Abstract
Obesity raises blood pressure(BP) in children and adults. Nevertheless, as obesity increased around the globe, population systolic(SBP) and diastolic blood pressures(DBP) were flat or fell. Examining children is insightful because pediatric trends are largely unconfounded by antihypertensive therapy. Decomposing BP into arterial types, large artery measures(pulse pressure, PP) increased in concert with obesity while small artery measures(mean arterial pressure, MAP) declined, suggesting small arteries are the locus of the countervailing temporal trends. Pediatric lead exposure declined as pediatric obesity rose. Over the period of rising obesity, we examined the association between lead exposure and temporal trends in BP. We analyzed anthropometric, BP, and laboratory data on 8–17 year old children from the serial cross-sectional National Health and Nutrition Examination Surveys (NHANES) 1976 through 2008. Multivariable adjusted survey regression was used to examine temporal trends in blood pressure in relation to blood lead concentrations (N= 13,501). As obesity prevalence rose from 5.3% to 24.5%, age-sex adjusted SBP was flat (−0.01[95%CI −0.06,0.04]mmHg/yr,p=0.8), DBP and MAP declined (respectively −0.28[−0.32,−0.24] and −0.19 [CI −0.23, −0.15], both p<0.0001) while PP increased (0.28 [0.23, 0.32], p<0.001). Accounting for blood lead concentration attenuated the declining MAP trend by 67%. In conclusion, the contrary trends in pediatric BP during the rise of pediatric obesity may be substantially attributable to decreasing lead exposure acting on small resistance arteries. These results have implications for globally observed BP trends in youth and adults. Environmental policy altering lead levels may have long-lasting cardiovascular benefits.
Keywords: blood pressure, pediatric obesity, child, NHANES, lead
INTRODUCTION
Obesity is associated with elevated blood pressure (BP) through changes at the small and large artery levels.1, 2 While increasing obesity prevalence should lead to higher BP measures, myriad population-based studies show decreasing systolic blood pressure (SBP) and diastolic blood pressure (DBP) and a lower than expected increase in proportion hypertensive.3–6 Longitudinal cohort data suggest the mechanism being a population BP change rather than just pharmacotherapy of hypertensive individuals.7 Children offer a window into this conundrum because few children are treated with BP lowering medicine, yet similar BP trends are present.1, 5, 6, 8 BP is usually assessed as SBP and DBP. Alternative decomposition into pulse pressure (PP) and mean arterial pressure (MAP) localizes the changes to large and small arteries, respectively, and predicts incident CVD events.9, 10 In prior National Health and Nutrition Examination Surveys (NHANES) work, in children over the past 3 decades PP increased but MAP actually declined, resulting in a nearly flat SBP trend.1 Obesity accounted for a portion of the rise in PP, but actually steepened the MAP trend, suggesting an alternate explanation is needed for the MAP trend. Coincident with rising pediatric obesity, lead concentrations declined, perhaps related to United States statutes reducing lead utilization and creation of the Environmental Protection Agency.11 Even low level lead exposure predicts future CVD events.12 Lead is known to raise BP.13 Given the population-wide changes in the lead exposure, we hypothesized that blood lead concentrations would be associated with the temporal BP trends and would localize to large(as PP) versus small(as MAP) arteries.
METHODS
All data and materials used herein are from the NHANES and are publicly available through the Centers for Disease Control at https://www.cdc.gov/nchs/nhanes/index.htm. The NHANES collects cross-sectional data on the civilian, non-institutionalized population of the United States. Since 1999, the survey has used a multistage probability sampling design including oversampling of Non-Hispanic blacks, Mexican-Americans, and the 12–19 year age range.14 We extracted data from the Centers for Disease Control and Prevention’s National Center for Health Statistics for all 8–17 year old children in each survey from NHANES II (1976–1984) through NHANES 2007–2008 to focus on the period of precipitous rise in pediatric obesity. We collected data on sex, age, race/ethnicity, height, weight, heart rate, SBP, DBP, sodium intake, and serum lead concentration from each survey. Participants without at least 2 SBP and DBP measurements or with missing data were excluded, except for NHANES II in which only 1 BP was obtained. Daily sodium intake was estimated from the first day of 24 hour dietary recall. This project as a secondary analysis of deidentified data was deemed exempt from formal review by the Institutional Review Board.
