Abstract
The use of plant essential oils has been shown to efficiently control insect pests of stored beans, significantly reducing the threats associated with synthetic insecticides. Here, we evaluated the potential of applications of essential oils of clove, Syzygium aromaticum L., and cinnamon, Cinnamomum zeylanicum L., to control Callosobruchus maculatus, considered as one of the most cosmopolitan pests of stored beans. Using four combinations of couples (i.e., unexposed couples, exposed females, exposed males, and exposed couples), we also evaluated how sublethal exposure to these essential oils impacted C. maculatus oviposition. Bioassays results revealed that both essential oils exhibited insecticidal activities similar to the synthetic pyrethroid insecticide deltamethrin. Furthermore, oil dosage increments proportionately decreased the growth rate and reduced the losses in bean weight caused by cowpea weevils, and offspring emergence was almost abolished when parents were exposed to the LD20 of each essential oil. Finally, significant oviposition impairments were perceived only in couples where females were exposed (i.e., females exposed and exposed couples) to the LD20 of cinnamon and clove essential oils. Thus, by exhibiting similar insecticidal activities as synthetic insecticides and by significantly affecting the oviposition of sublethally exposed C. maculatus females, the cinnamon and clove essential oils represent valuable tools with potential of integration into the management of C. maculatus infestations.
Introduction
Plant essential oils have gained a reputation as being potentially bioactive compounds against many insect species, which has portrayed them as safer tools in terms of the environment and human health [1–6]. Despite the potential of essential oils to control pests of stored products, few studies have addressed the physiological and biological responses of stored product pests when the exposure occurred at sublethal levels.
It has been well recognized that stored product pests when sublethally exposed to synthetic insecticides can exhibit not only detrimental (what is somehow expected), but under certain circumstances also positive responses on their physiology and behavior [7–11]. Although the mechanisms explaining such sublethal responses (i.e., positive or detrimental) are still not well understood, it has been described that individuals sublethally exposed to synthetic insecticides show alterations in relevant life traits (e.g., the development time, longevity, fertility, fecundity, immune capacities, locomotion, navigation, sexual communication, oviposition and feeding) [7]. It is worthy to note, however, that similar life trait alterations seem to be elicited by sublethal exposure to botanical insecticides, which in their turn can trigger insect responses that either increase [12–16] or compromise the efficacy of these alternative control tools [9, 17, 18].
Among the essential oils that have been shown to adequately control insect pests, the oils extracted from clove, Syzygium aromaticum (L.), and cinnamon, Cinnamomum zeylanicum (L.), plants have drawn particular interest because of their promising insecticidal activities against various pests of stored products such as the maize weevil Sitophilus zeamais and the red flour beetle Tribolium castaneum [2, 12–16]. In insects, these essential oils have neurotoxic action both as fumigants and or contact insecticides and there metabolites can act upon variety of molecular targets including inhibition of acetylcholinesterase or disturbing the functions of GABAergic and aminergic systems [19].
The cowpea weevil, Callosobruchus maculatus Fabricius (Coleoptera: Chrysomelidae: Bruchinae), is a cosmopolitan pest of legume seeds and is among the most serious pests of stored products in tropical countries [20–22]. The insect larvae represent the most destructive stage, as adult cowpea bruchid do not feed [23, 24]. However, as the availability of a specific host is highly discontinuous and because these adult insects have to live in hosts that are normally treated with insecticides [25–27], these insects might have to face insecticidal sublethal exposures prior to deciding where they are going to lay eggs.
Most of the cowpea, Vigna unguiculata (L.) Walp, production occurs in tropical countries with high contribution of small farmers. In this context, the control of C. maculatus, when accomplished, is mainly achieved by the application of a small group of synthetic molecules (e.g., phosphine and pyrethroid insecticides, such as deltamethrin). Dependence on a small group of synthetic molecules raises the risk of selecting resistant populations as well as increases the hazard risks to human health and to the environment [2, 28].
Thus, it is urgently needed to develop alternatives to the chemical control of C. maculatus that not only can reduce the concerns outlined above but also can be prone of embracing the actual trend in developing new botanical-derived insecticides based on the inclusion of the active ingredient (i.e. EOs) in stable delivery systems (nanoparticles, nanoemulsion, etc) [29, 30]. Therefore, we investigated the chemical composition of clove and cinnamon essential oils and evaluated whether these oils would adequately control C. maculatus. We also characterized the biological responses (e.g., oviposition, offspring emergence and population growth) of C. maculatus exposed to sublethal amounts of each type of essential oil.
Materials and methods
Insect rearing
The original population of C. maculatus was field-collected from small farms in the Viçosa region (Minas Gerais State, Brazil) during the year 2015, and the population was maintained on pest- and insecticide-free cowpea beans under laboratory conditions (27 ± 2°C, 75 ± 5% RH, 12 h scotophase). The bean grains had a water content of 12% and were offered ad libitum. The farm-owners gave permission to collect samples of C. maculatus from their fields and since C. maculatus is not an endangered or protected species in Brazil, no specific permissions were required for such collection.
Extraction and chemical characterization of essential oils
Locally purchased cinnamon bark and dried flower buds of clove were used for essential oil extraction, as described by [31]. Briefly, the primary material was ground and sieved to obtain a fine powder (less than 1 mm) that was extracted at room temperature by constant percolation with hexane, followed by hydrodistillation for 6 h. Then, the distillate was extracted twice with dichloromethane and dried over anhydrous sodium sulfate. The distilled oils were stored in airtight screw-capped vials at -10°C until use.
The components of the cinnamon and clove essential oils were determined by gas chromatography-mass spectrometry (GCMS-QP2010, Shimadzu). The separation was done on a capillary column of fused silica (30 m × 0.22 mm) with stationary phase RTX5 (0.25-μm-thick film). The initial column temperature was 60°C for 2 min, followed by increase of 3°C min-1 up to 240°C, and this temperature was maintained for 15 min. The temperatures of the injector and detector were maintained at 220°C and 240°C, respectively.
