Abstract
Background:
Although pharmacotherapies are available for alcohol (EtOH) or tobacco use disorders individually, it may be possible to develop a single pharmacotherapy to treat heavy drinking tobacco smokers by capitalizing on the commonalities in their mechanisms of action.
Methods:
Female alcohol-preferring (P) rats were trained for EtOH drinking and nicotine self-administration in two phases: (1) EtOH alone (0 vs. 15% EtOH, 2-bottle choice) and (2) concomitant access, during which EtOH access continued with access to nicotine (0.03 mg/kg/infusion, i.v.) using a 2-lever choice procedure (active vs. inactive lever) in which the fixed ratio (FR) requirement was gradually increased to FR30. When stable co-use was obtained, rats were pretreated with varying doses of naltrexone, varenicline, or r-bPiDI, an α6β2* subtype-selective nicotinic acetylcholine receptor antagonist shown previously to reduce nicotine self-administration.
Results:
While EtOH intake was initially suppressed in phase 2 (co-use), pharmacologically relevant intake for both substances was achieved by raising the “price” of nicotine to FR30. In phase 2, naltrexone decreased EtOH and water consumption but not nicotine intake; in contrast, naltrexone in phase 1 (EtOH only) did not significantly alter EtOH intake. Varenicline and r-bPiDI in phase 2 both decreased nicotine self-administration and inactive lever pressing, but neither altered EtOH or water consumption.
Conclusions:
These results indicate that increasing the “price” of nicotine increases EtOH intake during co-use. Additionally, the efficacy of naltrexone, varenicline, and r-bPiDI was specific to either EtOH or nicotine, with no efficacy for co-use. Nevertheless, future studies on combining these treatments may reveal synergistic efficacy.
Keywords: Alcohol, Nicotine, Co-Use, Varenicline, Naltrexone, r-bPiDI
1. Introduction
Approximately 14% of the U.S. population meets criteria for alcohol use disorder (AUD) (Grant et al., 2015), and approximately 70% of these individuals meet criteria for tobacco use disorder (TUD) (Falk et al., 2006), making this combination (AUD-TUD polysubstance abuse) highly prevalent in the United States and worldwide (for review see Van Skike et al., 2016). Although pharmacotherapies are available for AUD or TUD individually, it may be possible to develop a single pharmacotherapeutic agent to treat heavy drinking tobacco smokers through targeting common mechanisms mediating AUDs and TUDs (Roche et al., 2016).
A recent study from our laboratory (Maggio et al., 2018) used alcohol-preferring (P) rats, a translational genetic model of AUD (Bell et al., 2012; McBride et al., 2014), to develop a novel model of EtOH and nicotine co-use. That study used a two-bottle choice (EtOH vs water) procedure combined with a two-lever operant (active vs inactive for i.v. nicotine on an FR5 operant schedule) procedure. Under those co-use conditions, we determined the effects of two potential pharmacotherapies that target nicotinic acetylcholine receptors (nAChRs), i.e., varenicline and r-bPiDI. Varenicline is a clinically available partial agonist with high affinity for α4β2* nAChRs that reduces nicotine self-administration in rats (George et al., 2011; Rollema et al., 2007) and increases smoking abstinence in humans (Ebbert et al., 2016; Nides et al., 2006). However, the effects of varenicline on EtOH consumption have been mixed in laboratory animals (Hauser et al., 2017; Steensland et al., 2007) and humans (de Bejczy et al., 2015; Plebani et al., 2013; Schacht et al., 2014; Verplaetse et al., 2016). r-bPiDI is the reduced form of the potent and selective quaternary ammonium antagonist for α6β2* nAChRs, N,N’-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI), a compound that decreases both EtOH consumption (Srisontiyakul et al., 2016) and nicotine self-administration (Wooters et al., 2011). It has physiochemical properties which confer greater brain penetration than bPiDI and also reduce nicotine self-administration (Beckmann et al., 2015). However, one limitation of the study by Maggio et al. (2018) was that while the FR5 schedule maintained high levels of nicotine intake (>20 infusions of 0.03 mg/kg/infusion in 60 min), co-use of EtOH was relatively low (~0.5 g/kg in 60 min). Thus, the ability of varenicline and r-bPiDI to selectively decrease nicotine intake may have been due to low consumption of EtOH in the co-use phase (i.e., floor effect) or the absence/limitations of neuroadaptations associated with chronic EtOH.
To mitigate this problem, during the co-use phase, the current study increased the “price” of nicotine by gradually increasing the FR requirement from an FR5 to FR30. We hypothesized that this change would increase EtOH consumption without markedly decreasing nicotine intake, thus allowing assessment of the effects of varenicline and r-bPiDI in our co-use model when intake of both substances is pharmacologically relevant. In addition to assessing the effects of varenicline and r-bPiDI, the current study also determined the effect of naltrexone on EtOH and nicotine co-use. Naltrexone is a clinically available mu-opioid receptor antagonist used to treat AUD (Heilig and Egli, 2006), but it has also been examined as a treatment for TUD and co-use of EtOH and nicotine. Naltrexone has been demonstrated in preclinical studies to reduce EtOH intake in rats (Dhaher et al., 2012; Williams and Broadbridge, 2009) with a higher efficacy in rats exposed to both EtOH and nicotine (Lê et al., 2014). Additionally, treatment with naltrexone reduces EtOH use in heavy drinking smokers but not in non-smokers (Fridberg et al., 2014; Fucito et al., 2012).