During the physical examination component of the NHANES visit auscultatory BP measurements were obtained. In NHANES, SBP is defined as Korotkoff phase 1 which is the pressure where audible tapping begins. DBP is defined as Korotkoff phase 5 where tapping ceases. PP is calculated as the difference between SBP and DBP. MAP is calculated as the sum of DBP and one-third of the PP. The examining physicians were trained in a standardized measurement protocol beginning with NHANES III including a full range of blood pressure cuff sizes and quality control oversight. BP cuffs were selected so that the bladder length encircled 50 to 80% of the mid-arm circumference. For NHANES II standard adult and child cuffs were available. We have previously demonstrated temporal trends in MAP and PP are robust to exclusion of NHANES II as an outlier, digit preference, cuff size discrepancies, and other exam specific alterations.1 Overall, only 101 participants had valid entries regarding antihypertensive medications and so were included in our analysis without adjustment. Participant weight was measured on a digital scale with clothing limited to foam slippers, a disposable gown and underwear. Standing height was measured using a fixed stadiometer with vertical backboard and movable headboard. Body mass index (BMI) is calculated as weight in kg divided by the square of height in meters. BMI was treated as the age-sex referenced Z score as calculated from Centers for Disease Control normative values.15 Tobacco has been previously analyzed and did not substantially contribute to the population BP trends in children so is not included in this analysis.1 Venous blood sampling was used to obtain blood lead levels. Blood lead concentrations were determined using inductively coupled plasma mass spectrometry on aerosolized diluted whole blood samples based on quadrupole ICP-MS technology on more recent exams and atomic absorption spectroscopy on NHANES II with r values of 0.99.16 Detailed descriptions of techniques, verified consistency between techniques, and lack of bias from differential response rates have been documented by NHANES and others.14
Primary analyses used survey weighted linear regression to model differences in SBP, DBP, MAP or PP separately over time. National Center for Health Statistics guidelines on survey weighting were applied.14 The primary outcome of interest was MAP, with SBP, DBP, and PP as the secondary outcomes of interest. The primary predictor was year as calculated by the number of years from midpoint of NHANES II to midpoint of each subsequent survey. Adjustment covariates in the first step included age and sex. Height, heart rate, race/ethnicity, BMI Z score, sodium intake were added on the second step. For PP only, MAP was also added in the second step to account for the effect of distending pressure on arterial stiffness. Finally in the third step of the model, lead concentration was added as a continuous variable. To maintain consistency with a significant examination year-sex interaction term in the MAP temporal trend, pooled-sex and sex-specific analyses are reported across all model results. White versus other race/ethnicities had a nonsignificant interaction term, so race pooled results are presented. SAS 9.3 (IBM, Cary NC) was used for statistical analyses. P values less than 0.05 were deemed significant.