The carrier gas was helium with a flow of 1.8 mL min-1. Samples were diluted in dichloromethane and injected in a 1.0 μL split ratio of 1:20. Data acquisition was made in full-scan mode, with a scanning range between 29 and 400 m/z. Experimental mass spectra were compared with known mass spectra (The National Institute of Standards and Technology (NIST 14) Mass Spectra Library, 2017). The arithmetic index (AI) was calculated according to [32], using the retention times of the essential oil compounds and a homologous series of C8-C26 n-alkane standards following the formula: AI (x) = 100 Pz + 100[(t (x) − t (Pz))/t (Pz+1) − t (Pz))]; where x: compound at time t; Pz: alkane before x; and Pz+1: alkane after x. The relative percentage of each compound was calculated by the integral ratio of its respective peak area and the total area of all the compounds of the sample. The calculated AI for each compound was compared with values reported in the literature [32].
Insecticidal activity
We conducted dose-mortality bioassays to determine the lethal doses of the cinnamon and clove essential oils to adult C. maculatus. These bioassays followed procedures previously described elsewhere [33]. Briefly, each essential oil was pure (i.e., without diluents) and was applied using a 25-μL microsyringe (Hamilton, Reno, NV, USA) to 50 g of beans that were placed in 0.8-L glass jars. After the application, the jars were manually shaken for 60 s to ensure a complete distribution of the essential oils. Twenty unsexed 1-2-day-old C. maculatus adults were placed in each jar, and the jars were sealed with a fine porous cloth to allow ventilation; jars were kept under controlled conditions (27 ± 2°C, 75 ± 5% relative humidity, 12 h scotophase). The insect mortality was recorded after a 24-h exposure period. Insects were considered dead if they did not respond to fine paintbrush stimuli (i.e, two subsequent touches in 2 min intervals). Five doses of each essential oil were tested in the bioassays (e.g., Cinnamon: 20, 60, 120, 160 and 200 μl kg-1. Clove: 20, 40, 80, 120, 160 μl kg-1). Five replications were used per dose, and the control treatment did not receive any oil application. As a positive control, we used the pyrethroid insecticide deltamethrin (25 g L-1; EC; Bayer Crop Science, SP, Brazil) diluted in distilled water to obtain the desired doses (e.g., 64, 72, 80, 88, 96, 104 μl a.i kg-1). The application and the conditions of bioassays were similar to those described for the essential oils.
Effects of essential oils on the biological development and bean-mass losses
Effects on instantaneous rate of population growth (ri) and bean-mass losses
The instantaneous rate of increase (ri) test was carried out in 0.8-L glass jars, where 20 unsexed 1-2-day-old adults of C. maculatus were allowed to colonize 50 g of beans treated with an essential oil based on the concentration-mortality results previously obtained (see Results section). We measured the instantaneous rate of increase (ri) of groups of C. maculatus that were subjected to different sublethal exposures to cinnamon (LD20 = 106.2, LD40 = 123.0, LD60 = 139.4, LD80 = 161.4 μL kg-1 of bean) and clove (LD20 = 48.6, LD40 = 67.6, LD60 = 90.2, LD80 = 125.8 μL kg-1 of bean) essential oils. Five replicates were used for each combination of concentration and essential oil. All the glass jars were maintained at 27 ± 2°C, 75 ± 5% relative humidity and 12 h scotophase. The control treatment did not receive any essential oil application. The number of F1 insects was counted after 45 days, and the instantaneous rate of increase for each population was calculated as follows: ri = ln (Nf/Ni)/Δt, where Nf is the final number of observed adults, Ni is the initial number of C. maculatus, and Δt is the duration of the experiment [34].
The grain masses provided for insect colonization were weighed at the start (day 0) and at the end (day 45) of the bioassays to calculate the percentage of grain loss.
Effects on average and cumulative emergence
The bioassays for the average emergence were conducted using the same experimental procedures described for the instantaneous rate of increase (ri). The progeny formed by the adult C. maculatus emerging from the beans were assessed in two days intervals starting from the 20th day after treatments began until no adult emergence was observed (i.e., 20 days after emergence of the 1st adults). After each assessment, the emerged adults were removed.
Effects of sublethal exposure to essential oils on the C. maculatus oviposition
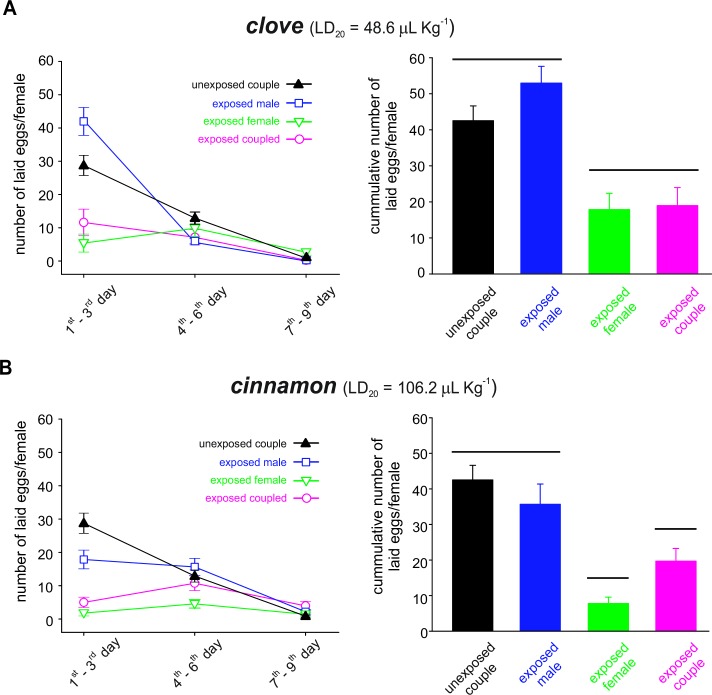
Newly emerged (< 48 h old) groups of C. maculatus adult males and females were exposed separately to clove- and cinnamon-essential-oil-treated beans at the LD20 values for clove (i.e., 48.6 μL kg-1 of beans) and for cinnamon (i.e., 106.2 μL kg-1 of beans) essential oils. After a 24-h exposure period, we paired C. maculatus couples in four combinations (i.e., unexposed couple, exposed female, exposed male, and exposed couple) and allowed each couple to oviposit in 20 g of untreated beans. At 3-day intervals, the couples were moved to new 20-g bean masses, and this process was repeated for a total period of 9 days. The number of oviposited eggs was assessed under microscope after 3, 6 and 9 days. Twenty couples were used for each treatment combination.
