Abstract
Haloacetamides (HAMs) are cytotoxic, genotoxic, and mutagenic byproducts of drinking water disinfection. They are soft electrophilic compounds that form covalent bonds with the free thiol/thiolate in cysteine residues through an SN2 reaction mechanism. Toxicity of the monohalogenated HAMs (iodoacetamide, IAM; bromoacetamide, BAM; or chloroacetamide, CAM) varied depending on the halogen substituent. The aim of this research was to investigate how the halogen atom affects the reactivity and toxicological properties of HAMs, measured as induction of oxidative/electrophilic stress response and genotoxicity. Additionally, we wanted to determine how well in silico estimates of electrophilic softness matched thiol/thiolate reactivity and in vitro toxicological endpoints. Each of the HAMs significantly induced nuclear Rad 51 accumulation and ARE signaling activity compared to a negative control. In each case the rank order of effect was IAM > BAM > CAM for Rad 51, and BAM ≈ IAM > CAM for ARE. In general, electrophilic softness and in chemico thiol/thiolate reactivity provided a qualitative indicator of toxicity, as the softer electrophiles IAM and BAM were more thiol/thiolate reactive and were more toxic.
Keywords: Drinking water disinfection byproducts, haloacetamide, toxicity, soft electrophile, oxidative stress, DNA damage
1. Introduction
Disinfection of municipal drinking water is an essential safeguard of public health (Calderon, 2000). However, the addition of chlorine based disinfectants to source waters containing organic and inorganic precursor molecules generate a mixture of toxic halogenated byproducts (Krasner et al., 1989). Many of these disinfection byproducts (DBPs) are cytotoxic, genotoxic, and mutagenic in vitro, and some are carcinogenic in rodents (Richardson et al., 2007). Moreover, epidemiologic studies suggested long-term exposures to DBPs increased risk of bladder cancer (Michaud et al., 2007; Villanueva et al., 2007). Still, the mechanisms of toxicity leading to the observed adverse effects are not fully understood, making it difficult to establish effective interventions. Additionally, since DBPs are formed at concentrations below their individual adverse effect levels, it is unlikely that any individual DBP can account for the aforementioned observed adverse effects highlighting the need for understanding the toxic effect of the whole mixture (Bull, 2006). Identification of chemical groups that share a common mechanism within the DBP mixture would provide a better understanding of the mixture toxicity (U.S. EPA, 2002) and could provide valuable insight on how to improve chemical regulation.
Several DBPs can be classified as soft electrophilic compounds (highly polarizable chemicals deficient in electrons) that preferentially react with biological soft nucleophiles (e.g. thiol/ thiolate groups) forming covalent bonds (Pearson, 1963). For example, many DBPs share a common motif (α-brominated carbonyl) that was identified as a structural predictor of thiol (glutathione (GSH)) reactivity (Hughes et al., 2015). Additionally, the cytotoxicity and genotoxicity properties of several α-halogenated carbonyl (or nitrile) containing DBP chemical classes (e.g. haloacetonitriles (HANs), haloacetamides (HAMs), and haloacetic acids (HAAs) correlated with predicted SN2 reactivity (Muellner et al., 2007; Plewa et al., 2008; Plewa et al., 2010). Genotoxicity induced by the α-halogenated carbonyl (or nitrile) containing DBPs, bromoacetamide (BAM), and bromoacetonitrile (BAN) were significantly reduced by co-treating with N-acetylcysteine (NAC) (Pals et al., 2016). Therefore, reactivity seems to play a significant role in the toxicity of DBPs directly perturbing the intracellular thiol pool through alkylation of cellular thiol/thiolates leading to a toxic response by disrupting various cellular processes (Schultz et al., 2006). Moreover, glutathione depletion is observed in the progression of several diseases including cancer, cardiovascular, and neurodegenerative diseases (Ballatori et al., 2009).
Furthermore, reactive electrophiles with common cellular targets present an ideal opportunity for additive or synergistic toxicity (U.S. EPA, 2002). In fact, experiments with α,β-unsaturated carbonyl derivatives, showed additive toxicity in binary and ternary mixtures in vitro and in an in vivo model (Zhang et al., 2016). Work by Dawson and colleagues showed that HANs and halogenated ethyl acetates (α-halogenated carbonyl containing compounds) generated, in some cases, dose additive toxicity within and among these chemical classes (Dawson et al., 2010; Dawson et al., 2011; Dawson et al., 2014). Additive genotoxicity was observed in binary mixtures of BAM and BAN (Pals et al., 2016).
