Abstract
Background:
A risk score identifying patients at high risk for veno-occlusive disease (VOD) may aid efforts to study preventive strategies for this uncommon complication of hematopoietic cell transplantation (HCT).
Methods:
Patients receiving a first allogeneic HCT between 2008–2013 as reported to the Center for International Blood & Marrow Transplant Research (N=13,097) were randomly divided into training and validation sets. Independent prognostic factors for development of VOD by day+100 post HCT were identified with a multivariate logistic regression model. A risk score was constructed in the training set using the significant factors and confirmed in the validation set.
Results:
Baseline characteristics of the training and validation sets were balanced. In total, 637 patients (4.9%) developed VOD by day+100. Younger age, positive hepatitis B/C serology (HBV, HCV), lower Karnofsky performance score (KPS), use of sirolimus, disease, disease status at transplant, and conditioning regimen were independent prognostic factors. Myeloablative (MAC) regimens were associated with higher risk of VOD. Busulfan-based MAC regimens guided by pharmacokinetic monitoring (PK) were associated with higher risk than those without PK. The patients were stratified into 4 distinct, statistically significantly different groups by their risk score percentile.
Conclusion:
This pre-transplant risk score successfully stratified allogeneic HCT patients by risk of developing VOD, was validated in an independent set, and demonstrated strong discriminatory ability to identify a high-risk cohort.
Keywords: Veno-occlusive disease, Hematopoietic Cell Transplantation, conditioning regimen, busulfan
INTRODUCTION
Veno-occlusive disease of the liver (VOD) is an uncommon, early complication of hematopoietic cell transplantation (HCT) that is associated with significant mortality1,2.
The pathophysiology of VOD is thought to be related to hepatic endothelial damage from the HCT conditioning regimen3. Myeloablative regimens (MAC) are associated with higher risk than reduced intensity regimens (RIC)3,4, and regimens containing busulfan5–8 have been associated with a particularly high risk. However, a multitude of other patient-, disease- and treatment-related factors have been proposed to modify the risk, including the patient’s age5,9, performance status10, pre-existing liver dysfunction6,10, and use of sirolimus11 or methotrexate12 for graft-versus-host disease (GVHD) prophylaxis. Quantifying the relative contributions of these risk factors has been challenging, owing to VOD’s rarity. Recent large retrospective analyses of patients undergoing allogeneic HCTs have reported incidences of 3.3–10%1,13. Identifying higher risk populations of patients may facilitate the systematic evaluation of prophylactic approaches for this relatively infrequent but potentially life threatening complication, and would be highly valuable in clinical decision making for patients being considered for allogeneic HCT.
The present study used contemporary data from the Center for International Blood and Marrow Transplant Research (CIBMTR) to develop and validate a pre-transplant risk score to stratify patients per their risk for developing VOD during the first 100 days post-HCT.
METHODS:
Data Source:
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program / Be The Match. CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a centralized Statistical Center. Observational studies conducted by CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information issued in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Additional details regarding the data source are described elsewhere14.
Patient Selection
All recipients of a first allogeneic HCT between 2008 and 2013 were identified from the CIBMTR database. Patients who received syngeneic twin transplants, did not provide signed informed consent, had inadequate follow-up, or received defibrotide as prophylaxis were excluded. Likewise, patients with missing data on critical variables were excluded. Due to the occasional clinical ambiguity associated with the diagnosis of VOD, a cut-off was established to reduce ascertainment bias introduced by center-to-center variance in the recognition of the syndrome. Based on a conservative estimate of VOD incidence of 5%, a center that has performed at least 31 transplants would have a less than 20% probability of having zero cases of VOD. Thus, patients transplanted at centers reporting at least 31 transplants during the study period, but reporting no cases of VOD, were excluded from the study.
Study Variables and Definitions:
Parameters relating to patient-, disease- and transplant-related variables were tested in the multivariate analysis (table 1). Patient related variables included age, gender, race, Karnofsky performance score (KPS), Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI), recipient cytomegalovirus (CMV), hepatitis B (HBV) and C (HCV) virus serology, and history of a prior autologous HCT. Disease related variables included number of prior lines of chemotherapy, disease type and disease status at transplant. Transplant related variables included anti-thymoglobulin (ATG)/alemtuzumab use, donor type and graft source, conditioning regimen (in busulfan containing regimens, whether pharmacokinetic [PK] monitoring was used to guide dosing was included), GVHD prophylaxis, sirolimus use, and ursodiol use. Established definitions were used to categorize the intensity of conditioning regimens15, and the degree of human leukocyte antigen (HLA) match of unrelated donors16. Categorical variables are presented as numbers and percentages. Continuous variables are presented as medians and ranges in table 1.
Table 1.
