Abstract
Locoregional recurrence negatively impacts both long-term survival and quality of life for a number of malignancies. For appropriate-risk patients with an isolated, resectable local recurrence, surgery represents the only potentially curative therapy. However, oncologic outcomes remain inferior for patients with locally recurrent disease even after macroscopically complete resection. Unfortunately, these operations are often extensive with significant perioperative morbidity and mortality. This review highlights selected malignancies (mesothelioma, sarcoma, lung cancer, breast cancer, rectal cancer, peritoneal surface malignancies) in which surgical resection is a key treatment modality and where local recurrence plays a significant role in overall oncologic outcome with regards to survival and quality of life. For each type of cancer, the current, state-of-the-art treatment strategies and their outcomes are assessed. The need for additional therapeutic options is presented given the limitations of the current standard therapies. New and emerging treatment modalities, including polymer films and nanoparticles, are highlighted as potential future solutions for both prevention and treatment of locally recurrent cancers. Finally, we identify additional clinical and research opportunities, and propose future research strategies based on the varying patterns of local recurrence among the different cancers.
Keywords: local recurrence, mesothelioma, sarcoma, lung cancer, breast cancer, rectal cancer, peritoneal surface malignancies, drug delivery, polymers, nanoparticles
Introduction
In 2018, there will be a projected 1.7 million new cancer cases and approximately 600,000 cancer deaths in the United States.1 Advances in surgery, chemotherapy, radiotherapy, and newer modalities such as molecularly targeted therapies and immunotherapy, have significantly reduced morbidity and improved survival over the past decades. Despite this progress, cancer remains the second leading cause of death in the United States, after cardiovascular disease.2 Most of the recent attention in antineoplastic therapy focuses on systemic treatments for metastatic disease, a point at which long-term cure is rarely possible. To reliably cure cancer, local control is essential. However, locoregional recurrence (LRR) is a clinically relevant, predominant pattern of failure in many malignancies. This review describes current therapeutic strategies to both prevent and treat local cancer recurrence in six selected malignancies: rectal cancer, breast cancer, mesothelioma, non-small cell lung cancer, retroperitoneal sarcoma, and peritoneal surface malignancies (Figure 1, Table 1). Local recurrence after surgical resection plays a major role in decreased survival and quality of life for patients with these cancers. Furthermore, these malignancies represent a range of important oncologic principles to consider when assessing present and future therapies to treat and prevent local recurrences. Patterns and risk factors for locoregional recurrence are highlighted. Finally, opportunities for clinical improvement are identified, and novel therapeutic strategies in preclinical development are discussed, specifically in the context of these six malignancies, but potentially applicable broadly.
Figure 1.
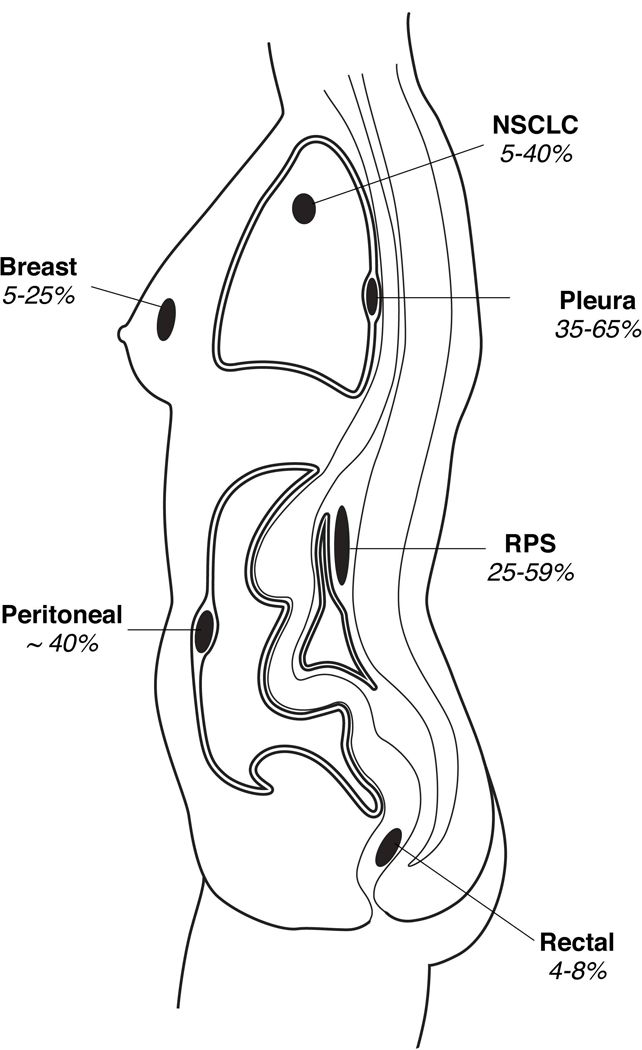
A Summary of Local Recurrence Rates for Various Common Malignancies After Surgery. 3D indicates 3-dimensional; n/a, not applicable.
Table 1.
Currently available and experimental therapies to reduce risk of local recurrence and treat locally recurrent cancer.
| Cancer | Treatments to Prevent Local Recurrence |
Treatment Options After Local Recurrence |
Experimental/Investigational (Phase, if applicable) |
|---|---|---|---|
| Rectal Cancer | •Surgery •Neoadjuvant •chemotherapy (FOLFOX, FOLFIRI) •Neoadjuvant radiotherapy |
•Chemotherapy •(FOLFOX, FOLFIRI) •Surgery •Radiotherapy |
•Intraoperative radiation therapy •Bevacizumab (II/III) •Cetuximab (II) •Trans-anal excision (III) •Immunotherapy (I/II) •Carbon ion radiation therapy •Aspirin (II), Celecoxib (II) |
| Breast Cancer | •Surgery (mastectomy, •lumpectomy) •Chemotherapy •Radiotherapy •Hormonal therapy •Herceptin |
•Surgery (mastectomy, •radical resection) •Chemotherapy •Radiotherapy •Hormonal therapy •Herceptin |
•TKI (III) •Nab-paclitaxel (III) •mTOR inhibitor (III) •Palbociclib (III) •NeuVax vaccine (II) •Cryoablation •Intraoperative radiotherapy •High-intensity focused •ultrasound |
| Mesothelioma | •Surgery •Chemotherapy (cisplatin, •pemetrexed, gemcitabine) •Radiotherapy |
•Surgery •Chemotherapy •Radiotherapy |
•Immunotherapy (II) •Photodynamic therapy •Intensity-modulated radiotherapy •(I/II) •Heated intraoperative •chemotherapy (II) |
| Non-Small Cell Lung Cancer |
•Surgery (lobectomy, •segmentectomy, wedge) •Chemotherapy •SBRT •Bevacizumab |
•Surgical re-resection •Chemotherapy •Radiotherapy •Nab-paclitaxel |
•ALK inhibitors (III) •Immunotherapy (III) •TKI (II/III) •Brachytherapy (III) |
| Retroperitoneal Sarcoma |
•Surgery •Chemotherapy •(doxorubicin, ifosfamide) •Radiotherapy |
•Surgery •Radiotherapy •Chemotherapy |
•Proton therapy (I/II) •Intraoperative radiotherapy (I/II) •Vaccine therapy (I) |
| Peritoneal Surface Malignancies |
•Cytoreductive surgery •HIPEC •Chemotherapy |
•Surgery •Chemotherapy •Bevacizumab •PARP inhibitor |
•Vaccine therapy (II) •Immunotherapy (II) •mTOR inhibitor (II) •Intraperitoneal NK cells (I) |
FOLFOX, leucovorin, 5-fluorouracil, oxaliplatin; FOLFIRI, leucovorin, 5-fluorouracil, irinotecan; TKI, tyrosine kinase inhibitor; Nab, nanoparticle-albumin bound; mTOR, mammalian target of rapamycin; SBRT, stereotactic body radiation therapy; ALK, anaplastic lymphoma kinase; HIPEC, hyperthermic intraperitoneal chemotherapy; PARP, poly (ADP-ribose) polymerase
Rectal Cancer
Approximately 43,000 cases of rectal cancer are diagnosed in the United States annually.1 Advances in surgical techniques and neoadjuvant/adjuvant therapies have significantly decreased LRR rates. Half of rectal cancer recurrences are locoregional (anastomotic, extravisceral pelvic, nodal) without distant metastatic disease.3 Large surgical series from the 1980’s reported LRR rates ranging from 10–32%,4 with approximately one-third of patients being candidates for re-resection. While patients undergoing re-operation achieved a 5-year overall survival (OS) of 18–49%, 3–8% died perioperatively. Non-surgical candidates fared worse with 5-year OS under 5%.5 Risk factors for local recurrence include anastomotic leak, non-R0 resection, intraoperative tumor perforation, high-grade pathology, and lack of adjuvant therapies.6 Local recurrence in rectal cancer is associated with lifestyle-altering symptoms such as rectal bleeding, bowel obstruction, chronic pain, fistulas, malodorous tumor discharge, tenesmus, and pelvic sepsis.7
The two main strategies to reduce LRR in rectal cancer are more extensive surgery and neoadjuvant (chemo)radiation (Table 2). In Japan, the standard abdominoperineal resection includes a wide perineal skin incision and resection of the levator ani muscle and ischiorectal adipose tissue, with additional dissection of the obturator and iliac lymph nodes when stage II/III disease is suspected. In a randomized trial enrolling 701 patients, the LRR rate after this more extensive surgery was 7.4% (versus 12.6% with mesorectal excision alone, p=0.024).8 More extensive surgery resulted in less presacral LRR; however, up to 96% of patients developed some degree of sexual dysfunction.9 In contrast, total mesorectal excision (TME), the standard surgical approach in Western countries, employs a dissection between the parietal and visceral endopelvic fascial layers. TME preserves the pelvic autonomic nerves, with lower rates of sexual dysfunction, ranging from 19% to 69%.10
Table 2.