Soft electrophile reactivity can be predicted using in silico methods based on Hard Soft Acid Base (HSAB) theory (Karelson et al., 1996). HSAB theory makes use of frontier molecular orbital (FMO) energies to predict reactivity between electrophile-nucleophile pairs (Karleson et al., 1996). Several parameters for reactivity based on FMO energies were developed and utilized in QSAR applications (Schwobel et al., 2011); however, in its basic form, HSAB theory suggests that an electrophile is soft if it has a low energy lowest unoccupied molecular orbital (ELUMO) (Karelson et al., 1996). Estimations of ELUMO, therefore, provide a theoretical method for identifying soft electrophiles among identified DBPs. Our previous work showed that ELUMO was a useful predictor of thiol reactivity and genotoxicity in a set of mono-brominated DBPs (bromoacetic acid, BAM, and BAN) (Pals et al., 2016). However, because reactivity of alkyl halides is dependent on halogen leaving efficiency, the effect of variable halogen substitution requires additional investigation. The set of monoHAMs, iodoacetamide (IAM), BAM, and toxicity, and the ability of in silico softness parameters to predict these effects. In this study we measured the ability of each monoHAM to generate an ARE driven stress response, and to generate chloroacetamide (CAM) isolates the effect the halogen substituent asserts on thiol/thiolate reactivity and toxicity as DNA double strand breaks. We then compared toxic potencies with in chemico reactivity and in silico parameters derived from FMO energies to determine their ability to predict in vitro effects.
2. Experimental
2.1. General Reagents
General laboratory reagents were purchased from Fisher Scientific Co. (Itasca, IL) or Sigma Aldrich Co. (St. Louis, MO). IAM, BAM, CAM, DTNB (5,5′-dithiobis (2-nitrobenzoic acid) and NAC were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. In Silico Estimates of FMO Energies
FMO energies, including energy of the highest occupied molecular orbital (EHOMO) and ELUMO were estimated using density function B3LYP 6–311+G**, from Hartree Fock 6–311+G** equilibrium geometries with Spartan 10 software (Wavefunction Inc., Irvine, CA). Electrophilic hardness (η) softness (σ), chemical potential (µ), and electrophilic index (ω) were calculated using equations 1–4 respectively.
| (1) |
| (2) |
| (3) |
| (4) |
2.3. N-Acetylcysteine Reactivity
NAC served as a model soft nucleophile to determine DBP electrophilic reactivity. After exposure to DBPs (30 min), the remaining free thiol was quantified with Ellman’s reagent (Ellman, 1959) with minor modifications of the previously published protocol (Pals et al., 2016). For these experiments, Ellman’s reagent was prepared from stock solutions of 100 mM DTNB in dimethyl sulfoxide (DMSO), and 200 mM EDTA in deionized water diluted to 1 mM and 0.1 mM, respectively, in 200 mM Tris buffered deionized water (pH 8.0). Fresh 1 M stock solutions of NAC or DBP were prepared in DMSO for each experiment. DBPs and NAC were further diluted into 200 mM Tris pH 8.0. Each DBP, in a range of concentrations from 0–2000 µM, was mixed with 400 µM NAC in a total volume of 50 µL 200 mM Tris pH 8.0 in a 96 well microplate. Reactions occurred at room temperature with orbital shaking at 250 rpm. After the reaction time expired, 50 µL of Ellman’s reagent was added to the wells. After 3 min at 250 rpm the absorbance at 412 nm (A412) was measured for each well with a Spectramax Paradigm plate reader (Molecular Devices, Sunnyvale, CA). Blank reactions (NAC + Tris buffer + Ellman’s reagent) were subtracted from the data. The blank adjusted A412 for the negative control was set to 100% free thiol, and the remaining data normalized to its concurrent negative control. Experiments were repeated three times.
