Summary
The general transcription factor P-TEFb stimulates RNA polymerase II elongation and co-transcriptional processing of pre-mRNA. Contributing to a functional equilibrium important for growth control, a reservoir of P-TEFb is maintained in an inactive snRNP where 7SK snRNA is a central scaffold. Here, we identify PIP7S as a La-related protein stably associated with and required for 7SK snRNP integrity. PIP7S binds and stabilizes nearly all the nuclear 7SK via 3' UUU-OH, leading to the sequestration and inactivation of P-TEFb. This function requires its La domain and intact C-terminus. The latter is frequently deleted in human tumors due to microsatellite instability-associated mutations. Consistent with the tumor suppressor role of a Drosophila homolog of PIP7S, loss of PIP7S function shifts the P-TEFb equilibrium toward the active state, disrupts epithelial differentiation and causes P-TEFb-dependent malignant transformation. Through PIP7S modulation of P-TEFb, our data thus link a general elongation factor to growth control and tumorigenesis.
Keywords: 7SK snRNA, La protein, P-TEFb, cancer, transcriptional elongation
Introduction
For many genes from flies to humans, transcriptional elongation is a key step to control their expression. During the transcription of these genes, RNA polymerase (Pol) II is paused early after initiation by negative transcription elongation factors (N-TEF). Reversing this block requires the positive transcription elongation factor b (P-TEFb), a kinase that phosphorylates the C-terminal domain (CTD) of the largest subunit of Pol II and N-TEF, and allows Pol II to produce full-length transcripts (Peterlin and Price, 2006). This function of P-TEFb also facilitates the coupling of elongation with pre-mRNA processing (Zhou and Yik, 2006). P-TEFb is a heterodimer composed of Cdk9 and cyclin T1 (CycT1) (or the minor forms T2 or K) (Peterlin and Price, 2006). Experiments employing RNAi or specific Cdk9 inhibitors suggest that P-TEFb is a general transcription factor important for the expression of a large number of genes (Chao and Price, 2001; Shim et al., 2002).
Recent studies indicate that most of P-TEFb in the nucleus exists in two distinct functional states (Zhou and Yik, 2006). In HeLa cells, about half of P-TEFb is in a catalytically inactive complex (referred herein as 7SK snRNP) that contains the 7SK snRNA and the HEXIM1 (or the minor isoform HEXIM2) protein (Michels et al., 2003; Nguyen et al., 2001; Yang et al., 2001; Yik et al., 2003). Within this complex, 7SK mediates the interaction of P-TEFb with HEXIM1, which in turn inhibits P-TEFb kinase activity. Transcribed by RNA Pol III, 7SK is an abundant non-coding RNA of 331 nucleotides that is highly conserved in higher eukaryotes (Wassarman and Steitz, 1991). In HeLa cells, approximately the other half of P-TEFb exists in an active complex containing the bromodomain protein Brd4 (Jang et al., 2005; Yang et al., 2005). Brd4 recruits P-TEFb to chromatin templates through interacting with acetylated histones and the mediator complex, and this function is important for general elongation. Notably, P-TEFb can also be recruited by a number of gene-specific transcription factors (e.g. the HIV-1 Tat protein) to activate their target genes (Peterlin and Price, 2006; Zhou and Yik, 2006).
Through alternately interacting with its positive (Brd4) and negative (HEXIM1/7SK) regulators, P-TEFb is maintained in a functional equilibrium (Zhou and Yik, 2006). Recent studies suggest that shifts in this equilibrium may underlie alternative pathways toward unrestricted growth or terminal division/differentiation (He et al., 2006; Sano et al., 2002; Turano et al., 2006). According to this model, the 7SK snRNP represents a reservoir of activity that can respond to demand for P-TEFb-dependent transcription and cell proliferation by rapidly releasing active P-TEFb (Zhou and Yik, 2006).
Toward the goal of identifying other factors involved in P-TEFb regulation, we report here the identification and characterization of a new protein, termed PIP7S, which is intimately associated with all the nuclear 7SK and required for 7SK stability and 7SK snRNP integrity. PIP7S is homologous to human La protein, a UUU-OH sequence-specific RNA-binding protein that associates with nascent Pol III transcripts and protects them from degradation by 3' exonucleases. We show that PIP7S indeed binds 7SK RNA in a UUU-OH-dependent manner and confers RNA 3' end protection activity in an established in vivo assay. This function of PIP7S is required for 7SK stability, 7SK snRNP assembly and inhibition of P-TEFb-dependent transcription. In addition to the La domain (the La and RRM motifs) in the PIP7S N-terminal region, sequestration and inactivation of P-TEFb also require the C-terminus, which is frequently deleted in human tumors with microsatellite instability. Consistent with the demonstration that the Drosophila homolog of PIP7S is a tumor suppressor, PIP7S knockdown shifts the P-TEFb equilibrium toward the active Brd4-bound state and causes P-TEFb-dependent malignant transformation and activation of key tumor-related genes. Together, these observations link the PIP7S-dependent modulation of P-TEFb activity to the global control of cell growth and tumorigenesis.
Results
Affinity-purification and identification of PIP7S as a Cdk9-associated protein
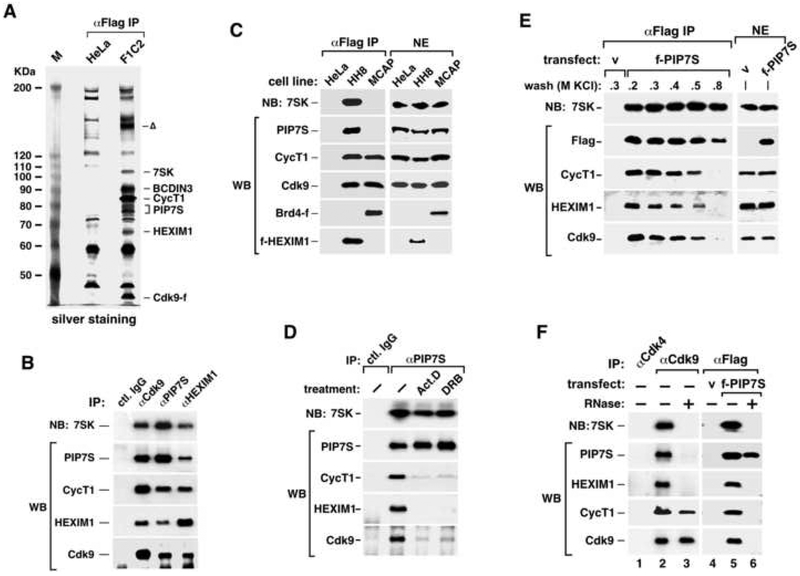
To identify new factors that can regulate P-TEFb, Cdk9 and its associated factors were affinity-purified from nuclear extract (NE) of F1C2 cells, a HeLa-based cell line stably expressing Cdk9-Flag (Cdk9-f) (Yang et al., 2001). Inspection by silver staining reveals two new bands (one above and one below CycT1) besides the known Cdk9-associated factors (CycT1, 7SK and HEXIM1) and the non-specific bands that were also in the negative control (Fig. 1A).
Figure 1. PIP7S is a component of 7SK snRNP.
A. Cdk9-f and its associated factors (αFlag IP) were affinity-purified from F1C2 NE and analyzed on a silver-stained SDS-gel, with their identities indicated on the right. HeLa NE was used in a parallel procedure for control. The band marked with “Δ” was not reproducibly seen and contained no identifiable protein. B. Immunoprecipitates (IP) obtained with the indicated antibodies from HeLa NE were analyzed by western (WB) and northern blotting (NB). C. The Cdk9-CycT1 heterodimers and their associated factors were isolated by anti-Flag immunoprecipitation (IP, left panel) from NEs (right panel) of two HeLa-based cell lines expressing Brd4-f (MCAP) and f-HEXIM1 (HH8), respectively, and analyzed as in B. D. HeLa cells were treated with the indicated compounds. IP with the indicated antibodies were analyzed as in B. E. NEs (right panels) from HeLa cells, which were transfected with a vector expressing no protein (v) or f-PIP7S, were subjected to anti-Flag IP. The immobilized immune-complexes were washed with buffer D containing the indicated amounts of KCl. f-PIP7S and its associated factors were eluted and analyzed (left panels) as in B. F. The same NEs in E were subjected to IP with the indicated antibodies. The immobilized immune complexes were incubated with (+) or without (−) RNase A before washing. The eluted complexes were analyzed as in B.
