Abstract
Sulfogalactosylglycerolipid (SGG, aka seminolipid) is selectively synthesized in high amounts in mammalian testicular germ cells (TGCs). SGG is an ordered lipid and directly involved in cell adhesion. SGG is indispensable for spermatogenesis, a process that greatly depends on interaction between Sertoli cells and TGCs. Spermatogenesis is disrupted in mice null for Cgt and Cst, encoding two enzymes essential for SGG biosynthesis. Sperm surface SGG also plays roles in fertilization. All of these results indicate the significance of SGG in male reproduction. SGG homeostasis is also important in male fertility. Approximately 50% of TGCs become apoptotic and phagocytosed by Sertoli cells. SGG in apoptotic remnants needs to be degraded by Sertoli lysosomal enzymes to the lipid backbone. Failure in this event leads to a lysosomal storage disorder and sub-functionality of Sertoli cells, including their support for TGC development, and consequently subfertility. Significantly, both biosynthesis and degradation pathways of the galactosylsulfate head group of SGG are the same as those of sulfogalactosylceramide (SGC), a structurally related sulfoglycolipid important for brain functions. If subfertility in males with gene mutations in SGG/SGC metabolism pathways manifests prior to neurological disorder, sperm SGG levels might be used as a reporting/predicting index of the neurological status.
Keywords: sulfogalactosylglycerolipid, seminolipid, lipidomics, mass spectrometry, lipid rafts, male reproduction, male fertility
1. Introduction
Sulfogalactosylglycerolipid (SGG, aka seminolipid) is present very selectively in mammalian male germ cells at 10 mole% of total lipids [1,2]. This uniqueness is from its active synthesis as well as its minimal turnover in live testicular germ cells (TGCs). These findings are described in detail below starting with the use of conventional detection and quantitation methods of SGG in the 1970’s and 1980’s to more recent times where mass spectrometry analysis is employed. As an ordered lipid, SGG is an integral component of lipid rafts, membrane domains that are platforms of cell interaction and signaling [3–5], and like other glycolipids, its involvement in cell adhesion is through its direct affinity to a number of cell surface proteins, extracellular proteins and extracellular matrix proteins [2, 6–10]. These properties are fundamental to spermatogenesis, which is greatly dependent on interaction between developing TGCs themselves as well as between TGCs and Sertoli cells [11–13]. In fact, studies in transgenic male mice null for Cst and Cgt, encoding two enzymes in the SGG biosynthesis pathway, indicate a spermatogenesis disruption, implicating the indispensability of SGG in this process [14, 15]. Furthermore, SGG in sperm lipid rafts, which are platforms on the sperm surface for egg binding, contributes to gamete interaction via its direct affinity for the zona pellucida (ZP), the egg extracellular matrix [3, 4]. This document relates these research studies together with emerging molecular mechanisms through which SGG regulates spermatogenesis.
Developing TGCs reside in the adluminal compartments between adjacent Sertoli cells, somatic cells that regulate spermatogenesis. Approximately 50% of TGCs, however, become apoptotic en route during spermatogenesis and are phagocytosed by Sertoli cells. SGG molecules in phagocytosed apoptotic corpses are catabolized by Sertoli cell lysosomal enzymes presumably into the lipid backbone. Arylsulfatase A (ARSA) is the first enzyme in the SGG degradation pathway, which removes sulfate from the galactosylsulfate head group of SGG. In the last part of this document, we describe a lysosomal storage disorder observed in Sertoli cells of aging Arsa null male mice, which leads to marked reduction in spermatogenesis and sperm fertilizing ability [16]. Notably, SGG in testicular germ cells has the same hydrophilic galactosylsulfate head group as sulfogalactosylceramide (SGC, aka sulfatide and cerebroside-3sulfate) present in the brain and kidney [17]. Therefore, the synthesis and degradation pathways of this head group of SGG and SGC utilize the same enzymes [2, 17–24].
Accumulated evidence reveals the roles of SGC in myelination. In fact, neurological disorder due to demyelination is observed in humans with natural mutations and transgenic mice null for enzymes in either the biosynthesis or degradation pathway of SGC/SGG [14, 15, 25–27]. Therefore, the interrelationship and temporal manifestation between neurological disorder and male infertility/subfertility caused by deficiency or decreased levels of enzymes in the biosynthesis and degradation pathways of SGC/SGG are discussed.
2. Discovery of SGG: past and present characterization methods
In the early 1970s, two glycolipid research groups independently described the presence of SGG in boar and rat testes and boar sperm, as revealed by a unique glycolipid spot following thin layer chromatography (TLC) of extracted testicular and sperm lipids and post-staining with a sugar detecting dye [1, 28]. This novel TLC spot reacted positively with reagents used for carbohydrate detection, and it had an Rf value similar to that of SGC (chemical name: (2S, 3R, 4E)-1-[3-O-(oxysulfonyl)- D-galactopyranosyloxy]-2-acylamino-3-hydroxy-octadec-4-ene) present in the brain (especially around the myelin sheath in the white matter) and epithelial cells of various organs [1]. SGC was first described by JLW Thudichum in 1884 (see review: [29]) and subsequently discerned for its chemical structure by G Blix in 1933 [30]. Similar approaches were then used to identify the chemical structure of this novel testicular glycolipid. These included specific biochemical assays for the sulfate group, an enzymatic assay for the galactose group, gas liquid chromatography (GLC) of methanolysis products (the fatty acyl chain and the lipid backbone), infrared spectroscopy and NMR [1, 28, 31]. Results from these analyses revealed the structure of this novel testicular glycolipid to be 1-O-alkyl-2-O-acyl-3-O-[3-O-oxysulfonyl-β-D-galactopyranosyl]-sn-glycerol, with the alkyl and acyl chains predominantly determined to be C16:0 (palmityl and palmitoyl groups, respectively) (Fig. 1). For simplicity, this testicular sulfoglycolipid is referred to as sulfogalactosylglycerolipid (SGG) [2]. Subsequently, SGG was found to be present in testes and sperm of other sexually mature mammals including humans [31–36]. In fact, SGG is the very predominant sulfoglycolipid in all mammalian testes and sperm, and hence Ishizuka named it “seminolipid” [1,31, 36]. The chemical structure of SGG has been confirmed by high-resolution ID and 2D NMR as well as fast atom bombardment mass spectrometry (FAB-MS) [37,38] As expected, the ion at m/z 795 in the negative ion mode is the major signal in the FAB mass spectra of SGG isolated from testicular sources [37, 38], confirming the prevalent existence of C16:0 alkyl and acyl chains. However, other less intense signals are also detected, assigned to SGG species with C17:0/C16:0, C16:0/C18:0, C 16:0/C 14:0, C16:0/C14:1, C15:0/C16:0 and C15:1/C16:0 alkyl/acyl chains [38]
Figure 1. Structures of SGG and SGC.
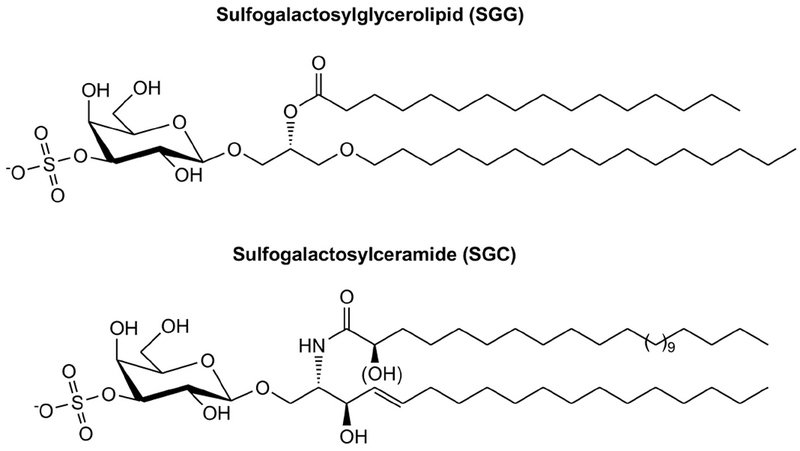
Although the two sulfoglycolipids have different lipid backbones, alkylated glycerol for SGG and sphingosine for SGC, their overall conformation is similar to each other. Both SGG and SGC have the same galactose-3’-sulfate head group and each has two hydrocarbon chains, which insert into the lipid bilayers. For SGG, an alkyl and an acyl chain are present at the sn-1 and sn-2 position of glycerol, respectively. For SGC, an acyl chain is N-linked to the long chain base d18:1 sphingosine. The trans double bond in the sphingosine d18:1 still allows tight chain packing as in a saturated hydrocarbon chain. As shown here, C16:0/C16:0 SGG is a prevalent molecular species in male germ cells, whereas (hydroxylated) C24:0 SGC is one of the abundant molecular species of SGC found in the brain and other tissues.
In general, mass spectrometry is the most direct and convenient method to detect SGG with molecular specificity, thus readily revealing its molecular species. However, FAB-MS used in early days of SGG identification requires a substantial amount of material for the analysis. With the development of electrospray ionization (ESI) mass spectrometry and advancement of MS-based lipidomic analyses, liquid chromatography-ESI tandem mass spectrometry (LC-ESI-MS/MS) has been recently used with supreme sensitivity and specificity to detect lipid molecular species across various classes [39–46], including SGG [16, 47–49]. Quantification for each specific lipid species is also possible via the multiple reaction monitoring (MRM) approach, and can achieve superb accuracy and reproducibility when performed with an appropriately labeled isotopic internal standard [44, 50–52]. With a chemically synthesized deuterated SGG internal standard [53], we have quantified SGG in mouse testis and sperm lipid extracts, by LC-ESI-MS/MS-MRM in the negative ion mode, to be of 500-700 μg or 630-880 nmole per gram testis and 0.15-0.2 μg or 190 -250 pmole per 106 sperm. In these ESI-MS/MS-MRM analyses, transitions of m/z 795 (belonging to SGG most predominant species (C 16:0/C 16:0)) to m/z 97 and 539 were monitored in parallel to corresponding ions of the synthetic internal standard, i.e., m/z 798 (2H3-C16:0/C16:0 SGG) to m/z 97 and 542) (Fig. 2). At the time the limit of detection of LC-ESI-MS/MS-MRM for SGG was 1 pmole (0.8 ng) [49], although this limit has likely been surpassed with the latest generation of instruments.
Figure 2. SGG quantification by multiple reaction monitoring (MRM) in triple quadrupoles (Q).

Upper panel: Schematic representation of the multiple reaction monitoring experiment in which parent ions (P in the lower panel) of interest (in this case the ions at m/z 798 and 795) are sequentially selected in the first mass analyzer (Q1). These ions are allowed to collide with gas molecules in the collision cell (Q2); the emerging ions are separated in the second mass analyzer (Q3), and their relative intensity is recorded by the detector. The arrows indicate the sites at which fragmentation takes place, and the proposed structures of the fragment are shown. Lower panel: Fragment ion mass spectra of deuterated SGG internal standard (Top) and natural (unlabeled) SGG (Bottom). The deuterated SGG contains a terminal C2H3 (CD3) moiety instead of CH3 in the natural SGG form, thus making the m/z values of the parent, and any fragment ions containing the CD3 moiety, 3 Daltons heavier than the corresponding ions from the natural SGG form. Both observed and calculated ion masses are shown as the integer values. The mass spectra presented are taken from Franchini et al. [53].
The advent of mass spectrometry imaging, which is based on the MALDI-TOF (matrix-assisted laser desorption/ionization) approach, allows detection of macromolecules with molecular specificity in tissue sections attached to a glass surface [54, 55]. MS imaging visualizes molecular distributions of individual m/z ions with a spatial resolution of 5-10 μm [56]. Therefore, this MS-based imaging approach supersedes the immuno-detection procedure, which often is not molecularly specific. Particular to the SGG work, antibodies that react with the sulfoglycolipid cannot differentiate between SGG molecular species, and most of these antibodies react with both SGG and SGC (see below). With MS imaging, we were able to detect the major species (C16:0/C16:0) of SGG and other molecular species in mouse testis sections [55, 57]. In addition, when the SGG degradation pathway was genetically disrupted, our MS imaging revealed the accumulation of various SGG molecular species, which were usually not present in wild type testes, as well as an unexpected presence of C16:0 SGC in mouse testicular seminiferous tubules [16] (see more in Section 6).
As shown in Figure 1, SGG and SGC possess the same sulfated galactose head group. While the lipid backbones of the two sulfoglycolipids are different (alkylated glycerol for SGG and sphingosine for SGC), their CPK (Corey-Pauling-Koltun) space-filling molecular models are similar [18]. Thermodynamically, the hydrocarbon chains of both sulfoglycolipids would have the propensity for embedding into the biomembrane lipid bilayers with their polar head groups exposed to the extracellular environment. The sulfated galactose moiety is therefore potentially antigenic. Not surprisingly a number of antibodies produced against SGC have cross-reactivity with SGG [6, 36, 58–62], although they do not recognize galactose sulfate. SGC has been more widely and longer studied for its chemical and physiological properties due to the discovery that its accumulation in the brain, because of natural mutations in the ARSA gene (see Section 6), is responsible for the lysosomal storage disorder, metachromatic leukodystrophy (MLD). Therefore, accumulated efforts have been invested into the purification of SGC from animal brains as well as its antibody production, including an O4 monoclonal antibody [60], which is now commercially available. Since SGC is either undetectable or present at minimal levels in the mammalian male reproductive tract, anti-SGC/SGG antibodies, including O4 antibody, have been conveniently used to localize SGG in testes and sperm [6, 16, 35, 61, 63–65]. In addition, they have been used to demonstrate the functions of SGG in male fertility [6, 35, 63, 66].
3. Biosynthesis and presence of SGG in male reproductive cells
3.1. Early studies on biosynthesis and presence of SGG in testicular germ cells
Most of these studies were performed during the 1970s and 1980s using basic biochemical techniques (as described above) as well as metabolic radiolabeling and immuno-detection approaches to detect and quantify SGG. The early demonstration of SGG in spermatozoa by Ishizuka [1] led to a speculation that SGG was synthesized in TGCs. However, defining the specific TGCs in which SGG originated was not an easy task, as spermatogenesis is a complicated and ongoing process with TGCs in the seminiferous tubule epithelium of adult testes comprising spermatogonial stem cells and spermatogenic cells of various developmental stages (Fig. 3B). Testosterone, secreted by Leydig cells present in the interstitial space of seminiferous tubules, regulates development of TGCs. In addition, Sertoli cells, which are somatic cells occupying the whole thickness of the seminiferous tubule epithelium play important roles in supporting development of germ cells both nutritionally and spatially. Developing TGCs in fact reside in the space, the so-called adluminal compartment, between two adjacent Sertoli cells. The adluminal compartment has a specific milieu due to tight junctions between the adjacent Sertoli cells. These tight junctions together with basal ectoplasmic specializations are the basis of the blood-testis barriers, which restrict passage of humoral components into the adluminal compartments above, where the “non-self’ developing germ cells (non-existing in the body at birth) reside (Fig. 3A). Spermatogonial stem cells are present underneath the blood-testis barriers, whereas diploid primary spermatocytes (developed from differentiated spermatognonia), haploid secondary spermatocytes (generated from the first phase of the meiotic division of primary spermatocytes) and haploid round spermatids (generated from the second phase of the meiotic division of secondary spermatocytes) (Fig. 3B) organize themselves in layers occupying the adluminal compartment, with the least developed germ cells-primary spermatocytes existing above the blood-testis barriers and the most developed ones-round spermatids closest to the tubule lumen. Round spermatids then undergo a specific differentiation process, called spermiogenesis, to become elongated spermatids, which then impregnate their heads into deep recesses of Sertoli cells. Finally, anatomically mature spermatozoa are developed from elongated spermatids and released by Sertoli cells into the lumen (Fig. 3A). The kinetics of the development of TGCs is amazingly precise, and spermatogenesis is continuously ongoing through adulthood. However, the spermatogenesis process is not synchronous in an adult testis. Namely, differentiated spermatogonia individually commit to enter spermatogenesis at different times, thus resulting in the presence of TGCs at various developmental stages in an adult testis [67].
Figure 3.
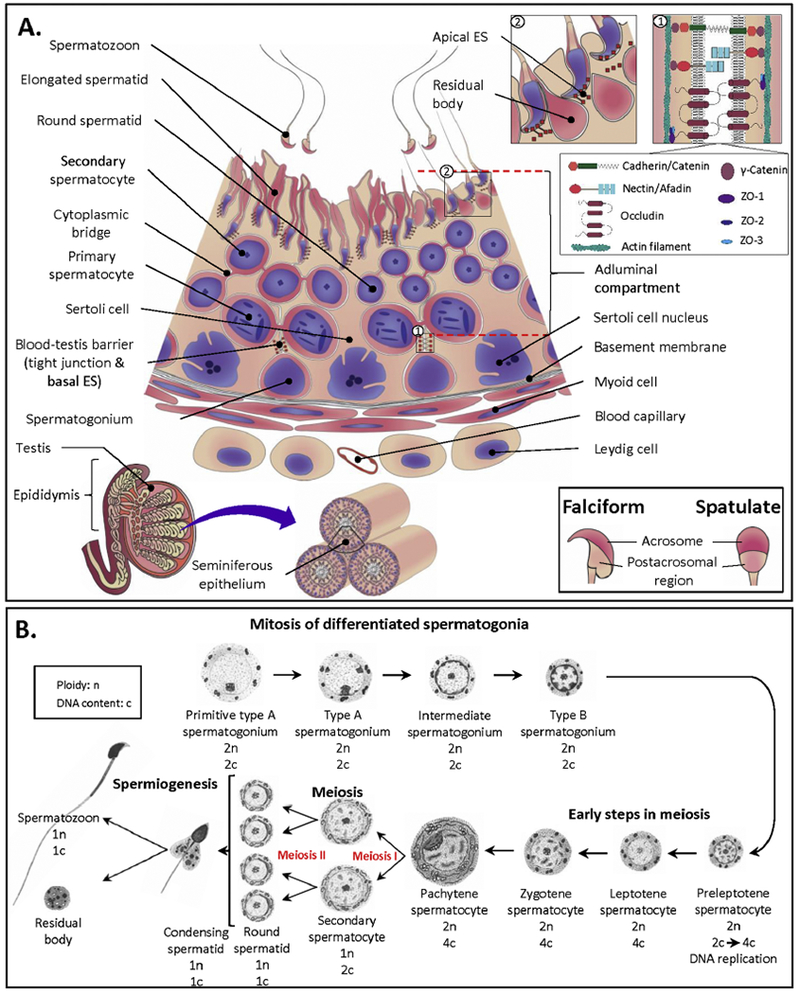
A: The seminiferous epithelium (SE), the site of spermatogenesis. Somatic Sertoli cells span through the whole thickness of the SE. Between adjacent Sertoli cells towards the basement membrane, there exist tight junctions (TJs) and basal ectoplasmic specializations (ESs), which together form the blood-testis barrier. Both TJs and ESs are bordered by actin filaments with TJ and ES proteins interconnecting between the two adjacent Sertoli cells, as shown in the top inset 1 (drawn based on the previous publication [239]). TJ proteins include occludin, ZO1, ZO2 and ZO3, whereas nectin/afadin, cadherin/catenin and γ-catenin constitute ES junctions. Spermatogonia undergoing mitosis and preleptotene spermatocytes are localized underneath these junctions, whereas other developing testicular germ cells (TGCs) in meiotic and morphogenetic phases of development are in the adluminal compartment above. During the course of their development, TGCs move towards the lumen, with the most developed (i.e., spermatids) being closest to the lumen. Each successive layer of TGCs can be referred to as a distinct generation arising from amplifying progenitor spermatogonia at precisely timed intervals depending on the species (16 days in human, 12 days in mice). Note that secondary spermatocytes are short lived and therefore are not always present in the SE. Finally, elongating/elongated spermatid heads are inserted into the recesses of the Sertoli cell apical membrane. Apical ESs in Sertoli cells surround these elongated spermatids, holding them in place. As part of maturation to become testicular spermatozoa, the residual bodies (extra cytoplasm) are shed from the elongated spermatids and phagocytosed by Sertoli cells (see the top inset 2). Testicular spermatozoa are then released into the lumen. In mammals, there are two major forms of sperm heads, falciform and spatulate (see bottom inset). Note that this is a simplified drawing, which does not include all types of primary spermatocytes and various steps of spermatids. Information on the former aspect is described in B. More information on the second aspect as well as information on how various TGCs associate with each other in SE layers during different time intervals of spermatogenesis can be obtained from the recent review [11].
B: Steps in spermatogenesis. Differentiated spermatogonia undergo mitosis divisions and transform in a number of steps to become preleptotene primary spermatocytes, in which DNA replication takes place. With syntheses of more macromolecules, the more developed primary spermatocytes become enlarged and their various stages are classified by the increasing chromatin condensation (i.e., leptotene, zygotene and pachytene spermatocytes). Pachytene spermatocytes (distributed at the highest percentage among all primary spermatocytes in the seminiferous epithelium) then enter the first meiotic division to generate haploid secondary spermatocytes, which in turn undergo the second meiotic division to produce haploid round spermatids with DNA content being one half of that of secondary spermatocytes. Finally, round spermatids are differentiated through a number of steps to become elongated spermatids and then testicular spermatozoa. Therefore, one pachytene spermatocyte should generate four testicular spermatozoa. However, apoptosis usually occurs en route [96], thus yielding a lower number of spermatozoa than expected. It takes ~45 days in mice for one Type A spermatogonium to develop into a testicular spermatozoon. Drawing was adapted from Bellve el al. [72].