2.4. ARE Signaling
Cellular oxidative/electrophilic stress responses generated by the HAMs were measured using the CellSensor ARE-bla HepG2 cell line (Life Technologies, Madison, WI). This cell line contains a stably integrated β-lactamase gene whose transcription is controlled by cis acting ARE coding regions. Briefly, cells were plated at 2000/well/5 µL in 1536 well black-clear bottom plates (Greiner Bio-One North America, Monroe, NC) and incubated at 37°C, 5% CO2 for 5 h. Twenty three nL of compounds or a positive control, β-napthoflavone, were transferred to each well using a pintool (Kalypsys, San Diego, CA). Cells in assay plates were incubated at 37 °C, 5% CO2 for 16 h. After the incubation period, 1 µL of CCF4 dye (Life Technologies) was added to each well and the plate was incubated at room temperature for 2 h. Fluorescence intensity at 460 and 530 nm emissions was measured after 405 nm excitation by an Envision plate reader (PerkinElmer, Boston, MA) followed by an addition of 4 µL/well of cell viability reagent (CellTiter-Glo, Promega, Madison, WI). The plates were incubated for 30 min at room temperature and luminescence intensity was read using a ViewLux plate reader (Perkin-Elmer). For the ARE-bla assay, data were expressed as the ratio of the 460/530 emission values, normalized to the positive control response (23 µM β-napthoflavone, 100% and DMSO, 0%). For cytotoxicity, data were normalized to 100% for the DMSO control, and to 0% for the cytotoxic control (92 µM tetraoctyl ammonium bromide).
2.5. Rad51 Redistribution Assay
The Rad51 Redistribution Assay (Thermo Scientific, Rockford, IL) evaluates DNA double strand breaks by measuring the accumulation of Rad51 in nuclear foci in recombinant SW480 cells stably expressing human Rad51 fused to the C-terminus of enhanced green fluorescent protein. SW480 cells containing the Rad51 construct (1500 cells/well/5 µl) were plated overnight and then treated with compounds for 24 h. After that, cells were fixed and Hoechst stain was added for 30 min. After 3 washes with phosphate buffered saline (PBS), plates were sealed and kept at 4°C until read. Cells were read using Arrayscan™ system (ThermoFisher Scientific, Waltham, MA) (400 cells minimum) 2 colors (blue and green). Data was analyzed using compartmental analysis.
2.6. Statistics
All statistical analyses were performed using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA). To determine significant reduction of free thiol with respect to negative controls, a one way analysis of variance (ANOVA) was conducted. When a significant (P ≤ 0.05) F value was obtained, a Holm Sidak comparison was used to compare to the negative control values. Before linear regression, data with < 50 % thiol were removed to eliminate plateau effects from diminished reactants. The reactive potency (RC50) values were calculated from the linear regression equations.
ARE activity and Rad51 pit counts were normalized as a percent of a positive control (23 µM β-napthoflavone or 10 µM camptothecin, respectively) and plotted vs. HAM concentration. For each HAM, 3-parameter logistic regression was fit to the data and EC50 values were calculated from the corresponding regression equation.
3. Results and Discussion
This work aimed to evaluate in silico parameters as predictors of thiol/thiolate reactivity, antioxidant/electrophilic stress response, and genotoxicity in a set of three monoHAMs. Compounds containing an α-halogenated carbonyl group are predicted to be thiol reactive (Hughes et al., 2015) to varying degrees based on the leaving efficiency of the halogen substituent (Plewa et al., 2008; Schultz et al., 2007). IAM, BAM, and CAM, which are genotoxic and mutagenic DBPs, contain this α-halogenated carbonyl motif and showed toxicity dependent on SN2 reactivity (Plewa et al., 2008). Selecting these three compounds for analysis allowed for the isolation of the halogen substituent as a single variable in a study of the relationship between reactivity and toxicity.
3.1. In Chemico Reactivity
Non-enzymatic reactivity was measured as the percent reduction of free thiol for the selected HAMs normalized to a negative control. Figure 1 shows the depletion of free thiol in the NAC standard after a 30 min reaction period. Reactivity followed the expected pattern, with the rank order of IAM > BAM >> CAM. IAM and BAM significantly (P < 0.001) reduced percent free thiol at all assayed concentrations (200 – 2000 µM); CAM did not deplete free thiol at any concentration. Linear regression equations (R2 ≥ 0.99) for IAM and BAM were used to calculate RC50. The RC50 values, concentrations that eliminated 50% of the initial free thiol, were 0.559 mM and 0.973 mM for IAM and BAM respectively (Table 1). These data were consistent with the I > Br > Cl pattern of reactivity in a larger group of α-halogenated carbonyl compounds (Dawson et al., 2010; Dawson et al., 2011; Schultz et al., 2007).
Figure 1.