2. Methods
2.1. Animals
Female P rats (n=14, selectively bred generations 79-81) were obtained from Indiana University School of Medicine (provided by NIAAA/NIH) and began training between PND 50-60. Females were used because they voluntarily drink more EtOH compared to male P rats (Bell et al., 2011). Rats were housed individually in a temperature-controlled colony room under a 12:12 hr. light/dark cycle. All testing procedures occurred during the light phase (7:00 am – 7:00 pm), were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011), and were approved by the IACUC at the University of Kentucky.
2.2. Drugs
EtOH was prepared in a concentration of 15% v/v 190 proof EtOH (Pharmco-AAPER, Shelbyville, KY) and diluted in distilled water. Nicotine hydrogen tartrate (Sigma-Aldrich, San Diego, CA) was dissolved in a 0.9% NaCl (saline) solution, to which NaOH was added to obtain a pH of 7.0 ± 0.05; nicotine dosage was based on freebase weight. Naltrexone (5α)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one) and varenicline (6,7,8,9-tetrahydro-6,10-methano-6H pyrazino[2,3-h][3]benzazepine tartrate), supplied by the National Institute on Drug Abuse (NIDA, Bethesda, MD), were dissolved in saline. r-bPiDI (1,10-bis(3-methyl-5,6-dihydropyridin-1 (2H)-yl)decane) was synthesized at the University of Arkansas for Medical Sciences (Little Rock, AK) and dissolved in saline. All test drug solutions were prepared fresh daily and administered s.c. 15 min prior to the start of the session with doses based on formula weights. For surgery, rats were anesthetized via i.p. injections of 55/7.5/7.5 mg/kg ketamine (Henry Schein Animal Health, Dublin, OH)/xylazine (LLOYD Laboratories, Shenandoah, IA)/sterile water.
2.3. Apparatus
All training and testing sessions were conducted in standard two-lever operant conditioning chambers (ENV-001; MED Associates, St. Albans VT). Two response levers were located on either side of a recessed food tray. Located above each lever was a white cue light. Nicotine infusions were delivered by a syringe pump, and food pellets were delivered by a pellet dispenser. A computer, linked to a MED Associates interface, recorded responses and controlled infusions during sessions. Each chamber was modified to allow access to two 100 mL Richter feeding tube glass bottles (Model 900010; Dyets, Inc., Bethlehem PA) on the wall of the chamber opposite the levers. The design of the bottles allowed them to be fixed to the chambers with lipped feeding tube holders (Model 901100; Dyets, Inc., Bethlehem, PA) such that only the drinking spout could be accessed by rats while inside the chambers.
2.4. Procedures
Pre-training and EtOH access (Phase 1) were conducted using procedures similar to those described by Maggio et al. (2018). Briefly, during pre-training, to allow for acclimation to the taste and smell of EtOH, rats were given one bottle of 20% EtOH as the sole source of liquid for 72 consecutive hours in the home cage (Simms et al., 2010); food was available ad libitum. Following pre-training, rats were trained during daily 60-min sessions in which rats were given free-choice access to two bottles in the operant chamber; one bottle contained water and the other 15% EtOH (v/v), counterbalanced for side daily. Animals were trained in this phase for at least 15 days until the average EtOH consumption stabilized, i.e., there were no significant differences in average consumption across 5 consecutive sessions (Mean = 20 days). After stable EtOH drinking was achieved, one group of animals (n=6) was pretreated with naltrexone (Experiment 1); results evaluating varenicline and r-bPiDI were reported previously (Maggio et al., 2018). A second group of animals (n=8) advanced to training for Experiment 2 (concurrent access, Phase 2) without drug pretreatment during Phase 1. In Experiment 2, naltrexone, varenicline, and r-bPiDI were each tested separately using a within-subject design.
During the concurrent access phase (Phase 2; Experiment 2), animals were first trained to acquire lever pressing for palatable food pellets (45 mg Dustless Precision Pellets, Bio-Serv, Frenchtown NJ) using the general methods described previously (Maggio et al., 2018) with some modifications. Rats were trained to lever press for food pellets using a standard 2-lever operant procedure (active vs inactive levers) with 2-bottle choice for EtOH (0% vs 15%) concurrently available during sessions. Rats then underwent surgery under anesthesia to implant a chronic indwelling catheter into the jugular vein, followed by 5-7 days of recovery with ad libitum access to food, water, and one bottle of 15% EtOH in the home cage.
Following the recovery period, rats were trained to self-administer nicotine (0.03 mg/kg/infusion, with a 20-sec time-out period following each infusion) using a 2-lever procedure, with both 15% EtOH and water access restricted to daily operant sessions. The FR requirement for nicotine was increased incrementally and maintained for 3 consecutive sessions before the FR value was increased. The FR progression was 1, 3, 5, 8, 12, 20, and 30. Each rat underwent at least 5 consecutive training sessions at FR30, during which there were no significant differences in average EtOH, water, or nicotine intake across 5 sessions of the experiment (Mean = 5 days).
2.5. Drug pretreatments
After operant responding and EtOH drinking stabilized in Phase 1 (Experiment 1) or Phase 2 (Experiment 2), pretreatments were given 15 min prior to test sessions. For naltrexone, test doses were 0.15, 0.3, or 0.6 mg/kg; for varenicline, test doses were 1.5 or 3 mg/kg; for r-bPiDI, test doses were 10, 20, or 40 mg/kg. Doses were selected based on previous literature (e.g., Beckmann et al., 2015; George et al., 2011; Williams and Broadbridge, 2009). Each animal in Experiment 2 received each drug and each dose in counterbalanced order, including the appropriate vehicle control. A minimum of 2 maintenance sessions (no pretreatment) separated each pretreatment test session.
