Abstract
Background:
Group 3 tumors account for approximately 25–30% of medulloblastomas and have the worst prognosis. UAB30 is a novel synthetic rexinoid shown to have limited toxicities in humans and significant efficacy in the pediatric neuroectodermal tumor, neuroblastoma. We hypothesized that treatment with UAB30 would decrease tumorigenicity in medulloblastoma patient-derived xenografts (PDXs).
Methods:
Three group 3 medulloblastoma PDXs (D341, D384 and D425) were utilized. Cell viability, proliferation, migration and invasion assays were performed after treatment with UAB30 or 13-cis-retinoic acid (RA). Cell cycle analysis was completed using flow cytometry. A flank model, a cerebellar model, and a model of leptomeningeal metastasis using human medulloblastoma PDX cells was used to assess the in vivo effects of UAB30 and RA.
Results:
UAB30 treatment led to cell differentiation and decreased medulloblastoma PDX cell viability, proliferation, migration and invasion and G1 cell cycle arrest in all three PDXs similar to RA. UAB30 and RA treatment of mice bearing medulloblastoma PDX tumors resulted in a significant decrease in tumor growth and metastasis compared to vehicle treated animals.
Conclusions:
UAB30 decreased viability, proliferation, and motility in group 3 medulloblastoma PDX cells and significantly decreased tumor growth in vivo in a fashion similar to RA, suggesting that further investigations into the potential therapeutic application of UAB30 for medulloblastoma are warranted.
Keywords: medulloblastoma, PDX, UAB30, retinoic acid
INTRODUCTION
Medulloblastoma (MB), a primitive neuroectodermal tumor, is the most common malignant brain tumor in children. MB is classified into four molecular subtypes [(WNT, Sonic Hedgehog (SHH), group 3, group 4), each with unique biologic and clinical characteristics [1]. Group 3 tumors account for approximately 25% of medulloblastomas and have the worst prognosis of all subtypes [1–3]. Despite current multimodal treatment with surgery, radiotherapy, and multiple drug chemotherapy, overall mortality remains high for group 3 tumors at approximately 50% [1–4], and survivors often suffer from significant post-treatment neurocognitive defects, hormone dysfunction, and hearing loss [5 6].
Retinoids are vitamin A-related compounds that induce cell differentiation and cause growth arrest. Retinoids have demonstrated promising anti-tumor effects in numerous human malignancies, including both adult and pediatric brain cancers [7–11]. Previous studies have demonstrated that 9-cis-retinoic acid (9-cis-RA) decreased medulloblastoma cell growth in vitro and in vivo in a flank tumor model [8, 12]. However, the use of 9-cis-RA and other related retinoids in cancer therapy has been limited in the clinical setting by their toxicities [13].
9-cis-UAB30 (UAB30) [8-(3′,4′-dihydro-1′(2′H)-naphthalen-1′-ylidene)-3,7-dimethyl2,4,6-octatrienoic acid] is a synthetic analog of 9-cis-RA that potently and selectively binds the retinoid X receptor (RXR) with limited or antagonistic retinoic acid receptor alpha (RARα) binding activity [14]. This novel rexinoid has minimal toxicity while maintaining the ability to activate genes involved in differentiation and apoptosis [15, 16, 17]. A pilot clinical trial of UAB30 in humans demonstrated a favorable toxicity and pharmacokinetic profile with no significant change in serum triglycerides [18].
We have previously demonstrated the efficacy of UAB30 in other pediatric solid tumors, including the neuroectodermal tumor, neuroblastoma [19, 20]. This knowledge, combined with effects of other retinoids on medulloblastoma noted by others, has led us to hypothesize that treatment of group 3 medulloblastoma patient-derived xenografts (PDXs) with UAB30 would lead to decreased tumorigenesis in vitro and in vivo. The following studies focused on group 3 tumors, as they afford the greatest treatment challenge to clinicians and these patients have the poorest prognosis with current regimens, and therefore may benefit the most from novel treatment options.
MATERIALS AND METHODS
Patient-Derived Medulloblastoma Xenograft Cell Lines
Three group 3 medulloblastoma xenografts [21, 22] established from pediatric patients were used for experiments: D341 Med (D341), D384 Med (D384), and D425 Med (D425); kindly provided by Darell D. Bigner, MD, PhD, Duke University Medical Center [23, 24]. The xenografts were maintained through serial passage in athymic nude mice (Envigo, Pratville, AL). Tumors were harvested and cells were dissociated using a Tumor Dissociation Kit (Miltenyi Biotec, San Diego, CA) per manufacturer’s protocol. Cells were then washed in Roswell Park Memorial Institute (RPMI) 1640 medium, spun (150 × g × 6 minutes), and debris removed using a 70 μm cell strainer (Corning Inc., Corning, NY). The cells were maintained under standard culture conditions at 37°C and 5% CO2, in neurobasal (NB) medium (Life Technologies, Carlsbad, CA) supplemented with B-27 supplement without Vitamin A (Life Technologies), N2 supplement (Life Technologies), amphotericin B (250 μg/mL), gentamicin (50 μg/mL), L-glutamine (2 mM), epidermal growth factor (10 ng/mL; Miltenyi Biotec), and fibroblast growth factor (10 ng/mL; Miltenyi Biotec). All three medulloblastoma human PDXs were verified within the last 12 months using short tandem repeat analysis (Heflin Center for Genomic Sciences, UAB, Birmingham, AL).
Antibodies and Reagents
13-cis-retinoic acid (13-cis-RA, RA) was purchased from Sigma Aldrich (St. Louis, MO) (R3255 Sigma, CAS Number 4759–48-2). 9-cis-UAB30 (UAB30) was synthesized as previously described [25]. Anti-phospho-AKT (9271), anti-AKT (9272) and antip44/42 MAP Kinase [ERK1/2 (9102)] were obtained from Cell Signaling Technology (Danvers, MA). Anti-retinoid X receptor β (MOK13–17, ab2815) and anti-retinoic acid receptor (ab53161) were from Abcam (Abcam, Inc. Cambridge, MA). Mouse monoclonal anti-c-myc antibody was from Santa Cruz (sc-40, Santa Cruz Biotechnology, Dallas, TX). Mouse monoclonal anti-β-actin was purchased from Sigma (A1978) and mouse monoclonal GAPDH (MAB374, clone 6C5) and rabbit polyclonal anti-cleaved PARP (ab3565) were from Millipore (EMD Millipore, Billerica, MA).