Statistical analyses
Dose-mortality data were subjected to probit analysis [35], and 95% confidence intervals for toxicity ratios were estimated following [36]; the values were considered significant if the range did not include the value 1. Regression analyses were performed to detect trends in cumulative and average emergence that resulted in each treatment over time. Regression analysis was performed using the curve-fitting procedure of Sigma Plot 12.0. The regression model was chosen based on parsimony, lower standard errors, and steep increases in R2 with increases in model complexity. The regression models for each treatment were considered different from each other if the confidence limits of their parameters did not overlap. We also conducted linear regression to assess the effects of increasing lethal exposure to essential oils on the ri and grain-mass losses of C. maculatus. The data on the number of eggs used in each treatment combination were submitted to repeated measures ANOVA. The assumptions of normality and homogeneity of variance were tested for all parameters, and no data transformations were necessary (PROC UNIVARIATE, SAS Institute Inc., Cary, NC, USA).
Results
Chemical composition of the essential oils
The chemical analyses showed that the two main components of cinnamon and clove essential oils were eugenol and β-caryophylene (Table 1). However, the cinnamon essential oil additionally contained a wide range of other compounds in smaller amounts, including acetyleugenol, benzyl benzoate, linalool, cinnamyl acetate and cinnamaldehyde.
Table 1. Chemical composition of clove and cinnamon essentials oils.
| Constituents | Arithmetic index | Concentration | |||||
|---|---|---|---|---|---|---|---|
| S. aromaticum | C. zeylanicum | S. aromaticum | C. zeylanicum | ||||
| a | b | a | b | (%) | |||
| eugenol | 1363 | 1356 | 1364 | 1356 | 87.4 | 73.1 | |
| β-caryophylene | 1415 | 1417 | 1414 | 1417 | 11.5 | 7.7 | |
| α-humulene | 1447 | 1452 | 1447 | 1452 | 1.1 | 0.4 | |
| α-pinene | - | - | 931 | 932 | - | 0.7 | |
| α-phellandrene | - | - | 1004 | 1002 | - | 0.3 | |
| p-cymene | - | - | 1022 | 1020 | - | 1.0 | |
| limonene | - | - | 1026 | 1024 | - | 0.5 | |
| eucalyptol | - | - | 1028 | 1026 | - | 0.7 | |
| linalool | - | - | 1100 | 1095 | - | 2.6 | |
| E-cinnamaldehyde | - | - | 1268 | 1267 | - | 2.3 | |
| methyleugenol | - | - | 1405 | 1403 | - | 0.6 | |
| cinnamyl acetate | - | - | 1444 | 1443 | - | 2.5 | |
| acetyleugenol | - | - | 1528 | 1521 | - | 3.6 | |
| caryophyllene oxide | - | - | 1576 | 1582 | - | 0.5 | |
| benzyl benzoate | - | - | 1760 | 1759 | - | 3.4 | |
a calculated
b tabulated.
Insecticidal activity
The mortality levels obtained in the dose-mortality bioassays were satisfactorily described by the probit model [goodness-of-fit tests exhibiting low χ2-values (<10) and high P-values (>0.05)]. The toxicity ratios (TR) were estimated relative to the LD50 for deltamethrin. The toxicities of the clove and cinnamon essential oils were similar to the pyrethroid-based insecticide deltamethrin (Table 2).
Table 2. Toxicity of clove and cinnamon essential oils and deltamethrin on adults of Callosobruchus maculatus.
| Insecticide | Slope ± SD | LD20 (95% FL) | LD40(95% FL) | LD50 (95% FL) | LD60 (95% FL) | χ2 | P | TR50 (95% CL) |
|---|---|---|---|---|---|---|---|---|
| Clove | 4.0 ± 0.32 | 48.6 (42.0–54.0) | 68.0 (62.0–74.0) | 78.2 (71.6–84.8) | 90.0 (84.0–98.0) | 5.25 | 0.15 | 0.94 (0.9–1.0) |
| Cinnamon | 9.3 ± 1.02 | 106.4 (96.0–114.0) | 124.0 (116.0–130.0) | 131.0 (124.0–137.0) | 138.0 (132.0–146.0) | 3.90 | 0.14 | 1.56 (1.5–1.6) |
| Deltamethrin | 13.9 ± 1.25 | 72.8 (68.8–76.0) | 80.0 (76.8–83.2) | 83.7 (80.6–86.6) | 87.2 (84.0–90.3) | 9.91 | 0.08 | 1.00 (0.9–1.0) |
SD standard deviation; LD: Lethal dose (μL kg-1); FL = Fiducial limits; χ2 = Chi-square for lack-of-fit to the probit model, and P = Probability associated with the chi-square statistic; TR50 = Toxicity ratio determined by LD50 of each the essential oil /LD50 of deltamethrin; CL = Confidence limits of TR50.