2.6. Data analysis
All statistical analyses were conducted using Prism 5.0 software (Graph Pad Software Inc., San Diego, CA, USA). Consumption from EtOH and water bottles was measured in g/kg body weight, and the numbers of active lever presses for nicotine and inactive lever presses were recorded automatically. Consumption differences in EtOH and water across sessions were analyzed by one-way repeated measure ANOVA during Phase 1 and at each FR value during Phase 2. Active and inactive lever presses for Phase 2 were also evaluated by one-way repeated measures ANOVA. Effects of naltrexone, varenicline, and r-bPiDI on EtOH and water consumption and on lever presses (active for nicotine vs inactive) earned during concurrent access sessions were analyzed by one-way, repeated-measure ANOVA. A priori multiple comparison analyses using Dunnett’s 2-tailed t-test comparing each dose to the vehicle control (α = 0.05) were conducted when appropriate.
3. Results
3.1. Baseline EtOH and nicotine intake in phase 1 (experiment 1) and phase 2 (experiment 2)
Figure 1 shows differences in EtOH consumption in Phase 1 in Experiment 1 (session 20) as well as EtOH and nicotine intake during Phase 2 in Experiment 2 under the FR5 and FR30 schedules (session 3 of each FR requirement) of nicotine self-administration. For Experiment 1, EtOH intake resulted in pharmacologically relevant levels (2 g/kg/hr). Results revealed a significant decrease in EtOH consumption in Phase 2 (concurrent access) compared to Phase 1 (EtOH access only), F(2, 17) = 30.07, p < 0.05. Posttests revealed that EtOH consumption was significantly lower in Phase 2 compared to Phase 1 during both FR5 and FR30 response requirements for nicotine. However, for Experiment 2, analyses revealed a significant increase in EtOH consumption at FR30 for nicotine compared to FR5, t(5) = 2.74, p < 0.05 (Figure 1A). Additionally, EtOH consumption at FR30 for nicotine reached pharmacologically relevant levels (~0.80 g/kg/hr). Analyses for nicotine revealed no significant difference in nicotine intake at FR30 compared to FR5, and the amount infused was pharmacologically relevant (~0.33 mg/kg in the 1 hr session; Figure 1B). Increases in the FR requirement for nicotine had no significant effect on water consumption or inactive lever pressing (data not shown).
Figure 1.
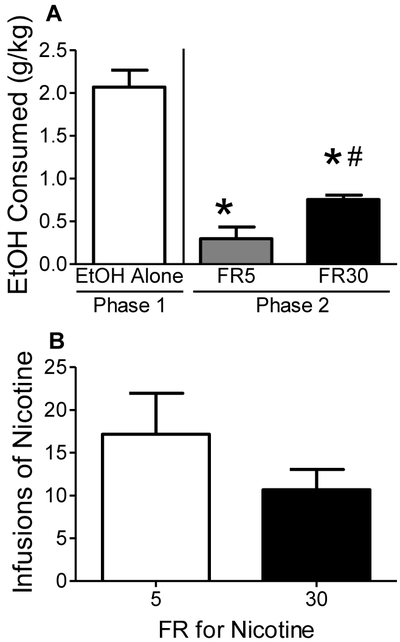
Baseline EtOH intake and nicotine self-administration in Phase 1 (EtOH alone) and Phase 2 (co-use) of Experiments 1 and 2. Panel A: EtOH consumed in Phase 1 (Experiment 1) and Phase 2 under either FR5 or FR30 schedule of nicotine self-administration (Experiment 2). Panel B: Nicotine infusions earned in Phase 2 under either FR5 or FR30 schedules of nicotine self-administration (Experiment 2). Values represent mean ± SEM. *p < 0.05 vs Phase 1, #p < 0.05 vs FR5.
3.2. Effects of naltrexone in phase 1 (experiment 1) and phase 2 (experiment 2)
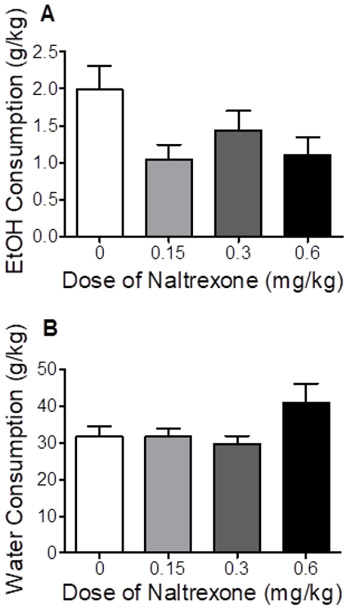
As shown in Figure 2, in Phase 1 (EtOH access), analyses revealed that naltrexone had no significant effect on EtOH or water consumption.
Figure 2.
Effect of naltrexone on EtOH consumed (Panel A) and water consumed (Panel B) in Phase 1 of Experiment 1 (EtOH alone). Values represent mean±SEM.
