Abstract
The binary approach to the diagnosis of Chronic Bronchitis (CB) is a major barrier to the study of the disease. We investigated whether severity of productive cough can be graded using symptoms and presence of fixed airflow obstruction (FAO), and whether the severity correlates with health status, exposures injurious to the lung, biomarkers of inflammation, and measures of airway wall thickening. Findings from a cross-sectional sample of 1,422 participants from the Lovelace Smokers Cohort (LSC) were validated in 4,488 participants from the COPDGene cohort (COPDGene). Health status was based on the St. George’s Respiratory Questionnaire, and Medical Outcomes Study 36-Item Short Form Health Survey. Circulating CC16 levels were quantified by ELISA (LSC), and airway wall thickening was measured using computed tomography (COPDGene). FAO was defined as postbronchodilator FEV1/FVC <0.7. The presence and duration of productive cough and presence of FAO or wheeze were graded into Healthy Smokers, Productive Cough (PC), Chronic PC, PC with Signs of Airflow Obstruction, and Chronic PC with Signs of Airflow Obstruction. In both cohorts, higher grade of severity correlated with lower health status, greater frequency of injurious exposures, greater airway wall thickening, and lower circulating CC16 levels. Further, longitudinal follow-up suggested that disease resolution can occur at every grade of severity but is more common in groups of lower severity and least common once airway remodeling develops. Therefore, severity of productive cough can be graded based on symptoms and FAO and early intervention may benefit patients by changing the natural history of disease.
Introduction
Chronic bronchitis (CB), also known as chronic mucous hypersecretion, is a major public health burden worldwide(1) and is variably defined, based on the presence and duration of cough, phlegm, or their combination(2,3). Partly due to varying definitions, the prevalence of CB in population studies ranges from 0.7% to 30% of adults(4,5). CB can lead to the development of chronic obstructive pulmonary disease (COPD), characterized by airflow obstruction (1). Even in the absence of fixed airflow obstruction (FAO), CB is associated with poor quality of life(6), acute respiratory exacerbations(7)(8), and mortality(9). Because patients with CB alone present with worse symptoms than patients with FAO alone(6), a high proportion of CB patients without FAO are treated with inhalers and even oral corticosteroids(8).
Despite the adverse impact of productive cough, there is a paucity of guidance on its evaluation and treatment, and in the absence of FAO, it has been excluded from professional COPD guidelines (GOLD, ATS). Smoking cessation and injurious inhalant avoidance may prevent and/or resolve symptoms of productive cough(10), but the neutrophilic airway inflamatory process can persist(11). Thus, FAO may result from seemingly resolved CB episodes(7). Unanswered questions in the field include the minimal duration of productive cough symptoms that are associated with long term outcomes and whether the early initiation of therapies during the evolution of the disease can prevent chronicity. To further characterize smokers with symptoms, the field would benefit from a more granular severity grading of productive cough (PC), encompassing all its common manifestations, instead of the current binary diagnosis that defines CB (2).
Previous attempts to grade the severity of CB have included pathological and bronchoscopic classifications(12,13), but their invasiveness precluded wide acceptance. The large-scale attempt to include CB in a classification scheme occurred in 2001 Global Obstructive Lung Disease strategy of using GOLD stage 0(14). Its inclusion was however debated (15,16), and removed from subsequent editions because of paucity of evidence at the time for the progression of patients from GOLD 0 to GOLD ≥1 stages(16). Currently, COPD guidelines fail to address the treatment of patients having CB without FAO(17).
Grading COPD severity using spirometry is justified because of its association with morbidity and mortality(18). These classifications summarize complex information to guide practitioners to prognosticate, prescribe stage-appropriate therapies, develop research protocols, compare performance and study disease processes(14). In this study, we investigated whether PC can similarly be graded in severity, based on the presence and duration of symptoms (i.e., cough, phlegm and wheeze), and the presence of fixed airflow obstruction.
Methods
Study design, setting, and populations
The study used the Lovelace Smokers Cohort (LSC) data for cross sectional grading of severity of PC and for longitudinal transition analyses among the resulting states. The mean follow-up duration was 6 years (19). Results were validated in the COPDGene cohort (COPDGene) with a mean follow-up for 5 years. The characteristics of both cohorts have been previously published(20–22), and a brief description is in the Online Data Supplement.
Internal validation for the associations with the categories related to SGRQ impact and activity subscales was obtained using the quality of life scale SF36. Further validation was obtained from spirometry results, the number of exacerbations, levels of the inflammatory biomarker CC16(23) that was available only in the LSC and radiographical measures of airway wall thickness that was available only in the COPD Gene cohort.
Definitions
We defined the 5 severity categories based on the presence and duration of symptoms (i.e., cough, phlegm, and wheeze) and presence of fixed airflow obstruction. Questions regarding symptoms were abstracted from the American Thoracic Society Division of Lung Disease (ATSDLD) 1978 Questionnaire 1 (2), FAO was defined as post-bronchodilator forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) ratio <0.7, and the resulting severity categories are listed in the Online Data Supplement and in the STROBE population flowcharts (Figures e1 and e2).