Local recurrence rates in rectal cancer associated with either more extensive surgery or after neoadjuvant radiotherapy, based on randomized trials data
| Clinical Trial | Treatment Group (number of patients) |
Local Recurrence Rate |
p-value |
|---|---|---|---|
| Japanese Trial8 | Mesorectal excision alone (350) | 17.6% (5-year) | p=0.024 |
| Mesorectal excision plus lateral lymph node dissection (351) | 9.8% (5-year) | ||
| Dutch TME Trial11 | TME alone (701) | 11.0% (5-year) | p<0.001 |
| Preoperative radiotherapy plus TME (713) | 4.6% (5-year) | ||
| MRC CR07 Trial12 | TME with selective postoperative chemoradiotherapy (676) | 10.6% (3-year) | p<0.0001 |
| Preoperative radiotherapy plus TME (674) | 4.4% (3-year) |
TME, total mesorectal excision
The use of neoadjuvant (chemo)radiation therapy is generally considered standard for stage II and III rectal cancers following two large randomized controlled trials, the Dutch TME study11 and the MRC CR07 trial.12 The Dutch trial randomized 1,417 patients to either receive preoperative radiotherapy and TME or TME alone. The 5-year LRR rates were 4.6% in the combined therapy group and 11.0% in the surgery alone group (p<0.001), with the largest reduction noted in anastomotic recurrence rates (0.7% versus 2.7%, p=0.003).11 The CR07 trial randomized 1,350 patients with resectable rectal cancers to receive either preoperative radiotherapy or selective postoperative chemoradiation with intravenous (IV) 5-fluorouracil. Preoperative radiotherapy significantly lowered 3-year LRR compared to selective adjuvant therapy (4.4% versus 10.6%, p<0.0001) and improved disease-free survival (DFS), but did not impact OS, thus demonstrating an important role for additional local therapy in the prevention of locoregional recurrence.12
However, neoadjuvant chemoradiation significantly increases morbidity. To reduce this risk, patients undergoing a low anterior resection after preoperative radiotherapy often receive a proximal diverting ileostomy for anastomotic protection.13 In a randomized trial of 166 patients, diversion was associated with significantly lower anastomotic leak rates (6.4% vs. 16.7%, p=0.0443) and lower re-operation rates for leaks (2.1% vs. 15.2%, p=0.0217).14 However, diverting ileostomies can have their own complications including readmission for dehydration, stoma prolapse, small bowel obstruction,15 and patients need a second operation to reverse the ostomy. Furthermore, pelvic radiotherapy independently adds significant morbidity, with a meta-analysis on long-term function showing significantly higher rates of stool incontinence (risk ratio 1.67, p<0.0001).16 In the Dutch trial,11 patients receiving preoperative radiotherapy experienced significantly more long-term sexual and bowel dysfunction.17,18
More recently, a phase II clinical trial of 32 patients with stage II to III rectal cancer evaluated selective use of neoadjuvant chemoradiation for patients who do not respond to chemotherapy alone.19 Thirty of the patients (94%) who received neoadjuvant FOLFOX/bevacizumab (6 cycles) achieved a radiographic response and proceeded to TME without radiotherapy. The 4-year local recurrence rate was 0% and the 4-year DFS was 84%. A multicenter, randomized phase III trial (PROSPECT) is currently recruiting to further explore this potential therapeutic option.
An alternative strategy for patients with locally advanced rectal cancers is the use of intraoperative radiotherapy (IORT). While two randomized trials showed no LRR or OS benefit with addition of IORT,20,21 a pooled retrospective analysis of over 600 patients reported that IORT significantly reduced LRR rates in patients with margin-positive resections.22 Complication rates with IORT range from 5% to 60%, including wound complications in up to 40% of patients and gastrointestinal fistulae and ureteral injury in 2–12%.23
Despite the notable advances in surgical and adjuvant therapy over the past few decades, LRR rates for rectal cancers remain between 4% and 8%, and the more extensive surgery and adjuvant therapies all come with increased patient morbidity. Without treatment, patients with LRR suffer a shortened life expectancy and often a poor quality of life.24 Chemoradiation therapy alone extends OS to 12–15 months, but it is not curative.25 Approximately 40% of patients with LRR are candidates for surgery with curative-intent, though surgery can be challenging.26 Although a macroscopically complete resection with negative margins (R0) is the goal, a macroscopically complete resection with positive margins (R1) or a macroscopically incomplete resection (R2) may be inevitable, with survival consequences. A review of 583 patients with locally recurrent rectal cancer who underwent surgery (approximately 60% R0) reported 5-year OS of 44%, 26%, and 10% for R0, R1, and R2 resections, respectively.27 Furthermore, anastomotic recurrences, for which re-resection is more straightforward, are less common in the era of neoadjuvant therapy. Thus, LRR often requires extensive pelvic exenterations, with lower rates of success.27
One newer area of active investigation for this disease is immunotherapy. Programmed death-ligand 1 (PD-L1) expression is upregulated in rectal adenocarcinomas treated with chemoradiation. Thus, checkpoint inhibitor immunotherapy may impact outcomes and redefine the role for surgery in the future.28
Breast Cancer
Breast cancer is the second leading cause of cancer mortality in females, accounting for 40,920 deaths in 2018.1 The National Surgical Adjuvant Breast and Bowel Project (NSABP) conducted randomized controlled trials in the 1970’s and 1980’s that drastically changed surgical management. The NSABP B-04 trial randomized 1,665 women to receive a radical mastectomy, a total mastectomy alone (removal of all breast tissue with limited axillary lymphadenectomy), or total mastectomy plus irradiation. The 25-year follow-up results from this trial showed no significant difference in DFS or OS among the three groups.29 Subsequently, NSABP B-06 randomized 1,851 women with stage I/II breast tumors to total mastectomy, partial mastectomy, or partial mastectomy plus breast irradiation. With 20 years of follow-up, there was no difference in OS or DFS.30 However, only 10% of women who underwent mastectomy developed LRR (chest wall), compared to 39% after partial mastectomy alone and 14% partial mastectomy with postoperative radiotherapy (p<0.001).30 Together, these studies have confirmed that less extensive surgery with appropriate use of local therapy, in this case radiotherapy, results in equal survival outcomes as more radical surgery, though the LRR rate may be higher.
The extent of axillary surgery for breast cancer also changed. NSABP B-32 randomized 5,611 women undergoing surgery for invasive breast cancer and clinically negative axillary lymph nodes to axillary lymph node dissection (ALND) or sentinel lymph node (SLN) biopsy, with completion axillary dissection performed in patients found to have a positive SLN.31 There was no difference in LRR, OS, or DFS. Based on this study, SLN biopsy replaced routine ALND for women with clinically node-negative breast cancer. More recently, the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial randomized 891 women with T1-T2 invasive breast cancers, clinically negative nodes, and 1–2 positive SLNs to either undergo completion axillary dissection or no further surgery. There was no difference in 5-year OS, DFS, or LRR rates, thereby further limiting indications for completion ALND.32
Local recurrence patterns differ based on initial surgical approach and may involve chest wall, residual breast after breast conservation therapy (BCT), or lymph nodes. Not surprisingly, management of LRR depends on initial therapy. Neoadjuvant chemotherapy is often used to enable BCT (partial mastectomy followed by whole-breast irradiation).33 The two most significant risk factors for LRR after BCT are lack of local radiotherapy and positive margins.34 Other risk factors include younger patient age, large tumor size, high grade, and absence of hormone receptors.35 A meta-analysis of 17 randomized trials including 10,801 women reported a 10-year LRR of 25% in those not receiving radiotherapy and 8% in those that did receive radiotherapy.36 Recurrences were noted later after BCT than after mastectomy (median 3–4 years versus 2–3 years).37 The increased use of partial breast irradiation,38 induction chemotherapy,33 and SLN biopsy may impact the future rate and management of LRR.
For women who develop an isolated LRR within the breast after BCT, the current standard of care is further surgery, generally a mastectomy. The 5- and 10-year OS rates after salvage mastectomy of a locally recurrent breast cancer are 84% and 71%, respectively.39 LRR within the breast parenchyma is associated with better survival than extramammary LRR (hazard ratio [HR] 2.58 versus 5.85; 5-year OS 60% versus 24%).35 The 3-year OS is worse for women with a shorter LRR disease-free interval (<2 years; 61.9% vs. 89.3%, 95% CI for HR 0.435–0.668).35 A prospective trial of 39 patients who underwent a repeat partial mastectomy with interstitial brachytherapy reported a 5-year LRR rate of 7% and 5- and 10-year OS of 87% and 77%, respectively.40 In a randomized trial of 162 women undergoing surgery for locally recurrent breast cancer, those receiving adjuvant chemotherapy had significantly better 5-year DFS than those who did not (69% vs 57%, p=0.046).41
Regional nodal recurrences after BCT are less common than isolated intramammary LRR (1–6% of patients), thus arguing that approaches to sterilize the local tumor bed and intramammary tissue could be improved.35,42 Surgery for regional nodal recurrences in the absence of distant metastatic disease may improve survival.42 Among 165 patients with extramammary LRR enrolled in NSABP trials, recurrences were confined to the axillary in 47 patients, supraclavicular nodes in 81, chest wall or scar in 20, or multiple or other sites in 17.35 Those with an isolated axillary nodal LRR had better 5-year DFS than other extramammary LRR (31.5% versus 12.1%; p>0.05).