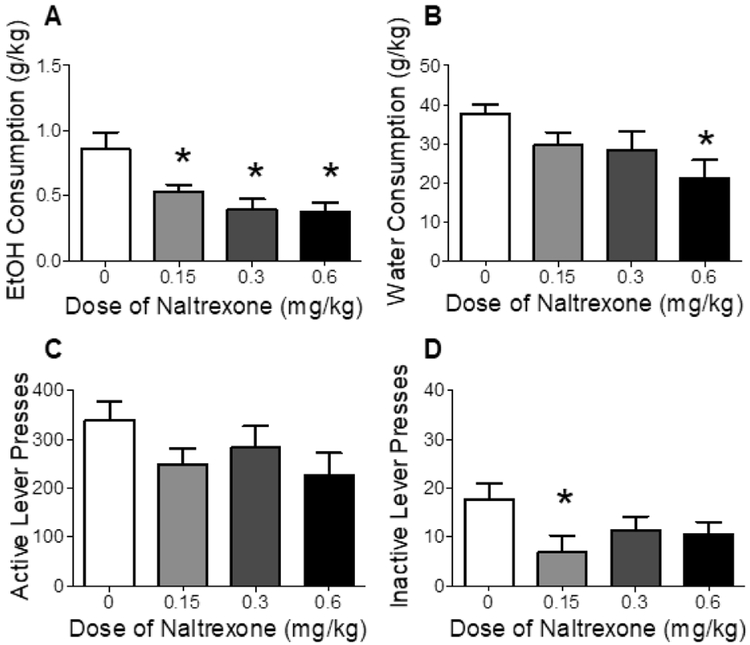
As shown in Figure 3, in Phase 2, there was a significant decrease in EtOH consumption following naltrexone pretreatment, F(3, 18) = 1.57, p < 0.05 (Figure 3A). Posttests revealed that EtOH consumption was decreased at all doses of naltrexone vs vehicle. Analyses also showed a decrease in water consumption following naltrexone pretreatment, F(3, 18) = 5.65, p < 0.05 (Figure 3B), with posttests showing that water consumption was decreased only at the highest dose (0.6 mg/kg vs vehicle). Naltrexone treatment had no effect on active lever presses for nicotine (Figure 3C) but significantly decreased inactive lever pressing, F(3, 18) = 3.38, p < 0.05 (Figure 3D). Posttests revealed that inactive lever pressing was decreased only at the lowest dose (0.15 mg/kg vs vehicle).
Figure 3.
Effect of naltrexone on EtOH consumption (Panel A), water consumption (Panel B), number of active lever presses for nicotine (Panel C), and number of inactive lever presses (Panel D) in Phase 2 of Experiment 2 (co-use). Values represent mean ± SEM. *p < 0.05 vs vehicle (0).
3.3. Effects of varenicline and r-bPiDI pretreatments in phase 2 (experiment 2)
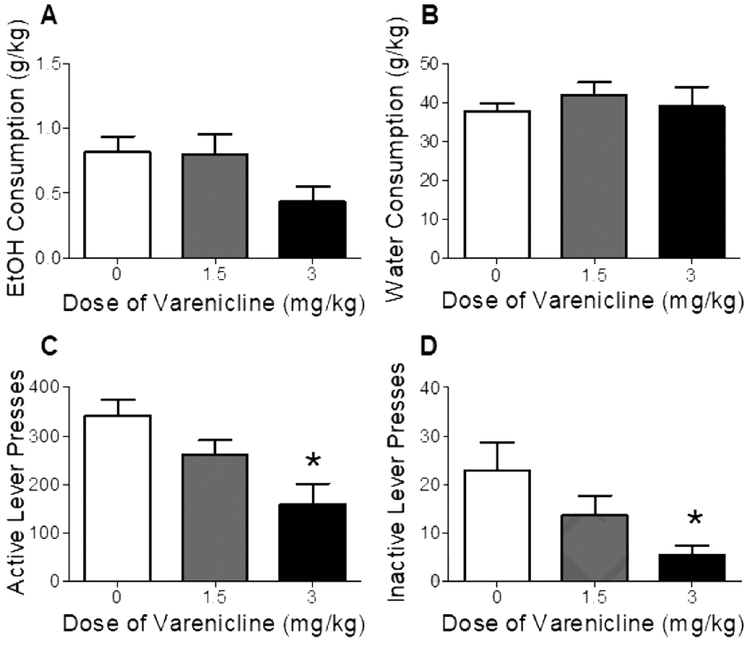
Figure 4 shows EtOH and water consumption as well as active and inactive lever presses after varenicline pretreatment. Analyses revealed that varenicline had no significant effect on EtOH or water consumption (Figs 4A and 4B). However, active and inactive lever responding was significantly decreased by varenicline treatment; F(2, 14) = 2.89, p < 0.01 and F(2, 14) = 3.29, p < 0.05, respectively (Figs 4C and 4D). Posttests showed that only the highest dose (3 mg/kg) decreased both active and inactive lever presses (vs vehicle).
Figure 4.
Effect of varenicline on EtOH consumption (Panel A), water consumption (Panel B), number of active lever presses for nicotine (Panel C), and number of inactive lever presses (Panel D) in Phase 2 of Experiment 2 (co-use). Values represent mean ± SEM. *p < 0.05 vs vehicle (0).