RESULTS
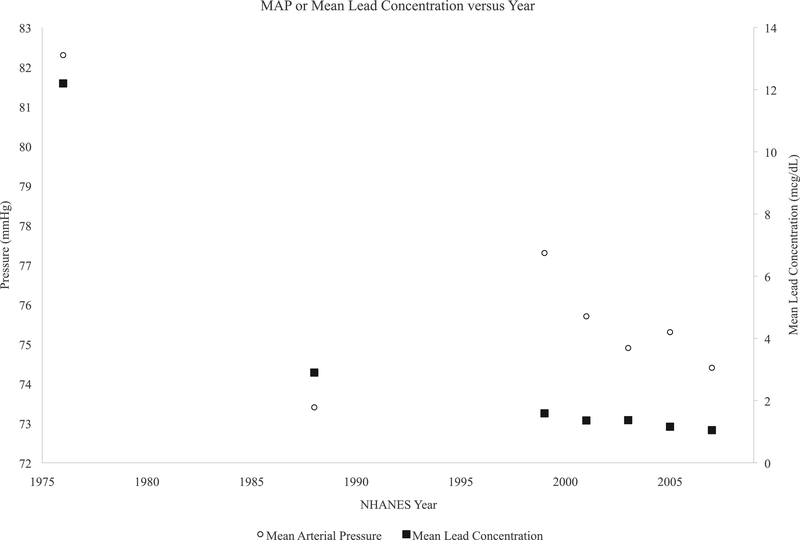
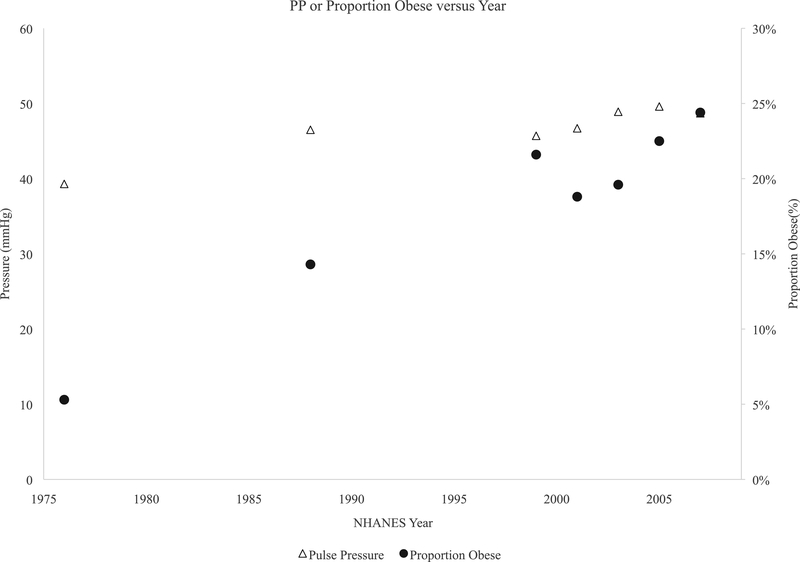
Sample characteristics of the lead analysis sample are detailed in Table 1. The proportion of children with obesity more than tripled over the analysis period from NHANES II 1976–80 to NHANES 2007–2008. The proportion of Mexican-Americans more than tripled. Population mean lead concentration declined 90% over the observation period and the prevalence of elevated lead levels declined from 60% of the population to less than 1%. Population means were flat for SBP, declined for DBP, and MAP, while PP increased. Figures 1 show SBP and PP versus population mean lead concentration over time, while Figure 2 shows DBP and MAP versus lead. The proportion with elevated BP as defined by the Fourth Report also decreased substantially over time.
TABLE 1.
Sample Characteristics and Unadjusted Primary Results by Survey Year
| NHANES | 1976–1980 | 1988–1994 | 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 |
|---|---|---|---|---|---|---|---|
| N examined | 1368 | 3342 | 2014 | 2147 | 1710 | 1719 | 1201 |
| N excluded | 1716 | 514 | 286 | 291 | 422 | 447 | 338 |
| Age (Years) | 12.7±2.8 | 12.4±2.9 | 13.2±2.7 | 13.1±2.7 | 13.46± 2.61 | 13.23±2.68 | 12.56±2.84 |
| Girls | 638 (46.6%) | 1728 (51.7%) | 1003 (49.8%) | 1102 (51.3%) | 860 (50.3%) | 891 (51.8%) | 615 (49.1%) |
| Non-Hispanic White | 940 (68.9%) | 902 (27.0%) | 408 (20.3%) | 666 (31.0%) | 447 (26.1%) | 463 (26.9%) | 393 (31.4%) |
| Non-Hispanic Black | 202 (14.8%) | 1161 (34.7%) | 592 (29.4%) | 660 (30.7%) | 605 (35.4%) | 556 (32.3%) | 293 (23.4%) |
| Mexican American | 71 (5.2%) | 1115 (33.4%) | 858 (42.6%) | 639 (29.8%) | 538 (31.5%) | 561 (32.6%) | 341 (27.2%) |
| Height (centimeters) | 154.9±15.7 | 153.7±14.8 | 157.9±14.0 | 157.9±14.2 | 159.9±13.49 | 158.4±14.16 | 155.2±14.97 |
| Weight (kilograms) | 48.61±16.04 | 50.73±18.05 | 57.44±20.33 | 56.27±19.42 | 58.94±19.33 | 57.89±20.59 | 54.83±20.15 |
| BMI (kilograms per meter squared) | 19.68±3.76 | 20.89±4.89 | 22.51±5.73 | 22.04±5.47 | 22.62±5.45 | 22.56±5.85 | 22.14±5.56 |
| BMI > 95th percentile | 72 (5.3%) | 472 (14.3%) | 434 (21.6%) | 400 (18.8%) | 333 (19.6%) | 386 (22.5%) | 304 (24.4%) |
| Heart rate per minute | 82±13 | 77±12 | 77±12 | 78±12 | 78±12 | 79±13 | 79±13 |
| Daily Sodium Intake (milligrams) | 2922±1598 | 3524±1998 | 3274±1790 | 3350±1818 | 3348±1726 | 3376±1846 | 3221±1746 |