Baseline characteristics of patients reported to the CIBMTR from 2008–2013
| Number of patients | 6523 | 6574 | |
| Age at HCT, years | |||
| Median (range) | 45 (< 1–79) | 46(<1–80) | |
| <10 | 1046(16) | 1047(16) | |
| 10–19 | 635(10) | 611(9) | |
| 20–39 | 1184(18) | 1123(17) | |
| 40–59 | 2333 (35) | 2421 (37) | |
| ≥60 | 1325(20) | 1372(20) | |
| Male Sex | 3790 (58) | 3828 (58) | |
| Karnofsky score 90–100% | 4426 (68) | 4476(68) | |
| Missing | 118(2) | 128(2) | |
| HCT-CI | |||
| 0 | 3656 (56) | 3645 (55) | |
| 1–2 | 1246(19) | 1279(19) | |
| 3 or more | 1473 (23) | 1511 (23) | |
| Missing | 148 (2) | 139(2) | |
| Disease / Disease Status at Time of Transplant | |||
| Advanced3/Missing | 518(8) | 566(9) | |
| Advanced3 | 75(1) | 75(1) | |
| Advanced6 | 55(1) | 59(1) | |
| Myelodysplastic Syndrome | 910(14) | 921 (14) | |
| REL resistant | 54 (1) | 53(1) | |
| Other leukemias7 | 280 (4) | 300 (5) | |
| Multiple Myeloma | 63(1) | 69(1) | |
| Myeloproliferative Syndromes | 361 (6) | 348 (5) | |
| Aplastic Anemia/Abnormalities of Erythrocytes | 422 (6) | 395(6) | |
| Histiocytic disorders | 85(1) | 71(1) | |
| Inherited problems of metabolism | 131 (2) | 128 (2) | |
| Severe Combined Immunodeficiency Disease | 233 (4) | 228 (3) | |
| Prior Autologous Transplant | 314(5) | 330(5) | |
| Recipient Hepatitis B/C status | |||
| Hepatitis B positive or Hepatitis B and C positive | 272 (4) | 249 (4) | |
| Hepatitis C positive | 45(1) | 47(1) | |
| Hepatitis B and C negative | 5981 (92) | 6041 (92) | |
| Not tested/Inconclusive | 225(3) | 237 (4) | |
| Recipient CMV Status Positive | 3918(60) | 3899 (59) | |
| Not tested/Inconclusive | 84 (<1) | 87 (<1) | |
| Lines of chemotherapy prior to HCT | |||
| Median (range) | 2(0–13) | 2(0–12) | |
| 08 | 1235(19) | 1195(18) | |
| 1 | 1564(24) | 1614(25) | |
| 2+ | 3616(55) | 3638 (55) | |
| Missing | 108(2) | 127(2) | |
| Donor and Graft Type | |||
| Cord Blood | |||
| HLA unknown | 254 (4) | 231 (4) | |
| Bone Marrow | |||
| Other Relative | 163(2) | 158(2) | |
| Peripheral Blood | |||
| Other Relative | 182(3) | 181(3) | |
| Conditioning Intensity/Regimens | |||
| MAC-TBI | |||
| <=12 Gy TBI/Cy | 514(8) | 533(8) | |
| >12GyTBI/Cy | 340 (5) | 348 (5) | |
| <=12GyTBI/Cy/Others | 244 (4) | 256 (4) | |
| >12GyTBI/Cy/Others | 348 (5) | 365(6) | |
| <=12 Gy TBI ± Others | 112(2) | 92(1) | |
| >12GyTBI± Others | 114(2) | 135(2) | |
| MAC-Chemo | |||
| Bu/Cy with PK monitoring | 574 (9) | 556(8) | |
| Bu/Cy without PK monitoring | 433 (7) | 480 (7) | |
| Bu/Cy/Others with PK monitoring | 91(1) | 88(1) | |
| Bu/Cy/Others without PK monitoring | 54(1) | 55(1) | |
| Bu/Flu with PK monitoring | 451 (7) | 453 (7) | |
| Bu/Flu without PK monitoring | 307 (5) | 326 (5) | |
| Bu/Flu/Others with PK monitoring | 48(1) | 51(1) | |
| Bu/Flu/Others without PK monitoring | 46(1) | 45(1) | |
| Bu/Mcl/Othcrs with PK monitoring | 46(1) | 48(1) | |
| Bu/Mel/Others without PK monitoring | 15 (<1) | 17 (<1) | |
| Bu/Others with PK monitoring | 28 (<1) | 38(1) | |
| Bu/Others without PK monitoring | 51(1) | 36(1) | |
| Thio/Others (non Bu) | 125 (2) | 120 (2) | |
| Others | 83(1) | 67(1) | |
| Bu-containing missing PK information | 2(<1) | 4(<1) | |
| R1C-TB1 | 185(3) | 188(3) | |
| RIC-Chemo | |||
| Mel/Others or BEAM/CBV-like | 618(9) | 612(9) | |
| Bu/Others | 666(10) | 685(10) | |
| NST or No regimen given9 | 1028(16) | 976(15) | |
| ATG/Alemtuzumab | |||
| ATG | 2192 (34) | 2129(32) | |
| Alemtuzumab | 374 (6) | 357(5) | |
| Both ATG and Alemtuzumab | 1(<1) | 1(<1) | |
| Neither ATG or Alemtuzumab | 3956(61) | 4087(62) | |
| GVHD prophylaxis | |||
| Tac | 530 (8) | 565 (9) | |
| CSA | 426 (7) | 438(7) | |
| Ex-vivo T-cell Depletion | 90(1) | 68(1) | |
| CD34 selection | 105(2) | 119(2) | |
| Post-transplant Cyclophosphamide | 69(1) | 74(1) | |
| Others | 81(1) | 93(1) | |
| Sinilimus used in GVHD prophylaxis | 514(8) | 556 (8) | |
| Ursodiol used in GVHD prophylaxis | 383 (6) | 381 (6) | |
| Median follow-up of survivors (range), months | 43 (3–79) | 38 (3–79) | |
Abbreviations: ATG=anti-thymoglobulin; BEAM= carmustine, etoposide, cytarabine, melphalan;Bu=busulfan; BM=bone marrow; CBV=cyclophosphamide, carmustine, etoposide;CMV=cytomegalovirus; Cy=cyclophosphamide; CSA=cyclosporine A; Flu=fludarabine; GVHD=graft vs host disease; HCT=hematopoietic cell transplantation; HCT-CI=HCT-Comorbidity index; MAC=myeloablative conditioning; Mel=melphalan; MMF=mycophenolate mofetil; MTX=methotrexate; NST=Non-myeloablative stem cell transplant; PB=peripheral blood; PIF=primary induction failure; PK=pharmacokinetic; REL=relapsed; RIC=reduced intensity conditioning; Thio=thiotepa; Tac=tacrolimus; TBI=total body irradiation; URD=unrelated donor.
