Summary
Consistent with its location on the ribosome, reporter assays demonstrate a role for Rps26 in recognition of the Kozak sequence. Consequently, Rps26-deficient ribosomes display specificity for mRNAs encoding components of the high salt and high pH stress response pathways and accumulate in yeast exposed to high salt or pH. Here we use this information to reprogram the cellular response to high salt by introducing point mutations in the Kozak sequence of key regulators for the cell wall MAP-kinase, filamentation, or DNA repair pathways. This stimulates their translation upon genetic, or salt-induced Rps26 depletion from ribosomes. Stress resistance assays show activation of the targeted pathways in an Rps26 and salt-dependent manner. Genomic alterations in diverse yeast populations indicate that analogous tuning occurs during adaptation to ecological niches. Thus, evolution shapes translational control across the genome by taking advantage of the accumulation of diverse ribosome populations.
Abstract Graphic

eTOC Blurb:
Ferretti et al. introduced point mutations into the Kozak sequence of key regulators of three yeast signaling pathways to render their translation responsive to Rps26 occupancy in ribosomes. This mechanism for ribosome-composition-mediated translational control – ribo-tuning – is facile to achieve and evidence for its use is found in nature.
Introduction
To thrive organisms must adapt to their environment. While more complex organisms can dynamically change the function of tissues (e.g. sweating in the heat), unicellular lifeforms must reshape their proteome to survive. Initial characterization of the yeast stress response painted a rich picture of the key proteins and signaling networks involved, but these changes were largely attributed to transcriptional regulation (Gasch et al., 2000). However, gene expression is regulated at every opportunity in the cell, and regulation at the translational level is no exception (Bhattacharyya et al., 2014; Dever et al., 2016; Hinnebusch et al., 2016; Lanctôt et al., 2007).
Despite their central role in protein production, ribosomes have not been thought to play an active role in the regulation of gene expression. Recent lines of evidence have begun to challenge this view: heterogeneity of ribosomal protein (RP) composition has been found across cell types (Shi et al., 2017; Slavov et al., 2015) and there is evidence that the composition of a ribosome can modulate its function (Ferretti et al., 2017; Shi and Barna, 2015; Xue and Barna, 2012). Mice lacking Rpl38 cannot effectively translate a subset of Hox gene mRNAs, resulting in tissue patterning defects (Kondrashov et al., 2011; Xue et al., 2015). In yeast, deletion of the ribosomal protein Asc1 (RACK1 in humans) results in less efficient translation of short mRNAs and increased expression of certain autophagy-inducing proteins (Kim et al., 2017; Thompson et al., 2016). Loss of certain RP paralogues in yeast disrupts translation of certain mitochondrial proteins (Segev and Gerst, 2018). Changes in ribosome composition also have implications for human health. Expression of RPs is dysregulated in multiple cancers and, loss of ribosome stoichiometry is correlated with poor clinical outcomes (Ajore et al., 2017; Kulkarni et al., 2017; Vlachos, 2017). Furthermore, diseases caused by RP-haploinsufficiency increase the risk of cancer, consistent with a link between ribosome composition and dysregulated translation of specific mRNAs (Armistead and Triggs-Raine, 2014; Sulima et al., 2017; Wong et al., 2014).
We have previously isolated ribosomes lacking Rps26 (ΔRps26) and used high-throughput sequencing to compare their bound mRNAs to those bound to ribosomes containing Rps26. These data, together with luciferase reporter assays, demonstrated a role for Rps26 in recognition of adenosine residues in the Kozak sequence, at position −4 upstream of the start codon; mutations at the −2 position were also Rps26-sensitive (Ferretti et al., 2017). Intriguingly, the Hog1 MAPK and the Rim101 pathways cluster mRNAs preferentially bound by ΔRps26 ribosomes, and accumulation of ΔRps26 ribosomes activates these pathways, which enables cells to respond to high salt and high pH stress, respectively (Ferretti et al., 2017). Furthermore, under these stress conditions ΔRps26 ribosomes accumulate.
Here, we extend our previous findings by introducing point mutations in the Kozak sequences of individual genes, thus ‘reprogramming’ several cellular pathways to become responsive to the levels of ΔRps26 ribosomes. We show that the activity of entire cellular pathways, and thereby the biological response to stress, can be modified via ribosome composition-mediated translational control, which we term “ribo-tuning”. These data demonstrate the importance of sequence-specific mRNA recognition by ribosome-bound Rps26 and show how evolution may take advantage of the relatively small fitness cost of modifying untranslated regions in mRNAs to allow for rapid adaptation to novel environmental stresses.
Results
Reprogramming the Cell Wall Integrity pathway
We have previously shown that the Cell Wall Integrity (CWI) pathway was not activated by accumulation of ΔRps26 ribosomes, even though many of the pathway’s mRNAs were enriched in ΔRps26 ribosomes. This behavior was likely due to the enrichment of a key pathway activator, the Rho1 GTPase, on Rps26-containing (wt) ribosomes, and the enrichment of its repressors (Sac7, Bag7, Bem2 and Lrg1) on ΔRps26 ribosomes (Figure 1A).