| Lead (micrograms per deciliter) | 12.20±4.48 | 2.90±2.80 | 1.59±1.19 | 1.36±1.16 | 1.37±1.31 | 1.16± 1.10 | 1.05±0.72 |
| Lead > 10 micrograms per deciliter | 822 (60.1%) | 85 (2.5%) | 4 (0.2%) | 4 (0.2%) | 2 (0.1%) | 3 (0.2%) | --- |
| PP (millimeters mercury) | 39.3±11.6 | 46.5±12.0 | 45.7±12.5 | 46.7±13.2 | 48.9±12.9 | 49.6±12.9 | 48.7±12.5 |
| MAP (millimeters mercury) | 82.3±10.1 | 73.4±8.9 | 77.3±8.1 | 75.7±8.0 | 74.9±7.8 | 75.3±8.2 | 74.4±8.3 |
| Systolic BP (millimeters mercury) | 108.6±13.7 | 104.4±10.3 | 107.8±10.3 | 106.8±10.3 | 107.5±9.8 | 108.4±10.2 | 106.8±10.1 |
| Diastolic BP (millimeters mercury) | 69.2±10.2 | 57.9±10.7 | 62.1±9.9 | 60.1±10.0 | 58.6±10.0 | 58.8±10.1 | 58.1±10.2 |
| Elevated BP | 82 (6.0%) | 38 (1.1%) | 24 (1.2%) | 23 (1.1%) | 14 (0.8%) | 30 (1.7%) | 21 (1.8%) |
All measurements given as Mean ± standard deviation or count and proportion of total as appropriate. Abbreviations: N-sample population number; SD standard deviation; cm- centimeter; kg- kilogram; m2-meter squared; BMI-body mass index; min-1- per minute; mcg- micrograms; dL- deciliter; PP- pulse pressure; MAP- mean arterial pressure; BP- blood pressure
Figure 1:
Temporal trends in MAP (open circle), and population mean lead concentration (closed squares) in children. Left y-axis is pressure in millimeters mercury(mmHg) while right y-axis is the population mean blood lead concentration in micrograms per deciliter(mcg/dL). Values are plotted against the midpoint of each NHANES along the X axis.
Figure 2:
Temporal trends in pooled sex PP (open triangle) and proportion of children obese (closed circles). Left y-axis is pressure in millimeters mercury(mmHg) while right y-axis is the proportion obese (%).Values are plotted against the midpoint of each NHANES along the X axis
In individual participant survey regression analyses examining yearly trends during the rapid rise of obesity in American children, age-sex adjusted SBP showed no temporal trend (Table 2). After multiple covariate adjustment including BMI and sodium intake, a significant decreasing trend over time was noted. When blood lead concentration was added to the model, the temporal trend reversed to a marginally significant increasing trend. Consistent with the temporal trend results, lead was noted to be strongly associated with higher SBP (regression coefficient 0.36 [95%CI 0.23,0.50] mmHg per mcg/dL of lead, p<0.001).