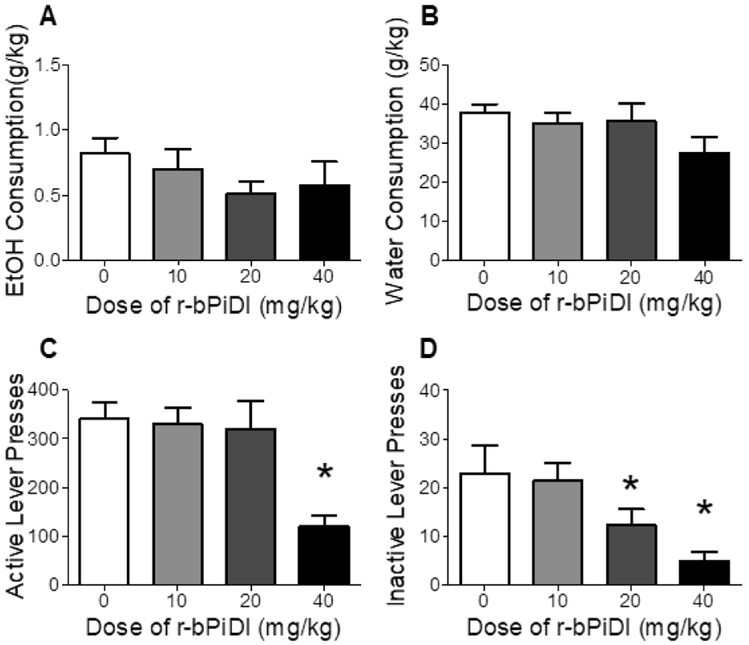
Figure 5 shows EtOH and water consumption as well as active and inactive lever presses after r-bPiDI pretreatment. Analyses revealed that r-bPiDI had no significant effect on EtOH or water consumption (Figs 5A and 5B). In contrast, the number of active and inactive lever presses were significantly decreased by r-bPiDI treatment; F(3, 21) = 3.34 p < 0.01 and F(3, 21) = 5.21, p < 0.01, respectively (Figs 5C and 5D). Posttests revealed that the highest dose of r-bPiDI (40 mg/kg) significantly decreased both active and inactive lever presses, whereas 20 mg/kg r-bPiDI decreased inactive lever presses only.
Figure 5.
Effect of r-bPiDI on EtOH consumption (Panel A), water consumption (Panel B), number of active lever presses for nicotine (Panel C), and number of inactive lever presses (Panel D) in Phase 2 of Experiment 2 (co-use). Values represent mean±SEM. *p < 0.05 vs vehicle (0).
3.4. Within-session nicotine self-administration
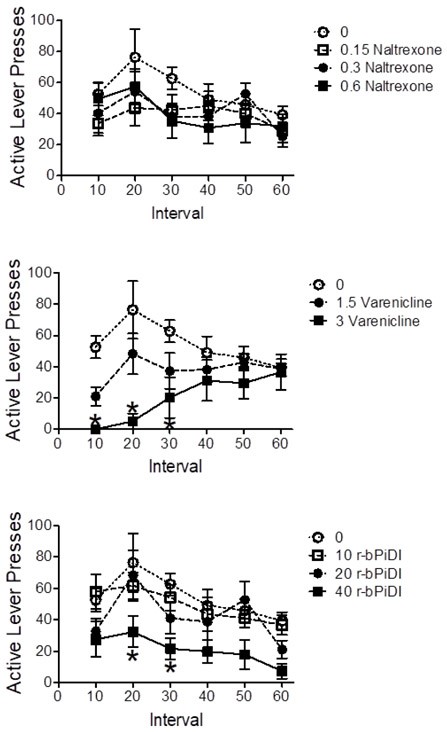
Figure 6 shows the number of active lever presses for nicotine in 10-min intervals during Phase 2. Analysis of the naltrexone data revealed a significant main effect of time interval, F(5, 20) = 4.83, p < 0.05, but no significant differences among the doses at any interval (Bonferroni - hoc test p > 0.05) (Figure 6A). Analysis of the varenicline data revealed a significant main effect of dose, F(2, 15) = 7.01, p < 0.05, and a significant dose x interval interaction, F(10, 75) = 2.82, p < 0.05. Varenicline (3.0 mg/kg) decreased responding compared to vehicle during the first three 10-min intervals; Bonferroni t(75) = 3.85, t(75) = 5.22, and t(75) = 3.11, respectively, p ’s < 0.05 (Figure 6B). Analysis of the r-bPiDI data revealed a significant main effect for interval, F(5, 20) = 28.93, p < 0.05, and for dose, F(3, 20) = 4.30, p < 0.05, with no significant dose x interval interaction. r-bPiDI (40 mg/kg) decreased responding compared to vehicle during the 20- and 30-min intervals; Bonferroni t(100) = 3.23 and t(100) = 3.01, respectively, p ’s < 0.05 (Figure 6C).
Figure 6.
Experiment 2 results showing within-session number of active lever presses for nicotine per 10-min interval following pretreatments with naltrexone (Panel A), varenicline (Panel B), and r-bPiDI (Panel C). Values represent mean ± SEM. *p < 0.05 vs vehicle (0) at same time point.
Unfortunately, the operant chambers did not provide a means to monitor cumulative EtOH consumption during the 10-min intervals across the session.
4. Discussion
The present findings show that our modified EtOH-nicotine co-use protocol resulted in pharmacologically relevant levels of concurrent EtOH intake and nicotine self-administration. When nicotine self-administration was on an FR30, EtOH consumption was ~0.80 g/kg/hr, an amount that is comparable to humans drinking ~3-4 standard alcoholic drinks/hr. (Grant and Bennett, 2003; McKee et al., 2008; Udo et al., 2013), which is defined as binge drinking (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2004). Although increasing the schedule requirement to FR30 tended to decrease nicotine self-administration, the decrease was not statistically significant. At the FR30, rats earned ~11 infusions, each containing 0.03 mg/kg of nicotine, yielding a dose of ~0.33 mg/kg nicotine in each 60-min session. This level of responding exceeds the number of infusions (i.e., 10) traditionally used as a criterion for demonstrating robust nicotine self-administration during a 60-min limited access session (Corrigall and Coen, 1989). Previous research has also shown that 10 infusions of 0.03 mg/kg i.v. nicotine produces nicotine plasma levels of ~65 ng/mL in male hooded Lister rats (Shoaib and Stolerman, 1999), well above peak plasma levels (~15-40 ng/mL) found in human chronic smokers (Feyerabend et al., 1985; Yamazaki et al., 2010). Thus, when using the selectively bred P rat line, the protocol described herein results in pharmacologically relevant intake of both EtOH and nicotine during concurrent availability, supporting previous observations that the P rat line can serve as a genetic animal model of poly-drug abuse (Bell et al., 2016).