The 10-year LRR risk is lower after mastectomy than BCT (8–12% vs. 15–20%).35,43,44 Post-mastectomy LRRs are usually confined to the chest wall and diagnosed on physical exam. The remainder present with local and regional nodal recurrence, most commonly supraclavicular nodes.45 Approximately two-thirds of these LRR are isolated without evidence of synchronous distant metastases,46 highlighting the potential importance of locoregional treatment strategies. The two major risk factors for post-mastectomy LRR are primary tumor size greater than 4 cm and 4 or more positive axillary lymph nodes.45 Other risk factors include younger age, negative hormone receptor status, and lymphovascular invasion (Table 3).46 In a meta-analysis of 1,314 women with positive axillary lymph nodes, adjuvant radiotherapy to the chest wall and axilla reduced overall recurrence (RR 0.68; p=0.00006) and breast cancer mortality (RR 0.80; p=0.01).47 Five-year OS varies based on the site of the post-mastectomy LRR after: chest wall (52%), axilla (50%), supraclavicular nodes (28%), chest wall and axilla (28%), and supraclavicular nodes and chest wall or axilla (7%).48 When a second surgery is required, the cosmetic outcomes and quality of life are often reduced.
Table 3.
Major significant risk factors for developing locoregional recurrence in breast cancer patients
| Primary Risk Factors for Locoregional Recurrence in Breast Cancer |
|---|
| Primary tumor size over 4 cm |
| 4 or more positive axillary lymph nodes |
| Lack of radiotherapy treatment |
| Younger age |
| Negative hormone receptor status |
| Lymphovascular invasion |
Patients with isolated chest wall recurrences should be evaluated for resection, which are often quite extensive and require complex reconstruction to achieve local control. Approximately 20% experience significant 30-day postoperative morbidity and 5-year OS is 40.8%.49 In a randomized trial of 165 patients with post-mastectomy LRR, addition of tamoxifen to surgery and radiotherapy resulted in significantly lower 5-year LRR (10% vs. 28%, p<0.001), but similar 5-year OS (74% vs. 76%).50 There is no consensus on the management of the axillary nodes in the setting of an isolated LRR within the breast or chest wall in patients who previously underwent SLN biopsy; management should be discussed in a multidisciplinary setting. Patients with axillary nodal LRR who previously underwent SLN biopsy should undergo completion ALND with or without axillary radiotherapy. The role of systemic therapy for LRR has not been the subject of large trials; however, many clinicians treat estrogen-receptor positive disease with tamoxifen, HER2-positive disease with trastuzumab, and hormone receptor negative disease with systemic chemotherapy.51
Malignant Pleural Mesothelioma
Malignant pleural mesothelioma (MPM) is a primary pleural malignancy arising from mesothelial cells, mostly related to asbestos exposure. In the United States, there are about 3,000 new cases diagnosed each year52 with a median OS of only 9–12 months with supportive care alone.53 There are three major histologic subtypes of MPM, with distinctly different outcomes: epithelioid (median OS 20 months), biphasic or mixed (median OS 13 months), and sarcomatoid (median OS 8 months) (HR 1.70; p<0.0001).54 Other adverse risk factors include lymph node involvement, increased patient age, and non-curative surgery.54 The high rate of LRR in MPM is due to its preponderance for diffuse involvement of the serosal surfaces as well as the difficulty in achieving a R0 resection. Morbidity and mortality from MPM is due to LRR, commonly resulting in respiratory compromise, dysphagia, and superior vena cava compression.
The surgical options for MPM are extrapleural pneumonectomy (EPP) and pleurectomy/ decortication (P/D). EPP involves en bloc resection of the lung, parietal and visceral pleura, pericardium, and diaphragm, whereas P/D consists of parietal and visceral pleurectomy and resection of diaphragm and/or pericardium if tumor involvement is noted intraoperatively.55 P/D is associated with lower perioperative morbidity, similar survival rates, but significantly higher rates of LRR (65% vs. 33%).56 Mortality rates are 5–10% for EPP and 3–6% for P/D. Major complications include diaphragm patch dehiscence, bronchopleural fistula, prolonged air leak, chylothorax, and empyema, with over 10% requiring reoperation.57
Multiple prospective trials have evaluated heated intraoperative chemotherapy (HIOC). In a phase I/II trial of 44 patients treated with P/D and HIOC (high-dose cisplatin), median OS and DFS were 18 months and 9 months, respectively, but 54% of patients still experienced LRR.58 In a phase II trial of 92 patients treated with EPP and HIOC with cisplatin, sodium thiosulfate, and amifostine, the LRR rate was 17%.59 However, HIOC increased rates of diaphragmatic patch failure, deep venous thrombosis, atrial fibrillation, and acute respiratory distress syndrome .60
Several other less successful intraoperative/perioperative adjuvant therapies have also been evaluated, including heated povidone-iodine (PVP-I), intensity-modulated radiation therapy (IMRT), and photodynamic therapy. The largest reported cohort investigating PVP-I included 102 patients who underwent P/D, hyperthermic PVP-I, chest wall radiotherapy, and systemic chemotherapy, with a median OS of 32 months, overall recurrence rate of 74.5%, and LRR rate of 90%.61 IMRT plus EPP yielded a 1-year LRR rate of 12%; however, the 1-year OS was only 55%, and 6% of patients experienced fatal (grade 5) pulmonary toxicity.62 Lastly, photodynamic therapy is a light-based adjuvant therapy which produces reactive oxygen singlets.63 A 73 patient trial with epithelioid histology receiving photodynamic therapy during P/D reported median OS and DFS of 3 years and 1.2 years, respectively. However, 74% of patients experienced local (+/− distant) recurrence, demonstrating a long interval between disease recurrence and death.64
Given the high rates of recurrence, systemic chemotherapy is often employed as part of multimodality treatment regimens for MPM. A phase III trial randomized 456 patients with unresectable MPM to receive single-agent cisplatin or cisplatin plus pemetrexed. The addition of pemetrexed improved response rates (41% vs. 17%, p<0.0001), median progression-free survival (PFS; 5.7 months vs. 3.9 months, p=0.001) and median OS (12.1 months vs. 9.3 months, p=0.020).65 Another phase III trial of 448 patients randomized to receive cisplatin and pemetrexed with or without bevacizumab showed that the addition of bevacizumab also increased PFS (9.2 vs. 7.3 months, p<0.0001) and OS (18.8 vs. 16.1 months, p=0.017).66 Outcomes of the various treatment options are summarized in Table 4.
Table 4.
Outcomes associated with various treatment options for patients with locally recurrent malignant pleural mesothelioma
| Treatment | Locoregional Recurrence |
Survival | Complications |
|---|---|---|---|
| Supportive Care | n/a | Median OS 9–12 mo | n/a |
| Extrapleural Pneumonectomy | 33–42% (site of first recurrence) | Median OS 12–32 mo | 5–10% perioperative mortality |
| Pleurectomy/Decortication | 53–65% (site of first recurrence) | Median OS 12–26 mo | 3–6% perioperative mortality |
| Heated Intraoperative Chemotherapy | 17–54% (site of first recurrence) | Median OS 12–26 mo | Increased rates of diaphragmatic patch failure, DVT, ARDS, and atrial fibrillation |
| Systemic Chemotherapy | Median PFS 5.7–10 mo | Median OS 12.1–29.1 mo (if extrapleural penumonectomy + radiotherapy completed) | 27.2% grade III or higher complications |
| Heated Povidone-Iodine | Median PFS 12 mo (89.5% local-first recurrence) | Median OS 32 mo (with pneumonectomy/decortication, radiation therapy, and adjuvant chemo) | Persistent air leak, chylothorax, pneumonia, empyema, ARDS |
| Intensity Modulated Radiation Therapy | 12% at 1 year | 1-year OS 55% | 6% fatal pulmonary toxicity |
| Photodynamic Therapy | Median PFS 9.6 mo | Median OS 15–31.7 mo (with surgery) | Skin burning, pain, cellulitis |
OS, overall survival; PFS progression free survival; DVT, deep venous thrombosis; ARDS, acute respiratory distress syndrome
Although LRR remains the predominant pattern of failure for MPM patients, there is no standardized treatment approach, with both local and systemic approaches utilized. Isolated chest wall recurrences may be resected, with one series demonstrating median OS was 20.4 months for patients with recurrent epithelioid histology and 7.4 months for those with mixed histology.67 Palliative radiotherapy provides short-term symptomatic relief for approximately 4 months.68 A recent series of 24 patients with recurrent MPM undergoing 110 cryoablation treatments reported freedom from local recurrence of 91% at 1 year and 74% at 3 years.69 Systemic chemotherapy should be considered, particularly for patients with synchronous local and distant recurrence. For recurrent MPM with a malignant pleural effusion, talc pleurodesis and pleural catheter placement are viable palliative options without anti-neoplastic effects. Immunotherapy agents such as pembrolizumab70 and nivolumab/ipilimumab71 reported disease control rates of 72% and 52% in phase I/II trials, respectively; larger trials are needed to further evaluate efficacy.
Non-Small Cell Lung Cancer
Lung cancer is by far the leading cause of cancer death in the United States, accounting for an estimated 154,050 deaths in 2018.1 Lobar resection is the standard of care for stage I, II, and select III non-small cell lung cancers (NSCLC). However, many patients present with poor baseline pulmonary function, limiting standard treatment options to sublobar resections or radiotherapy for early stage cancers. The oncologic superiority of lobectomy to smaller, non-anatomic resections for NSCLC is supported by multiple studies (Table 5). An early trial randomized 247 patients to lobectomy or limited resection (segmentectomy or wedge resection) demonstrated a tripling of LRR in limited resections (p=0.008) with a trend towards lower OS.72 A trial with 975 resectable NSCLC patients reported that sublobar resection, stage >IA, squamous or large cell histology, and presence of lymphovascular invasion were independently associated with higher rates of LRR on multivariate analysis.73 Even negative margins after wedge resections, if less than a centimeter, are associated with increased LRR and worse OS rates.74 Sublobar resections are associated with higher rates of positive margins (4.0% in wedge, 2.1% in segmentectomy, 1.4% in lobectomy, p<0.001), inadequate nodal evaluation (fewer than 4 lymph nodes), and decreased median OS (67.9 months with wedge, 73.7 months with segmentectomy, and 94.5 months with lobectomy; p=0.0008).75
Table 5.