Early: CR1
Intermediate: CR2+
Advanced: Active Leukemia
Early: Chronic phase 1, hematologic complete remission
Intermediate: Chronic phase 2+
Advanced: Accelerated phase, blast crisis
Other Leukemias included: CLL, NOS; hairy cell leukemia; other leukemia, specify; CLL B-cell; CLL T-cell; PLL B cell; PLL T cell
Includes N=1693 non-malignant diseases
All patients who received no conditioning regimen had Severe Combined Immunodeficiency Diseases
Age was analyzed as a continuous variable within five strata as shown in table 1. Very few patients had co-infection of HBV and HCV (n= 37), and they were grouped for analysis with the HBV positive patients. Patients were considered positive for hepatitis B if they were reported to have hepatitis B core antibody, surface antigen or DNA positive. Patients were considered positive for hepatitis C if they were reported to have hepatitis C antibody or nucleic acid amplification testing positive. Disease and disease status were analyzed as a composite variable, as were donor type and graft type. Conditioning regimens were categorized as shown in table 1.
Outcomes:
The primary outcome was development of VOD by Day+100 with or without multi-organ failure as reported by the reporting institution to the CIBMTR. Severe VOD was defined as VOD with concurrent renal failure or noninfectious pulmonary abnormalities. Secondary outcomes included overall survival rates at day+100, six months and one year.
Statistics:
The full cohort was randomly divided into a training and a validation set. Prognostic factors for VOD were identified using logistic regression with stepwise elimination on the training set. Risk factors tested in the logistic regression model are listed above under Study Variables and Definitions. Age was analyzed continuously within the following groups: <10, 10–19, 20–39, 40–59, and ≥60 years. Risk factors with a p<0.05 were considered significant. The potential interaction of sirolimus use with use of busulfan-based MAC regimens was tested in the model; no other interactions were tested. The following risk factors emerged as independent predictors and were included in the final model: age, KPS, hepatitis B/C serology, sirolimus use, conditioning regimen, disease, and disease status at time of transplant. Once the final model was built, the risk score and predicted probability of developing VOD could be calculated for each individual patient. Four risk groups were identified using maximum likelihood estimates based on predicted VOD rate. The risk score was then examined and validated using the validation set. Overall survival was calculated using Kaplan Meier limit estimation, and the curves were compared using the log rank test.
RESULTS
Study Cohort
The study cohort included 13,097 total eligible patients. Their patient-, disease-, and transplant-related characteristics are shown in table 1. The majority had KPS of 90–100% (68%) and HCTCI of 0–2 (75%). The median age at HCT was 46 years (range <1–80 years). Overall, malignant diseases comprised 87% of cases. The most common donor type was a matched unrelated donor (32%). Peripheral blood stem cell grafts were the most common graft type (56%). Most patients received MAC regimens (62%). Sirolimus was used in only 8% of cases.
In total, 637 patients (4.86%) were reported to have developed VOD by day +100. VOD diagnosis was established in 85% of patients (n=543) by either biopsy/autopsy (n=139), Baltimore criteria17 (n=289), or modified Seattle criteria2 (n=115). The remaining patients were diagnosed using other clinical evidence (e.g. ascites, ultrasonographic findings). Day+100 survival in the total cohort was 86% (95% confidence interval [CI]: 86–87%). The day+100 survival for patient with severe VOD, with non-severe VOD, and without VOD was 44% (95%CI: 39–50%), 77% (95%CI: 73–82%) and 87% (95%CI: 87–88%), respectively (p<0.001) (table 4).
Table 4:
Overall Survival
| Overall Survival | ||||
|---|---|---|---|---|
| No VOD (n=12460) | VOD without MOD* (n=349) | VOD with MOD* (n=288) | p-value | |
| Day+100-%(95%CI) | 87 (87–88)% | 77 (73–82)% | 44 (39–50)% | 0.001 |
| 6 months - % (95%CI) | 77 (77–78)% | 66 (60–70)% | 32 (27–38)% | <0.001 |
| 12 months - % (95%CI) | 65 (65–66)% | 57 (52–62)% | 24(19–29)% | 0.001 |
MOD: multi-organ dysfunction (pulmonary and/or renal)
The randomly selected training and validation sets were compared across all study variables, and no major differences were identified (table 1).
Multivariate Model
The training set was used to construct a multivariate model, which identified 6 variables significantly associated with development of VOD: age, Karnofsky score, hepatitis serology, disease/disease status, conditioning regimen, and sirolimus use (table 2). Dysfunction of individual organ systems as defined by the HCT-CI were initially included in the analysis. However, no single comorbidity achieved statistical significance, including baseline liver dysfunction. Thus, total aggregate HCT-CI was used in the final analysis, though notably did not have a statistically significant association.
Table 2.