Analyses by mass spectrometry revealed that the upper band contained a recently described protein called BCDIN3, which resides in 7SK snRNP and functions as a methylphosphate capping enzyme for 7SK RNA (Jeronimo et al., 2007). The lower band together with minor ones in its immediate vicinity contained a 582-amino acid protein formerly known as HDCMA18p or DKFZp564K112 and now renamed as PIP7S (P-TEFb-interaction protein for 7SK stability). Notably, the same protein was also identified independently as an associated protein of HEXIM1 and 7SK (data not shown).
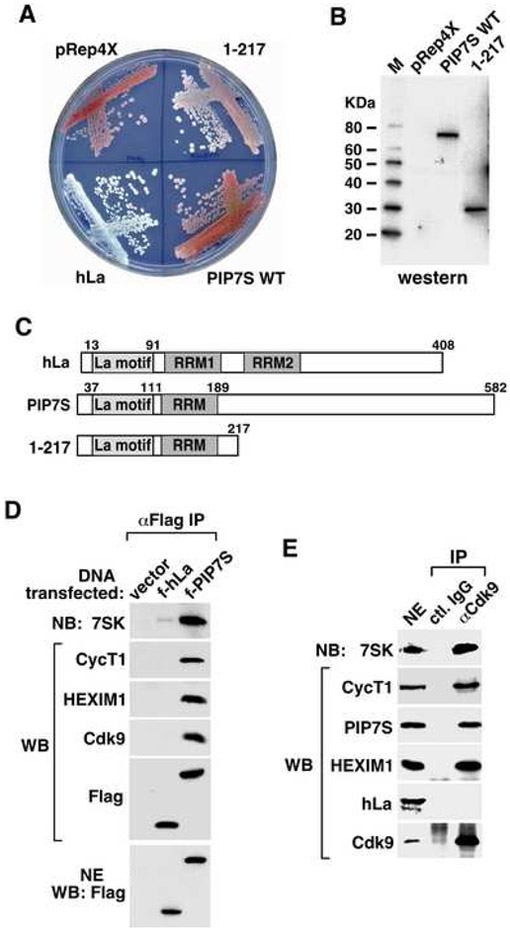
Sequence analysis revealed that the N-terminal region of PIP7S is homologous to human La (hLa) protein via the La and RRM motifs (called the La domain, see Fig. 5C). In addition, the Drosophila ortholog of PIP7S, termed multi-sex-combs (mxc), encodes a known tumor suppressor (Remillieux-Leschelle et al., 2002). Finally, in an unbiased and comprehensive screen of gastric tumors with microsatellite instability, the PIP7S gene was identified as having the second highest frequency (41.2%) of frame shift mutations (Mori et al., 2002). The mutations occur at an oligoadenylate stretch in the sequence encoding the C-terminal region of PIP7S, resulting in truncated proteins.
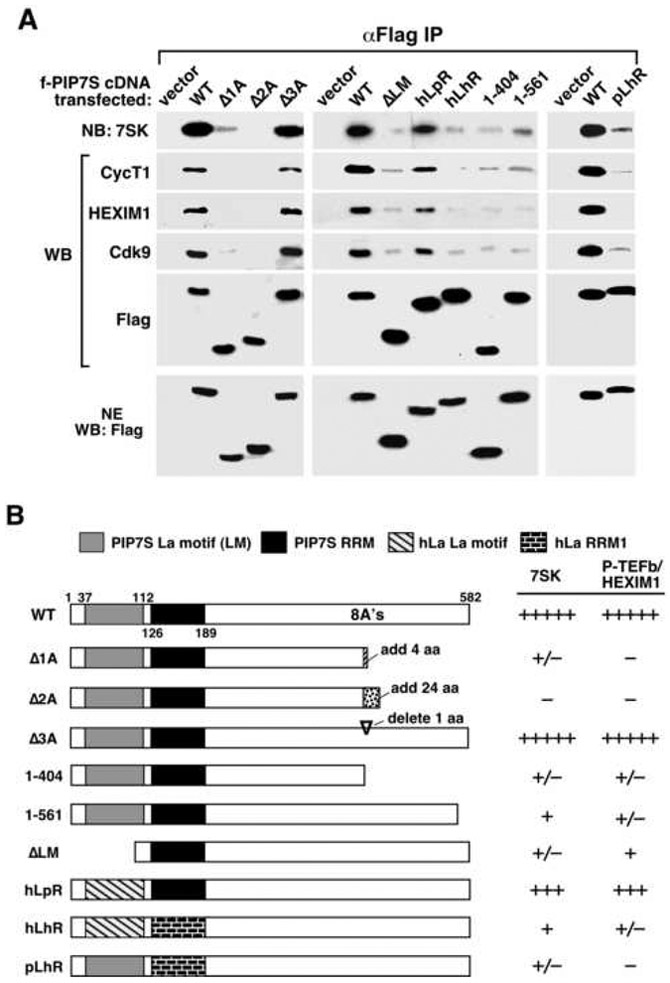
Figure 5. The N-terminal La domain and the C-terminus missing in cancer cells are both important for PIP7S to interact with 7SK snRNP.
A series of Flag-tagged PIP7S mutants as diagrammed in B were expressed in HeLa cells. Anti-Flag IP derived from NEs were analyzed by western (WB) and northern blotting (NB) as indicated in A, with quantification of the binding data summarized in the last two columns in B. hLa: human La protein. RRM: RNA recognition motif.
PIP7S is a component of 7SK snRNP
To determine the relationship between PIP7S and the other known Cdk9-associated factors, we performed immunoprecipitations in HeLa NE with anti-Cdk9, anti-PIP7S, or anti-HEXIM1 antibodies and analyzed the immune complexes by western and northern blotting (WB and NB, Fig. 1B). All three antibodies precipitated PIP7S together with 7SK, HEXIM1, Cdk9 and CycT1, all known components of 7SK snRNP. Furthermore, anti-Flag immunoprecipitations in NEs of two HeLa-based cell lines expressing either Brd4-f (MCAP) (Jang et al., 2005; Yang et al., 2005) or f-HEXIM (HH8) (Yik et al., 2003) reveal that Brd4 and PIP7S/HEXIM1/7SK exist in two mutually exclusive P-TEFb-containing complexes (Fig. 1C). Together, these data indicate PIP7S as a new subunit of 7SK snRNP.
The PIP7S-7SK interaction is stable under conditions of stress and high salt treatments
How does PIP7S interact with the rest of 7SK snRNP? HEXIM1 and 7SK have been shown to dissociate from P-TEFb in cells treated with certain stress-inducing agents (e.g. actinomycin D and DRB) that globally block transcription (Nguyen et al., 2001; Yang et al., 2001; Yik et al., 2003). Notably, both compounds also dissociated HEXIM1, Cdk9 and CycT1 but not 7SK from immunoprecipitated PIP7S (Fig. 1D). In a separate experiment, immunoprecipitated f-PIP7S remained tightly bound to 7SK in high salt (e.g. 0.8M KCl), whereas Cdk9, CycT1 and HEXIM1 were washed away (Fig. 1E). These data suggest that the PIP7S-7SK binding is independent of P-TEFb/HEXIM1 and stable under conditions of stress and high salt treatments.
PIP7S interacts with P-TEFb and HEXIM1 in a 7SK-dependent manner
7SK mediates the HEXIM1-P-TEFb interaction in 7SK snRNP (Michels et al., 2003; Yik et al., 2003). To extend the analysis to PIP7S, we degraded 7SK in the immobilized, anti-Cdk9 and anti-f-PIP7S immune complexes by RNase A before washing and elution. This procedure disrupted the interactions of PIP7S with both P-TEFb and HEXIM1 (Fig. 1F). Thus, 7SK is required for stable interactions among all the protein components (i.e. PIP7S, HEXIM1 and P-TEFb dimer) within 7SK snRNP. Consistently, the Cdk9 T-loop and the CycT1 cyclin-box, both of which are essential for the 7SK-P-TEFb binding (Chen et al., 2004; Yik et al., 2003), are also required for the PIP7S-P-TEFb interaction (supplemental Fig. S1).