Outcomes associated with various treatment options for patients with locally recurrent early stage non-small cell lung cancer
| Treatment | Locoregional Recurrence | Positive Margin Rate | |
|---|---|---|---|
| Surgery | Lobectomy | 4.9–7% | 1.4% |
| Segmentectomy | 9.1–16% | 2.1% | |
| Wedge resection | 11–27.8% | 4.0% | |
| Stereotactic body radiotherapya | 8–15% | n/a |
Only includes early stage non-small cell lung cancers
Stereotactic body radiation therapy (SBRT) is an alternative treatment option to surgery for patients with stage I NSCLC, especially those with medical comorbidities precluding surgery. Phase II trials of early NSCLC report local control rates of 85–92% and OS of 43–60% at 3 years.76 Two randomized phase III trials of SBRT versus surgery for operable stage I NSCLC are currently recruiting. Presently, for standard operative risk patients with stage I NSCLC, the American Society of Clinical Oncology does not recommend SBRT outside of a clinical trial.77
LRR after surgery for NSCLC primarily occurs in two peaks, the first at 6–8 months and the second at 22–24 months.78 Analysis of 74 patients that developed local recurrence after surgery for stage I NSCLC reported that patients who underwent re-resection via surgery survived significantly longer than patients treated with chemotherapy or radiotherapy, though the 5-year post-recurrence survival after repeat surgery remained dismal at only 15%.79 In a recent study of 40 patients treated with salvage SBRT for locally recurrent NSCLC deemed inoperable, 18-month OS and local control were 77.6% and 87%, respectively.80
Rates of LRR increase with increasing primary stage (5–19% for stage I, 11–27% for stage II and 24–40% for stage IIIA).81,82 In a meta-analysis of 2,385 patients, neoadjuvant chemotherapy significantly improved OS (HR 0.87, p=0.007), but there was only a trend towards improved local control (HR 0.88, p=0.20).83
The location of the recurrence impacts median OS, with LRR (intrathoracic) being 25.5 months vs. distant (extrathoracic) being 10.1 months vs. both at 4.8 months (p=0.003).84 Patients presenting with an isolated, resectable LRR should be considered for surgery if medically appropriate and other local ablative therapies, such as SBRT, cryoablation, or more standard radiotherapy, if not a surgical candidate. Potential surgical options include completion lobectomy, pneumonectomy, and chest wall resection. There are no trials directly comparing radiotherapy and surgery for LRR, and therefore it remains unclear which treatment option is superior.73,84
Molecularly targeted agents (i.e. tyrosine kinase inhibitors for EGFR mutated cancer, BRAF inhibitors, ROS1 inhibitors, etc) and immunotherapy agents (pembrolizumab, nivolumab, ipilimumab, atezolizumab, etc) are being used to treat more advanced NSCLC and thus are being used in cases of non-resectable LRR.85 Perhaps more importantly are the emerging technologies being developed to prevent LRR in NSCLC, namely more accurate staging using near-infrared lymphatic mapping to more accurately identify SLNs86 and the placement of brachytherapy seeds at the staple line following sublobar resections.87 Though identifying SLNs is not technically a therapeutic intervention, no LRR occurred in patients with a negative SLN identified using this technique and DFS is superior compared to patients staged with standard lymphadenectomy (p=0.036).86 The ACOSOG Z4032 phase III trial randomized 222 patients with stage I NSCLC to sublobar resection vs. sublobar resection with brachytherapy seeds placed at the suture line, with a non-significant trend towards decreased local recurrence with brachytherapy in patients with positive staple line cytology (HR 0.22, p>0.05).87
Retroperitoneal Sarcoma
Soft-tissue sarcomas are malignant mesenchymal tumors with an estimated 12,390 new sarcoma cases and 4,990 deaths in the United States in 2017 with a 5-year OS of 64%.88 Approximately 15% of soft-tissue sarcomas arise in the retroperitoneum. Patients often present with large tumors that are characterized by complex anatomic relationships to vital surrounding organs, posing a significant surgical challenge.
The primary pattern of treatment failure for most retroperitoneal sarcomas (RPS) is LRR, and reported 5-year LRR rates range from 26–59%.89–91 Death in RPS is typically from direct sequelae of LRR. Completeness of resection, histologic subtype, and tumor grade are the major determinants of survival.89,92 Table 6 shows rates of local and distant recurrence for the most common histologic RPS subtypes. Key prognostic factors predictive for LRR are patient age, tumor size, completeness of resection, grade, tumor rupture, multifocality, histologic subtype, and prior radiotherapy.90 The predominant RPS histologies are liposarcoma and leiomyosarcoma, with liposarcomas exhibiting the highest rate of LRR.90,92 The median OS after LRR is 15–33 months.90,93
Table 6.
Rates of local and distant recurrence in the most common histologic subtypes of retroperitoneal sarcoma
| Retroperitoneal Sarcoma Histologic Subtype | Overall Percentage of Patients |
Local Recurrence Rate |
Distant Recurrence Rate |
|---|---|---|---|
| Dedifferentiated liposarcoma | 30–40% | 40–85% | 10–20% |
| Well-differentiated liposarcoma | 25–30% | 5–45% | 0–5% |
| Leiomyosarcoma | 15–25% | 10–30% | 40–50% |
| Malignant peripheral-nerve sheath tumor | 2–5% | 35–65% | 40–70% |
| Solitary fibrous tumor | 2–5% | 5–10% | 20–30% |
A macroscopically complete (R0/R1) resection represents the only potentially curative treatment. Aggressive, multi-visceral organ resections (most commonly kidney and colon, but also occasionally psoas muscle, spleen, diaphragm, abdominal wall muscles, pancreas, and major vessels) are more commonly performed at referral centers.90 The ideal extent of resection for RPS is unclear, with some centers advocating complete compartmental resection or liberal en bloc resection of uninvolved organs. Other sarcoma centers recommend limiting organ resection to those with evidence of local invasion or selection of more aggressive surgeries based on histologic subtype (i.e., more aggressive for dedifferentiated liposarcoma).94 More extensive surgery at the time of the initial operation is associated in some but not all series with decreased LRR, though this is still a subject of significant debate as impact on OS is less consistent.89,92,95
Chemotherapy and radiotherapy are used as adjuvant therapies in an attempt to decrease LRR after resection, though their use remains investigational. Doxorubicin, either alone or in combination with ifosfamide, is considered first-line chemotherapy for advanced or metastatic sarcomas but its utility in the adjuvant or neoadjuvant setting for RPS is uncertain.96 While radiotherapy may reduce local recurrence, the requirement for large field sizes and the close proximity to radiosensitive organs (i.e., intestines, liver) can result in significant toxicity.97 Low rates of local recurrence and reasonable survival rates have been reported in prospective non-randomized trials using preoperative radiotherapy, but these studies were not compared to surgery-alone controls.98,99 The results of an international, multi-center, phase III trial enrolling 256 patients evaluating the role of neoadjuvant radiotherapy are expected in 2019. Intraoperative radiotherapy results in 5-year local control rates of roughly 51–83% in retrospective series, but approximately 10% of patients experienced severe gastrointestinal toxicity as well as high rates of neuropathy and ureteral stenosis.100 An analysis of the SEER database study demonstrated that postoperative radiotherapy showed no survival benefit.101 Regional hyperthermia, in addition to chemotherapy, has been shown to significantly reduce 5-year LRR after macroscopically complete resection (44% vs. 55%, p=0.044) but results in similar 5-year OS (57% versus 55%, p=0.82).102 The combination of hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery has been explored, but there is currently no proven efficacy.103
The most significant predictor of outcome following a local recurrence is resectability of recurrent disease. Patients most likely to derive benefit from re-resection are those with longer disease-free intervals, no history of tumor rupture at initial operation, low-grade tumors, and unifocal recurrences.90 Rates of resectability decrease with each recurrence, from 57% at first local recurrence to 33% at second recurrence and 14% at third recurrence.91 In borderline resectable recurrences, neoadjuvant chemotherapy or radiotherapy may be utilized in an attempt to downsize the recurrent tumor.93,104 However, there is no defined role for chemotherapy for locally recurrent disease, with numerous trials showing no survival benefit for doxorubin-based105 or second-line96 regimens. Immunotherapy studies, including vaccines, checkpoint inhibitor, and adoptive T-cell therapy, are under early-stage investigation.106
Peritoneal Surface Malignancies
The peritoneum itself can serve as the site of origin for malignancies such as peritoneal mesothelioma and primary peritoneal carcinoma. In addition, appendiceal pseudomyxoma peritonei is a unique condition characterized by peritoneal surface mucinous implants producing gelatinous material, presenting a similar oncologic challenge. Patients with peritoneal surface malignancies frequently die of complications of locoregional disease, most often due to intestinal obstruction. While some patients can be treated with selective removal of gross disease, patients with more diffuse disease often require a more extensive debulking including complete peritonectomy.107
In addition to cytoreductive surgery (CS), HIPEC is frequently used. Thorough cytoreduction is imperative as the depth of HIPEC drug penetration is estimated to be only 3–5 mm.108 While the addition of HIPEC seemed to improve OS compared to historic controls, randomized clinical trial data are lacking, and the added benefit of HIPEC to CS at baseline is uncertain.109,110 The most common HIPEC regimens use mitomycin C, doxorubicin, taxanes and/or platinum-based chemotherapy.108 The benefits of locally delivered chemotherapy are particularly evident following treatment of pseudomyxoma peritonei from appendiceal origin, with CS-HIPEC achieving a median OS of 16.3 years and median PFS of 8.2 years in a multi-institutional registry analysis of 2,298 patients111 In this cohort, 51% of patients achieved a complete cytoreduction.111 For peritoneal mesothelioma, a meta-analysis of CS-HIPEC reported 84% 1-year survival and 42% OS at 5 years,112 whereas a separate trial using systemic chemotherapy with pemetrexed and cisplatin yielded a 1-year survival of only 57.4%.113 The median DFS for peritoneal mesothelioma ranged from 7.2 to 40 months.112 Despite this success, the use of HIPEC for PC is associated with significant morbidity and mortality, with mortality rates being 0.9–5.8% (mostly gastrointestinal, pulmonary, and hematologic) of 12–52% being reported even as tertiary centers with significant expertise in these techniques.114
Emerging Therapeutic Drug-Delivery Strategies for Locoregional Recurrence
This review demonstrates that there is a critical unmet need to improve the prevention and treatment of LRR across a number of major malignancies. Fortunately, several novel treatment strategies are emerging to improve local drug delivery to the tumor bed (Figure 2). One appealing approach centers on therapies that can deliver high local doses of chemotherapy with minimal systemic side effects. Such adjuvant treatment options can better target appropriate cell cycle regulators through sustained delivery of high-dose cytotoxic therapy. These novel drug delivery systems fall into two major categories: (1) those intended for intravenous administration and (2) those designed for peritumoral injection or direct implantation into the tumor bed. Anatomic considerations as well as patterns of LRR among different cancers provide insight into the therapeutic strategies that might be most efficacious for a particular patient.