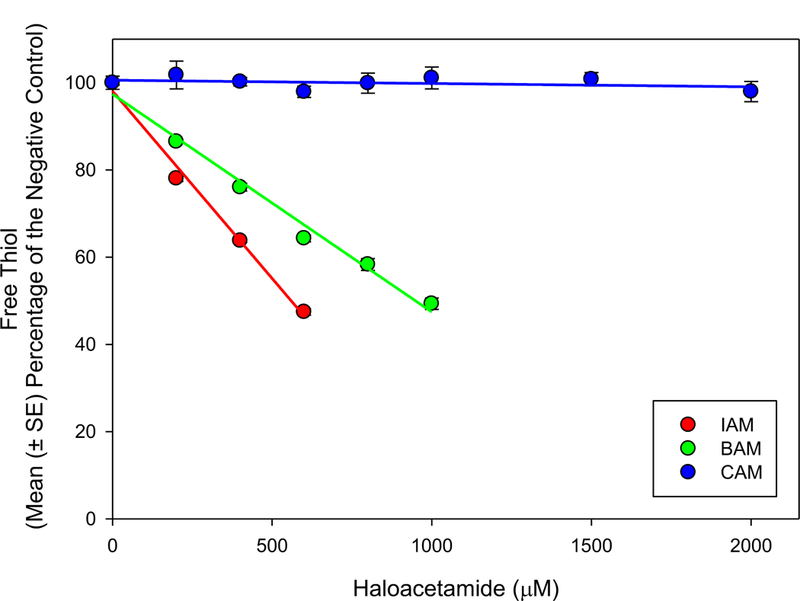
Thiol/thiolate reactivity for each of the monoHAMs after 30 min reaction with NAC at pH 8.0. Data are reported as mean (± SE) percent of the concurrent negative control from 3 independent experiments.
Table 1.
Summary of in silico reactivity parameters, in chemico RC50, and EC50 values for ARE signaling activity and Rad51 foci induction.
| Compound | ELUMO (eV) |
Softness (eV) |
Electrophilic Index (eV) |
RC50 (mM) |
Rad51 EC50 (M) |
ARE EC50 (M) |
|---|---|---|---|---|---|---|
| IAM | −1.89 | 0.370 | 3.90 | 0.559 | 3.80 × 10−6 | 2.05 × 10−6 |
| BAM | −0.82 | 0.298 | 2.60 | 0.973 | 5.69 × 10−6 | 1.92 × 10−6 |
| CAM | −0.22 | 0.271 | 2.07 | N/A | 3.00 × 10−4 | 9.47 × 10−6 |
Schultz and coworkers reported CAM depleted free thiol using GSH as a model nucleophile and a 2 h reaction time; in their study the reactive potency was much greater for BAM (EC50, 0.26 mM) than CAM (EC50, 16.99 mM) (Schultz et al., 2007). The lack of reactivity of CAM in the present study may be due to the shorter reaction time (30 min). Since the treatment time for the ARE and Rad51 assays were longer than 2 h, we expect CAM to be weakly reactive with free thiols/thiolates in our in vitro assays.
3.2. ARE Activation
Cellular responses to antioxidant and electrophilic stresses are largely mediated through transcriptional regulation at cis acting ARE sequences in the genome. Nuclear factor erythroid 2-related factor 2 (Nrf2), an ARE binding transcription factor is, under physiological conditions, sequestered in the cytosol by Kelch ECH associating protein 1 (Keap1) (Itoh et al., 1999). The interactions between Nrf2 and Keap1 are mediated in part by cysteine thiols (Levonen et al., 2004; Yamamoto et al., 2008). Oxidative or electrophilic modifications to these regulatory thiols release Nrf2 and allow its translocation into the nucleus where it binds AREs and induces transcription of stress response genes (Kensler, 2007). This phenomenon can be exploited by linking a reporter gene to AREs so that cellular stress can be measured in live cells. Here we used an ARE controlled β-lactamase reporter gene to measure induction of cellular stress response as a function of monoHAM concentration (Shukla et al., 2012).