Western Blotting
Briefly, cells were lysed on ice for 30 minutes in either RIPA buffer consisting of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton x-100, 1% sodium deoxcycholate, 0.1% sodium dodecyl sulfate (SDS), phosphatase inhibitor (P5726, Sigma Aldrich), protease inhibitor (P8340, Sigma Aldrich), and phenylmethylsulfonyl fluoride (PMSF, P7626, Sigma Aldrich), or mTOR buffer (for immunoblotting for AKT, phospho-AKT, ERK1/2) consisting of 40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate (P8010, Sigma Aldrich), 10 mM glyceropyrophosphate (G9891, Sigma Aldrich), 50 nM NaF, 1.5 mM Na vanadate, 0.3% CHAPS, protease inhibitors (1:100 dilution, P8340, Sigma Aldrich). The lysates were then centrifuged at 14,000 rpm for 30 minutes at 4C. After determination of protein concentrations using a Micro BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL), the proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to a Immobilon®-P polyvinylidene fluoride (PVDF) transfer membrane (EMD Millipore, Billerica, MA). Precision Plus Protein Kaleidoscope Standards (161–0375, Bio-Rad, Hercules, CA) molecular weight markers were used to confirm expected size of target proteins. Antibodies were used in accordance with the manufacturers’ recommended protocols. Samples were visualized by enhanced chemiluminescence (ECL) using Luminata Classico and Luminata Crescendo Western horseradish peroxidase (HPR) substrates (EMD Millipore). Anti-β-actin was used an internal control to ensure equal protein loading between samples.
Cell Differentiation
Medulloblastoma cells (1 × 103) were plated in a 96-well culture plate with neurobasal media and treated with vehicle, 13-cis-RA (RA, 30 μM), or 9-cis-UAB30 (UAB30, 30 μM) for 72 hours. Pictures [Photometrics CoolSNAP HQ2 CCD camera (Tucson, AZ) attached to a Nikon Eclipse Ti microscope (Tokyo, Japan)] of the cells were obtained and number of neurite outgrowths per cell were counted using the image software SPOT Basic 5.2 (Diagnostic Instruments Inc., Sterling Heights, MI) and reported as mean neurite outgrowths [26].
In Vitro Cell Viability, Apoptosis and Proliferation Assays
Cell viability was determined using an alamarBlue® assay at 72 hours after treatment with RA or UAB30 at increasing concentrations. The cells (1.5 × 103) were plated in 96well culture plates and after 5 days incubation, 10 μL of sterile alamarBlue® dye (Thermo Fisher) was added to each well. After 6 hours, the absorbance at 570 nm and 600 nm (reference) was performed using a microplate reader (Epoch Microplate Spectrophotometer, BioTek Instruments, Winooski, VT). Experiments were completed in triplicate and viability reported as fold change ± SEM. In a separate experiment, D425 cells (1.5 × 103) were incubated in standard media (bFGF, 10 ng/mL, described above) or media without bFGF added. Cells were treated with RA or UAB 30 for 72 hours and viability assessed with alamarBlue® assay.
Apoptosis was evaluated by immunoblotting for cleaved PARP. Cells were treated with RA (0, 30 μM) or UAB30 (0, 30 μM) for D341 and D425 and RA (0, 10 μM) or (0, 10 μM) UAB30 for D384 cells for 72 hours and whole cell lysates were obtained. Immunoblotting for cleaved PARP was performed as described above. Increasing presence of cleaved PARP products indicated apoptosis.
Proliferation was assessed using the CellTiter96® Aqueous One Solution Cell Proliferation assay (Promega, Madison, WI). MB cells (5 × 103 cells) were plated in 96-well plates and treated with RA or UAB30 in increasing concentrations. After 5 days, 10μL CellTiter96® dye was added to each well and the absorbance was measured at 490 nm using a microplate reader (Epoch Microplate Spectrophotometer). Experiments were repeated in triplicate and proliferation reported as fold change ± SEM.
In Vitro Limiting Dilution Assay
Cells were plated in 96 well plates with decreasing number of cells per well (1000, 100, 50, 20, 10, 1) in conditioned neurobasal media with vehicle, RA, or UAB30. After 5 days, the number of wells containing neurospheres was counted for each concentration. Extreme limiting dilution assay analysis was performed to determine significance using the online software available at http://bioinf.wehi.edu.au/software/elda/. Experiments were repeated in triplicate.
Cell Cycle Analysis
Cells were plated (1.5 × 106 cells) and treated with RA or UAB30 (0 μM, 5 μM) for 48 hours. Neurospheres were dissociated with accutase, washed with PBS, and fixed in 100% ethanol overnight. The cells underwent a second PBS wash and were stained for 1 hour with 200 μL of staining solution consisting of 20 μg/mL propidium iodide (Invitrogen), 0.1% Triton X (Active Motif, Carlsbad, CA) and RNAse A (0.1 mg/mL, Qiagen, Valencia, CA). The samples were analyzed with fluorescence activated cell sorting (FACS) using a FACSCalibur™ Flow Cytometer (BD Biosciences, San Jose, CA). Data were analyzed using the ModFit LT software (Verity Software House Inc., Topsham, ME).
Cell Motility Assays
Transwell assays for migration and invasion were performed. Micropore culture inserts (8 μm, Transwell®, Corning Inc., Corning, NY) were coated with laminin (10 μg/mL) on the bottom. Cells (1 × 106) were plated in a 6 well culture plates and treated with RA or UAB30 (0 μM, 10 μM, 30 μM) for 24 hours. Treated cells (1.5 × 105) were plated in the upper well with neurobasal media; 10% fetal bovine serum was added to the lower chamber as a chemoattractant. The cells were allowed to migrate or invade for 24 hours. The cells on the top surface were removed from the insert using a cotton swab. The inserts were then fixed in 3% paraformaldehyde and stained with crystal violet. Pictures of the inserts (×7) were taken using the image software SPOT Basic 5.2 (Diagnostic Instruments Inc., Sterling Heights, MI). The cells were then counted using ImageJ software (Ver 1.49, http://imagej.nih.gov/ij))http://imagej.nih.gov/ij)[27]. The invasion assay was performed as described above, except the invasion inserts were also coated on the top side with an extracellular matrix (ECM) gel (Matrigel 50μL, Corning Inc.) for 4 hours at 37°C. Experiments were repeated in triplicate and migration and invasion reported as fold change ± SEM.
In Vivo Tumor Growth
D425 cells (2.5 × 106 cells in 25% Matrigel™; Corning Inc.) were injected into the right flank of 6-week-old, female, athymic nude mice (Envigo). After injection, the mice were randomized to receive vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 10 mice per group). These dosages were based upon previous in vivo experiments [19, 20, 28]. The flank tumors were measured twice weekly using calipers. Tumor volume was calculated using the formula (width2 × length)/2, where length was the largest measurement. The mice were treated for 3 weeks and all animals euthanized at that time when the majority of the control tumors had reached IACUC parameters for euthanasia.