Factors independently predictive of development of VOD in multivariate analysis
| Variable | N | OR (95% CI) | p-value |
|---|---|---|---|
| Aee: OR of developing VOD corresponding to a 1 year decrease in age within each stratum | |||
| <10 years age group | 944 | 1.10(1.03–1.17) | 0.004 |
| 10–19 years age group | 604 | 1.01 (0.95–1.07) | 0.81 |
| 20–39 years age group | 1128 | 1.04(1.01–1.08) | 0.01 |
| 40–59 years age group | 2184 | 1.01(0.98–1.04) | 0.61 |
| ≥60 years age group | 1242 | 1.05(0.96–1.16) | 0.28 |
| Karnofsky | 0.007 | ||
| <90 | 1901 | 1.47(1.11–1.94) | |
| Hepatitis Serology | 0.005 | ||
| Hepatitis B/C negative | 5799 | 1.00 | |
| Hepatitis C positive | 42 | 0.45 (0.06–3.68) | 0.46 |
| Hepatitis B/C positive or Hep B positive | 261 | 2.19(1.35–3.56) | 0.002 |
| Disease/Disease Status | 0.04 | ||
| Inherited problems of metabolism | 119 | 1.00 | |
| Acute Lymphoblastic Leukemia Early1/Missing | 427 | 1.96(0.73–5.25) | 0.18 |
| Acute Lymphoblastic Leukemia Intermediate2 | 316 | 2.03 (0.75–5.50) | 0.16 |
| Acute Lymphoblastic Leukemia Advanced3 | 70 | 2.04 (0.47–8.83) | 0.34 |
| Severe Combined Immunodeficiency Disease | 177 | 2.21 (0.87–5.63) | 0.10 |
| Other leukemias4 | 256 | 2.23 (0.65–7.67) | 0.21 |
| Chronic Myeloid Leukemia Early5/lntermediate6 | 164 | 2.50(0.77–8.12) | 0.13 |
| Multiple Myeloma | 59 | 2.52 (0.28–22.26) | 0.41 |
| NHL/HD Primary Induction Failure-Resistant | 39 | 2.61(0.29–23.24) | 0.39 |
| NHL/HD PIF/REL Sensitive, CR, untreated | 448 | 2.68 (0.92–7.78) | 0.07 |
| Acute Myeloid Leukemia Early1/Intermediate2 | 1760 | 2.84(1.21 −6.64) | 0.02 |
| Acute Myeloid Leukemia Advanced3/ Missing | 494 | 3.06(1.19–7.87) | 0.02 |
| Histiocytic disorders | 80 | 3.34(1.17–9.48) | 0.02 |
| Aplastic Anemia/Abnormalities of Erythrocytes | 398 | 3.37(1.33–8.54) | 0.01 |
| Myelodysplastic syndromes Early/Advanced | 853 | 4.15(1.67–10.33) | 0.002 |
| Chronic Myeloid Leukemia Advanced7 | 53 | 5.22(1.37–19.97) | 0.02 |
| Myeloproliferative Syndromes | 339 | 6.24(2.47–15.78) | <0.001 |
| NHL/HD Relapse Resistant | 50 | 7.34(1.66–32.32) | 0.009 |
| Conditioning Regimen | <0.001 | ||
| RIC-Chemo: Bu ± others | 637 | 1.00 | |
| RIC-Chemo: Mel ± Others or BEAM/CBV like | 589 | 1.01(0.42–2.45) | 0.98 |
| MAC-Chemo: Thio ± others | 118 | 1.06(0.27–4.08) | 0.94 |
| NST or No regimen | 961 | 1.08(0.47–2.52) | 0.85 |
| RIC-TBI | 170 | 1.22(0.36–4.11) | 0.75 |
| MAC-Chemo: Bu/Cy no PK monitoring | 409 | 1.90(0.79–4.59) | 0.15 |
| MAC-TBI: <=12 Gy TBI/Cy | 495 | 2.04 (0.84–4.97) | 0.12 |
| MAC-TBI: >12 Gy TBI/Cy | 321 | 2.13(0.87–5.23) | 0.10 |
| MAC-Chemo: Bu/Cy/Others no PK | 53 | 2.38 (0.72–7.96) | 0.16 |
| MAC-Chemo: Bu/Mel ± Others no PK | 15 | 2.45 (0.27–22.30) | 0.42 |
| MAC-TBI: <=12 Gy TBI ± Others | 108 | 2.56 (0.80–8.20) | 0.11 |
| MAC-Chemo: Bu/Flu/Others no PK | 46 | 2.69(0.55–13.22) | k 0.22 |
| MAC-TBI: >12 Gy TBI/Cy/Others | 336 | 3.07(1.30–7.22) | 0.01 |
| MAC-Chemo: Bu/Flu no PK | 292 | 3.52(1.51 −8.25) | 0.003 |
| MAC-TBI: >12 Gy TBI ± Others | 107 | 3.56(1.16–10.92) | 0.02 |
| MAC-Chemo: Bu ± Others no PK | 47 | 3.89(0.96–15.70) | 0.06 |
| MAC-Chemo: Bu/Flu with PK | 413 | 4.01(1.83–8.76) | <0.001 |
| MAC-TBI: <=12Gy TBI/Cy/Others | 236 | 4.37(1.82–10.48) | 0.001 |
| MAC-Chemo: Bu/Cy with PK | 552 | 4.52(2.10–9.74) | <0.001 |
| MAC-Chemo: Bu/Cy/Others with PK | 85 | 5.22(2.09–13.07) | <0.001 |
| MAC-Chemo: Bu ± Others with PK | 27 | 6.74(1.93–23.56) | 0.003 |
| MAC-Chemo: Bu/Mel ± Others with PK | 45 | 7.44 (2.49–22.28) | O.001 |
| MAC-Chemo: Bu/Flu/Others with PK | 40 | 8.09(2.46–26.59) | <0.001 |
| Sirolimus Use | |||
| Yes | 478 | 2.39(1.54–3.71) | <0.001 |
Abbreviations: BEAM=carmustine, etoposide, cytarabine, melphalan; Bu=busulfan; CBV= cyclophosphamide, carmustine, etoposide; Cy=cyclophosphamide; Flu=fludarabine; HD=Hodgkin Disease; MAC=myeloablative conditioning; Mel=melphalan; NHL=Non-hodgkin Lymphoma; NST=Nonmyeloablative stem cell transplantation; PIF=primary induction failure; PK=pharmacokinetic; RIC=reduced intensity conditioning; Thio=thiotepa; TBI=total body irradiation;
Early: CR1
Intermediate: CR2+
Advanced: Active Leukemia
Other Leukemias included: CLL, NOS; hairy cell leukemia; other leukemia, specify; CLL B-cell; CLL T-cell; PLL B cell; PLL T cell
Early: Chronic phase 1, hematologic complete remission
Intermediate: Chronic phase 2+
Advanced: Accelerated phase, blast crisis
“Hepatitis B/C negative” was used as the reference group (odds ratio [OR] = 1.00) and “hepatitis B positivity with or without hepatitis C positivity” was associated with high risk of VOD (OR = 2.19; 95% CI 1.35–3.56; p=0.005). Additionally, KPS < 90 (OR 1.47; 95% CI 1.11–1.94; p = 0.007) and use of sirolimus (OR 2.39; 95% CI 1.54–3.71; p<0.001) were also associated with higher risk of VOD.
