Abstract
Background:
Black women with endometrial cancer are more likely to die from their disease compared to white women with endometrial cancer. These survival disparities persist even when disproportionately worse tumor characteristics among black women are accounted. Receipt of less complete adjuvant treatment among black endometrial cancer patients could contribute to this disparity.
Objective:
We assessed the hypothesis that black women with endometrial cancer are less likely than their white counterparts to receive adjuvant treatment within subgroups defined by tumor characteristics in the NRG Oncology/Gynecology Oncology Group 210 Study.
Study design:
Our analysis included 615 black and 4,283 white women with endometrial cancer who underwent hysterectomy. Women completed a questionnaire that assessed race and endometrial cancer risk factors. Tumor characteristics were available from pathology reports and central review. We categorized women as low-, intermediate-, or high-risk based on the European Society for Medical Oncology definition. Adjuvant treatment was documented during postoperative visits and was categorized as no adjuvant treatment (54.3%), radiotherapy only (16.5%), chemotherapy only (15.2%), and radiotherapy plus chemotherapy (14.0%). We used polytomous logistic regression to estimate odds ratios and 95% confidence intervals for multivariable-adjusted associations between race and adjuvant treatment in the overall study population and stratified by tumor subtype, stage, or European Society for Medical Oncology risk category.
Results:
Overall, black women were more likely to have received chemotherapy-only (odds ratio=1.40, 95% confidence interval=1.04-1.86) or radiotherapy plus chemotherapy (odds ratio=2.01, 95% confidence interval=1.54-2.62) compared to white women in multivariable-adjusted models. No racial difference in receipt of radiotherapy-only was observed. In tumor subtype-stratified models, black women had higher odds of receiving radiotherapy plus chemotherapy than white women when diagnosed with low-grade endometrioid (odds ratio=2.04, 95% confidence interval=1.06-3.93) or serous tumors (odds ratio=1.81, 95% confidence interval=1.07-3.08). Race was not associated with adjuvant treatment among women diagnosed with other tumor subtypes. In stage-stratified models we observed no racial differences in receipt of adjuvant treatment. In models stratified by European Society for Medical Oncology risk group, black women with high-risk cancer were more likely to receive radiotherapy plus chemotherapy compared to white women (odds ratio=1.41, 95% confidence interval=1.03-1.94).
Conclusion:
Contrary to our hypothesis we observed higher odds of specific adjuvant treatment regimens among black as compared to white women within specific subgroups of endometrial cancer characteristics.
Introduction
Endometrial cancer (EC) is the fourth most common cancer among women in the United States, with 63,230 new cases expected in 2018 (1). Racial disparities in EC mortality are well-documented, with black women experiencing a 93% higher mortality compared to white women with EC. This represents one of the largest racial disparities in mortality among common cancers (2). Although black women more commonly present with aggressive tumor characteristics (e.g. non-endometrioid subtypes and later stage disease) (3-7), which contributes to their worse prognosis (8), racial differences in survival within these subgroups are apparent. For example, in an analysis restricted to EC patients with advanced stage or recurrent disease, Maxwell et al. (9) reported significantly worse survival among black as compared to white women. In a study of EC patients < 50 years of age with stage I or II disease, black women had a 24% higher risk of death than white women (10). Apart from a higher prevalence of aggressive tumor characteristics (11), other factors, including lower socioeconomic status (12), presence of comorbidities (13), and receipt of less complete treatment among black EC patients could contribute to racial disparities in EC survival (14).
The majority of EC patients begin treatment with hysterectomy, bilateral salpingo-oophorectomy, and lymph node sampling (15). Following surgery, adjuvant treatment is recommended on the basis of tumor characteristics determined at the time of hysterectomy (16). Poor tumor differentiation, non-endometrioid histology, and tumors with invasion into the myometrial wall are clinical indications for adjuvant treatment with radiotherapy (RT) and/or chemotherapy (CT). As mentioned, these aggressive tumor features are more common among black women – therefore, observations that black women more commonly receive adjuvant treatment than white EC cases are expected (12, 17-20). Still, others report no difference in receipt of adjuvant treatment between black and white EC patients (19, 21-23).