Orthotopic Tumor Growth
D341 cells (5 × 105 cells) were injected into the cerebellum of athymic nude mice. Briefly, the mice were anesthetized with ketamine (100 mg/kg) and buprenorphine (0.5 mg/kg) and then fixed into a stereotactic frame. Injections were performed using a 250 uL Hamilton syringe with a 30-gauge needle at a rate of 2.5 uL/minute, using stereotactic coordinates 1 mm to the right of midline, 2 mm posterior to the lambdoid suture at a depth of 2 mm below the skull surface.
On the day of injection, the mice were randomized to receive vehicle-treated, UAB30treated (100 mg/kg/day), or RA-treated (53 mg/kg/day) chow (n = 9 per group). These doses were based upon previous data in the literature [19, 20, 28]. The animals were monitored twice daily for neurologic symptoms and followed for overall survival. They were euthanized once they developed neurologic symptoms (e.g. hemiparesis, lethargy, anorexia) or when Institutional Animal Care and Use Committee (IACUC) criteria for euthanasia were reached. H&E staining was performed to confirm the presence of tumorigenic cells in the cerebellum of the animals.
In Vivo Disseminated Tumor Model with Bioluminescence Imaging
Six-week-old, female, athymic nude mice (Envigo) underwent injection of D341-luc cells (5 × 105 in 5 uL methylcellulose), that had been previously infected with lentiviral particles generated from the pGreenFire1 vector (System Biosciences, Palo Alto, CA) to stably express GFP luciferase [29], into the right lateral ventricle using stereotactic guidance as a model of cerebrospinal fluid (CSF) dissemination [30]. The mice received ketamine (100 mg/kg) and buprenorphine (0.5 mg/kg) for anesthesia. Injections were performed using a 250 uL Hamilton syringe with a 30-gauge needle at 2.5 uL/minute; the needle was placed 1 mm to the right and 0.5 mm posterior to the bregma, and 2.3 mm below the skull surface. Beginning on the day of injection, mice were randomized (n = 8 per group) to receive either vehicle-treated, RA-treated (53 mg/kg/day), or UAB30treated (100 mg/kg/day) chow, as described above. Bioluminescence imaging was performed one week following injection, and then again on days 11, 14, 18, and 20. Bioluminescence imaging was performed using the IVIS Lumina Series III Imaging System (Perkin Elmer, Waltham, WA). All mice were euthanized on post-injection day 20 when the majority of the animals had begun to develop neurologic symptoms. Tumor growth was evaluated by quantifying the bioluminescence signals using the integrated fluxes of photons within each area of interest using the Living Images Software Package 3.2 (Xenogen-Perkin Elmer). H&E staining was performed to confirm the presence of tumorigenic cells tracking along the spinal column of the mice.
Immunohistochemistry
Formalin-fixed, paraffin-embedded animal tumor specimens were cut (6 μm sections), baked at 70 °C for 1 hour, deparaffinized, rehydrated and steamed. Standard hematoxylin and eosin staining was completed.
Statistical Analyses
Experiments were performed at a minimum of triplicate. Data reported are the mean ± standard error of the mean. Data between groups was compared using an analysis of variance or Student’s t test as appropriate. Survival curves were generated using SigmaPlot v12.0 (Systat Software Inc, San Jose, CA) and Kaplan-Meier analysis was performed. Log-rank test was used to determine survival significance. Statistical significance was defined as p ≤ 0.05.
RESULTS
Retinoids cause morphologic changes in MB cells.
UAB30 selectively binds to the retinoid X receptor (RXR) [17]. Immunoblotting was utilized to detect RXR expression in the MB PDX cells to be studied. Whole cell lysates of D425, D384, and D341 MB PDX cells were examined and it was noted that all 3 PDXs expressed protein for the RXR receptor (Fig 1 a). Although not necessary for the function of UAB30, RAR is required for RA function. Immunoblotting also showed protein expression of this receptor in all three MD PDXs (Online Resource Figure 1). Retinoids function through cell differentiation [31]. The appearance of neurite outgrowths on neural cells is indicative of cell differentiation [32–34]. We analyzed MB cells for morphologic changes following treatment with RA (30 μM) and UAB30 (30 μM). After 72 hours the control cells remained relatively rounded without significant growth of neurite processes (Fig 1 b, left panels). In the MB PDX cell lines treated with RA or UAB30, the cells showed signs of differentiation including the development of elongated cell bodies and neurite outgrowths (Fig 1 b, middle and right panels, black arrows). Neurite outgrowths were quantified and reported in graphic form (Fig 1 c), and were significantly increased in all 3 PDXs following treatment.
Figure 1. RA and UAB30 induced cellular differentiation in MB cell lines.

a Whole cell lysates from human MB PDXs D425, D384, and D341 were examined with immunoblotting to determine RXR expression. All three PDXs had protein for RXR detected. b Human MB PDX cells were treated with RA or UAB30 at 30 μM for 72 hours and photographs were obtained. Cells were examined for the presence of neurite outgrowths indicating differentiation. In all cell lines, neurite outgrowths (black arrows) were seen following RA and UAB30 treatment. c Neurite outgrowth from three independent experiments was quantitated and presented in graphic form as mean ± SEM.
RA and UAB30 decreased cell viability and proliferation and increased apoptosis.
Although primarily known as differentiating agents, retinoids have been shown to decrease viability in some cancer cell lines. Viability was evaluated in MB PDX cells following RA or UAB30 treatment. MB PDX cells were treated with increasing concentrations of RA or UAB30 for 72 hours and cell viability assessed. Treatment with both RA and UAB30 resulted in a significant decrease in cell viability in all three PDXs (Fig 2 a). The D384 PDX was the most sensitive to retinoid therapy (Fig 2 a, middle panel). In the D341 cells, viability decreased in a dose-dependent fashion with an LD50 of approximately 30 μM for both RA and UAB30 (Fig 2 a, left panel). The D425 cells were the least sensitive to retinoid therapy, but viability was still affected significantly with doses as low as 10 μM of both RA and UAB30 (1.0 vs. 0.76, and 1.0 vs. 0.49, p ≤ 0.02, respectively) (Fig 2 a, right panel).
Figure 2. RA and UAB30 treatment led to decreased MB cell survival and proliferation and increased apoptosis.

a D341, D384, D425 MB PDX cells were treated with RA or UAB30 at increasing concentrations. After 72 hours of treatment, cell viability was measured with alamarBlue® assays and data reported as mean ± standard error of the mean. There was a statistically significant decrease in viability in all cell lines following RA and UAB30 treatment starting at 10 μM. b Since these compounds resulted in decreased cell survival, we examined the effects of RA and UAB30 on apoptosis in the MB cell. MB PDX cells were treated with RA or UAB30 30 μM for 72 hours and lysates were examined with immunoblotting for PARP cleavage products. There was an increase in cleaved PARP in all cell lines, indicating apoptosis was occurring. c Cell proliferation was detected utilizing CellTiter96® assays. MB PDX cells were treated with increasing concentrations of RA or UAB30 for 5 days. There was a significant decrease in proliferation in all three PDX’s with both RA and UAB30. Bars represent mean ± SEM.