Each of the monoHAMs generated a concentration dependent increase in ARE signaling activity with concentrations that were not acutely cytotoxic (Figure 2). These data were fit with 3-parameter logistic regression curves (R2 > 0.98). The potencies of the compounds, calculated from the regression equations, reported here as the concentration that generated half maximum effect (EC50), were 2.05 ×10−6, 1.92 × 10−6, and 9.47 × 10−6 for IAM, BAM, and CAM respectively. Comparing these data to reactivity (Table 1.) show similar patterns; IAM and BAM were the most reactive in chemico, and were also more potent activators of ARE than CAM. BAM was less reactive than IAM in chemico but was essentially an equally potent inducer of ARE activity. This observation could indicate that BAM reactivity is sufficient to alkylate susceptible thiols/thiolates to generate maximum effect, thus the increased reactive potential of IAM is not reflected as increased stress response or toxicity. It could also indicate other factors independent of reactivity (e.g. log P) could play an important role in monoHAM induced toxicity.
Figure 2.
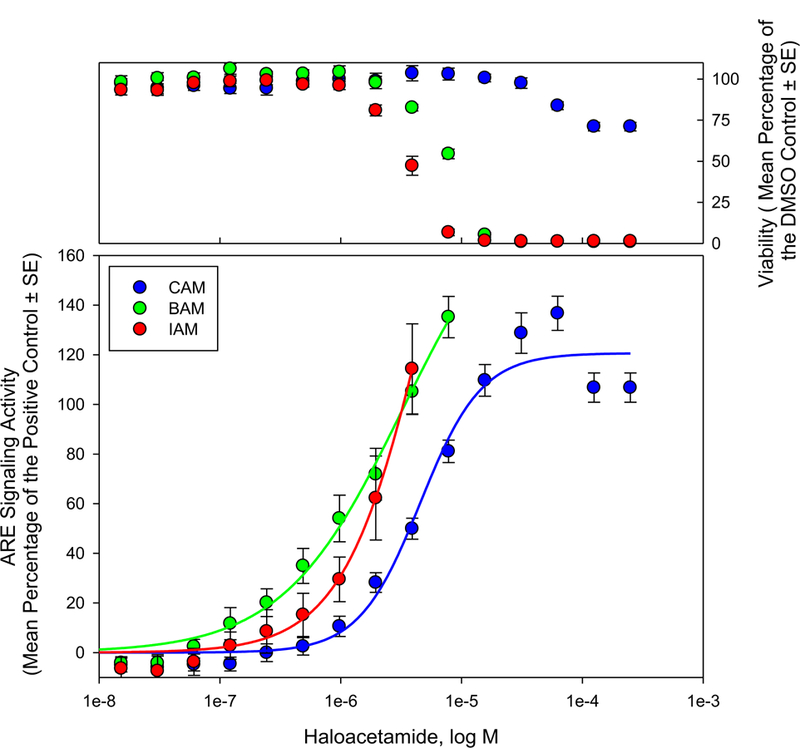
ARE signaling activity reported as a function of HAM concentration (bottom panel). ARE signaling activity at various HAM concentrations was reported as the mean (± SE) percent of the positive control (23 µM β-napthoflavone) from 4 independent experiments. Acute cytotoxicity (top panel) was measured as a function of HAM concentration. Data are reported as mean (± SE) percent of the DMSO control from 4 independent experiments.
Similar to the monoHAMs, the mono-halogenated acetic acids (monoHAAs) activated ARE signaling with an I > Br > Cl pattern of potency (Pals et al., 2013). Many additional DBPs with GSH reactive molecular motifs activated ARE signaling (Stalter et al., 2016). Futhermore, ARE activity correlated with increased mixture toxicity as water moved through stages of the disinfection process (Neale et al., 2012), suggesting that oxidative or electrophilic stress is related to total toxicity within the DBP mixture.
3.3. Rad51 Foci Formation
As part of a multicomponent system to initiate and facilitate DNA damage signaling and repair, Rad51 accumulates as foci near DNA double strand breaks (Haaf et al., 1995). Accumulation of nuclear Rad51, therefore, can be used as a measure of DNA damage. Figure 3 displays nuclear Rad51 foci with respect to HAM concentration normalized to a positive control (10 µM camptothecin). Each of the monoHAMs generated a concentration dependent increase of nuclear Rad51 foci. IAM, BAM and CAM generated EC50 values of 3.80 × 10−6, 5.69 × 10−6, and 3.00 × 10−4 M, respectively. These data were consistent with DNA damage generated in Chinese hamster ovary cells; genotoxic potency (equivalent to EC50) values of 3.41 × 10−5, 3.68 × 10−5, and 1.38 × 10−3 M were reported for IAM, BAM, and CAM, respectively (Plewa et al., 2008). These data showed a similar pattern where BAM and IAM generated similar toxicity with CAM significantly less genotoxic. Genotoxicity (indirectly measured as Rad51 foci formation) for these compounds seems to be related to thiol/thiolate reactivity with a threshold above which additional reactivity no longer generates additional in vitro effects.