PIP7S interacts with about half of P-TEFb but all of 7SK molecules in vivo
To determine the percentages of 7SK and P-TEFb associated with PIP7S in HeLa cells, PIP7S was immunodepleted from HeLa NE. The specificity and efficiency of the depletion were illustrated by the amounts of α-tubulin in the depleted NEs and the effect of non-specific IgG in a mock-depletion (Fig. 2A). Remarkably, this procedure caused nearly complete co-depletion of 7SK (Fig. 2A & 2B), suggesting that PIP7S interacts with almost all of this RNA in vivo. Besides 7SK, 48% of CycT1 and 20% of HEXIM1 were also removed. These numbers are consistent with the previous reports that about half of nuclear P-TEFb and 15-20% of HEXIM1 are sequestered in 7SK snRNP (Yang et al., 2001). In a separate experiment to immunodeplete Cdk9 from HeLa NE, about 20% of PIP7S was also co-depleted (Supplemental Fig. S2), suggesting that like 7SK, the majority of PIP7S exist outside the 7SK-P-TEFb snRNP.
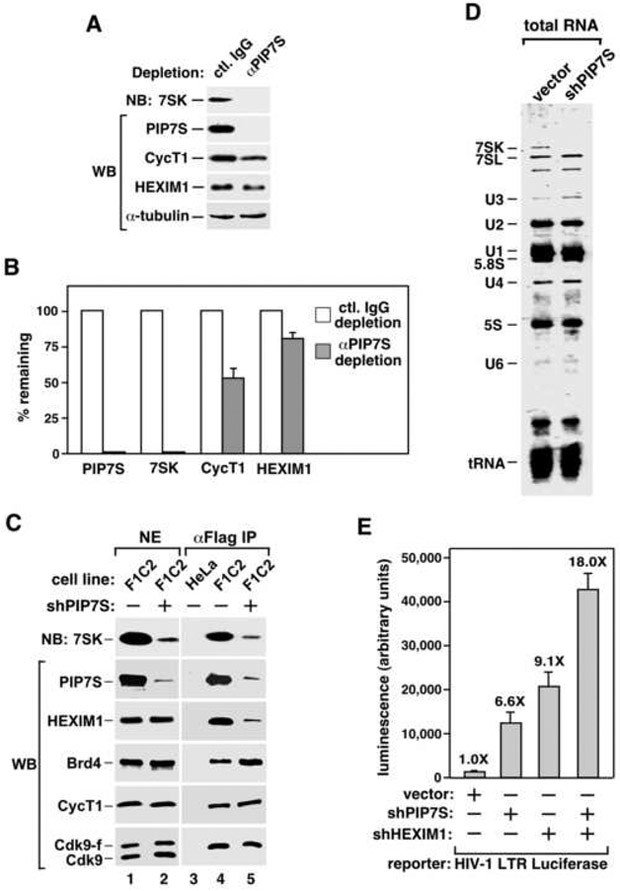
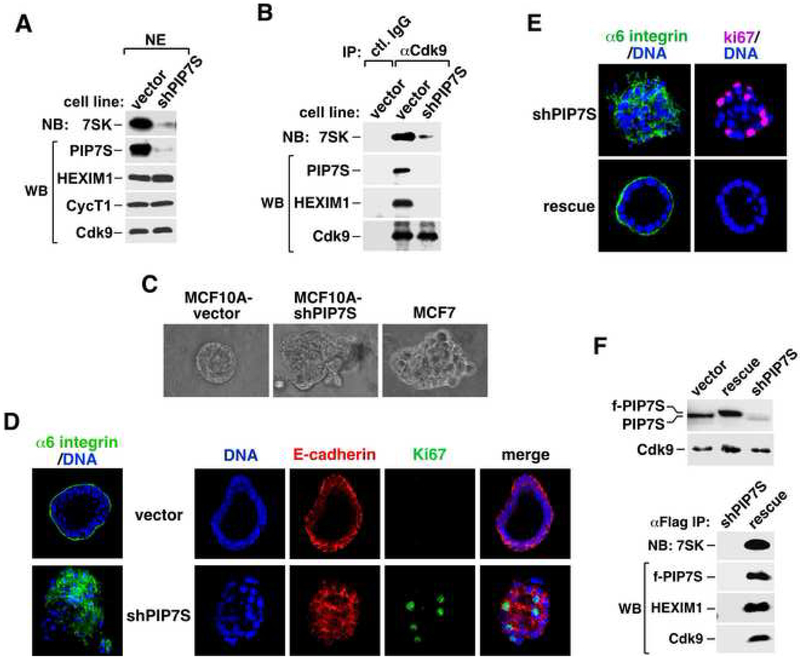
Figure 2. PIP7S knockdown decreases nuclear levels of 7SK RNA and 7SK snRNP but increases the Brd4-bound P-TEFb and P-TEFb-dependent transcription.
A. NEs were subjected to immunodepletion with the indicated antibodies and then analyzed by northern (NB) and western blotting (WB). B. The levels of the indicated components in the depleted NEs were normalized to those of α-tubulin and quantified based on serial dilutions, with those in mock-depleted NE artificially set to 100%. The error bars represent mean +/− SD. C. Stable expression of shPIP7S from a Cre-controlled cassette was induced (+) or uninduced (−) in HeLa or F1C2 cells expressing Cdk9-f. The indicated factors in NEs (lanes 1 & 2) and anti-Flag IP (lanes 3-5) were analyzed as in A. D. Total RNA was extracted from NEs of HeLa cells expressing or not expressing shPIP7S, resolved on a denaturing gel, and stained with GelRed. E. HeLa cells were co-transfected with the HIV-1 LTR-luciferase reporter construct and a vector expressing no RNA, shPIP7S, shHEXIM1 or both. Luciferase activities were measured and the error bars represent mean +/− SD.
PIP7S knockdown markedly decreases nuclear levels of 7SK RNA and 7SK snRNP
Consistent with the observation that nearly all the nuclear 7SK were associated with PIP7S, stable knockdown of PIP7S by a short hairpin (sh)RNA, shPIP7S, reduced the levels of not only PIP7S but also 7SK by more than 90% in F1C2 cells as determined by serial dilution and quantification (Fig. 2C, lanes 1 & 2; also data not shown). Thus, nearly all 7SK molecules relied on PIP7S for stability. Moreover, underscoring the structural role of 7SK RNA in 7SK snRNP, the globally reduced 7SK level caused by shPIP7S also prevented the association of HEXIM1 with immunoprecipitated Cdk9-f/CycT1 and thus the formation of 7SK snRNP (Fig. 2C, lane 5). Significantly, however, the amount of Brd4 bound to P-TEFb increased about 2-fold at the same time (compare lanes 4 & 5). This is most likely due to a shift of the P-TEFb equilibrium from about half to nearly all in the Brd4-bound state (Yang et al., 2005) in knockdown cells. Correlating with this change, P-TEFb affinity-purified from knockdown cells also displayed ~2-fold increase in kinase activity toward GST-CTD (Supplemental Fig. S3).
To illustrate the specificity of shPIP7S action, 7SK was shown to be the only species among total nuclear RNA that was drastically reduced in PIP7S knockdown cells (Fig. 2D). Moreover, the elimination of 7SK RNA/snRNP could be achieved by more than one shRNAs that target different regions of PIP7S (data not shown), effectively ruling it out as an off-target effect caused by a single shRNA sequence.