Individuals with current or past smoking history and normal spirometry and without cough or phlegm were classified as Healthy Smokers. PC and Chronic PC included those with productive cough for most days for at least 3 months annually, for either less than 2 years or for greater than 2 years, respectively. This division was based on the hypothesis that duration of symptoms was associated with severity of CB. Additional groups included PC with Signs of Airflow Obstruction and Chronic PC with Signs of Airflow Obstruction, if in addition to the above criteria, patients had self-reported wheeze and/or FAO. This classification was based on our previous findings that the presence of self-reported wheeze was strongly associated with worse respiratory health status of subjects with CB(6), and finding of others that the presence of wheeze predicted FAO(24). We considered to subdivide PC and Chronic PC with Signs of Airflow Obstruction based on the individual components of wheeze and FAO, but the individual subgroups were not large enough to justify their categorization (Figure e3, for example, substate Productive cough + FAO without wheeze was infrequent, i.e., 0–2.7%% in both cohorts).
Exclusion criteria
Initially subjects were excluded if they had self-reported, provider-diagnosed asthma or a postbronchodilator improvement of ≥200ml and/or ≥12% in FEV1 and/or FVC. These parameters were used to decrease heterogeneity of the study population to ensure that the presence of self-reported wheeze was not related to asthma, even at the cost of excluding some subjects with FAO with bronchodilator response. Further, patients with FAO only (defined as postbronchodilator FEV1/FVC<0.7 and the absence of cough and phlegm production) were excluded from the cross-sectional analysis to focus on severity states (this group was however included in the longitudinal analysis as described below). Because cough can be present for reasons other than CB(25), participants who reported cough without phlegm production, were also excluded from the cross-sectional analysis.
Measurements
In both cohorts, demographics including smoking history, past medical history and medication use were based on the ATS-DLD 1978 questionnaire(2). Because the groups were defined by symptoms, which are also included in the St. George’s Respiratory Questionnaire (SGRQ) total scale and symptom subscale, we used the SGRQ impact and activity subscales for validation (26), The Medical Ou tcomes Study 36-Item Short Form Health Survey (SF-36)(27) was used to evaluate general quality of life, and to confirm the findings from the SGRQ, with lower SF-36 scores indicating worse health status. FAO, as defined previously, was established by postbronchodilator spirometry test. Both cohorts adhered to strict spirometry quality control guidelines(21,22). Circulating club cell protein 16 (CC16) levels were quantified in LSC participants by ELISA as described(23). In COPDGene, airway wall thickness was measured using high-resolution inspiratory and expiratory computed tomography (CT) chest images, as previously described using wall area percent and Pi10 (the square root of the area of a standardized airway with an internal perimeter of 10mm)(22).
Statistical Analysis
The final portion of the cross-sectional analysis included multivariable ordinal regression modeling for predictors of PC disease severity. Candidate variables for the model were determined a priory: age, gender, race, cumulative smoking history, current smoker, wood smoke exposure, dust and fumes exposure, obesity, hypertension, FEV1, wall area %, Pi10 and CC16 levels.
Statistics included multivariate analysis of variance (MANOVA) with post hoc Tukey’s test for continuous variables and Jonckheere-Terpstra tests for categorical variables to address differences between PC states. Multivariable adjusted ordinal logistic regression models with groups ranked according to respiratory health status impairment as the outcome were also performed in both cohorts.
To follow changes in the severity of PC states over time, we used a multi-state Markov-like model similar to our recently published study(28). In the Markov chain literature, going from one state (prior state) to another (current state), even if they return to their original state, is called a transition. I n cases where individuals go from a disease state to a healthy state, this is termed a good or beneficial transition, whereas going from a healthy or a low severity state to a high severity state is considered a poor or harmful transition(28). Disease resolution was defined as a transition from any PC state to the ‘Healthy Smoker’ state. Disease stability was defined as a ‘transition’ to the same state (i.e., no change in state from the prior measurement). For transition probabilities to be a reliable representation of disease evolution, all possible outcome states have to be represented in the model; therefore, the two non-PC states, (i.e., patients with FAO only and smokers with cough only but without phlegm production), were included in longitudinal models (these states were excluded in the original cross-sectional analysis). For this reason, this analysis included 1,710 LSC and 2,761 COPDGene participants. Additional information on this analysis can be found in the Online Data Supplement.
Finally, we performed a sensitivity analysis with alternate PC state definitions as detailed in the Online Data Supplement. Statistical Analysis Software SAS 9.4 (Cary, NC) was used for analysis. P values < 0.05 were considered to be significant.
RESULTS
A comparison of demographic characteristics of the LSC and COPDGene cohorts can be found in Table e-1 in the Online Data Supplement. After exclusions, 1,422 LSC and 4,488 COPDGene participants were analyzed (STROBE diagrams 1 and 2 in the Online Data Supplement). Healthy smokers accounted for 61.2% and 60.5%; PC for 5.7% and 6.2%; chronic PC for 4.9% and 3.1%; PC with signs of airflow obstruction for 5.8% and 9.9%; and chronic PC with signs of airflow obstruction for 22.4% and 20.3% of the baseline LSC and COPDGene cohorts, respectively.
The SGRQ impact and activity subscales showed progressively and significantly worse scores from ‘Healthy Smokers’ to ‘Chronic PC with Signs of Airflow Obstruction’ states in both cohorts (Table 1). Additionally, most post hoc pairwise comparisons between the states were statistically significant in both cohorts, even though COPDGene demonstrated higher absolute scores than LSC for all PC states. As expected, the scoring for the total SGRQ score and symptoms subscale across the states showed a similar pattern as the impact and activity subscales, as shown in the Online Data Supplement. The eight SF-36 subscale scores generally decreased (i.e., worsened) with increasing PC severity, including the SF-36 Mental Health and SF-36 General Health, (Tables e-2a and e-2b).