Notably, at all phases of germ cell development, direct interactions, either through transient or prolonged adhesion, and communications between Sertoli cells and germ cells are essential [12, 13, 68–70], and it is likely that SGG, as a glycolipid, plays roles in these adhesion and communication processes. Therefore, information on TGCs in which SGG is synthesized and turned over (if any) is essential for a further understanding of the roles of SGG in these spermatogenesis-related adhesion processes. These research goals were, however, challenged by the difficulty in isolating each TGC population from adult testes with purity for the biochemical assay of SGG. It was not until 1976, when the sedimentation-velocity based STA-PUT method, which separated different types of loose TGCs, prepared from collagenase/trypsin treated seminiferous tubule, was described [71]. Regardless, this STA-PUT separation method not only is tedious but also requires expertise in identifying various types of TGCs.
A simpler approach to identify the TGC type in which SGG synthesis takes place is to use postnatal rodents in the study. Neonatal mice/rats (5-7 days old) possess only spermatogonial stem cells and Sertoli cells in the seminiferous tubules, with a small number of Leydig cells in the interstitial space (Table 1). As the animals develop towards puberty, primary spermatocytes first start to be present in the seminiferous tubule epithelium followed by secondary spermatocytes, round spermatids, elongating and elongated spermatids and finally testicular spermatozoa at puberty. The kinetics of these sequential increases of TGCs is precise in each species. The presence of early primary spermatocytes (called preleptotene and leptotene, see Fig. 3B) in the rodent seminiferous tubule epithelium starts on day 10 after birth. By postnatal day 15-16, the majority of TGCs are pachytene spermatocytes, the most developed form of primary spermatocytes. By day 20, round spermatids start to be present in the seminiferous tubule epithelium, although pachytene spermatocytes are still the major type of TGCs at this age (see Table 1)[72]. The number of round spermatids keeps on rising and by postnatal day 25, their proportion in the seminiferous tubule epithelium exceeds that of primary spermatocytes. Elongating and elongated spermatids then start to appear around day 30. Completion of the first round of spermatogenesis in mice/rats is hallmarked by the appearance of testicular spermatozoa by 35 days after birth [73, 74]. The increasing number of TGCs in the seminiferous tubule epithelium from postnatal day 10 to day 35 is therefore tremendous. However, in subsequent rounds of spermatogenesis, the seminiferous cycle (time required to generate a testicular spermatozoon from a spermatogonium Type A) is usually longer, i.e., about 45 days in mice [75–78]. In contrast, Sertoli cells stop dividing when the rodents are around 16-17 days old [79–81], rendering them to constitute only 5% of total cells in the seminiferous tubules of adult animals [82].
Table 1:
Percentages of various spermatogenic cell types and Sertoli cells in neonatal and prepubertal mice (adapted from Bellve et al. [72])
| Cell type | Postnatal day | |||||||
|---|---|---|---|---|---|---|---|---|
| 6d | 8d | 10d | 12d | 14d | 16d | 18d | 20d | |
| Early differentiated spermatogonia | 16 | - | - | - | - | - | - | - |
| Differentiated spermatogonia | - | 27 | 18 | 15 | 12 | 17 | 10 | 10 |
| Preleptotene spermatocyes | - | - | 15 | 11 | 9 | 5 | 10 | 7 |
| Leptotene spermatocytes | - | - | 15 | 12 | 13 | 5 | 5 | 9 |
| Zygotene spermatocytes | - | - | - | 23 | 14 | 7 | 8 | 8 |
| Pachytene spermatocytes | - | - | - | - | 15 | 27 | 36 | 33 |
| Secondary spermatocytes | - | - | - | - | - | - | 1 | 1 |
| Round spermatids | - | - | - | - | - | - | 1 | 4 |
| Sertoli cells | 84 | 73 | 52 | 39 | 37 | 39 | 29 | 28 |
Two independent research groups (M Kornblatt, H Schachter & RK Murray in Canada and I Ishizuka, A Suzuki & T Yamakawa in Japan) used postnatal rodents to determine the testicular germ cell type that first synthesized SGG. Basically, lipids isolated from the whole testis homogenate from postnatal mice were used for SGG assays and the SGG amounts obtained were ascribed to specific TGC types existing during the prepubertal and pubertal ages. Taking the advantage that SGG is a predominant sulfolipid in the testis, Kornblatt et al. [31] quantified SGG in rat testicular lipids through a colorimetric azure A assay, which measures the lipid-bound sulfate [83]. On the other hand, Handa et al. [84] (in the group of Ishizuka, Suzuki and Yamakawa) metabolically radiolabeled SGG through testicular injection of Na235SO4 into mice of increasing ages. One hour after the injection, mice were sacrificed and lipids isolated from the testis were scintillation-counted for the 35S radiolabel. These 35S counts were presumed to belong to SGG, the predominant testicular sulfolipid.
Kornblatt et al. [31] demonstrated that SGG was not detectable in testes of 7- and 10-day old rats, indicating that spermatogonia, Sertoli cells, early primary spermatocytes and Leydig cells did not contain and/or synthesize SGG. The first appearance of testicular SGG was observed in rats at 15 days of age, implicating that SGG was first synthesized in pachytene spermatocytes (Table 1). The levels of SGG/mg protein in the testis homogenate further increased to the maximum 30 days after birth. These SGG values then declined to about 50% of the peak value in rats 45 days of age, after the first spermatogenesis round had been completed. The increase in SGG levels at postnatal day 30 reflected the continuing SGG synthesis in the new generation of pachytene spermatocytes, while the first set of these primary spermatocytes with SGG already synthesized had become round spermatids (see Fig. 3B), with a minimal turnover of SGG. Although the authors did not offer an explanation on a 50% decrease of SGG in the seminiferous tubules of animals 45 days of age, the decrease could be considered to be from the substantial rate of TGC apoptosis even in the normal spermatogenesis process [12, 85–87] (see more in Section 6).
Around the same time, Handa et al. [84] demonstrated that 35S incorporation into testicular lipids was observed in mice of 10 days of age and older following an intraperitoneal injection of Na235SO4. However, this radioactive incorporation was minimal in 5-day old mice. At increasing ages until adulthood, the radioactive incorporation into testicular lipids persisted. Handa et al. [84] interpreted that SGG biosynthesis activity started when early primary spermatocytes were present in the testis and absent in spermatogonia, Sertoli cells and Leydig cells. These results were similar to those of Komblatt et al. [31] except that Handa et al. [84] described the first detectable SGG synthesis to be in early primary spermatocytes instead of mature primary pachytene spermatocytes described by Kornblatt et al. [31]. It is likely that the colorimetric assay used by Kornblatt et al. [31] to detect SGG was not as sensitive as the radiolabeling detection of 35SGG employed by Handa et al. [84], thus failing to detect the newly synthesized SGG in early primary spermatocytes.
Subsequently, Letts et al. [88] (in Schachter’s group) confirmed that the biosynthesis of SGG occurred in primary spermatocytes. Their experimentation involved injection of Na235SO4 into adult rats. Primary spermatocytes (mainly pachytene) and round spermatids, the two major populations of round spermatogenic cells, were then isolated by the STA-PUT technique from collagenase/trypsin digested seminiferous tubules. Lipids extracted from primary spermatocytes and round spermatids were subjected to TLC to separate SGG from other lipid types. This allowed quantification of 35S specifically incorporated into SGG. This experimentation revealed that primary spermatocytes possessed high levels of 35S-SGG. Although some 35S-SGG was detected in isolated round spermatids, the level of incorporation was much reduced, compared with those in primary spermatocytes. Altogether, the results indicated that primary spermatocytes were the main site of SGG biosynthesis, although round spermatids also appeared to synthesize SGG, but at a much lower rate than primary spermatocytes.
The biosynthesis of SGG in early primary spermatocytes was also demonstrated in situ by Lingwood [89]. Sexually mature male rats were fed Na235SO4 laced chow over an 18-h period prior to sacrifice. The removed testes were lightly fixed with aldehyde and frozen for preparation of cryosections, which were then subjected to autoradiography. Light microscopic assessment of 35S radioactive silver grains in cryosections indicated their localization to the perimeter of seminiferous tubules, just above the basement membrane- a location enriched in early primary spermatocytes (Fig. 3). These 35S-labeled radioactive grains disappeared upon dehydration of the sections with organic solvent, consistent with what would be expected for a radiolabeled sulfated lipid, i.e., SGG. The author identified these primary spermatocytes with SGG synthesis activity to be zygotene and early pachytene spermatocytes.
In humans, Ueno et al. [90] reported a similar result in which SGG was present only in the testis of adult males. The analyses of SGG were based on its TLC separation from other testicular lipids, followed by specific quantification of galactose in the SGG band, recovered from the TLC plate. Significantly, SGG was not detected in parallel analyses of lipids extracted from testes of 2-year old infants and 9-year old children.
Kornblatt [91] further showed that SGG, once biosynthesized, remained stable in live germ cells. This was demonstrated by a dual-labeling “pulse-chase” experiment in which adult rats were injected intra-testicularly with Na235SO4 and 3H-thymidine. While 35S would be incorporated into SGG during its biosynthesis, 3H-thymidine would be incorporated into DNA of primary preleptotene spermatocytes during DNA replication in the interphase before meiosis I (see Fig. 3B). Following injections, a selected number of animals were sacrificed at various time points (“chase” periods) for the preparation of testis homogenates. 3H and 35S were scintillation-counted in DNA and lipids extracted from the homogenates, respectively, with the 35S counts presumed to belong to SGG. In the first four weeks of the chase period, 35S-SGG and 3H-DNA showed parallel rates of decline, and by day 28, both radiolabels in DNA and SGG were half of those in mice sacrificed on day 1. The 50% decrease of 3H-DNA during these first 4 weeks likely reflected apoptosis of TGCs [12, 85–87], which would have been phagocytosed by Sertoli cells [92–96] for further molecular degradation (see more in Section 6). This interpretation would explain the simultaneous decrease of 35S-SGG. In contrast, the parallel existence of 35S-SGG and 3H-DNA in the testis homogenate suggested that 35S-SGG did not turn over in live germ cells. However, in the fifth and sixth weeks of the chase period, radioactive counts of 35S-SGG and 3H-DNA in the testis dropped dramatically but with a concurrent appearance of both radioactive counts in the cauda epididymis and vas deferens. In rats, it takes about 35 to 40 days for early primary spermatocytes to develop in a stepwise manner into testicular spermatozoa, which then move to the cauda epididymis and vas deferens [67]. Therefore, the author concluded that SGG was synthesized in early primary spermatocytes and because of its stability in TGCs and testicular sperm, 35S-SGG could be detected in epididymal/vas deferens sperm 5-7 weeks post-radiolabel injection. The results also suggested that sperm did not have SGG biosynthetic capacity and the presence of SGG in mature sperm was due to its prior synthesis in primary spermatocytes [91].
The availability of antibodies that recognize SGG (either produced against SGG or directed against SGC but with cross-reactivity to SGG) allows immuno-detection of SGG at the subcellular level. With modern imaging technology, immuno-detection is not only easier to perform but also more sensitive than the radioactive or colorimetric detection of SGG used in the early studies described above. Immunofluorescence performed by various investigators confirms that SGG is present in isolated primary spermatocytes, round spermatids, elongating and elongated spermatids, testicular sperm, epididymal sperm and ejaculated sperm. In addition, when unfixed cells were used for immuno-detection, the results indicated that SGG was localized to the cell surface [2, 6, 35, 61, 63, 97–99]. However, it is a challenge to detect SGG on sectioned tissues embedded in paraffin or plastic, as the embedding process requires dehydration by an organic solvent, which extracts SGG.
3.2. Mass spectrometry based quantification of SGG in testicular germ cells and epididymal sperm
In the seminiferous tubule epithelium of adult mice, pachytene spermatocytes and round spermatids constitute the bulk of TGCs, the former contributing ~20% and the latter ~80%. Pure populations of pachytene spermatocytes and round spermatids can be readily prepared by the STA-PUT technique. Our LC-ESI-MS/MS analyses of lipids extracted from pachytene spermatocytes and round spermatids as well as caudal epididymal/vas deferens mouse spermatozoa indicated that in all samples C16:0/C16:0 SGG with m/z 795.5 was the main SGG molecular species (Fig. 2), as expected. By MRM, SGG was quantified to be 1.912 ± 0.591, 0.712 ± 0.036, and 0.120 ± 0.006 nmole/106 cells, for pachytene spermatocytes, round spermatids and spermatozoa, respectively (Fig. 4). According to Kornblatt [91]. SGG does not turn over in live TGCs. Through meiosis one pachytene spermatocyte produces four spermatids (Fig. 3B). Based on this meiotic division, relative molar abundance of SGG in round spermatids is expected to be ¼ value of SGG in pachytene spermatocytes (i.e., 0.478 nmole/106 cells), should there be no new synthesis after that occurring in primary spermatocytes. Therefore, the observed value of 0.712 ± 0.036 nmole/106 round spermatids, a value 1.5 χ higher than the expected value, indicates that an additional round of SGG synthesis likely takes place in round spermatids. Our recent observation corroborates the results described by Letts et al. [88], who showed the presence of 35S-labeled SGG in isolated round spermatids following injection of Na235SO4 into adult male rats.
Figure 4. SGG levels in mouse testicular germ cells and sperm.

Purified populations of primary spermatocytes, round spermatids and caudal epididymal/vas deferens sperm isolated from CD-I male mice (kindly provided by Dr. Stuart Moss, University of Pennsylvania) were subjected to lipid extraction and SGG quantification by ESI-MS/MS MRM. The data were expressed as mean ± S.D. of results from triplicate experiments and are taken from K Kongmanas’ Ph.D. thesis, 2015, University of Ottawa, which has been published on line (https://ruor.uottawa.ca/handle/10393/32509).
Notably, our LC-ESI-MS/MS MRM indicated that SGG levels of caudal epididymal/vas deferens sperm were only ~17% of those of round spermatids. This reduction suggested that the majority of SGG in spermatids was possibly removed or metabolized, as part of their differentiation process (spermiogenesis). It is likely that SGG exists in the residual bodies, the bulk of excess cytoplasm that is extruded from spermatids during their last phase of shape elongation (see Fig. 3 A); these extruded residual bodies are immediately phagocytosed by Sertoli cells [100, 101].
Table 2 shows the calculated density of SGG per unit surface area of pachytene spermatocytes, round spermatids and caudal epididymal/vas deferens mouse sperm. The calculation of the spherical surface area of pachytene spermatocytes (706.5 μm2) and round spermatids (254.3 μm2) was based on their average diameters of 15 and 9 μm, respectively. On the other hand, the density of SGG on the sperm surface was calculated per unit area of the sperm head surface, since SGG in all mammalian sperm is present only in the head region [6, 35, 61, 63–65, 102]. Considering a cone-like shape of the mouse sperm head with a height and diameter of 10 and 5 μm, its surface area was calculated to be 28 μm2. Notably, the densities of SGG on the surface of pachytene spermatocytes and round spermatids are similar (Table 2). The results suggest that SGG might be essential throughout the spermatogenesis process and that its adequate and stable surface density is required across the various phases of testicular germ cell development. We have shown that SGG is an ordered lipid endowing rigidity to biomembranes [38]. These features may be important to maintain the physiological properties of pachytene spermatocytes and round spermatids, and this may be the reason why additional SGG is synthesized in round spermatids.
Table 2:
Amounts of SGG per surface area of mouse primary spermatocytes, round spermatids and sperm heads
| Male germ cells | SGG amounts/cell (fmole/cell) | Surface area/cell (μm2/cell) | SGG amounts/μm2 (amole/μm2) |
|---|---|---|---|
| Primary spermatocyte | 1.912 ± 0.591 | 706.5 | 2.7 ± 0.8 |
| Round spermatid | 0.712 ± 0.036 | 254.3 | 2.8 ± 0.1 |
| Sperm head | 0.120 ± 0.006 | 28 | 4.3 ± 0.2 |
In the head of mature sperm, the cell surface density of SGG is 1.6 times higher than that in pachytene spermatocytes and round spermatids. This high density would likely endow additional tensile strength and rigidity to the sperm head. Considering that sperm travel through both male and female reproductive tracts prior to fertilizing the egg, membrane tensile strength and rigidity would be required to protect sperm from physical impact during this journey. The multiplicity of surface SGG molecules is also relevant for the interaction of sperm with the egg vestment, which will be discussed more in Section 5.
3.3. Presence of SGG in Sertoli cells of 20-day old and adult mice
Previous findings from experiments using neonatal/postnatal animals [31, 84] indicate the absence of SGG in 5-day old rats and mice. Since only Sertoli cells and spermatogonia are present in seminiferous tubules at this age, the results suggest that SGG is not present in Sertoli cells and spermatogonia. We have confirmed these results in Sertoli cells and spermatogonia isolated from collagenase/trypsin/hyaluronidase/DNase digested seminiferous tubules of 5-day old mice. Loose Sertoli cells and spermatogonia obtained after the enzyme digestion were then separated from each other via the preferential adherence of Sertoli cells to the substratum [103]. After Sertoli cells were attached to the glass slide, spermatogonia remaining in the supernatant were dried under a gentle air stream onto another glass slide. The matrix, 2,5-dihydroxybenzoic acid was then sprayed over both glass slides for mass spectrometry imaging. The MS imaging results (Fig. 5A, top and middle panels) indicate that SGG was not detected in either Sertoli cells or spermatogonia isolated from 5-day old mice. Furthermore, TLC of lipids extracted from Sertoli cells of 5-day old mice did not reveal the purple SGG band upon sugar staining with orcinol [104] (Fig. 5B). In contrast, when Sertoli cells were isolated from enzyme-digested seminiferous tubules of 20-day old male mice following a similar method as described above for 5-day old mice [103]. SGG was clearly apparent. This was revealed by both a positive orcinol stained band on TLC (Fig. 5B) and an ion peak at m/z 795 in LC-ESI-MS/MS (via sulfate precursor ion scanning) in lipids extracted from these Sertoli cells (Fig. 5C, panel a). In 20-day old mice, primary spermatocytes constitute the majority of TGCs (Table 1). MS imaging of these TGCs revealed the presence of SGG, in agreement with previous findings (see above) (Fig. 5A).
Figure 5. Presence of SGG in Sertoli cells and testicular germ cells in mice of different ages.

A: Mass spectra and reconstructed ion images (insets) from MS imaging showing the absence of SGG (m/z 795) in Sertoli cells (top panel) and spermatogonia cells (middle panel), both isolated from 5-day-old mice, but its presence in TGCs of 20-day-old mice (bottom panel). B: HPTLC showing the presence of SGG in Sertoli cells of 20-day-old mice but not in those of the 5-day-old animals. Lipids extracted from 2 million Sertoli cells isolated from 5-day-old or 20-day-old mice were loaded onto a HPTLC plate, and stained with orcinol solution which specifically reacted with glycolipids to yield purple bands. SGG was found as the major glycolipid in the Sertoli cells of 20-day-old mice (where the first round of spermatogenesis has initiated) but it was not detectable in Sertoli cells of neonatal 5-day-old mice. C: Panel a: Mass spectra from precursor ion scanning of m/z 97 (sulfate group) showing the presence of SGG (m/z 795) as the major sulfolipid in Sertoli cells isolated from 20-day-old (top) and 10-week-old (bottom) mice. Panel b: Table showing the amounts of SGG in Sertoli cells of 20-day-old and 10-week-old mice quantified by ESI-MS/MS-MRM analysis. Data are expressed as mean ± S.D. of triplicate results. All data presented are taken from K Kongmanas’ Ph.D. thesis, 2015, University of Ottawa, which has been published on line (https://mor.uottawa.ca/handle/10393/32509).
Isolation of a pure population of Sertoli cells from adult animals is a daunting task, since Sertoli cells constitute only 5% of the total population of seminiferous tubules. However, with some modifications to the protocol used for 20-day old mice, we successfully prepared a primary culture of Sertoli cells (entirely free from TGCs) from adult mice (our unpublished data). Sulfate precursor ion scanning of lipids extracted from these cells indicated the presence of SGG (Fig. 5C, panel a). Further quantification of SGG in lipids of Sertoli cells from 20-day old and 10-week old (adult) mice indicated a higher amount of SGG in Sertoli cells from adult mice (1.383 ± 0.054 nmole/106 cells versus 0.063 ± 0.016 nmole/106 cells in 20-day old mice).
It is unlikely that the presence of SGG in Sertoli cells of mice that are 20 days of age and older reflects de novo biosynthesis. Rather, since TGCs undergo apoptosis at a high rate (−50%) [12, 85–87] and Sertoli cells are professional phagocytes [92–96]. SGG present in Sertoli cells of pubertal and adult mice is likely a molecular component derived from apoptotic germ cell corpses and remnants that are phagocytosed by Sertoli cells [92–96]. The number of apoptotic germ cells in adult testes is expectedly higher than that in testes of 20-day old mice, since at the adult age, seminiferous tubules contain more germ cells, comprising primary and secondary spermatocytes, round spermatids and elongating/elongated spermatids (Fig. 3). Furthermore, Sertoli cells in adult animals are responsible for phagocytosing residual bodies shed from elongating spermatids [100, 101], which likely contain SGG. This residual body phagocytosis event has not taken place in Sertoli cells of 20-day old mice because their seminiferous tubule epithelia do not contain any elongating spermatids (Table 1). Altogether, Sertoli cells from adult animals would possess more of the phagocytosed SGG-containing materials than Sertoli cells from 20-day old mice.