PIP7S knockdown enhances the P-TEFb-dependent HIV-1 transcription
Because PIP7S knockdown liberated P-TEFb from 7SK snRNP and shifted the P-TEFb equilibrium to the active Brd4-bound state, it is expected to increase HIV-1 transcription, which is highly responsive to P-TEFb’s activity (Yang et al., 2001; Yik et al., 2003). Indeed, shPIP7S significantly increased (6.6-fold) the HIV-1 LTR-driven luciferase expression, and shPIP7S plus shHEXIM1 further enhanced (18-fold) this effect (Fig. 2E). It is worth noting that besides HIV-1 transcription in HeLa cells, PIP7S knockdown also stimulated P-TEFb occupancy on and transcription from two endogenous genes in a different cell type (see below). Thus, despite the fact that PIP7S is not a kinase inhibitor (data not shown), it contributes to the sequestration and inactivation of P-TEFb by maintaining the integrity of both 7SK RNA and 7SK snRNP in vivo.
The 3’ oligouridylate tail of 7SK as well as the La and RRM motifs of PIP7S are all required for the PIP7S-7SK binding
How does PIP7S control the stability of 7SK snRNA? hLa binds nascent Pol III transcripts via the 3’ -UUU-OH sequence and sequester them away from exonucleases (Maraia and Bayfield, 2006; Wolin and Cedervall, 2002). The La motif confers the binding specificity for -UUU-OH. Coincidentally, 7SK is a Pol III transcript with the signature 3’ oligouridylate tail (-UUUU-OH). Moreover, PIP7S is homologous to hLa and its La domain has all of the invariant and highly conserved amino acids involved in recognition of 3’ UUU-OH (Teplova et al., 2006). Based on these facts as well as the observation that nearly all the nuclear 7SK were associated with PIP7S, we postulated that PIP7S displays intrinsic La activity, which is responsible for 7SK’s stability in vivo.
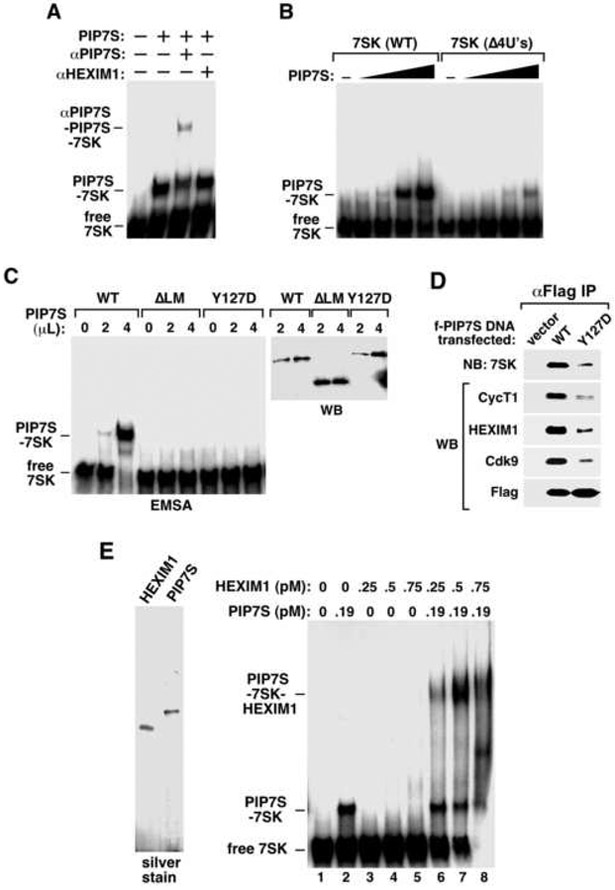
Three lines of evidence exist to support this notion. First, the 3’ -UUUU-OH of 7SK and the La domain of PIP7S are both required for the PIP7S-7SK binding. In an electrophoretic mobility shift assay (EMSA), homogeneously purified PIP7S protein (Materials and Methods; also Fig. 3E) readily formed a complex with 32P-labeled 7SK, which could be partially super-shifted by anti-PIP7S but not anti-HEXIM1 antibody (Fig. 3A). More importantly, deletion of the 7SK 3’ –UUUU-OH (Δ4U's, Fig. 3B), removal of the PIP7S La motif (ΔLM), or substitution of a highly conserved residue (Y127D) in the PIP7S RRM (Fig. 3C) all dramatically reduced the PIP7S-7SK binding. Moreover, the ΔLM and Y127D mutations also significantly blocked the 7SK snRNP formation in vivo (Fig. 3D and Fig. 5). These data agree completely with the description of interactions between authentic La proteins and Pol III transcripts with 3’ UUU-OH.
Figure 3. The 7SK-PIP7S binding requires both the poly(U) tail of 7SK and the La domain of PIP7S and recruits HEXIM1 to 7SK snRNP.
A. Affinity-purified PIP7S, anti-PIP7S (αPIP7S) and anti-HEXIM1 (aHEXIM1) antibodies were incubated as indicated with 32P-labeled 7SK and analyzed by EMSA. B. Wild-type 7SK or its mutant Δ4U’s was added to EMSA reactions containing increasing amounts of PIP7S (in 2-fold increments). C. Flag-tagged wild-type PIP7S and its mutants ΔLM and Y127D were affinity-purified, adjusted to similar concentrations by anti-Flag western blotting (right), and analyzed for their interactions with 7SK by EMSA (left). D. Flag-tagged wild-type PIP7S or Y127D was expressed in HeLa cells. Anti-Flag IP derived from NEs was analyzed by western (WB) and northern blotting (NB) as indicated. E. Left: Flag-tagged HEXIM1 and PIP7S were affinity-purified from transfected HeLa cells and examined on a silver-stained SDS-gel. Right: The two proteins were added at the indicated concentrations to EMSA reactions containing 32P-labeled 7SK.
PIP7S enhances the binding of HEXIM1 to 7SK
Further evidence implicating a La-like function in PIP7S is the demonstration by EMSA that PIP7S markedly enhanced the binding of HEXIM1 to 7SK to form a robust, slow-migrating complex (Fig. 3E, lanes 6-8), which could be super-shifted or disrupted by anti-PIP7S or anti-HEXIM1 antibodies, respectively (data not shown). Thus, besides its requirement for 7SK stability, the strong and independent PIP7S-7SK binding (Fig. 1D & 1E) may help recruit HEXIM1 to 7SK snRNP. This is reminiscent of classic La proteins that can facilitate the assembly of U snRNPs (Xue et al., 2000).
The PIP7S N-terminal region partially compensates for the loss of La function to provide RNA 3' end protection
The final evidence in support of PIP7S's La-like activity is the demonstration that PIP7S partially compensated for the loss of La function in an established S. pombe-based assay that depends on ectopic La protein for RNA 3’ end protection against exonuclease digestion (Huang et al., 2006; Intine et al., 2000). In this system, La activity, monitored by a red-white colony assay, is required for the 3' end protection-dependent maturation of a suppressor tRNASerUGA that suppresses a premature UGA stop codon in ade6-704. Under limiting adenine conditions, unsuppressed cells accumulated red pigment (e.g. cells with vector pRep4X, Fig. 4A). Expression of hLa, however, suppressed the stop codon and turned colonies white. Although wild-type PIP7S displayed little activity, PIP7S 1-217 containing just the La and RRM motifs (Fig. 4C) significantly reduced red pigment (Fig. 4A). Thus, when separated from the C-terminal region, the PIP7S La domain could indeed display RNA 3' protection activity that is characteristic of authentic La proteins. However, the PIP7S C-terminal region may determine functional specificity, allowing PIP7S to focus on 7SK rather than pre-tRNAs as its major or perhaps sole target in vivo (see Discussion below).
Figure 4. While PIP7S displays authentic La activity to protect RNA 3’ end from exonuclease digestion, hLa cannot substitute for PIP7S in 7SK snRNP.