Table 1:
St George’s Respiratory Questionnaire activity and impact subscales differences between rank-ordered productive cough severity states in the Lovelace Smokers’ Cohort (n=1422) and COPDGene Cohort (n=4488).
| Cohort | SGRQ | Healthy Smokers | Productive Cough | Chronic Productive Cough | Productive Cough with Signs of Airflow Obstruction | Chronic Productive Cough with Signs of Airflow Obstruction | p value |
|---|---|---|---|---|---|---|---|
| LSC | Activity | 22.2 ± 21.4d,e | 25.2 ± 20.0e | 27.5 ± 19.9e | 32.0 ± 20.4a,e | 41.2 ± 24.1a,b,c,d | p<0.001 |
| Impact | 5.6 ± 8.9d,e | 7.6 ± 10.2e | 9.1 ± 10.5e | 11.8 ± 11.0a,e | 19.1 ± 15.4a,b,c,d | p<0.001 | |
| COPDGene | Activity | 17.6 ± 21.3b,c,d,e | 28.7 ± 24.9a,d,e | 29.3 ± 25.4a,d,e | 43.6 ± 25.6a,b,c,e | 54.5 ± 27.5a,b,c,d | p<0.001 |
| Impact | 4.9 ± 9.6b,c,d,e | 10.5 ± 13.6a,d,e | 13.0 ± 14.6a,d,e | 20.7 ± 17.7a,b,c,e | 31.7 ± 21.8a,b,c,d | p<0.001 |
St George’s Respiratory Questionnaire (SGRQ) measures’ mean score ± standard deviation for each rank-ordered productive cough severity state.
LSC: Lovelace Smokers Cohort
p values for ANOVA results.
different from Healthy Smokers
different from Productive Cough
different from Chronic Productive Cough
different from Productive Cough with Signs of Airflow Obstruction
different from Chronic Productive Cough with Signs of Airflow Obstruction, respectively in post hoc Tukey’s tests
The baseline characteristics of the rank-ordered PC states for the two cohorts are described in the univariate analysis in Tables 2a and 2b. For both cohorts, significant differences were observed among the groups in the distribution of males, current smokers, those exposed to dust and fumes, in the mean pack-years smoked, and spirometric parameters (FEV1, FVC, and FEV1/FVC). The proportion of males and dust/fume exposures increased, and spirometric parameters decreased with increasing severity. In the LSC, wood smoke exposure was more frequently reported; and plasma CC16 levels were lower in the higher severity groups (Table 2a and 3). Exacerbations were overall infrequent in the LSC, but a gradual increase in prevalence of respiratory exacerbations with increasing severity was observed in the COPDGene cohort. In the COPDGene Cohort, measures for airway wall thickening and Pi10 values were greater with higher severity (Table 2b and 3).
Table 2a.
Baseline characteristics of productive cough severity states, determined by SGRQ activity and impact score, in the Lovelace Smokers Cohort, NM (n=1422)
| Healthy Smokers n=870 | Productive Cough n=81 | Chronic Productive Cough n=69 | Productive Cough with Signs of Airflow Obstruction n=83 | Chronic Productive Cough with Signs of Airflow Obstruction n=319 | |
|---|---|---|---|---|---|
| Age (years) | 55.0 ± 8.9 | 56.0 ± 10.5 | 54.0 ± 9.1 | 51.0 ± 7.3 | 55.0 ± 9.3 |
| Male (%)* | 18.2 | 28.4 | 23.2 | 18.1 | 37.9 |
| Hispanic (%) | 17.9 | 22.2 | 18.8 | 19.3 | 18.8 |
| ≥ High school education (%)* | 75.2 | 67.9 | 60.9 | 68.7 | 65.8 |
| BMI (kg/m2) † | 28.5 ± 6.4 | 28.1 ± 4.8 | 28.1 ± 7.2 | 28.1 ± 5.8 | 27.7 ± 5.8 |
| Obese (%) | 32.6 | 28.4 | 24.6 | 28.9 | 30.7 |
| Current Smoker (%)* | 50.3 | 60.5 | 79.7 | 81.9 | 84 |
| Pack-years* | 36.0 ± 19.4 | 34.0 ± 16.8 | 40.1 ± 18.8 | 33.8 ± 16.0 | 43.3 ± 22.2 |
| Dust and Fume exposure (%)* | 23.4 | 29.6 | 20.3 | 37.3 | 46.7 |
| Wood smoke exposure (%)* | 22.9 | 24.1 | 17.4 | 32.9 | 36.1 |
| FEV1/FVC* | 78.7 ± 4.7 | 79.0 ± 4.8 | 78.0 ± 4.6 | 78.8 ± 4.5 | 69.7 ± 11.8 |
| FEV1 (liters) † | 2.7 ± 0.7 | 2.8 ± 0.7 | 2.8 ± 0.8 | 2.8 ± 0.7 | 2.6 ± 0.8 |
| FEV1% predicted* | 94.5 ± 13.7 | 96.6 ± 15.2 | 93.0 ± 13.1 | 94.0 ± 13.2 | 83.5 ± 19.8 |
| FVC (liters)* | 3.5 ± 0.8 | 3.5 ± 0.9 | 3.6 ± 1.0 | 3.6 ± 0.9 | 3.7 ± 1.1 |
| FVC% predicted | 94.3 ± 13.4 | 95.7 ± 14.2 | 93.8 ± 13.1 | 94.4 ± 12.5 | 93.2 ± 16.4 |
| tCC16(ng/ml)* | 1.23 ± 0.6 | 1.29 ± 0.7 | 0.68 ± 1.1 | 0.97 ± 0.6 | 0.95 ± 0.7 |
| Exacerbations (%) | 6 | 6.2 | 8.7 | 7.2 | 6.3 |
Results are presented as mean ± standard deviation for continuous variables and percentage for proportions.