Table 2:
Multivariable Adjusted Survey Regression results
| Model | Pooled Sex Regression Coefficient (95% CI) | p-value | Model R square | Boys Regression Coefficient (95% CI) | p-value | Girls Regression Coefficient (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| SBP | |||||||
| Model 1 | −0.01 (−0.06,0.04) | 0.78 | 0.15 | −0.02 (−0.07,0.03) | 0.45 | 0.01 (−0.05,0.07) | 0.8 |
| Model 2 | −0.06 (−0.10,−0.01) | 0.02 | 0.27 | −0.08 (−0.13,−0.03) | 0.003 | −0.03 (−0.09,0.03) | 0.27 |
| Model 3 | 0.06 (0,0.12) | 0.05 | 0.28 | 0.05 (−0.02,0.11) | 0.14 | 0.07 (0.0, 0.13) | 0.04 |
| DBP | |||||||
| Model 1 | −0.28 (−0.32,−0.24) | <0.001 | 0.12 | −0.35 (−0.40,−0.31) | <0.001 | −0.21 (−0.26,−0.15) | <0.001 |
| Model 2 | −0.28 (−0.32,−0.24) | <0.001 | 0.14 | −0.36 (−0.40,−0.31) | <0.001 | −0.20 (−0.25,−0.15) | <0.001 |
| Model 3 | −0.13 (−0.19,−0.07) | <0.001 | 0.15 | −0.20 (−0.27,−0.14) | <0.001 | −0.08 (−0.15,−0.01) | 0.02 |
| PP | |||||||
| Model 1 | 0.28 (0.23, 0.32) | <0.001 | 0.07 | 0.33 ( 0.28, 0.38) | <0.001 | 0.22 ( 0.16, 0.27) | <0.001 |
| Model 2 | 0.12 (0.07, 0.17) | <0.001 | 0.23 | 0.14 ( 0.09, 0.20) | <0.001 | 0.09 ( 0.03, 0.15) | 0.002 |
| Model 3 | 0.16 (0.09, 0.22) | <0.001 | 0.23 | 0.19 ( 0.12, 0.26) | <0.001 | 0.13 ( 0.06, 0.20) | <0.001 |
| MAP | |||||||
| Model 1 | −0.19 (−0.23,−0.15) | <0.001 | 0.15 | −0.24 (−0.28,−0.20) | <0.001 | −0.14 (−0.19,−0.09) | <0.001 |
| Model 2 | −0.21 (−0.24,−0.17) | <0.001 | 0.20 | −0.26 (−0.30,−0.22) | <0.001 | −0.14 (−0.19,−0.09) | <0.001 |
| Model 3 | −0.07 (−0.12,−0.02) | 0.01 | 0.22 | −0.12 (−0.18,−0.06) | <0.001 | −0.03 (−0.09, 0.03) | 0.31 |
Regression coefficient indicates mmHg change in SBP, DBP, PP, or MAP per year. Model R square indicates the proportion of variance accounted for in the outcome by included variables at each step.
Model 1 includes variables Age, Sex, and Yearly trend.
Model 2 includes Model 1 variables and race/ethnicity, height, heart rate, continuous BMI- Z-Score, daily sodium intake (and MAP for PP model only).
Model 3 includes Model 2 variables and blood lead concentration as a continuous variable.
Abbreviations: 95%CI- 95% confidence intervals; SBP- systolic blood pressure; DBP- diastolic blood pressure; PP- pulse pressure; MAP- mean arterial pressure; BMI- body mass index
For DBP, age-sex models revealed a declining trend over time. Multiple covariate adjustment had minimal effect on the trend. Addition of lead to the model attenuated the temporal trend by 54%. Again consistent with the temporal trend, lead was strongly related to DBP (0.47 [0.33,0.61],p<0.001).