A. PIP7S N-terminal region partially rescues defective tRNA processing in S. pombe lacking endogenous La function. ySH9 cells containing a La-dependent suppressor tRNA as well as a premature stop codon in ade6-704 were transformed with pRep4X containing no or the indicated inserts. Transformants were streaked onto plates supplemented with adenine. B. Expression of the His-tagged PIP7S was confirmed by anti-His6 western blotting. C. A diagram showing the domain structures of hLa, wild-type PIP7S and PIP7S 1-217. D. HeLa cells were transfected with a vector expressing nothing, f-PIP7S or f-hLa. Anti-Flag immunoprecipitates (IP) from NEs (bottom panel) were analyzed by western (WB) and northern blotting (NB) as indicated. E. IP obtained with the indicated antibodies from HeLa NE were analyzed as in D.
hLa cannot substitute for PIP7S in 7SK snRNP
If the PIP7S N-terminal region can function like hLa to protect RNA 3’ ends, we asked whether hLa could also replace PIP7S to enter 7SK snRNP. Data in Figure 4D indicate that in sharp contrast to f-PIP7S, f-hLa was only able to co-precipitate less than 5% of total 7SK RNA but not any known protein components of 7SK snRNP. Consistently, endogenous 7SK snRNP obtained by anti-Cdk9 IP contained PIP7S but not hLa, despite the fact that the latter is highly abundant and readily detectable in NE (Fig. 4E). Thus, although hLa is homologous to PIP7S, it cannot substitute for PIP7S to enter 7SK snRNP.
The PIP7S C-terminus deleted in human gastric tumors and absent in hLa is required for binding to 7SK snRNP
Given that PIP7S is required for sequestering P-TEFb in inactive 7SK snRNP, we tested how the PIP7S mutations detected in gastric tumors (Mori et al., 2002) would affect this ability. To mimic these mutations, Δ1A, Δ2A and Δ3A, with a deletion of 1, 2 and 3 adenosines, respectively, from a microsatellite repeat of 8 A's (nucleotides 1206-1213) in the PIP7S C-terminal region, were created (Fig. 5B). While Δ3A, which has a restored open reading frame after skipping one amino acid, interacted with the 7SK snRNP components normally, Δ1A and Δ2A, both of which acquire premature stop codons, almost completely lost this ability (Fig. 5A). Moreover, deletion of just the last 21 amino acids also largely abolished the interaction (Fig. 5). Thus, the PIP7S C-terminus deleted in tumors and absent in hLa is essential for the PIP7S-dependent sequestration of P-TEFb. This observation, together with the demonstration that replacing the PIP7S RRM motif, and to a lesser extent the La motif, with those of hLa prevented the interactions with 7SK snRNP by PIP7S mutants pLhR and hLpR, respectively (Fig. 5), explain why hLa was not detected in 7SK snRNP (Fig. 4D & E).
PIP7S knockdown in MCF10A cells disrupts the formation of highly organized, multi-cellular acini with a well-defined border
The observation that the tumor-derived PIP7S mutants failed to sequester and inactivate P-TEFb, which would otherwise promote cell growth (Zhou and Yik, 2006), implicates the PIP7S deficiency as a potential contributor to cancer. Further support of this idea comes from PIP7S’s homology to the Drosophila tumor suppressor MXC. Overall, the two proteins are 25% identical and 43% similar (6e-29). An even stronger homology (40% identity and 62% similarity; 3e-24) exists in their N-terminal regions that contain the La domain. Based on these observations, we postulated that like Drosophila MXC, PIP7S is a human tumor suppressor.
To test this hypothesis, we stably expressed shPIP7S in human normal mammary epithelial cell line MCF10A, which is an accepted model system for studying transformation (Shaw et al., 2004). PIP7S knockdown significantly decreased the levels of both 7SK RNA and 7SK snRNP (Fig. 6A&B) as in HeLa cells. When cultured in a three-dimensional (3D) reconstituted basement membrane (Matrigel) (Shaw et al., 2004), MCF10A with an empty vector underwent morphological differentiation to form a multi-cellular structure with an organized spherical arrangement reminiscent of breast acinus in vivo (Fig. 6C). In contrast, colonies formed by knockdown cells were disorganized and showed irregular borders similar to those of the breast cancer cell line MCF7 (Fig. 6C).
Figure 6. PIP7S knockdown in MCF10A cells disrupts 7SK snRNP and mammary epithelial cell differentiation and causes transformation.
A. NEs from stable MCF10A clones containing the vector expressing nothing or shPIP7S were examined by western (WB) and northern blotting (NB) for the indicated factors. B. Immunoprecipitates (IP) obtained with the indicated antibodies were analyzed as in A. C. Microscopic examination of the morphology of colonies formed in Matrigel from the MCF10A vector control or PIP7S knockdown cells, or the MCF7 cells. D. Colonies formed in Matrigel from the MCF10A vector control (vector) or PIP7S knockdown (shPIP7S) cells were harvested at day 12 and stained with the indicated antibodies or the DNA dye. E. PIP7S knockdown cells (shPIP7S) and their derivatives expressing shRNA-resistant f-PIP7S (rescue) were cultured in Matrigel and analyzed as in D. F. NEs from the indicated MCF10A clones were examined by WB (upper panel). f-PIP7S and its associated factors in αFlag IP were analyzed as in A.
PIP7S knockdown disrupts cell polarity, blocks mammary epithelial cell differentiation and causes transformation
To test whether PIP7S knockdown would induce morphological changes often associated with malignant transformation, the localizations of several key protein markers within the 3D colonies were examined by immunofluorescence. First, α6 integrin, a marker for epithelial polarity, was found predominantly at the basal and to a lesser extent the lateral side of a control colony, which also displayed a well-formed lumen (Fig. 6D). In contrast, it was completely mislocalized within the colony of knockdown cells and detected at the apical side and among cells that filled the central cavity (Fig. 6D), indicating a severe disruption of apicobasal polarity. Furthermore, E-cadherin, normally restricted to cell-cell junction, was also dramatically disorganized in the knockdown colony (Fig. 6D). Finally, Ki67, a proliferation marker, was detected much more frequently in the knockdown colony than in the control (Fig. 6D), indicating a lack of cell cycle withdrawal and apoptosis for many knockdown cells at this stage.
Importantly, all these shPIP7S-mediated changes could be completely reversed by the introduction of a siRNA-resistant PIP7S cDNA that expresses wild-type f-PIP7S (Fig. 6E), which was properly incorporated into 7SK snRNP as expected (Fig. 6F). Thus, the observed changes are unlikely to be off-target effects caused by a particular shPIP7S sequence.
Elevated P-TEFb activity is essential for shPIP7S-induced MCF10A transformation
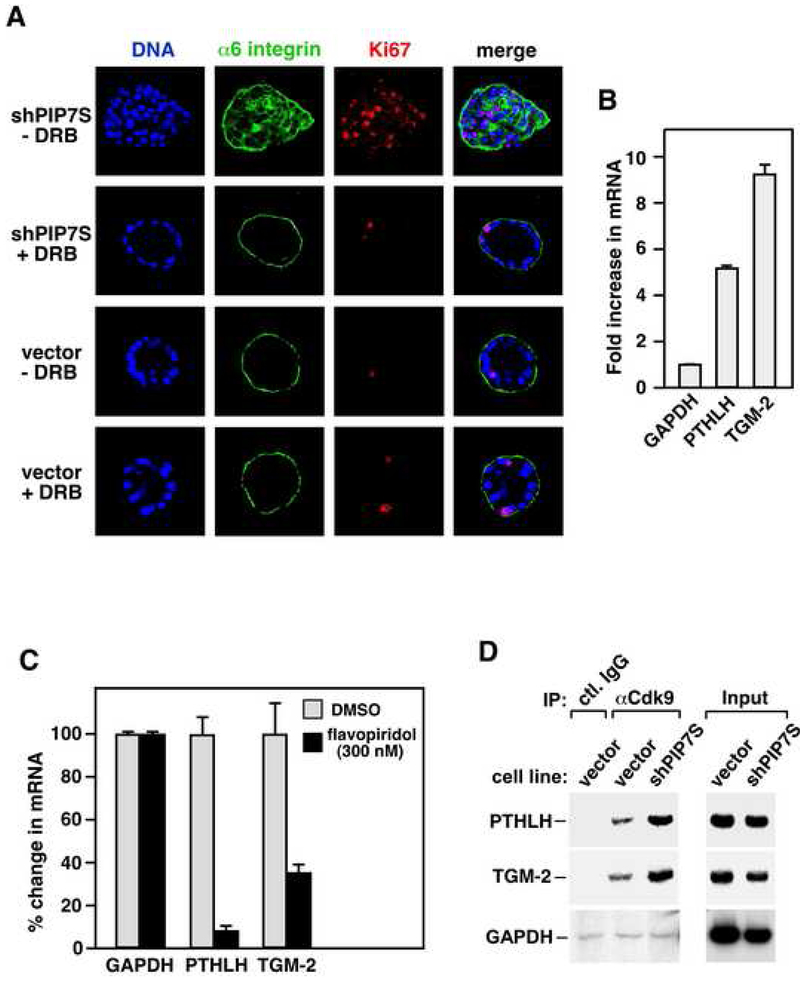
To determine whether increased P-TEFb activity is required for shPIP7S to induce MCF10A transformation, we incubated DRB (5,6-dichloro-1-β-D-ribofuranosylbenzimidazole), a well-documented inhibitor of P-TEFb kinase (Marshall et al., 1996; Zhu et al., 1997), with the vector control and knockdown cells grown in Matrigel (Fig. 7A). Although P-TEFb and Pol II elongation are efficiently blocked by greater than 50 μM DRB (Marshall et al., 1996; Zhu et al., 1997), the ability of control cells to form acini-like structure was minimally affected by 15-20 μM of the drug (18 μM in Fig. 7A). In contrast, the same amount of DRB completely blocked transformation of PIP7S knockdown cells, which formed colonies with well-organized structures like control cells (Fig. 7A). Thus, the DRB-mediated partial reduction in P-TEFb activity effectively reversed the shPIP7S-induced transformation, consistent with the notion that P-TEFb is the target of PIP7S suppression of tumorigenesis.