BMI: body mass index
FEV1: forced expiratory volume in liters during the first second.
FVC: forced vital capacity in liters.
tCC16: Club cell protein 16 (logarithmically transformed because of non-normal distribution)
SGRQ: St. George’s Respiratory Questionnaire.
p<0.001
p<0.05
Table 2b.
Baseline characteristics of productive cough severity states, determined by SGRQ total score, in the COPDGene cohort (n=4488).
| Healthy smokers n=2717 | Productive Cough n=278 | Chronic Productive Cough n=137 | Productive Cough with Signs of Airflow Obstruction n=444 | Chronic Productive Cough with Signs of Airflow Obstruction n=912 | |
|---|---|---|---|---|---|
| Age (years) | 58 ± 8.6 | 56.0 ± 8.3 | 56.0 ± 8.3 | 55.0 ± 7.4 | 59.0 ± 8.6 |
| Male (%)* | 52.6 | 59.4 | 58.4 | 57.9 | 63.9 |
| African American (%) | 35.1 | 53.2 | 36.5 | 48 | 26.2 |
| ≥ High school education (%)* | 89.3 | 86.7 | 91.2 | 84 | 84.6 |
| BMI (kg/m2) | 29.0 ± 5.9 | 28.5 ± 5.8 | 27.7 ± 5.9 | 29.5 ± 6.2 | 28.8 ± 6.6 |
| Obese (%) | 37.5 | 37.4 | 27.7 | 40.8 | 35.9 |
| Current smoker (%)* | 46.4 | 76.3 | 76.6 | 79.5 | 69.8 |
| Pack-years* | 36.7 ± 20.5 | 38.8 ± 20.1 | 38.6 ± 18.0 | 39.4 ± 20.6 | 51.7 ± 27.1 |
| Dust and fumes exposed (%)* | 50.8 | 59.4 | 65.7 | 65.3 | 69.1 |
| FEV1/FVC* | 78.7 ± 5.1 | 78.9 ± 5.4 | 78.4 ± 5.5 | 77.8 ± 6.6 | 62.9 ± 16.6 |
| FEV1 (liters)* | 2.8 ± 0.7 | 2.8 ± 0.7 | 3.0 ± 0.7 | 2.7 ± 0.8 | 2.1 ± 0.9 |
| FEV1% predicted* | 93.6 ± 15.2 | 94.2 ± 14.5 | 95.0 ± 15.3 | 89.1 ± 16.6 | 69.5 ± 25.5 |
| FVC (liters)* | 3.6 ± 0.9 | 3.6 ± 0.9 | 3.8 ± 0.9 | 3.5 ± 1.0 | 3.3 ± 1.1 |
| FVC% predicted* | 92.4 ± 14.6 | 93.4 ± 14.3 | 94.5 ± 15.2 | 89.5 ± 15.6 | 83.5 ± 19.0 |
| Wall area % * | 63.0 ± 2.2 | 63.5 ± 2.3 | 62.9 ± 2.3 | 63.5 ± 2.2 | 64.5 ± 2.7 |
| Pi10 (X 100)* | 364.4 ± 11.3 | 364.8 ± 10.8 | 366.9 ± 12.1 | 366.4 ± 11.7 | 369.2 ± 14.3 |
| CT defined bronchiectasis | 404(16.8) | 38(15.6) | 19(16.8) | 50(13.2) | 169(20.7) |
| Exacerbations (%)† | 5 | 5.8 | 7.3 | 15.1 | 30.4 |
Results are presented as mean ± standard deviation for continuous variables and percentage for proportions.
BMI: body mass index
FEV1: forced expiratory volume in liters during the first second.
FVC: forced vital capacity in liters.
Wall area %: (wall area/total bronchial area) x100
Pi10: square root of the wall area of an airway with an internal perimeter of 10mm.
SGRQ: St. George’s Respiratory Questionnaire.
p<0.001
p<0.05
Table 3.
Select predictors of higher productive cough severity states, using multivariable ordinal logistic regression for the Lovelace Smokers’ Cohort (LSC) and the COPDGene Cohort.
| Statistical Models | Predictor variable | LSC OR (95% CI) | p value | COPDGene OR (95% CI) | p value |
|---|---|---|---|---|---|
| Model 1 | Age (in years) | 1.01 (0.99, 1.02) | 0.3 | 1.02 (1.01, 1.03) | <0.001 |
| Male sex | 1.78 (1.38, 2.3) | <0.001 | 1.11 (0.97, 1.26) | 0.13 | |
| African American race | - | - | 0.58 (0.5, 0.67) | <0.001 | |
| Hispanic ethnicity | 0.77 (0.58, 1.12) | 0.25 | - | - | |
| Obese | 1.02 (0.8, 1.31) | 0.88 | 1.05 (0.92,1.19) | 0.48 | |
| Pack-years/10 | 1.12 (1.05, 1.19) | <0.001 | 1.18 (1.15, 1.22) | <0.001 | |
| Current smoker | 5 (3.82, 6.54) | <0.001 | 4.19 (3.58, 4.91) | <0.001 | |
| Dust and fume exposure | 1.99 (1.58, 2.52) | <0.001 | 1.69 (1.48, 1.92) | <0.001 | |
| Hypertension | 1.34(1.04, 1.71) | 0.023 | 1.3(1.14, 1.48) | <0.001 | |
| Model 1 + Wall area % subsegmental | 1.2 (1.14, 1.26) | <0.001 | |||
| Model 1 + Pi10 × 100 | 1.02 (1.02, 1.03) | <0.001 | |||
| Model 1 +FEV1 (in L/sec.) | 0.46 (0.37, 0.58) | <0.001 | 0.29 (0.26, 0.32) | <0.001 | |
| Model 1 + Wood smoke exposure | 1.33 (1.01, 1.75) | 0.02 | |||
| Model 1 + tCc16 (ng/mL) | 0.72 (0.57, 0.92) | 0.01 | |||
Multivariable ordinal logistic regression including preselected baseline characteristics and exposure variables as predictors and rank ordered chronic bronchitis severity groups as dependent variable. Standard covariates included age, sex, predominant minority, obesity, pack-years current smoker, dust and fumes exposure and the comorbidity hypertension. The odds ratio for wall area %, Pi10, FEV1, wood smoke, and tCC16 were obtained from separate models adjusted for model 1 covariates.