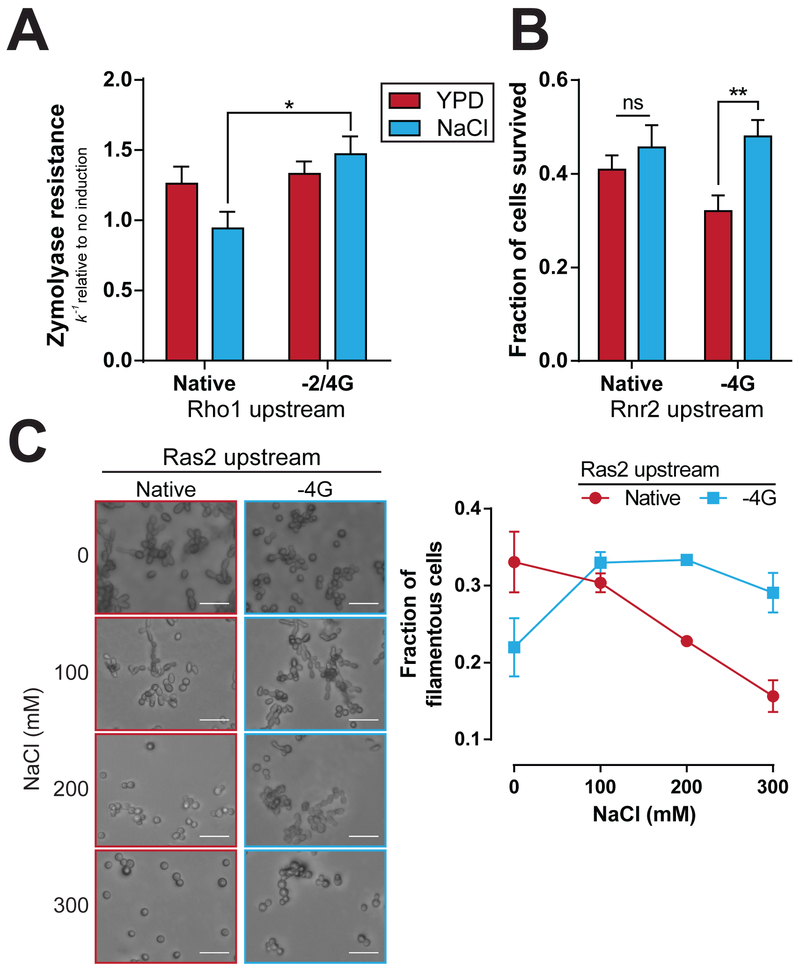
Figure 1. Modification of the Rho1 upstream sequence causes Rps26-dependent changes in translation.
A) Diagram of the CWI pathway. mRNAs in blue circles are enriched on ΔRps26 ribosomes, while those in orange/red rectangles are enriched on +Rps26 ribosomes (Ferretti et al., 2017). B) The Rho1 Kozak sequence was mutated as indicated. C) TET:Rps26 cells containing Rho1 with a native or −2/4 G upstream sequence were challenged with a zymolyase digestion assay. Cells were assayed before and after incubation with 20mM caffeine. ** is p=0.0072, ns is p=0.8862 by t-test; n=6. Rho1 protein levels in strains from C were analyzed by western blot (D) and quantified (E). Each of the samples is a biological replicate. ** is p=0.0017, ns is p=0.1526 by t-test; n=4. All error bars = SEM. See also Figure S1.
Preferential translation of Rho1 mRNA by wt ribosomes was also consistent with our mechanistic analysis, which had shown that recognition of −4 adenosine upstream of the start codon required Rps26, and suggested a role for Rps26 in recognition of adenosine at the −2 position (Ferretti et al., 2017). The Rho1 mRNA contains adenosine at both positions (Figure 1B). Given the large predisposition of the CWI pathway components to ΔRps26 ribosomes, we reasoned that by modification of the −2 and −4 residues upstream of Rho1, we should be able to ‘reprogram’ the CWI pathway, making it Rps26-responsive.
We cloned Rho1, including the 21bp upstream of the start codon into a constitutive yeast expression plasmid, and used site-directed mutagenesis to generate −2/4 A→G Rho1 (Figure 1B). The wt and mutant Rho1 plasmids were transformed into our previously described and validated yeast cells (TET:Rps26), in which Rps26 was under the control of a doxycycline (dox-) repressible promoter, where Rps26 deficiency is induced by addition of 100 ng/ml dox. Additionally, Rho1 was under GAL1 control, ensuring that, in glucose media, Rho1 was produced only from the plasmid. Similar to the parent strain (Ferretti et al., 2017), about 70% of Rps26 was depleted in this system (Figure S1A-B). To assay CWI signaling, we adapted a previously published method to measure cell wall strength (Kuranda et al., 2006). This assay takes advantage of the observation that the caffeine directly activates the CWI pathway through Rho1 (Kuranda et al., 2006; Levin, 2011; Martin et al., 2000), leading to increased resistance to zymolyase, which digests the cell wall and produces spheroblasts. In a hypotonic solution spheroblasts are sensitive to lysis, which is monitored by tracking the drop in OD600 of the culture (Figure S1C).
We compared the zymolyase resistance of cells expressing Rho1 preceded by its native sequence to those with a −2/4G in the presence or absence of Rps26. As expected from our previous data (Ferretti et al., 2017), the cells with the native Rho1 sequence showed no response to dox-induced Rps26-depletion. In contrast, the cells containing the −2/4G sequence exhibited a marked change in phenotype upon Rps26-depletion (Figure 1C). In the Rps26-replete cells (0 dox), the - 2/4G sequence imparted a defect in caffeine-induced zymolyase resistance. In contrast, Rps26 depletion increases the zymolyase resistance of cells with −2/4G Rho1 (Figure 1C). Thus, by changing two nucleotides upstream of the Rho1 start codon, we have rendered the CWI pathway Rps26-responsive.
To exclude the possibility that our data are the result of a ribosome depletion, and not specific to Rps26 depletion, we generated a control yeast strain in which Rps17 is under the control of the TET-repressible promoter and genomic Rho1 expression is under the control of GAL1. When we tested zymolyase resistance in this strain there was no Rps17-dependent difference for either Rho1 construct (Figure S1E). These findings support a model in which Rps26 is acting specifically through recognition of the −2 and −4 positions upstream of the start codon.