Effects of essential oils on the biological development and bean-mass losses
Effect on instantaneous rate of population increase (ri) and bean weight loss
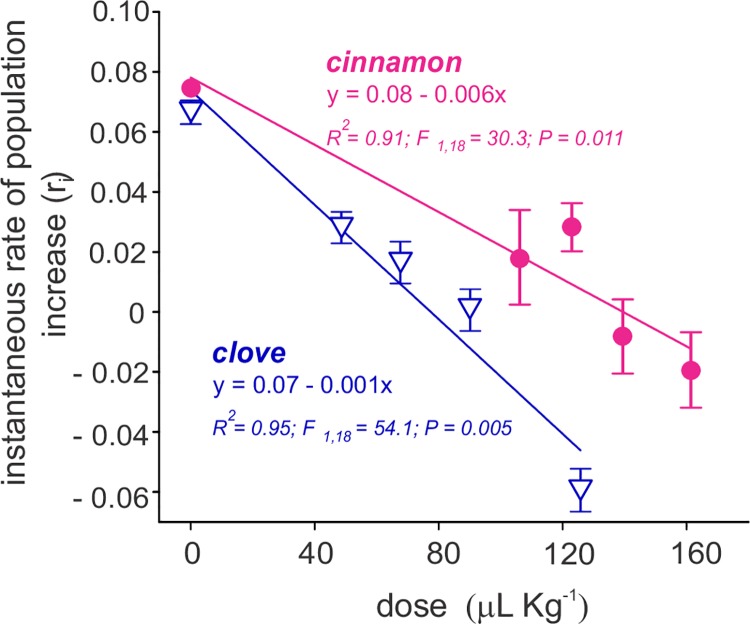
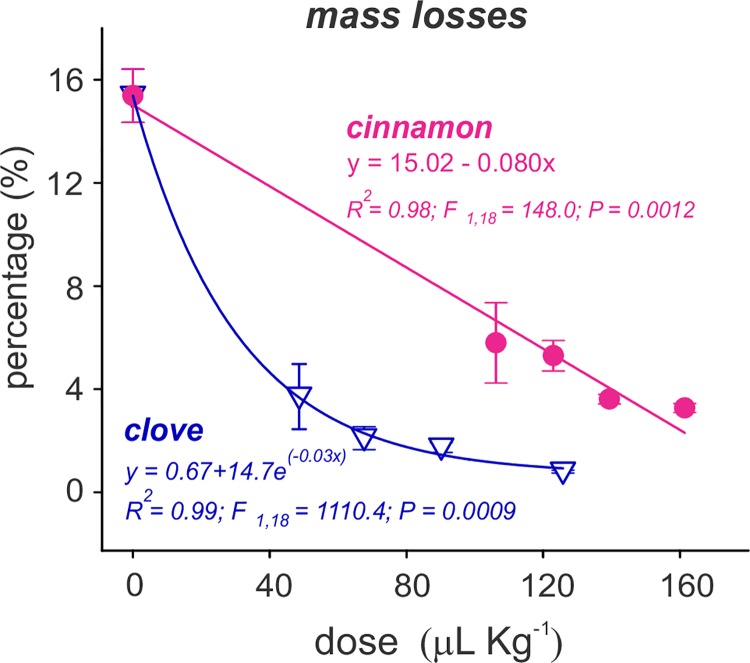
The instantaneous rate of population increase (ri) decreased in a dose-dependent manner for the two essential oils used (Fig 1). The extinction stage (negative ri) was reached when the C. maculatus insects were in contact with concentrations equal to or higher than LD60 for clove (i.e., 67.6 μL kg-1 of bean) and cinnamon (i.e., 139.4 μL kg-1 of bean) essential oils. Similar trends were observed for the bean mass losses, where the LD20 concentrations of clove (F = 21.3; P < 0.001) and cinnamon (F = 69.8; P < 0.001) essential oils significantly reduced grain loss when applied as a contact treatment (Fig 2). These losses were reduced from approximately 15%, when the beans were incubated with C. maculatus insects in the absence of essential oils, to less than 6%, when the bean was treated with clove and cinnamon essential oils.
Fig 1. Instantaneous rate of population increase of C. maculatus exposed to clove and cinnamon essential oils.
The symbols represent the means of five replicates of the LD0 (control), LD20, LD40, LD60 and LD80 for each oil. The doses are expressed in μL of essential oil/kg beans. The vertical bars represent the SD.
Fig 2. Bean weight losses caused by C. maculatus exposed to clove and cinnamon essential oils.
The symbols represent the means of five replicates of the LD0 (control), LD20, LD40, LD60 and LD80 for each oil. The doses are expressed in μL of essential oil/kg beans. The vertical bars represent the SD.
Positive correlations were observed between the instantaneous rate of increase of C. maculatus and the mass losses in grain masses treated with clove (R2 = 0.72; P < 0.001) and cinnamon (R2 = 0.83; P < 0.001) essential oils, between the instantaneous rate of increase and the total number of emerged adult of C. maculatus for clove (R2 = 0.75; P < 0.001) and cinnamon (R2 = 0.85; P < 0.001) essential oils, and between the total number of emerged adult of C. maculatus and the grain mass losses for clove (R2 = 0.98; P = 0.03) and cinnamon (R2 = 0.99; P < 0.001) essential oils. All of the fitting parameters for the curves in Figs 1 and 2 are presented in supplementary S1 Table.
Effects on average and cumulative emergence
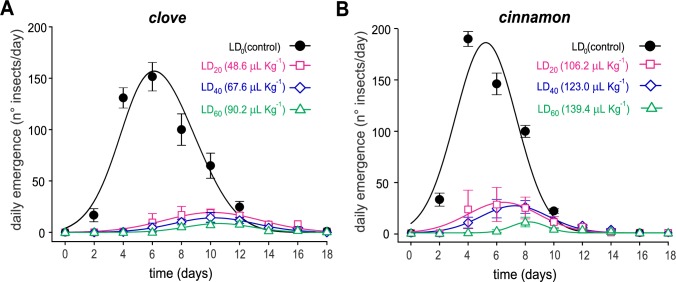
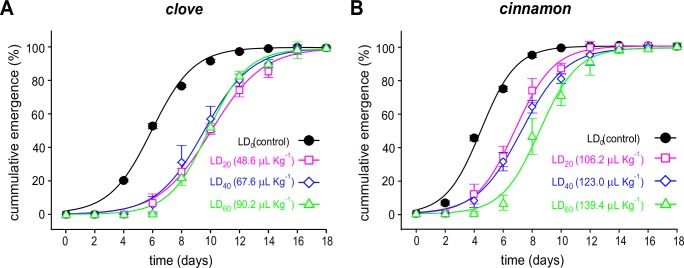
The average emergence of new C. maculatus insects was negatively and severely impacted after treatment by either clove or cinnamon essential oil, as observed in the differences between the emergence curves (Fig 3). Treatments with concentrations starting at the LD20 for both essential oils resulted in a near abolition of emergence. Moreover, the total emergence of C. maculatus was significantly delayed by treatments of almost all concentrations of the two essential oils when compared to the control, as shown by the lack of overlap between the cumulative emergence curves (Fig 4). All of the fitting parameters for the curves in Figs 3 and 4 are presented in supplementary S2 and S3 Tables.
Fig 3.
Average emergence of C. maculatus exposed to clove (A) and cinnamon (B) essential oils. The symbols represent the means of four replicates of the LD0 (control), LD20, LD40, and LD60 for each oil. The doses are expressed in μL of essential oil/kg beans. The vertical bars represent the SD.
Fig 4.