An important limitation of studies that use hospital registry data is the ascertainment of adjuvant treatment. Although details of hospital-based treatments, including surgery, and to a lesser extent radiotherapy, are reliably collected (24, 25), systemic treatments are typically delivered in outpatient settings and are more susceptible to measurement error. We undertook the current analysis to clarify associations between race and receipt of specific adjuvant treatment regimens according to tumor characteristics that guide treatment assignment. This information will be useful to clinicians treating diverse populations of EC patients. Based on poorer survival among black EC patients within subgroups defined by tumor characteristics, we hypothesized that receipt of adjuvant treatment would be lower among black compared to white patients within subgroups defined by tumor features in the large NRG Oncology/Gynecologic Oncology Group (GOG) 210 study.
Materials and Methods
The NRG Oncology/GOG 210 Study was conducted from September 22, 2003 to December 1, 2011, at 62 U.S. institutions. Eligible patients included women with pre-surgical diagnoses of EC or carcinosarcoma who were eligible for surgery and had not undergone prior retroperitoneal surgery or pelvic/abdominal radiation. Prior to surgery (hysterectomy, bilateral salpingo-oophorectomy, and lymph node sampling at the discretion of the treating provider), consenting patients completed a self-administered questionnaire that collected demographic and risk factor information (4). On September 23, 2007, eligibility criteria in NRG Oncology/GOG 210 changed from unrestricted enrollment to restricted enrollment of poor prognosis cancers (e.g., non-endometrioid) and cancers occurring among non-obese and non-white patients.
Of 6,124 women enrolled, 632 (10%) did not complete questionnaires and were excluded from further analyses. We excluded women for the following reasons: incomplete surgical staging (n=20), final diagnosis not EC (n=53), benign diagnoses (n=6), diagnosis of a second primary (n=2), misclassified pathologic diagnosis based on central pathology review (n=49), inadequate material for pathology review (n=22), protocol deviations (n=17), and improper pre-protocol treatment (n=1). We excluded women with missing grade (n=23), mucinous tumors (n=18), unusual histologic types (including squamous cell, undifferentiated, and de-differentiated histologies) (n=111), missing stage (n=5), self-reported race other than black or white (n=231), and unknown adjuvant treatment status (n=36) leaving 4,898 patients for analysis. This study was approved by Institutional Review Boards at the National Cancer Institute and participating study centers. All participants provided informed, written consent prior to participation.
Questionnaires assessed information on demographic characteristics (age, race, annual income, highest level education attained) and established EC risk factors including height, weight, reproductive factors, diabetes, smoking status, oral contraceptive use, menopausal hormone use, tamoxifen use, and history of breast cancer.
Pathology information was available from participating NRG Oncology/GOG institutions and through specialized reviews [previously described in (4)] performed by the NRG Oncology/GOG Pathology Committee. Specifically, diagnoses of high-grade endometrioid carcinoma, serous carcinoma, clear cell carcinoma, carcinosarcoma and tumors involving the cervix or with non-nodal metastases were reviewed by a panel of gynecologic pathologists. Endometrial tumor subtypes were classified as low-grade (grades 1 and 2) endometrioid carcinoma (n=2,614), high-grade (grade 3) endometrioid carcinoma (n=593), serous carcinoma (n=677), mixed epithelial (n=536), carcinosarcoma (n=312), or clear cell carcinoma (n=166). Depth of myometrial invasion (negative, inner half, outer half, serosal involvement), stage according to International Federation for Gynecology and Obstetrics 1988 criteria (26), pelvic and/or aortic lymph node involvement, peritoneal cytology and biopsy results and extra-uterine sites of metastasis were recorded. We assessed complete surgical staging on the basis of lymph node sampling and peritoneal cytology. Women who had sampling of the pelvic and para-aortic lymph nodes and had peritoneal washings (until 2009) were considered to have complete surgical staging. In 2009, peritoneal washings were removed from surgical staging criteria(27).