Figure 7. Elevated P-TEFb activity in PIP7S knockdown cells enhances P-TEFb’s occupancy on and transcription from two key tumor-related genes and is essential for transformation.
A. MCF10A vector control (vector) and PIP7S knockdown (shPIP7S) cells were allowed to form colonies in Matrigel in the presence of DRB and then stained with the indicated antibodies or the DNA dye. DMSO was used for the DRB (−) samples. B. qRT-PCR was used to examine the fold induction in mRNA levels from the indicated genes. The error bars represent mean +/− SD. C. PIP7S knockdown cells were treated with either DMSO or 300 nM flavopiridol for 5 hr. mRNA levels of the indicated genes were analyzed by qRT-PCR, with those in DMSO-treated cells artificially set at 100%. The error bars represent mean +/− SD. D. Chromatin immunoprecipitation with the indicated antibodies was performed. The regions close to the 3' end of the indicated genes were PCR-amplified from the precipitated DNA. Amplified signals from 10% of the input chromatin were also shown. The GAPDH signals were exposed 50% longer to reveal weak bands.
PIP7S knockdown enhances P-TEFb’s occupancy on and transcription from two key tumor-related genes
Finally, we tested whether the shPIP7S-mediated release of P-TEFb from 7SK snRNP would enhance P-TEFb’s occupancy on and transcription from endogenous genes in PIP7S knockdown MCF10A cells. To this end, the PTHLH (parathyroid hormone-like hormone) and TGM-2 (transglutaminase 2) genes were selected because of their demonstrated roles in breast cancer development and the availability of reagents in the lab. Both genes display elevated expression in breast cancer tissues and cell lines and are strongly correlated with tumor invasion and metastasis (Torricelli et al., 2006; Mehta et al., 2004).
Consistent with the transformed phenotypes of PIP7S knockdown cells, a significant increase in transcription from both PTHLH and TGM-2 but not the P-TEFb-independent housekeeping gene GAPDH (glyseraldehyde-3-phosphate dehydrogenase) (He et al., 2006) was detected by quantitative real-time PCR (qRT-PCR) in these cells (Fig. 7B). Importantly, the elevated PTHLH and TGM-2 expression was inhibited by flavopiridol (Fig. 7C), a specific Cdk9 inhibitor (Chao and Price, 2001), indicating these two genes as P-TEFb-dependent. Consistently, an enhanced occupancy of P-TEFb at the PTHLH and TGM-2 loci near the 3' end of the ORFs was also revealed by chromatin immunoprecipitation (ChIP, Fig. 7D). In contrast, no more than the background level of P-TEFb (obtained via control IgG) was detected at the GAPDH locus and this situation was unchanged by PIP7S knockdown. Together, these data are consistent with the model that PIP7S knockdown disrupts mammary epithelial differentiation and causes transformation through activating P-TEFb and increasing P-TEFb-dependent expression of key tumor-promoting genes.
Discussion
The 7SK snRNA has been likened to a molecular scaffold that holds all the protein components together within 7SK snRNP (Michels et al., 2003; Yik et al., 2003). Without this RNA, HEXIM1 is unable to interact stably with P-TEFb and inhibit Cdk9’s kinase activity. For such an important function ascribed to 7SK, one would imagine that there must exist cellular mechanisms to ensure that its stability, and in turn, the integrity of 7SK snRNP are not compromised. Indeed, nature finds an effective, and in hindsight, rather obvious solution in PIP7S, a La-related protein, which binds 7SK through its 3’ poly(U) tail and is requited for the stable accumulation of this RNA and formation of 7SK snRNP. Further underscoring the importance of 7SK, a recent study (Jeronimo et al., 2007) demonstrates that the 5’ end of this RNA is also protected in 7SK snRNP by a specific methylphosphate capping enzyme called BCDIN3.
Within 7SK snRNP, the PIP7S-7SK binding is direct, strong and independent of other proteins (Fig.1D, 1E & 3). This observation raises the possibility that the PIP7S-7SK sub-complex functions as a preexisting unit to nucleate the formation of 7SK snRNP in vivo. It has been suggested that while there are probably two copies each of Cdk9, CycT1 and HEXIM1 (or HEXIM2) proteins in 7SK snRNP, there is only one copy of 7SK RNA in this complex (Li et al., 2005; Yik et al., 2005). Given our inability to detect any PIP7S dimers (data not shown), we suspect that the PIP7S-7SK sub-complex exists as a monomeric unit within 7SK snRNP.
In contrast to PIP7S, the prototypical hLa, which is highly abundant and homologous to PIP7S, was not detected in 7SK snRNP (Fig. 4D & E). Moreover, only less than 5% of 7SK RNA were associated with hLA in HeLa NE (Fig. 4D), whereas nearly all were bound by PIP7S (Fig. 2A & 2B). Given the strong homology between PIP7S and hLa (their La motifs are 40% identical and 65% similar), why would 7SK snRNA/snRNP preferentially employ PIP7S but not hLa to maintain their stability in vivo? We believe that part of the answer may lie in the unique C-terminal region of PIP7S that is frequently deleted in human cancers and absent in hLa. In support of this idea, PIP7S mutants lacking the C-terminal region were unable to bind 7SK and enter 7SK snRNP (Fig. 5). Besides the C-terminal region, certain amino acids that distinguish the La domains of PIP7S and hLa also contribute to the functional difference between these two proteins. This is illustrated by the demonstration that replacing the PIP7S La and RRM motifs with those of hLa almost completely disrupted the interactions of PIP7S with 7SK snRNA/snRNP (Fig. 5). Together, these data provide a likely explanation for the specific involvement of PIP7S but not hLa in controlling 7SK stability in vivo.
Interestingly, the functional difference between PIP7S and hLa is also evident when it comes to their roles in tRNA processing. While hLa was fully capable of promoting tRNA maturation thereby compensating for the loss of La function in the fission yeast strain ySH9, only the C-terminally truncated PIP7S but not the full-length protein displayed a partial activity (Fig. 4A). It is possible that the C-terminal region and the unique residues in the N-terminal half of PIP7S render the protection of pre-tRNA 3’ ends not a top priority of PIP7S under normal conditions. However, in gastric tumors with PIP7S frame shift mutations (Mori et al., 2002), the situation could be quite different. Here, the C-terminally truncated PIP7S may increase tRNA levels as has been implicated in fission yeast (Fig. 4A), which could potentially contribute to malignant transformation according to the current model that increased tRNAs and other pol III transcripts promote tumorigenesis (White, 2004). If this hypothesis is proven, it will indicate a dual role for the PIP7S frame shift mutations in cancer. On one hand, the truncated proteins are unable to sequester P-TEFb into inactive 7SK snRNP (Fig. 5) and thus would lead to a loss of tumor suppressor activity. On the other hand, the mutant PIP7S may also acquire a gain-of-function in promoting tumorigenesis in a manner reminiscent of that of activated oncogenes.