Normalized cumulative emergence of C. maculatus exposed to clove (A) and cinnamon (B) essential oils. The symbols represent the means of four replicates of the LD0 (control), LD20, LD40 and LD60 for each oil. The doses are expressed in μL of essential oil/kg beans. The vertical bars represent the SD.
Effects of sublethal concentrations of essential oils on C. maculatus oviposition
The results of repeated measures ANOVA showed that there was a significant interaction (Wilks’ lambda = 11.77; df = 6; P < 0.001) between oil types, couple combinations and evaluation times (Fig 5). The sublethal exposure to clove and cinnamon essential oils mediated the effects on C. maculatus oviposition, resulting in significant (F = 25.1; df = 3; P < 0.001) differences in the total number of eggs among the four combinations of treatments. When only females were exposed (i.e., exposed females or exposed couples) or when both females and males were exposed (i.e., exposed couples), the total number of laid eggs decreased dramatically in comparison with both the untreated couples and the couples where only males were treated. The two essential oils showed significantly (F = 4.19; df = 1; P = 0.04) different inhibiting effects on oviposition, and this difference was more evident in the oviposition of couples where only females were treated, as the decrease was more important for cinnamon compared to clove and untreated couples. Moreover, the differences between the effects of sublethal exposure to both essential oils were significantly (F = 136.2; df = 2; P < 0.001) higher during the first 3 days of the oviposition period.
Fig 5. Effects of sublethal exposure to essential oils on C. maculatus oviposition.
Number of eggs of C. maculatus females that were sublethally exposed to clove (A) or cinnamon (B) essential oils and coupled with essential oil-treated or essential oil-untreated partners. The symbols represent the means of 20 replicates (± SD) for the number of eggs laid by females of C. maculatus at three-day intervals (left panels) and the cumulative number of eggs (right panels) laid on cowpea bean masses during the first 9 days of adulthood. On the right panels, the treatments grouped by the same horizontal line did not differ according to a Tukey HSD test (P < 0.05).
Discussion
Plant essential oils are among the most interesting options for cheaper, safer and eco-friendly replacements (or to be used as adjuvants) for synthetic insecticides [2–4, 6]. Here, we demonstrated that applications of clove and cinnamon essential oils not only adequately controlled C. maculatus on stored cowpea beans but also were capable of reducing the oviposition and population growth of C. maculatus even at sublethal dosages.
Essential oils, such as clove and cinnamon oils, are very complex natural mixtures and can contain various compounds at different concentrations with two or three major components that will determine the biological properties of the essential oil [37]. However, synergistic effects between the components of essential oils have been frequently reported in previous studies [38–43]. Our chemical analyses of cinnamon and clove essential oils revealed that their primarily components were eugenol (>70.0%), followed by the sesquiterpene β-caryophyllene (between 7.0 and 12%). These results are in concordance with previous studies that reported similar compositions [33, 37, 44–48]. It is worth noting that cinnamon essential oil, despite its major components, also contained a range of other compounds, including acetyleugenol, benzyl benzoate, linalool, cinnamyl acetate and cinnamaldehyde (between 2 and 4%), lending more evidence to the hypothesis that essential oil biological activities may be shaped by the potential synergistic and antagonistic interactions among all these molecules and not only by major essential oil compounds [37, 49].
Several studies have reported the insecticidal toxicity of clove and cinnamon essential oils and their primary compounds that successfully control stored product pests [9, 18, 26, 33, 50–52] and other insects [48, 53–56]. The vast majority of these investigations have attributed these essential oil insecticidal activities to their major constituents (i.e., eugenol and β-caryophyllene), as these compounds are known to act on the insects’ nervous system by disturbing the functions of GABAergic [57, 58] and aminergic [59–61] systems and by inhibiting the actions of acetylcholinesterase [62–64].
Negative effects on developmental traits, such as rates of growth and progeny emergence of bruchid insects such as C. maculatus, have been reported for various essential oils and their components [22, 23, 26, 52, 65–69]. The present investigation demonstrated that treating cowpea bean masses with sublethal dosages (i.e., as lower as their LD20) of these essential oils leds to significant decreases in the C. maculatus instantaneous rates of population growth and the bean mass losses, and the application of the essential oils almost abolished C. maculatus offspring emergence. Such biological impairments caused by clove and cinnamon essential oils on bruchids can be the result of the direct mortality of adults, repellency, oviposition deterrence or progeny and growth inhibition [51]. However, our oviposition results (i.e., females sublethally exposed to these essential oils decreased their ability to lay eggs even when they were offered to mate with untreated sexual partners in untreated bean masses) revealed an even more complex scenario and potential effects on sexual fitness (e.g., locomotory activities, mating behavior) or on the physiological basis of oviposition (e.g., respiratory activities, oogenesis or hormonal disruption) may also contribute to the reduced performance of essential oil sublethally exposed insects. For instance, similar physiological impairments (e.g., repellence, emergence inhibition, altered respiratory activities and transgenerational behavior changes) have been reported in stored product pests (e.g., S. zeamais) exposed to essential oils of cinnamon and clove [9, 13, 17, 18].
Furthermore, as biosynthesis and release of mating signals as well as the production of eggs may be influenced by atmospheric volatiles and gases [70, 71],. plant extracts, such as terpenes, can influence the site-choice of egg-laying female insects [70]. Although future experiments are required to isolate the effects of the exposure to essential oils on the physiological basis of oviposition and on the mating behavior, a potential energy trade-off between the detoxification process and oogenesis might be an explanation for the inhibited oviposition observed here [72–74].
In our study we have used drops of pure essential oils on bean masses and although such technique showed good biological activities on C.maculatus under laboratory conditions and may have potential applications at small farmer’s level, this delivery system may suffer from draw backs inherent to the volatile nature of essential oils in larger storage facilities. In fact, rapid biodegradation of these compounds due to their poor physicochemical stability, high volatility, and thermal decomposition will require some controlled-release system such as nanotechnological formulations to optimize the action of their active ingredients [75, 76].