To confirm that CWI activation reflects Rho1 protein levels, as implied, we analyzed whole cell lysates via western blot. Consistent with the zymolyase assay, cells with the native Rho1 construct exhibited no change in Rho1 levels after Rps26 depletion. In contrast, in cells containing −2/4G Rho1 protein levels increased after Rps26 depletion (Figure 1D-E). These changes were due to translational reprogramming and not the result of transcriptional changes, as indicated by qPCR (Figure S1F). Additionally, cells with the −2/4G Rho1 construct contained less Rho1 in the presence of Rps26, but more after Rps26 depletion (Figure S1G-H).
The increase in Rho1 protein, combined with the zymolyase resistance, collectively support a model whereby the accumulation of ΔRps26 ribosomes increases the translation of the −2/4G Rho1 construct. Thus, we have reprogrammed the CWI pathway to become Rps26-responsive.
Reprogramming the DNA damage pathway
We next wanted to see if reprogramming could be achieved for other pathways. After examining our sequencing enrichment data, we found two other targets among well characterized yeast pathways that appeared amenable to reprogramming: the DNA-damage response pathway and the haploid filamentation pathway.
While GO-term analysis of mRNAs preferentially bound to ΔRps26 ribosomes indicated an enrichment of genes that are part of the DNA damage response pathway (Ferretti et al., 2017), careful analysis of the Rad53/Chek2-mediated pathway revealed that the mRNA encoding the key downstream effector Rnr2 is depleted from ΔRps26 ribosomes (Figure 2A). Rnr2 is part of the ribonucleotide reductase complex (RNR). RNR components are upregulated in response to DNA damaging agents and the complex is essential in generating the dNTP pool required for DNA replication and repair (Chabes et al., 2003; Desany et al., 1998; Elledge et al., 1992). Additionally, increased Rnr2 protein levels confer resistance to DNA damage (Abid et al., 1999; Rittberg and Wright, 1989), making it an ideal target for reprogramming. Thus, we produced Gal::Rnr2; TET:Rps26 cells and used site directed mutagenesis of plasmid-encoded Rnr2 to change the −4 thymine to a guanine (Figure 2B). This and the wild type plasmid were then transformed into our yeast strain and tested for their ability to confer resistance against methyl methanesulfonate (MMS), a DNA damaging agent. Cells expressing Rnr2 preceded either by the native or the −4G sequence were exposed to 0.4% MMS for 20 minutes with or without predepletion of Rps26, and their survival was measured by plating cells and comparing the difference in colony forming units.
Figure 2. Modification of the Rnr2 upstream sequence causes Rps26-dependent changes in translation.
A) Diagram of the DNA damage response pathway, color coded as in Figure 1 (Ferretti et al., 2017). B) The Rnr2 Kozak sequence was mutated as indicated. C) TET:Rps26 cells with the native or −4G Rnr2 upstream sequence were subjected to MMS, either with or without Rps26 depletion. After treatment, cells were plated and surviving colony forming units were counted. ** is p=0.0027, ns is p=0.1485 by t-test; for native, n=7 and for −4G n=6. Cells with HA tagged Rnr2 were grown as in C and analyzed by Western blot (D) and quantified (E). Each of the samples is a biological replicate. *** is p=0.00013, * is p=0.0384 by t-test; n=5. All error bars = SEM. See also Figure S2.
Cells with −4G Rnr2 showed a significant increase in MMS tolerance after depletion of Rps26, whereas cells containing the native Rnr2 upstream sequence showed no significant change in survival (Figure 2C). Again, control experiments confirmed that this effect was specific to depletion of Rps26 (Figure S2A). Furthermore, Western blotting and qPCR (Figure 2E and S2B) show that the survival effect was the result of an Rps26-dependent increase in Rnr2 translation in the −4G cells, while Rnr2 with the native Kozak sequence is down-regulated upon Rps26 depletion, as expected based on our previous mRNA enrichment (Ferretti et al., 2017). Thus, modification of the −4 residue upstream of Rnr2 produces Rps26-dependent changes in Rnr2 translation, which rewires the DNA damage repair pathway, making it inducible by depletion of Rps26.
Reprogramming the filamentation pathway
When S. cerevisiae cells growing on solid media begin to exhaust their carbon or nitrogen source, they activate a signaling pathway that induces a developmental program leading to pseudohyphal (for diploid cells) or filamentous (haploid) morphology (Blacketer et al., 1995; Cullen and Sprague, 2000). Analysis of this signaling pathway indicates that while the mRNAs encoding most components are enriched on ΔRps26 ribosomes, one of its key upstream regulators, the Ras2 GTPase, was depleted from ΔRps26 ribosomes (Figure 3A). Furthermore, the upstream sequence of Ras2 is highly enriched for adenosines, making it an attractive target for reprogramming via a −4 A→G mutation. The TET:Rps26 cells were based on the BY4741 parent strain, which contains a premature stop codon in Flo8, a gene critical to the pathway (Figure 3A), and is therefore unable to filament (Liu et al., 1996). Therefore, we deleted Rps26A in the filamentation-competent Σ1278b strain. Rps26A was then either reintroduced under the control of the strong, constitutively active, TEF promoter (TEF:Rps26) or cells were transformed with the vector only (VO). In addition, we placed Ras2 under GAL1 control and then reintroduced the gene on a plasmid under the control of its native promoter and terminator. In this genetic background, Rps26 levels in the VO cells are about 80% reduced relative to the Rps26-containing cells (Figure S3A-B). As before, we used site directed mutagenesis to produce a −4G Ras2 (Figure 3B) and used a standard agar invasion assay to test for adhesive growth (Blacketer et al., 1995; Cullen and Sprague, 2000). As previously described (Strittmatter et al., 2006), cells lacking Rps26A were severely limited in their ability to undergo adhesive growth and were washed off the plate almost entirely (Figure 3C and data not shown). However, the cells which remained on the plate displayed striking changes in morphology that were dependent on the Ras2 upstream sequence and Rps26 levels (Figure 3C-D). While cells with the native Ras2 sequence showed no major Rps26-dependent change in filamentous morphology, the cells containing −4G Ras2 are nearly devoid of filamentous morphology when Rps26 is replete, while the fraction of filamenting cells increased upon Rps26 depletion (Figure 3C-D).