Figure 2.
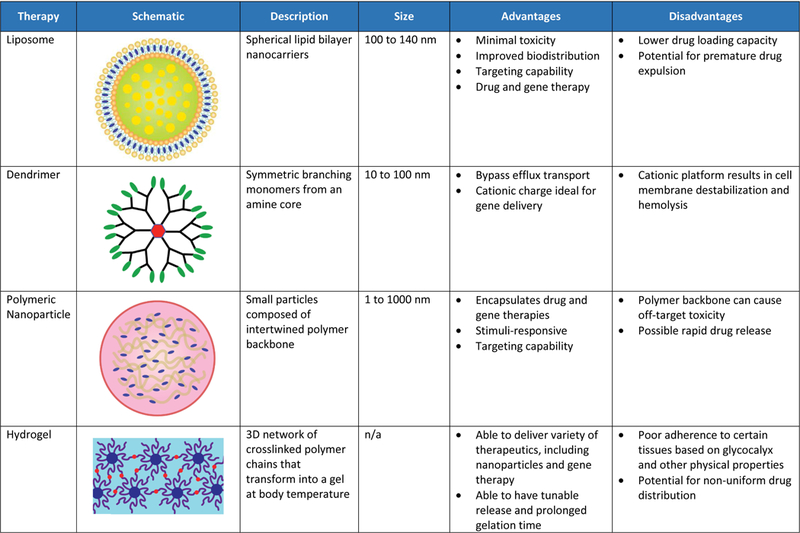
Leading Novel Drug-Delivery Options in Preclinical and Clinical Testing. NSCLC indicates non-small cell lung cancer; RPS, retroperitoneal sarcoma.
Intravenous delivery of nanoparticle systems, including liposomes, dendrimers, and polymeric nanoparticles are designed to target cancer cells by passive diffusion, conjugation to a specific targeting ligand, or physiologically triggered release, such as pH or temperature.115 Steric stabilization results both in improved efficacy by limiting in vivo opsonization in the bloodstream and in resultant increased half-life with fewer systemic side effects. While ligand-targeting has shown enhanced anti-tumor efficacy in vivo, its use in clinical studies has thus far been limited by a combination of tumor-dependent physiological barriers and formulation stability.116 Triggering adjuncts have the potential to enhance efficacy in locoregional control. The combination of targeting ligand and heat-triggered release was shown to deliver 3-fold higher intratumoral quantity of chemotherapy drug in an in vivo model.117 Targeting mechanisms could allow for higher therapeutic efficacy and decreased off-target toxicity.118
Liposomes contain concentric phospholipid bilayers that enclose a discrete aqueous space, allowing for the delivery of both hydrophobic and hydrophilic compounds. Their biochemical properties can be modified with antibodies, ligands, or other small molecules to allow specific targeting, and their circulation stability and ability to evade the reticuloendothelial system can be prolonged with polyethylene glycol (PEG).116 Although clinical studies of liposomal agents for treatment of LRR are limited, PEGylated liposomal doxorubicin exhibits a better safety profile than non-liposomal doxorubicin and in a phase II trial of elderly and cardiotoxicity-prone patients with high-risk breast cancer, liposomal doxorubicin resulted in a 5-year PFS of 58%.119 While liposomal doxorubicin is the best characterized and most commonly used liposome to date, liposomal formulations of other chemotherapy drugs are being evaluated in clinical trials, including paclitaxel for breast and ovarian cancers and irinotecan for colorectal cancer.
Dendrimers are well-organized, multi-branched macromolecules being investigated for intravenous delivery of drugs, siRNAs, shRNAs, and other therapeutics.120 Dendrimers are most commonly derived from polyamidoamine, affording a hydrophilic delivery system. Although no clinical data is available, a pluronic-attached polyamidoamine dendrimer conjugates for doxorubicin successfully overcame multi-drug resistance in in vitro and in vivo breast cancer models.121 Further, a folate receptor-targeted dendrimer loaded with an siRNA and cis-diamine platinum exhibits enhanced therapeutic efficacy in vitro for lung cancer.122 While dendrimers are typically formulated for intravenous delivery, they can also be made in aerosol formulations, providing potential for targeted lung delivery.123
Nanoparticles (NP) are a microscopic delivery system measuring <100 nM in diameter that can be optimized for tumor localization and efficacy by varying their size, shape, or surface charge.124 Nanoparticles can be coupled with targeting ligands (i.e., transferrin, LDL, integrin, folate receptor, and epidermal growth factor receptor).125 The most well-studied NP example is the clinical use of nanoparticle albumin-bound paclitaxel (nab-pax) for a variety of solid tumor malignancies such as NSCLC, breast, and pancreatic cancers. A phase I trial in advanced NSCLC showed that the combination of radiotherapy, nanoparticle-albumin-bound paclitaxel (nab-PTX) and carboplatin produced an objective response in 71% of patients with acceptable toxicity.126 A phase II trial using nab-PTX in 42 patients with recurrent, refractory ovarian cancer reported a 23% response rate, 36% stable disease rate, median OS of 17.4 months, and no grade 4 or higher adverse events.127 Polymeric NP are particularly of interest as they can be designed to be responsive to various triggering stimuli, including microenvironmental changes in temperature, pH or hypoxia as well as magnetic or ultrasound activation.124 Furthermore, radiotherapy alters the tumor microenvironment immune-cell composition, vasculature, and cell-signaling to enhance intra-tumoral accumulation of NP and the presence of NP may also enhance the efficacy of ionizing radiotherapy.128
Our group reported decreased LRR with peritumoral administration of paclitaxel-loaded expansile-nanoparticles (PTX-eNPs) in multiple cancer types. PTX-eNPs delivered intraperitoneally decreased recurrence and improve survival in murine models of peritoneal mesothelioma,129 decreased lymph node metastases in a murine breast cancer model,130 and demonstrated >20 cm lymphatic migration to the sentinel lymph node in a large animal model.131 Interestingly, eNP can be loaded in vivo drawing paclitaxel into the tumor, with co-administration of intravenous paclitaxel and local eNPs, resulting in a 5-fold higher intratumoral paclitaxel concentration than intravenous paclitaxel alone.132 Another group utilized CD133-targeted paclitaxel nanoparticles and showed significant inhibition of local tumor recurrence in a murine model of CD133-expressing breast cancer.133
An alternative option for the prevention of LRR within the tumor bed is an implantable drug-delivery platform, such as drug-eluting polymer films, wafers, hydrogels, etc. Carmustine-impregnated (Gliadel®) wafers improve OS in patients with glioblastoma multiforme and were the first FDA-approved local polymer chemotherapy.134 Prolonged local drug delivery through such a platform is an attractive therapeutic option for RPS, rectal cancer, and sublobar resections for NSCLC based on their patterns of LRR. Such local chemotherapy delivery achieves higher drug concentrations than the low drug concentrations achieved at the tumor bed or in regional lymph nodes with systemic therapy.
Local delivery platforms for adjuvant chemotherapy delivery after surgery are being examined, but pose unique challenges. One delivery platform of interest is chemotherapy-eluting polymeric films adhered to collagen-based buttressing material, which allow for flexible surgical fixation and result in sustained, controlled drug release in the resected tumor bed (Figure 3). Paclitaxel-loaded films directly implanted immediately following resection in murine tumor models of lung (LLC) and sarcoma (CS-1) tumors prevented LRR in over 80% of mice for both tumor types. 134,135 In contrast, only 22.2% of LLC and 11% of CS-1 control mice were free from local recurrence at 60 days, after receiving an equivalent dose of intravenous paclitaxel.135,136 While paclitaxel-films led to 50–300 fold higher paclitaxel levels within the local tissues compared to intravenous delivery, systemic paclitaxel levels were minimal, confirming a decreased risk of systemic side effects.135 Similarly, a superhydrophobic mesh loaded with cisplatin implanted immediately after surgery also significantly decreased local lung cancer recurrence in vivo in a murine model (Figure 4).137
Figure 3.