Naltrexone was the only drug pretreatment given during EtOH access (Phase 1), as we previously reported that there are no effects of varenicline or r-bPiDI on EtOH consumption when tested in this phase (Maggio et al., 2018). The current findings indicate that while naltrexone was ineffective in reducing EtOH consumption in Phase 1, it reduced EtOH consumption during Phase 2 (co-use). The fact that subthreshold doses of naltrexone (null finding in Phase 1) resulted in significant decreases in EtOH consumption during Phase 2 provides some pharmacological validity for the co-use model described above. The present finding that doses of naltrexone have no efficacy when EtOH is given alone but can reduce EtOH intake when nicotine is available concurrently parallels similar findings in outbred Long Evans rats trained to self-administer both EtOH and nicotine in a 2-lever alternating choice test (Lê et al., 2014). The current results are also congruent with previous clinical research showing that treatment with naltrexone is more effective in heavy drinkers or alcoholics who are nicotine-dependent (Fucito et al., 2012; King et al., 2009). In contrast, other reports have shown that naltrexone can reduce EtOH consumption in rats without nicotine exposure, which may be due to differences in sex and rat line, including male Wistar (Lê et al., 1999; Steensland et al., 2007) and male Long-Evans Hooded rats (Steensland et al., 2007; Williams and Broadbridge, 2009). Methodological differences, including differences in dose, may also play a role (Henderson-Redmond and Czachowski, 2014). Importantly, in each of these latter studies, EtOH delivery was contingent on operant lever-press responding, whereas in the current experiment EtOH was freely available. The finding that naltrexone had no effect on nicotine intake is consistent with previous preclinical studies (Corrigall and Coen, 1991; Lê et al., 2014). In contrast, clinical research suggests that alcohol use promotes the increased effectiveness of naltrexone in reducing smoking (King et al., 2009). However, these latter clinical results were obtained with repeated treatments over one month which contrast with the acute pretreatments used in the current preclinical study. Thus, chronic treatment with naltrexone may yield positive results when applied to preclinical basic research.
During co-use (Phase 2), varenicline significantly reduced nicotine self-administration but not EtOH consumption. However, in contrast to previous studies showing a selective effect of varenicline on active lever pressing for nicotine alone (Maggio et al., 2018), the present results indicated that varenicline also decreased inactive lever pressing. It is possible that the high FR requirement (FR30) in the current study may have enhanced the sensitivity of the rats to nonspecific suppressant effects of varenicline. Nevertheless, the varenicline-induced decrease in nicotine intake observed here is consistent with previous preclinical results (Funk et al., 2016; Maggio et al., 2018; Scuppa et al., 2015). Although several preclinical studies have shown decreases in EtOH consumption following pretreatment with varenicline (Czachowski et al., 2018; Froehlich et al., 2017; Steensland et al., 2007), those studies only examined EtOH consumption in the absence of nicotine. Overall, preclinical evidence provides limited support for varenicline’s efficacy as a pharmacotherapeutic for co-users of EtOH and nicotine.
Also consistent with our previous findings (Maggio et al., 2018), when tested in the couse phase, r-bPiDI decreased nicotine intake but not EtOH consumption. However, similar to the effect of varenicline, there was also a nonspecific decrease in inactive lever pressing. In combination with previous investigations of the neuropharmacology of r-bPiDI (Beckmann et al., 2015), these results suggest that α6β2* nAChRs play an important role in the maintenance of nicotine intake but not necessarily EtOH intake. Furthermore, as r-bPiDI did not disrupt EtOH or water drinking in the current experiment, and previous research has shown similar doses of r-bPiDI do not disrupt operant responding for food (Beckmann et al., 2015), it is unlikely that r-bPiDI disrupted motor function or caused general sedation. While previous research shows that less selective nAChR antagonists such as mecamylamine have the potential to reduce nicotine intake in animals (DeNoble and Mele, 2006; Glick et al., 1996) and in humans (Rose, 2006; Rose et al., 1994), aversive peripheral side effects decrease their utility in clinical trials (Bevins and Caggiula, 2009; Shytle et al., 2002). Since r-bPiDI is selective for central α6β2* nAChRs, it is possible that peripheral side effects would be reduced compared to those seen with previously tested nAChR antagonists, indicating further research is needed.
One limitation of the current study is that we only used female P rats. It is well-documented that there are sex differences in consumption of EtOH and related behaviors (Erol and Karpyak, 2015; Schulte et al., 2009) as well as in nicotine use (Torchalla et al., 2011). Additionally, previous clinical research suggests that there are sex differences in the efficacy of varenicline, with efficacy being greater in females (McKee et al., 2016). However, previous research with mice shows that there are no sex differences in the effects of varenicline on EtOH drinking (Kamens et al., 2018). Clinical research with naltrexone has also indicated differences in effects for men and women, but overall efficacy appears similar for both sexes (Baros et al., 2008). Nonetheless, given the evidence for sex differences in several studies, it will be important to use both male and female subjects in future research on treatment of EtOH and nicotine co-use.