Turning to large artery measures, age-sex adjusted PP showed a significant increase in PP over time which attenuated 57% with covariate adjustment including obesity and sodium. The addition of lead to the PP model modestly increased the temporal trend. Blood lead concentration was marginally related to PP overall (regression coefficient 0.13mmHg per mcg/dL [95% CI −0.01, 0.26]; p=0.07).
For small artery measures, MAP age-sex adjusted models showed a marked decline in MAP over time. Covariate adjustment had minimal effect of the temporal trend. The addition of lead concentration to the regression models markedly attenuated the temporal trend in MAP by 67%. Blood lead concentration was again strongly associated with MAP (0.43mmHg per mcg/dL [95%CI 0.31,0.55];p<0.001).
As to effect modification, boys appeared to have a steeper decline than girls only in DBP and MAP (each p<0.001). Race did not modify the BP trends and the effect of lead on any BP trend did not vary by sex (data not shown).
DISCUSSION
Our analyses showed that during the rise of pediatric obesity, SBP was flat due to its components PP increasing while MAP declined. Lead was significantly associated with higher SBP, DBP, MAP but not PP. Declining lead exposure would be inferred to relate to declining SBP, DBP, and MAP. Adjusting for secular trends in BMI and sodium intake indeed uncovered a decline in SBP. Addition of lead to the model abolished the decline in SBP. Decomposing SBP into PP and MAP suggested changes in obesity, sodium and other secular trends raised PP but may have been counteracted by the effects declining lead exposure on MAP, thus resulting in a flat SBP. Since the effect of a given degree of lead on BP measures did not vary by sex, the observed sex differences in DBP and MAP is likely due to lead concentrations varying by sex.11
Our results suggest a key role for the decline in MAP which was markedly attenuated after adjustment for lead. Less lead exposure in the population may have a key role in the declining MAP and population SBP. Higher lead levels are associated with higher BP on meta-analysis of epidemiological cohort studies.17 Biological plausibility of this association is supported by mechanistic studies.18 Implicated mechanisms include effects on regulatory molecules, direct interaction with ion-channel effects, and tissue level effect. Lead facilitates reactive oxygen species production which in turn lead to oxidative stress on vital tissues such as endothelial cells and vascular smooth muscle cells which predominate in resistance small arteries.19 Other work suggests the oxidative stress may have effects on nitric oxide-dependent vascular health.20 Oxidative stress promotes inflammation, fibrosis, and apoptosis in small artery tissue types.18 In addition lead appears to activate the adrenergic system, which can change the arterial tone and thus increase MAP.21 Lead may also activate endothelin which can also have vasoconstrictive effects.22 Alterations in the renin-angiotensin-aldosterone system are also implicated in lead-induced vascular change.23 Lead appears to have direct effects on vascular tone likely through interaction with calcium ions.24 Interestingly, animal data found that ion related effects varied by artery type such that vascular tone in aortic tissue is not affected by lead, consistent with our finding of marginal association to PP.25 Finally lead may interfere with normal vascular cell repair mechanisms and angiogenesis thereby interfering with mechanisms that maintain and increase the cross sectional area of small arteries ready to receive pulsatile flow.26 Therefore the association between decreasing lead concentrations and declining MAP is biologically plausible.
Less straightforward are the PP related trends. Major determinants of PP are known to include flow volume, arterial diameter, and aortic tissue stiffness.27 Existing adult studies are conflicting wherein some find that lead levels were not related to vascular parameters while other suggest a relation to arterial stiffness.28, 29 These contrasting findings may be the result of differing exposure profiles, baseline characteristics, or vascular testing methods. Large artery relations are different in children compared to adults.1 In children, PP modestly declines with age for girls while in boys PP is higher with age.1 During childhood, somatic growth entails increases in blood volume and thereby pulsatile flow.2 In turn, the aortic diameter remodels to accommodate.27 A larger diameter reduces pulsatile impedance to flow but also increases pulsatile tension on the artery wall.1 Higher wall tension can induce increased wall stiffness via extracellular matrix remodeling specifically as collagen deposition in support of fragmented elastin fibers.27 Thus, aortic accommodation may transfer load from fragmented elastin to intact, stiffer collagen and thereby increase arterial stiffness. Before adulthood, maximum aortic size endowment is achieved and any further remodeling relies on this matrix remodeling to counteracting wall tension.27 Somatic growth can therefore be a key difference between observations on vascular properties in adults versus children. On the whole, the present data suggests lead either does not affect the key drivers of arterial size, stiffness, nor volume flow or that lead has divergent and offsetting effects on these drivers.