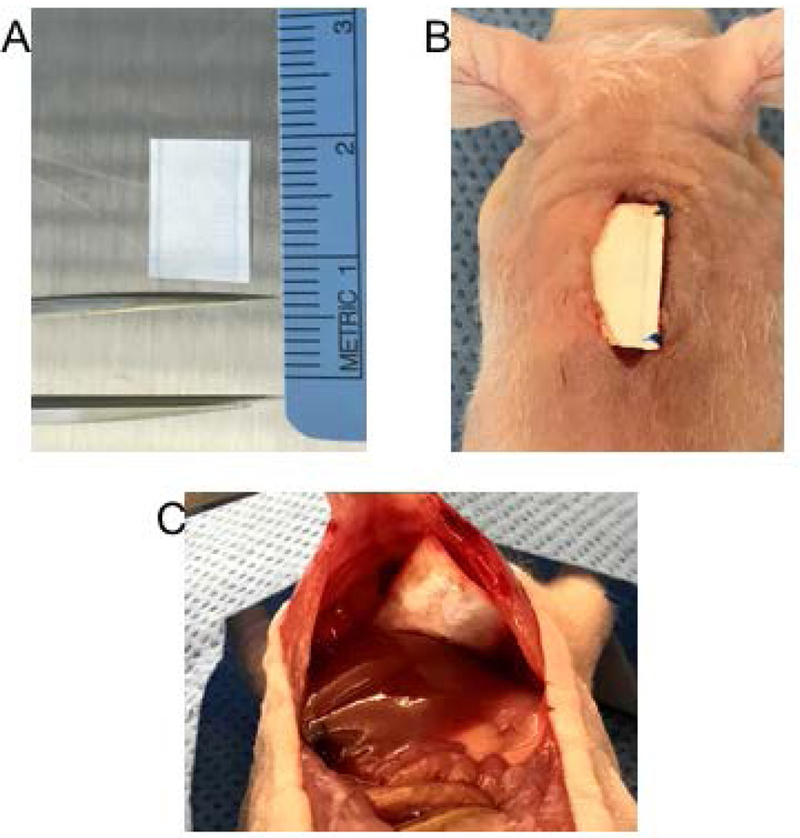
(A) A 1 × 1 cm Poly(Glycerol Monostearate-Co--Caprolactone) Polymer Mesh, (B) Polymer Mesh Implanted After Subcutaneous Sarcoma Resection With Prolene Suture, and (C) Polymer Mesh Secured to the Diaphragm Superior to the Liver.
Figure 4.
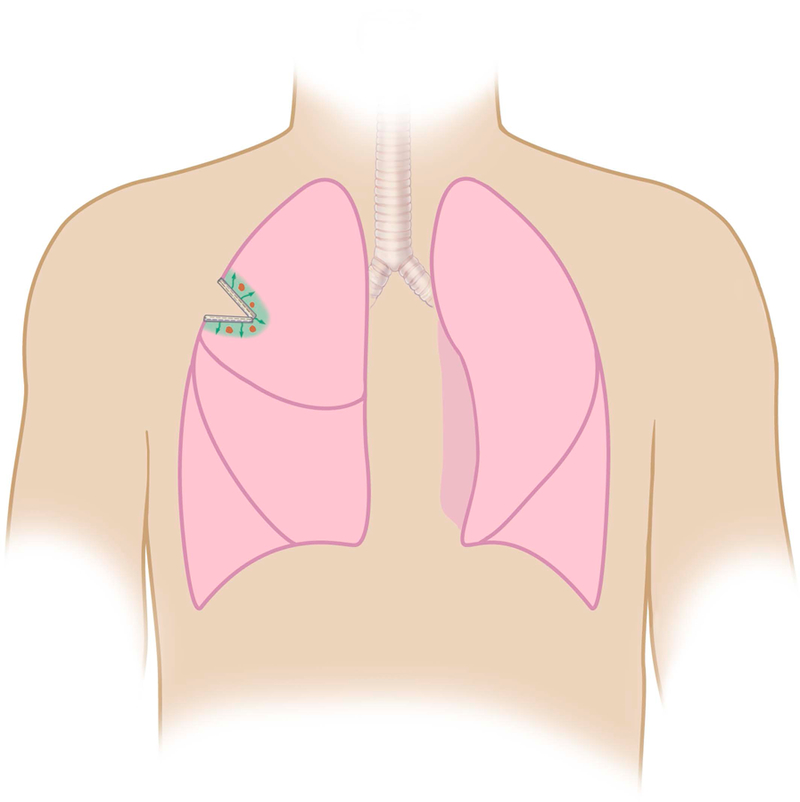
Polymer Film Containing Cisplatin Can Be Stapled to Lung Tissue During Surgery for Local Drug Delivery.
Another potential local delivery platform under investigation are hydrogels. Hydrogels are a scaffold of crosslinked hydrophilic polymer chains which form gels when injected in situ and can serve as a local drug delivery platform.138 A PEG-based hydrogel loaded with paclitaxel significantly decreased LRR in a murine model following resection of a primary breast tumor (9.1% vs. 77.8% with IV paclitaxel).139 Recently, a thermosensitive chitosan hydrogel loaded with 5-fluorouracil micelles and cisplatin showed prolonged survival and decreased lung and liver metastases in a mouse model of colorectal peritoneal carcinomatosis.140 Intrapleural delivery of cisplatin coupled with a fibrin glue showed sustained concentrations of cisplatin at the site of resection 1 week after application during extrapleural pneumonectomy without systemic toxicity in a pig model. This approach is currently under evaluation for mesothelioma in a phase I/II trial.141 Currently in early stages of development, inhalational delivery of multiple nanoparticle subtypes (i.e. polymeric NP, liposomes, polymeric micelles) hold promise for treatment of lung cancers.142
While the majority of the above novel drug-delivery platforms remain in pre-clinical testing, the promise of precise tumor-targeting, prolonged therapeutic efficacy, and minimization of off-target side effects holds significant promise. A keen understanding of patterns of local recurrence inherent in particular tumor subtypes will allow for optimal development and selection of the ideal delivery platform to decrease local recurrence for a given patient (Table 7).
Table 7.
Ideal deal drug delivery platforms based on patterns of locoregional recurrence
| Cancer | Ideal Novel Drug Delivery Platform |
|---|---|
| Rectal Cancer | •Nanoparticle (intravenous or intraperitoneal) •Hydrogel at anastomosis •Polymer film along pelvic side wall |
| Breast Cancer | •Nanoparticle (intravenous or peritumoral) •Polymer film in resection bed |
| Mesothelioma | •Intrapleural nanoparticle •Polymer film (after pneumonectomy/decortication) |
| Non-Small Cell Lung Cancer | •Polymer film along staple line •Systemic nanoparticle/dendrimer/liposome |
| Retroperitoneal Sarcoma | •Polymer film in resection bed |
| Peritoneal Surface Malignancies | •Intraperitoneal nanoparticle |
Conclusions
In several of the most common malignancies, local recurrence portends a significantly worse oncologic outcome and quality of life. Remarkable advances in surgical techniques, systemic chemotherapy, and radiotherapy are decreasing locoregional recurrence rates and improving overall survival. However, these therapies are often complicated by significant local and systemic morbidity as well as treatment-related mortality, highlighting the need for better local strategies. Over the next decade, one of the next frontiers to reduce local recurrence, with minimization of systemic side effects, will involve local drug-delivery platforms. Further translational and clinical research is imperative to investigate the safety and efficacy of these various emerging therapeutic strategies.