3.4. The biosynthesis pathway of the galactosylsulfate head group of SGG
The biosynthesis pathway of the galactosylsulfate head group of SGC was discerned before that of SGG. In the brain and kidney, ceramide, the lipid building block of SGC is galactosylated by UDP-galactose:ceramide galactosyltransferase (CGT, aka UDP galactosyltransferase 8A, UGT8A) to form galactosylceramide (GC, aka cerebroside), which is then sulfated by 3’-phosphoadenosine-5’-phosphosulfate (PAPS):cerebroside sulfotransferase (CST, aka galactose-3-O-sulfotransferase 1, GAL3ST1) to become SGC (Fig. 6) [2, 18, 19, 21, 105–108]. Since SGC and SGG are structurally related, the biosynthetic pathway of SGG was presumed to be identical or similar to that of SGC. The presumption on the sulfation process was validated by the presence of sulfotransferase in homogenates of the testis and seminiferous tubules, which utilized PAPS (a sulfate donor) to sulfate both GC and galactosylglycerol (GG, the desulfated form of SGG), as well as lactosylceramide, but not glucosylceramide [28, 31, 84, 109]. Like purified CST in rat kidneys and brains [110, 111], the testicular sulfotransferase exists in the Golgi apparatus and uses GG, GC and lactosylceramide as their substrates [109]. The peak activity of testicular sulfotransferase also precedes the presence of SGG in primary spermatocytes, a result suggesting its relevance to the SGG formation [28, 31, 84]. Lingwood’s group purified this sulfotransferase enzyme to homogeneity (a single band of 56 kDa on SDS-PAGE) and further showed that the enzyme was stimulated upon phosphorylation [112]. A soluble factor in the testis homogenate, subsequently identified as phosphoinositol glycerolipid [89, 113], was shown to be an inhibitor of the testicular sulfotransferase. This inhibitor was first detected in the rat testis on day 25 after birth (after the appearance of primary spermatocytes), a finding that would explain at least in part the much lower SGG synthetic capacity of round spermatids [88]. Collectively, results from these studies indicated that the testicular galactolipid sulfotransferase enzyme had similar enzymatic properties to the counterpart enzyme in kidneys and brains with the exception of its regulation by an inhibitor specifically present in the testis.
Figure 6. Biosynthesis pathway of the galactosylsulfate head group of SGG and SGC.

The two sulfogalactolipids utilize the same biosynthesis pathway for their head group, which was discerned mainly from studies on Cgt and Cst null mice (see text for more details). CGT: UDP-galactose:ceramide galactosyltransferase; CST: PAPS: cerebroside sulfotransferase. PAPS: 3’-phosphoadenosine-5’-phosphosulfate.
The ultimate proof that CST is the enzyme that sulfates GG in the testis came from molecular cloning and transgenic mouse studies of Honke and colleagues [15]. CST was first purified from a human renal cell line to homogeneity (appearing as a single band of 54 kDa on SDS-PAGE) [114]. Information on its peptide sequence was then used to retrieve and sequence the human CST cDNA [115], which in turn was used as a probe to obtain the mouse kidney cDNA orthologue. RT-PCR analyses indicated that the Cst gene was transcribed in various tissues, including the digestive tract, kidney, brain, lung and testis, with an identical encoding sequence [116]. Honke et al. [115] further provided evidence that there were no other homologous genes of CST. These results indicated that SGG produced through GG sulfation in TGCs was catalyzed by CST and the purified testicular sulfotransferase described by Sakac et al. [112] was in fact CST. Finally, in transgenic mice globally deleted of the Cst gene, SGC and SGG were not detected in the brain and testis, respectively, confirming that CST was responsible for the sulfation step in the biosynthesis of these two sulfogalactolipids. The observation that GG was accumulated in the testis of these knockout mice further indicated that it was the substrate of CST to generate SGG (Fig. 6) [15].
Unlike research studies on CST, attempts were not made in early days by researchers in the male reproduction field to isolate and/or characterize the enzyme that galactosylates the lipid building block, alkylacylglycerol, of SGG. This was despite the fact that UDP-galactose:CGT with galactosylation activity on ceramide, the lipid building block of SGC, was purified from the rat brain and its cDNA clone was isolated and sequenced [117]. In addition, CGT was localized in various cultured cell lines (MDCK, CHO, HepG2 and D6P2T cells) to be in both the endoplasmic reticulum and Golgi complex [118]. The first study to implicate galactosylation as part of the biosynthetic pathway of SGG was the in vivo metabolic radiolabeling experiment of Hsu et al. [119]. In this experiment, adult rats were intratesticularly injected with D-[1-14C]galactose. Lipids extracted from testes of the sacrificed animals were then subjected to TLC to separate GG and SGG from other components. Incorporation of 14C into the GG TLC spot peaked within 3 h after the D-[1-14C]galactose injection, and then declined to the minimal level by 168 h post-injection. The decrease in the levels 14C-labeled GG was concurrent with an increase in the 14C counts in the SGG TLC spot, and at 168 h post-injection the radioactivity in SGG reached a plateau. These results suggested that galactosylation was a process through which GG was generated and GG was likely a precursor of SGG.
The definitive proof that UDP-galactose:CGT is the enzyme that produces GC and GG came from studies using mice with a global genetic deletion of Cgt (aka Ugt8). These knockout mice were generated by neuroscientists for studying the functions of GC in myelin formation. Both GC and SGC were absent in the brain of these mice [14], a result that further validated that CGT was the enzyme that was responsible for ceramide galactosylation.
Two-dimensional TLC was performed on total testicular lipids extracted from adult wild type and Cgt null mice. Temporal profiles of GG and SGG in wild type mice of increasing ages were also demonstrated. Both GG and SGG were present at low amounts in the testis of mice at 10 days of age [120], a result corroborating the earlier findings [31, 84]. On day 12 after birth, both GG and SGG amounts in the wild type testis were much higher than those in 10-day old mice. However, the level of SGG increased from day 12 to day 17 after birth, whereas that of GG remained the same. Consequently, the ratio of GG to SGG was about 3:7 in testes of 17-day old mice. In adult testes, however, the amount of GG decreased dramatically to be about 5% of SGG. These results in wild type mice suggested that GG was a precursor of SGG and its conversion to SGG occurred rapidly [120]. Results on the absence of SGG in Cst−/− mouse testes with a concurrent accumulation of GG [121] validated the former aspect of the preceding statement. However, since both SGG and GG were absent in testes of adult Cgt null mice, CGT was most likely responsible for the formation of GG [120]. Subsequently, we demonstrated that mouse testes contained CGT activity. In the presence of UDP-galactose, the post-nuclear supernatant of the testis homogenate catalyzed the conversion of C6-NBD-ceramide (N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] hexanoyl]-D-erythro-sphingosine to NBD-GC, which was detected by TLC with Typhoon imaging [49]. Production of GG using endogenous alkylacylglycerol by Chinese hamster ovary (CHO) cells and Mop8 fibroblast cells, both transfected with Cgt-containing plasmids, was also documented [122, 123].
Collectively, results from all these studies indicate that the biosynthesis of the galactosylsulfate head group of SGG and SGC utilizes the same enzymes. CGT first galactosylates alkylacylglycerol (mainly palmitylpalmitoylglycerol (PPG)) to form GG, which then becomes sulfated by CST to form SGG (Fig. 6).
Since CST is localized in the Golgi apparatus [31], newly synthesized SGG has to be transported to the plasma membrane of germ cells. Results in ENU (N-ethyl-N-nitrosourea)-induced mutant Stx2Repro34 mice suggested that t-SNARE syntaxin 2 protein (STX2) may be involved in SGG transport [124]. In somatic cells, STX2 functions in secretory granule fusion [125] and cytokinesis [126]. In primary spermatocytes of wild type mice, STX2 was immuno-localized to the Golgi apparatus as well as the plasma membrane. In addition, a fraction of STX2 was present in the intercellular bridges (see Fig. 3A) of all developing germ cells. Immunofluorescence studies in wild type males also revealed the presence of SGG on the plasma membrane and intercellular bridges between developing germ cells. Like Cgt and Cst null male mice [14, 15] (see more in Section 4), Stx2Repro34 males were infertile with a spermatogenesis arrest at the meiotic prophase and concurrently formation of syncytial multinucleated primary spermatocytes. In these mutant mice, SGG was no longer present either on the germ cell plasma membrane or in the intercellular bridges [124]. These results suggest that STX2 may be engaged in transporting SGG to the cell plasma membrane and intercellular bridges. Nonetheless, direct or indirect affinity between STX2 and SGG needs to be demonstrated to validate this postulation.
4. Indispensability of SGG in spermatogenesis
Transgenic deletion of the Cgt or Cst gene leads to similar phenotypes. Both Cgt and Cst knockout mice exhibit neurological disorders including hind limb paralysis, severe tremor and progressive ataxia [14, 15, 107]. These disorders are caused by myelin abnormalities, in particular, at the CNS nodes of Ranvier [15, 21, 105]. However, the severity of these disorders is more pronounced in Cgt null mice, lacking both GC and SGC, in contrast to Cst null mice, which are deficient in only SGC [21, 107]. The results therefore suggest that both GC and SGC are involved in formation of functional myelin membranes.
Significantly, all male Cgt and Cst knockout mice are infertile with an inability to produce sperm. Histological and genetic studies revealed that a spermatogenesis arrest occurred in primary spermatocytes. A number of multinucleated primary spermatocytes were also observed in testes of both Cgt−/− and Cst−/− mice, indicating the blockade of meiosis and subsequent fusion between adjacent primary spermatocytes, which then became apoptotic [15, 120]. However, the spermatogenesis arrest in Cst null mice took place in late primary spermatocytes, whereas that in Cgt null mice was manifested in earlier primary spermatocytes [21]. In Cst knockout mice, GG was still present in the seminiferous tubules, but both SGG and GG were completely absent in the testis of Cgt null mice [15, 120]. Therefore, the results suggest that both GG (despite its small amount in the testis) and SGG play roles in the progression of primary spermatocytes through the first meiosis, but SGG alone is indispensable for the completion of spermatogenesis [15, 21, 107].
The molecular mechanisms underlying the functions of SGG in germ cell development are still unknown. This development is dependent on interactive communication between Sertoli cells and germ cells [12, 13, 68–70]. Nonetheless, the depletion of SGG as in Cst null mice, while resulting in spermatogenesis arrest, did not affect the function of Sertoli cells, which still remained in the seminiferous tubules, to support spermatogenesis of wild type spermatogonia, following their transplant by direct microinjection into the seminiferous tubule portal (the efferent duct) [127]. In this experiment, Zhang et al. [127] pre-injected Cst−/− mice intraperitoneally with busulfan to destroy all endogenous germ cells (including spermatogonia) in the seminiferous tubules before the testicular microinjection of wild type spermatogonia, isolated from Green mice (globally expressing GFP) [128]. Forty-five days after the Green spermatogonia transplantation, the seminiferous tubules of these Cst−/− mice were populated with all stages of TGCs in the epithelium and testicular sperm in the lumen. These TGCs developed from the transplanted Green spermatogonia as they expressed GFP as well as SGG (detected by immunofluorescence staining using a monoclonal antibody that recognizes both SGG and SGC). Since spermatogenesis is tightly regulated by Sertoli cells [12, 13, 68–70], the results indicated that Sertoli cells of Cst−/− mice were still functioning [127]. Nonetheless, testicular sperm produced in the Cst−/−-seminiferous tubules from transplanted Green spermatogonia could not enter the epididymis and thus these “spermatogenesis-rescued” mice were still infertile. Zhang et al. [127] suggested that the epididymal epithelium in Cst−/− mice might be defective, thus not allowing testicular sperm entry. The authors further demonstrated immunofluorescence signals upon exposure of the epididymis sections of wild type mice to the anti-SGC/SGG antibody, a result indicating the presence of SGG or SGC in the epididymal epithelium. In contrast, epididymal epithelial cells of Cst−/− mice showed no reactivity with anti-SGC/SGG. It was suggested that SGC or SGG in the epididymal epithelium might contribute to the induction of sperm entry into the epididymis [127]. While this suggestion is interesting, the presence of SGC or SGG in epididymal epithelial cells needs to be verified with a more definitive approach such as mass spectrometry and subsequently the roles of SGC/SGG in endowing the ability of epididymal epithelial cells to induce the entry of testicular sperm into the lumen need to be thoroughly investigated.
Using Cgt+/− male mice, whose spermatogenesis rate, sperm fertilizing ability and fecundity were not different from those of the age-matched wild types, we determined the amounts of testicular SGG and sperm SGG required to maintain these male fertility related processes [49]. Our ESI-MS/MS-MRM demonstrated that the testicular SGG level in these heterozygous males was −78% of the wild type values (406.06 ± 23.63 versus 516.65 ± 30.62 μg per gram of testis), a result corroborating the finding that the CGT polypeptide, with normal enzymatic activity, was expressed at ~80% wild type level in Cgt+/− mice. On the other hand, sperm SGG levels in Cgt+/− and wild type mice were not different from each other [49]. This adjustment was possible, since the sperm SGG levels in wild type animals were only 17% of those in round spermatids (see Fig. 3 and Table 2). During differentiation of round spermatids to become testicular sperm, a significant proportion of the cytoplasm enveloped by the plasma membrane is removed from round spermatids in the form of residual bodies [67] (Fig. 3). It is likely that 83% of SGG in round spermatids become part of the residual bodies. In Cgt+/− mice, SGG shed from round spermatids into residual bodies may be slightly less than the corresponding wild type value, resulting in the same levels of sperm SGG in Cgt+/− and wild type mice. The regulatory mechanism in the reduction of SGG levels during the differentiation of round spermatids to sperm is a matter of further investigation.
4.1. Possible mechanisms on the roles of SGG in spermatogenesis
While it is definitive that SGG on TGCs is indispensable for the completion of spermatogenesis, the molecular mechanisms underlying the action of SGG in this process are unclear. As described above, SGG was detected in the membrane of the intercellular bridges linking adjacent spermatogenic cells (see Fig. 3A) [124]. As an ordered lipid and a lipid raft component [2–4] (see more in Section 5.2), SGG likely endows stability to these bridges, which are deemed to be relevant in intercellular communication between adjacent germ cells [129]. Consequently, the presence of intracellular bridges is essential for the completion of the meiotic division [129, 130]. In the case when SGG was absent, the intracellular bridges were structurally abnormal concurrently with the formation of multinucleated primary spermatocytes and an arrest of the meiotic division [124].
The ongoing interaction between Sertoli cells and spermatogenic cells is also essential for spermatogenesis [12, 13, 68–70]. Developing male germ cells in the adluminal compartment (located above the blood-testis barrier between adjacent Sertoli cells, see Fig. 3A) depend on lactate as their main energy source. The lactate metabolite is in fact produced from glucose and secreted by Sertoli cells into the microenvironment of the adluminal compartment [131]. The transport and uptake of lactate into spermatocytes are then mediated by a lactate transporter (monocarboxylate transporter (MCT)). However, Cst null spermatocytes, lacking SGG, failed to uptake lactate, leading to energy deprivation and then apoptosis. Honke [21] postulates that SGG may regulate MCT traffic in male germ cells. This postulation, however, needs to be experimentally validated.
The significance of the Sertoli cell-germ cell interaction led us to investigate whether SGG has direct affinity for Sertoli cell plasma membrane (PM) proteins. Sertoli cells were isolated from 20-day old mice as previously described [103]. A primary culture of these Sertoli cells were essentially free from TGCs, and contained less than 5% of co-isolated peritubular myoid cells (single-layered flattened cells present underneath the seminiferous tubule basement membrane, see Fig. 3A). Octylglucoside (2%) was used to extract Sertoli cell PM proteins, which were then co-incubated with dipalmitoyl phosphatidylcholine (DPPC) multilamellar liposomes. Phosphatidylcholine (PC) is the most abundant lipid on the mammalian cell PM [132]. Therefore, this incubation step was to remove Sertoli cell PM proteins that bound to PC but not specifically to SGG. The DPPC-PM protein complexes were then pelleted by ultracentrifugation. The supernatant obtained was then used for co-incubation with SGG multilamellar liposomes followed by ultracentrifugation to collect the SGG-PM protein complexes as a pellet. Both the DPPC-bound and SGG-bound proteins were extracted from the liposome complexes with SDS-PAGE sample buffer for gel loading (Fig. 7).
Figure 7. Preparation of Sertoli cell plasma membrane proteins with specific affinity to SGG.

Plasma membrane proteins were extracted from the primary culture of Sertoli cells by 2% octylglucoside in PBS. Following removal of the detergent by dialysis, the extracted proteins were incubated (37°C, 1 h) with multilamellar liposomes of DPPC/cholesterol (molar ratio, 2:1) in PBS. DPPC/cholesterol liposomes with bound proteins were then pelleted by ultracentrifugation. The supernatant obtained was further incubated (37°C, 1 h) with multilamellar liposomes of SGG/cholesterol (molar ratio, 2:1) in PBS, and SGG/cholesterol liposomes with bound proteins were likewise pelleted by ultracentrifugation. Proteins bound to DPPC/cholesterol liposomes and SGG/cholesterol liposomes were then solubilized in Laemmli’s sample buffer and subjected to SDS-PAGE. Both DPPC and SGG multilamellar liposomes were prepared as previously described Attar et al. [38].
When the Sertoli cell PM proteins that bound to PC and SGG liposomes were compared on SDS-PAGE by staining with either silver nitrate or Coomassie Blue, two protein bands with apparent molecular weights of 86,000 and 91,000 appeared to be present specifically in the SGG liposome-bound samples. Tryptic peptides were then prepared from these two excised bands and subjected to bottom-up MS analyses [133]. Over 100 proteins were identified from each protein gel band. After eliminating proteins with ion scores less than 100 and those without possible localization on the cell surface, the list of SGG liposome-bound proteins was significantly shortened, containing two major families of proteins, i.e., the ERM (erzin/radixin/moesin)-actin binding proteins and chaperone proteins (Fig. 8A). Notably, the sequences of erzin, radixin and moesin are highly homologous to each other [134], making it difficult to generate antibodies that specifically recognize erzin, radixin or moesin individually. Regardless, the presence of ERM in the whole Sertoli cell PM extract and in the SGG bound liposomes was further confirmed by immunoblotting using an antibody recognizing the three proteins (Fig. 8B).
Figure 8.
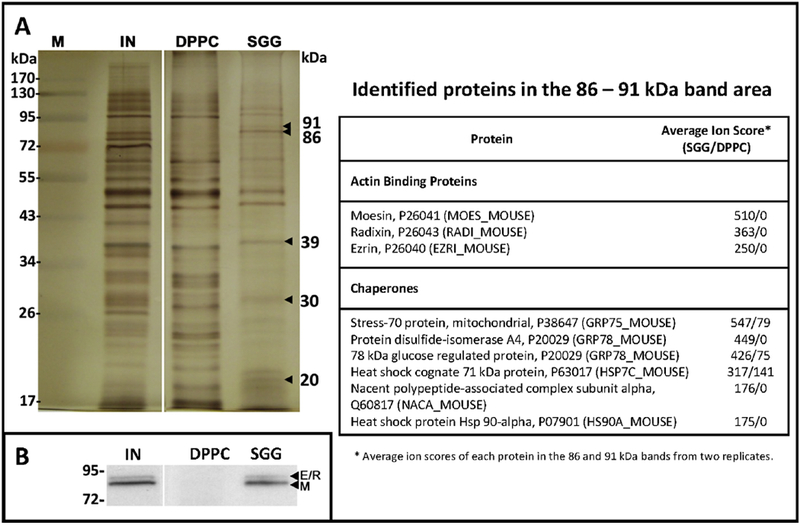
A: Identification of Sertoli cell plasma membrane proteins with SGG affinity. Proteins bound to DPPC and SGG liposomes prepared as described in the legend of Figure 7 were subjected to SDS-PAGE followed by silver staining. Since the amounts of DPPC bound proteins were much higher than those of SGG bound proteins in one preparation, only 1/10 by weight of DPPC bound proteins were loaded as compared with SGG bound proteins. Comparison of the stained gel profile indicates that protein bands of 91, 86, 39, 30 and 20 kDa were present specifically in SGG bound protein samples. The 91 and 86 kDa bands were excised from both SGG bound and DPPC bound protein lanes and in-gel digested with trypsin. Tryptic peptides obtained were subjected to linear ion trap based Fourier transform MS analyses. Identities of proteins (UniProt nomenclature) present in the two excised gel bands were shown in the table along with their average ion scores in the SGG bound and DPPC bound protein samples. B: Immunoblotting of erzin (E), radixin (R) and moesin (M) proteins. The whole Sertoli cell plasma membrane extract (IN) was loaded as a positive control. SGG and DPPC denote proteins that were bound to SGG and DPPC liposomes, respectively. Note that ERM proteins were specifically present in the SGG bound protein sample. All data presented are unpublished and obtained from the study performed by B Doyle, K Kongmanas and N Tanphaichitr, University of Ottawa, and J Whitelegge, UCLA.