Additionally, one caveat in interpreting the naltrexone results is that naltrexone produced a non-significant decreasing trend in EtOH consumption in Phase 1, suggestive of an effect similar to what was obtained in Phase 2. While Phase 1 and 2 data were collected in separate experiments, an exploratory analysis comparing the percent change from control in each experiment revealed no significant differences in the effect of naltrexone on EtOH consumption in Phase 1 vs. Phase 2, which may be interpreted to reflect a similar sensitivity to naltrexone in both phases. However, since these groups were run at separate times and baseline rates of intake (vehicle control) were significantly different from each other, we have not included this exploratory analysis in the graphic presentation of results.
When nicotine self-administration results were examined across time within the session, naltrexone showed no effect during any 10-min time interval. In contrast, the highest doses of varenicline (3 mg/kg) and r-bPiDI (40 mg/kg) decreased active lever responding for nicotine early in the session but not later in the session. Importantly, responding for nicotine in the absence of pretreatment was higher during the early portion of the session, an effect that is sometimes referred to “loading” under limited access conditions (Williams and Broadbridge, 2009). Thus, the high rate of responding observed early in the session appears to be more sensitive to disruption by these compounds compared to lower response rates later in the session. Alternatively, it could be that the lack of effect late in the session may reflect attenuation of efficacy due to pharmacokinetics. This latter interpretation is not likely, however, as the half-life of varenicline is about 4 hours in rats (Obach et al., 2006). Thus, taken together, these results indicate that therapeutics which may be useful for treating AUD via opioid receptor antagonism and those that may be useful for smoking cessation via selective inhibition of α4β2* or α6β2* nAChRs may not be sufficient to treat EtOH and nicotine co-use. Alternatively, a combination of these compounds, titrated for effective dose ranges, may yield synergistic or additive efficacy, which will require continued research.
5. Conclusions
The procedures used in our previous co-use study in female P rats (Maggio et al., 2018) revealed that, while robust nicotine self-administration was achieved, EtOH intake was relatively low and thus hampered our ability to assess drug pretreatment effects on EtOH intake. The current study modified the procedures by increasing the FR requirement for a nicotine infusion from an FR5 to FR30. As modified, the present co-use procedures resulted in increased levels of EtOH intake with no significant diminution in nicotine intake. Since interactions between EtOH and nicotine have been postulated to arise from neural substrates common to both drugs (for review see Van Skike et al., 2016), we assessed the effects of opiate and nicotine receptor-selective drugs. Naltrexone significantly decreased EtOH intake when nicotine reinforcement was concurrently available but not when EtOH was available alone. Varenicline and r-bPiDI both reduced nicotine self-administration but not EtOH drinking in a dose-dependent manner. Thus, under the current procedures, these results suggest that none of the drugs tested are effective as a monotherapy for co-use of EtOH and nicotine.
Highlights.
Levels of EtOH and nicotine intake during co-use were pharmacologically relevant.
Increasing the fixed ratio (FR) requirement for nicotine increases EtOH intake during co-use.
Naltrexone decreased EtOH and water intake, but not nicotine intake during co-use.
Varenicline and r-bPiDI decreased active and inactive lever pressing for nicotine.
Acknowledgments
Role of the Funding Source
This work was supported by the National Institutes of Health [grant numbers UL1 TR000117, U19 DA17548, P50 DA05312, T32 DA016176, and U24AA015512].
Footnotes
Conflict of Interest
The University of Kentucky holds a patent on r-bPiDI, and a potential royalty stream to Dwoskin and Crooks may occur consistent with University of Kentucky policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baros A, Latham P, Anton R, 2008. Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: Do sex differences exist? Alcohol. Clin. Exp. Res 32, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Meyer AC, Pivavarchyk M, Horton DB, Zheng G, Smith AM, Wooters TE, McIntosh JM, Crooks PA, Bardo MT, Dwoskin LP, 2015. r-bPiDI, an alpha6beta2* nicotinic receptor antagonist, decreases nicotine-evoked dopamine release and nicotine reinforcement. Neurochem. Res 40, 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser S, Rodd ZA, Liang T, Sari Y, McClintick J, Rahman S, Engleman EA, 2016. A genetic animal model of alcoholism for screening medications to treat addiction. Int. Rev. Neurobiol 126, 179–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ, 2011. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol. Biochem. Behav 100, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L, 2012. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity. Pharmacol. Biochem. Behav 103, 119–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Caggiula AR, 2009. The motivational impact of nicotine and its role in tobacco use. Springer, New York. [Google Scholar]
- Corrigall WA, Coen KM, 1989. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99, 473–478. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, 1991. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology 104, 167–170. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Froehlich JC, DeLory M, 2018. The effects of long-term varenicline administration on ethanol and sucrose seeking and self-administration in male P rats. Alcohol. Clin. Exp. Res 42, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bejczy A, Lof E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Franck J, Isaksson A, Soderpalm B, 2015. Varenicline for treatment of alcohol dependence: A randomized, placebo-controlled trial. Alcohol. Clin. Exp. Res 39, 2189–2199. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC, 2006. Intravenous nicotine self-administration in rats: Effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology 184, 266–272. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Toalston JE, Hauser SR, Bell RL, McKinzie DL, McBride WJ, Rodd ZA, 2012. Effects of naltrexone and LY255582 on ethanol maintenance, seeking, and relapse responding by alcohol-preferring (P) rats. Alcohol 46, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Hurt RT, Schroeder DR, Hays JT, 2016. Varenicline for smoking cessation in light smokers. Nicotine Tob. Res 18, 2031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM, 2015. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 156, 1–13. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Alcohol Res. Health 29, 162–171. [PMC free article] [PubMed] [Google Scholar]
