cell Physiol. Biochem. 36, 2263–2273 10.1159/000430190 . [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y., Li Y., Ye J., Jiang R., Yan H., Yang X.. et al. (2013) MicroRNA-491 is involved in metastasis of hepatocellular carcinoma by inhibitions of matrix metalloproteinase and epithelial to mesenchymal transition. Liver Int. 33, 1271–1280 10.1111/liv.12190 . [DOI] [PubMed] [Google Scholar]