Acknowledgments
Funding for this research study: NIH R01 EB017722, CA131044–01A1 and CA227433
Footnotes
Conflict of Interest Disclosures: DAM – none; RL–none; MWG- has ownership interest in AcuityBio and Ionic Pharmaceuticals; YLC – none; CPR – none
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin 2018;68(1):7–30 [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD: 2017. [PubMed] [Google Scholar]
- 3.Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis 2006;8:733–747 [DOI] [PubMed] [Google Scholar]
- 4.Sagar PM, Pemberton JH. Surgical management of locally recurrent rectal cancer. Brit Jour Surg 1996;83:293–304 [DOI] [PubMed] [Google Scholar]
- 5.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007;14(2):447–54 [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Li Z, Gu X, et al. Prognostic factors associated with locally recurrent rectal cancer following primary surgery (Review). Oncol Letters 2014;7:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harji DP, Sagar PM. Advancing the surgical treatment of locally recurrent rectal cancer. Brit Jour Surg 2012;99:1169–71 [DOI] [PubMed] [Google Scholar]
- 8.Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg 2017;266(2):201–7 [DOI] [PubMed] [Google Scholar]
- 9.Kusters M, Beets GL, van de Velde CJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg 2009;249:229–235 [DOI] [PubMed] [Google Scholar]
- 10.Ho VP, Lee Y, Stein SL, Temple LK. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum 2011;54(1):113–25. [DOI] [PubMed] [Google Scholar]
- 11.Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur Jour Surg Oncol 2010;36:470–476 [DOI] [PubMed] [Google Scholar]
- 12.Sebag-Mentefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and Ncic-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu W, Wu S. Meta-analysis of defunctioning stoma in low anterior resection with total mesorectal excision for rectal cancer: evidence based on thirteen studies. World J Surg Oncol 2015;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrak K, Uranitsch S, Pedross F, et al. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery 2016;159(4):1129–39. [DOI] [PubMed] [Google Scholar]
- 15.Phatak UR, Kao LS, You YN, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol 2014. 21 (2): 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loos M, Quentmeier P, Schuster T, et al. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20(6):1816–28. [DOI] [PubMed] [Google Scholar]
- 17.Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 2005;23(9):1847–58. [DOI] [PubMed] [Google Scholar]
- 18.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol 2005;23(25):199–206. [DOI] [PubMed] [Google Scholar]
- 19.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant Chemotherapy Without Routine Use of Radiation Therapy for Patients With Locally Advanced Rectal Cancer: A Pilot Trial. J Clin Oncol 2014;32(6): 513–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masaki T, Takayama M, Matsuoka H, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg 2008;393(2):173–80 [DOI] [PubMed] [Google Scholar]
- 21.Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol 2011;98(3):298–303 [DOI] [PubMed] [Google Scholar]
- 22.Kusters M, Velntini V, Calvo FA, et al. Results of the European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastasis. Ann Oncol 2010;21(6):1279–84 [DOI] [PubMed] [Google Scholar]
- 23.Pilar A, Gupta M, Ghosh Laskar S, Laskar S et al. Intraoperative radiotherapy: review of techniques and results. Ecancermedicalscience 2017;11:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol 2001;27(4):349–53 [DOI] [PubMed] [Google Scholar]
- 25.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007;14:447–54 [DOI] [PubMed] [Google Scholar]
- 26.Tanis PJ, Doeksen A, van Lanschot JJ. Intentionally curative treatment of locally recurrent rectal cancer: a systematic review. Can J Surg 2013;56(2):135–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris CA, Solomon MJ, Heriot AG, et al. The Outcomes and Patterns of Treatment Failure After Surgery for Locally Recurrent Rectal Cancer. Ann Surg 2016;264:323–9 [DOI] [PubMed] [Google Scholar]
- 28.Hecht M, Büttner-Herold M, Erlenbach-Wünsch K, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer 2016;65:52–60 [DOI] [PubMed] [Google Scholar]
- 29.Fisher B, Jeong JH, Anderson S, et al. Twenty-Five-Year Follow Up of a Randomized Trial Comparing Radical Mastectomy, Total Mastectomy, and Total Mastectomy Followed by Irradiation. N Engl J Med 2002;347(8):567–75 [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Anderson S, Bryant J, et al. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N Engl J Med 2002;347:1233–41 [DOI] [PubMed] [Google Scholar]
- 31.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11(10):927–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized trial. JAMA 2011;305(6):569–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007;18(2):CD005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 2014;21(3):704–16 [DOI] [PubMed] [Google Scholar]
- 35.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 2006;24(13):2028–37 [DOI] [PubMed] [Google Scholar]
- 36.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804):1707–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 1999;35(1):32–8 [DOI] [PubMed] [Google Scholar]
- 38.Lehman M, Hickey BE, Francis DP, See AM. Partial breast irradiation for early breast cancer. Cochrane Database Syst Rev 2014;18(6):CD007077. [DOI] [PubMed] [Google Scholar]
- 39.Fredriksson I, Liljegren G, Arnesson LG, et al. Local recurrence in the breast after conservative surgery—a study of prognosis and prognostic factors in 391 women. Eur J Canc 2002;38:1860–70 [DOI] [PubMed] [Google Scholar]
- 40.Kauer-Dorner D, Pötter R, Resch A, et al. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother Oncol 2012;102(1):96–101 [DOI] [PubMed] [Google Scholar]
- 41.Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol 2014;15(2):156–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman LA, Hunt KK, Buchholz T, et al. Presentation, management, and outcome of axillary recurrence from breast cancer. Am J Surg 2000;180(4):252–6 [DOI] [PubMed] [Google Scholar]
- 43.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000. 92; 1143–50 [DOI] [PubMed] [Google Scholar]
- 44.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995;332(14):907–11 [DOI] [PubMed] [Google Scholar]
- 45.Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 2000;18:2817–27 [DOI] [PubMed] [Google Scholar]
- 46.Cheng SH, Horng CF, Clarke JL, et al. Prognostic index score and clinical prediction model of local recurrence after mastectomy in breast cancer patients. Int J Radait Oncol Biol Phys 2006;64(5):1401–9 [DOI] [PubMed] [Google Scholar]
- 47.Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after mastectomy and axially surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet 2014;383(9935):2127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willner J, Kiricuta IC, Kölbl O. Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys 1997;37(4):853–63 [DOI] [PubMed] [Google Scholar]
- 49.Wakeam E, Acuna SA, Keshavjee S. Chest Wall Resection for Recurrent Breast Cancer in the Modern Era: A Systematic Review and Meta-analysis. Ann Surg 2018;267(4):646–55 [DOI] [PubMed] [Google Scholar]
- 50.Borner M, Bacchi M, Goldhirsch A, et al. First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. Swiss Group for Clinical Cancer Research. J Clin Oncol 1994;12(10):2071–7 [DOI] [PubMed] [Google Scholar]
- 51.Sirohi B, Leary A, Johnston SR. Ipsilateral breast tumor recurrence: Is there any evidence for benefit of further systemic therapy. Breast J 2009;15(3):268–78 [DOI] [PubMed] [Google Scholar]
- 52.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39(7):576–88 [DOI] [PubMed] [Google Scholar]
- 53.Ruffie PA. Pleural mesothelioma. Curr Opin Oncol 1991. 3: 328–34 [DOI] [PubMed] [Google Scholar]
- 54.Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg 2012;1(4):438–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batirel HF. Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D). Ann Transl Med 2017;5(11):232–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135(3):620–6 [DOI] [PubMed] [Google Scholar]
- 57.Sharkey AJ, Tenconi S, Nakas A, Waller DA. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632–41 [DOI] [PubMed] [Google Scholar]
- 58.Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561–7 [DOI] [PubMed] [Google Scholar]
- 59.Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405–11 [DOI] [PubMed] [Google Scholar]
- 60.Chang MY, Sugarbaker DJ. Innovative therapies: intraoperative intracavitary chemotherapy. Thorac Surg Clin 2004;14(4):549–56 [DOI] [PubMed] [Google Scholar]
- 61.Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 2015;149(2):558–66 [DOI] [PubMed] [Google Scholar]
- 62.Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 2013;8:238–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simone CB 2nd, Cengel KA. Photodynamic therapy for lung cancer and malignant pleural mesothelioma. Semin Oncol 2014;41:820–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103(3):912–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2003;21:2636–44 [DOI] [PubMed] [Google Scholar]
- 66.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405–14 [DOI] [PubMed] [Google Scholar]
- 67.Burt BM, Ali SO, DaSilva MC, et al. Clinical indications and results after chest wall resection for recurrent mesothelioma. J Thorac and Cardiovasc Surg 2013;146(6):1373–80 [DOI] [PubMed] [Google Scholar]
- 68.Bissett D, Macbeth FR, Cram I. The role of palliative radiotherapy in malignant mesothelioma. Clin Oncol 1991;3:315–7 [DOI] [PubMed] [Google Scholar]
- 69.Abtin F, Quirk MT, Suh RD, et al. Percutaneous Cryoablation for the Treatment of Recurrent Malignant Pleural Mesothelioma: Safety, Early-Term Efficacy, and Predictors of Local Recurrence. J Vasc Interv Radiol 2017;28(2):213–21 [DOI] [PubMed] [Google Scholar]
- 70.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18(5):623–30 [DOI] [PubMed] [Google Scholar]
- 71.Scherpereel A, Mazieres J, Greillier L, et al. Second- or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Results of the IFCT-1501 MAPS2 randomized phase II trial. J Clin Oncol 35, no. 18_suppl - published online before print [Google Scholar]
- 72.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60(3):615–22 [DOI] [PubMed] [Google Scholar]
- 73.Kelsey CR, Marks LB, Hollis D, et al. Local Recurrence After Surgery for Early Stage Lung Cancer. An 11-Year Experience With 975 Patients. Cancer 2009;115(220):5218–27 [DOI] [PubMed] [Google Scholar]
- 74.Wolf AS, Swanson SJ, Yip R, et al. The Impact of Margins on Outcomes After Wedge Resection for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104(4):1171–8 [DOI] [PubMed] [Google Scholar]
- 75.Khullar OV, Liu Y, Gilliespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10(11):1625–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tandberg DJ, Tong BC, Ackerson BG, Kelsey CR. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer 2018. 124(4): 667–78 [DOI] [PubMed] [Google Scholar]
- 77.Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018. 36(7): 710–9 [DOI] [PubMed] [Google Scholar]