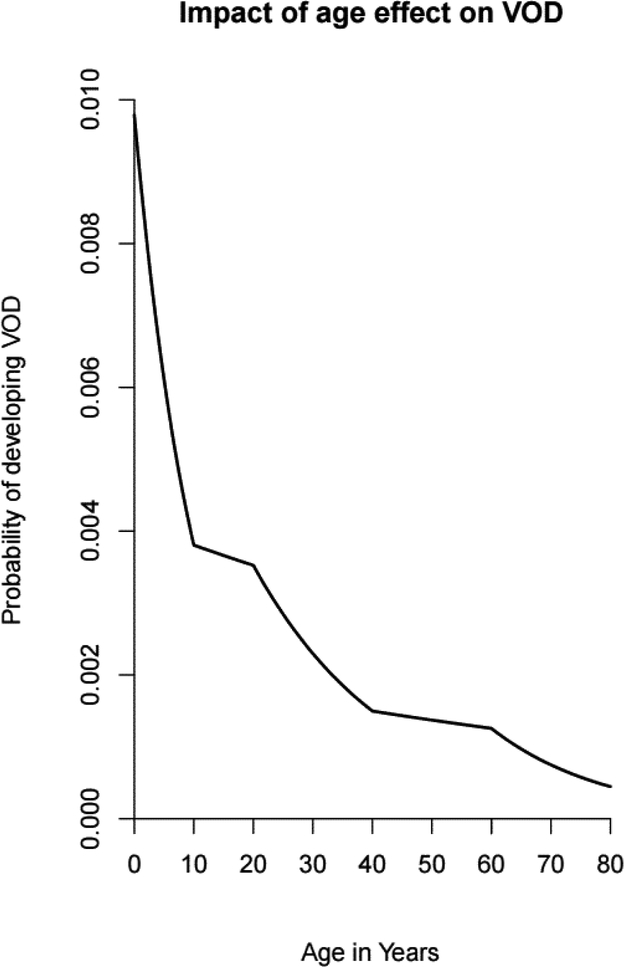
Younger age was associated with development of VOD across all strata (figure 1). Within the 0–10 years age stratum, the odds ratio was 1.10, corresponding to a 10% increased risk per year younger between years 0–10. Similarly, for ages 10–20, 20–40, 40–60, and 60–80 the ORs were 1.01, 1.04, 1.01, and 1.05, corresponding to 1%, 4%, 1% and 5% increased risk of VOD for each year younger within their respective strata (table 2). Patients who were younger than 10 years of age had the most significant relative age effect on risk of developing VOD.
Figure 1: Impact of Age Effect on VOD.
Figure 1 shows the relationship between age and risk of VOD. Age was analyzed as a continuous variable stratified within 5 age strata (<10, 10–19, 20–39, 40–59, and ≥60 years)
There was a wide diversity of conditioning regimens reported to the CIBMTR, with many occurring very infrequently. The low event rate necessitated grouping the infrequently reported conditioning regimens with other regimens of similar intensity for the analysis. A sensitivity analysis of conditioning regimens was performed, which excluded patients receiving some infrequently used conditioning regimens; no significant changes to the ORs were found (data not shown).
The regimen found to have the lowest odds ratio was “busulfan based RIC”, and this regimen was used as the reference group for the chemotherapy regimens (OR = 1.00). “Busulfan and fludarabine based MAC regimens with an additional agent and with PK monitoring” was associated with the highest odds ratio (OR= 8.09; 95% CI 2.46–26.59; p<0.001). The most common additional agent in this group was total body irradiation (62%), followed by clofarabine (17.3%). This group was followed by “busulfan and melphalan based MAC regimens with or without an additional agent and with PK monitoring” (OR = 7.44; 95% CI 2.49–22.28; p<0.001) (table 2, and supplemental appendix 1 figure s1). We tested whether the risk of VOD with use of busulfan was differentially higher when sirolimus was concurrently given by testing an interaction term, but this was found to have no statistically significant association (p=0.12).
The disease group with the lowest odds ratio was “inherited problems of metabolism”, and this group was used as the reference group for the disease type/statuses (OR = 1.00). “Relapsed/resistant non-Hodgkin lymphoma / Hodgkin disease” was associated with the highest odds ratio (OR = 7.34; 95%CI 1.66–32.32; p=0.009), followed by “myeloproliferative syndromes” (OR = 6.24; 95% CI 2.47–15.78; p<0.001) (table 2, and supplemental appendix 1 figure s2).
Risk Score
The six variables identified as significant were used to build the VOD risk score (supplemental appendix 2, table s1). The magnitude of the odds ratios determined the magnitude of the risk score term coefficients for each variable. Each patient’s risk score was calculated by addition of the risk score coefficients corresponding to the patient’s characteristics (example calculation shown insupplemental appendix 2, table s2). Each patient’s expected probability of developing VOD may be calculated from the model using the following equation: probability = e^{score}/(1+e^{score}).
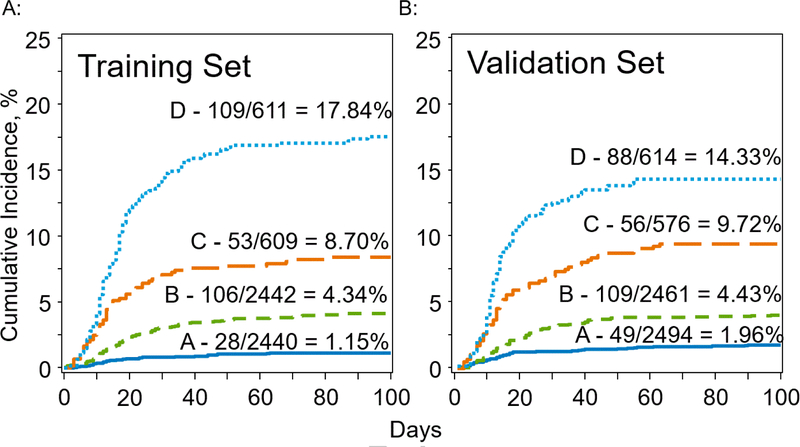
Four risk groups were established in the training set as described in the statistics section. Groups A (n=2440) and B (n=2442) each contained 40% of the training set, comprising percentiles 0–40 and 40–80, respectively. Groups C (n=609) and D (n=611) each contained 10% of the training set, comprising percentiles 80–90 and >90, respectively. The incidence of VOD in the training set in each group was 1.15%, 4.34%, 8.70% and 17.84% for groups A, B, C, and D, respectively (table 3A, figure 2A). The odds ratios for development of VOD in the training set for group B vs A, group C vs B and group D vs C were 3.91 (95% CI: 2.57–5.95, p<0.001), 2.10 (95% CI: 1.49–2.96, p<0.001), and 2.28 (95% CI: 1.8–3.23, p<0.001), respectively (table 3B). The discriminative ability of the model was measured by calculating the c statistic, a measure of concordance between a model’s risk estimates and the observed events. C statistic values range from 0.5, indicating random concordance, to 1.0, indicating perfect concordance18. The c statistic for the training set was 0.76 (standard error = 0.014).