Figure 3. Modification of the Ras2 upstream sequence causes Rps26-dependent changes in translation.
A) Diagram of the Ras2-dependent arm of the haploid filamentation pathway, color coded as in Figure 1 (Ferretti et al., 2017). B) The Ras2 Kozak sequence was changed as indicated. C) Σ1278b ΔRps26A cells with the native, or reprogrammed Rnr2 and with an empty vector or a plasmid containing Rps26A under the control of the TEF promotor were plated on YPD. After 5 days of growth, plates were washed and the adherent colonies were imaged. Scale bars = 20μm D) Quantification of C was performed by counting cells from a minimum of 3 randomly chosen fields of view for each replicate. Cells were classified as yeast form (ovoid) or filamentous. Aggregations in which individual cells could not be distinguished were not counted. **** is p<0.0001, ns is p=0.2658 by t-test; for TEF:Rps26 cells, n=5 and for VO n=7. E) Cells containing Ras2-HA were analyzed by Western blot. Each of the samples is a biological replicate. F) Quantification of E. * is p=0.0245, ns is p=0.639 by t-test; n=5. All error bars = SEM. See also Figure S3.
Next, Ras2 levels were examined by western blot via an HA-tag fused to Ras2. In cells depleted of Rps26, expression of −4G Ras2 is significantly increased. In contrast, expression of Ras2 in its native context is insensitive to Rps26 levels (Figure 3E-F). As before, control experiments demonstrate that this effect is specific to Rps26 depletion, and that it is not due to changes in Ras2 mRNA levels (Figures S3C-E), but instead the result of Rps26-mediated translational reprogramming of Ras2.
Collectively, these data show that modifications of just one or two bases within the Kozak sequence of a gene can render it translationally responsive to accumulation of ΔRps26 ribosomes in the cell, thereby activating an entire signaling pathway. We refer to this as “ribo-tuning”, as it requires changes in the ribosome composition that modulate transcriptional responses.
Salt-induced depletion of Rps26 from ribosomes activates reprogrammed pathways
Wild-type yeast cells exposed to high salt accumulate ΔRps26 ribosomes as part of an adaptive response (Ferretti et al., 2017). This enables the preferential translation of mRNAs from the Hog1 pathway, thus providing for a translational reinforcement of the well-characterized transcriptional changes upon high salt exposure (Ferretti et al., 2017). Given the accumulation of ΔRps26 ribosomes at high salt, we reasoned that the reprogrammed pathways should be activated in high salt as well.
To test this hypothesis, the inducible TET:Rps26A plasmid from our reprogrammed strains was swapped for a strong, constitutively active TEF:Rps26A construct, and the key assays for each pathway were repeated. Importantly, TEF:Rps26 cells grow as well as wild type yeast (Figure S4), indicating that they are Rps26 replete.
In testing the CWI pathway, we found that cells containing the native and −2/4G Rho1 show opposite responses to 1M NaCl (Figure 4A). When Rho1 is preceded by its native upstream sequence, exposure to salt has no significant effect on zymolyase resistance with a trend towards decreased resistance. With the −4G context, this trend is reversed, and resistance to zymolyase shows a trend towards improvement.
Figure 4. High salt stress recapitulates activation of reprogrammed pathways.
A) Cells expressing Rps26A from the constitutive TEF promoter (TEF:Rps26) and native or −2/4G Rho1 were grown in YPD with or without addition of 1M NaCl and then subjected to the zymolyase digestion assay as in Figure 1C. * is p=0.0135 by two-way ANOVA; n=4. B) TEF:Rps26 cells with native or reprogrammed Rnr2, grown in YPD +/− 1M NaCl and then challenged with an MMS survival assay as in Figure 2C. ** is p=0.008, ns is p=0.3925 by t-test; for YPD, n=8 and for NaCl n=4. C) Σ1278b yeast with reprogrammed or native Ras2 and TEF:Rps26A were grown on plates containing 0 – 300mM NaCl for 5 days and then washed and imaged as in Figure 3C. For 0mM NaCl, n=8 and for all others, n=4. Scale bars = 20μm, quantification is on the right. All error bars = SEM. See also Figure S4.
Testing of the DNA damage response pathway via the MMS survival assay revealed a similar tendency. Cells with the native upstream sequence of Rnr2 showed no significant change in survival when pre-treated with 1M NaCl for 6 hours. The cells with −4G Rnr2, however, did show a significant improvement in survival after exposure to high salt (Figure 4B).
Finally, we tested if the Rps26-responsive filamentation pathway reacts to high salt (Figure 4C). At NaCl concentrations above 300mM filamentous cells were extraordinarily rare regardless of the Ras2 context and no cell growth was observed even after 10 days (data not shown). At lower NaCl concentrations, the difference between the cells expressing the native and −4G Ras2 constructs was evident. Without salt addition, approximately one third of cells with native Ras2 exhibit filamentous morphology. As NaCl concentration increases, however, the fraction of filamentous cells begins to fall rapidly. Conversely, only 21% of cells with −4G Ras2 show filamentous morphology in the absence of NaCl, but the fraction increases to 33% at 100mM NaCl and 200mM NaCl. Even at 300mM NaCl, nearly 30% of the cells expressing the −4G Ras2 continue to display filamentous morphology.