Thus, our findings revealed adequate insecticidal activities of clove and cinnamon essential oils against C. maculatus and demonstrated that, even at sublethal doses, these botanical compounds impaired the ability of C. maculatus to damage cowpea bean masses, which make them suitable tools that can be integrated into management programs of C. maculatus, especially for storage facilities. Further work is also needed to test the applicability and efficacy of nanofomulations of these essential oils under broader stored products conditions.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Grants from the CAPES Foundation to KH, from the National Council of Scientific and Technological Development (CNPq) to LRDF and EEO and from the Secretaria Nacional de Educación Superior Ciencia y Tecnologia of Ecuador (SENESCYT-Ecuador) to LOVJ supported this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Isman MB, Grieneisen ML. Botanical insecticide research: many publications, limited useful data. Trends in Plant Science. 2014;19(3):140–5. 10.1016/j.tplants.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: Low-risk products in a high-stakes world. Annual Review of Entomology. 2012;57(1):405–24. 10.1146/annurev-ento-120710-100554 . [DOI] [PubMed] [Google Scholar]
- 3.Stevenson PC, Isman MB, Belmain SR. Pesticidal plants in Africa: a global vision of new biological control products from local uses. Industrial Crops and Products. 2017. [Google Scholar]
- 4.Isman MB. Pesticides Based on Plant Essential Oils: Phytochemical and Practical Considerations Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization: ACS Publications; 2016. p. 13–26. [Google Scholar]
- 5.Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresource Technology. 2010;101(1):372–8. 10.1016/j.biortech.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 6.Pavela R. Essential oils for the development of eco-friendly mosquito larvicides: a review. Industrial Crops and Products. 2015;76:174–87. [Google Scholar]
- 7.Desneux N, Decourtye A, Delpuech J-M. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007;52:81–106. 10.1146/annurev.ento.52.110405.091440 [DOI] [PubMed] [Google Scholar]
- 8.Haddi K, Mendes MV, Barcellos MS, Lino-Neto J, Freitas HL, Guedes RNC, et al. Sexual success after stress? Imidacloprid-induced hormesis in males of the neotropical stink bug Euschistus heros. PloS one. 2016;11(6):e0156616 10.1371/journal.pone.0156616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales Correa YDCG, Faroni LR, Haddi K, Oliveira EE, Pereira EJG. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pesticide Biochemistry and Physiology. 2015;125:31–7. 10.1016/j.pestbp.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Guedes R, Smagghe G, Stark J, Desneux N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annual review of entomology. 2016;61:43–62. 10.1146/annurev-ento-010715-023646 [DOI] [PubMed] [Google Scholar]
- 11.Cutler GC, Guedes RN. Occurrence and significance of insecticide-induced hormesis in insects. Pesticide dose: effects on the environment and target and non-target organisms ACS, Washington. 2017:101–19. [Google Scholar]
- 12.Teodoro AV, Silva MDJDS, Filho JGDS, Oliveira EED, Galvão AS Silva SS. Bioactivity of cottonseed oil against the coconut mite Aceria guerreronis (Acari: Eriophyidae) and side effects on Typhlodromus ornatus (Acari: Phytoseiidae). Systematic and Applied Acarology. 2017;22(7):1037–47. [Google Scholar]
- 13.Freitas RCP, Faroni LRDA, Haddi K, Jumbo LOV, Oliveira EE. Allyl isothiocyanate actions on populations of Sitophilus zeamais resistant to phosphine: Toxicity, emergence inhibition and repellency. Journal of Stored Products Research. 2016;69:257–64. [Google Scholar]
- 14.Haddi K, Faroni LR, Oliveira EE. Cinnamon Oil In: Nollet LM, and Hamir Singh Rathore, editor. Green Pesticides Handbook: Essential Oils for Pest Control: CRC Press; 2017. p. 117–50. [Google Scholar]
- 15.Oliveira NN, Galvão AS, Amaral EA, Santos AW, Sena-Filho JG, Oliveira EE, et al. Toxicity of vegetable oils to the coconut mite Aceria guerreronis and selectivity against the predator Neoseiulus baraki. Experimental and Applied Acarology. 2017:1–12. [DOI] [PubMed] [Google Scholar]
- 16.de Araújo AMN, Faroni LRDA, de Oliveira JV, Navarro DMdAF, Breda MO, de França SM. Lethal and sublethal responses of Sitophilus zeamais populations to essential oils. Journal of Pest Science. 2017;90(2):589–600. [Google Scholar]
- 17.Haddi K, Oliveira EE, Faroni LR, Guedes DC, Miranda NN. Sublethal exposure to clove and cinnamon essential oils induces hormetic-like responses and disturbs behavioral and respiratory responses in Sitophilus zeamais (Coleoptera: Curculionidae). Journal of Economic Entomology. 2015;108(6):2815–22. 10.1093/jee/tov255 [DOI] [PubMed] [Google Scholar]
- 18.Silva S, Haddi K, Viteri Jumbo L, Oliveira E. Progeny of the maize weevil, Sitophilus zeamais, is affected by parental exposure to clove and cinnamon essential oils. Entomologia Experimentalis et Applicata. 2017;163(2):220–8. [Google Scholar]
- 19.Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules. 2017;23(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JK, Pittendrigh BR, Onstad DW. Insect resistance management for stored product pests: a case study of cowpea weevil (Coleoptera: Bruchidae). Journal of Economic Entomology. 2013;106(6):2473–90. [DOI] [PubMed] [Google Scholar]
- 21.Brisibe EA, Adugbo SE, Ekanem U, Brisibe F, Figueira GM. Controlling bruchid pests of stored cowpea seeds with dried leaves of Artemisia annua and two other common botanicals. African Journal of Biotechnology. 2011;10(47):9593–9. [Google Scholar]
- 22.Massango H, Faroni L, Haddi K, Heleno F, Jumbo LV, Oliveira E. Toxicity and metabolic mechanisms underlying the insecticidal activity of parsley essential oil on bean weevil, Callosobruchus maculatus. Journal of Pest Science. 2017;90(2):723–33. [Google Scholar]
