Abstract
Objective:
To identify facilitators and barriers to implementation of a Clostridium difficile (C. difficile) screening intervention among bone marrow transplant (BMT) patients and to evaluate the clinical effectiveness of the intervention on the rate of hospital-onset C. difficile infection (HO-CDI).
Design:
Before-after trial.
Setting:
505-bed tertiary-care medical center.
Participants:
5357 patients admitted to the BMT and general medicine wards from January 2014 to February 2017. Interview participants included three physicians, four nurses, and four administrators.
Intervention:
BMT patients were screened within 48-hours of admission. Colonized patients, as defined by a C. difficile positive PCR stool result, were placed in contact precautions for the duration of their hospital stay.
Methods:
Interview responses were coded according to the Systems Engineering Initiative for Patient Safety conceptual framework. HO-CDI rates were compared pre- and post-intervention implementation, on BMT and general internal medicine units, by time-series analysis.
Results:
Stakeholder engagement, at both the person and organizational level, facilitates standardization and optimization of intervention protocols. While the screening intervention was generally well-received, tools and technology were sources of concern. The mean incidence of HO-CDI decreased on the BMT service post-intervention (p<0.0001). However, the effect of the change in the trend post-intervention was not significantly different on BMT compared to the control wards (p=0.93).
Conclusions:
This is the first mixed-methods study to evaluate a C. difficile screening intervention among the BMT population. The positive nature by which the intervention was received by front line clinical staff, laboratory staff, and administrators is promising for future implementation studies.
Keywords: Bone marrow transplant, Clostridium difficile, screening intervention, mixed methods
Introduction
Clostridium difficile infection (C. difficile; CDI), with its resultant diarrhea and colitis, is the most common healthcare-associated infection in the United States [1]. Main sources of healthcare C. difficile transmission include environmental contamination, healthcare workers’ hands, equipment, or apparel, and a reservoir of undetected colonized patients [2,3]. Bone marrow transplant (BMT) recipients are particularly prone to CDI because of their prolonged hospital stays, immune compromised status, chemotherapy-related mucosal damage, and high rates of antibiotic use [4,5]. The incidence of CDI among BMT patients ranges from 6% to 25% in recent studies [6]. Novel, safe, and effective interventions are essential to reducing healthcare-associated CDI in this vulnerable population.
Hospitals typically place patients with known C. difficile infection under contact precautions to reduce subsequent transmission events [7]. However, whole genome studies have shown that many CDI cases cannot be attributed to transmission from known cases [3]. Thus, focusing only on symptomatic patients fails to control for the major asymptomatic reservoir of C. difficile transmission. Screening for asymptomatic C. difficile is not recommended as a routine practice in current CDI prevention guidelines, because the impact of infection control interventions on asymptomatic patients with C. difficile is unknown [7]. However, in very vulnerable populations such as BMT patients, where interventions are urgently needed, identifying patients with asymptomatic colonization may be an important mechanism for reducing CDI. Screening for asymptomatic colonization is complex and labor intensive and may lead to undesirable consequences, such as unnecessary treatment. Therefore, assessment of the risks and benefits of such an intervention is essential.
Hospital-wide screening for asymptomatic C. difficile colonization at admission has previously been shown to reduce the rate of healthcare-associated CDIs by up to 56% [8,9]. Given the high rates of CDI among BMT patients and the promising results of existing studies, we implemented a screening program for BMT patients at our facility and evaluated the intervention’s feasibility and clinical effectiveness. We aimed to identify facilitators and barriers to intervention implementation and significantly reduce the rate of hospital-onset CDIs in the BMT population.
Methods
We conducted a mixed methods study of an asymptomatic C. difficile screening intervention of patients on the BMT service at the 505-bed, tertiary care, University of Wisconsin Hospital in Madison, Wisconsin. Between 180 and 200 bone marrow transplants are performed at the facility each year, of which roughly one-third are autologous and two-thirds allogenic. The BMT unit is part of a mixed ward that also includes hematology and oncology patients, however BMT patients are cared for in a separate wing of the ward. The study was considered quality improvement and was exempt from review by the university’s institutional review board.