Table 3A:
Observed Day+100 VOD Incidence
| Patients, n | Patients developing VOD, n (%) | |
|---|---|---|
| Total Cohort | 13097 | 637 (4.86%) |
| Training Set | ||
| Group A | 2440 | 28(1.15%) |
| Group B | 2442 | 106 (4.34%) |
| Group C | 609 | 53 (8.70%) |
| Group D | 611 | 109(17.84%) |
| Validation Set | ||
| Group A | 2494 | 49 (1.96%) |
| Group B | 2461 | 109 (4.43%) |
| Group C | 576 | 56 (9.72%) |
| Group D | 614 | 88(14.33%) |
Figure 2A, 2B: VOD Incidence Among Four Risk Groups in Training and Validation Cohorts.
Figure 2A shows the incidence of VOD in the training set for each of the 4 risk groups (A-D). Figure 2B shows the incidence of VOD in the validation set for each of the 4 risk groups (A-D). The curves are labeled with the cumulative incidence by day+100 in each of the 4 risk groups (A-D).
Table 3B:
Intergroup Comparisons for Training and Validation Sets
| Intergroup Comparisons (OR [95% CI]) | p-value | ||
|---|---|---|---|
| Training set | |||
| B vs A | 3.91 (2.57–5.95) | <.001 | |
| C vs B | 2.10(1.49–2.96) | <.001 | |
| D vs C | 2.28(1.61 −3.23) | <.001 | |
| Validation set | |||
| BvsA | 2.31 (1.64–3.25) | <0.001 | |
| C vs B | 2.32(1.66–3.25) | <0.001 | |
| D vs C | 1.55(1.09–2.22) | 0.02 | |
Validation
The model was tested using the validation set. Risk score value cut offs for the four risk groups established in the training set were used to define the same four risk groups in the validation set. The proportion of patients in each group was 41% for group A (2494 patients), 40% for group B (2461 patients), 9% for group C (576 patients) and 10% for group D (614 patients). The incidence of VOD in each group was 1.96%, 4.43%, 9.72% and 14.33% for groups A, B, C, and D, respectively (table 3A, figure 2B). The odds ratios for development of VOD in the validation set for groups B vs A, C vs B, and D vs C were 2.31 (95% CI: 1.64–3.25, p<0.001), 2.32 (95% CI: 1.66–3.25, p<0.001), and 1.55 (95% CI: 1.09–2.22, p=0.02), respectively (table 3B). The c statistic for the validation set was 0.72 (SE 0.015). There was no statistical difference in c statistics between the training and validation sets (p=0.07).
DISCUSSION
The risk model described here demonstrates a strong predictive ability to identify patients at high risk of developing VOD (c statistic = 0.72–0.76). The factors found to be significantly associated with the development of VOD have been previously described as significant factors in the development of VOD3–11,19–22, however this is the first model developed to integrate these risk factors and use weighted coefficients for each factor to estimate the risk of VOD based on pre-transplant characteristics.
Myeloablative conditioning regimens have long been implicated in the pathogenesis of VOD, and this is corroborated by our model3. Myeloablative regimens were consistently associated with a higher odds ratios than reduced intensity regimens, which is suggestive of a dose-response relationship between total exposure to conditioning agents and risk of VOD, as has been previously reported8. Unexpectedly, use of pharmacokinetic monitoring of busulfan dosing was also found to be associated with higher risk of VOD. Though this analysis does not suggest a pathophysiologic mechanism to explain this finding, the association is unlikely to be spurious as it was tested using the validation set and found to be consistent. Use of PK monitoring of busulfan is intended to achieve two benefits through optimization of the busulfan dose–increased disease control and reduced toxicity. And while use of PK monitoring may result in improved overall outcomes in patients undergoing allogeneic HCT, it is possible that important associations between PK monitoring and relatively infrequent outcomes, such as VOD, may not have been identified in prior studies with less statistical power. One hypothesized mechanism to explain this association is that use of PK monitoring may generally result in higher exposure to busulfan, as reported by Weil et al. using data from a single institution23. In their adult population, the patients’ busulfan doses were increased in 96.7% of cases after the first dose level, which suggests that patients treated without PK monitoring generally are exposed to a lower level of busulfan. However, the applicability of this hypothesis to the rest of the cohort is not known. Though data was available for this study regarding whether or not PK monitoring was used for each patient in the registry, data on how the busulfan dose was modified was not available. Importantly, it should not be concluded that current practices regarding the use of PK monitoring should be changed on the basis of these data, as this study analyzed only one of the many important clinical outcomes which must be considered (such as disease relapse). Rather, these data may be helpful in identifying patients at high risk of VOD who may benefit from prophylactic pharmacologic approaches or careful post-HCT monitoring.
To evaluate for the possibility of a non-linear relationship between age and risk of VOD, patient age was analyzed as a continuous variable within 5 age strata. However, the association between younger age and development of VOD was robust and persistent across all age strata (figure 1). Similar associations have previously been described, primarily in studies of pediatric patients5,19. Our analysis does not suggest a pathophysiologic explanation for the association, though differences in hepatic physiology and anatomy in pediatric patients as compared to adults, such as smaller caliber hepatic venues more prone to obstruction, have been postulated as possible causes of such an association19. Alternatively, differences in exposure to busulfan between pediatric patients and adults might underlie this association. The pharmacokinetics of busulfan are particularly variable in pediatric patients24. In contrast to adult patients, one pharmacokinetic study of pediatric patients found that the initial busulfan dosing, which in some regimens represents a significant portion of the total dosing, exceeded the upper limit of target exposure in 16–23% of patients when weight based dosing was used25. Given the strength of the effect of busulfan exposure on development of VOD, unintentional overdosing of busulfan due to unpredictable pharmacokinetics among pediatric patients may increase risk of VOD.