- 78.Watanabe K, Tsuboi M, Sakamaki K, et al. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur J Cardiothorac Surg 2016;49(6):1624–31 [DOI] [PubMed] [Google Scholar]
- 79.Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64(3):192–6 [DOI] [PubMed] [Google Scholar]
- 80.Juloori A, Vassil AD, Woody NM, et al. Managing Local Recurrence After Primary Resection of Non-Small Cell Lung Cancer: The Role for Salvage Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;96(2):SE472 [Google Scholar]
- 81.Saynak M, Veeramachaneni NK, Hubbs JL, et al. Local failure after complete resection of N0–1 non-small cell lung cancer. Lung Cancer 2011. 71 (2): 156–65 [DOI] [PubMed] [Google Scholar]
- 82.Choi MS, Park JS, Kim HK, et al. Analysis of 1,067 cases of video-assisted thoracic surgery lobectomy. Korean J Thorac Cardiovasc Surg 2011;44(2):169–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383(9928):1561–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Endo C, Sakurada A, Notsuda H, et al. Results of a long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg 2012. 93 (4): 1061–8 [DOI] [PubMed] [Google Scholar]
- 85.Chuang JC, Liang Y, Wakelee HA. Neoadjuvant and Adjuvant Therapy for Non-Small Cell Lung Cancer. Hem Oncol Clin North Amer 2017;31(1):31–44 [DOI] [PubMed] [Google Scholar]
- 86.Digesu CS, Hachey KJ, Gilmore DM, et al. Long-term outcomes after near-infrared sentinel lymph node mapping in non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;155(3):1280–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of Brachytherapy on Local Recurrence Rates After Sublobar Resection: Results From ACOSOG Z4032 (Alliance), a Phase III Randomized Trial for High-Risk Operable Non-Small Cell Lung Cancer. J Clin Oncol 32(23): 2456–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.American Cancer Society. Cancer Facts and Figures 2017. American Cancer Society; 2017 [Google Scholar]
- 89.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol 2009;27:31–7 [DOI] [PubMed] [Google Scholar]
- 90.Gronchi A, Strauss DC, Miceli R, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS). A Report on 1007 Patients from the Multi-institutional Collaborative RPS Working Group. Ann Surg 2016;263(5):1002–9 [DOI] [PubMed] [Google Scholar]
- 91.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer 2001;92:359–68 [DOI] [PubMed] [Google Scholar]
- 92.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg 2003;238:358–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Houdt WJ, Zaidi S, Messiou C, et al. Treatment of retroperitoneal sarcoma: current standards and new developments. Curr Opin Oncol 2017;29(4):260–7 [DOI] [PubMed] [Google Scholar]
- 94.Fairweather M, Gonzalez RJ, Strauss D, Raut CP. Current principles of surgery for retroperitoneal sarcomas. J Surg Oncol 2018. 117(1):33–41 [DOI] [PubMed] [Google Scholar]
- 95.Gronchi A, Miceli R, Colombo C, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol 2012;23(4):1067–73 [DOI] [PubMed] [Google Scholar]
- 96.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15(4):415–23 [DOI] [PubMed] [Google Scholar]
- 97.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045–54 [DOI] [PubMed] [Google Scholar]
- 98.Raut CP, Pisters PW. Retroperitoneal Sarcomas: Combined-Modality Treatment Approaches. J Surg Oncol 2006;94:81–7 [DOI] [PubMed] [Google Scholar]
- 99.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol 2006;13(4):508–17 [DOI] [PubMed] [Google Scholar]
- 100.Roeder F, Krempien R. Intraoperative radiation therapy (IORT) in soft-tissue sarcoma. Radiat Oncol 2017;12(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tseng WH, Martinez SR, Do L, et al. Lack of survival benefit following adjuvant radiation in patients with retroperitoneal sarcoma: a SEER analysis. J Surg Res 2011;168:173–80 [DOI] [PubMed] [Google Scholar]
- 102.Angele MK, Albertsmeier M, Prix NJ, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg 2014;260(5):749–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colombo C, Baratti D, Kusamura S, et al. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) and isolated perfusion (ILP) interventions in sarcoma. J Surg Oncol 2015;111(5):570–9 [DOI] [PubMed] [Google Scholar]
- 104.Trans-Atlantic RPS Working Group. Management of Recurrent Retroperitoneal Sarcoma (RPS) in the Adult: A Consensus Approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol 2016;23(11):3531–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a phase III placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol 2016;24(32):3898–3905 [DOI] [PubMed] [Google Scholar]
- 106.Nathenson MJ, Conley AP, Sausville E. Immunotherapy: A New (and Old) Approach to Treatment of Soft Tissue and Bone Sarcomas. Oncologist 2018;23(1):71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol 2013;19(41):6979–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol 2010;2(2):68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glehen O, Gilly FN, Boutitie F, et al. Toward Curative Treatment of Peritoneal Carcinomatosis From Nonovarian Origin by Cytoreductive Surgery Combined With Perioperative Intraperitoneal Chemotherapy: A Multi-Institutional Study of 1290 Patients. Cancer 2010;116(24):5608–18 [DOI] [PubMed] [Google Scholar]
- 110.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gyn Oncol 2013;130(3):493–8 [DOI] [PubMed] [Google Scholar]
- 111.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30(20):2449–56 [DOI] [PubMed] [Google Scholar]
- 112.Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22(5):1686–93 [DOI] [PubMed] [Google Scholar]
- 113.Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma—Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64(2):211–8 [DOI] [PubMed] [Google Scholar]
- 114.Mehta SS, Gelli M, Agarwal D, Goéré D. Complications of Cytoreductive Surgery and HIPEC in the Treatment of Peritoneal Metastases. Indian J Surg Oncol 2016;7(2):225–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolinsky JB, Colson YL, Grinstaff MW. Local Drug Delivery Strategies for Cancer Treatments: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J Control Release 2012;159(1):14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sercombe L, Veerati T, Moheimani F, et al. Advances and Challenges of Liposome Assisted Drug Delivery. Frontiers Pharmacol 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dicheva BM, ten Hagen TL, Schipper D, et al. Targeted and heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. J Control Release 2014. 195: 37–48 [DOI] [PubMed] [Google Scholar]
- 118.Huang YW, Cambre M, Lee HJ. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int J Mol Sci 2017. 18(12): 2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gil-Gil MJ, Bellet M, Morales S, et al. Pegylated liposomal doxorubicin plus cyclophosphamide followed by paclitaxel as primary chemotherapy in elderly or cardiotoxicity-prone patients with high-risk breast cancer: results of the phase II CAPRICE study. Breast Cancer Res Treat 2015;151(3):597–606 [DOI] [PubMed] [Google Scholar]
- 120.Madaan K, Kumar S, Poonia N, et al. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci 2014;6(3):139–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang M, Li Y, HuangFu M, et al. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine (Lond) 2016;11(22):2917–34 [DOI] [PubMed] [Google Scholar]
- 122.Amreddy N, Babu A, Panneerselvam J, et al. Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimernanoparticles for lung cancer treatment. Nanomedicine 2017. S1549–9634(17)30205–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Conti DS, Brewer D, Grashik J, et al. Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol Pharm 2014. 11 (6): 1808–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Overchuk M, Zheng G. Overcoming Obstacles in the Tumor Microenvironment: Recent Advancements in Nanoparticle Delivery for Cancer Theranostics. Biomaterials 2017;156: 217–37 [DOI] [PubMed] [Google Scholar]
- 125.Bahrami B, Hojjat-Farsani M, Mohammadi H, et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett 2017. 190: 64–83 [DOI] [PubMed] [Google Scholar]
- 126.Kaira K, Tomizawa Y, Imai H, et al. Phase I study of nab-paclitaxel plus carboplatin and concurrent thoracic radiotherapy in patients with locally advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2017;79(1):165–71 [DOI] [PubMed] [Google Scholar]
- 127.Coleman RL, Brady WE, McMeekin DS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2011. 122(1): 111–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stapleton S, Jaffray D, Milosevic M. Radiation effects on the tumor microenvironment: Implications for nanomedicine delivery. Adv Drug Deliv Rev 2017;109:119–30 [DOI] [PubMed] [Google Scholar]
- 129.Liu R, Colby AH, Gilmore D, et al. Nanoparticle tumor localization, disruption of autophagosomal trafficking, and prolonged drug delivery improve survival in peritoneal mesothelioma. Biomaterials 2016;102:175–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu R, Gilmore DM, Zubris KA, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials 2013;34(7) :1810–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zubris KA, Khullar OV, Griset AP, et al. Ease of Synthesis, Controllable Sizes, and In Vivo Large Animal Lymph Migration of Polymeric Nanoparticles. ChemMedChem 2010;5(9):1435–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Colby AH, Liu R, Schulz MD, et al. Two-Step Delivery: Exploiting the Partition Coefficient Concept to Increase Intratumoral Paclitaxel Concentrations In vivo Using Responsive Nanoparticles. Sci Rep 2016;6:18720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swaminathan SK, Roger E, Toti U, et al. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release 2013. 171(3): 280–7 [DOI] [PubMed] [Google Scholar]
- 134.Xing WK, Saho C, Qi ZY, et al. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des Devel Ther 2015;9:3341–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu R, Wolinsky JB, Walpole J, et al. Prevention of Local Tumor Recurrence Following Surgery Using Low-Dose Chemotherapeutic Polymer Films. Ann Surg Oncol 2010;17(4):1203–13 [DOI] [PubMed] [Google Scholar]
- 136.Liu R, Wolinsky JB, Catalano PJ, et al. Paclitaxel-Eluting Polymer Film Reduces Locoregional Recurrence and Improves Survival in a Recurrent Sarcoma Model: A Novel Investigational Therapy. Ann Surg Oncol 2012;19(1):199–206 [DOI] [PubMed] [Google Scholar]
- 137.Kaplan JA, Liu R, Freedman JD, et al. Prevention of lung cancer recurrence using cisplatin-loaded superhydrophobic nanofiber meshes. Biomaterials 2016;76:273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discovery Today 2016;21(11):1835–49 [DOI] [PubMed] [Google Scholar]
- 139.Lei N, Gong C, Qian Z, et al. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale 2012;4(18):5686–93 [DOI] [PubMed] [Google Scholar]
- 140.Yun Q, Wang SS, Xu S, et al. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol Biosci 2017;17(4):10.1002/mabi.201600262 [DOI] [PubMed] [Google Scholar]
- 141.Opitz I, Erne BV, Demirbas S, et al. Optimized intrapleural cisplatin chemotherapy with a fibrin carrier after extrapleural pneumonectomy: A preclinical study. J Thorac Cardiovasc Surg 2011;141:165–71 [DOI] [PubMed] [Google Scholar]
- 142.Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Cont Rel 2015;219:500–18 [DOI] [PubMed] [Google Scholar]