- 23.Ileke KD, Bulus DS, Aladegoroye AY. Effects of three medicinal plant products on survival, oviposition and progeny development of cowpea bruchid, Callosobruchus maculatus (Fab.)[Coleoptera: Chrysomelidae] infesting cowpea seeds in storage. Jordan Journal of Biological Science. 2013;6(1):61–6. [Google Scholar]
- 24.Lima MPd, Oliveira JV, Barros R, Torres JB. Identification of cowpea Vigna unguiculata (L.) Walp. genotypes resistant to Callosobruchus maculatus (Fabr.)(Coleoptera: Bruchidae). Neotropical Entomology. 2001;30(2):289–95. [Google Scholar]
- 25.Gbaye OA, Millard JC, Holloway GJ. Legume type and temperature effects on the toxicity of insecticide to the genus Callosobruchus (Coleoptera: Bruchidae). Journal of Stored Products Research. 2011;47(1):8–12. [Google Scholar]
- 26.Haddi K, Jumbo LV, Costa M, Santos M, Faroni L, Serrão J, et al. Changes in the insecticide susceptibility and physiological trade-offs associated with a host change in the bean weevil Acanthoscelides obtectus. Journal of Pest Science. 2017:1–10. [Google Scholar]
- 27.Gbaye O, Millard J, Holloway G. Synergistic effects of geographical strain, temperature and larval food on insecticide tolerance in Callosobruchus maculatus (F.). Journal of Applied Entomology. 2012;136(4):282–91. [Google Scholar]
- 28.Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006;51:45–66. 10.1146/annurev.ento.51.110104.151146 [DOI] [PubMed] [Google Scholar]
- 29.Kah M, Kookana RS, Gogos A, Bucheli TD. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature nanotechnology. 2018:1. [DOI] [PubMed] [Google Scholar]
- 30.Campolo O, Cherif A, Ricupero M, Siscaro G, Grissa-Lebdi K, Russo A, et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Scientific Reports. 2017;7(1):13036 10.1038/s41598-017-13413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jham GN, Dhingra OD, Jardim CM, Valente VM. Identification of the major fungitoxic component of cinnamon bark oil. Fitopatologia Brasileira. 2005;30(4):404–8. [Google Scholar]
- 32.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Journal of the American Society for Mass Spectrometry. 1997;6(8):671–2. [Google Scholar]
- 33.Viteri Jumbo LO, Faroni LRA, Oliveira EE, Pimentel MA, Silva GN. Potential use of clove and cinnamon essential oils to control the bean weevil, Acanthoscelides obtectus Say, in small storage units. Industrial Crops and Products. 2014;56(0):27–34. 10.1016/j.indcrop.2014.02.038. [DOI] [Google Scholar]
- 34.Walthall WK, Stark JD. Comparison of two population‐level ecotoxicological endpoints: The intrinsic (rm) and instantaneous (ri) rates of increase. Environmental Toxicology and Chemistry. 1997;16(5):1068–73. [Google Scholar]
- 35.SAS Institute. SAS/STAT User’s Guide. SAS, editor. Cary, NC, USA: 2008. [Google Scholar]
- 36.Robertson JL, Preisler HK. Pesticide Bioassays with Arthopods. PCRC, editor. Boca Raton, FL, USA: 1992. [Google Scholar]
- 37.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food and Chemical Toxicology. 2008;46(2):446–75. 10.1016/j.fct.2007.09.106 [DOI] [PubMed] [Google Scholar]
- 38.Fornari T, Vicente G, Vázquez E, García-Risco MR, Reglero G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. Journal of Chromatography A. 2012;1250:34–48. 10.1016/j.chroma.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 39.Joffe T, Gunning RV, Allen GR, Kristensen M, Alptekin S, Field LM, et al. Investigating the potential of selected natural compounds to increase the potency of pyrethrum against houseflies Musca domestica (Diptera: Muscidae). Pest Management Science. 2012;68(2):178–84. 10.1002/ps.2241 [DOI] [PubMed] [Google Scholar]
- 40.Koul O, Singh R, Kaur B, Kanda D. Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Industrial Crops and Products. 2013;49:428–36. [Google Scholar]
- 41.Miresmailli S, Bradbury R, Isman MB. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Management Science. 2006;62(4):366–71. 10.1002/ps.1157 [DOI] [PubMed] [Google Scholar]
- 42.Omolo M, Okinyo D, Ndiege I, Lwande W, Hassanali A. Fumigant toxicity of the essential oils of some African plants against Anopheles gambiae sensu stricto. Phytomedicine. 2005;12(3):241–6. 10.1016/j.phymed.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 43.Kanda D, Kaur S, Koul O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: acute toxins or feeding deterrents. Journal of Pest Science. 2017;90(2):531–45. [Google Scholar]
- 44.Chaieb K, Hajlaoui H, Zmantar T, Kahla‐Nakbi AB, Rouabhia M, Mahdouani K, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytotherapy Research. 2007;21(6):501–6. 10.1002/ptr.2124 [DOI] [PubMed] [Google Scholar]
- 45.Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorganic & Medicinal Chemistry. 2009;17(12):4022–34. [DOI] [PubMed] [Google Scholar]
- 46.Fichi G, Flamini G, Giovanelli F, Otranto D, Perrucci S. Efficacy of an essential oil of Eugenia caryophyllata against Psoroptes cuniculi. Experimental Parasitology. 2007;115(2):168–72. 10.1016/j.exppara.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 47.Fichi G, Flamini G, Zaralli L, Perrucci S. Efficacy of an essentifal oil of Cinnamomum zeylanicum against Psoroptes cuniculi. Phytomedicine. 2007;14(2):227–31. [DOI] [PubMed] [Google Scholar]
- 48.Park I-K, Lee H-S, Lee S-G, Park J-D, Ahn Y-J. Insecticidal and fumigant activities of Cinnamomum cassia bark-derived materials against Mechoris ursulus (Coleoptera: Attelabidae). Journal of Agricultural and Food Chemistry. 2000;48(6):2528–31. [DOI] [PubMed] [Google Scholar]
- 49.Koul O, Walia S, Dhaliwal G. Essential oils as green pesticides: potential and constraints. Biopesticide International. 2008;4(1):63–84. [Google Scholar]
- 50.Brari J, Thakur D. Insecticidal efficacy of essential oil from Cinnamomum zeylanicum Blume and its two major constituents against Callosobruchus maculatus (F.) and Sitophilus oryzae (L.). Journal of Agricultural Technology. 2015;11(6):1323–36. [Google Scholar]