Following the post-surgical clinic visit, which occurred approximately six weeks after surgery, patients were followed every three months for the first two years, every six months for the next three years, and then annually for the next five years. All cancer-related adjuvant treatment was prospectively documented on standard follow-up forms. Treatment decisions were made by the treating clinician. We categorized adjuvant treatment as no adjuvant treatment (NAT), RT-only, CT-only, or RT plus CT. Detailed information on treatment (e.g. radiotherapy type, radiotherapy dose, chemotherapy agents, chemotherapy dose, etc.) was unavailable.
Tumor and treatment characteristics were compared between black and white women with EC using chi-square tests. We used unordered polytomous logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between race and receipt of adjuvant treatment, with NAT as the reference. We first examined an overall model with minimal adjustment for age at diagnosis, tumor subtype, stage, annual income, and educational attainment. Missing values for income and educational attainment were modeled as separate categories. We considered the epidemiological characteristics listed in Supplemental Table 1 as potential confounders by adding them to the minimally adjusted models. As inclusion of these factors did not change minimally adjusted estimates for the association between race and receipt of adjuvant treatment by more than 10%, no additional adjustments were made. We also examined the same polytomous logistic regression model stratified by tumor subtype, stage, or European Society for Medical Oncology (ESMO) risk category. The ESMO classifier includes three groups (low-, intermediate-, or high-risk) based on stage (including depth of myometrial invasion) grade, and histology (28). This classifier was selected because it does not incorporate lymphovascular space invasion and we lacked information on this variable. As the high-risk category includes a heterogeneous group (all stages with non-endometrioid histology), we adjusted this model for stage and tumor subtype.
When we observed significant differences in receipt of adjuvant therapy in stratified models, we compared distributions of other tumor characteristics (e.g. peritoneal cytology, peritoneal metastasis, aortic lymph node involvement, and pelvic lymph node involvement) between black and white women within those strata to determine if these factors contributed to racial differences in receipt of adjuvant therapy. Statistical analyses were performed using SAS (version 9.3, SAS Institute, Cary, NC, USA). All P values were two-sided; statistical significance was set at P less than 0.05.
Results
Of the 4,898 study participants, 12% (n = 615) self-reported black race. Distributions of tumor and treatment characteristics among black and white women with EC are shown in Table 1. Compared to white women with EC, black women had a higher prevalence of aggressive tumor characteristics, including positive pelvic and/or aortic lymph node involvement, positive peritoneal cytology, advanced stage, histologic subtypes other than low-grade endometrioid, high-risk tumors as defined by ESMO, and a lower prevalence of complete surgical staging. Adjuvant CT-only and RT plus CT regimens were more common among black than white women. Supplemental Table 1 shows distributions of epidemiological characteristics according to race. Compared to white women with EC, black women were more likely to have less than high school education, have lower annual incomes, be non-users of menopausal hormones, be obese, have more live births, and have a history of diabetes.