- Feyerabend C, Ings RM, Russel MA, 1985. Nicotine pharmacokinetics and its application to intake from smoking. Br. J. Clin. Pharmacol 19, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Cao D, Grant JE, King AC, 2014. Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcohol. Clin. Exp. Res 38, 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Nicholson ER, Dilley JE, Filosa NJ, Rademacher LC, Smith TN, 2017. Varenicline reduces alcohol intake during repeated cycles of alcohol reaccess following deprivation in alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res 41, 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O’Malley SS, 2012. Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol. Psychiatry 72, 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Lo S, Coen K, Le AD, 2016. Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse. Behav. Brain. Res 296, 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF, 2011. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology 213, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S, Visker K, Maisonneuve I, 1996. An oral self-administration model of nicotine preference in rats: Effects of mecamylamine. Psychopharmacology 128, 426–431. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ, 2003. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol. Ther 100, 235–255. [DOI] [PubMed] [Google Scholar]
- Hauser S, Waeiss R, Knight C, Pratt L, Bell R, McBride W, Rodd Z, 2017. Varenicline reduces nicotine self-administration, naltrexone inhibits alcohol consumption, but both fail to alter concurrent ethanol and nicotine self-administration. Neuropsychopharmacology 43, S279–S280. [Google Scholar]
- Heilig M, Egli M, 2006. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol. Ther 111, 855–876. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C, 2014. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology 231, 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Silva C, Peck C, Miller CN, 2018. Varenicline modulates ethanol and saccharin consumption in adolescent male and female C57BL/6J mice. Brain Res. Bull 138, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, Wilcox T, 2009. Naltrexone decreases heavy drinking rates in smoking cessation treatment: An exploratory study. Alcohol. Clin. Exp. Res 33, 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Lo S, Coen K, 2014. Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology 231, 4019–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Poulos C, Harding S, Watchus J, Juzytsch W, Shaham Y, 1999. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology 21, 435–444. [DOI] [PubMed] [Google Scholar]
- Maggio SE, Saunders MA, Baxter TA, Nixon K, Prendergast MA, Zheng G, Crooks P, Dwoskin LP, Slack RD, Newman AH, Bell RL, Bardo MT, 2018. Effects of the nicotinic agonist varenicline, nicotinic antagonist r-bPiDI, and DAT inhibitor (R)-modafinil on co-use of ethanol and nicotine in female P rats. Psychopharmacology 235, 1439–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK, 2014. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats— Animal models of alcoholism. Alcohol 48, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S, 2008. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology 196, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH, 2016. Sex differences in varenicline efficacy for smoking cessation: A meta-analysis. Nicotine Tob. Res 18, 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR, 2006. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: Results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch. Intern. Med 166, 1561–1568. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, Miller S, Coe JW, 2006. Metabolism and disposition of varenicline, a selective α4β2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab. Dispos 34, 121–130. [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion, 2015. Dietary Guidelines for Americans, 2015–2020. Office of Disease Prevention and Health Promotion, Rockville, MD. Appendix 9. [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM, 2013. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 133, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Ray LA, Yardley MM, King AC, 2016. Current insights into the mechanisms and development of treatments for heavy drinking cigarette smokers. Curr. Addict. Rep 3, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley Iii FD, Williams KE, 2007. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994. [DOI] [PubMed] [Google Scholar]
- Rose JE, 2006. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 184, 274–285. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV, 1994. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin. Pharmacol. Ther 56, 86–99. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H, 2014. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231, 3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA, 2009. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin. Psychol. Rev 29, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuppa G, Cippitelli A, Toll L, Ciccocioppo R, Ubaldi M, 2015. Varenicline decreases nicotine but not alcohol self-administration in genetically selected Marchigian Sardinian alcohol-preferring (msP) rats. Drug Alcohol Depend. 156, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, 1999. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology 143, 318–321. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR, 2002. Mecamylamine (Inversine): An old antihypertensive with new research directions. J. Hum. Hypertens 16, 453–457. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE, 2010. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology 35, 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisontiyakul J, Kastman HE, Krstew EV, Govitrapong P, Lawrence AJ, 2016. The nicotinic alpha6-subunit selective antagonist bPiDI reduces alcohol self-administration in alcohol-preferring rats. Neurochem. Res 41, 3206–3214. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE, 2007. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc. Natl. Acad. Sci. USA 104, 12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchalla I, Okoli CTC, Malchy L, Johnson JL, 2011. Nicotine dependence and gender differences in smokers accessing community mental health services. J. Psychiatr. Mental Health Nurs 18, 349–358. [DOI] [PubMed] [Google Scholar]
- Udo T, Harrison EL, Shi J, Tetrault J, McKee SA, 2013. A preliminary study on the effect of combined nicotine replacement therapy on alcohol responses and alcohol self-administration. Am. J. Addict 22, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA, 2016. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J. Addict. Med 10, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL, 2009. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol 43, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, Crooks PA, Bardo MT, Dwoskin LP, 2011. bPiDI: A novel selective alpha6beta2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br. J. Pharmacol 163, 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Horiuchi K, Takano R, Nagano T, Shimizu M, Kitajima M, Murayama N, Shono F, 2010. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int. J. Environ. Res. Public Health 7, 3406–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]