Ezrin and radixin have been shown to be present in Sertoli cells [135, 136]). By an in vivo knockdown study, ezrin has been demonstrated to be involved in formation of both apical and basal ectoplasmic specializations (ES) (see Fig. 3A). Both of these adherens junctions require surrounding clusters of actin filaments for which ERM proteins have affinity [134]. The apical ES is important for the interaction between Sertoli cells and elongated spermatid heads, whereas the basal ES contributes to the formation of the blood-testis barrier [134, 136]. However, ERM proteins are not present in the PM as such. They are adaptor proteins localized underneath the PM. They bind to integral PM proteins at their N-terminal region, whereas the affinity for actin filaments is at the C-terminal region [134]. Our Sertoli cell PM extract likely contained the ERM-integral PM complexes and future studies are required to determine whether SGG on the elongated spermatid heads in the apical ES directly binds to ERM or the associated integral PM protein(s) in the apical surface of Sertoli cells.
Chaperone proteins identified for their affinity for SGG liposomes in our study include various Hsp70 family members (i.e., Grp75 (HSPA9), Grp78 (HSPA5) and Hsc70 (HSPA8)), NACA and HS90A. These results, in fact, confirm the previous findings describing the direct affinity of SGG/SGC to various purified Hsp70 family members [137–139]. Notably, HSPA8 has been previously shown in vitro to associate strongly with ezrin by ELISA and pull down assays [135]. HSPA8-ezrin complexes may synergistically bind to SGG and if their existence on the Sertoli cell surface is confirmed, these HSPA8-ezrin complexes would contribute to the interaction between Sertoli cells and developing TGCs, essential for the spermatogenesis process. However, involvement of other Hsp70 family members in Sertoli cell functions is still unclear. In mouse spermatogenic cells and human sperm, the physiological relevance of an Hsp70 isoform, Hsp70-2 (HSPA2) has been documented. Hsp70-2 is essential for formation of synaptonemal complexes [140] and HSPA2 likely plays roles in “chaperoning” egg binding proteins into the high molecular weight (HMW) complexes beneficial for sperm-egg interaction [141, 142] (see Section 5.2). Future localization studies are required to verify that all chaperone proteins identified by our MS analyses are on the Sertoli cell surface and engage in “chaperoning” other surface proteins for the interaction with TGCs. In addition, molecular mechanisms of how SGG interaction with Hsp70 can regulate the chaperone functions of Hsp70 family members should be discerned. Biochemical studies indicate that both SGG and SGC bind to the ATPase domain of Hsp70 [138]. Another research group further demonstrates that SGC interaction with this ATPase domain promotes Hsp70 clustering and stabilizes binding to unfolded proteins [143]. How these biochemical results relate to observations on SGG-Hsp70 interaction in Sertoli cells, TGCs and sperm described above is still unclear. Future investigations on this matter should also consider involvement of other heat shock proteins such as Hsp40 and Hsp90, which usually co-function with Hsp70 [144].
5. Roles of sperm surface SGG in fertilization
Although testicular sperm are anatomically mature with a polarized head and tail structure, they do not have fertilizing ability. This ability is gained in two sequential processes, sperm maturation and sperm capacitation, taking place during sperm transport and storage in the epididymis, and during sperm movement through the female reproductive tract, respectively [145, 146] (Fig. 9). A number of egg binding proteins secreted by epididymal epithelial cells into the lumen deposit onto sperm that move through the epididymis, thus accruing their egg binding ability. In addition, sperm gain their forward movement during their residence in the epididymis [145]. Subsequently, sperm undergo capacitation, the process through which they acquire full fertilizing ability [146, 147]. Only capacitated sperm can interact and fertilize ovulated mature eggs in the oviduct.
Figure 9.

A: Steps in sperm maturation and sperm-egg interaction. Testicular sperm cannot fertilize eggs. They acquire fertilizing ability in a stepwise manner. 1. They undergo “maturation” during their transit through the proximal part of the epididymis and during their storage in the distal (cauda) epididymis. Epididymal sperm acquire forward movement. As well, a number of egg binding proteins present in the epididymal lumen deposit onto their head surface. 2. Sperm gain full fertilizing ability in the female reproductive tract through the so-called “capacitation” process, which involves cholesterol efflux, leading to an increase in membrane fluidity and signal transduction. As a result, their motility becomes hyperactivated with whiplash patterns. Sperm protein tyrosine phosphorylation is distinctively increased and ZP binding proteins are fully exposed on the sperm head surface. Despite cholesterol efflux, sperm lipid rafts increase in amount following capacitation. 3. During capacitated sperm movement through the cumulus cell layers surrounding mature eggs, acrosomal exocytosis is usually initiated in mice. Released acrosomal hydrolytic enzymes likely digest the egg vestments facilitating sperm to swim to the ZP. Sperm-ZP interaction then takes place in a species-specific manner, involving a number of ZP binding proteins present on the sperm surface as well as in the acrosomal matrix. 4. Finally, acrosome reacted sperm that have penetrated through the ZP layer bind to the egg plasma membrane. Following sperm-egg plasma membrane interaction, one acrosome reacted sperm enters the egg cytoplasm, signifying that fertilization has occurred.
B: Presence of SGG in both acrosome intact and reacted sperm. SGG has direct affinity for the ZP [6]. Therefore, it is involved in sperm-ZP interaction according to the model presented in A, step 3. The role of sperm SGG in sperm-ZP binding also fits into the earlier model describing that only acrosome intact sperm bind to the ZP [167].
Biochemical and physiological changes occur on capacitated sperm. Release of cholesterol from the sperm head surface, as induced by HDL and albumin, present in the uterine and ovarian follicular fluid [148–151], is one of the relevant capacitation processes, which leads to an increase in sperm head plasma membrane fluidity [152], essential for downstream membrane fusion events, acrosomal exocytosis and sperm-egg plasma membrane fusion. Entry of calcium into sperm from the surrounding milieu also induces signaling pathways with two obvious consequences. First, there is a specific increase in sperm tyrosine phosphorylation. Second, sperm acquire hyperactivated whiplash motility patterns (Fig. 9). This hyperactivated motility endows the thrust force for capacitated sperm to move through egg vestments into the egg cytoplasm to achieve fertilization [146, 153]. The success in fertilization, however, also depends on the initial proper interaction between capacitated sperm and the extracellular matrix, the ZP, of unfertilized eggs in a species-specific manner [146, 154, 155] (Fig. 9). A number of egg binding molecules on the sperm surface (mainly proteins) have been described so far. This redundancy is likely for securing the fertilization process [146, 156, 157]. Interestingly, egg binding molecules appear to co-exist in lipid raft membrane domains [2–4, 158–160] as high molecular weight complexes [156, 161–163].
Sperm capacitation can also be induced in vitro by simply incubating sperm in medium containing calcium, bicarbonate and albumin [146, 149]. This ability has opened up research on molecular mechanisms of sperm capacitation as well as research and clinical translation on in vitro fertilization. In this in vitro system, capacitated acrosome intact sperm have been shown to bind with high efficiency to the ZP of mature eggs. The acrosome is a sperm specific membrane enclosed organelle, which houses a number of hydrolytic enzymes, believed to play roles in digesting the egg vestments. In addition, a number of ZP binding proteins are present in the acrosome. The acrosome is localized in the sperm head anterior, the site of ZP interaction (Figs. 3A and 9). In vitro, solubilized ZP is able to induce acrosomal exocytosis, which initially involves interval fusion between the sperm plasma membrane overlying the acrosome and the outer acrosomal membrane, followed by a release of the acrosomal content through the pores created from the membrane fusion. Completion of acrosomal exocytosis, the so-called acrosome reaction, is essential for successful fertilization and thus male fertility [164–166]). For a long period of time it was accepted, at least in the rodent system, that the onset of acrosomal exocytosis was when sperm bound to the ZP [146, 167]. However, more recent studies utilizing high-performance videomicroscopy and sperm with GFP-labeled acrosome indicate that acrosomal exocytosis has started before sperm interaction with the egg ZP, i.e., during sperm passaging through cumulus cell layers that surround the egg [168, 169]. Nonetheless, the roles of the ZP in initial sperm-egg interaction as well as its ability to further induce acrosomal exocytosis cannot be discounted. Further, a number of ZP binding molecules on the sperm head surface would still be relevant in sperm-egg interaction provided they still exist in acrosome reacting/reacted sperm.
In sections below, we describe the roles of sperm SGG in the epididymal maturation process and sperm-egg interaction. In the latter process, we have shown that SGG exists in both acrosome intact [6, 170] and reacted [102] sperm. Therefore, the description of its involvement in sperm-egg interaction should hold true regardless as to when the acrosomal exocytosis starts.
5.1. Roles of SGG and its binding protein, arylsulfatase A, on the sperm head surface on egg ZP binding
In vitro binding studies reveal that both SGG and SGC have affinity for a number of extracellular proteins (e.g., selectin, laminin, thrombospondin, Von Willebrand Factor) (see reviews: [18, 36]). Therefore, our discovery that fluorescently labelled unilamellar SGG or SGC liposomes can bind specifically to the intact ZP, the egg extracellular matrix, of unfertilized eggs, is not surprising [6]. This is in contrast to a minimal ZP affinity of unilamellar liposomes of GG (desulfated form of SGG) and DPPS (dipalmitoylphosphatidylserine, negatively charged like SGG), which were fluorescently labelled in the same manner as SGG liposomes. These results indicate that the sulfate in the head group of SGG is significant in this ZP interaction, since GG does not contain this sulfate. On the other hand, the binding of SGG to the ZP is not exclusively dependent on its negative charge as the anionic DPPS does not have affinity for the ZP. However, SGG liposomes do not bind to the ZP of fertilized eggs, which is structurally modified and refractory to additional sperm binding following entry of one sperm into the egg cytoplasm. Therefore, this result indicates the significance of the interaction between sperm SGG and the ZP of unfertilized eggs in the fertilization event [6]. As expected, when capacitated mouse sperm were pretreated with anti-SGG IgG antibody before co-incubation with mature mouse eggs, the levels of sperm binding to the ZP were decreased in a concentration dependent manner. Similar results were obtained when anti-SGG IgG treated human sperm were co-incubated with human eggs, which had failed to be fertilized in a human in vitro fertilization procedure, but still contained the ZP with sperm binding ability [63]. In the mouse system, the monovalent anti-SGG Fab fragment exhibited the same inhibition on sperm-ZP binding as anti-SGG IgG, indicating that the inhibition observed was not due to a steric hindrance of the bivalent anti-SGG IgG over ZP-binding proteins locating nearby SGG on the sperm head surface. However, the inhibition of sperm-ZP binding by anti-SGG IgG/Fab reached a plateau at 40% of the positive control values. This result suggested that SGG may act with another ZP binding molecule(s), co-existing on the sperm head surface, for the full adherence of sperm to the ZP [6]. In particular, SGG may also have affinity for this ZP binding molecule.
An SGG binding protein with an apparent molecular mass of 68 kDa, named SLIP1 (sulfolipidimmobilizing protein 1), was in fact described in the rat testicular homogenate [171]. Subsequently, we demonstrated that SLIP1 was co-localized with SGG on the mouse and human sperm head surface and SLIP1 itself also played roles in sperm-ZP binding [172–174]. Following purification to homogeneity from the pig sperm plasma membrane extract, SLIP1 was renamed P68 and was shown to have affinity for the intact ZP of unfertilized eggs from various species (mouse, rat, cat, dog, pig and human). Like SGG liposomes, P68 did not bind to the ZP of fertilized mouse eggs or preimplantation embryos [175].
Tryptic peptide mass finger printing revealed the identity of P68 to be arylsulfatase A (ARSA) and this was further confirmed by molecular cloning [176]. This finding corroborates the previous observation describing ARSA activity in pig sperm [177]. We further demonstrated the roles of ARSA in pig and mouse sperm-ZP interaction and mouse in vivo fertilization [170, 176], as well as direct binding of ARSA to mouse ZP3 and ZP2 glycoproteins [102]. Moreover, we revealed that ARSA and SGG had a strong affinity for each other (Kd = 9 nM) and ARSA was localized at the same site as SGG on the sperm head [178]. We were initially puzzled by this finding, since ARSA is the first enzyme in the SGG degradation pathway, expected to act with its co-enzyme, saposin B, to desulfate SGG into GG (see Section 6 for more details). ARSA on intact mouse sperm also could desulfate an artificial substrate, nitrocatechol sulfate (NCS), indicating that its active site pocket [179] was unoccupied. However, GG was never found on the head surface of intact non-capacitated and capacitated sperm [178]. This puzzle was resolved by our structural modeling results using a known crystal structure of ARSA with the identified active site pocket [102, 180]. In addition to the active site to which the substrate sulfogalactolipid binds and becomes desulfated, there is another cleft on the molecular surface of ARSA into which SGG/SGC can dock with affinity. It is this latter site on ARSA to which SGG on the sperm surface adheres, and therefore this ARSA-SGG interaction does not result in SGG desulfation [102]. In fact, testicular sperm do not possess ARSA on their head surface. As described above, SGG is synthesized in TGCs, and in testicular sperm it becomes accumulated on the head surface (the convex ridge in mouse sperm). It is the caudal epididymal epithelial cells that synthesize and secrete ARSA into the epididymal lumen. When sperm move into the epididymis, ARSA then deposits onto the sperm surface due to the high affinity between its surface cleft and the sperm surface SGG [64, 178]. Although the active site pocket of ARSA can also capture an individual SGG molecule with high affinity [180]. SGG in the sperm surface membrane would not be able to reach this buried active site pocket of ARSA. As ARSA is a ZP binding protein, SGG on the sperm surface plays a role in sperm maturation by adding egg binding ability to epididymal sperm.
SGG and ARSA on the capacitated sperm head surface likely bind to the ZP in a synergistic manner. SGG and ARSA both have affinity for the ZP and also for one another. ARSA is a peripheral plasma membrane protein and therefore it rises about 100 angstroms above the sperm head plasma membrane. The protruded ARSA is at a much higher position than SGG with only the galactosylsulfate moiety rising above the bilayers of the sperm plasma membrane (less than 10 angstroms). We propose that ARSA first captures the ZP glycans and draws them next to the sperm head plasma membrane where they interact with SGG molecules. Although the carbohydrate-carbohydrate interaction is not strong [181, 182], the interaction would be strengthened by the multiplicity of SGG molecules on the sperm head surface (see more description and our model in our previous reviews: [3, 4]). Without ARSA on the sperm head surface, it would be problematic for SGG molecules to effectively capture the ZP glycans for proper binding. This may explain why testicular sperm in spite of having SGG on their sperm surface cannot bind to the ZP of intact eggs even when they are placed together in close proximity. ARSA is co-localized with SGG in the head of not only acrosome intact sperm but also acrosome reacting/reacted sperm [102, 170]. Therefore, our model of the synergistic interaction between sperm SGG and ARSA and the egg ZP should be applicable to sperm of both acrosomal statuses.
5.2. Roles of SGG in formation of sperm lipid rafts housing ZP binding proteins
When total lipids extracted from non-capacitated and capacitated pig sperm were subjected to TLC followed by Coomassie blue staining, the SGG band accounted for about 10% of total lipids in both samples [35]. However, since SGG exists only in the sperm head, its percentage in this sperm area is higher than 10%. Therefore, SGG would contribute to the biophysical property of the sperm head plasma membrane. Our infrared spectroscopy reveals that SGG is an ordered lipid with a Tm of the transition from the gel phase to the liquid crystalline phase of 45°C [38], a property similar to that of SGC [183]. SGG also has a propensity to interact with cholesterol and saturated phospholipids, and thus it is integral to the sperm lipid rafts formation [35, 38, 184]. Lipid rafts are liquid-ordered cholesterol containing membrane domains, which are surface platforms of adhesion and signalling molecules [4, 5, 185] and are operationally defined as isolated detergent resistant membranes (DRMs) following treatment of cells with a low percentage of a non-ionic detergent (e.g., Triton X-100) at 4°C [186]. We have shown for the first time that DRMs from both non-capacitated and capacitated sperm have direct affinity for isolated pig ZP glycoproteins, but the latter having a higher affinity. Furthermore, the DRM levels in capacitated sperm are about twofold higher than those in non-capacitated sperm [35]. These observations corroborate the fact that capacitated sperm naturally bind to the egg ZP better than non-capacitated sperm [146]. Our lipidomic analyses indicate that SGG is a component of sperm lipid rafts [35]. This result is not surprising considering that SGG is a structural analog of SGC, which has been repeatedly identified to be a lipid raft component [184, 186–190]. However, the very high distribution of SGG in sperm DRMs is astounding. Namely, 30% and 70% of total SGG in sperm is localized in non-capacitated and capacitated sperm DRMs, respectively [4, 35]. Since SGG has direct affinity for the ZP, its high amount in sperm DRMs would contribute not only to the ordered structure of these membrane domains but also to their ZP binding ability [35]. We and several other investigators also have demonstrated that ZP binding proteins including ARSA (SGG binding protein) are components of mammalian sperm DRMs [158–160, 191, 192]. It is a matter of further investigation to determine whether other ZP binding proteins present in isolated sperm DRMs have affinity for SGG or not.
In the late 2000’s, concerns were voiced by researchers in the lipid rafts field that the architecture of isolated DRMs could be artifacts of protein aggregation induced by the detergent [193]. Alternative methods such as the use of physical force (e.g., nitrogen cavitation) were suggested for the isolation of lipid rafts [194]. Nitrogen cavitation at 650 psi has actually been used to isolate anterior head plasma membrane vesicles (APMs) from pig sperm [195]. These isolated APMs have affinity for the ZP [163, 196], and contain SGG as a lipid component [184]. Atomic force microscopy analyses further indicate the ordered structure of APMs, atypical feature of lipid rafts [184,197]. Proteomic analyses of capacitated pig sperm APMs reveal the presence of several ZP binding proteins (e.g., SED1, zonadhesin) together with signalling proteins and chaperone proteins (heat shock 70 kDa protein I-like and heat shock protein HSP90-alpha) [163]. The chaperone proteins may function in escorting proteins relevant for the ZP binding and subsequent sperm cell signalling into the APMs to form high molecular weight (HMW) protein complexes therein. In fact, a number of heat shock proteins including heat shock 70 kDa protein 5 (HSPA5) are present in isolated mouse sperm DRMs [160] and also in HMW protein complexes isolated from mouse and human sperm [141, 142]. In pig sperm APMs, we have shown that their HMW protein complexes bind to the ZP at a higher efficiency than individual ZP binding proteins in the monomeric form [156, 163]. Of note is the accumulated evidence that a number of heat shock 70 kDa proteins have a specific affinity for SGG and SGC [138, 139, 143, 198–200]. Therefore, SGG on the sperm surface also plays a role in orchestrating ZP binding proteins on the sperm surface to be in appropriate ensembles for the most efficient ZP interaction.
6. Degradation of SGG: significance of SGG homeostasis in male fertility
6.1. Degradation of sulfogalactosylceramide (SGC) in the brain: manifestation of lysosomal storage diseases due to failure in SGC degradation
While the physiological relevance of SGG biosynthesis has been validated by the studies in Cgt and Cst knockout mice (see Section 3.4), it was unclear for decades whether SGG was degraded at all. As described above, Kornblatt [91] demonstrated that SGG did not turn over in live TGCs. However, the fate of SGG in apoptotic germ cells was not discerned in this study. Surprisingly apoptosis occurs at 50% rate in TGCs in normal spermatogenesis [12, 85–87] and Sertoli cells play an important role in “cleaning up” these apoptotic germ cells from the seminiferous tubule epithelium through their phagocytic activity [92–96]. For sphingolipids including SGC and GC, intracellular accumulation due to their failure to become catabolized to the backbone lipids by lysosomal enzymes leads to cytotoxicity, cellular dysfunction and clinical abnormalities, the so-called lysosomal storage diseases (LSDs) [24,201]. Therefore, it is expected that Sertoli cells would also have various lysosomal enzymatic activities to degrade SGG (see Fig. 5 for its presence in adult Sertoli cells) in the phagocytosed apoptotic germ cells and residual bodies [92–96, 100, 101] into a non-toxic neutral lipid backbone.
The degradation pathway of SGG in vivo was considered to mirror that of SGC, which was uncovered due to natural mutations in humans of the lysosomal enzymes in the pathway. Mutations of ARSA and GALC lead to intracellular accumulation of SGC and GC (and/or lyso-GC, aka psychosine), and subsequently structural and clinical abnormalities –the LSDs, i.e., metachromatic leukodystrophy (MLD) and globoid cell leukodystrophy (aka Krabbe disease), respectively [24–26, 202–204]. Biochemical studies further demonstrated that arylsulfatase A (ARSA) (aka cerebroside sulfatase, cerebroside-3-sulfate-3-sulfohydrolase or sulfatase A) purified from tissue lysosomes together with co-enzyme saposin B (originally called activator protein of sulfatase A [205, 206]) could desulfate SGC (sulfatide) into GC [205, 207]. Likewise, purified lysosomal GALC + co-enzyme saposin A could degalactosylate GC into ceramide [208–210]. All of these results conclude that the degradation pathway of the galactosylsulfate head group of SGC in the brain is as shown in Figure 10 [19, 211]. Interestingly, SGG and GG were also shown to be substrates of ARSA + saposin B and GALC + saposin A, respectively [207, 210]. These results suggested that the SGG degradation pathway of its galactosylsulfate head group was the same as that of SGC. However, to date accumulation of SGG and GG has not been shown in testes of men with MLD and Krabbe disease, respectively. The lack of this information is likely from the fact that true MLD and Krabbe disease males die young. Rather, the degradation pathway of SGG in vivo was discerned in mouse models (Arsa null mice and mutant “twitcher” mice) (see Sections 6.2 and 6.3).