Table 1.
Tumor and treatment characteristics among 4,283 white and 615 black women with endometrial carcinoma in the NRG Oncology/GOG 210 Study
| Race, n (%) | |||
|---|---|---|---|
| White | Black | P1 | |
| n=4,283 | n=615 | ||
| Tumor subtype | <.0001 | ||
| Low-grade endometrioid | 2392 (55.9) | 221 (35.9) | |
| High-grade endometrioid | 525 (12.3) | 68 (11.1) | |
| Serous | 522 (12.2) | 155 (25.2) | |
| Mixed cell | 467 (10.9) | 69 (11.2) | |
| Carcinosarcoma | 241 (5.6) | 71 (11.5) | |
| Clear cell | 135 (3.2) | 31 (5.0) | |
| Stage | <.0001 | ||
| I | 3090 (72.2) | 377 (61.3) | |
| II | 299 (7.0) | 61 (9.9) | |
| III | 700 (16.3) | 137 (22.3) | |
| IV | 194 (4.5) | 40 (6.5) | |
| Myometrial invasion | 0.06 | ||
| Negative | 1060 (24.7) | 140 (22.8) | |
| Inner half | 1987 (46.4) | 310 (50.4) | |
| Outer half | 1018 (23.8) | 131 (21.3) | |
| Serosa | 95 (2.2) | 21 (3.4) | |
| Not assessed/not reported | 123 (2.9) | 13 (2.1) | |
| Pelvic lymph node involvement | <.0001 | ||
| No | 3512 (82.0) | 458 (74.5) | |
| Yes | 482 (11.2) | 102 (16.6) | |
| Not assessed/not reported | 289 (6.8) | 55 (8.9) | |
| Aortic lymph node involvement | <.0001 | ||
| No | 3537 (82.6) | 455 (74.0) | |
| Yes | 262 (6.1) | 68 (11.1) | |
| Not assessed/not reported | 484 (11.3) | 92 (15.0) | |
| Peritoneal cytology | 0.09 | ||
| Negative | 3557 (83.0) | 489 (79.5) | |
| Positive | 447 (10.4) | 80 (13.0) | |
| Not assessed/not reported | 279 (6.5) | 46 (7.5) | |
| Peritoneal biopsy | <.0001 | ||
| Negative | 1716 (40.1) | 319 (51.9) | |
| Positive | 129 (3.0) | 22 (3.6) | |
| Not assessed/not reported | 2438 (56.9) | 274 (44.5) | |
| Complete surgical staging | 0.01 | ||
| No | 642 (15.0) | 116 (18.9) | |
| Yes | 3641 (85.0) | 499 (81.1) | |
| European Society for Medical Oncology risk category | <.0001 | ||
| Low-risk | 1687 (39.9) | 163 (26.7) | |
| Intermediate-risk | 525 (12.4) | 47 (7.7) | |
| High-risk | 2011 (47.6) | 401 (65.6) | |
| Unknown2 | 60 (1.4) | 4 (0.6) | |
| Adjuvant treatment | <.0001 | ||
| None | 2381 (55.6) | 278 (45.2) | |
| Radiotherapy only | 714 (16.7) | 92 (15.0) | |
| Chemotherapy only | 635 (14.8) | 110 (17.9) | |
| Radiotherapy plus chemotherapy | 553 (12.9) | 135 (22.0) | |
P value was from two-sided χ2 test
64 women were missing information on myometrial invasion and could not be categorized
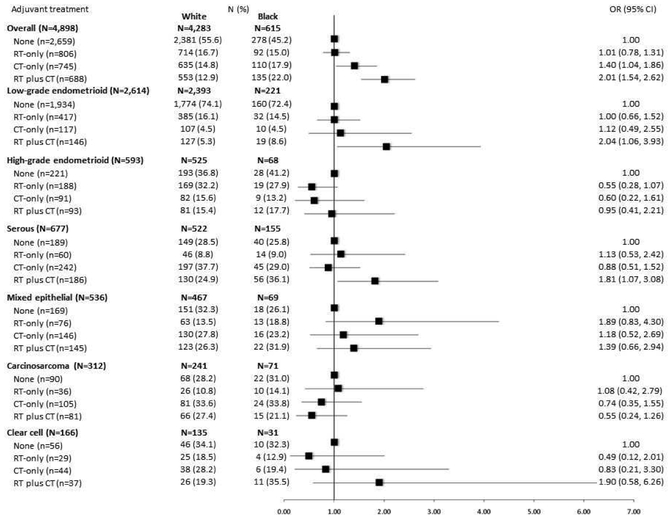
Overall, more than half of women with EC received NAT (54.3%), while 16.5% received RT-only, 15.2% received CT-only, and 14% received RT plus CT. Figure 1 shows multivariable-adjusted associations between race and receipt of adjuvant treatment overall and stratified by tumor subtype. In the overall model, black women were more likely to receive CT-only (OR=1.40, 95% CI=1.04-1.86) or RT plus CT (OR=2.01, 95% CI=1.54-2.62) compared to white women. Race was not associated with receipt of RT-only compared to NAT. In analyses stratified by tumor subtype, we noted racial differences in the receipt of RT plus CT among women diagnosed with low-grade endometrioid tumors (OR=2.04, 95% CI=1.06-3.93) or serous tumors (OR=1.81, 95% CI=1.07-3.08). No racial differences in receipt of RT-only or CT-only regimens were observed in the tumor subtype–stratified models.