Among the study limitations, NHANES datasets do not permit causal inferences due to the serial cross sectional nature of the exams. NHANES are uniquely suited to describe temporal trends in US children as they are a nationally representative sample. While population means are presented as summary variables, it is critical to note the regression analyses presented are individual-level survey regression data whereby we avoid the so-called ecological fallacy. Unmeasured confounders can be a concern with observational data. We have previously considered measurement issues like the NHANES cycle-specific technical artifacts, end-digit preferences, and the effects of smoking or obesity.1 The only relevant issue has been obesity which was included in this analysis. Lead was measured in two different ways during this observation period, but the two methods are in virtual identity. We cannot account for the total body lead exposure since blood lead represents only a part of the total body lead content.18 Given this misclassification should bias to the null, our findings appear robust to this measurement concern. One study identified association between higher lead and lower BMI in adults and children.30 We adjusted for obesity and sodium, but such an adjustment could be inappropriate if obesity is actually along the causal chain.
In conclusion, contrasting trends have been noted in populations around the globe wherein obesity has increased but BP has stayed flat or declined. Children offer important insights because they are largely untouched with antihypertensive medicine. In our work from the nationally representative NHANES during the rapid rise of pediatric obesity, SBP was flat, due to PP rising while MAP fell. Expected relations were found between obesity and sodium with BP measures. But blood lead concentrations accounted for the bulk of declining MAP or DBP trends. These results suggest small arteries are the location of the countervailing trend and may be the consequence of lower lead exposure. Environmental toxin mitigation policies may have BP consequences at the population level and should be accounted for in epidemiological investigations.
Acknowledgements:
This work was supported by NHLBI HL111335 (JPZ).
Footnotes
DISCLOSURES: We declare we have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zachariah JP, Graham DA, de Ferranti SD, Vasan RS, Newburger JW, Mitchell GF. Temporal trends in pulse pressure and mean arterial pressure during the rise of pediatric obesity in us children. Journal of the American Heart Association 2014;3:e000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, Frohlich ED. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med 1981;141:81–85 [DOI] [PubMed] [Google Scholar]
- 3.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: Systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 2011;377:568–577 [DOI] [PubMed] [Google Scholar]
- 4.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiolero A, Paradis G, Madeleine G, Hanley JA, Paccaud F, Bovet P. Discordant secular trends in elevated blood pressure and obesity in children and adolescents in a rapidly developing country. Circulation 2009;119:558–565 [DOI] [PubMed] [Google Scholar]
- 6.Khang YH, Lynch JW. Exploring determinants of secular decreases in childhood blood pressure and hypertension. Circulation 2011;124:397–405 [DOI] [PubMed] [Google Scholar]
- 7.Hopstock LA, Bonaa KH, Eggen AE, Grimsgaard S, Jacobsen BK, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T. Longitudinal and secular trends in blood pressure among women and men in birth cohorts born between 1905 and 1977: The tromso study 1979 to 2008. Hypertension 2015;66:496–501 [DOI] [PubMed] [Google Scholar]
- 8.Dobson CP, Eide M, Nylund CM. Hypertension prevalence, cardiac complications, and antihypertensive medication use in children. J Pediatr 2015;167:92–97 e91 [DOI] [PubMed] [Google Scholar]
- 9.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: The framingham heart study. Circulation 2009;119:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundstrom J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: Cohort study of swedish male conscripts. BMJ 2011;342:d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the united states. The national health and nutrition examination surveys (nhanes). JAMA 1994;272:284–291 [PubMed] [Google Scholar]