Together these data show that single point mutations in the Kozak sequence can tune the physiological response to high salt stress to include a translational upregulation of the CWI, DNA repair or filamentation pathways in yeast. This is because in high salt media, ribosome populations are remodeled, leading to the accumulation of ΔRps26 ribosomes, which have a distinct mRNA preference (Ferretti et al., 2017).
Evolutionary co-opting of ribo-tuning
Given that single point mutations in several pathways can lead to their activation in a salt- (or Rps26-) dependent manner, it is possible that organisms may leverage this response to assist in adaptation to extreme environments. To test this idea, we queried a recently published data set of 1,011 Saccharomyces cerevisiae isolates collected from diverse geographic and ecological origins (Peter et al., 2018). While single nucleotide polymorphisms (SNPs) were not more common at the −4 position than at other positions upstream of the start codon (Figure S2C), the GO-term “oxidation-reduction process” was enriched among the genes with −4 SNPs (Table S1). This gene list covered enzymes involved in amino acid biosynthesis, protection against oxidative stress and DNA repair, and included Rnr4 (Figure 2 & Table S1). Furthermore, Mec3, Mrc1 and Rad53, which are all part of the DNA repair pathway outlined in Figure 2, also had SNPs at −4, changing from a −4A to a −4G in the cases of Rnr4 and Mec3, from a −4A to a −4T in the case of Mrc1, and from a −4T to a −4C in the case of Rad53 (Figure S2D & Table S2). In both reporters and endogenous proteins, the −4G mutation leads to reduced protein levels in the presence of Rps26 (Figure S1E & Ferretti et al. 2017), and indeed the reported phenotypic data (Peter et al., 2018) show that the organisms with mutations at the −4 of the DNA repair genes Rnr4, Mec3, Mrc1 and Rad53 all have decreased resistance to the DNA-damage inducing agents hydroxyurea and arsenite (Figure S2E). Both in reporter assays (Ferretti et al., 2017) as well as in endogenous genes, including the Rnr4-binding partner Rnr2 (see above), the −4A→G mutation enhances translation upon genetic, salt, or pH-dependent Rps26 depletion. Thus, we expect that, as shown here for Rnr2, −4G mutations in the related genes Rnr4, Mec3, Mrc1 and Rad53 also activate the DNA-repair pathway in an Rps26-dependent manner. Thus, our analysis of genotypic and phenotypic data from yeast isolates (Peter et al., 2018) suggests that ribo-tuning might occur naturally in yeast existing in defined ecological niches, taking advantage of pools of ribosomes with distinct composition and mRNA-specificity.
Discussion
In this work we extend and further validate our previous findings that Rps26 recognizes the −2/−4 positions upstream of the Kozak sequence. Point mutations in those two residues can tune protein translation in a predictable and programmable manner, such that it becomes Rps26-responsive. Because in yeast ΔRps26 ribosomes are produced under high salt stress, these changes also lead to salt-stress dependent changes in protein translation.
This work also demonstrates that several, perhaps many, signaling pathways are poised for this activation, as mutation of a single protein’s Kozak sequence can produce Rps26- or salt-dependent induction of entire signaling pathways, leading to programmed changes in the biological response to high salt stress. We believe that the “preactivation” for such reprogramming arises, because many signaling proteins are poorly expressed as they often contain suboptimal residues in their Kozak sequence. This renders them more poorly translated, and less likely to be preferentially translated by wt ribosomes (Ferretti et al., 2017).
Advantages of reshaping gene expression by ribo-tuning, as described here, include a potentially rapid and energy-conserving response, as transcription is not required. Furthermore, because many pathways appear poised for this activation, only a single mutation must be incurred while transcriptional changes tend to involve entire pathways and are thus much harder to orchestrate.
Importantly, our analysis of phenotypic and genotypic data from a large group of yeast isolates from different ecological niches (Peter et al., 2018) strongly suggests that yeast are poised for ribo-tuning to take advantage of changes in ribosome composition, as they show mutations in the DNA repair pathway, akin to the ones we have explored here (Figure S2D). It is likely that such rapid adaptations to specialized growth environments based on ribosome-mediated translational control are not confined to yeast cells, but due to the advantages described above, extend to higher organisms, and likely involve many different ribosomal proteins. Indeed, from the analysis of genomic changes, mRNA levels, and protein content in cancer cells it has recently become apparent that ribosomes from cancer cells often lack stoichiometry of all ribosomal proteins, and indeed contain ribosomes lacking individual proteins (Ajore et al., 2017; Guimaraes and Zavolan, 2016; Kulkarni et al., 2017; Vlachos, 2017). Both the wide-spread occurrence of this phenomenon as well as its correlation with poor patient outcomes (Guimaraes and Zavolan, 2016; Kulkarni et al., 2017), suggests that the evolution of these ribosomal protein-deficient ribosome populations provides advantages to the cancer cells. Our previous work on mRNA-specific translation suggests that these advantages might include changes in protein homeostasis (Ferretti et al., 2017). In addition, we have also recently shown that ribosomal protein-deficient ribosomes can have defects in translational fidelity, which promote survival under stress, presumably due to the low-level production of novel proteins, some of which might evolve to have stress-protective functions (Collins et al., 2018, in submission).
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Katrin Karbstein.
EXPERIMENTAL MODEL DETAILS
Genetic Modification of Yeast Strains.
Strains were either obtained from the GE Dharmacon Yeast Knock-Out Collection or were created by standard recombination techniques (Longtine et al., 1998). The identity of strains was verified by PCR and western blotting, if antibodies were available. For ‘reprogrammed’ cells, the gene of interest was placed under the control of the GAL1 promoter and cells were transformed with a centromeric plasmid containing the gene of interest. Site directed mutagenesis was used to modify the Kozak sequence. Plasmids were propagated in XL1 Blue cells.