Figure 10. Degradation pathway of the galactosylsulfate head group of SGG and SGC.
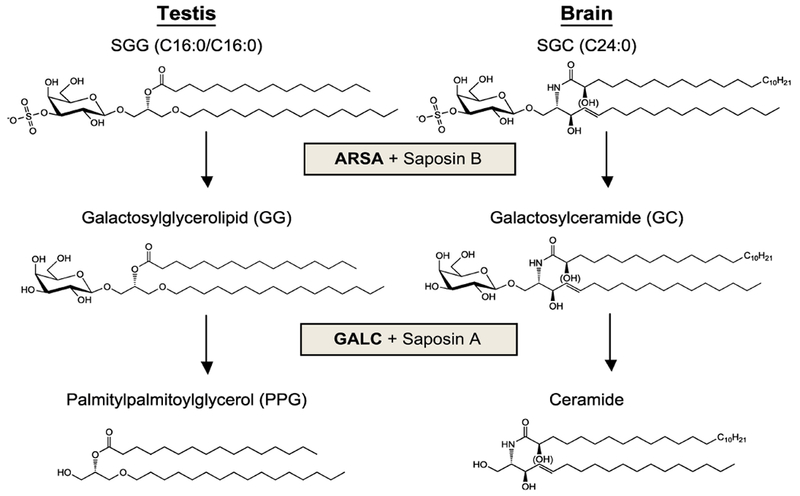
The degradation pathway of the galactosylsulfate head group of SGC in the brain was discerned mainly from samples of humans with natural mutations of the catabolic enzymes, ARSA and GALC, and their co-enzymes, SAP-B and SAP-A, respectively. The degradation pathway of SGG was subsequently revealed through studies employing Arsa knockout mice and twitcher mice (lacking GALC due to gene mutation by ENU).
Degradation of sulfoglycosphingolipids and glycosphingolipids also serves the purpose of generating the lipid backbone/building block to be recycled for their biosynthesis, usually in the same cell [212, 213]. In the case of SGC and GC, their sequential degradation yields ceramide (see Figs. 6 and 10). The disruption of the degradation pathway caused by mutation of lysosomal enzymes involved therefore induces cacostasis of the glycosphingolipids [201].
A proper amount of SGC and GC is essential in maintaining the structure and function of the myelin sheath and cacostasis of these sphingolipids results in its degradation in the white matter (leukodystrophy) of the brain as well as in the peripheral nervous system [25, 26]. In the case of ARSA mutation, intracellular accumulation of SGC also results in metachromatic staining with a dye of the brain sections. For GALC mutation, infiltration of multinucleated macrophages (globoid cells) also occurs at the demyelination sites. This infiltration, however, is due to accumulation of psychosine (lyso-GC), which is formed in myelinated cells and also a GALC substrate. The very cytotoxic psychosine also accelerates the destruction of myelin-forming cells [26]. Damages to the myelin sheath and myelin-forming cells leads to neurological and motor neuron disorders. As expected, the severity of MLD and Krabbe disease symptoms is variable depending on the degree of gene mutations. Severe symptoms of the infantile and juvenile forms of MLD are similar but the symptoms develop much faster in infants. These include muscle wasting and weakness, gait disturbances, increasing loss of vision until blindness, impaired swallowing, dementia, convulsions and paralysis. In the adult form with an onset at age 16, the apparent symptoms of MLD are psychiatric disorder and dementia [25, 214]. Symptoms and progression of Krabbe disease are similar to those observed in MLD [26, 215].
In addition to mutation of ARSA and GALC, MLD and Krabbe disease phenotypes have been observed in individuals having inherited defects of saposin B (Sap-B) and saposin A (Sap-A), respectively [211, 216–220]. Sap-A, Sap-B and Sap-C (aka sphingolipid activator proteins) act as physiological detergents, plucking out the (sulfo)glycolipids from the membrane and delivering them to the active sites of their degrading enzymes [220]. Sap-A and Sap-B are co-enzymes of not only GALC and ARSA, respectively, but also lysosomal enzymes in the degradation pathways of other glycosphingolipids [20, 211]. Therefore, higher levels of other glycosphingolipids are also present in these individuals deficient in Sap-A and Sap-B.
6.2. Demonstration of the significance in SGG degradation on male fertility using Arsa null mice
Although both MLD and Krabbe disease are considered rare inherited metabolic diseases, the severity of the symptoms has demanded research studies for effective therapy. Animal models were therefore produced including Arsa knockout mice (generated by Volkmar Gieselmann, University of Bonn for the MLD research [221]). Unfortunately, these knockout mice exhibit mild MLD symptoms despite the accumulation of SGC in their brain (Fig. 11) [222, 223]. These Arsa null mice can live for over one year. Cryopreserved embryos of mice with natural Galc mutations were also available from the Jackson Laboratory, but these Galc mutant mice cannot live past 50 days [27]. They are also called “twitcher (Twi/Twi)” mice due to their body tremors.
Figure 11. Increased levels of SGC in the brain of Arsa knockout mice relative to age-matched wild type mice.

Lipids were extracted from brain tissues of wild type and Arsa null mice of 5 months and 8 months of age and subjected to ESI-MS/MS-MRM of SGC of various molecular species (with m/z values shown in the top right comer). Data are expressed as means ± SDs from three replicate experiments. * denotes a statistical difference of the level of each SGC molecular species in the brain between Arsa null mice and age-matched wild types. Note the increases in SGC levels in the knockout mice. Although there was no statistical difference of the levels of C24:1 and C24:0 SGC between 8-month old Arsa knockout mice and the wild type counterparts, the trend showing higher levels of these SGC molecular species in the knockout mice was obvious. Data presented are taken from K Kongmanas’ Ph.D. thesis, 2015, University of Ottawa, which has been published on line (https://ruor.uottawa.ca/handle/10393/325091).
Our results indicate that primary cultures of Sertoli cells and TGCs possess ARSA enzymatic activity, assayed by using an artificial substrate, NCS [170]. The ARSA activity is, however, much higher in Sertoli cells than in TGCs, i.e., 164.5 ± 36.8 mU/106 Sertoli cells versus 2.6 ± 1.3 mU/106 germ cells. While the role of the ARSA enzymatic activity in TGCs still needs to be discerned, our studies reveal that ARSA in Sertoli cells is localized specifically in lysosomes and it is responsible for SGG desulfation. The role of Sertoli lysosomal ARSA was in fact revealed in studies using Arsa knockout mice. Uniquely, SGG accumulated in Sertoli cell lysosomes in Arsa−/− male mice 5 months of age and older, concurrently with lysosomal swelling and accumulation of lipid droplets, which were markedly apparent when these knockout mice became 8 months of age and older (equivalent to >40 years old men). These abnormalities are an obvious sign of a lysosomal storage disorder and to our best knowledge, this is the first incident reported in Sertoli cells [102].
As previously shown [221], Arsa knockout mice at all ages do not show any obvious neurological disorder. However, fecundity of aging Arsa−/− male mice is significantly reduced [102]. In our natural mating experiments, where Arsa−/− males and wild type males were mated with wild type fertile females in parallel, starting from 2 months to 10 months of age, the average litter size that Arsa−/− males produced was 6.2 ± 2.4 pups, as compared with 7.9 ±1.3 pups from the age-matched wild type males (P < 0.05). The decrease in fecundity of Arsa−/− males was attributed to a much lower rate of spermatogenesis as well as an increased production of abnormal sperm. Evidence towards the former aspect included the 50% average testis weight and the 60% seminiferous tubule epithelium area of adult Arsa−/− males relative to the corresponding wild type values. Accordingly, the numbers of sperm retrieved from the cauda epididymis and vas deferens of Arsa−/− mice were only half of those collected from age-matched wild type males. It was likely that the numbers of spermatogonia entering meiosis in Arsa−/− and wild type animals were the same, but spermatogenic cells in the knockout mice could not develop normally. This was evident by a 20-fold increase in the numbers of apoptotic germ cells in Arsa−/− males, relative to the wild type counterpart. Furthermore, >50% of caudal epididymal/vas deferens sperm of the knockout mice exhibit abnormal morphology. These caudal epididymal/vas deferens sperm retrieved from 8-month old Arsa−/− males could minimally fertilize eggs in vitro.
Interestingly, levels of SGG in TGCs and sperm in Arsa−/− males at this age were also 50% of the wild type values [102]. As described above, SGG plays important roles in spermatogenesis and sperm-egg interaction, their decreased levels likely attribute to the lower rate of spermatogenesis and a minimal sperm fertilizing ability, and finally reduced fecundity in Arsa null males.
Considering that ARSA is an enzyme in the SGG degradation pathway, the decrease of SGG amounts in TGCs and sperm of 8-month old Arsa−/− males was unexpected, and even more so with our results showing the normal levels of Cgt and Cst transcripts in these knockout mice. Since Sertoli cells play a pivotal role in supporting spermatogenesis (see Fig. 3A), it is likely that the lysosomal storage disorder observed in Sertoli cells of Arsa−/− males was the prime cause that led to various adverse effects on TGCs. These included the reduced SGG synthesis activity, which may have reflected decreases in the translation or catalyzing abilities of CGT and CST and/or availability of their substrates, i.e., GG for CST and PPG for CGT (see Fig. 6 for SGG biosynthesis pathway); these possibilities need to be validated experimentally. Ultimately, the lipid building block PPG has to be available for SGG synthesis. In wild type animals, PPG in TGCs may come from both de novo synthesis and transport of the recycling pool from Sertoli cells. The fact that TGCs have the ability to synthesize PPG was derived from the observation that SGG was still present in TGCs and sperm at the normal and 50% of the wild type levels in 5-month old and 8-month old Arsa−/− males, respectively [102]. Although the recycling pool of PPG from Sertoli cells may contribute to SGG synthesis in wild type animals, the de novo synthesis of PPG in 5-month old Arsa−/− mice may have been enhanced to compensate for the loss of the recycling PPG pool with final production of SGG at the wild type levels. On the other hand, the 50% decrease in SGG levels in TGCs and sperm in 8-month old Arsa−/− males suggested that TGCs in these aging knockout mice could not synthesize PPG at an elevated rate like TGCs in younger Arsa−/− mice. In addition, the possibility that CGT and CST in TGCs of 8-month old knockout males may not function at the wild type capacity has to be considered, and this may include an elevated level or activity of the CST inhibitor, glycosyl phosphoinositide [113]. The extent of the recycling PPG pool to participate in SGG biosynthesis, which would depend on the transport of PPG across from Sertoli cells to TGCs, is still a matter of further investigation. The possibility that PPG is first deacylated in the Sertoli cell lysosomes to generate palmitylglycerol, which may form micelles with better transportability through Sertoli cell and TGC plasma membranes, should also be considered. Notably, C16:0 SGC was also unexpectedly present in TGCs of 8-month old Arsa−/− males [102], a result that indicated that CGT and CST in these ARSA depleted TGCs could alternatively use ceramide as the lipid building block to synthesize SGC. Perhaps TGCs in these knockout mice attempted, but unsuccessfully, to adjust the level of the sulfogalactolipid to the normal values. This explanation may also apply to the presence of unusual SGG molecular species (e.g., C16:0/C14:0, C18:1/C16:0, C18:0/C16:0) in the seminiferous tubules of Arsa−/− males at eight months of age [102].
The reduced synthesis activities of SGG and perhaps also other macromolecules by TGCs may occur concurrently with increasing rates of their apoptosis, and these changes finally manifested in the subfertility of these knockout mice at older ages [102]. The reduced functionality of TGCs, however, gradually took place, commensurate with the degree of SGG accumulation and corresponding lysosomal swelling in Sertoli cells of Arsa−/− mice (see our model of a pictorial explanation in Fig. 12). By 8 months of age, Arsa−/− Sertoli cells themselves also exhibited abnormal morphology (smaller size with dislocated nuclei) suggesting the severity of their dysfunctions. Regardless of the mechanisms, our results indicate that ARSA is the enzyme in the SGG degradation pathway in vivo in Sertoli cells (Fig. 10). Saposin B, the co-enzyme of ARSA, is presumably produced also in Sertoli cells via proteolytic processing from its precursor, prosaposin, which is de novo synthesized at a substantial amount in these cells [224].
Figure 12. Metabolisms of SGG in seminiferous tubules: interrelationship between testicular germ cells and Sertoli cells.

Top panel: In normal testes, Sertoli cells degrade SGG in phagocytosed residual bodies and apoptotic TGC remnants to GG, and then to PPG by lysosomal ARSA and GALC, respectively Note that in Sertoli cells of wild type animals, phagocytosed apoptotic germ cells or remnants thereof are not evident indicating the rapid pace of their degradation by lysosomal enzymes. Likewise, late residual bodies disappear in due time. PPG produced from the SGG degradation pathway may be shuttled to developing pachytene spermatocytes (pSc) for new SGG synthesis, although pSc themselves would also have the ability to synthesize PPG de novo. See box for the SGG degradation and synthesis pathways. Bottom left panel: In 5-month old Arsa knockout mice, SGG is accumulated in Sertoli cells due to the lack of ARSA, although these higher levels of intracellular SGG have not yet reached the cytotoxic threshold. With no PPG produced in Sertoli cells for recycling, pSc would likely have to increase the de novo synthesis of PPG, and therefore the amounts of SGG in TGCs of these knockout mice are the same as those in age-matched wild type males. Bottom right panel: In 8-month old Arsa null mice, the much higher levels of accumulated SGG exert cytotoxicity to Sertoli cells, which then become smaller in size with their nuclei (N) displaced to be on top of the basement membrane. Sertoli cells in these aging Arsa null mice presumably are much less functional having reduced support for the development of TGCs. pSc in turn synthesize PPG at reduced rates and the subsequent levels of SGG in TGCs in these knockout mice are only 50% of the corresponding wild type levels. Also, the shaping of the elongated spermatid head, as regulated by Sertoli cells, is aberrant, resulting in production of sperm with abnormal morphology. BTB: blood-testis barrier, Ly: lysosome, Rb: residual body, SG: spermatogonium, lSc: leptotene spermatocyte, RS: round spermatid, ES: elongated spermatid. Adapted from Xu et al. [16].
6.3. Presence of galactosylceramidase (GALC) in the SGG degradation pathway downstream to ARSA
Using 6-hexadecanoylamino-4-methylumbelliferyl-β-D-galactoside (HMU-Gal) as an artificial substrate, we have preliminarily described the activity of GALC in Sertoli cells, which like that of ARSA, is about 80× higher than that in TGCs. Accumulation of GG in Sertoli cells of twitcher mice (deficient in GALC) has also been reported [225, 226], confirming the presence of GALC in wild type Sertoli cells, and implication of its involvement in converting GG to PPG. Twitcher mice can live to up to only 50 days or less, and the males are infertile due to the production of abnormal sperm as well as impairment of the epididymis architecture [225, 226]. However, the cause of abnormalities in spermatogenesis at least in this first round in twitcher mice is not from direct effects of accumulated GG in the testis. Rather it arises from the decreased expression in the brain of peptide hormones relevant for reproduction, i.e., gonadotrophin-releasing hormone (GnRH) from the hypothalamus, and LH and FSH from the pituitary [227]. LH regulates Leydig cells to produce androgen essential for spermatogenesis, while FSH supports the functionality of Sertoli cells [228]. As described above, GALC deficiency also results in accumulation of the very cytotoxic psychosine in the brain with the plausible consequences on the hypothalamus and pituitary dysfunction. Regardless, results from experiments in twitcher and Arsa−/− mice indicate that the degradation pathway of the galactosylsulfate head group of SGG in the testis mirrors that of SGC in the brain (Fig. 10).
7. Concluding remarks and future studies
Accumulated lines of evidence indicate that SGG is synthesized in TGCs, mainly in primary spermatocytes, and to a lesser extent in round spermatids (Sections 3.1 & 3.2). Results from Cgt and Cst knockout mice further reveal that the biosynthesis pathway of SGG in the testis is the same as that of SGC in the brain and kidney, except that alkylacylglycerol (mainly PPG) is the lipid building block for the synthesis of SGG instead of ceramide for SGC (Fig. 6). The enzymatic activities of CGT and CST have also been demonstrated in the testis. In particular, CST from the testis has been purified to homogeneity. Once SGG is synthesized in the Golgi apparatus, it is transported by STX2 to the surface of TGCs as well as their intercellular bridges that are communicating conduits between them. The exact mechanisms on how newly synthesized SGG molecules in TGCs are transported to the cell surface are still unknown. Like SGC and other glycolipids, SGG may be delivered to the cell surface through a vesicular transport [189, 229–231]. The fact that STX2 is essential for this SGG trafficking to the TGC plasma membrane [124] supports this postulation. Since SGG has affinity for cholesterol [35], which has propensity, upon being synthesized, to target to the plasma membrane [232], cholesterol is likely to be co-present in the transporting vesicles [233] and it may help direct the SGG containing vesicles to the TGC plasma membrane. It is expected that STX2 (a t-SNARE protein) is relevant for the fusion between the transporting vesicle and the plasma membrane. However, it is still a matter of further investigation whether a glycolipid transfer protein (GLTP) [234, 235] is involved in the ultimate transfer of SGG into the plasma membrane. Considering that a GLTP for SGC has been isolated [236], investigation whether this GLTP has a membrane transferring activity on SGG could be readily performed. The presence of cell surface SGG on TGCs is likely important for the progression of spermatogenesis as discussed below. When TGCs develop into mature sperm, SGG still remains on the sperm surface. However, it is localized exclusively in the sperm head, in the liquid-ordered raft platforms with ZP binding ability (see Section 5.2). Further investigation should be performed to discern how SGG molecules become segregated into the sperm head entity.
SGG appears to be essential for male fertility. Depletion of SGG in TGCs induced by targeted genetic deletion of Cgt and Cst resulted in a spermatogenesis arrest with inability of primary spermatocytes to go through meiosis. However, impairment in the brain of both Cgt and Cst knockout mice with subsequent neurological disorder is an obvious phenotype of these mice. Therefore, the possibility that defects in the expression of peptide hormones that regulate spermatogenesis from the hypothalamus and pituitary (GnRH and FSH/LH, respectively) cannot be ruled out and warrants further investigation. Conditional Cgt and Cst knockout or knockdown mice specific to the testis should be produced and assessed for the male fertility status. Alternatively, small inhibitors of testicular CST and CGT should be sought, a pursuit that can be immediately initiated on testicular CST, with an already described purification method and at least one inhibitor, phosphoinositol glycerolipid, described [113]. Male mice with their seminiferous tubules specifically treated with these inhibitors can be evaluated whether or not spermatogenesis is arrested. Positive results should give an aspiration towards development of these inhibitors into male contraceptives. Along with this attempt, molecular mechanisms on SGG involvement in spermatogenesis need to be clearly discerned. As described in Section 4.1, the impact of SGG on maintaining the TGC intercellular bridges for proper communication between them and progress in spermatogenesis should be further investigated. As TGCs of Cst null mice (with no SGG) could not uptake lactate, further investigation must be made to understand how SGG regulates functionality of a lactate transporter (MCT). Our pilot study in defining SGG binding proteins on the Sertoli cell surface through proteomic analyses is the first step towards understanding the molecular mechanisms through which SGG on TGCs regulates TGC-Sertoli cell interaction, deemed to be essential for completion of spermatogenesis (see Section 4.1). Further studies on this subject at the cellular levels are required, although a lack of a Sertoli-TGC co-culture system that mimics the dynamics of spermatogenesis in vivo is a challenge to these studies.
Depletion of ARSA, the first enzyme in the SGG degradation pathway also leads to subfertility in aging Arsa null mice. This subfertility is likely due to intracellular accumulation of SGG in Sertoli cells, which show marked swelling of their lysosomes, typical of a lysosomal storage disease. Although SGC is accumulated in the brain of these transgenic mice, as seen in adult MLD men with natural mutation of ARSA, Arsa null mice exhibit no neurological disorder. Together with the significance of Sertoli cells in spermatogenesis regulation, the prime cause of subfertility in these aging knockout mice is likely due to cytotoxicity exerted from abnormally high levels of SGG inside Sertoli cells (see Section 6). Our ongoing studies indicate an increased production of reactive oxygen species (ROS) in ARSA depleted Sertoli cells, a plausible cause of cytotoxicity, which in aging Arsa−/− mice manifests in a 50% reduction of SGG levels in TGCs and sperm as well as spermatogenesis. This cytotoxicity is being confirmed in wild type Sertoli cells uploaded with a sulfonate analogue of SGG [237], which cannot be desulfated by ARSA. The marked decrease in SGG levels in aging ARSA deficient sperm is likely the cause of their minimal ability to fertilize eggs in vitro. Since the efficiency of SGG synthesis depends on the availability of PPG, the lipid building block, the decreased levels of SGG in TGCs in aging Arsa−/− mice are possibly attributed to their reduced ability to synthesize PPG de novo as well as the lack of the recycling pool of PPG from SGG breakdown in Sertoli cells (see Fig. 12). Nonetheless, for the latter possibility, experiments to demonstrate PPG transport through Sertoli cells into primary spermatocytes and round spermatids (the sites of SGG synthesis) need to be performed.