Pi10: standardized airway wall thickness of an airway with an internal diameter of 10mm.
Data for wall area % subsegmental was available in only 1,444 subjects of the COPDGene Cohort.
FEV1: forced expiratory volume in liters during the first second. tCC16: logarithmically transformed club cell protein 16.
In multivariable ordinal logistic regression analyses (Table 3) with rank-ordered PC states according to increasing SGRQ as the outcome, there was notable concordance in strength and direction of association for predictor variables as the univariate analysis (Tables 2a and 2b). Among comorbidities, hypertension was the only one included because its baseline prevalence was greater than 10% in both cohorts. The predominant minority populations included in the LSC and COPDGene cohorts were Hispanics and African American respectively, with both showing protective associations, which was significant for African Americans. Male sex was associated with increasing severity in the LSC while it only showed a trend in COPDGene (Table 3). The two way interactions between male sex and African American race and between male sex and pack years on severity were significant, indicating that men compared to women may be differentially impacted by heavy smoking and protected by African American ancestry (Figure e4A, B).
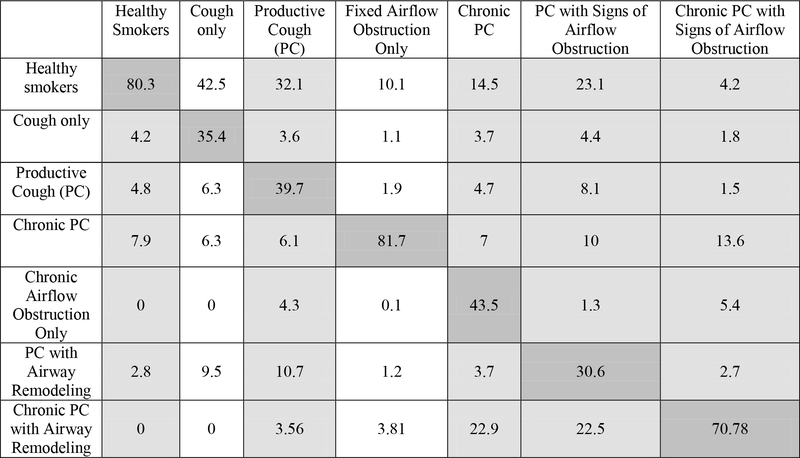
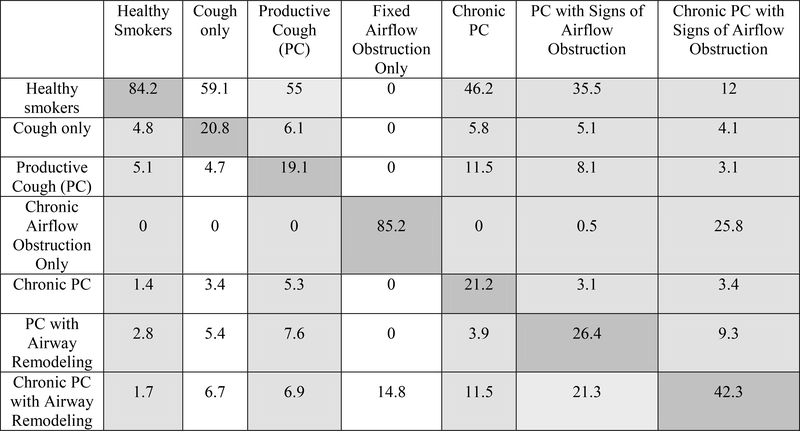
In the longitudinal evaluation of transitions, including 1,710 and 2,761 participants from LSC and COPDGene respectively, the probability of staying within the same severity stage group (i.e., diagnostic stability) was highest among Healthy Smokers and Chronic PC with Signs of Airflow Obstruction, the extreme severity grades (Figure 1a and 1b). Further, resolution to healthy smoker (i.e., diagnostic reversal) occurred from all severity categories, but was more frequent among subjects in lower severity groups (i.e., PC), and least among those in the most severe groups (i.e., Chronic PC with Signs of Airflow Obstruction).
Figure 1a.
Transition probability matrix in the Lovelace Smokers Cohort - included in the longitudinal analysis was the ‘Cough only’ and ‘Fixed airflow obstruction only’ states which were not included in the cross-sectional analysis. (n=1710)
Multi-state Markov-like model analyzing longitudinal transition probabilities between productive cough disease severity states over approximately 5 years in the Lovelace Smokers’ Cohort. The table is read vertically with the header representing the baseline state and each column in the vertical axis the transition probabilities to all possible states. Strength of transition probabilities is reflected by the percentage in each cell. Light grey cells denote the PC stage and white cells the non-PC stages. Dark grey cells following the diagonal axis of the table represent transition probabilities of staying within the same severity stage between study examination visits at 18-month intervals
Figure 1b.