Apoptosis is one mechanism of cell death. There was a significant increase in the expression of cleaved PARP protein in all 3 MB PDXs following treatment with RA or UAB30 (Fig 2 b). These data suggest that treatment with the retinoids studied led to apoptosis.
Since retinoids are known to cause cells to differentiate and thereby decrease proliferation, the effects of RA and UAB30 on MB PDX proliferation was investigated. RA and UAB30 treatment resulted in diminished proliferation in all three MB PDXs (Fig 2 c). Similar to the pattern seen with viability, the D384 cells were the most sensitive to RA and UAB30 (Fig 2 c, middle panel).
RA and UAB30 decreased sphere forming capacity.
The ability of cells to form neurospheres in vitro is a hallmark of cell self-renewal [35]. We evaluated the effect of RA and UAB30 on neurosphere formation using an in vitro extreme limiting dilution assay (ELDA) and analysis. The ELDA demonstrated a significant decrease in neurosphere formation in the D341, D384, and D425 PDX cells following treatment with vehicle compared to RA or vehicle compared to UAB30 (Fig 3 a-c). There was no difference in neurosphere formation between RA and UAB30 treatment groups in any of the PDX cell lines. This decrease in neurosphere formation indicated a loss of self-renewal or stemness with retinoid treatment.
Figure 3. RA and UAB30 decrease sphere forming capacity in MB PDXs.

a b c PDX cells were plated in 96 well plates with decreasing number of cells per well (1000, 100, 50, 20, 10, 1) in conditioned neurobasal media with vehicle, RA, or UAB30. After 5 days, the number of wells containing spheres was counted for each concentration. Extreme limiting dilution assay analysis was performed and RA and UAB30 both significantly decreased sphere forming capacity in a D341, b D384, and c D425 MB PDX cells. There was no difference in neurosphere formation between RA and UAB30 treatment groups in any PDX. Representative photographs of plates are presented on the graphs.
RA and UAB30 resulted in cell cycle changes.
Retinoids have been shown to lead to cell cycle arrest through an increase G0/G1 phase of the cell cycle [36, 37]. After 48 hours of treatment of RA (0, 5 μM) or UAB30 (0, 5 μM), MB PDX cells were found to have a lack of progression through the cell cycle with an increased percentage of cells in G1 phase and decreased percentage of cells in S phase. Representative histograms from each PDX have been displayed (Fig 4 a). The data from all replications have been demonstrated in graphic form (Fig 4 b) and in Table 1.
Figure 4. RA and UAB30 treatment of MB PDXs led to cell cycle arrest.

a Representative histograms for cell cycle analysis of D341, D384 and D425 human MB PDX cells following treatment with RA or UAB30 (0 μM, 5 μM) for 48 hours. Cells were analyzed by flow cytometry following staining with propidium iodine. There was an increase in the percentage of cells in the G1 phase and a decrease in the percentage in S phase following treatment. b Graphic representation of cell cycle analysis in D341, D384 and D425 MB PDX cells treated with RA or UAB30. There was a significant increase in the G1 phase in cells from all three PDXs (*p ≤ 0.05) and a significant decrease in S phase in all 3 PDXs (*p ≤ 0.05) after treatment, indicating failure to progress through the cell cycle. Experiments were repeated at least in triplicate and reported as mean ± SEM.
Table 1.
Cell cycle analysis following treatment of MB human PDX cells with RA or UAB30.
| G1 (mean ± SEM) | S (mean ± SEM) | G2 (mean ± SEM) | |
|---|---|---|---|
| D341 | |||
| Control (%) | 50.6 ± 0.7 | 45.2 ± 1.5 | 4.2 ± 0.8 |
| RA (%) | 60.7 ± 0.9* | 35.7 ± 0.8* | 3.6 ±0.5 |
| UAB30 (%) | 64.4 ± 0.6* | 33.3 ± 0.7* | 4.8 ± .2 |
| D384 | |||
| Control (%) | 37.9 ± 0.8 | 28.4 ± 1.5 | 33.7 ± 0.7 |
| RA (%) | 72.1 ± 0.9* | 12.5 ±0.2* | 15.4 ±0.9* |
| UAB30 (%) | 66.5 ± 1.9* | 9.9 ± 1.7* | 23.6 ± 0.4 |
| D425 | |||
| Control (%) | 54.5 ± 1.0 | 34.0 ± 0.8 | 11.5 ± 0.9 |
| RA (%) | 67.1 ± 0.9* | 21.3 ± 1.3* | 11.5 ± 0.9 |
| UAB30 (%) | 69.6 ±2.2* | 20.9 ± 0.8* | 9.5 ± 1.5 |
p ≤ 0.05 versus Control
RA and UAB30 decreased cell migration and invasion
We have previously demonstrated that UAB30 causes decreased cell migration and invasion in other pediatric solid tumors [19, 20], prompting us to investigate whether similar phenotypic changes occur in MB PDX cells following RA or UAB30 treatment. Using Transwell™ plates, we noted that both RA and UAB30 significantly decreased cell migration and invasion in all three MB PDXs. Representative photomicrographs of the migration and invasion inserts have been shown (Fig 5 a, b, panels below graphs). Cell counts were obtained from at least three independent experiments using ImageJ Software and the fold change in migration and invasion has been displayed graphically in figure 5 a, b.
Figure 5. RA and UAB30 decreased MB PDX cell invasion and migration.

a For cell migration, cells (1 × 106) were plated in a 6 well culture plates and treated with RA or UAB30 (0 μM, 10 μM, 30 μM) for 24 hours. Treated cells (1.5 × 105) were plated in Transwell® culture plates in the upper well with neurobasal media; 10% fetal bovine serum was added to the lower chamber as a chemoattractant. The cells were allowed to migrate for 24 hours, then were fixed, stained and counted and migration reported as fold change ± SEM. Migration was significantly inhibited in all 3 PDXs with 10 μM RA and UAB30. Representative photomicrographs of the 10 μM treated migration plates are provided below the migration graphs. b Invasion was assessed in a similar fashion. Treated cells were plated in Transwell® culture plates with Matrigel™ coating the top side of the insert. Cells were allowed to invade for 24 hours, and then fixed, stained and counted. Invasion was reported as fold change ± SEM. Invasion was significantly decreased in all 3 MB PDXs beginning at 10 μM RA or UAB30. Representative photomicrographs of the 10 μM treated invasion plates are provided below the graphs.