Figure 3.
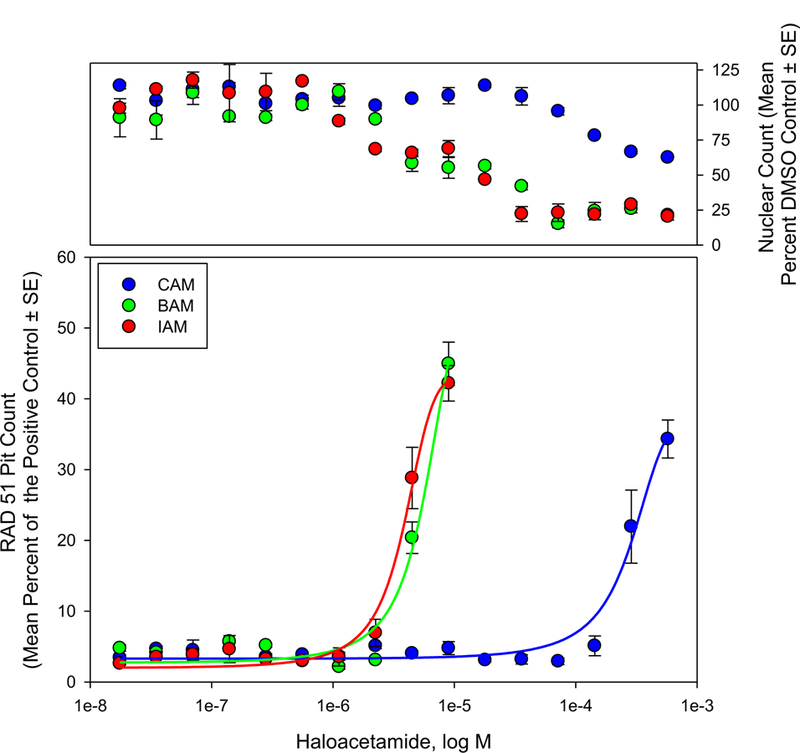
Nuclear Rad51 foci accumulation measured as a function of HAM concentration (bottom). Rad51 pit count at various HAM concentrations was reported as the mean (± SE) percent of the positive control (10 µM camptothecin) from 2 independent experiments. Acute cytotoxicity (nuclear count) was measured as a function of HAM concentration (top panel). Nuclear counts are reported as mean (± SE) percentage of the DMSO control from 2 independent experiments.
3.4. In Silico Estimates of FMO Energies, Electrophilic Softness, and Reactivity
Selected molecular parameters along with potency values for in chemico reactivity, activation of ARE signaling activity, and Rad51 foci formation are listed in Table 1. The in silico parameters (Table 1.) identified an IAM > BAM > CAM rank order of electrophilic softness. This pattern matched the measured in chemico reactivity; a similar trend was previously observed where the low ELUMO compounds BAN and BAM were more thiol reactive than bromoacetate, which had a higher LUMO energy (Pals et al, 2016). These observations suggest electrophilic softness is a useful indicator of thiol/thiolate reactivity in chemico for α-halogenated carbonyl/nitrile containing DBPs.
The softer and more reactive electrophiles IAM and BAM were significantly more potent inducers of ARE signaling activity and Rad51 foci than the less reactive CAM, however, the rank order of softness (or in chemico reactivity) was not maintained in vitro, as BAM and IAM were equally potent inducers of ARE signaling activity. LoPachin and others showed a direct relationship between in silico predictions of electrophilic softness and cytotoxicity for α,β-unsaturated carbonyl compounds (Chan et al., 2008; LoPachin et al., 2009). These compounds react through a Michael addition mechanism that may be better suited for modeling with FMO energies. The SN2 reaction mechanism favored by the monoHAMs is dependent on the leaving efficiency of the halogen (Plewa et al., 2008). This along with additional variables that affect reactivity, or bioavailability independent of FMO energies, such as steric hindrance, or log P, likely modify the relationship between electrophilic softness and in vitro effects (Tuppurainen, 1999) . In general we observe that in silico estimates of electrophilic softness provide a useful qualitative predictor of thiol/thiolate reactivity in chemico and toxicity for electrophilic compounds, however, additional consideration should be given to variables that contribute to reactivity and or toxicity independent of FMO energy.