Intervention
The screening intervention was implemented in December 2015 and is currently ongoing. We consider a 23-month pre-intervention period from January 1, 2014 to November 31, 2015 and a 14-month post-intervention period from January 1, 2016 to February 28, 2017. Data from December 2015, the intervention phase-in period, was excluded. All 793 patients admitted as an inpatient to the hospital’s BMT, hematology, and oncology ward under the BMT clinical service were included in the intervention group. All 4564 patients admitted as an inpatient to one of the hospital’s general internal medicine units were included in the control group, regardless of their clinical service.
Throughout the study, the presence of C. difficile was evaluated using a polymerase chain reaction (PCR) assay [GeneXpert (Cepheid, Sunnyvale, CA)] for the tcdB gene. For BMT patients screened in the post-intervention period, PCR analysis was conducted on a patient’s first stool collected within 48 hours of admission. Testing was done irrespective of whether the sample was formed, unformed, or watery. Patients who did not produce a stool sample within 48-hours were subsequently excluded from the study.
Patients identified with C. difficile colonization, as defined by a positive PCR result, were placed under contact precautions for the duration of their hospital stay. Hospital-wide policies for contact precautions included the use of gowns and gloves for all healthcare workers and visitors to the patient’s room and hand hygiene with soap and water. These policies were well established prior to initiation of the pre-intervention study period. No treatment was provided to asymptomatic patients and no changes were made to infection control protocols for symptomatic patients.
A new hospital-wide testing algorithm was introduced during the study time-period and ran concurrently with the screening intervention. The algorithm details that in the first 48-hours, patients with unexplained loose stools prior to admission should be placed in contact precautions and tested for C. difficile. After 48-hours, high-risk patients experiencing three or more stools than their baseline may be tested for C. difficile if there is no other potential known cause of diarrhea. Testing is limited to once every seven days.
Qualitative methodology
We used the Systems Engineering Initiative for Patient Safety (SEIPS) conceptual framework to evaluate the feasibility of the intervention [10]. The SEIPS model conceptualizes hospitals in terms of interactions between processes, outcomes, and five work system elements: person, task, technology and tools, environment, and organization. SEIPS has been widely used to evaluate infection control and other patient safety interventions, including implementation of C. difficile contact precautions [11]. The SEIPS model guided our development of interview questions and organization of the data.
We conducted thirteen semi-structured interviews to identify barriers and facilitators to the C. difficile screening intervention. Participants were selected by convenience sampling and included three attending physicians, four nurses, and four administrators selected from nursing, laboratory, and environmental services staff. Verbal informed consent was obtained from all interview participants before data was collected. Interview questions assessed participants’ perceptions of C. difficile risk, infection control policies, and intervention implementation.
Participants were interviewed individually, except for two environmental services administrators. All interviews were audio recorded and transcribed. Qualitative analysis was conducted using line-by-line structural coding [12]. Participant statements with supporting quotations were organized into key themes corresponding to the subcategories of the SEIPS conceptual framework [10].
Quantitative outcomes
The primary quantitative outcome was hospital-onset CDI (HO-CDI) per 10,000 patient days. HO-CDI was defined according to the Centers for Disease Control’s C. difficile reporting guidelines for LabID events [13], as a C. difficile positive diagnostic laboratory test result of a loose stool sample collected more than three days after facility admission. The HO-CDI rate was calculated from internal infection control data.
Secondary outcomes included length of stay and mortality rate, derived from administrative data extracted from our institution’s internal data warehouse, and oral vancomycin usage. These outcomes were selected, because C. difficile prolongs patients’ length of stay and causes mortality [14,15]. We sought to address both benefits and potential harms from this intervention. Oral vancomycin usage was selected as a secondary outcome, because it can disrupt the gastrointestinal microbiota, resulting in higher risks long-term of colonization by pathogenic organisms such as vancomycin-resistant enterococcus [16]. Oral vancomycin usage was calculated using internal pharmacy data as days of therapy per 1,000 patient days.
Statistical analyses
Pairwise comparisons between aggregate pre- and post-intervention measures were performed using the two-sample t-test. We conducted time series analyses to evaluate the effect of the screening intervention on the HO-CDI rate and oral vancomycin usage over time. We used the Prais-Winsten regression with robust standard errors estimated using the Huber/White variance estimator [17,18]. Prais-Winsten regression was utilized to account for first-order autocorrelation between monthly serial measurements. We considered statistical significance as a p-value of ≤ 0.05. All statistical analyses were conducted using Stata version 14 (Stata Corp, College Station, Texas, USA).