Figure 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between race and adjuvant treatment in the NRG Oncology/GOG 210 Study, overall and stratified by tumor subtype
Figure 1 shows that black women were more likely to receive CT-only or RT plus CT compared to white women in the overall study population. The tumor subtype-stratified models showed racial differences in the receipt of RT plus CT among women diagnosed with low-grade endometrioid tumors or serous tumors.
We also explored distributions of other tumor characteristics (peritoneal cytology, aortic lymph node involvement, etc.) among white and black women with low-grade endometrioid or serous tumors. Among women with low-grade endometrioid tumors, we observed a higher proportion of invasion into the outer half of the myometrium among white compared to black women (18.3% vs. 12.7%). We observed no racial differences in prevalence of tumor characteristics among women with serous tumors. Complete surgical staging did not vary between black and white EC patients within these strata.
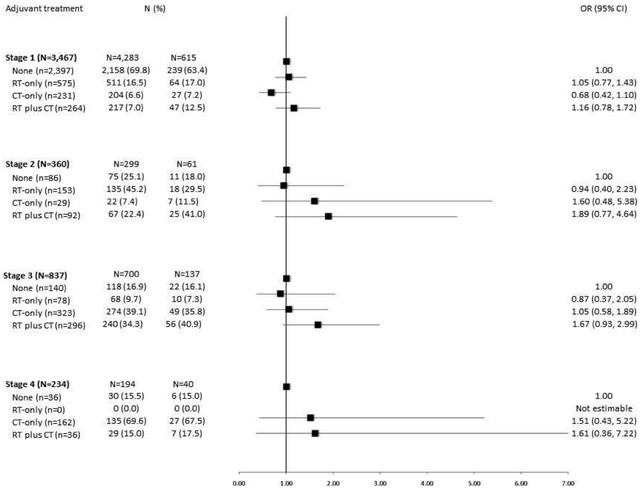
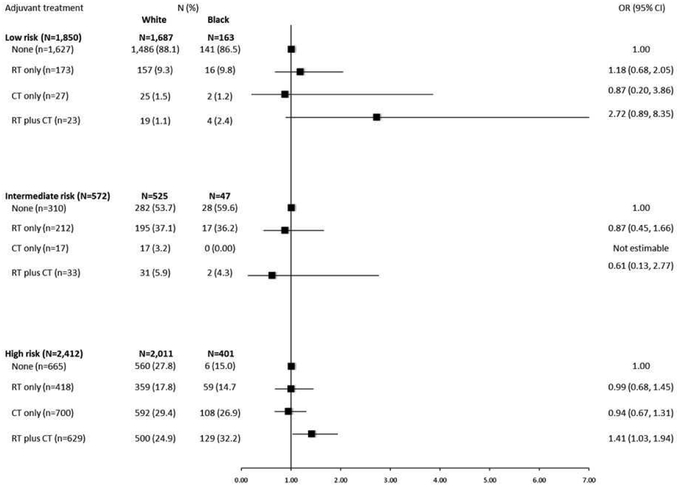
We observed no differences in receipt of adjuvant treatment according to race in models stratified by stage (Figure 2). In models stratified by ESMO risk category (Figure 3), no racial differences in receipt of adjuvant treatment were noted for women in the low- or intermediate risk groups; however, among women in the high-risk category, we observed higher odds of receiving RT plus CT among black as compared with white EC patients (OR=1.41, 95% CI=1.03-1.94). In this stratum, no racial differences in receipt of RT-only or CT- only regimens were observed. Aortic lymph node involvement was significantly more common among black than white EC patients in the high-risk group (17% vs. 13%, p=0.002).
Figure 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between race and adjuvant treatment in the NRG Oncology/GOG 210 Study stratified by stage
Figure 2 shows no racial differences in receipt of adjuvant treatment in models stratified by stage.
Figure 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between race and adjuvant treatment in the NRG Oncology/GOG 210 Study, stratified by ESMO risk group
Figure 3 shows that black women were more likely to receive RT plus CT compared to white women in the subgroup of women with high-risk EC.