Effects of RA or UAB30 on AKT, ERK, and c-myc
Various pathways have been implicated as the mechanism of action of retinoids. Some authors have postulated that changes in AKT or ERK activation may be responsible for the differentiating effects of retinoids. It has been shown that RA and UAB30 affected both AKT and ERK dependent pathways in neuroblastoma cell lines, another neuroectodermal pediatric tumor [19, 33, 34, 38]. Additionally, Chang and colleagues found that c-myc expression was decreased in DAOY MB cells following treatment with all-trans-retinoic acid (ATRA) [37]. Therefore, we wished to determine if any of these mechanisms came into play with RA or UAB30 in the 3 MB PDX cell lines under study. MB PDX cells were treated with RA or UAB30 (0, 10, 30, 50 μM) for 48 hours and immunoblotting was performed to detect total and phosphorylated AKT, total ERK1/2 and c-myc. There were no consistent changes in total or phosphorylated AKT expression (Fig. 6 a). ERK1/2 was consistently decreased in all three PDX cell lines with RA or UAB30 treatment. We also studied c-myc expression in treated D425 and D384 cell lysates and found a decrease in c-myc protein in both the D384 and D425 cells with both RA and UAB30 (Fig. 6 c). Expression of c-myc was not detected in D341 cell lysates. These findings suggested that the changes seen in differentiation and cellular survival induced by RA or UAB30 likely did not involve AKT pathways, but that changes in ERK and c-myc may contribute to some of the phenotypic changes noted.
Figure 6. AKT, ERK1/2 and c-myc expression in MB PDX cells with RA or UAB30 treatment.

D341, D384, and D425 cells were treated with RA or UAB30 (0, 10, 30, 50 μM) for 48 hours and whole cell lysates obtained. a Immunoblotting revealed a variable change in AKT phosphorylation following treatment with RA or UAB30; AKT phosphorylation was increased in D341 cells, unchanged in D384 cells, and increased in D425 cells. Total AKT expression was unchanged. b Immunoblotting for ERK1/2 and demonstrated a decrease in total ERK1/2 in D341, D384, and D425 MB PDX cells with both RA and UAB30. c Immunoblotting for c-myc in D384 and D425 cell lysates showed decreased protein expression in both MB PDX cell lysates after RA or UAB30 treatment.
MB flank xenografts
Several in vivo models were utilized to evaluate changes in MB growth following retinoid therapy. First, we evaluated flank tumor growth using the D425 cell line, the least sensitive cell line of the three in in vitro studies. This model allowed us to accurately follow change in tumor size over time with tumor volume measurements every two days. Following injection of D425 cells (2.5 × 106) into the right flank of athymic nude mice, animals were randomized to receive vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 10 mice per group). These doses were based on previous animal studies and have been shown to be well-tolerated in this species [19, 20]. Flank tumors were measured twice per week and animals were euthanized when tumors reached IACUC parameters. Animals treated with UAB30 had significantly smaller tumors than vehicle-treated controls beginning at day 13 of treatment (Fig 7 a). Tumors in RA-treated animals were smaller than controls, but only reached statistical significance at 17 days (Fig 7 a). There were no significant differences in changes in tumor volumes between RA and UAB30 treated animals (Fig 7 a).
Figure 7. RA and UAB30 decreased tumor growth in MB tumor bearing mice.
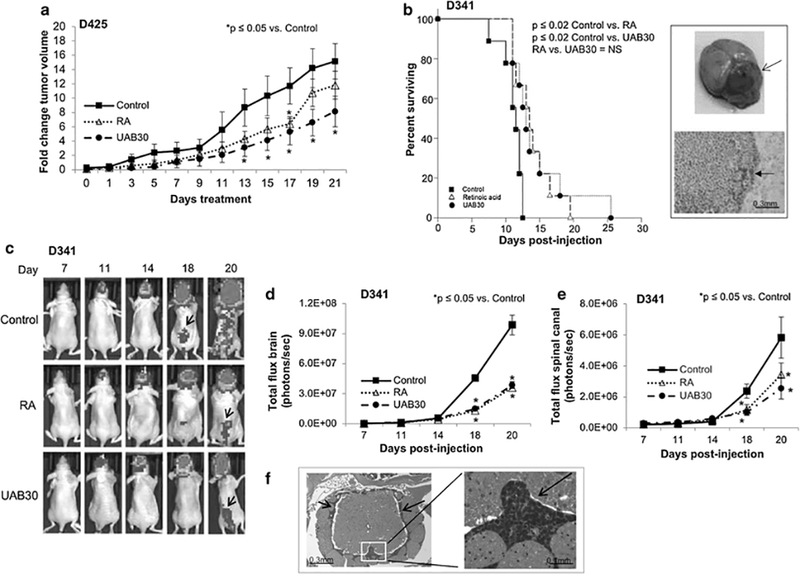
a D425 cells (2.5 × 106) were injected into the right flank of athymic nude mice and animals were randomized to receive vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 10 mice per group). Flank tumors were measured twice per week. Animals treated with UAB30 had significantly smaller tumors than vehicle treated controls beginning at day 13. Tumors in RA treated animals were smaller than controls, but only reached statistical significance at 17 days. There were no significant differences in changes in tumor volumes between RA and UAB30 treated animals. b D341 cells (5 × 105 cells) were injected into the cerebellum of nude mice and the animals were randomized to receive treatment with vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 9 mice per group). The mice were monitored twice daily for the development of neurologic symptoms (e.g. hemiparesis, lethargy). Animals receiving RA and UAB30 had significantly prolonged survival compared to animals receiving control chow. Open arrow in upper insert demonstrates intracerebellar location of the tumor and H&E staining shown in the bottom insert shows infiltrating MB tumor cells in the cerebellum (bottom panel, closed arrow). c Following intraventricular injection of D341 cells (5×105 cells), animals were randomized to receive treatment with vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 8 mice per group). The mice were monitored daily for the development of neurologic symptoms twice daily. Bioluminescence imaging was utilized to follow development of tumors and representative bioluminescence images are shown. There are marked differences in tumor burden in the brains and spinal canals (black arrows) of the animals treated with vehicle alone compared to RA or UAB30 treated animals. There was a statistically significant difference in the tumor burden found in the brain and spinal canal between the animals treated with RA or UAB30 compared to control vehicle-treated animals. There was no significant difference in brain or spinal canal tumor burden between the RA or UAB30 groups. d Bioluminescence data for tumor burden in the brain. There were significant decreases in total flux in animals treated with RA or UAB30. There were no differences between RA and UAB30 treated animals. e Bioluminescence data for tumor burden in the spinal canal. There were significant decreases in total flux in animals treated with RA or UAB30 compared to control animals. There were no differences between RA and UAB30 treated animals. f H&E staining confirmed tumor cells were present in the spinal canal (left panel, black arrows, right panel black arrows).