Results
Qualitative results
Interview results were organized into the five elements of the work system component of the SEIPS model: person, task, technology and tools, environment, and organization (Figure 1).
Figure 1: Conceptualization of the screening intervention using the SEIPS conceptual framework;
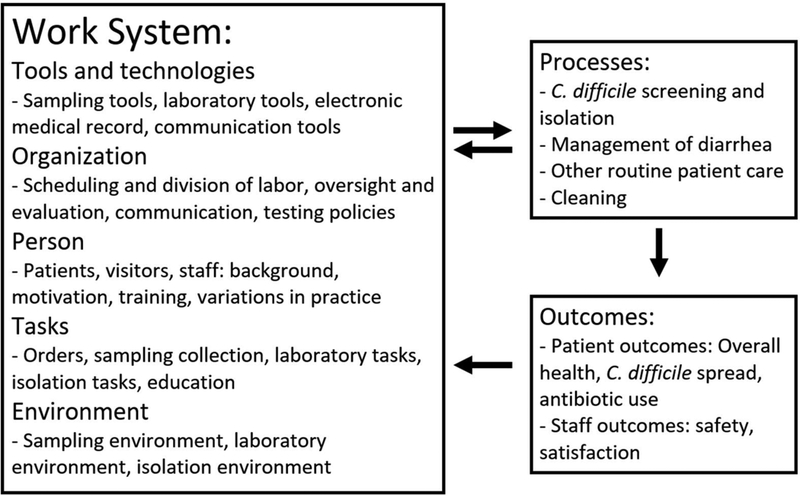
The overall SEIPS model, including the five conceptual divisions of the work system and the relation of processes and outcomes to the work system is adopted from reference [10].
Person
All participants identified CDI as a major concern, with transmission to patients considered a greater problem than transmission to clinical staff. Most study participants expressed support for the screening intervention, believing that it had reduced C. difficile transmission and improved patient health and worker safety. However, communicating screening and isolation policies to new hires or temporary staff was a barrier to success. It was particularly difficult to educate new team members during shift changes.
Patient engagement was a major facilitator to the intervention (Table 1, Quote 1). Some patients were informed of the screening procedure before their hospitalization, which made sample collection easier and more timely (Table 1, Quote 2). The C. difficile education component of the intervention was more difficult when conducted for the first time at admission (Table 1, Quote 3). Clinical staff reported that patient reactions to a positive screening result varied. Some felt dirty or blamed healthcare workers, straining patient-provider relationships. However, most patients were generally compliant (Table 1, Quote 4).
Table 1:
Representative participant quotes.
| Quote | Participant |
|---|---|
| 1. “Once [patients] understand [the reasons for isolation], they are usually very in tune with the enforcement of it. They will start watching people for breaks in policy” | Nursing staff |
| 2. “Some patients actually know that we are going to want this [stool] sample, so they wait to go to the bathroom until they come in. […] I would like it if before they [all patients] came in, they were told that this was going to happen.” | Nursing staff |
| 3. “When they get admitted they are bombarded with questions. I think they either do not remember the brief education on C. difficile, or there are times when they do not understand it. […] We just say we need to get a stool sample from you, like we say we need to get a urine sample. There is not any explanation, or not enough.” | Nursing staff |
| 4. “Every once in a while we have an occasional patient that kind of rebels against it, saying ‘I don’t want to do this, this is inconvenient.’ I would say that is very rare.” | Nursing staff |
| 5. “Visitors keep changing. That is another barrier to it. It is not like the same visitor is the only one that I have to educate. Every time there are new visitors, they have to be educated.” | Nursing staff |
| 6. “For the most part their visitors know that [the condition of BMT patients] is very serious, and they do not want to spread anything to anyone else in this area. I think their visitors are kind of different than other visitors.” | Nursing staff |
| 7. “They [the nurses] just thought it [stool collection] was onerous. […] I don’t think they really understood the purpose. Nobody likes to deal with stool.” | Physician |
| 8. “It is really annoying to have to leave the room, get something, go back, re-gown up, come in, and then have to leave the room again. […] I think it does affect the amount of time that you are in there.” | Nursing staff |
| 9. “We talk to the [physician] team every day about bowel movements. We also talk about it as nursing staff. [… If] there is a change, and they [the patient] start having diarrhea, then we would discuss with it the doctors.” | Nursing staff |
| 10. “It is a molecular test, so it costs more. It is also not cleared by the Food and Drug Administration for formed stool, which is what we are currently testing it on.” | Laboratory administrator |
| 11. “My biggest frustration about the whole contact isolation process is how we allow families to have so much stuff in their room. If you have a tray table full of stuff, it is hard to clean that tray table.” | Nursing practitioner |
| 12. “Even outside the foot pedal [problem], we are touching the soap dispensers. We need automatic soap dispensers, like in the airports, that they have everywhere.” | Physician |