Transition probabilities in the COPDGene Cohort - included in the longitudinal analysis were the ‘Cough only’ and ‘Fixed airflow obstruction only’ states which were not included in the cross-sectional analysis.(n=2761)
Multi-state Markov-like model analyzing longitudinal transition probabilities between productive cough disease severity states over approximately 5 years in the COPDGene cohort. The table is read vertically with the header representing the baseline state and each column in the vertical axis the transition probabilities to all possible states. Strength of transition probabilities is reflected by the percentage in each cell. Light grey cells denote the PC stage and white cells the non-PC stages. Dark grey cells following the diagonal axis of the table represent transition probabilities of staying within the same severity stage between study examination visits at 5-year intervals.
DISCUSSION
The present study shows that the severity of Productive Cough (PC) can be graded based on duration and presence of symptoms and airflow obstruction into groups of progressively greater impairment. Greater severity of PC was associated with greater wood smoke and cigarette smoke exposure, greater airway wall thickness, and lower plasma CC16 levels. On longitudinal follow up, beneficial transitions, including disease resolution, were more likely to occur in the less severe PC states, highlighting the importance of early intervention (Figure 2).
Figure 2:
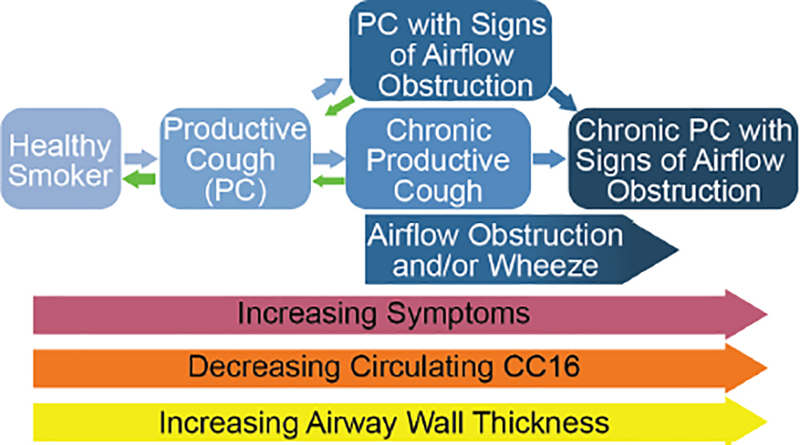
Schematic overview of PC by severity and the associated symptoms, circulating CC16 levels, and airway wall thickness. Note that transition to a healthier state drops once the most severe state is reached.
After Fletcher (29) failed to demonstrate a clear association between the presence of CB and the development of FAO and despite later findings to the contrary by other investigators(30), CB was described as a ‘benign’ condition. However, PC, even in the absence of FAO, are associated with impaired respiratory health status and general quality of life, and with respiratory exacerbations, and can lead to the development of COPD(6–9). Grading the severity of PC is a novel approach to the study of CB and attempts to capture the entire spectrum of PC symptoms, including those too mild to have been considered in the classic binary definition to the most severe cases. The resulting grading could be used to measure disease severity and highlights the importance of inquiring about the presence of wheeze in smokers.
We included self-reported wheeze as a marker of PC severity because wheezing adversely impacts the quality of life in patients with COPD and CB(6). Also, several studies on COPD have mentioned wheeze as one of the predicting factors to help identify patients who are candidates for further evaluation via spirometry (24). Although “wheeze” is traditionally associated with asthma, it is also present in patients with CB and CAO phenotypes of COPD. In our analyses, after excluding participants with reversible airflow obstruction or a history of asthma, many included participants (11% in LSC and 45% in COPDGene) reported wheeze. The report of wheeze represents perceived audible change in breathing (either transient or persistent), likely associated with the narrowing of airway lumen. The latter is likely due to the presence of inflammation-induced airway remodeling causing smooth muscle and basement membrane thickening, along with increased mucus secretion that further obstructs the airflow. In COPD subjects, the airway smooth muscle layer is thicker than in control subjects and characterized by increased extracellular matrix (31). We propose that the wheeze observed independent of asthma is a result of fixed small airway remodeling observed in COPD subjects. Our analysis supports the inclusion of wheeze as a simple marker of PC severity and airway remodeling. After adjusting for PC severity and FAO, linear regression analyses with PC states, FEV1/FVC and self-reported wheeze as predictors and the radiographic measures of airway remodeling, wall area % at the sub-segmental and segmental levels and Pi10 consistently reported wheeze as an independent and direct predictor of airway wall thickness (Table e3, Online Data Supplement). Also, our sensitivity analysis showed that the severity staging was not altered even after the inclusion of participants with reversible airway obstruction or a history of asthma (Table e4).
In our study, men demonstrated greater severity of PC in the LSC than women, and there was a similar trend in the COPDGene cohort. Although not entirely comparable to our severity grading, the role of gender as a risk factor for CB is conflicting across the published literature(5,32–34).
Our proposed grading of severity for PC is consistent with previous reports that circulating levels of CC16 are associated with emphysema (35) and CB(23), and the loss of CC16 is closely associated with increasing COPD severity(36). Further, other studies have proposed that airway wall thickening is a marker for CB as determined by pathological examination of tissues (13) and CT imaging of patients with CB(37). Therefore, objective measures known to be associated with CB correlate with our proposed severity staging.