Intracerebellar MB murine model
We further tested the in vivo effects of UAB30 using an orthotopic intracerebellar MB tumor model in athymic nude mice. Following intracerebellar injection with D341 cells (5 × 105 cells), the mice were randomized to receive treatment with vehicle-treated, RAtreated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 9 mice per group). The mice were monitored daily for the development of neurologic symptoms (e.g. hemiparesis, lethargy). Mice were euthanized once they developed neurologic symptoms or met other IACUC criteria. Kaplan-Meier curves were generated and survival was compared using a log-rank analysis. Animals receiving RA and UAB30 had significantly prolonged survival compared to animals receiving control chow (p < 0.02, RA vs. control and p < 0.02, UAB30 vs. control) (Fig 7 b). The median survival increased from 11.5 days in the control group to 13 days with RA and 13.5 days with UAB30. H&E staining confirmed intracerebellar location of the tumors and that they recapitulated histology seen in human medulloblastoma. Insert in figure 7 b demonstrates intracerebellar location of the D341 tumor (Fig 7 b, top panel open arrow). The bottom insert is a photomicrograph of an H&E stained slide showing infiltrating medulloblastoma tumor cells in the cerebellum (Fig 7 b, bottom panel, closed arrow).
Disseminated MB murine model
Since MB has a propensity for leptomeningeal spread via the cerebral spinal fluid, and nearly one third of MB patients present with evidence of metastatic disease [39], we next chose to utilize an intraventricular model of MB to evaluate the effects of RA and UAB30 on leptomeningeal metastasis. Following intraventricular injection of D341 human MB PDX cells (5 × 105 cells), animals were randomized to receive treatment with vehicle-treated, RA-treated (53 mg/kg/day), or UAB30-treated chow (100 mg/kg/day) (n = 8 mice per group). The mice were monitored daily for the development of neurologic symptoms (e.g. hemiparesis, lethargy). Mice were euthanized once they developed neurologic symptoms or met other IACUC criteria. Bioluminescence imaging was utilized to follow development of tumors in the brain and spinal canal. Representative bioluminescence images are shown in figure 7 c. There were marked differences in tumor burden in the brains and spinal canals (Fig 7 c, black arrows) of the animals treated with vehicle alone compared to RA or UAB30 treated animals. There was a statistically significant difference in the brain and spinal canal tumor burden in the animals treated with RA or UAB30 compared to control vehicle-treated animals. There was no significant difference in brain or spinal canal tumor burden between the RA or UAB30 groups. Data are presented in graphic form in figure 7 d, e. Animals in the control group consistently lost more weight than those in the treatment groups, likely related to results of increasing tumor burden. There were no statistically significant differences between weights in the animals treated with RA versus UAB30 (Online Resource Figure 2). H&E staining confirmed tumor cells were present in the spinal column (Fig 7 f, left panel, black arrows, right panel black arrows).
DISCUSSION
Despite advances in therapy, the outcomes for children with group 3 MB remains poor and long term survivors suffer significant morbidities secondary to treatment [5, 6]. Retinoids induce cell differentiation and are used as standard therapy in other pediatric neuroectodermal tumors such as neuroblastoma [40, 41]. Other groups have also investigated the role of retinoids for use in MB [8]. In the current study, we have investigated the role of a novel RXR agonist (UAB30), with a vastly improved toxicity profile, in group 3 MB and demonstrated that UAB30 treatment resulted in cell differentiation, decreased tumor motility and decreased tumor proliferation both in vitro and in vivo.
13-cis-retinoic acid (isotretinoin, RA) was chosen as the comparison with UAB30 in this study as RA is the compound currently utilized in the treatment of the pediatric neuroectodermal solid tumor, neuroblastoma [42]. The three MB PDX cell lines had varying sensitivities to RA and UAB30. These results were consistent with findings of other authors. Bai and colleagues investigated 6 different MB cell lines and found varying retinoid sensitivities among them, but attributed this variance to overexpression of OTX2. Further, they showed that 9-cis-retinoic acid was more effective than 13-cisretinoic acid in decreasing MB growth [8]. Di and others also showed that OTX2 expression was essential for ATRA-induced apoptosis in MB cells [9]. Since all three of the PDXs used in the current study expressed OTX2 [9], this explanation does not likely completely account for our findings. Since available therapies for MB result in significant toxicities for long-term survivors, and considering that the side effect profile for UAB30 may be more benign than that of RA [18, 43], these findings would portend well for the further investigation of UAB30 as a potential agent for MB therapy.
Some researchers have hypothesized that the effects of retinoids are dependent upon the expression of the RARs and RXRs. These receptors are sequence-specific, ligandactivated transcription factors [44]. ATRA and 13-cis-retinoic acid activates RARs, UAB30 primarily activates RXRs [15, 19], and 9-cis-retinoic acid activate both [45]. In the current study, we noted that D384, D341 and D425, express both RAR and RXR receptors. These findings have not been reported previously in D341 and D384, but other investigators have shown similar findings in D425 cells [46] and Hallahan and colleagues found RAR to be expressed in D341 cells [47]. Proliferation and viability were most affected in D384 cells by UAB30 and these cells had more pronounced expression of RXR expression when compared to D341 and D425 (Fig 1 a), implying that RXR expression may influence the effects of UAB30 on MBs. However, the expression of RXR does not fully explain the current findings, as D341 and D425 cells were also sensitive to treatment with UAB30, suggesting that there are likely other mechanisms involved in the effects of rexinoids upon cell viability and proliferation. These results are similar to those reported previously in neuroblastoma cell lines, where amount of expression of RXR did not necessarily correlate with phenotypic changes noted in the treated cell lines, and it was only important that the RXR was expressed [19]. Further, Fu and colleagues have shown in MB cell lines that RXR receptors were present in both retinoic acid sensitive (Med-3) and resistant (UW228–2 and UW228–3) cell lines [48].
In these studies, there were varying degrees of apoptosis with UAB30 treatment depending upon which cell line was being investigated. Although strong increases in PARP cleavage were noted in the D341 and D425 PDX cell lines, it was not seen in the D384 cell line, despite this PDX showing increased sensitivity to RA and UAB30 from the standpoint of viability. When another marker of apoptosis was studied, cleaved caspase 3, apoptosis was evident in the D384 cells (Online Resource Figure 3). These findings are not unique. Celay et al noted variations in apoptosis in neuroblastoma cell lines that were treated with ATRA that were not only cell line dependent, but also time dependent. In their study, one cell line took up to nine days of treatment before showing changes in TUNEL assay and another cell line showed no change in TUNEL assay even after 9 days of treatment [49].