METHOD DETAILS
Media and growth conditions.
Cells were grown at 30°C in YPD media: 2% w/v peptone, 1% yeast extract, 2% glucose. For Rps26-depleted cells, cultures were inoculated from mid-log phase cultures into YPD + 100ng/ml doxycycline hydrochloride at an OD of 0.01 and were then grown for ~16 hours to mid-log phase.
MMS survival assay.
This assay was performed as previously described (Lee et al., 2002) with slight modifications. Cells at mid-log phase were washed and resuspended in Phosphate Buffered Saline at a concentration of 6×106 cells/ml. After addition of water (for controls) or methyl methanesulfonate (MMS) to a final concentration of 0.4%, cells were incubated while shaking at 30°C for 20 minutes after which the MMS was neutralized with 2 volumes of 10% sodium thiosulfate. Appropriate dilutions were then plates on YPD plates (+ or − dox) in technical triplicate and the number of colony forming units was counted using OpenCFU v3.9.0 (Geissmann, 2013). For each cell type, an identical experiment was performed in parallel except that MMS was excluded and was used to calculate the fraction of cells which survived.
Zymolyase digestion assay.
This assay was performed as described previously (Kuranda et al., 2006). Cells were grown to mid-log phase and then inoculated into YPD + 20mM caffeine at an OD of 0.3-0.5. Caffeine exposure causes yeast to remodel cell walls resulting in resistance to digestion by Zymolyase. For cells depleted of ribosomal proteins, this process takes significantly longer than for RP-replete cells, as they grow more slowly, and so we determined the time period for which the maximum response to caffeine was achieved in both conditions. We exposed TET:Rps26 cells containing plasmid-borne Rho1 under the control of its native upstream sequence to 20mM caffeine over 7.5 hours to find the point at which the cell wall remodeling is complete and resistance to Zymolyase plateaus (Figure S1D, left). We repeated this timecourse for cells grown in the presence of 100 ng/ml of doxycycline (dox) but extended it out to ~27 hours to account for the slow-growth phenotype caused by depletion of Rps26 (Figure S1D, right). These two experiments determined that the time-to-maximum response for cells exposed to 20mM caffeine was 4.5 hours for Rps26-replete cells and 15.5 hours for Rps26-depleted cells.
After being grown for the either 4.5 (RP-replete) or 15.5 (RP-depleted) hours, each corresponding to less than one cellular division, cells were washed and resuspended in 10mM Tris-Cl pH 7.4 + 40mM 2-mercaptoethanol at 3×107 cells per ml and incubated for 20 minutes. Zymolyase 20T was then added to a final concentration of 50 μg/ml and the drop in the OD600 relative to the initial value was measured using a Synergy 2 multi-mode microplate reader (BioTek). Each digestion was performed in technical duplicate. Occasionally, certain wells experienced large spikes in OD, possibly due to protein aggregations from lysed cells or condensation on the plate lid, and these points were either removed individually or (in a few extreme cases) the entire technical duplicate was excluded. Individual points were only removed if they exceeded four times the minimum value of all previous points in the decay graph. Finally, data points were fit to an exponential decay equation using GraphPad Prism 6 to determine the time constant, k.
Filamentation assay.
Cells were grown to mid-log phase and plated onto thinly poured YPD plates by carefully adding 20ul of cells (at 1×107 cells/ml) per replicate onto the plate without touching the pipette tip to the agar surface. Cells were grown for 5 days at 30°C. An invasive growth assay was then performed as previously described (Blacketer et al., 1995; Cullen and Sprague, 2000). Briefly, plates were gently washed and rubbed, with care taken not to break the plane of the agar. Plates were imaged using a EVOS FL (Invitrogen) microscope at 400X magnification. Fields of view were chosen randomly for each cell patch (though some fields were skipped when a plane of focus or very dense patch of non-distinguishable adherent cells obscured quantification). A minimum of 3 images were quantified per plate. For strains where there were fewer cells, all adherent cells were imaged. Cells were then counted by a blinded scorer who classified cell morphology as either ovoid or filamentous.
qPCR analysis.
Total RNA was extracted from cells with the hot acid-phenol method and precipitated with sodium acetate and ethanol. Resuspended RNA was treated with RQ1 DNase per manufacturer’s instructions and then extracted again by phenol-chloroform-isoamyl alcohol and precipitated as above. ProtoScriptII was used for reverse transcription on 1ug of total RNA with Oligo dT18. qPCR was performed in a BioRad IQ5 with 2x Excella SYBR MasterMix per manufacturer’s instructions using primers listed in Table S4.
Western blot analysis.
Cells used for Western Blot analysis were harvested at mid-log phase OD600 0.4-0.6. Cells depleted of Rps26 were inoculated at OD 0.01 in the presence of 100 ng/ml of dox and allowed to grow to mid-log phase. Blots were imaged using a BioRad ChemiDoc XRS and quantified using BioRad QuantityOne 4.6.9 software.
Antibodies.
The Rcl1, Fap7 and Rps26 antibodies were tested against yeast lysates and either recombinant protein or purified 40S ribosomal subunits.
QUANTIFICATION AND STATISTICAL ANALYSIS
In all figures where the t-test was used, the Holm-Sidak correction for multiple comparisons was applied with an alpha set at 0.05. For two-way ANOVA, the Sidak correction for multiple comparisons was used to calculate an adjusted p value. Data analysis was performed in GraphPad Prism6. Data management was performed in R 3.5.0 (R Core Team, 2018) using the dplyr (Wickham et al., 2017) and sqldf (Grothendieck, 2017) packages and in Microsoft Excel.