Comment
On the whole, studies that evaluate survival following a cancer diagnosis demonstrate lower survival rates among blacks compared to whites (29). Morris and colleagues (30) describe several mechanisms that likely contribute to this observation, including: aggressive clinical characteristics, lower socioeconomic status, higher prevalence of comorbid conditions, poor patient-provider interactions, and inferior treatment. In this large cohort of women with EC, we examined receipt of specific adjuvant treatments among black and white women in the overall study population and according to tumor characteristics that guide treatment recommendations. Contrary to our hypothesis, we observed that black women had higher odds of receiving certain adjuvant treatment regimens, which varied according to tumor subtype and ESMO risk category.
We noted that black women had higher odds of receiving CT-only or RT plus CT compared to white women. Our large study population with central review of tumor characteristics also provided an opportunity to explore in depth whether the association between race and receipt of specific adjuvant treatment regimens varied within categories defined by tumor characteristics, which is a notable extension of the prior literature. We noted some racial differences in receipt of adjuvant treatment: namely, RT plus CT was more common among black women diagnosed with low-grade endometrioid EC or serous EC. In addition, among women with high-risk EC, we noted a higher odds of receiving RT plus CT among black women. No racial differences in receipt of adjuvant treatment were observed in models stratified by stage alone, which is in contrast to an NCDB analysis where black women with advanced stage disease had a 12% higher odds of receiving adjuvant treatment compared to similarly-staged white EC patients (20).
Our findings are distinct from prior studies that reported no racial differences in receipt of adjuvant treatment (19, 21-23). Findings from our study and the NCDB analysis might reflect that other factors, including overall health of the patient and treatment preference, also guide a clinician’s decision to provide adjuvant treatment, factors that were unaccounted for in the respective analyses. Moreover, we speculate that these unanticipated findings may partially be attributable to increased clinician awareness of poorer survival among black as compared with white EC patients, which may have motivated more intense treatment in the absence of clinical indications. Over the past two decades, several reviews describing the survival disparity among black and white EC patients have been published (31-33), prompting national attention to this problem.
The National Comprehensive Cancer Network (NCCN) guidelines warrant adjuvant RT plus CT for women with low-grade endometrioid tumors when the tumor invades into the serosa or beyond (stage IIIA or higher) (16). Although black EC patients have a higher prevalence of advanced stage disease than white patients, our tumor subtype-stratified analyses were adjusted for stage. Our finding of increased use of RT plus CT among low-grade endometrioid black women suggests that clinicians are potentially over-prescribing this treatment. This pattern of over-treatment could contribute to deleterious survival outcomes observed among black women. On the other hand, adjuvant treatment with any combination of RT and/or CT is indicated among women with serous tumors, regardless of stage. As survival from serous EC is poor for all women, regardless of race, it is unclear what additional factors might lead to increased use of RT plus CT among black women with this aggressive subtype. We did not observe a higher prevalence of other aggressive tumor characteristics among black women with low-grade endometrioid or serous tumors that would explain our observations. In the high-risk ESMO group, we did note that black women had greater involvement of the aortic lymph nodes, possibly contributing to increased use of RT plus CT.
Our data are limited in their ability to provide explanations for the intriguing observations noted in this study population. Additional hypotheses of interest that we cannot directly address with these data include the possibility of racial differences in treatment preference/adherence, quality of patient-provider communications, and regional variations in treatment patterns. This last point may be particularly relevant in our study, given the national recruitment for this study. In the NCDB analysis, striking regional differences in prescribing EC adjuvant treatment were observed: patients from the Northeast, Midwest, Great Lakes, and Atlantic regions had higher odds of receiving adjuvant treatment compared with patients in the South (20). As the racial distribution of EC patients differs by region, there is likely an important interaction between geographical residence and race that could impact treatment receipt. In this secondary data analysis, we lack information on patient residence and cannot directly address this hypothesis with our data.