The exact mechanisms involved in UAB30-induced MB cell differentiation are unknown and remain to be elucidated. Evidence exists that RA-induced neuroblastoma cell differentiation is dependent upon AKT activation [32, 33, 38]. Other researchers were not able to demonstrate this finding [19, 50]. In the present study, we did not consistently demonstrate AKT activation following RA or UAB30 treatment. The D341 PDX did show an increase in AKT phosphorylation, but the D425 PDX had decreased AKT phosphorylation following treatment and AKT phosphorylation was unchanged in the D384 cell line (Fig 6 a). Previous studies have also shown that the ERK pathway may be involved. Cheepala and colleagues showed that ATRA decreased total ERK expression in skin cancer studies [51]. Fenretinide, another retinoid, was found to decrease ERK expression in ONS-76 MB cells [52]. In the current study, total ERK expression was diminished in all 3 MB PDX cell lines following RA or UAB30 treatment. More in-depth investigations of the mechanisms involved will be the subject of subsequent studies.
Retinoids are known to affect progression through the cell cycle and in the current study we examined the effects of UAB30 and RA on the cell cycle in 3 MB PDX cell lines. Both UAB30 and RA led to changes in the percentage of cells in the G1 and S phases of the cell cycle in all 3 PDXs. Previous investigators noted cell cycle arrest in DAOY cells after treatment with ATRA. They did not, however, see G1/S cell cycle arrest in D384 or D283 cells treated with ATRA. Again, the mechanisms responsible for their findings were not clear [37], but hypothesized that decreased c-myc may be responsible in the DAOY cells. Likewise, we noted decreased c-myc in the D425 and D384 PDX cells with both RA and UAB30 treatment, and did find a lack of progression through the cell cycle in these cells. Clearly, the delineation of these mechanisms will be the subject of intense future research.
A flank tumor model was utilized to examine the effects of UAB30 upon the growth of D425 PDX cells. This model was chosen rather than an intracranial model to provide the opportunity to more closely and objectively follow the growth of the tumors over time, as D425 tumors grow exceedingly quickly in the orthotopic position leading to early and rapid demise of the animals. There was a significant decrease in tumor growth in the animals treated with UAB30 compared to controls, but not in the animals treated with RA compared to controls (Fig 7 a). This finding is not entirely unique. Bai et al noted that 9-cis-retinoic acid was effective in decreasing D425 flank tumors in mice but not intracranial growth of D425 tumors. They surmised that it was the presence of bFGF in the brain that rendered the 9-cis-retinoic acid ineffective [8]. We examined cell survival following treatment of D425 cells by RA and UAB30 in media with and without the addition of bFGF, and saw no statistically significant difference in cell survival, and cell survival was actually slightly more affected by the compounds when treated in bFGF containing media (Online Resource Figure 4).
There has been a recent trial through the Children’s Oncology Group (ACNS0332) using isotretinoin (RA) for the treatment of high-risk MB and supratentorial PNET’s. At the time of trial inception, there was a lack of understanding of tumor heterogeneity. Subsequent genomic data have revealed that the heterogeneity of the tumors enrolled in the study was so significant that the supratentorial PNET arm closed. Out of 31 nonpineal tumors included in the study, 22 represented tumors that were thought to be supratentorial PNETs but genomics actually revealed that they were high-grade gliomas (18), ependymomas (2), and atypical teratoid/rhabdoid tumors [ATRT, (2)]. In addition, the eligibility criteria for MB patients on this study only included tumors with residual disease > 1.5 cm3, metastatic disease or diffusely anaplastic disease. By 2012, consensus emerged that there are at least four distinct molecular subgroups of MB that differ in their demographics, transcriptomes, somatic genetic events, and clinical outcomes [53]. More recent data have emerged to indicate that significant molecular heterogeneity exists within the four subgroups [54]. These findings are leading to major changes in risk stratification of MB patients and clinical trial designs have already begun to be altered centered on these changes. Therefore, based on lack of genomic-based eligibility criteria used for ACNS0332, it is not surprising that a futility analysis performed in 2014 concluded that treatment of MB with RA as performed in the context of the study would not lead to a significant survival advantage. Because of the current genetic data, it is possible that RA will provide clinical benefit to patients with a specific molecular subgroup or subgroups of MB. Our study suggests that subgroup 3, which portends the worst prognosis, may benefit from this therapy.
In the current study we demonstrated that group 3 MB cell survival was decreased by a novel RXR agonist, UAB30. UAB30 also led to alterations in the cell phenotype including differentiation, failure of cell cycle progression, and decreased motility. Most importantly, UAB30 and RA treatment resulted in a decrease in tumor growth and increased survival in mice bearing human MB PDX tumors in the flank and in an intracranial location. This study also employed the novel model of intraventricular tumor injection to study leptomeningeal MB spread, and showed significantly decreased tumor burden in the spinal canals of animals treated with RA or UAB30. These findings suggest that UAB30 should be further investigated as a potential therapeutic adjunct for MB treatment.
Supplementary Material
IMPORTANCE OF THE STUDY.
Molecular group 3 pediatric medulloblastomas portend a poor prognosis with overall survival of only 50% despite multimodal therapy including surgery, craniospinal radiation, and cytotoxic chemotherapy. 13-cis-retinoic acid (RA) has been studied as an apoptotic agent to target high-risk medulloblastoma and supratentorial primitive neuroectodermal tumors, but the diversity of tumors tested and the added toxicities caused by RA in these heavily-treated patients limited its use. 9-cis-UAB30 (UAB30), a novel synthetic analog of 9-cis-RA, has minimal toxicity in humans while maintaining the ability to activate genes involved in differentiation and apoptosis. In three patient-derived group 3 medulloblastoma xenograft models, we demonstrate that UAB30 resulted in cell differentiation; decreased viability, proliferation, migration and invasion; and G1 cell cycle arrest. Further, UAB30 treatment of mice bearing medulloblastoma tumors resulted in a significant decrease in tumor growth and leptomeningeal metastases compared to controls. These data support therapeutic application of UAB30 to target group 3 medulloblastoma.
ACKNOWLEDGMENTS:
Funding provided by the University of Alabama, Birmingham, Comprehensive Cancer Center to G.K.F. and E.A.B. National Institutes of Health (T32 CA091078 to E.F.G. and L.L.S.) (T32 CA183926 to A.P.W.) and (P30 AR048311 and P30 AI27667 to the University of Alabama Flow Cytometry Core).