A number of previously described risk factors for development of VOD did not achieve statistical significance in our model, particularly the effect of underlying liver disease on development of VOD6,26. This finding may reflect a change in patient selection for HCT, as physicians may opt for RIC regimens rather than MAC regimens in patients with numerous comorbidities.
The low event rate of VOD necessitated grouping many infrequently used conditioning regimens with other similar regimens. Though an analysis excluding these regimens was performed and did not result in significantly different ORs, the risk model may have less applicability in these infrequently used regimens. Similarly, because patients with uncommon diseases were excluded from the analysis, the findings of the model may have less applicability in these cases. Notably, the analysis does not analyze data on exposure to recently approved therapies, such as inotuzumab ozogamicin, blinatumomab or gemtuzumab ozogamicin (GO), and thus the applicability of the model to patients receiving these agents is not known. Because VOD remains a relatively infrequent event, it was necessary in the design of this study to carefully limit the number of subsets included in the analysis to preserve the model’s statistical power. For example, there was a small number of patients in the cohort who had prior exposure to gemtuzumab ozogamicin. Including this exposure in the analysis was considered. However, the inclusion of such subsets would reduce the model’s ability to concurrently integrate all the risk factors into the risk estimate, as it would reduce the events per variable (EPV) ratio of the model27. Furthermore, in the interest of creating a risk score with wide applicability to all patients undergoing first allogeneic transplant, exposure to GO was not included in the analysis, as only patients with acute myeloid leukemia would have been exposed to GO and the exposure would thus act as a surrogate for that specific disease type. Analyses of the relationship between exposure to GO and VOD in adult and pediatric patients have recently been reported by the CIBMTR. The authors identified an increased risk of VOD associated with exposure to GO in pediatric patients, but not in adult patients28,29. It is our expectation that the risk score analysis will periodically need to be repeated to incorporate future practice changes. Due to the limitations of the registry data set, other exposures which may significantly contribute to the risk of VOD, such as involved field radiation exposure to the liver, were not able to be included in the model and the risk score should thus be used with caution in such situations.
Another important limitation of the multivariate analysis presented here is its limited ability to compare individual variables head-to-head. For example, it is inappropriate to conclude from the data presented in Table 2 that “MAC busulfan/fludarabine/others based regimens with PK monitoring” are associated with higher risk of VOD than “MAC busulfan/cyclophosphamide/others based regimens with PK monitoring”. This is because the purpose of the multivariate analysis was to concurrently integrate a large number of pre-transplant patient-related, disease-related and transplant-related factors to generate a global estimate of an individual’s risk of VOD. The odds ratios presented in Table 2 represent the comparison between each group and the reference groups (in the case of conditioning regimens, the reference group was reduced intensity busulfan ± others) and were used in the construction of the risk score model. Pairwise comparisons were not performed, and no interaction testing was performed, as it would have made the analysis difficult to interpret and was beyond the scope of the study. Additionally, the large number of multiple comparisons that would have been necessary would have severely inflated the type I error rate. Thus, head-to-head comparisons of individual conditioning regimens using the odds ratios presented in table 2 will tend to produce erroneous conclusions and should be avoided. Despite these limitations, the odds ratios produced by the multivariate analysis were successfully integrated into the risk score, which has demonstrated high concordance (c statistic) within the training set and the independent validation set.
The validity of the model was assessed via use of a randomly selected validation set. Because it was developed using a multicenter, international cohort treated using modern transplant techniques, the model likely has strong external applicability. The risk calculation integrates highly granular pre-transplant patient information to produce as precise a risk estimate as possible, as shown in Supplemental Appendix 2. Construction of a simplified risk model based on these analyses was considered. However, considering the wide range of risk associated with the heterogeneous conditioning regimens in use in clinical practice, as well as the range of risk associated with the disease/disease statuses observed, we felt it would be inaccurate and statistically inappropriate to attempt to simplify the risk model. The model will be best integrated into clinical practice with an online risk calculator, which is hosted by the CIBMTR and is publicly accessible (https://www.cibmtr.org/ReferenceCenter/Statistical/Tools/Pages/VOD.aspx). Such online calculators have found increasing clinical application in modern practice, for example the Disease Risk Index (DRI) Assignment Tool30. Patients found by the VOD risk prediction model to be at high risk of VOD may warrant closer monitoring for development of VOD, or modification of the conditioning regimen. Identifying high risk patients may also facilitate earlier initiation of defibrotide upon the onset of VOD, which has been shown to improve survival from this complication31. However, it must be emphasized that this study and risk model focused solely on the incidence of VOD and did not consider other clinically important outcomes, such as disease relapse or overall mortality, which also must be taken into account when considering the treatment plan. The prospective application of the risk model should be the focus of future investigation.
Supplementary Material
Highlights.
The incidence of VOD was 4.86% in allogeneic transplant patients.
Age, conditioning regimen, and disease type contributed to risk of VOD
Sirolimus use, performance status and hepatitis B status also contributed to risk
A risk score based on pre-transplant clinical factors was developed and validated
The highest risk cohort of patients had incidence of VOD of 14.3–17.8%.
ACKNOWLEDGEMENTS
The study was funded by Jazz Pharmaceuticals, Inc. The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–17-1–2388 and N00014–16-1–2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd.–Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Corporate Members
Conflicts of Interest Disclosure: K.F.V. is an employee of Jazz Pharmaceuticals, who in the course of employment received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Authors C.S., Y.Z., M.-J.Z, A.G., S.L., M.R., W.C., A.L., L.B., A.A. and W.S. have no relevant disclosures.