Despite unclear mechanisms linking race with adjuvant treatment receipt, it is apparent that black women with EC continue to experience worse outcomes than their white counterparts (20, 34). In our NRG Oncology/GOG 210 Study population, black EC patients have significantly higher risks of all-cause mortality [hazard ratio (HR) =1.21], EC-specific mortality (HR=1.27), and recurrence risk (HR=1.36) (35). These outcomes are independent of tumor characteristics, socioeconomic factors, and treatment. That the survival metrics for black EC patients have somewhat improved over the past two decades suggests progress (18, 20); however, the persistent gap in survival for black women should be addressed with additional prospectively designed research. Moreover, research on other minority groups is clearly needed.
Our study has several strengths including the prospective design, long follow-up, large sample size, and prospective collection of adjuvant treatment information. This last feature limits the extent to which our results are explained by differential misclassification of treatment between black and white patients. Like other previous studies, we were unable to examine associations among women of other racial minority groups, whom we excluded from this analysis due to extremely small sample sizes. We also lacked data on type of radiotherapy (e.g. brachytherapy, external beam, combination), which would have allowed for an assessment of whether women received treatment in line with NCCN recommendations. Finally, women enrolled in this study were treated at academic specialty centers, limiting our ability to generalize to women treated in community settings. Nonetheless, this case-series is one of the largest and includes a diverse group of well-characterized endometrial tumors among black and white women with centrally reviewed tumors, availability of epidemiological risk factor information, and prospectively collected treatment data.
In summary, we observed that black women with EC had higher odds of receiving certain adjuvant treatment regimens compared to white women. In the setting of low-grade, early stage disease, this may represent over-prescription, potentially due to clinician concerns about racial disparities in EC survival. Prospective studies focusing on contextual patient-level factors, patient-provider interactions, and treatment facility characteristics are warranted to increase our understanding of adjuvant treatment receipt in EC.
Supplementary Material
AJOG at a Glance.
A. Why was this study conducted?
• To assess the hypothesis that black women with endometrial cancer are less likely than their white counterparts to receive adjuvant treatment in models stratified by tumor characteristics.
B. What are the key findings?
• In histology-stratified models, black women with low-grade endometrioid or serous tumors were more likely to receive chemotherapy-only or radiotherapy plus chemotherapy compared to white women. In models stratified by the European Society for Medical Oncology definition of risk, black women with high-risk cancer were more likely to receive radiotherapy plus chemotherapy compared to white women.
C. What does this study add to what is already known?
• Our focus on specific types of adjuvant treatment regimens along with subgroup analyses provides greater clarity in understanding racial differences in receipt of adjuvant treatment.
Acknowledgements
This work is funded by National Cancer Institute grants U10 CA180868 (NRG Oncology Operations), U10 CA180822 (NRG SDMC), CA27469 (GOG Administrative Office and Tissue Bank), and CA37517 (GOG Statistical and Data Center). The authors would like to acknowledge the significant contributions of the late Dr. D. Scott McMeekin who worked extensively on the GOG-210 study and Drs. Wei Deng and Shamshad Ali for their contributions to the GOG-210 study.
The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Northwestern University, University of Mississippi, University of Colorado-Anschutz Cancer Pavilion, University of California at Los Angeles, Fred Hutchinson Cancer Research Center, Penn State Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center, Indiana University Medical Center, Wake Forest University Health Sciences, University of California Medical Center at Irvine – Orange Campus, Magee Women’s Hospital – University of Pittsburgh Medical Center, University of New Mexico, Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council/Ohio State University, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, Women's Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago, Mayo Clinic, Case Western Reserve University, Moffitt Cancer Center and Research Institute, Yale University, University of Wisconsin Hospital, Women and Infants’ Hospital of Rhode Island, The Hospital of Central Connecticut at New Britain General, GYN Oncology of West Michigan, PLLC and Community Clinical Oncology Program.
Financial Support: This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517) and the NRG Oncology Grant number: 1 U10 CA180822 and U10 CA180868. In addition, this research was supported in part by funds provided by the intramural research program of the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation: Black women with endometrial cancer are more likely to receive certain adjuvant treatment regimens than their white counterparts.