- 51.Athanassiou CG, Rani PU, Kavallieratos NG. The use of plant extracts for stored product protection Advances in Plant Biopesticides: Springer; 2014. p. 131–47. [Google Scholar]
- 52.Pérez S, Ramos-López M, Zavala-Sánchez M, Cárdenas-Ortega N. Activity of essential oils as a biorational alternative to control coleopteran insects in stored grains. Journal of Medicinal Plants Research. 2010;4(25):2827–35. [Google Scholar]
- 53.Chang S-T, Cheng S-S. Antitermitic activity of leaf essential oils and components from Cinnamomum osmophleum. Journal of Agricultural and Food Chemistry. 2002;50(6):1389–92. [DOI] [PubMed] [Google Scholar]
- 54.Cheng S-S, Liu J-Y, Huang C-G, Hsui Y-R, Chen W-J, Chang S-T. Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Bioresource Technology. 2009;100(1):457–64. 10.1016/j.biortech.2008.02.030 [DOI] [PubMed] [Google Scholar]
- 55.Khater HF, Ramadan MY, El-Madawy RS. Lousicidal, ovicidal and repellent efficacy of some essential oils against lice and flies infesting water buffaloes in Egypt. Veterinary Parasitology. 2009;164(2):257–66. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Kim H-K, Tao W, Wang M, Ahn Y-J. Contact and Fumigant Toxicity of Cinnamaldehyde and Cinnamic Acid and Related Compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). Journal of Medical Entomology. 2011;48(2):366–71. [DOI] [PubMed] [Google Scholar]
- 57.Tong F, Coats JR. Quantitative structure–activity relationships of monoterpenoid binding activities to the housefly GABA receptor. Pest Management Science. 2012;68(8):1122–9. 10.1002/ps.3280 [DOI] [PubMed] [Google Scholar]
- 58.Bloomquist JR, Boina DR, Chow E, Carlier PR, Reina M, Gonzalez-Coloma A. Mode of action of the plant-derived silphinenes on insect and mammalian GABA A receptor/chloride channel complex. Pesticide Biochemistry and Physiology. 2008;91(1):17–23. [Google Scholar]
- 59.Enan EE. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Archives of Insect Biochemistry and Physiology. 2005;59(3):161–71. 10.1002/arch.20076 [DOI] [PubMed] [Google Scholar]
- 60.Enan EE. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochemistry and Molecular Biology. 2005;35(4):309–21. 10.1016/j.ibmb.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 61.Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Shaaya E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Management Science. 2002;58(11):1101–6. 10.1002/ps.548 [DOI] [PubMed] [Google Scholar]
- 62.Keane S, Ryan M. Purification, characterisation, and inhibition by monoterpenes of acetylcholinesterase from the waxmoth, Galleria mellonella (L.). Insect Biochemistry and Molecular Biology. 1999;29(12):1097–104. [Google Scholar]
- 63.Lopes KV, Silva LB, Reis AP, Oliveira MG, Guedes RN. Modified alpha-amylase activity among insecticide-resistant and -susceptible strains of the maize weevil, Sitophilus zeamais. Journal of insect physiology. 2010;56(9):1050–7. 10.1016/j.jinsphys.2010.02.020 . [DOI] [PubMed] [Google Scholar]
- 64.Abdelgaleil SA, Mohamed MI, Shawir MS, Abou-Taleb HK. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. Journal of Pest Science. 2016;89(1):219–29. [Google Scholar]
- 65.Paranagama P, Gunasekera J. The efficacy of the essential oils of Sri Lankan Cinnamomum zeylanicum fruit and Micromelum minutum leaf against Callosobruchus maculatus (F.)(Coleoptera: Bruchidae). Journal of Essential Oil Research. 2011;23(1):75–82. [Google Scholar]
- 66.Jumbo LOV, Faroni LR, Oliveira EE, Pimentel MA, Silva GN. Potential use of clove and cinnamon essential oils to control the bean weevil, Acanthoscelides obtectus Say, in small storage units. Industrial Crops and Products. 2014;56:27–34. [Google Scholar]
- 67.Elhag EA. Deterrent effects of some botanical products on oviposition of the cowpea bruchid Callosobruchus maculatus (F.)(Coleoptera: Bruchidae). International Journal of Pest Management. 2000;46(2):109–13. [Google Scholar]
- 68.Singh R. Evaluation of some plant products for their oviposition deterrent properties against the Callosobruchus maculatus (F.) on chick pea seeds. Journal of Agricultural Science and Technology. 2011;7(5):1363–7. [Google Scholar]
- 69.Ekeh FN, Oleru KI, Ivoke N, Nwani CD, Eyo JE. Effects of Citrus sinensis peel oil on the oviposition and development of cowpea beetle Callosobruchus maculatus (Coleoptera: Chrysomelidae) in some legume grains. Pakistan Journal of Zoology. 2013;45(4):967–74. [Google Scholar]
- 70.Hilker M, Meiners T. Plants and insect eggs: how do they affect each other? Phytochemistry. 2011;72(13):1612–23. 10.1016/j.phytochem.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 71.Städler E, Hilker M, Meiners T. Plant chemical cues important for egg deposition by herbivorous insects. Chemoecology of Insect Eggs and Egg Deposition. 2002:171–204. [Google Scholar]
- 72.Hashem MY, Ismail II, Lutfallah AF, El-Rahman SFA. Effects of carbon dioxide on Sitotroga cerealella (Olivier) larvae and their enzyme activity. Journal of Stored Products Research. 2014;59:17–23. [Google Scholar]
- 73.Kaur AP, Sohal SK, Kaur M, Singh J. Monitoring growth, survival and enzyme system of melon fruit fly Bactrocera cucurbitae (Coquillett) under the influence of affinity purified pea lectin. Entomological Science. 2013;16(1):91–9. [Google Scholar]
- 74.Oliveira DM, Gomes FM, Carvalho DB, Ramos I, Carneiro AB, Silva-Neto MA, et al. Yolk hydrolases in the eggs of Anticarsia gemmatalis hubner (Lepidoptera: Noctuidae): a role for inorganic polyphosphate towards yolk mobilization. Journal of insect physiology. 2013;59(12):1242–9. 10.1016/j.jinsphys.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 75.Pavela R, Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends in plant science. 2016;21(12):1000–7. 10.1016/j.tplants.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 76.de Oliveira JL, Campos EVR, Bakshi M, Abhilash P, Fraceto LF. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotechnology advances. 2014;32(8):1550–61. 10.1016/j.biotechadv.2014.10.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.