CB with the presence of severe FAO (partly equivalent to Chronic PC with Signs of Airflow Obstruction) is already partly addressed by clinical guidelines with therapies such as phosphodiesterase 4 inhibitors (38), and long acting bronchodilators(39–41). By including the dimensions of time and presence of complicating factors - wheeze and airflow obstruction-, the proposed classification attempts to capture the entire spectrum of PC symptoms. We believe these findings bridge the gap between real world patients, and current COPD guidelines. As smokers with normal spirometry were shown to have symptomatic exacerbations, questions were raised whether these patients should be included as COPD patients or as a different entity(8). Our findings provide supporting evidence for exploring this group more carefully.
Our longitudinal analyses found that disease resolution is possible, especially among the milder PC (i.e., Productive Cough and Chronic Productive Cough). We and others have recently reported that spirometrically-defined COPD states, may not be uniformly progressive and can improve or resolve over time(28)(42)(43). Treatment with inhaler medications was more frequent in the higher severity PC states from which resolution of disease was less likely (Table e.5.). Overall, the implication of these findings is that the early stages of airflow obstruction or PC are the optimal stages of COPD for initiating interventions to reverse or change the natural history.
The strengths of our analysis include the innovative approach to a common disease, external validation with large sample sizes, representation from most geographical areas in the United States, inclusion of two different minorities, good female representation, and a strong internal validation provided by the congruence of findings from radiological anatomy, spirometry, and inflammatory markers.
Our study has several limitations. First, the remitting-relapsing nature of the disease and the occurrence of disease in a minority of those exposed may explain the relatively small numbers of patients in the Productive Cough, Chronic Productive Cough and Productive Cough with Signs of Airflow Obstruction states in both cohorts.. Second, we excluded patients with reversible airflow obstruction and a history of asthma but are unable to exclude patients with productive cough originating from outside the lower respiratory tract (such as chronic rhino-sinusitis) or patients with bronchiectasis. However, using CT presence of bronchiectasis in the COPDGene cohort, we demonstrate no significant difference in bronchiectasis between the various PC states in Table 2b. We used a FEV1/FVC < 0.7 in a post bronchodilator spirometry to define fixed airflow obstruction. This approach is associated with age bias with overdiagnosis in the elderly and underdiagnoses in younger populations. However as shown in Table e.6 the misclassification from our approach, in comparison to use of lower limit of normal criterion to define FAO, was minimal and does not modify our findings. Finally, both cohorts recruited individuals not from the general population at random but by using telephone and radio advertisement representing a less severe group (LSC) and from patients in pulmonary clinics with more severe disease (COPDGene). Although it is possible that both cohorts had a selection bias, our external validation process confirms the generalizability of our study results. Our staging will need to be externally validated in additional cohorts and should be improved and amended as new knowledge is generated to better reflect the natural history of the disease. However, once validated, the proposed severity staging may be useful in developing new biomarkers of disease severity and to start analyzing the appropriate timing of interventions such as smoking cessation or appropriate management strategies, including inhaler prescriptions.
Supplementary Material
Acknowledgements
We thank Dr. Clifford Qualls, University of New Mexico Health Sciences Center, for help with the interpretation of the multi-state Markov-like model analyses, and Ms. Suzanne C Lareau RN, MS, FAAN for the careful editing of the manuscript.
Sources of Support: This work was supported from funding by the State of New Mexico (appropriation from the Tobacco Settlement Fund), and from the National Institutes of Health (RO1 ES015482 and RO1 HL068111 to YT).
Footnotes
Declaration of interest
The authors report no conflict of interest.
References
- 1.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006. September;28(3):523–32. [DOI] [PubMed] [Google Scholar]
- 2.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978. December;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 3.de Oca MM, Halbert RJ, Lopez MV, Perez-Padilla R, Tálamo C, Moreno D, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012. July;40(1):28–36. [DOI] [PubMed] [Google Scholar]
- 4.Cerveri I, Accordini S, Verlato G, Corsico A, Zoia MC, Casali L, et al. Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adults. Eur Respir J. 2001. July 1;18(1):85–92. [DOI] [PubMed] [Google Scholar]
- 5.Lu M, Yao W, Zhong N, Zhou Y, Wang C, Chen P, et al. Chronic obstructive pulmonary disease in the absence of chronic bronchitis in China. Respirology. 2010. October 1;15(7):1072–8. [DOI] [PubMed] [Google Scholar]
- 6.Meek PM, Petersen H, Washko GR, Diaz AA, Kim V, Sood A, et al. Chronic Bronchitis Is Associated With Worse Symptoms and Quality of Life Than Chronic Airflow Obstruction. Chest. 2015. August;148(2):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The Presence of Chronic Mucus Hypersecretion across Adult Life in Relation to Chronic Obstructive Pulmonary Disease Development. Am J Respir Crit Care Med. 2016. March 15;193(6):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med. 2016. May 12;374(19):1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelkonen M, Notkola I-L, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006. October;130(4):1129–37. [DOI] [PubMed] [Google Scholar]
- 10.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999. April;106(4):410–6. [DOI] [PubMed] [Google Scholar]