| 13. “If a patient is in contact isolation and then they are placed in enhanced contact, infection control tells us we have to have both signs prominently displayed on the door. One sign says use alcohol gel, while one sign says use soap and water.” | Nursing staff |
| 14. “I kind of think that [retesting at admission] is a little overboard, if they were already negative. The patient is not going to go home, get C. difficile, and come right back.” | Nursing staff |
Visitors were perceived to exhibit the least compliance with contact precautions after a positive result to screening, with glove use especially difficult to enforce (Table 1, Quote 5). However, in general, visitors to BMT patients were thought to be more compliant with infection control policies than visitors of non-BMT patients (Table 1, Quote 6).
Tasks
Overall, most intervention related tasks were positively received. All physician and nurse participants reported that sample collection was straightforward, although one physician described initial pushback to the policy (Table 1, Quote 7). Sample collection was most difficult in the case of constipated patients. There was variability regarding who was responsible for placing the sample collection order, although this task was not considered burdensome. Multiple nursing participants felt that screening should be added to standard admission order sets.
The most time-consuming intervention-related task was the introduction of contact precautions for asymptomatic positive patients. Both soap and water and gown and glove use were perceived to require additional time to preformed correctly (Table 1, Quote 8). Some nurses also believed that contact precautions strain the patient-provider relationship.
Tools and Technologies
The electronic health record was vital to facilitating order placement, communication between clinical providers and laboratory staff, and reviewing screening results. It also issued reminders for providers to collect stool samples that had been ordered but not collected. However, the electronic health record did not distinguish between C. difficile screening tests and diagnostic tests ordered in the context of patient symptoms. Positive laboratory results prompted an automatic best practice alert that recommended initiating antibiotics, which required additional communication between staff to determine whether treatment was necessary (Table 1, Quote 9). Patients who screened positive were typically treated with oral vancomycin if any post-chemotherapy diarrhea developed. Because of the high rate of non-infectious diarrhea in this population, several physicians expressed concerns about the impact of the screening intervention on inappropriate vancomycin usage on the unit. However, advance knowledge of a C. difficile negative patient’s status was also credited with allowing faster symptomatic treatment with antimotility agents.
Increased stool processing was not reported to be a strain on laboratory facilities, as the additional burden from implementing the intervention on the BMT service alone was minimal. However, there is currently no standardized method for C. difficile testing on a formed stool. The laboratory administrator identified this lack of protocol and high cost as two barriers of implementing the screening intervention (Table 1, Quote 10).
Environment
The effect of C. difficile screening on daily cleaning practices was minimal, as all rooms in the hospital are already treated daily with sporicidal products as standard practice. The rooms of patients who screened positive, regardless of symptoms, were prioritized for ultraviolet light treatment as part of terminal cleaning upon patient discharge or room transfer.
The initiative of unit personnel to prepare a room for isolation prior to receiving an order facilitated the isolation process. However, once patients moved into a room, medical equipment and patient belongings made it became more difficult for staff to effectively clean (Table 1, Quote 11).
Sink location and accessibility were also reported concerns. Several participants were reluctant to clean their hands using sinks in patient rooms, out of respect for the patient or fear of contamination. The availability of pedal-operated sinks outside patient rooms is limited and automatic soap dispensers were non-existent (Table 1, Quote 12). A nursing staff member thought the signage on patient doors could be confusing and favored streamlining it (Table 1, Quote 13).