- 12.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in us adults: A population-based cohort study. The Lancet Public health 2018 [DOI] [PubMed] [Google Scholar]
- 13.Hara A, Thijs L, Asayama K, Gu YM, Jacobs L, Zhang ZY, Liu YP, Nawrot TS, Staessen JA. Blood pressure in relation to environmental lead exposure in the national health and nutrition examination survey 2003 to 2010. Hypertension 2015;65:62–69 [DOI] [PubMed] [Google Scholar]
- 14.Statistics CfDC-NCFH. Analytic and reporting guidelines: The national health and nutrition examination survey (nhanes) National Center for Health Statistics; 2011 [Google Scholar]
- 15.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for disease control and prevention 2000 growth charts for the united states: Improvements to the 1977 national center for health statistics version. Pediatrics 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 16.White MA. A comparison of inductively coupled plasma mass spectrometry with electrothermal atomic absorption spectrophotometry for the determination of trace elements in blood and urine from non occupationally exposed populations. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements 1999;13:93–101 [DOI] [PubMed] [Google Scholar]
- 17.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. J Hum Hypertens 2002;16:123–131 [DOI] [PubMed] [Google Scholar]
- 18.Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. American journal of physiology. Heart and circulatory physiology 2008;295:H454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni Z, Hou S, Barton CH, Vaziri ND. Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney international 2004;66:2329–2336 [DOI] [PubMed] [Google Scholar]
- 20.Ding Y, Vaziri ND, Gonick HC. Lead-induced hypertension. Ii. Response to sequential infusions of l-arginine, superoxide dismutase, and nitroprusside. Environmental research 1998;76:107–113 [DOI] [PubMed] [Google Scholar]
- 21.Tsao DA, Yu HS, Cheng JT, Ho CK, Chang HR. The change of beta-adrenergic system in lead-induced hypertension. Toxicology and applied pharmacology 2000;164:127–133 [DOI] [PubMed] [Google Scholar]
- 22.Molero L, Carrasco C, Marques M, Vaziri ND, Mateos-Caceres PJ, Casado S, Macaya C, Barrientos A, Lopez-Farre AJ. Involvement of endothelium and endothelin-1 in lead-induced smooth muscle cell dysfunction in rats. Kidney international 2006;69:685–690 [DOI] [PubMed] [Google Scholar]
- 23.Vander AJ. Chronic effects of lead on the renin-angiotensin system. Environmental health perspectives 1988;78:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valencia I, Castillo EE, Chamorro G, Bobadilla RA, Castillo C. Lead induces endothelium- and ca2+-independent contraction in rat aortic rings. Pharmacology & toxicology 2001;89:177–182 [DOI] [PubMed] [Google Scholar]
- 25.Shelkovnikov SA, Gonick HC. Influence of lead on rat thoracic aorta contraction and relaxation. Am J Hypertens 2001;14:873–878 [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara Y, Watanabe S, Sakamoto M, Kaji T. Repair of wounded monolayers of cultured vascular endothelial cells after simultaneous exposure to lead and zinc. Toxicology letters 1998;94:181–188 [DOI] [PubMed] [Google Scholar]
- 27.Ben Driss A, Benessiano J, Poitevin P, Levy BI, Michel JB. Arterial expansive remodeling induced by high flow rates. Am J Physiol 1997;272:H851–858 [DOI] [PubMed] [Google Scholar]
- 28.Karakulak UN, Yilmaz OH, Tutkun E, Ates I, Bal C, Gunduzoz M. Evaluation of the ambulatory arterial stiffness index in lead-exposed workers. Anatolian journal of cardiology 2017;18:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozturk MT, Yavuz B, Ozkan S, Ayturk M, Akkan T, Ozkan E, Tutkun E, Yilmaz OH. Lead exposure is related to impairment of aortic elasticity parameters. Journal of clinical hypertension 2014;16:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scinicariello F, Buser MC, Mevissen M, Portier CJ. Blood lead level association with lower body weight in nhanes 1999–2006. Toxicology and applied pharmacology 2013;273:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]