A key observation of the current study is that PIP7S are associated with nearly all the 7SK RNA and required to maintain their stability in vivo (Fig. 2). Considering that 7SK is a very abundant RNA species (~2 × 105 per cell) (Wassarman and Steitz, 1991), PIP7S is expected to be as abundant as 7SK. Importantly, stable and simultaneous knockdown of both molecules to less than 10% of their normal levels in HeLa and MCF10A cells not only failed to decrease cell viability, it even caused transformation of MCF10A cells (Fig. 6 & 7). These observations suggest that PIP7S and 7SK are not required for cell survival. Rather, they contribute to the suppression of growth and transformation. We noticed that this conclusion apparently contradicts a previous claim that 7SK depletion by transfected siRNA leads to apoptosis (Haaland et al., 2005). Since only one siRNA sequence was tested and there was no attempt to reverse the phenotype with functional, siRNA-resistant 7SK, the possibility of an off-target effect caused by the siRNA cannot be ruled out. Alternatively, the difference could be due to the fact that both PIP7S and 7SK were co-depleted in our system, whereas the previous study involves the depletion of 7SK only.
Notably, the anti-growth/anti-tumor function of PIP7S agrees well with the previous demonstration that MXC, the Drosophila homolog of PIP7S, is a confirmed tumor suppressor for preventing the overproliferation of lymph glands and circulating hemocytes in larvae (Remillieux-Leschelle et al., 2002). As for 7SK, previous studies have also assigned a growth inhibitory role for at least a portion of this RNA that are associated with P-TEFb. For example, induction of hypertrophic growth of cardiac myocytes has been shown to involve the dissociation of 7SK from P-TEFb and activation of Cdk9 kinase, which is limiting for cell growth (Sano et al., 2002). Conversely, in murine erythroleukemia cells (MELC) that are induced to undergo terminal division and differentiation by HMBA, the P-TEFb equilibrium is overwhelmingly shifted toward the inactive 7SK snRNP, where 7SK mediates the binding and inhibition of P-TEFb by HEXIM1 (He et al., 2006). These findings, coupled with the demonstrations that both Brd4 and HEXIM1 affect cell growth albeit in opposite manners, all point to P-TEFb as a common target for the global control of cell growth and differentiation by its associated regulators (Zhou and Yik, 2006). Since a key characteristic of cancer cells is unchecked growth, it is conceivable that the loss of PIP7S/7SK function in human cells results in a significant increase in the active pool of P-TEFb, leading to the elevated expression of key tumor-promoting genes such as PTHLH and TGM-2, unsuppressed cell growth and ultimately transformation. Our results thus implicate an important and direct role for P-TEFb and its control of transcriptional elongation in tumorigenesis.
Experimental Procedures
Immunological reagents
The rabbit polyclonal anti-PIP7S antibodies were raised against the PIP7S C-terminal peptide (TQQASKHIRFSEYD; aa 569-582) and affinity-purified. Antibodies against α6 integrin (rat), E-cadherin (mouse) and Ki-67 (rabbit) were from Chemicon, BD and Zymed, respectively. The anti-HEXIM1 antibodies have been described previously (Yik et al., 2003). All other antibodies were from Santa Cruz Biotechnology.
Immunodepletion of PIP7S or Cdk9 from HeLa NE
Depletion was performed by incubating 100 μl of HeLa NE (~7 mg/ml) containing 0.2% NP-40 and 0.35 M NaCl with 7 μg of anti-PIP7S or anti-Cdk9 antibodies at 4°C for 30 min, followed by the incubation with 30 μl of protein A-Sepharose beads (Amersham Biosciences) for 30 min. Upon the removal of the beads, 7 μg of fresh antibodies were added to the NE for 30 min at 4°C followed by three successive rounds of incubation with 30 μl each of fresh protein A-Sepharose beads.
7SK RNA gel shift assay (EMSA)
32P-labeled wild-type 7SK and 7SK(Δ4U's) were synthesized by T7 RNA polymerase from PCR-amplified DNA templates. For EMSA, 20 μl reactions were carried out in buffer D (20 mM HEPES-KOH [pH 7.9], 15% glycerol, 0.2 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride) supplemented with 100 mM KCl, 5 mM MgCl2, 1 μg BSA, 150 ng poly(rG), 2,000 cpm of radiolabeled 7SK, and 0.2 pM of affinity-purified wild-type or mutant f-PIP7S proteins as indicated. For antibody supershift of the PIP7S-7SK complex, 0.3 μg of anti-PIP7S or anti-HEXIM1 antibodies were also included. After incubating at RT for 20 min, the reactions were resolved on a 4% polyacrylamide (19:1, acrylamide:bisacrylamide) gel in 0.5x Tris-glycine at 4°C for 3 h at 250 V.
To purify wild type and mutant f-PIP7S proteins free of any associated factors for EMSA, anti-Flag immunoprecipitation was performed in micrococcal nuclease (MN)-treated NEs from transiently transfected HeLa cells. The immunoprecipitates were washed extensively with buffer D containing 1.0 M KCl (D1.0) and then buffer D0.1 (0.1 M KCl) before elution with the Flag peptide as described previously (Chen et al., 2004). To treat NE with MN (Roche Applied Science), 75 units of MN were incubated with 1 ml of NE and 1mM CaCl2 for 15 min at RT. The reaction was stopped with 10 mM EDTA. Purity of the proteins was confirmed by silver staining and the concentrations estimated by comparing with the BSA standards.
Generation of PIP7S knockdown cell lines
The Cre-induced RNA interference (RNAi) was used to generate the HeLa-based PIP7S knockdown cell lines. The lentiviral vector pSico (Ventura et al., 2004) was a gift from Dr. Tyler Jacks at MIT. Either of the following two shRNA sequences: 5’-AAGTTAATCACCAAAGCTGAATTCAAGAGATTCAGCTTTGGTGATTAACTTTTT-3’ and 5’-AATCACAGCTGGATTGAAAGATTCAAGAGATCTTTCAATCCAGCTGTGATTTTT-3’ was cloned into pSico for knocking down PIP7S. The procedures for the generation of recombinant lentiviruses, infection of F1C2 cells, and selection of knockdown clones were as described (Ventura et al., 2004). To induce the expression of shPIP7S, two rounds of infection 24 hr apart were performed by using 5-10 PFU/cell of the adenovirus expressing Cre recombinase (Gene Transfer Vector Core facility of University of Iowa).
To establish MCF10A-based PIP7S knockdown cells, retroviruses, generated with the pSUPER vector (Oligoengine, WA) containing the shPIP7S-expressing cassette, were produced in the GP2-293 packaging cell line (Clontech, CA). Infected cells were selected with 0.4 μg/ml puromycin for two weeks to obtain individual clones.
To rescue PIP7S expression in MCF10A knockdown cells, nucleotides A, C and C at positions 1671, 1674 and 1677 were changed to G, T and G, respectively, to obtain the shPIP7S-resistant f-PIP7S cDNA encoding wild-type PIP7S, which was stably transduced via lentiviral infection.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed with Applied Biosystem 7300 Real-Time PCR System and Finnyzme F-410L SYBR Green RT-PCR reagents following the manufacturers’ instructions. PCR primers were designed using Integrated DNA Technologies' Primer Quest. PCR conditions include an initial denaturing step at 94°C for 2 min and 40 cycles of 94°C for 30 sec and 60°C for 90 sec. Threshold values (Ct) were calculated to obtain the relative folds of induction. All reactions were run in triplicates.