4. Conclusion
The aim of this research was to investigate the relationship between thiol/thiolate reactivity, activation of ARE regulated stress response, and Rad51 foci formation. Within the selected group of HAMs, reactivity parameters derived from FMO energies as well as in chemico thiol/thiolate reactivity were able to qualitatively predict activation of antioxidant/electrophilic stress response and genotoxicity (measured as Rad51 foci formation). The softer electrophiles, IAM and BAM, were more reactive with our model soft nucleophile and were more potent inducers of cellular stress and Rad51 foci when compared to a less reactive CAM.
The ability to identify mechanisms of toxic activity from molecular or physicochemical descriptors provides a useful tool in mixture toxicity. Both in silico and in chemico reactivity are useful descriptors to screen/identify toxicants that act by disrupting cellular processes dependent on free thiol/thiolate chemistry. However, this approach carries its own limitations. In the case of in silico parameters, additional consideration should be given to factors independent of ELUMO when predicting electrophilic reactivity, and in the case of in chemico reactivity, kinetics should be considered to avoid false negative classification.
Acknowledgments
We wish to thank the Chemistry Department at Eastern Illinois University for access to and assistance using Spartan 10 software. This work was funded in part by DOD grant Army CESU W9132T-16-2-0005 (MJP). This work was partly supported by the interagency agreement IAG #NTR 12003 from the National Institute of Environmental Health Sciences/Division of the National Toxicology Program to the National Center for Advancing Translational Sciences, National Institutes of Health.
References
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL, 2009. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem 390:191–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RJ, 2006. Use of toxicological and chemical models to prioritize DBP research American Water Works Association. [Google Scholar]
- Calderon RL, 2000. The epidemiology of chemical contaminants of drinking water. Food Chem. Toxicol, 38, Supplement 1:S13–S20. [DOI] [PubMed] [Google Scholar]
- Chan K, Jensen N, O’Brien PJ, 2008. Structure–activity relationships for thiol reactivity and rat or human hepatocyte toxicity induced by substituted p-benzoquinone compounds. J. Appl. Toxicol, 28:608–620. [DOI] [PubMed] [Google Scholar]
- Dawson D, Jeyaratnam J, Mooneyham T, Pöch G, Schultz TW, 2010. Mixture toxicity of SN2-reactive soft electrophiles: 1. Evaluation of mixtures containing α-halogenated acetonitriles. Arch. Environ. Contam. Toxicol, 59:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D, Mooneyham T, Jeyaratnam J, Schultz T, Pöch G, 2011. Mixture toxicity of SN2-reactive soft electrophiles: 2. Evaluation of mixtures containing ethyl α-halogenated acetates. Arch. Environ. Contam. Toxicol, 61:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D, Pöch G, Schultz T, 2014. Mixture toxicity of SN2-reactive soft electrophiles: 3. Evaluation of ethyl α-halogenated acetates with α-halogenated acetonitriles. Arch. Environ. Contam. Toxicol, 66:248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, 1959. Tissue sulfhydryl groups. Arc. Biochem. Biophys 82:70–77. [DOI] [PubMed] [Google Scholar]
- Haaf T, Golub EI, Reddy G, Radding CM, Ward DC, 1995. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc. Natl. Acad. Sci, 92:2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TB, Miller GP, Swamidass SJ, 2015. Site of reactivity models predict molecular reactivity of diverse chemicals with glutathione. Chem. Res. Toxicol, 28:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M, 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev, 13:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelson M, Lobanov VS, Katritzky AR, 1996. Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev, 96:1027–1044. [DOI] [PubMed] [Google Scholar]
- Kensler T, 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol, 47:89–116. [DOI] [PubMed] [Google Scholar]
- Krasner SW, McGuire MJ, Jacangelo JG, Patania NL, Reagan KM, Aieta EM, 1989. The occurrence of disinfection by-products in US drinking water. J. Am. Water Works Assoc, 81:41–53. [Google Scholar]
- Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM, 2004. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J, 378:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Geohagen BC, Gavin T, 2009. Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal. Toxicol. Sci, 107:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Kogevinas M, Cantor KP, Villanueva CM, Garcia-Closas M, Rothman N, Malats N, Real FX, Serra C, Garcia-Closas R, Tardon A, Carrato A, Dosemeci M, Silverman DT, 2007. Total fluid and water consumption and the joint effect of exposure to disinfection by-products on risk of bladder cancer. Environ. Health Perspect, 115:1569–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner MG, Wagner ED, McCalla K, Richardson SD, Woo YT, Plewa MJ, 2007. Haloacetonitriles vs. regulated haloacetic acids: are nitrogen-containing DBPs more toxic? Environ. Sci. Technol, 41:645–651. [DOI] [PubMed] [Google Scholar]
- Neale PA, Antony A, Bartkow ME, Farré MJ, Heitz A, Kristiana I, Tang JYM, Escher BI, 2012. Bioanalytical assessment of the formation of disinfection byproducts in a drinking water treatment plant. Environ. Sci. Technol, 46:10317–10325. [DOI] [PubMed] [Google Scholar]
- Pals J, Attene-Ramos MS, Xia M, Wagner ED, Plewa MJ, 2013. Human cell toxicogenomic analysis linking reactive oxygen species to the toxicity of monohaloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol 47:12514–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pals JA, Wagner ED, Plewa MJ, 2016. Energy of the lowest unoccupied molecular orbital, thiol reactivity, and toxicity of three monobrominated water disinfection byproducts. Environ. Sci. Technol, 50:3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RG, 1963. Hard and soft acids and bases. J. Am. Chem. Soc, 85:3533–3539. [Google Scholar]
- Plewa MJ, Muellner MG, Richardson SD, Fasano F, Buettner KM, Woo YT, McKague AB, Wagner ED, 2008. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection byproducts. Environ. Sci. Technol, 42:955–961. [DOI] [PubMed] [Google Scholar]
- Plewa MJ, Simmons JE, Richardson SD, Wagner ED, 2010. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ. Mol. Mutagen, 51:871–878. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM, 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res, 636:178–242. [DOI] [PubMed] [Google Scholar]
- Schultz TW, Carlson R, Cronin M, Hermens J, Johnson R, O’Brien P, Roberts D, Siraki A, Wallace K, Veith G, 2006. A conceptual framework for predicting the toxicity of reactive chemicals: modeling soft electrophilicity. SAR QSAR Environ. Res, 17:413–428. [DOI] [PubMed] [Google Scholar]
- Schultz TW, Ralston KE, Roberts DW, Veith GD, Aptula AO, 2007. Structure–activity relationships for abiotic thiol reactivity and aquatic toxicity of halo-substituted carbonyl compounds. SAR QSAR Environ. Res, 18:21–29. [DOI] [PubMed] [Google Scholar]
- Schwöbel JA, Koleva YK, Enoch SJ, Bajot F, Hewitt M, Madden JC, Roberts DW, Schultz TW, Cronin MT, 2011. Measurement and estimation of electrophilic reactivity for predictive toxicology. Chem. Rev 111:2562–2596. [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Huang R, Simmons SO, Tice RR, Witt KL, VanLeer D, Ramabhadran R, Austin CP, Xia M, 2012. Profiling environmental chemicals for activity in the antioxidant response element signaling pathway using a high throughput screening approach. Environ. Health Perspect 120:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalter D, O’Malley E, von Gunten U, Escher BI, 2016. Fingerprinting the reactive toxicity pathways of 50 drinking water disinfection by-products. Water Research 91:19–30. [DOI] [PubMed] [Google Scholar]
- Tuppurainen K, 1999. Frontier orbital energies, hydrophobicity and steric factors as physical QSAR descriptors of molecular mutagenicity. A review with a case study: MX compounds. Chemosphere, 38:3015–3030. [DOI] [PubMed] [Google Scholar]
- U.S. Environemental Protection Agency, 2002. Guidance on cumulative risk assessment of pesticide chemicals that have a common mechanism of toxicity, Washington, DC: US Environmental Protection Agency, Office of Pesticide Programs, Office of Prevention, Pesticides, and Toxic Substances. [Google Scholar]
- Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castano-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M, 2007. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am. J. Epidemiol, 165:148–156. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M, 2008. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol, 28:2758–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Geohagen BC, Gavin T, LoPachin RM, 2016. Joint toxic effects of the type-2 alkene electrophiles. Chem. –Biol. Interact, 254:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