DATA AND SOFTWARE AVAILABILITY
Data are available upon request to the Lead contact.
Supplementary Material
List of genes with SNPs at −4 position in data from Peter et al., 2018. Related to Figure 2.
Organisms with SNPs in genes from the DNA damage pathway. Related to Figure 2.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-HA [HA.C5] | Abcam | CAT# ab18181 |
| Mouse monoclonal anti-Rpl3 | Deposited to the Developmental Studies Hybridoma Bank by Warner, J.R. | CAT# ScRPL3 |
| Rabbit polyclonal anti-Rps8 | Giorgio Dieci, University of Parma (Dieci et al., 2005) | N/A |
| Rabbit polyclonal anti-Rho1 | Gary Eitzen, University of Alberta (Drgonová et al., 1999) | N/A |
| Rabbit polyclonal anti-Tsr2/S26 | Vikram Panse, University of Zurich (Schütz et al., 2014) | N/A |
| Rabbit polyclonal anti-Fap7 | Raised against purified recombinant proteins by Josman, LLC (Strunk et al., 2012) | N/A |
| Rabbit polyclonal anti-Rcl1 | Raised against purified recombinant proteins by Josman, LLC (Ferretti et al., 2017) | N/A |
| Rabbit polyclonal anti-Rps26 | Raised against a peptide fragment by New England Peptide (Ferretti et al., 2017) | N/A |
| Bacterial and Virus Strains | ||
| XL1 Blue Supercompetent | Agilent | CAT#200249 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Yeast Extract | HiMedia | CAT# RM027 |
| Peptone, bacteriological | HiMedia | CAT# RM001 |
| Glucose | Sigma | CAT# G8270 |
| Doxycycline hydrochloride | Fisher Scientific | CAT# BP26531 |
| Caffeine | MP Biomedical | CAT# 02150114 |
| Zymolyase 20T | Seikagaku Biobusiness | CAT# 120491-1 |
| 2-Mercaptoethanol | Sigma | CAT# 516732 |
| Phosphate Buffered Saline | G-Biosciences | CAT# 786-027 |
| Methyl Methanesulfonate | Sigma | CAT# 129925 |
| Sodium Thiosulfate | Fisher Scientific | CAT# S445 |
| Tris-HCL | Fisher Scientific | CAT# BP1757 |
| RQ1 DNase | Promega | CAT# M6101 |
| ProtoScriptII | New England Biolabs | CAT# M0368S |
| Oligo dT | New England Biolabs | CAT# S1316S |
| Excella™ SYBR MasterMix IQ | Worldwide Medical Products | CAT# 61071103 |
| Experimental Models: Cell Lines | ||
| Saccharomyces Cerevisiae | Table S3 | N/A |
| Experimental Models: Organisms/Strains | ||
| Saccharomyces Cerevisiae | Table S3 | N/A |
| Oligonucleotides | ||
| All oligonucleotides are listed in Table S4 | ||
| Recombinant DNA | ||
| All plasmids used in this study are listed in Table S4 | ||
| Software and Algorithms | ||
| Graphpad Prism 6 | Graphpad Corporation | Version 6.01 |
| QuantityOne | BioRad | Version 4.6.9 |
| R | R Foundation | Version 3.5.0 |
| dplyr (R package) | Version 0.7.4 | |
| sqldf (R package) | Version 0.4.11 | |
Significance.
Here we show that by introducing point mutations into the Kozak sequence of individual genes, we render their translation responsive to the accumulation of Rps26-deficient ribosomes, which are produced either through genetic depletion or by growth in high salt media. Furthermore, this translational regulation of individual mRNAs leads to activation of the pathways in which they are involved. In this manner, we have reprogrammed the cellular response to high salt stress to include filamentation, activation of DNA repair, or cell wall strengthening. Analysis of genotypic and phenotypic changes in yeast populations from diverse ecological niches indicates that ribo-tuning, ribosome-composition-mediated translational control, occurs during evolution, where it appears to activate DNA repair upon Rps26-depletion. This finding, together with the ease by which reprogramming was achieved, suggests that ribo-tuning could be a facile way to effect adaptation, likely because signaling pathways are a priori poised for such control. Consistent with this notion, recent results show that cancer cells often have heterogeneous ribosome populations, which can confer a growth advantage. Thus, the directed reprogramming we have undertaken here is a blueprint for evolution, which is exploited both on an organismal level as well as during malignancy.
Highlights:
Single point mutations in the Kozak sequence renders translation Rps26-responsive.
Ribo-tuning can activate entire pathways in response to high salt.
Signaling cascades might be poised for ribo-tuning.
Analysis of natural yeast samples provides evidence for ribo-tuning.