Chromatin immunoprecipitation (ChIP) asay
The assay conditions were as described (Yang et al., 2005). After DNA purification, PCRs containing α-[32P]dCTP (800 Ci/mmol) were performed for 24 cycles. Immunoprecipitated chromatin was analyzed first in pilot experiments to ensure that PCRs occurred in the linear range of amplification. The primers for amplifying the TGM-2 gene are: TGM-2-1: 5’-ACCTGAACAAACTGGCCGAG and TGM-2-2: 5’-CAGAGAAAGGCTCCAGGTTG; for PTHLH: PTHLH-1: 5’-TACAAAGAGCAGCCGCTCA and PTHLH-2: 5’-TTACCGTGAATCGAGCTCCAG; and for GAPDH: GAPDH-1: 5’-ACTGCCAACGTGTCAGTGGT and GAPDH-2: 5’-CATACCAGGAAATGAGCTTGAC.
3D culture and immunofluorescence staining of MCF10A cells in Matrigel (BD Biosciences)
Morphogenesis analysis of stable MCF10A vector control or PIP7S knockdown clones was performed as described (Debnath et al., 2003). 3D structures were harvested from the wells where they were grown after 12-16 days. Microscopy was performed on Zeiss LSM 510 Meta at the Berkeley Biological Imaging Facility. The localizations of the markers were viewed in serial confocal cross sections (x–y axis) through each colony. For the DRB treatment, the drug (from 100 mM stock solution in 40% DMSO and 60% DMEM) was added at the final concentration of 18 μM to the 3D cultures at day 4 and then replaced with fresh DRB on day 8.
S. pombe red-white colony assay for detecting La activity
S. pombe ySH9 cells containing the La-dependent suppressor tRNA allele, tRNASerUGA-C37:10 (Huang et al., 2006), were transformed to the ura4+ phenotype with pRep4X containing no or the indicated inserts. Transformants were streaked onto plates containing Edinburgh minimal media (EMM) supplemented with adenine (10 mg/L) and amino acids lacking leucine (Intine et al., 2000). Immunoblotting with anti-His6 antibody was performed as described (Intine et al., 2000).
Supplementary Material
Acknowledgements
We thank Wanichaya Ramey for technical assistance and JiaDe Yu for qRT-PCR analysis. Supported by grants from the National Institutes of Health (AI41757) and the Susan G. Komen Breast Cancer Foundation (BCTR71506) to Q.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chao SH, and Price DH (2001). Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem 276, 31793–31799. [DOI] [PubMed] [Google Scholar]
- Chen R, Yang Z, and Zhou Q (2004). Phosphorylated positive transcription elongation factor b (P-TEFb) is tagged for inhibition through association with 7SK snRNA. J Biol Chem 279, 4153–4160. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, and Brugge JS (2003). Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268. [DOI] [PubMed] [Google Scholar]
- Haaland RE, Herrmann CH, Rice AP (2005). siRNA depletion of 7SK snRNA induces apoptosis but does not affect expression of the HIV-1 LTR or P-TEFb-dependent cellular genes. J Cell Physiol 205, 463–470. [DOI] [PubMed] [Google Scholar]
- He N, Pezda AC, and Zhou Q (2006). Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Mol Cell Biol 26, 7068–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Bayfield MA, Intine RV, and Maraia RJ (2006). Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat Struct Mol Biol 13, 611–618. [DOI] [PubMed] [Google Scholar]
- Intine RV, Sakulich AL, Koduru SB, Huang Y, Pierstorff E, Goodier JL, Phan L, and Maraia RJ (2000). Control of transfer RNA maturation by phosphorylation of the human La antigen on serine 366. Mol Cell 6, 339–348. [DOI] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, and Ozato K (2005). The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 19, 523–534. [DOI] [PubMed] [Google Scholar]
- Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, Therien C, Bergeron D, Bourassa S, Greenblatt J, Chabot B, Poirier GG, Hughes TR, Blanchette M, Price DH, Coulombe B (2007). Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol Cell 27, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Price JP, Byers SA, Cheng D, Peng J, and Price DH (2005). Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing Cdk9 phosphorylated at threonine 186. J Biol Chem 280, 28819–28826. [DOI] [PubMed] [Google Scholar]
- Maraia RJ, and Bayfield MA (2006). The La protein-RNA complex surfaces. Mol Cell 21, 149–152. [DOI] [PubMed] [Google Scholar]
- Marshall NF, Peng J, Xie Z, Price DH (1996). Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem 271, 27176–27183. [DOI] [PubMed] [Google Scholar]
- Mehta K, Fok J, Miller FR, Koul D Sahin AA (2004). Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res 10, 8068–8076. [DOI] [PubMed] [Google Scholar]
- Michels AA, Nguyen VT, Fraldi A, Labas V, Edwards M, Bonnet F, Lania L, and Bensaude O (2003). MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol Cell Biol 23, 4859–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Sato F, Selaru FM, Olaru A, Perry K, Kimos MC, Tamura G, Matsubara N, Wang S, Xu Y, et al. (2002). Instabilotyping reveals unique mutational spectra in microsatellite-unstable gastric cancers. Cancer Res 62, 3641–3645. [PubMed] [Google Scholar]
- Nguyen VT, Kiss T, Michels AA, and Bensaude O (2001). 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322–325. [DOI] [PubMed] [Google Scholar]
- Peterlin BM, and Price DH (2006). Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23, 297–305. [DOI] [PubMed] [Google Scholar]
- Remillieux-Leschelle N, Santamaria P, and Randsholt NB (2002). Regulation of larval hematopoiesis in Drosophila melanogaster: a role for the multi sex combs gene. Genetics 162, 1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, and Schneider MD (2002). Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat Med 8, 1310–1317. [DOI] [PubMed] [Google Scholar]
- Shaw KR, Wrobel CN, and Brugge JS (2004). Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J Mammary Gland Biol Neoplasia 9, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim EY, Walker AK, Shi Y, and Blackwell TK (2002). CDK-9/cyclin T (P-TEFb) is required in two postinitiation pathways for transcription in the C. elegans embryo. Genes Dev 16, 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplova M, Yuan YR, Phan AT, Malinina L, Ilin S, Teplov A, and Patel DJ (2006). Structural basis for recognition and sequestration of UUU(OH) 3' temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol Cell 21, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli C, Fortino V, Capurro E, Sacchi G, Ponzo P, Pacini A, Muscettola M, Maioli E (2006). Role of PTHrp and PTHrp-engaged pathways in MCF-7 cells migration/invasion. Matrix Biol 25, 104–111. [DOI] [PubMed] [Google Scholar]
- Turano M, Napolitano G, Dulac C, Majello B, Bensaude O, and Lania L (2006). Increased HEXIM1 expression during erythroleukemia and neuroblastoma cell differentiation. J Cell Physiol 206, 603–610. [DOI] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, and Jacks T (2004). Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A 101, 10380–10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman DA, and Steitz JA (1991). Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol Cell Biol 11, 3432–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ (2004). RNA polymerase III transcription--a battleground for tumour suppressors and oncogenes. Eur J Cancer 40, 21–27. [DOI] [PubMed] [Google Scholar]
- Wolin SL, and Cedervall T (2002). The La protein. Annu Rev Biochem 71, 375–403. [DOI] [PubMed] [Google Scholar]
- Xue D, Rubinson DA, Pannone BK, Yoo CJ, and Wolin SL (2000). U snRNP assembly in yeast involves the La protein. EMBO J 19, 1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, and Zhou Q (2005). Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 19, 535–545. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhu Q, Luo K, and Zhou Q (2001). The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317–322. [DOI] [PubMed] [Google Scholar]
- Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, and Zhou Q (2003). Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell 12, 971–982. [DOI] [PubMed] [Google Scholar]
- Yik JH, Chen R, Pezda AC, and Zhou Q (2005). Compensatory contributions of HEXIM1 and HEXIM2 in maintaining the balance of active and inactive positive transcription elongation factor b complexes for control of transcription. J Biol Chem 280, 16368–16376. [DOI] [PubMed] [Google Scholar]
- Zhou Q, and Yik JH (2006). The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev 70, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews MB, Price DH (1997). Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev 11, 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.