Organization
The hospital has prioritized clear communication between key intervention stakeholders. The screening status of patients is communicated between nurses in verbal and written sign-outs. Close communication between the BMT service and clinical laboratory enabled formed stools to be sent for C. difficile testing, despite hospital norms against this practice. Formed stools were required to be sent with a card explaining the screening nature of the sample. While participants reported that in practice these cards were not always included, no one was aware of an instance in which this had caused a significant problem with screening.
Nursing administration on the BMT unit provides oversight for the intervention and facilitates screening by monitoring order placement, sample collection, and the time limit on testing. A new C. difficile diagnostic testing algorithm was implemented hospital-wide and ran concurrently with the BMT screening intervention. This complicated screening ordering decisions, especially in the context of repeat testing of discharged patients who were rapidly readmitted to the service (Table 1, Quote 14).
Quantitative results
Before the intervention, 10.3% of BMT patients underwent diagnostic testing for C. difficile at the time of admission (Table 2). With the introduction of screening, the proportion of patients tested at admission rose to 74.5% (p<0.0001). During the study period, the rate of HO-CDI ranged from 107.0 to 0.0 per 10,000 patient days on the BMT service and 14.3 to 0.0 per 10,000 patient days on general medicine control unit (Figure 2). The mean incidence of HO-CDI dropped significantly on the BMT service post-intervention (p<0.0001; Table 3), while remaining unchanged on the control ward. However, time series analysis showed that the effect of the change in the trend after the start of the intervention was not significantly different in the BMT service compared to the control ward (p=0.93; Table 4).
Table 2:
Results of C. difficile testing among BMT patients
| Pre-intervention | Post-intervention | |
|---|---|---|
| Total months | 23 | 14 |
| Total admitted patients | 499 | 294 |
| All time total tests (screening and diagnostic) | 461 | 367 |
| Total tests at admission (screening and diagnostic) | 53 | 216 |
| Positive tests at admission, n (%) | 6 (11.3) | 32 (14.8) |
| Tests after 48-hours (diagnostic) | 408 | 151 |
| HO-CDI cases detected after 48-hours, n (%) | 41 (10.0) | 7 (4.6) |
HO-CDI: Hospital-onset Clostridium difficile infection
Figure 2:
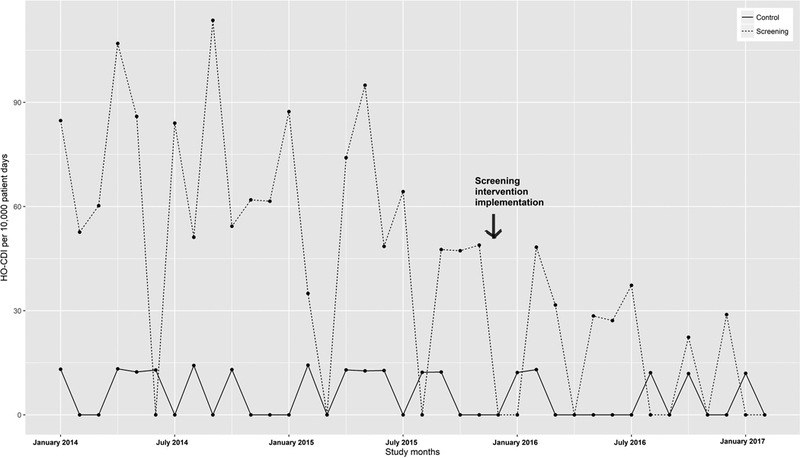
Hospital-onset CDI rates pre- and post-intervention.