REFERENCES
- 1.Coppell JA, Richardson PG, Soiffer R, et al. Hepatic Veno-Occlusive Disease following Stem Cell Transplantation: Incidence, Clinical Course, and Outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168. doi:10.1016/j.bbmt.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatol Baltim Md. 1984;4(1):116–122. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Ho VT, Cutler C, Glotzbecker B, Antin JH, Soiffer R. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Novel Insights to Pathogenesis, Current Status of Treatment, and Future Directions. Biol Blood Marrow Transplant. 2013;19(1):S88–S90. doi:10.1016/j.bbmt.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 4.Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The Incidence of Veno-Occlusive Disease Following Allogeneic Hematopoietic Stem Cell Transplantation Has Diminished and the Outcome Improved over the Last Decade. Biol Blood Marrow Transplant. 2011;17(11):1713–1720. doi:10.1016/j.bbmt.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Cesaro S, Pillon M, Talenti E, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. haematologica. 2005;90(10):1396–1404. [PubMed] [Google Scholar]
- 6.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. [DOI] [PubMed] [Google Scholar]
- 7.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17(2):225–230. [PubMed] [Google Scholar]
- 8.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25(1):55–61. doi:10.1007/BF00694339 [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Deleve LD, Kamath PS, Tefferi A. Hepatic Veno-occlusive Disease (Sinusoidal Obstruction Syndrome) After Hematopoietic Stem Cell Transplantation. Mayo Clin Proc. 2003;78(5):589–598. doi:10.4065/78.5.589 [DOI] [PubMed] [Google Scholar]
- 10.Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92(10):3599–3604. [PubMed] [Google Scholar]
- 11.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–4431. doi:10.1182/blood-2008-07-169342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essell JH, Thompson JM, Harman GS, et al. Marked increase in veno-occlusive disease of the liver associated with methotrexate use for graft-versus-host disease prophylaxis in patients receiving busulfan/cyclophosphamide. Blood. 1992;79(10):2784–2788. [PubMed] [Google Scholar]
- 13.Strouse C, Richardson P, Prentice G, et al. Defibrotide for Treatment of Severe Veno-Occlusive Disease in Pediatrics and Adults: An Exploratory Analysis Using Data from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2016;22(7):1306–1312. doi:10.1016/j.bbmt.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz MM. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42:S1–S2. doi:10.1038/bmt.2008.101 [DOI] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi:10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-Matching for Retrospective Analysis of Unrelated Donor Transplantation: Revised Definitions to Predict Survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi:10.1016/j.bbmt.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–783. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB. Evaluating Discrimination of Risk Prediction Models: The C Statistic. JAMA. 2015;314(10):1063. doi:10.1001/jama.2015.11082 [DOI] [PubMed] [Google Scholar]
- 19.Cheuk DKL, Wang P, Lee TL, et al. Risk factors and mortality predictors of hepatic venoocclusive disease after pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40(10):935–944. [DOI] [PubMed] [Google Scholar]
- 20.Reiss U, Cowan M, McMillan A, Horn B. Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol. 2002;24(9):746–750. [DOI] [PubMed] [Google Scholar]
- 21.Dalle J-H, Giralt SA. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Risk Factors and Stratification, Prophylaxis, and Treatment. Biol Blood Marrow Transplant. 2016;22(3):400–409. doi:10.1016/j.bbmt.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 22.Maximova N, Ferrara G, Minute M, et al. Experience from a single paediatric transplant centre with identification of some protective and risk factors concerning the development of hepatic veno-occlusive disease in children after allogeneic hematopoietic stem cell transplant. Int J Hematol. 2014;99(6):766–772. doi:10.1007/s12185-014-1578-y [DOI] [PubMed] [Google Scholar]
- 23.Weil E, Zook F, Oxencis C, et al. Evaluation of the Pharmacokinetics and Efficacy of a Busulfan Test Dose in Adult Patients Undergoing Myeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(6):952–957. doi:10.1016/j.bbmt.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Russell JA, Kangarloo SB. Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des. 2008;14(20):1936–1949. [DOI] [PubMed] [Google Scholar]
- 25.Booth BP, Rahman A, Dagher R, et al. Population Pharmacokinetic-Based Dosing of Intravenous Busulfan in Pediatric Patients. J Clin Pharmacol. 2007;47(1):101–111. doi:10.1177/0091270006295789 [DOI] [PubMed] [Google Scholar]
- 26.Ozkaynak MF, Weinberg K, Kohn D, Sender L, Parkman R, Lenarsky C. Hepatic venoocclusive disease post-bone marrow transplantation in children conditioned with busulfan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant. 1991;7(6):467–474. [PubMed] [Google Scholar]
- 27.Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. 2016;76:175–182. doi:10.1016/j.jclinepi.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan C, St Martin A, Perez W, et al. Veno-Occlusive Disease Characteristics in Pediatric Patients with Acute Myeloid Leukemia Receiving Gemtuzumab Ozogamicin before Allogeneic Stem Cell Transplant. Biol Blood Marrow Transplant. 2018;24(3):S302. doi:10.1016/j.bbmt.2017.12.347 [Google Scholar]
- 29.Ho VT, Martin AS, Perez W, et al. Characterization of Veno-Occlusive Disease (VOD) in Adult Patients (Pts) with Acute Myeloid Leukemia (AML) Receiving Gemtuzumab Ozogamicin (GO) before Allogeneic Stem Cell Transplant (SCT). Biol Blood Marrow Transplant. 2018;24(3):S301–S302. doi:10.1016/j.bbmt.2017.12.346 [Google Scholar]
- 30.Center for International Blood and Marrow Transplant and Research. Disease Risk Index (DRI) Assignment Tool. https://www.cibmtr.org/ReferenceCenter/Statistical/Tools/Pages/DRI.aspx. Accessed September 20, 2017.
- 31.Richardson PG, Smith AR, Triplett BM, et al. Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation. Br J Haematol. 2017;178(1):112–118. doi:10.1111/bjh.14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.