The most interesting question arising from studies in Arsa knockout mice is whether male subfertility precedes the neurological disorder in adult MLD in humans. Retroactive demographic studies should be performed in adult MLD males to answer this question. However, prospective studies would be more meaningful. In these studies, sperm SGG from individual males should be quantified and attempts should be made to temporally correlate these sperm SGG levels with neurological disorders and infertility (clinically defined as inability of their female partners to become pregnant after one year of unprotected sexual activities). These proposed studies may be initiated in men with familial history of genetic mutations on the enzymes involved in SGG metabolisms. However, for the correlation between sperm SGG levels and male infertility, studies should be conducted in a larger male population, as the results should reveal the threshold level of sperm SGG that segregates between fertile and infertile men. Although this study will be time and resource consuming, the multi-functions of SGG in male reproduction as already shown in animals and in vitro studies in human sperm (see Sections 4 & 5) are already an endorsement of the benefit and validity of using sperm SGG levels as a bio-index of male fertility. Such a reliable sperm bio-index is much needed at the present time, since the currently used WHO semen analysis guideline based on sperm parameters such as motility, morphology and concentration [238] do not always give accurate prediction on sperm fertilizing ability and male fertility.
Acknowledgement
This work was mainly funded by grants from Canadian Institutes of Health Research (MOP-119438 and MOP-84420) and Natural Science and Engineering Research Council of Canada (RGPIN/183958 −2012 and RGPIN-183958-2004) (all given to NT) as well as partially supported by an NIH grant (P30 DK063491) (to JPW) and a grant from the Naito Foundation (to NG-I). HX and KK were recipients of an Ontario Graduate Scholarship and a scholarship from Development and Promotion of Science and Technology Talented Project (DPST), Ministry of Education, Thailand, respectively. Both HX and KK were also awarded a scholarship from CIHR-Strategic Training Initiative in Health Research (STIHR). The authors thank Dr. Jodie V Johnson, University of Florida, for help in assigning likely structures to the fragment ions depicted in Figure 2. We also thank Ms. Terri Van Gulik for her assistance in the manuscript preparation. This manuscript is dedicated to the late Dr. Morris Kates, University of Ottawa, who tirelessly gave us advice for almost three decades on both intellectual and technical aspects of glycolipid research studies.
Abbreviations
- APMs
anterior head plasma membrane vesicles
- ARSA
arylsulfatase A
- CGT
ceramide galactosyltransferase
- CHO
Chinese hamster ovary cells
- CPK
Corey-Pauling-Koltun
- CST
cerebroside sulfotransferase
- DPPC
dipalmitoyl phosphatidylcholine
- DRMs
detergent resistant membranes
- ENU
N-ethyl-N-nitrosourea
- ERM
erzin radixin moesin
- ES
ectoplasmic specialization
- ESI
electrospray ionization
- FAB-MS
fast atom bombardment mass spectrometry
- GALC
galactosylceramidase
- GC
galactosylceramide
- GLC
gas liquid chromatography
- GG
galactosylglycerol
- HMW
high molecular weight
- LC-ESI-MS/MS
liquid chromatography-ESI tandem mass spectrometry
- MALDI-TOF
matrix-assisted laser desorption/ionization
- MCT
monocarboxylate transporter
- MLD
metachromatic leukodystrophy
- MRM
multiple reaction monitoring
- NCS
nitrocatechol sulfate
- PAPS
3’-phosphoadenosine-5’-phosphosulfate
- PC
phosphatidylcholine
- PM
plasma membrane
- PPG
palmitylpalmitoylglycerol
- SE
seminiferous epithelium
- SLIP1
sulfolipidimmobilizing protein 1
- SGG
sulfogalactosylglycerolipid
- STX2
syntaxin 2 protein
- TGCs
testicular germ cells
- SGC
sulfgalactosylceramide
- TLC
Thin layer chromatography
- ZP
zona pellucida
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None
References
- [1].Ishizuka I, Suzuki M, Yamakawa T, Isolation and characterization of a novel sulfoglycolipid, seminolipid, from boar testis and spermatozoa, J. Biochem 73 (1973) 77–87. [PubMed] [Google Scholar]
- [2].Tanphaichitr N, Bou Khalil M, Weerachatyanukul W, Kates M, Xu H, Carmona E, Attar M, Carrier D, Physiological and biophysical properties of male germ cell sulfogalactosylglycerolipid, in: De Vriese S (Ed.), Lipid Metabolism and Male Fertility, AOCS Press, Champaign, IL, 2003, pp. 125–148. [Google Scholar]
- [3].Tanphaichitr N, Carmona E, Bou Khalil M, Xu H, Berger T, Gerton GL, New insights into sperm-zona pellucida interaction: involvement of sperm lipid rafts, Front. Biosci 12 (2007) 1748–1766. [DOI] [PubMed] [Google Scholar]
- [4].Tanphaichitr N, Faull KF, Yaghoubian A, Xu H, Lipid rafts and sulfogalactosylglycerolipid (SGG) in sperm functions: consensus and controversy, Trends Glycosci. Glycotech 19(106) (2007) 67–83. [Google Scholar]
- [5].Rajendran L, Simons K, Lipid rafts and membrane dynamics, J. Cell Sci 118(Pt 6) (2005) 1099–1102. [DOI] [PubMed] [Google Scholar]
- [6].White D, Weerachatyanukul W, Gadella B, Kamolvarin N, Attar M, Tanphaichitr N, Role of sperm sulfogalactosylglycerolipid in mouse sperm-zona pellucida binding, Biol. Reprod 63(1) (2000) 147–155. [DOI] [PubMed] [Google Scholar]
- [7].Gadella BM, Hammache D, Pieroni G, Colenbrander B, Van Golde LM, Fantini J, Glycolipids as potential binding sites for HIV: topology in the sperm plasma membrane in relation to the regulation of membrane fusion, J. Reprod. Immunol 41(1–2) (1998) 233–253. [DOI] [PubMed] [Google Scholar]
- [8].Roberts DD, Wewer UM, Liotta LA, Ginsburg V, Laminin-dependent and laminin-independent adhesion of human melanoma cells to sulfatides, Cancer Res 48(12) (1988) 3367–3373. [PubMed] [Google Scholar]
- [9].Suzuki Y, Toda Y, Tamatani T, Watanabe T, Suzuki T, Nakao T, Murase K, Kiso M, Hasegawa A, Tadano-Aritomi K, Ishizuka I, Miyasaka M, Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), binding epitope in sulfated sugar chain, Biochem. Biophys. Res. Commun 190 (1993) 426–434. [DOI] [PubMed] [Google Scholar]
- [10].Regina Todeschini A, Hakomori SI, Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains, Biochim. Biophys. Acta 1780(3) (2008) 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Griswold MD, Spermatogenesis: The Commitment to Meiosis, Physiological reviews 96(1) (2016) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng CY, Mruk DD, A local autocrine axis in the testes that regulates spermatogenesis, Nat. Rev. Endocrinol 6(7) (2010) 380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Franca LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD, The Sertoli cell: one hundred fifty years of beauty and plasticity, Andrology 4(2) (2016) 189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B, Myelination in the absence of galactorcerebroside and sulfatide: Normal structure with abnormal function and regional instability, Cell 86 (1996) 209–219. [DOI] [PubMed] [Google Scholar]
- [15].Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N, Paranodal junction formation and spermatogenesis require sulfoglycolipids, Proc. Natl. Acad. Sci. U.S.A 99(7) (2002) 4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu H, Kongmanas K, Kadunganattil S, Smith CE, Rupar T, Goto-Inoue N, Hermo L, Faull KF, Tanphaichitr N, Arylsulfatase A deficiency causes seminolipid accumulation and a lysosomal storage disorder in Sertoli cells, J. Lipid Res 52(12) (2011) 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ishizuka I, Chemistry and functional distribution of sulfoglycolipids, Prog. Lipid Res 36(4) (1997) 245–319. [DOI] [PubMed] [Google Scholar]
- [18].Vos JP, Lopes-Cardozo M, Gadella BM, Metabolic and functional aspects of sulfogalactolipids, Biochim. Biophys. Acta 1211(2) (1994) 125–149. [DOI] [PubMed] [Google Scholar]
- [19].Eckhardt M, The role and metabolism of sulfatide in the nervous system, Mol. Neurobiol 37(2–3) (2008) 93–103. [DOI] [PubMed] [Google Scholar]
- [20].Xu YH, Barnes S, Sun Y, Grabowski GA, Multi-system disorders of glycosphingolipid and ganglioside metabolism, J. Lipid Res 51(7) (2010) 1643–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Honke K, Biosynthesis and biological function of sulfoglycolipids, Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 89(4) (2013) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xiao S, Finkielstein CV, Capelluto DG, The enigmatic role of sulfatides: new insights into cellular functions and mechanisms of protein recognition, Adv. Exp. Med. Biol 991 (2013) 27–40. [DOI] [PubMed] [Google Scholar]
- [23].Schulze H, Sandhoff K, Sphingolipids and lysosomal pathologies, Biochim. Biophys. Acta 1841(5) (2014) 799–810. [DOI] [PubMed] [Google Scholar]
- [24].Ferreira CR, Gahl WA, Lysosomal storage diseases, Transl. Sci. Rare Dis 2(1–2) (2017) 1–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gieselmann V, Metachromatic Leukodystrophy, in: Barranger JA, Cabrera-Salazar MA (Eds.), Lysosomal Storage Disorders, Springer, New York, 2007, pp. 285–306. [Google Scholar]
- [26].Matsuda J, Suzuki K, Krabbe Disease (Globoid Cell Leukodystrophy), in: Barranger JA, Cabrera-Salazar MA (Eds.), Lysosomal Storage Disorders, Springer, New York, 2007, pp. 269–283. [Google Scholar]
- [27].Rafi MA, Rao HZ, Luzi P, Curtis MT, Wenger DA, Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease, Mol. Ther 20(11) (2012) 2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kornblatt MJ, Schachter H, Murray RK, Partial characterization of a novel glycerogalactolipid from rat testis, Biochem. Biophys. Res. Commun 48(6) (1972) 1489–1494. [DOI] [PubMed] [Google Scholar]
- [29].Yamakawa T, A reflection on the early history of glycosphingolipids, Glycoconj. J 13(2) (1996) 123–126. [DOI] [PubMed] [Google Scholar]
- [30].Blix G, Zur Kenntnis der schwefelhaltigen Lipoidstoffe des Gehirns. Über Cerebronschweielsäure., Hoppe-Seyler’s Z. Physiol. Chem 219(1–2) (1933) 82–98. [Google Scholar]
- [31].Kornblatt MJ, Knapp A, Levine M, Schachter H, Murray RK, Studies on the structure and formation during spermatogenesis of the sulfoglycerogalactolipid of rat testis, Can. J. Biochem 52(8) (1974) 689–697. [DOI] [PubMed] [Google Scholar]
- [32].Levine M, Bain J, Narashimhan R, Palmer B, Yates AJ, Murray RK, A comparative study of the glycolipids of human, bird and fish testes and of human sperm, Biochim. Biophys. Acta 441(1) (1976) 134–145. [DOI] [PubMed] [Google Scholar]
- [33].Nikolopoulou M, Soucek D, Vary J, Changes in the lipid content of boar sperm plasma membranes during epididymal maturation, Biochim. Biophys. Acta 815(3) (1985) 486–498. [DOI] [PubMed] [Google Scholar]
- [34].Furimsky A, Vuong N, Xu H, Kumarathasan P, Xu M, Weerachatyanukul W, Bou KM, Kates M, Tanphaichitr N, Percoll gradient-centrifuged capacitated mouse sperm have increased fertilizing ability and higher contents of sulfogalactosylglycerolipid and docosahexaenoic acid-containing phosphatidylcholine compared to washed capacitated mouse sperm, Biol. Reprod 72(3) (2005) 574–583. [DOI] [PubMed] [Google Scholar]
- [35].Bou Khalil M, Chakrabandhu K, Xu H, Weerachatyanukul W, Buhr M, Berger T, Carmona E, Vuong N, Kumarathasan P, Wong PT, Carrier D, Tanphaichitr N, Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid, Dev. Biol 290(1) (2006) 220–235. [DOI] [PubMed] [Google Scholar]
- [36].Ishizuka I, Chemistry and functional distribution of sulfoglycolipids, Prog. Lipid Res 36(4) (1997) 245–319. [DOI] [PubMed] [Google Scholar]
- [37].Alvarez JG, Storey BT, Hemling ML, Grob RL, High-resolution proton nuclear magnetic resonance characterization of seminolipid from bovine spermatozoa, J. Lipid Res 31(6) (1990) 1073–1081. [PubMed] [Google Scholar]
- [38].Attar M, Kates M, Bou Khalil M, Carrier D, Wong PT, Tanphaichitr N, A Fourier-transform infrared study of the interaction between germ-cell specific sulfogalactosylglycerolipid and dimyristoylglycerophosphocholine, Chem. Phys. Lipids 106(2) (2000) 101–114. [DOI] [PubMed] [Google Scholar]
- [39].Hsu FF, Bohrer A, Turk J, Electrospray ionization tandem mass spectrometric analysis of sulfatide. Determination of fragmentation patterns and characterization of molecular species expressed in brain and in pancreatic islets, Biochim. Biophys. Acta 1392(2–3) (1998) 202–216. [DOI] [PubMed] [Google Scholar]
- [40].Xiao Y, Chen Y, Kennedy AW, Belinson J, Xu Y, Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses, Ann. N. Y. Acad. Sci 905 (2000) 242–259. [DOI] [PubMed] [Google Scholar]
- [41].Sandhoff R, Hepbildikler ST, Jennemann R, Geyer R, Gieselmann V, Proia RL, Wiegandt H, Grone HJ, Kidney sulfatides in mouse models of inherited glycosphingolipid disorders: determination by nano-electrospray ionization tandem mass spectrometry, J. Biol. Chem 277(23) (2002) 20386–20398. [DOI] [PubMed] [Google Scholar]
- [42].Ivanova PT, Milne SB, Myers DS, Brown HA, Lipidomics: a mass spectrometry based systems level analysis of cellular lipids, Curr. Opin. Chem. Biol 13(5–6) (2009) 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Whitehead SN, Hou W, Ethier M, Smith JC, Bourgeois A, Denis R, Bennett SA, Figeys D, Identification and quantitation of changes in the platelet activating factor family of glycerophospholipids over the course of neuronal differentiation by high-performance liquid chromatography electrospray ionization tandem mass spectrometry, Anal. Chem 79(22) (2007) 8539–8548. [DOI] [PubMed] [Google Scholar]
- [44].Spacil Z, Babu Kumar A, Liao HC, Auray-Blais C, Stark S, Suhr TR, Scott CR, Turecek F, Gelb MH, Sulfatide analysis by mass spectrometry for screening of metachromatic leukodystrophy in dried blood and urine samples, Clin. Chem 62(1) (2016) 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tumanov S, Kamphorst JJ, Recent advances in expanding the coverage of the lipidome, Curr. Opin. Biotechnol 43 (2017) 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pintado-Sierra M, Garcia-Alvarez I, Bribian A, Medina-Rodriguez EM, Lebron-Aguilar R, Garrido L, de Castro F, Fernandez-Mayoralas A, Quintanilla-Lopez JE, A comprehensive profiling of sulfatides in myelin from mouse brain using liquid chromatography coupled to high-resolution accurate tandem mass spectrometry, Anal. Chim. Acta 951 (2017) 89–98. [DOI] [PubMed] [Google Scholar]
- [47].Tadano-Aritomi K, Matsuda J, Fujimoto H, Suzuki K, Ishizuka I, Seminolipid and its precursor/degradative product, galactosylalkylacylglycerol, in the testis of saposin A- and prosaposin-deficient mice, J. Lipid Res 44(9) (2003) 1737–1743. [DOI] [PubMed] [Google Scholar]
- [48].Nagai K, Tadano-Aritomi K, Niimura Y, Ishizuka I, Development and application of a system for seminolipid metabolism using mouse seminiferous tubules, Glycoconj. J 27(1) (2010) 181–187. [DOI] [PubMed] [Google Scholar]
- [49].Kongmanas K, Xu H, Yaghoubian A, Franchini L, Panza L, Ronchetti F, Faull K, Tanphaichitr N, Quantification of seminolipid by LC-ESI-MS/MS-multiple reaction monitoring: compensatory levels in Cgt(+/(−)) mice, J. Lipid Res 51(12) (2010) 3548–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang K, Han X, Accurate quantification of lipid species by electrospray ionization mass spectrometry - Meet a key challenge in lipidomics, Metabolites 1(1) (2011) 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ikeda K, Shimizu T, Taguchi R, Targeted analysis of ganglioside and sulfatide molecular species by LC/ESI-MS/MS with theoretically expanded multiple reaction monitoring, J. Lipid Res 49(12) (2008) 2678–2689. [DOI] [PubMed] [Google Scholar]
- [52].Ikeda K, Taguchi R, Highly sensitive localization analysis of gangliosides and sulfatides including structural isomers in mouse cerebellum sections by combination of laser microdissection and hydrophilic interaction liquid chromatography/electrospray ionization mass spectrometry with theoretically expanded multiple reaction monitoring, Rapid Commun. Mass Spectrom 24(20) (2010) 2957–2965. [DOI] [PubMed] [Google Scholar]
- [53].Franchini L, Panza L, Kongmanas K, Tanphaichitr N, Faull KF, Ronchetti F, An efficient and convenient synthesis of deuterium-labelled seminolipid isotopomers and their ESI-MS characterization, Chem. Phys. Lipids 152(2) (2008) 78–85. [DOI] [PubMed] [Google Scholar]
- [54].Cornett DS, Reyzer ML, Chaurand P, Caprioli RM, MALDI imaging mass spectrometry: molecular snapshots of biochemical systems, Nat. Methods 4(10) (2007) 828–833. [DOI] [PubMed] [Google Scholar]
- [55].Goto-Inoue N, Hayasaka T, Zaima N, Setou M, Imaging mass spectrometry for lipidomics, Biochim. Biophys. Acta 1811(11) (2011) 961–969. [DOI] [PubMed] [Google Scholar]
- [56].Anderson DM, Spraggins JM, Rose KL, Schey KL, High spatial resolution imaging mass spectrometry of human optic nerve lipids and proteins, J. Am. Soc. Mass Spectrom 26(6) (2015) 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Goto-Inoue N, Hayasaka T, Zaima N, Setou M, The specific localization of seminolipid molecular species on mouse testis during testicular maturation revealed by imaging mass spectrometry, Glycobiology 19(9) (2009) 950–957. [DOI] [PubMed] [Google Scholar]
- [58].Fredman P, Mattsson L, Andersson K, Davidsson P, Ishizuka I, Jeansson S, Mansson JE, Svennerholm L, Characterization of the binding epitope of a monoclonal antibody to sulphatide, Biochem. J 251(1) (1988) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kotani M, Kawashima I, Ozawa H, Ogura K, Ishizuka I, Terashima T, Tai T, Immunohistochemical localization of minor gangliosides in the rat central nervous system, Glycobiology 4(6) (1994) 855–865. [DOI] [PubMed] [Google Scholar]
- [60].Sommer I, Schachner M, Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system, Dev. Biol 83(2) (1981) 311–327. [DOI] [PubMed] [Google Scholar]
- [61].Gadella BM, Gadella TW Jr., Colenbrander B, van Golde LM, Lopes-Cardozo M, Visualization and quantification of glycolipid polarity dynamics in the plasma membrane of the mammalian spermatozoon, J. Cell Sci 107 ( Pt 8) (1994) 2151–2163. [DOI] [PubMed] [Google Scholar]
- [62].Cheng X, Zhang Y, Kotani N, Watanabe T, Lee S, Wang X, Kawashima I, Tai T, Taniguchi N, Honke K, Production of a recombinant single-chain variable-fragment (scFv) antibody against sulfoglycolipid, J. Biochem 137(3) (2005) 415–421. [DOI] [PubMed] [Google Scholar]
- [63].Weerachatyanukul W, Rattanachaiyanont M, Carmona E, Furimsky A, Mai A, Shoushtarian A, Sirichotiyakul S, Ballakier H, Leader A, Tanphaichitr N, Sulfogalactosylglycerolipid is involved in human gamete interaction, Mol. Reprod. Devel 60(4) (2001) 569–578. [DOI] [PubMed] [Google Scholar]
- [64].Weerachatyanukul W, Xu H, Anupriwan A, Carmona E, Wade M, Hermo L, da Silva SM, Rippstein P, Sobhon P, Sretarugsa P, Tanphaichitr N, Acquisition of arylsulfatase A onto the mouse sperm surface during epididymal transit, Biol. Reprod 69(4) (2003) 1183–1192. [DOI] [PubMed] [Google Scholar]
- [65].Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E, New insights into the interaction between the gp120 and the HIV receptor in human sperm (human.sperm/gp120/galactoglycerolipid/antigalactosylceramide/seminolipid/spermatogonia), J. Reprod. Immunol 41(1–2) (1998) 213–231. [DOI] [PubMed] [Google Scholar]