Table 3:
Study characteristics and outcomes
| Variable | Bone marrow transplant service | General internal medicine control unit | ||||
|---|---|---|---|---|---|---|
| Pre-intervention, mean (SD) | Post-intervention, mean (SD) | p-value | Pre-intervention, mean (SD) | Post-intervention, mean (SD) | p-value | |
| Duration (months) | 23, Jan 2014 – Nov 2015 | 14, Jan 2016 – Feb 2017 | 23, Jan 2014 – Nov 2015 | 14, Jan 2016 – Feb 2017 | ||
| Hand hygiene compliance | 90.7% (n=2246) | 96.4% (n=1462) | <0.0001 | 98.5% (n=3790) | 98.6% (n=1313) | 0.17 |
| Length of stay, days | 15.0 (2.2) | 16.3 (1.9) | 0.07 | 5.4 (0.8) | 5.5 (0.8) | 0.77 |
| Case mix index1 | 5.14 (0.7) | 5.75 (0.7) | 0.01 | 1.50 (0.1) | 1.51 (0.1) | 0.66 |
| Admissions, per month | 22 (4) | 21 (4) | 0.64 | 121 (16) | 124 (10) | 0.50 |
| Patient days, per month | 311 (79) | 339 (69) | 0.27 | 509 (50) | 537 (58) | 0.16 |
| Mortality rate, percent | 4.7 (4.9) | 2.5 (3.4) | 0.12 | 2.3 (1.4) | 2.0 (1.2) | 0.42 |
| Total samples,2 per month | 20.0 (8.6) | 26.2 (5.0) | 0.01 | 18.9 (4.2) | 15.4 (3.0) | 0.005 |
| Samples at admission,2 per month | 2.3 (1.5) | 15.4 (3.1) | <0.0001 | 7.9 (2.5) | 6.0 (2.2) | 0.02 |
| HO-CDI rate, per 10,000 PD | 59.4 (31.1) | 16.0 (17.6) | <0.0001 | 6.80 (6.7) | 4.38 (6.1) | 0.27 |
| Oral vancomycin usage, DOT per 1,000 PD | 88.3 (54.1) | 147.3 (86.2) | 0.03 | 22.2 (12.6) | 27.1 (14.1) | 0.30 |
The case mix index reflects the complexity and resource needs of a given patient population, based on the average diagnosis-related group relative weight of population. Higher numbers reflect increased complexity and resource needs.
Includes tests ordered for both screening and diagnostic purposes. DOT: days of therapy; Feb: February; HO-CDI: hospital onset Clostridium difficile infection; Jan: January; Nov: November; PD: patient days; SD: standard deviation
Table 4:
Time series analysis for HO-CDI rates
| Factor | BMT service vs control | |
|---|---|---|
| Coefficient | p-value | |
| Intercept in control unit (HO-CDI per 10,000 patient days) | 7.29 | 0.004 |
| Slope in control unit pre-intervention (change in HO-CDI per 10,000 patient days per month) | −0.04 | 0.82 |
| Immediate effect in control unit at time of intervention (HO-CDI per 10,000 patient days) | −0.82 | 0.84 |
| Difference between pre- and post-intervention slopes in control unit (change in HO-CDI per 10,000 patient days per month) | −0.11 | 0.78 |
| Difference in intercept of transplant vs. control unit (HO-CDI per 10,000 patient days) | 69.45 | <0.001 |
| Difference in pre-intervention slope between transplant vs control units (change in HO-CDI per 10,000 patient days per month) | −1.42 | 0.048 |
| Difference in immediate effect at time of intervention between transplant vs. control units (HO-CDI per 10,000 patient days) | −11.83 | 0.37 |
| Difference between pre-and post-intervention slopes in the transplant vs. control unit, i.e. difference in differences of the slopes (change in HO-CDI per 10,000 patient days per month) | −0.11 | 0.93 |
BMT: Bone marrow transplant; HO-CDI: Hospital onset Clostridium difficile infection;
There was no significant change in length of stay and mortality rate on either unit after intervention implementation, despite a significant increase in the average case mix index on the BMT service. Average oral vancomycin usage increased on the BMT service in the post-intervention period (p = 0.03), with no significant change on the control ward (Figure 3, Table 3). However, as with HO-CDI rate, time series analysis showed that the effect of the change in the trend of vancomycin usage after the start of the intervention was not significantly different on the BMT service compared to the control ward (p=0.52; Table 5).