ClinicalTrials.gov Identifier: NCT00340808
REFERENCES:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21(11):1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman ME, Sturgeon S, Brinton LA, Potischman N, Kurman RJ, Berman ML, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10(10):963–8. [PubMed] [Google Scholar]
- 7.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–85. [DOI] [PubMed] [Google Scholar]
- 8.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S105–43. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell GL, Tian C, Risinger J, Brown CL, Rose GS, Thigpen JT, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107(9):2197–205. [DOI] [PubMed] [Google Scholar]
- 10.Mukerji B, Baptiste C, Chen L, Tergas AI, Hou JY, Ananth CV, et al. Racial disparities in young women with endometrial cancer. Gynecol Oncol. 2018;148(3):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, et al. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res. 2000;6(8):2999–3005. [PubMed] [Google Scholar]
- 12.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94(12):2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruterbusch JJ, Ali-Fehmi R, Olson SH, Sealy-Jefferson S, Rybicki BA, Hensley-Alford S, et al. The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol. 2014;211(6):627.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control. 2009;16(1):53–6. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute: Physicians Data Query 2017;Pageshttps://www.cancer.gov/types/uterine/hp/endometrial-treatment-pdq#section/36 on July 19, 2017. 2017.
- 16.National Comprehensive Cancer Network;Pageshttp://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf on November 30, 2015.
- 17.Lee CM, Szabo A, Shrieve DC, Macdonald OK, Tward JD, Skidmore TB, et al. Descriptive nomograms of adjuvant radiotherapy use and patterns of care analysis for stage I and II endometrial adenocarcinoma: A surveillance, epidemiology, and end results population study. Cancer. 2007;110(9):2092–100. [DOI] [PubMed] [Google Scholar]
- 18.Hicks ML, Phillips JL, Parham G, Andrews N, Jones WB, Shingleton HM, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83(12):2629–37. [DOI] [PubMed] [Google Scholar]
- 19.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, Clemmer JT, Schorge JO, Rice LW, et al. Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis. Gynecol Oncol. 2017;145(1):114–21. [DOI] [PubMed] [Google Scholar]
- 20.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: A contemporary national cancer database registry analysis. Gynecol Oncol. 2016;143(1):98–104. [DOI] [PubMed] [Google Scholar]
- 21.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21(22):4200–6. [DOI] [PubMed] [Google Scholar]
- 22.Trimble EL, Harlan LC, Clegg LX, Stevens JL. Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol. 2005;96(3):741–8. [DOI] [PubMed] [Google Scholar]
- 23.Elshaikh MA, Munkarah AR, Robbins JR, Laser BS, Bhatt N, Cogan C, et al. The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol. 2013;128(2):171–4. [DOI] [PubMed] [Google Scholar]
- 24.Bickell NA, Chassin MR. Determining the quality of breast cancer care: do tumor registries measure up? Ann Intern Med. 2000;132(9):705–10. [DOI] [PubMed] [Google Scholar]
- 25.Cress RD, Zaslavsky AM, West DW, Wolf RE, Felter MC, Ayanian JZ. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care. 2003;41(9):1006–12. [DOI] [PubMed] [Google Scholar]
- 26.Zaino RJ. FIGO staging of endometrial adenocarcinoma: a critical review and proposal. Int J Gynecol Pathol. 2009;28(1):1–9. [DOI] [PubMed] [Google Scholar]
- 27.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–4. [DOI] [PubMed] [Google Scholar]
- 28.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi33–8. [DOI] [PubMed] [Google Scholar]
- 29.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. [DOI] [PubMed] [Google Scholar]
- 30.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–13. [DOI] [PubMed] [Google Scholar]
- 31.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133(2):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110(2):234–43. [DOI] [PubMed] [Google Scholar]
- 33.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol. 2013;130(3):652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felix AS, Brasky TM, Cohn DE, Mutch DG, Creasman WT, Thaker PH, et al. Endometrial carcinoma recurrence according to race and ethnicity: An NRG Oncology/Gynecologic Oncology Group 210 Study. Int J Cancer. 2018;142(6):1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.