- [66].Srakaew N, Young CD, Sae-wu A, Xu H, Quesnel KL, di Brisco R, Kongmanas K, Fongmoon D, Hommalai G, Weerachatyanukul W, Hall SH, Zhang YL, Panza L, Franchini L, Compostella F, Pearson TW, Hancock RE, Oko RJ, Hermo LS, Tanphaichitr N, Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive, Hum. Reprod 29(4) (2014) 683–696. [DOI] [PubMed] [Google Scholar]
- [67].Valli H, Phillips B, Orwig K, Gassei K, Nagano M, Spermatogonial Stem Cells and Spermatogenesis, in: Tony MP, Anthony Z (Eds.), Knobil and Neill’s Physiology of Reproduction, Elsevier Inc., New York, 2015, pp. 595–635. [Google Scholar]
- [68].Jegou B, The Sertoli-germ cell communication network in mammals, Int. Rev. Cytol 147 (1993) 25–96. [PubMed] [Google Scholar]
- [69].Hermo L, Pelletier RM, Cyr DG, Smith CE, Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes, Microsc. Res. Tech 73(4) (2010) 241–278. [DOI] [PubMed] [Google Scholar]
- [70].Dimitriadis F, Tsiampali C, Chaliasos N, Tsounapi P, Takenaka A, Sofikitis N, The Sertoli cell as the orchestra conductor of spermatogenesis: spermatogenic cells dance to the tune of testosterone, Hormones 14(4) (2015) 479–503. [DOI] [PubMed] [Google Scholar]
- [71].Romrell LJ, Bellve AR, Fawcett DW, Separation of mouse spermatogenic cells by sedimentation velocity, Dev. Biol 49(1) (1976) 49–119. [DOI] [PubMed] [Google Scholar]
- [72].Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M, Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization, J. Cell Biol 74(1) (1977) 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Arkoun B, Dumont L, Milazzo JP, Way A, Bironneau A, Wils J, Mace B, Rives N, Retinol improves in vitro differentiation of pre-pubertal mouse spermatogonial stem cells into sperm during the first wave of spermatogenesis, PloS One 10(2) (2015) e0116660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chi YH, Cheng LI, Myers T, Ward JM, Williams E, Su Q, Faucette L, Wang JY, Jeang KT, Requirement for Sun1 in the expression of meiotic reproductive genes and piRNA, Development 136(6) (2009) 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van Haaster LH, de Rooij DG, Spermatogenesis is accelerated in the immature Djungarian and Chinese hamster and rat, Biol. Reprod 49(6) (1993) 1229–1235. [DOI] [PubMed] [Google Scholar]
- [76].Montoto LG, Arregui L, Sanchez NM, Gomendio M, Roldan ER, Postnatal testicular development in mouse species with different levels of sperm competition, Reproduction 143(3) (2012) 333–346. [DOI] [PubMed] [Google Scholar]
- [77].Murta D, Batista M, Trindade A, Silva E, Henrique D, Duarte A, Lopes-da-Costa L, In vivo notch signaling blockade induces abnormal spermatogenesis in the mouse, PloS One 9(11) (2014) e113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Johnson MH, Everitt BJ, Essential reproduction, 6th ed., Blackwell Pub., Malden, Mass., 2007. [Google Scholar]
- [79].Steinberger A, Steinberger E, Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture, Biol. Reprod 4(1) (1971) 84–87. [DOI] [PubMed] [Google Scholar]
- [80].Wang ZX, Wreford NG, De Kretser DM, Determination of Sertoli cell numbers in the developing rat testis by stereological methods, Int. J. Androl 12(1) (1989) 58–64. [DOI] [PubMed] [Google Scholar]
- [81].Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG, Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice, J. Reprod. Fertil 93(1) (1991) 233–243. [DOI] [PubMed] [Google Scholar]
- [82].Cheng CY, Mruk DD, The blood-testis barrier and its implications for male contraception, Pharmacol. Rev 64(1) (2012) 16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kean EL, Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides, J. Lipid Res 9 (1968) 319–327. [PubMed] [Google Scholar]
- [84].Handa S, Yamoto K, Ishizuka I, Suzuki A, Yamakawa T, Biosynthesis of seminolipid: Sulfation in vivo and in vitro, J. Biochem 75(1) (1974) 77–83. [DOI] [PubMed] [Google Scholar]
- [85].Dunkel L, Hirvonen V, Erkkila K, Clinical aspects of male germ cell apoptosis during testis development and spermatogenesis, Cell Death Differ 4(3) (1997) 171–179. [DOI] [PubMed] [Google Scholar]
- [86].Shaha C, Tripathi R, Mishra DP, Male germ cell apoptosis: regulation and biology, Philos. Trans. R. Soc. Lond. B Biol. Sci 365(1546) (2010) 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jeyaraj DA, Grossman G, Petrusz P, Dynamics of testicular germ cell apoptosis in normal mice and transgenic mice overexpressing rat androgen-binding protein, Reprod. Biol. Endocrinol 1 (2003) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Letts PJ, Hunt RC, Shirley MA, Pinteric L, Schachter H, Late spermatocytes from immature rat testis: Isolation, electron microscopy, lectin agglutinability and capacity for glycoprotein and sulfogalactoglycerolipid biosynthesis, Biochim. Biophys. Acta 541(1) (1978) 59–75. [Google Scholar]
- [89].Lingwood CA, Timing of sulphogalactolipid biosynthesis in the rat testis studied by tissue autoradiography, J. Cell Sci 75 (1985) 329–338. [DOI] [PubMed] [Google Scholar]
- [90].Ueno K, Ishizuka I, Yamakawa T, Glycolipid composition of human testis at different ages and the stereochemical configuration of seminolipid, Biochim. Biophys. Acta 487(1) (1977) 61–73. [PubMed] [Google Scholar]
- [91].Kornblatt MJ, Synthesis and turnover of sulfogalactoglycerolipid, a membrane lipid, during spermatogenesis, Can. J. Biochem 57(3) (1979) 255–258. [DOI] [PubMed] [Google Scholar]
- [92].Nakanishi Y, Shiratsuchi A, Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences, Biol. Pharm. Bull 27(1) (2004) 13–16. [DOI] [PubMed] [Google Scholar]
- [93].Wang H, Xiong W, Chen Y, Ma Q, Ma J, Ge Y, Han D, Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining, Reproduction 132(3) (2006) 485–492. [DOI] [PubMed] [Google Scholar]
- [94].Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS, Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo, Nature 467(7313) (2010) 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hai Y, Hou J, Liu Y, Yang H, Li Z, He Z, The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis, Semin. Cell Devel. Biol 29 (2014) 66–75. [DOI] [PubMed] [Google Scholar]
- [96].Qian X, Mruk DD, Cheng YH, Tang EI, Han D, Lee WM, Wong EW, Cheng CY, Actin binding proteins, spermatid transport and spermiation, Semin. Cell Devel. Biol 30 (2014) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lingwood C, Schachter H, Localization of sulfatoxygalactosylacylalkylglycerol at the surface of rat testicular germinal cells by immunocytochemical techniques: pH dependence of a nonimmunological reaction between immunoglobulin and germinal cells, J. Cell Biol 89(3) (1981) 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lingwood CA, Colocalization of sulfogalactosylacylalkylglycerol (SGG) and its binding protein during spermatogenesis and sperm maturation. Topology of SGG defines a new testicular germ cell membrane domain, Biochem. Cell Biol 64(10) (1986) 984–992. [DOI] [PubMed] [Google Scholar]
- [99].Eddy EM, Muller CH, Lingwood CA, Preparation of monoclonal antibody to sulfatoxygalactosylglycerolipd by in vitro immunization with a glycolipid-glass conjugate, J. Immunol. Meth 81(1) (1985) 137–146. [DOI] [PubMed] [Google Scholar]
- [100].Chemes H, The phagocytic function of Sertoli cells: a morphological, biochemical, and endocrinological study of lysosomes and acid phosphatase localization in the rat testis, Endocrinology 119(4) (1986) 1673–1681. [DOI] [PubMed] [Google Scholar]
- [101].Clermont Y, Morales C, Hermo L, Endocytic activities of Sertoli cells in the rat, Ann. N. Y. Acad. Sci 513 (1987) 1–15. [DOI] [PubMed] [Google Scholar]
- [102].Xu H, Liu F, Srakaew N, Koppisetty C, Nyholm PG, Carmona E, Tanphaichitr N, Sperm arylsulfatase A binds to mZP2 and mZP3 glycoproteins in a nonenzymatic manner, Reproduction 144(2) (2012) 209–219. [DOI] [PubMed] [Google Scholar]
- [103].Li JC, Lee TW, Mruk TD, Cheng CY, Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro, J. Androl 22(2) (2001) 266–277. [PubMed] [Google Scholar]
- [104].Kates M, Technique of lipidology: isolation, analysis and identification of lipids, in: Burdon RH (Ed.), Laboratory Techniques in Biochemistry and Molecular Biology, Elsevier, New York, 1986, pp. 100–278. [Google Scholar]
- [105].Dupree JL, Suzuki K, Popko B, Galactolipids in the formation and function of the myelin sheath, Microsc. Res. Tech 41(5) (1998) 431–440. [DOI] [PubMed] [Google Scholar]
- [106].Tadano-Aritomi K, Hikita T, Fujimoto H, Suzuki K, Motegi K, Ishizuka I, Kidney lipids in galactosylceramide synthase-deficient mice. Absence of galactosylsulfatide and compensatory increase in more polar sulfoglycolipids, J. Lipid Res 41(8) (2000) 1237–1243. [PubMed] [Google Scholar]
- [107].Honke K, Zhang Y, Cheng X, Kotani N, Taniguchi N, Biological roles of sulfoglycolipids and pathophysiology of their deficiency, Glycoconj. J 21(1–2) (2004) 59–62. [DOI] [PubMed] [Google Scholar]
- [108].Takahashi T, Suzuki T, Role of sulfatide in normal and pathological cells and tissues, J. Lipid Res 53(8) (2012) 1437–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Knapp A, Kornblatt MJ, Schachter H, Murray RK, Studies on the biosynthesis of testicular sulfoglycerogalactolipid: Demonstration of a golgi-associated sulfotransferase activity, Biochem. Biophys. Res. Commun 55(1) (1973) 179–186. [DOI] [PubMed] [Google Scholar]
- [110].Fleischer B, Zambrano F, Localization of cerebroside-sulfotransferase activity in the Golgi apparatus of rat kidney, Biochem. Biophys. Res. Commun 52(3) (1973) 951–958. [DOI] [PubMed] [Google Scholar]
- [111].Benjamins JA, Hadden T, Skoff RP, Cerebroside sulfotransferase in Golgi-enriched fractions from rat brain, J. Neurochem 38(1) (1982) 233–241. [DOI] [PubMed] [Google Scholar]
- [112].Sakac D, Zachos M, Lingwood CA, Purification of the testicular galactolipid: 3’-phosphoadenoisine 5’-phosphosulfate sulfotransferase, J. Biol. Chem 267(3) (1992) 1655–1659. [PubMed] [Google Scholar]
- [113].Lingwood C, Sakac K, Saltiel A, Developmentally regulated testicular galactolipid sulfotransferase inhibitor is a phosphoinositol glycerolipid and insulin-mimetic, Mol. Reprod. Devel 37(4) (1994) 462–466. [DOI] [PubMed] [Google Scholar]
- [114].Honke K, Yamane M, Ishii A, Kobayashi T, Makita A, Purification and characterization of 3’-phosphoadenosine-5’-phosphosulfate:GalCer sulfotransferase from human renal cancer cells, J. Biochem 119(3) (1996) 421–427. [DOI] [PubMed] [Google Scholar]
- [115].Honke K, Tsuda M, Hirahara Y, Ishii A, Makita A, Wada Y, Molecular cloning and expression of cDNA encoding human 3’-phosphoadenylylsulfate:galactosylceramide 3’-sulfotransferase, J. Biol. Chem 272(8) (1997) 4864–4868. [DOI] [PubMed] [Google Scholar]
- [116].Hirahara Y, Tsuda M, Wada Y, Honke K, cDNA cloning, genomic cloning, and tissue-specific regulation of mouse cerebroside sulfotransferase, Eur. J. Biochem 267(7) (2000) 1909–1916. [DOI] [PubMed] [Google Scholar]
- [117].Schulte S, Stoffel W, Ceramide UDPgalactosyltransferase from myelinating rat brain: purification, cloning, and expression, Proc. Natl. Acad. Sci. U. S. A 90(21) (1993) 10265–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Burger KN, van der Bijl P, van Meer G, Topology of sphingolipid galactosyltransferases in ER and Golgi: transbilayer movement of monohexosyl sphingolipids is required for higher glycosphingolipid biosynthesis, J. Cell Biol 133(1) (1996) 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hsu LH, Narasimhan R, Levine M, Norwich KH, Murray RK, Studies of the biosynthesis and metabolism of rat testicular galactoglycerolipids, Can. J. Biochem. Cell Biol 61(12) (1983) 1272–1281. [DOI] [PubMed] [Google Scholar]
- [120].Fujimoto H, Tadano-Aritomi K, Tokumasu A, Ito K, Hikita T, Suzuki K, Ishizuka I, Requirement of seminolipid in spermatogenesis revealed by UDP-galactose: ceramide galactosyltransferase-deficient mice, J. Biol. Chem 275(30) (2000) 22623–22626. [DOI] [PubMed] [Google Scholar]
- [121].Honke K, Tsuda M, Koyota S, Wada Y, Iida-Tanaka N, Ishizuka I, Nakayama J, Taniguchi N, Molecular cloning and characterization of a human - Gal-3’-sulfotransferase that acts on both type 1 and type 2 (Gal 1–3/1–4GlcNAc-R) oligosaccharides, J. Biol. Chem 276(1) (2001) 267–274. [DOI] [PubMed] [Google Scholar]
- [122].van der Bijl P, Strous GJ, Lopes-Cardozo M, Thomas-Oates J, van Meer G, Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase, Biochem. J 317 ( Pt 2) (1996) 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nagai K, Takahashi N, Niimura Y, Novel biosynthesis of monogalactosyl-alkylacyl glycerolipid in Mop8 fibroblast cells transfected with a ceramide galactosyltransferase gene, Biomed. Res. Clin. Prac 1(3) (2016) 103–108. [Google Scholar]
- [124].Fujiwara Y, Ogonuki N, Inoue K, Ogura A, Handel MA, Noguchi J, Kunieda T, t-SNARE Syntaxin2 (STX2) is implicated in intracellular transport of sulfoglycolipids during meiotic prophase in mouse spermatogenesis, Biol. Reprod 88(6) (2013) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hansen NJ, Antonin W, Edwardson JM, Identification of SNAREs involved in regulated exocytosis in the pancreatic acinar cell, J. Biol. Chem 274(32) (1999) 22871–22876. [DOI] [PubMed] [Google Scholar]
- [126].Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T, Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells, Dev. Cell 4(5) (2003) 753–759. [DOI] [PubMed] [Google Scholar]
- [127].Zhang Y, Hayashi Y, Cheng X, Watanabe T, Wang X, Taniguchi N, Honke K, Testis-specific sulfoglycolipid, seminolipid, is essential for germ cell function in spermatogenesis, Glycobiology 15(6) (2005) 649–654. [DOI] [PubMed] [Google Scholar]
- [128].Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y, ‘Green mice’ as a source of ubiquitous green cells, FEBS Lett 407(3) (1997) 313–319. [DOI] [PubMed] [Google Scholar]
- [129].Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM, Germ cell intercellular bridges, Cold Spring Harb. Perspect. Biol 3(8) (2011) a005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM, TEX14 is essential for intercellular bridges and fertility in male mice, Proc. Natl. Acad. Sci. U. S. A 103(13) (2006) 4982–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Boussouar F, Benahmed M, Lactate and energy metabolism in male germ cells, Trends in endocrinology and metabolism: Trends Endocrinol. Metabol 15(7) (2004) 345–350. [DOI] [PubMed] [Google Scholar]
- [132].Lagace TA, Ridgway ND, The role of phospholipids in the biological activity and structure of the endoplasmic reticulum, Biochim. Biophys. Acta 1833(11) (2013) 2499–2510. [DOI] [PubMed] [Google Scholar]
- [133].Tubaon RM, Haddad PR, Quirino JP, Sample Clean-up Strategies for ESI Mass Spectrometry Applications in Bottom-up Proteomics: Trends from 2012 to 2016, Proteomics 17(20) (2017). [DOI] [PubMed] [Google Scholar]
- [134].Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY, Ezrin: a regulator of actinmicrofilaments in cell junctions of the rat testis, Asian J. Androl 17(4) (2015) 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wakayama T, Nakata H, Kurobo M, Sai Y, Iseki S, Expression, localization, and binding activity of the ezrin/radixin/moesin proteins in the mouse testis, J. Histochem. Cytochem 57(4) (2009) 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY, Ezrin is an actin binding protein that regulates sertoli cell and spermatid adhesion during spermatogenesis, Endocrinology 155(10) (2014) 3981–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Boulanger J, Faulds D, Eddy EM, Lingwood CA, Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility, J. Cell Physiol 165(1) (1995) 7–17. [DOI] [PubMed] [Google Scholar]
- [138].Mamelak D, Mylvaganam M, Whetstone H, Hartmann E, Lennarz W, Wyrick PB, Raulston J, Han H, Hoffman P, Lingwood CA, Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members, Biochemistry 40(12) (2001) 3572–3582. [DOI] [PubMed] [Google Scholar]
- [139].Mamelak D, Lingwood C, Expression and sulfogalactolipid binding specificity of the recombinant testis-specific cognate heat shock protein 70, Glycoconj. J 14(6) (1997) 715–722. [DOI] [PubMed] [Google Scholar]
- [140].Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM, Targeted gene disruption of {IHsp70-2} results in failed meiosis, germ cell apoptosis, and male infertility, Proc. Natl. Acad. Sci. U. S. A 93(8) (1996) 3264–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, Liu DY, Aitken RJ, The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition, PloS One 7(11) (2012) e50851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nixon B, Bromfield EG, Dun MD, Redgrove KA, McLaughlin EA, Aitken RJ, The role of the molecular chaperone heat shock protein A2 (HSPA2) in regulating human sperm-egg recognition, Asian J. Androl 17(4) (2015) 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Harada Y, Sato C, Kitajima K, Sulfatide-Hsp70 interaction promotes Hsp70 clustering and stabilizes binding to unfolded protein, Biomolecules 5(2) (2015) 958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Benjamin IJ, McMillan DR, Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease, Circ. Res 83(2) (1998) 117–132. [DOI] [PubMed] [Google Scholar]
- [145].Robaire B, Hinton BT, The Epididymis, in: Tony MP, Anthony Z (Eds.), Knobil and Neill’s Physiology of Reproduction, New York, New York, 2015, pp. 691–764. [Google Scholar]
- [146].Florman H, Fissore R, Fertilization in Mammals, in: Tony MP, Anthony Z (Eds.), Knobil and Neill’s Physiology of Reproduction, Elsevier Inc., New York, 2015, pp. 149–195. [Google Scholar]
- [147].Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D, Sperm capacitation and acrosome reaction in mammalian sperm, Adv. Anat. Embryol. Cell Biol 220 (2016) 93–106. [DOI] [PubMed] [Google Scholar]
- [148].Go KJ, Wolf DP, Albumin-mediated changes in sperm sterol content during capacitation, Biol. Reprod 32(1) (1985) 145–153. [DOI] [PubMed] [Google Scholar]
- [149].Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS, Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation, J. Biol. Chem 274(5) (1999) 3235–3242. [DOI] [PubMed] [Google Scholar]
- [150].Davis BK, Uterine fluid proteins bind sperm cholesterol during capacitation in the rabbit, Experentia 38 (1982) 1063–1064. [DOI] [PubMed] [Google Scholar]
- [151].Langlais J, Kan FW, Granger L, Raymond L, Bleau G, Roberts KD, Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation, Gamete Res 20(2) (1988) 185–201. [DOI] [PubMed] [Google Scholar]
- [152].Leahy T, Gadella BM, New insights into the regulation of cholesterol efflux from the sperm membrane, Asian J. Androl 17(4) (2015) 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Suarez SS, Gamete and Zygote Transport, in: Plant TM, Zeleznik A (Eds.), Knobil and Neill’s Physiology of Reproduction: Two-Volume Set, Academic Press, London, UK, 2015, pp. 197–232. [Google Scholar]