Figure 3:
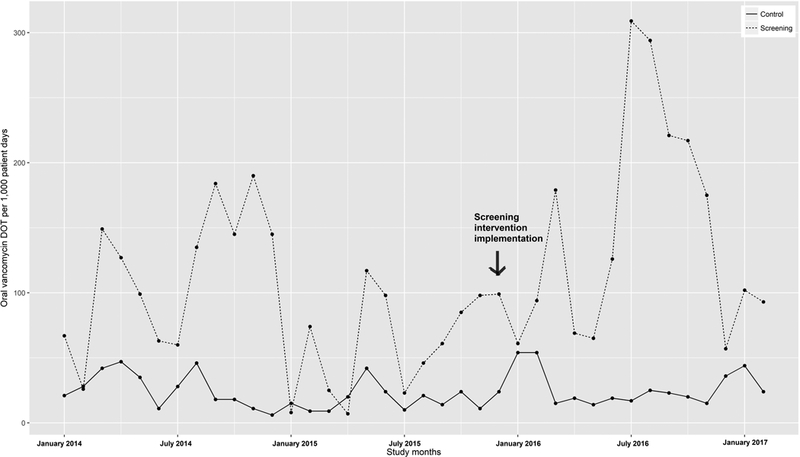
Oral vancomycin usage pre- and post-intervention.
Table 5:
Time series analysis for oral vancomycin usage on the BMT service
| Factor | BMT service vs control | |
|---|---|---|
| Coefficient | p-value | |
| Intercept in control unit (vancomycin DOT per 1,000 patient days) | 30.24 | <0.001 |
| Slope in control unit pre-intervention (change in vancomycin DOT per 1,000 patient days per month) | −0.71 | 0.19 |
| Immediate effect in control unit at time of intervention (vancomycin DOT per 1,000 patient days) | 23.73 | 0.17 |
| Difference between pre- and post-intervention slopes in control unit (change in vancomycin DOT per 1,000 patient days per month) | −0.43 | 0.80 |
| Difference in intercept of transplant vs. control unit (vancomycin DOT per 1,000 patient days) | 71.93 | 0.04 |
| Difference in pre-intervention slope between transplant vs control units (change in vancomycin DOT per 1,000 patient days per month) | −0.48 | 0.83 |
| Difference in immediate effect at time of intervention between transplant vs. control units (vancomycin DOT per 1,000 patient days) | 22.77 | 0.73 |
| Difference between pre-and post-intervention slopes in the transplant vs. control unit, i.e. difference in differences of the slopes (change in vancomycin DOT per 10,000 patient days per month) | 4.17 | 0.52 |
BMT: Bone marrow transplant; DOT: Days of therapy
Discussion
We found that stakeholder engagement, at both the person and organizational level, facilitates standardization and optimization of intervention protocols prior to the implementation of a larger, hospital-wide intervention. While the screening intervention was generally well-received, the tools and technology element of the work system was a source of concern. The implemented electronic medical record and ordering system did not differentiate between screening and diagnostic reasons for ordering a C. difficile test, which complicated subsequent follow-up of patient outcomes.
The implications of screening for future testing and empiric treatment of diarrhea recurred as major themes in these interviews. These are of crucial importance, because BMT patients are at high risk for developing chemotherapy-associated diarrhea [5]. While there was a post-intervention increase in vancomycin usage on the BMT unit, this occurred at a similar rate in our control unit. Thus, surveillance is unlikely to be related to changes in oral vancomycin usage, despite physician perceptions of increased overtreatment. In addition to vancomycin, BMT patients are frequently treated with other antibiotics that disrupt the gastrointestinal microbiome. This is especially problematic among colonized C. difficile patients, as it may predispose them to symptomatic CDI.
In our study, screening did not prohibitively burden the microbiology laboratory, nursing staff, or environmental services. It is unknown whether isolation of patients colonized by C. difficile reduces transmission [7], but given that isolation policies were generally well received, we believe that patients found positive on surveillance screening should be placed in contact precautions. The burden of a screening intervention is likely to be greater in hospital-wide interventions than on specific wards, especially among institutions that do not routinely utilize sporicidal products for daily disinfection.
Unlike the previous hospital-wide screening study [9], we did not find the intervention to be significantly associated with HO-CDI reduction in our time series analysis. The magnitude of HO-CDI reduction was similar between that and this study, when pre- and post-intervention mean estimates were compared. However, the baseline pre-intervention trends in HO-CDI rates at our institution were much larger. Thus, it is not unexpected that the difference between pre- and post-intervention reduction in our study was not statistically significant.