- 11.Willemse BWM, ten Hacken NHT, Rutgers B, Lesman-Leegte IG a. T, Postma DS, Timens W Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005. November;26(5):835–45. [DOI] [PubMed] [Google Scholar]
- 12.Thompson AB, Daughton D, Robbins RA, Ghafouri MA, Oehlerking M, Rennard SI. Intraluminal Airway Inflammation in Chronic Bronchitis: Characterization and Correlation with Clinical Parameters. Am Rev Respir Dis. 1989. December 1;140(6):1527–37. [DOI] [PubMed] [Google Scholar]
- 13.Reid L PATHOLOGY OF CHRONIC BRONCHITIS. The Lancet. 1954. February 6;263(6806):275–8. [PubMed] [Google Scholar]
- 14.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001. August;46(8):798–825. [PubMed] [Google Scholar]
- 15.Mannino DM. Gold stage 0 copd: Is it real? does it matter? Chest. 2006. August 1;130(2):309–10. [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J, Lange P. Can GOLD Stage 0 Provide Information of Prognostic Value in Chronic Obstructive Pulmonary Disease? Am J Respir Crit Care Med. 2002. August 1;166(3):329–32. [DOI] [PubMed] [Google Scholar]
- 17.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017. Report: GOLD Executive Summary | European Respiratory Society [Internet]. [cited 2017 Feb 27]. Available from: http://erj.ersjournals.com.libproxy.unm.edu/content/early/2017/01/30/13993003.002142017.1 [DOI] [PubMed]
- 18.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995. November;152(5 Pt 2):S77–121. [PubMed] [Google Scholar]
- 19.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015. July 9;373(2):111–22. [DOI] [PubMed] [Google Scholar]
- 20.Petersen H, Sood A, Meek PM, Shen X, Cheng Y, Belinsky SA, et al. Rapid lung function decline in smokers is a risk factor for COPD and is attenuated by angiotensin-converting enzyme inhibitor use. Chest. 2014. April;145(4):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood A, Petersen H, Blanchette CM, Meek P, Picchi MA, Belinsky SA, et al. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010. November 1;182(9):1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) Study Design. COPD. 2010. February;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen H, Leng S, Belinsky SA, Miller BE, Tal-Singer R, Owen CA, et al. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur Respir J. 2015. November 1;46(5):1501–3. [DOI] [PubMed] [Google Scholar]
- 24.Hanania NA, Mannino DM, Yawn BP, Mapel DW, Martinez FJ, Donohue JF, et al. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ). Respir Med. 2010. August;104(8):1160–70. [DOI] [PubMed] [Google Scholar]
- 25.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet Lond Engl. 2008. April 19;371(9621):1364–74. [DOI] [PubMed] [Google Scholar]
- 26.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther. 2000. September;22(9):1121–45. [DOI] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE, Rogers W, Raczek AE, Lu JFR. The Validity and Relative Precision of MOS Short- and Long-Form Health Status Scales and Dartmouth COOP Charts: Results from the Medical Outcomes Study. Med Care. 1992;30(5):MS253–MS265. [DOI] [PubMed] [Google Scholar]
- 28.Sood A, Petersen H, Qualls C, Meek PM, Vazquez-Guillamet R, Celli BR, et al. Spirometric variability in smokers: transitions in COPD diagnosis in a five-year longitudinal study. Respir Res. 2016;17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977. June 25;1(6077):1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherrill DL, Lebowitz MD, Knudson RJ, Burrows B. Longitudinal methods for describing the relationship between pulmonary function, respiratory symptoms and smoking in elderly subjects: the Tucson Study. Eur Respir J. 1993. March 1;6(3):342–8. [PubMed] [Google Scholar]
- 31.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004. June 24;350(26):2645–53. [DOI] [PubMed] [Google Scholar]
- 32.Accordini S, Corsico AG, Cerveri I, Antonicelli L, Attena F, Bono R, et al. Diverging trends of chronic bronchitis and smoking habits between 1998 and 2010. Respir Res. 2013. February 8;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meteran H, Backer V, Kyvik KO, Skytthe A, Thomsen SF. Heredity of chronic bronchitis: a registry-based twin study. Respir Med. 2014. September;108(9):1321–6. [DOI] [PubMed] [Google Scholar]
- 34.Ferré A, Fuhrman C, Zureik M, Chouaid C, Vergnenègre A, Huchon G, et al. Chronic bronchitis in the general population: influence of age, gender and socio-economic conditions. Respir Med. 2012. March;106(3):467–71. [DOI] [PubMed] [Google Scholar]
- 35.Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, et al. Genome-Wide Association Analysis of Blood Biomarkers in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2012. December 15;186(12):1238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015. June 1;45(6):1544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011. September;140(3):626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez FJ, Calverley PMA, Goehring U-M, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet Lond Engl. 2015. March 7;385(9971):857–66. [DOI] [PubMed] [Google Scholar]
- 39.Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N Engl J Med. 2007. February 22;356(8):775–89. [DOI] [PubMed] [Google Scholar]
- 40.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008. October 9;359(15):1543–54. [DOI] [PubMed] [Google Scholar]
- 41.Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016. September;374(23):2222–34. [DOI] [PubMed] [Google Scholar]
- 42.Aaron SD, Tan WC, Bourbeau J, Sin DD, Loves RH, MacNeil J, et al. Diagnostic Instability and Reversals of Chronic Obstructive Pulmonary Disease Diagnosis in Individuals with Mild to Moderate Airflow Obstruction. Am J Respir Crit Care Med. 2017. March 7;196(3):306–14. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez Guillamet R, Petersen H, Meek PM, Tesfaigzi Y, Sood A. Normalization of FEV1/FVC Ratio to >0.7 Does Not Equal Resolution of Disease. Am J Respir Crit Care Med [Internet]. 2017. November 6 [cited 2017 Nov 20]; Available from: http://www.atsjournals.org.libproxy.unm.edu/doi/abs/10.1164/rccm.201708-1693LE [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.