- [154].Dun MD, Mitchell LA, Aitken RJ, Nixon B, Sperm-zona pellucida interaction: molecular mechanisms and the potential for contraceptive intervention, Handb. Exp. Pharmacol (198) (2010) 139–178. [DOI] [PubMed] [Google Scholar]
- [155].Wassarman PM, Litscher ES, The multifunctional zona pellucida and mammalian fertilization, J. Reprod. Immunol 83(1–2) (2009) 45–49. [DOI] [PubMed] [Google Scholar]
- [156].Tanphaichitr N, Kongmanas K, Kruevaisayawan H, Saewu A, Sugeng C, Fernandes J, Souda P, Angel JB, Faull KF, Aitken RJ, Whitelegge J, Hardy D, Berger T, Baker M, Remodeling of the plasma membrane in preparation for sperm-egg recognition: roles of acrosomal proteins, Asian J. Androl 17(4) (2015) 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lyng R, Shur BD, Sperm-egg binding requires a multiplicity of receptor-ligand interactions: new insights into the nature of gamete receptors derived from reproductive tract secretions, Soc. Reprod. Fertil Suppl 65 (2007) 335–351. [PubMed] [Google Scholar]
- [158].Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, Scrable H, Herr JC, Visconti PE, Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation, Biol. Reprod 73(4) (2005) 721–729. [DOI] [PubMed] [Google Scholar]
- [159].Van Gestel RA, Brewis IA, Ashton PR, Helms JB, Brouwers JF, Gadella BM, Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells, Mol. Hum. Reprod 11(8) (2005) 583–590. [DOI] [PubMed] [Google Scholar]
- [160].Nixon B, Bielanowicz A, McLaughlin EA, Tanphaichitr N, Ensslin MA, Aitken RJ, Composition and significance of detergent resistant membranes in mouse spermatozoa, J. Cell Physiol 218(1) (2009) 122–134. [DOI] [PubMed] [Google Scholar]
- [161].Dun MD, Smith ND, Baker MA, Lin M, Aitken RJ, Nixon B, The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction, J. Biol. Chem 286(42) (2011) 36875–36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Redgrove KA, Anderson AL, Dun MD, McLaughlin EA, O’Bryan MK, Aitken RJ, Nixon B, Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae, Dev. Biol 356(2) (2011) 460–474. [DOI] [PubMed] [Google Scholar]
- [163].Kongmanas K, Kruevaisayawan H, Saewu A, Sugeng C, Fernandes J, Souda P, Angel JB, Faull KF, Aitken RJ, Whitelegge J, Hardy D, Berger T, Baker MA, Tanphaichitr N, Proteomic characterization of pig sperm anterior head plasma membrane reveals roles of acrosomal proteins in ZP3 binding, J. Cell Physiol 230(2) (2015) 449–463. [DOI] [PubMed] [Google Scholar]
- [164].Sotomayor RE, Handel MA, Failure of acrosome assembly in a male sterile mouse mutant, Biol. Reprod 34(1) (1986) 171–182. [DOI] [PubMed] [Google Scholar]
- [165].Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM, Lack of acrosome formation in Hrb-deficient mice, Science 294(5546) (2001) 1531–1533. [DOI] [PubMed] [Google Scholar]
- [166].Lin YN, Roy A, Yan W, Burns KH, Matzuk MM, Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis, Mol. Cell. Biol 27(19) (2007) 6794–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Litscher ES, Williams Z, Wassarman PM, Zona pellucida glycoprotein ZP3 and fertilization in mammals, Mol. Reprod. Dev 76(10) (2009) 933–941. [DOI] [PubMed] [Google Scholar]
- [168].Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N, Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization, Proc. Natl. Acad. Sci. U. S. A 108(12) (2011) 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Buffone MG, Hirohashi N, Gerton GL, Unresolved questions concerning mammalian sperm acrosomal exocytosis, Biol. Reprod 90(5) (2014) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tantibhedhyangkul J, Weerachatyanukul W, Carmona E, Xu H, Anupriwan A, Michaud D, Tanphaichitr N, Role of sperm sufrace arylsulfatase A in mouse sperm-zona pellucida binding, Biol. Reprod 67(1) (2002) 212–219. [DOI] [PubMed] [Google Scholar]
- [171].Law H, Itkonnen O, Lingwood CA, Sulfogalactolipid binding protein SLIP 1: A conserved function for a conserved protein, J. Cell. Physiol 137 (1988) 462–468. [DOI] [PubMed] [Google Scholar]
- [172].Tanphaichitr N, Smith J, Mongkolsirikieart S, Gradil C, Lingwood CA, Role of a gamete-specific sulfoglycolipid immobilizing protein on mouse sperm-egg binding, Dev. Biol 156(1) (1993) 164–175. [DOI] [PubMed] [Google Scholar]
- [173].Tanphaichitr N, Tayabali A, Gradil C, Juneja S, Leveille MC, Lingwood C, Role of germ cell-specific sulfolipidimmobilizing protein (SLIP1) in mouse in vivo fertilization, Mol. Reprod. Dev 32 (1992) 17–22. [DOI] [PubMed] [Google Scholar]
- [174].Rattanachaiyanont M, Weerachatyanukul W, Leveille MC, Taylor T, D’Amours D, Rivers D, Leader A, Tanphaichitr N, Anti-SLIP1-reactive proteins exist on human sperm and are involved in zona pellucida binding, Mol. Human Reprod 7(7)(2001) 633–640. [DOI] [PubMed] [Google Scholar]
- [175].Tanphaichitr N, Moase C, Taylor T, Surewicz K, Hansen C, Namking M, r.B. B, N. Kamolvarin, C.A. Lingwood, R. Sullivan, M. Rattanachaiyanont, D. White, Isolation of antiSLIP1-reactive boar sperm P68/62 and its binding to mammalian zona pellucida, Mol. Reprod. Dev 49(2) (1998) 203–216. [DOI] [PubMed] [Google Scholar]
- [176].Carmona E, Weerachatyanukul W, Soboloff T, Fluhary AL, White D, Promdee L, Ekker M, Berger T, Buhr M, Tanphaichitr N, Arylsulfatase A is present on the pig sperm surface and is involved in sperm-zona pellucida binding, Dev. Biol 247(1) (2002) 182–196. [DOI] [PubMed] [Google Scholar]
- [177].Gadella BM, Colenbrander B, Van Golde LMG, Lopes-Cardozo M, Characterization of three arylsulfatases in semen: seminolipid sulfohydrolase activity is present in seminal plasma, Biochim. Biophys. Acta 1128(2–3) (1992) 155–162. [DOI] [PubMed] [Google Scholar]
- [178].Carmona E, Weerachatyanukul W, Xu H, Fluharty A, Anupriwan A, Shoushtarian A, Chakrabandhu K, Tanphaichitr N, Binding of arylsulfatase A to mouse sperm inhibits gamete interaction and induces the acrosome reaction, Biol. Reprod 66(6) (2002) 1820–1827. [DOI] [PubMed] [Google Scholar]
- [179].Lukatela G, Krauss N, Theis K, Selmer T, Gieselmann V, von Figura K, Saenger W, Crystal structure of human arylsulfatase A: The aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis, Biochemistry 37(11) (1998) 3654–3664. [DOI] [PubMed] [Google Scholar]
- [180].Schenk M, Koppisetty CA, Santos DC, Carmona E, Bhatia S, Nyholm PG, Tanphaichitr N, Interaction of arylsulfatase-A (ASA) with its natural sulfoglycolipid substrates: a computational and site-directed mutagenesis study, Glycoconj. J 26(8) (2009) 1-20–1045. [DOI] [PubMed] [Google Scholar]
- [181].Hakomori S, Cell adhesion/recognition and signal transduction through glycosphingolipid microdomain, Glycoconj. J 17(3–4) (2000) 143–151. [DOI] [PubMed] [Google Scholar]
- [182].Hakomori S, Traveling for the glycosphingolipid path, Glycoconj. J 17(7–9) (2000) 627–647. [DOI] [PubMed] [Google Scholar]
- [183].Boggs JM, Koshy KM, Rangaraj G, Thermotropic phase behaviours of mixtures of long chain fatty acid species of cerebroside sulfate with different fatty acid chain length species of phospholipids, Biochemistry 32(34) (1993) 8908–8922. [DOI] [PubMed] [Google Scholar]
- [184].Weerachatyanukul W, Probodh I, Kongmanas K, Tanphaichiatr N, Johnston LJ, Visualizing the localization of sulfoglycolipids in lipid raft domains in model membranes and sperm membrane extracts, Biochim. Biophys. Acta 1768(2)(2007) 299–310. [DOI] [PubMed] [Google Scholar]
- [185].Simons K, Ehehalt R, Cholesterol, lipid rafts, and disease, J. Clin. Invest 110(5) (2002) 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Brown DA, Rose JK, Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface, Cell 68(3) (1992) 533–544. [DOI] [PubMed] [Google Scholar]
- [187].London E, Brown DA, Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts), Biochim. Biophys. Acta 1508(1–2) (2000) 182–195. [DOI] [PubMed] [Google Scholar]
- [188].Blomqvist M, Osterbye T, Mansson JE, Horn T, Buschard K, Fredman P, Sulfatide is associated with insulin granules and located to microdomains of a cultured beta cell line, Glycoconj. J 19(6) (2002) 403–413. [DOI] [PubMed] [Google Scholar]
- [189].Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G, Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells, J. Cell Biol 169(3) (2005) 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Saravanan K, Schaeren-Wiemers N, Klein D, Sandhoff R, Schwarz A, Yaghootfam A, Gieselmann V, Franken S, Specific downregulation and mistargeting of the lipid raft-associated protein MAL in a glycolipid storage disorder, Neurobiol. Dis 16(2) (2004) 396–406. [DOI] [PubMed] [Google Scholar]
- [191].Honda A, Yamagata K, Sugiura S, Watanabe K, Baba T, A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein, J. Biol. Chem 277(19) (2002) 16976–16984. [DOI] [PubMed] [Google Scholar]
- [192].Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P, Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin Dev. Biol 233(1) (2001) 204–213. [DOI] [PubMed] [Google Scholar]
- [193].Pike LJ, Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function, J Lipid Res 47(7) (2006) 1597–1598. [DOI] [PubMed] [Google Scholar]
- [194].Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RG, Mumby SM, Organization of G proteins and adenylyl cyclase at the plasma membrane, Mol. Biol. Cell 8(12) (1997) 2365–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Peterson R, Russell L, Bundman D, Freund M, Evaluation of the purity of boar sperm plasma membranes prepared by nitrogen cavitation, Biol. Reprod 23(3) (1980) 637–645. [DOI] [PubMed] [Google Scholar]
- [196].Yurewicz EC, Pack BA, Armant DR, Sacco AG, Porcine zona pellucida ZP3 … glycoprotein mediates binding of the biotin-labeled M{-r} 55,000 family (ZP3) to boar sperm membrane vesicles, Mol. Reprod. Dev 36(3) (1993) 382–389. [DOI] [PubMed] [Google Scholar]
- [197].Jones R, James PS, Howes L, Bruckbauer A, Klenerman D, Supramolecular organization of the sperm plasma membrane during maturation and capacitation, Asian J. Androl 9(4) (2007) 438–444. [DOI] [PubMed] [Google Scholar]
- [198].Boulanger J, Faulds D, Eddy EM, Lingwood CA, Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility, J. Cell. Physiol 165(1) (1995) 7–17. [DOI] [PubMed] [Google Scholar]
- [199].Mamelak D, Lingwood C, The ATPase domain of hsp70 possesses a unique binding specificity for 3’-sulfogalactolipids, J. Biol. Chem 276(1) (2001) 449–456. [DOI] [PubMed] [Google Scholar]
- [200].Mamelak D, Mylvaganam M, Tanahashi E, Ito H, Ishida H, Kiso M, Lingwood C, The aglycone of sulfogalactolipids can alter the sulfate ester substitution position required for hsc70 recognition, Carbohydr. Res 335(2) (2001) 91–100. [DOI] [PubMed] [Google Scholar]
- [201].Dodge JC, Lipid Involvement in Neurodegenerative Diseases of the Motor System: Insights from Lysosomal Storage Diseases, Front. Mol. Neurosci 10 (2017) 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Jatzkewitz H, Mehl E, Cerebroside-sulphatase and arylsulphatase A deficiency in metachromatic leukodystrophy (ML), J. Neurochem 16(1) (1969) 19–28. [DOI] [PubMed] [Google Scholar]
- [203].Mehl E, Jatzkewitz H, Evidence for the genetic block in metachromatic leucodystrophy (Ml), Biochem. Biophys. Res. Commun 19 (1965) 407–411. [DOI] [PubMed] [Google Scholar]
- [204].Suzuki K, Suzuki Y, Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside beta-galactosidase, Proc. Natl. Acad. Sci. U. S. A 66(2) (1970) 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Mehl E, Jatzkewitz H, Cerebroside 3-sulfate as a physiological substrate of arylsulfatase A, Biochim. Biophys. Acta 151(3) (1968) 619–627. [DOI] [PubMed] [Google Scholar]
- [206].Fischer G, Jatzkewitz H, The activator of cerebroside-sulphatase. A model of the activation, Biochim. Biophys. Acta 528(1) (1978) 69–76. [DOI] [PubMed] [Google Scholar]
- [207].Fischer G, Reiter S, Jatzkewitz H, Enzymic hydrolysis of sulphosphingolipids and sulphoglycerolipids by sulphatase A in the presence and absence of activator protein, Hoppe-Seyler’s Z. Physiol. Chem 359 (1978) 863–866. [PubMed] [Google Scholar]
- [208].Chen YQ, Wenger DA, Galactocerebrosidase from human urine: purification and partial characterization, Biochim. Biophys. Acta 1170(1) (1993) 53–61. [DOI] [PubMed] [Google Scholar]
- [209].Matsuda J, Vanier MT, Saito Y, Tohyama J, Suzuki K, A mutation in the saposin A domain of the sphingolipid activator protein (prosaposin) gene results in a late-onset, chronic form of globoid cell leukodystrophy in the mouse, Hum. Mol. Genet 10(11) (2001) 1191–1199. [DOI] [PubMed] [Google Scholar]
- [210].Matsuda J, Suzuki K, Krabbe Disease (Globoid Cell Leukodystrophy), in: Barranger JA, Cabrera-Salazar MA (Eds.), Lysosomal Storage Disorders, Springer, New York, NY, 2007, pp. 269–284. [Google Scholar]
- [211].Schulze H, Kolter T, Sandhoff K, Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation, Biochim. Biophys. Acta 1793(4) (2009) 674–683. [DOI] [PubMed] [Google Scholar]
- [212].Kolter T, Sandhoff K, Lysosomal degradation of membrane lipids, FEBS Lett 584(9) (2010) 1700–1712. [DOI] [PubMed] [Google Scholar]
- [213].Jaishy B, Abel ED, Lipids, lysosomes, and autophagy, J. Lipid Res 57(9) (2016) 1619–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].von Figura K, Gieselmann V, Jaeken J, Metachromatic Leukodystrophy, The Online Metabolic & Molecular Bases of Inherited Disease, The McGraw-Hill Companies, New York, 2001, pp. 3695–3724. [Google Scholar]
- [215].Wenger DA, Rafi MA, Luzi P, Krabbe disease: One hundred years from the bedside to the bench to the bedside, J. Neurosci. Res 94(11) (2016) 982–989. [DOI] [PubMed] [Google Scholar]
- [216].Deconinck N, Messaaoui A, Ziereisen F, Kadhim H, Sznajer Y, Pelc K, Nassogne MC, Vanier MT, Dan B, Metachromatic leukodystrophy without arylsulfatase A deficiency: a new case of saposin-B deficiency, Eur. J. Paediatr. Neurol 12(1) (2008) 46–50. [DOI] [PubMed] [Google Scholar]
- [217].Kuchar L, Ledvinova J, Hrebicek M, Myskova H, Dvorakova L, Berna L, Chrastina P, Asfaw B, Elleder M, Petermoller M, Mayrhofer H, Staudt M, Krageloh-Mann I, Paton BC, Harzer K, Prosaposin deficiency and saposin B deficiency (activator-deficient metachromatic leukodystrophy): report on two patients detected by analysis of urinary sphingolipids and carrying novel PSAP gene mutations, Am. J. Med. Genet. A 149A(4) (2009) 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Regis S, Filocamo M, Corsolini F, Caroli F, Keulemans JL, van Diggelen OP, Gatti R, An Asn > Lys substitution in saposin B involving a conserved amino acidic residue and leading to the loss of the single N-glycosylation site in a patient with metachromatic leukodystrophy and normal arylsulphatase A activity, Eur. J. Hum. Genet 7(2) (1999) 125–130. [DOI] [PubMed] [Google Scholar]
- [219].Spiegel R, Bach G, Sury V, Mengistu G, Meidan B, Shalev S, Shneor Y, Mandel H, Zeigler M, A mutation in the saposin A coding region of the prosaposin gene in an infant presenting as Krabbe disease: first report of saposin A deficiency in humans, Mol. Genet. Metabol 84(2) (2005) 160–166. [DOI] [PubMed] [Google Scholar]
- [220].Kolter T, Sandhoff K, Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids, Annu. Rev. Cell Dev. Biol 21 (2005) 81–103. [DOI] [PubMed] [Google Scholar]
- [221].Hess B, Saftig P, Hartmann D, Coenen R, Lullmann-Rauch R, Goebel HH, Evers M, von Figura K, D’Hooge R, Nagel G, De Deyn P, Peters C, Gieselmann V, Phenotype of arylsulfatse A-deficient mice: Relationship to human metachromatic leukodystrophy, Proc. Natl. Acad. Sci. U. S. A 93(25)(1996) 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Gieselmann V, Metachromatic leukodystrophy: recent research developments, J. Child Neurol 18(9) (2003) 591–594. [DOI] [PubMed] [Google Scholar]
- [223].Molander-Melin M, Pernber Z, Franken S, Gieselmann V, Mansson JE, Fredman P, Accumulation of sulfatide in neuronal and glial cells of arylsulfatase A deficient mice, J. Neurocytol 33(4) (2004) 417–427. [DOI] [PubMed] [Google Scholar]
- [224].Igdoura SA, Rasky A, Morales CR, Trafficking of sulfated glycoprotein-1 (prosaposin) to lysosomes or to the extracellular space in rat Sertoli cells, Cell Tiss. Res 283(3) (1996) 385–394. [DOI] [PubMed] [Google Scholar]
- [225].Luddi A, Strazza M, Carbone M, Moretti E, Costantino-Ceccarini E, Galactosylceramidase deficiency causes sperm abnormalities in the mouse model of globoid cell leukodystrophy, Exp. Cell Res 304(1) (2005) 59–68. [DOI] [PubMed] [Google Scholar]
- [226].Luddi A, Gori M, Crifasi L, Marrocco C, Belmonte G, Costantino-Ceccarini E, Piomboni P, Impaired spermatogenesis in the twitcher mouse: A morphological evaluation from the seminiferous tubules to epididymal transit, Syst. Biol. Reprod. Med 63(2) (2017) 77–85. [DOI] [PubMed] [Google Scholar]
- [227].Puggioni E, Governini L, Gori M, Belmonte G, Piomboni P, Costantino-Ceccarini E, Luddi A, Morphological and molecular characterisation of Twitcher mouse spermatogenesis: an update, Reprod. Fertil. Dev 28(9)(2015) 1258–1267. [DOI] [PubMed] [Google Scholar]
- [228].Smith LB, Walker WH, Hormone Signaling in the Testis, in: Tony MP, Anthony Z (Eds.), Knobil and Neill’s Physiology of Reproduction, Elsevier Inc., New York, 2015, pp. 637–690. [Google Scholar]
- [229].Burkart T, Caimi L, Siegrist H, Herschkowitz N, Wiesmann U, Vesicular transport of sulfatide in the myelinating mouse brain: Functional association with lysosomes, J. Biol. Chem 257(6) (1982) 3151–3156. [PubMed] [Google Scholar]
- [230].van Meer G, Voelker DR, Feigenson GW, Membrane lipids: where they are and how they behave, Nature reviews. Mol. Cell Biol 9(2) (2008) 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Blom T, Somerharju P, Ikonen E, Synthesis and biosynthetic trafficking of membrane lipids, Cold Spring Harb. Perspect. Biol 3(8) (2011) a004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Maxfield FR, Wustner D, Intracellular cholesterol transport, J. Clin. Invest 110(7) (2002) 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K, Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network, J. Cell Biol 185(4) (2009) 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Tuuf J, Mattjus P, Membranes and mammalian glycolipid transferring proteins, Chem. Phys. Lipids 178 (2014) 27–37. [DOI] [PubMed] [Google Scholar]
- [235].Mattjus P, Specificity of the mammalian glycolipid transfer proteins, Chem. Phys. Lipids 194 (2016) 72–78. [DOI] [PubMed] [Google Scholar]
- [236].Yamada K, Abe A, Sasaki T, Glycolipid transfer protein from pig brain transfers glycolipids with beta-linked sugars but not with alpha-linked sugars at the sugar-lipid linkage, Biochim. Biophys. Acta 879(3) (1986) 345–349. [PubMed] [Google Scholar]
- [237].Franchini L, Compostella F, Colombo D, Panza L, Ronchetti F, Synthesis of the sulfonate analogue of seminolipid via Horner-Wadsworth-Emmons olefination, J. Org. Chem 75(15) (2010) 5363–5366. [DOI] [PubMed] [Google Scholar]
- [238].World Health Organization, WHO laboratory manual for the examination and processing of human semen, 5 ed., WHO Press, Geneva, Switzerland: 2010. [Google Scholar]
- [239].Wong CH, Mruk DD, Lui WY, Cheng CY, Regulation of blood-testis barrier dynamics: an in vivo study, J. Cell Sci 117(Pt 5) (2004) 783–798. [DOI] [PubMed] [Google Scholar]