The decrease in HO-CDI in our study is due in part to a recategorization of cases previously defined as HO-CDI, rather than a total decline in overall CDIs. Screening at admission allowed for a subset of infections to be more appropriately labeled as community acquired or recurrent. Correctly identifying the source of C. difficile is essential and has implications for CDI epidemiology and prevention.
By design, the generalizability of this study is limited. We aimed to assess the impact of C. difficile screening at admission for high-risk BMT patients. Thus, findings may not be generalizable to a hospital-wide population.
Given the time-period of our study, we also did not account for seasonal effects in our analyses. Both C. difficile and antibiotic prescribing may be affected by seasonal variations, and it is possible that not accounting for these effects masks some of the reduction in HO-CDI due to the intervention. Future studies covering a longer time-period may benefit from accounting for seasonality in the analyses.
This study’s mixed-methods methodology offers a unique perspective on intervention feasibility and provides critical insight to infection control practitioners developing similar C. difficile screening interventions. The positive nature in which the intervention was received by front line clinical staff, laboratory staff, and administrators is promising for future implementation studies.
Acknowledgements
Financial support:
AKB received support as a pre-doctoral trainee from the National Institutes of Health [grant number TL1TR000429] administered by the University of Wisconsin, Madison Institute for Clinical and Translational Research, funded by the National Institutes of Health [grant number UL1TR000427]. NS is supported by a VA funded patient safety center of inquiry.
Footnotes
Conflict of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med 2015;372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 2010;38:S25–33. [DOI] [PubMed] [Google Scholar]
- 3.Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013;369:1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso CD, Treadway SB, Hanna DB, Huff CA, Neofytos D, Carroll KC, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2012;54:1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cózar-Llistó A, Ramos-Martinez A, Cobo J. Clostridium difficile Infection in Special High-Risk Populations. Infect Dis Ther 2016;5:253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balletto E, Mikulska M. Bacterial Infections in Hematopoietic Stem Cell Transplant Recipients. Mediterr J Hematol Infect Dis 2015;7:e2015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubberke ER, Carling P, Carrico R, Donskey CJ, Loo VG, McDonald LC, et al. Strategies to Prevent Clostridium difficile Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol 2014;35:S48–65. [DOI] [PubMed] [Google Scholar]
- 8.Lanzas C, Dubberke ER. Effectiveness of Screening Hospital Admissions to Detect Asymptomatic Carriers of Clostridium difficile: A Modeling Evaluation. Infect Control Hosp Epidemiol 2014;35:1043–50. [DOI] [PubMed] [Google Scholar]
- 9.Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, et al. Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections: A Quasi-Experimental Controlled Study. JAMA Intern Med 2016;176:796–804. [DOI] [PubMed] [Google Scholar]
- 10.Carayon P, Hundt AS, Karsh B, Gurses AP, Alvarado CJ, Smith M, et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care 2006;15:i50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanke E, Zellmer C, Van Hoof S, Moriarty H, Carayon P, Safdar N. Understanding the Current State of Infection Prevention to Prevent Clostridium difficile Infection: A Human Factors and Systems Engineering Approach. Am J Infect Control 2015;43:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles M, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: Sage Publishing, Inc.; 2014. [Google Scholar]
- 13.Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. Centers for Disease Control website. https://www.cdc.gov/nhsn/pdfs/pscmanual/12pscmdro_cdadcurrent.pdf. Published 2017. Accessed April 18, 2017.
- 14.Stevens VW, Khader K, Nelson RE, Jones M, Rubin MA, Brown KA, et al. Excess Length of Stay Attributable to Clostridium difficile Infection (CDI) in the Acute Care Setting: A Multistate Model. Infect Control Hosp Epidemiol 2015;36:1024–30. [DOI] [PubMed] [Google Scholar]
- 15.Hota SS, Achonu C, Crowcroft NS, Harvey BJ, Lauwers A, and Gardam MA. Determining Mortality Rates Attributable to Clostridium difficile Infection. Emerg Infect Dis 2012;18:305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, et al. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection With Oral Vancomycin Compared With Metronidazole. J Infect Dis 2015;212:1656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White H A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980;48:817–38. [Google Scholar]
- 18. Huber P The behavior of maximum likelihood estimates under nonstandard conditions In: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1:Statistics; Berkeley, CA: University of California Press; 1967:221–233. [Google Scholar]