Acknowledgments
This work was supported by NIH grants R01-GM086451 (to K.K.), F31-GM116406 (to MBF) and HHMI Faculty Scholar grant 55108536 (to K.K.). We thank G. Eitzen and V. Panse for providing antibodies, J. Collins for assistance with morphology scoring, A. Getzler & H. Diao from the Pipkin Lab for assistance with bioinformatics, and members of the Karbstein lab for comments on the manuscript.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abid MR, Li Y, Anthony C, and De Benedetti A (1999). Translational regulation of ribonucleotide reductase by eukaryotic initiation factor 4E links protein synthesis to the control of DNA replication. J. Biol. Chem. 274, 35991–35998. [DOI] [PubMed] [Google Scholar]
- Ajore R, Raiser D, Mcconkey M, Joud M, Boidol B, Mar B, Saksena G, Weinstock DM, Armstrong S, Ellis SR, et al. (2017). Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations. EMBO Mol Med 9, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead J, and Triggs-Raine B (2014). Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett. 588, 1491–1500. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Yu H, Mim C, and Matouschek A (2014). Regulated protein turnover: Snapshots of the proteasome in action. Nat. Rev. Mol. Cell Biol. 15, 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacketer M, Madaule P, and Myers A (1995). Mutational analysis of morphologic differentiation in Saccharomyces cerevisiae. Genetics 140, 1259–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, and Thelander L (2003). Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112, 391–401. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, and Sprague GF (2000). Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. U. S. A. 97, 13619–13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desany BA, Alcasabas AA, Bachant JB, and Elledge SJ (1998). Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 12, 2956–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Kinzy TG, and Pavitt GD (2016). Mechanism and regulation of protein synthesis in Saccharomyces cerevisiae. Genetics 203, 65–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge SJ, Zhou Z, and Allen JB (1992). Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem. Sci. 17, 119–123. [DOI] [PubMed] [Google Scholar]
- Ferretti MB, Ghalei H, Ward EA, Potts EL, and Karbstein K (2017). Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat. Struct. Mol. Biol. 24, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, and Brown PO (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann Q (2013). OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothendieck G (2017). sqldf: Manipulate R Data Frames Using SQL. R Packag. Version 0.4-11. [Google Scholar]
- Guimaraes JC, and Zavolan M (2016). Patterns of ribosomal protein expression specify normal and malignant human cells. Genome Biol 17, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Ivanov IP, and Sonenberg N (2016). Translational control by 5 ′-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Kong E, Kim Y, Chang J-S, and Kim J (2017). RACK1 depletion in the ribosome induces selective translation for non-canonical autophagy. Cell Death Dis. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, and Barna M (2011). Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Dolezal JM, Wang H, Jackson L, Lu J, Frodey BP, Dosunmu-Ogunbi A, Li Y, Fromherz M, Kang A, et al. (2017). Ribosomopathy-like properties of murine and human cancers. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda K, Leberre V, Sokol S, Palamarczyk G, and Françis J (2006). Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 61, 1147–1166. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Cheutin T, Cremer M, Cavalli G, and Cremer T (2007). Dynamic genome architecture in the nuclear space: Regulation of gene expression in three dimensions. Nat. Rev. Genet. 8, 104–115. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Yu S-L, Prakash L, and Prakash S (2002). Yeast RAD26, a homolog of the human CSB gene, functions independently of nucleotide excision repair and base excision repair in promoting transcription through damaged bases. Mol. Cell. Biol. 22, 4383–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE (2011). Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 189, 1145–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Styles CA, and Fink GR (1996). Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, and Pringle JR (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Martin H, Rodriguez-Pachon JM, Ruiz C, Nombela C, and Molina M (2000). Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275, 1511–1519. [DOI] [PubMed] [Google Scholar]
- Peter J, De Chiara M, Friedrich A, Yue J-X, Pflieger D, Bergstrom A, Sigwalt A, Barre B, Freel K, Llored A, et al. (2018). Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 556, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. [Google Scholar]
- Rittberg DA, and Wright JA (1989). Relationships between sensitivity to hydroxyurea and 4-methyl-5-amino-1-formylisoquinoline thiosemicarbazone (MAIO) and ribonucleotide reductase RNR2 mRNA levels in strains of Saccharomyces cerevisiae. Biochem Cell Biol 67, 352–357. [DOI] [PubMed] [Google Scholar]
- Segev N, and Gerst JE (2018). Specialized ribosomes and specific ribosomal protein paralogs control translation of mitochondrial proteins. J. Cell Biol. 1, jcb.201706059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, and Barna M (2015). Translating the Genome in Time and Space: Specialized Ribosomes, RNA Regulons, and RNA-Binding Proteins. Annu. Rev. Cell Dev. Biol. 31, 31–54. [DOI] [PubMed] [Google Scholar]
- Shi Z, Fujii K, Kovary KM, Genuth NR, Röst HL, Teruel MN, Barna M, Segal E, Mohammed AK, Hamon C, et al. (2017). Heterogeneous Ribosomes Preferentially Translate Distinct Subpools of mRNAs Genome-wide. Mol. Cell 32, 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov N, Semrau S, Budnik B, Slavov N, Semrau S, Airoldi E, Budnik B, and Oudenaarden A Van (2015). Differential Stoichiometry among Core Ribosomal Proteins. Cell Rep. 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter AW, Fischer C, Kleinschmidt M, and Braus GH (2006). FLO11 mediated filamentous growth of the yeast Saccharomyces cerevisiae depends on the expression of the ribosomal RPS26 genes. Mol. Genet. Genomics 276, 113–125. [DOI] [PubMed] [Google Scholar]
- Sulima SO, Hofman IJF, De Keersmaecker K, and Dinman JD (2017). How Ribosomes Translate Cancer. Cancer Discov. 7, 1069–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MK, Rojas-Duran MF, Gangaramani P, and Gilbert WV (2016). The ribosomal protein Asc1/RACK1 is required for efficient translation of short mRNAs. Elife 5, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A (2017). Acquired ribosomopathies in leukemia and solid tumors. Hematology 2017, 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, Francois R, Henry L, and Muller K (2017). dplyr: A Grammar of Data Manipulation. R Packag. Version 0.7.4. [Google Scholar]
- Wong QW-L, Li J, Ng SR, Lim SG, Yang H, and Vardy L a (2014). RPL39L is an example of a recently evolved ribosomal protein paralog that shows highly specific tissue expression patterns and is upregulated in ESCs and HCC tumors. RNA Biol. 11, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, and Barna M (2012). Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 13, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Tian S, Fujii K, Kladwang W, Das R, and Barna M (2015). RNA regulons in Hox 5’ UTRs confer ribosome specificity to gene regulation. Nature 517, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes with SNPs at −4 position in data from Peter et al., 2018. Related to Figure 2.
Organisms with SNPs in genes from the DNA damage pathway. Related to Figure 2.