Footnotes
Ethical Approval
All applicable international, national, and /or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The University of Alabama, Birmingham Institutional Animal Care and Use Committee approved all animal experiments (IACUC-09355, IACUC10299). This article does not contain any studies with human participants performed by any of the authors.
CONFLICT OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Bäcklund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM (2012) Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL (2011) Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberhart CG (2012) Three down and one to go: modeling medulloblastoma subgroups. Cancer Cell 21:137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer SL, Reddick WE, Gajjar A (2007) Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol 2:1040–1049. [DOI] [PubMed] [Google Scholar]
- 6.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, Leisenring WM, Meacham LR, Mertens AC, Mulrooney DA, Oeffinger KC, Packer RJ, Robison LL, Sklar CA (2009) Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 27:2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J, Xia S (2011) Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene 30: 3454–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai R, Siu IM, Tyler BM, Staedtke V, Gallia GL, Riggins GJ (2010) Evaluation of retinoic acid therapy for OTX2-positive medulloblastomas. Neuro Oncol 12:655663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di C, Liao S, Adamson DC, Parrett TJ, Broderick DK, Shi Q, Lengauer C, Cummins JM, Velculescu VE, Fults DW, McLendon RE, Bigner DD, Yan H (2005) Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res 65:919–924. [PubMed] [Google Scholar]
- 10.Karmakar S, Banik NL, Ray SK (2008) Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer 112:596–607. [DOI] [PubMed] [Google Scholar]
- 11.Karmakar S, Banik NL, Patel SJ, Ray SK (2007) Combination of all-trans retinoic acid and taxol regressed glioblastoma T98G xenografts in nude mice. Apoptosis 12:2077–2087. [DOI] [PubMed] [Google Scholar]
- 12.Spiller SE, Ditzler SH, Pullar BJ, Olson JM (2008) Response of preclinical medulloblastoma models to combination therapy with 13-cis-retinoic acid and suberoylanilide hydroxamic acid (SAHA). J Neurooncol 87:133–141. [DOI] [PubMed] [Google Scholar]
- 13.David M, Hodak E. Lowe NJ. Adverse effects of retinoids (1988) Med Toxicol Advers Drug Exp 3:273–288. [DOI] [PubMed] [Google Scholar]
- 14.Muccio DD, Brouillette WJ, Breitman TR, Taimi M, Emanuel PD, Zhang X, Chen G, Sani BP, Venepally P, Reddy L, Alam M, Simpson-Herren L, Hill DL (1998) Conformationally defined retinoic acid analogues. 4. Potential new agents for acute promyelocytic and juvenile myelomonocytic leukemias. J Med Chem 41:1679–1687. [DOI] [PubMed] [Google Scholar]
- 15.Grubbs CJ, Hill DL, Bland KI, Beenken SW, Lin TH, Eto I, Atigadda VR, Vines KK, Brouillette WJ, Muccio DD (2003) 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett 201:17–24. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Chaudhary SC, Atigadda VR, Belyaeva OV, Harville SR, Elmets CA, Muccio DD, Athar M, Kedishvili NY (2016) Retinoid X receptor agonists upregulate genes responsible for the biosynthesis of all-trans-retinoic acid in human epidermis. PLoS One 11:e0153556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vedell PT, Lu Y, Grubbs CJ, Yin Y, Jiang H, Bland KI, Muccio DD, Cvetkovic D, You M, Lubet R (2013) Effects on gene expression in rat liver after administration of RXR agonists: UAB30, 4-methyl-UAB30, and Targretin (Bexarotene). Mol Pharmacol 83:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolesar JM, Hoel R, Pomplun M, Havighurst T, Stublaski J, Wollmer B, Krontiras H, Brouillette W, Muccio D, Kim K, Grubbs CJ, Bailey HE (2010) A pilot, first-inhuman, pharmacokinetic study of 9cUAB30 in healthy volunteers. Cancer Prev Res (Phila) 3:1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters AM, Stewart JE, Atigadda VR, Mroczek-Musulman E, Muccio DD, Grubbs CJ, Beierle EA (2015) Preclinical Evaluation of a Novel RXR Agonist for the Treatment of Neuroblastoma. Mol Cancer Ther 14:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters AM, Stewart JE, Atigadda VR, Mroczek-Musulman E, Muccio DD, Grubbs CJ, Beierle EA(2016) Preclinical Evaluation of UAB30 in Pediatric Renal and Hepatic Malignancies. Mol Cancer Ther 15:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, Knevels E, Schmidt T, Farrar CT, Vakoc BJ, Mohan N, Chung E, Roberge S, Peterson T, Bais C, Zhelyazkova BH, Yip S, Hasselblatt M, Rossig C, Niemeyer E, Ferrara N, Klagsbrun M, Duda DG, Fukumura D, Xu L, Carmeliet P, Jain RK (2013) Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, Xu H, Han X, Markert JM, Beierle EA, Gillespie GY (2016) Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol 18;227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XM, Skapek SX, Wikstrand CJ, Friedman HS, Trojanowski JQ, Kemshead JT, Coakham HB, Bigner SH, Bigner DD (1989) Phenotypic analysis of four human medulloblastoma cell lines and transplantable xenografts. J Neuropathol Exp Neurol 48:48–68. [DOI] [PubMed] [Google Scholar]
- 24.He XM, Wikstrand CJ, Friedman HS, Bigner SH, Pleasure S, Trojanowski JQ, Bigner DD (1991) Differentiation characteristics of newly established medulloblastoma cell lines (D384 Med, D425 Med, and D458 Med) and their transplantable xenografts. Lab Invest 64:833–843. [PubMed] [Google Scholar]
- 25.Atigadda VR, Vines KK, Grubbs CJ, Hill DL, Beenken SL, Bland KI, Brouillette WJ, Muccio DD (2003) Conformationally defined retinoic acid analogues. 5. Large-scale synthesis and mammary cancer chemopreventive activity for (2E,4E,6Z,8E)-8-(3’,4’-dihydro-1’(2’H)-naphthalen-1’-ylidene)-3,7-dimethyl-2,4,6-octatrienoic acid (9cUAB30). J Med Chem 46:3766–3769. [DOI] [PubMed] [Google Scholar]
- 26.Shah N, Wang J, Selich-Anderson J, Graham G, Siddiqui H, Li X, Khan J, Toretsky J (2014) PBX1 is a favorable prognostic biomarker as it modulates 13cis retinoic acid-mediated differentiation in neuroblastoma. Clin Cancer Res 20:4400–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindeblad M, Kapetanovic IM, Kabirov KK, Dinger N, Mankovskaya I, Morrisey R, Martín-Jiménez T, Lyubimov A (2011) Assessment of oral toxicity and safety of 9-cis-UAB30, a potential chemopreventive agent, in rat and dog studies. Drug Chem Toxicol 34:300–310. [DOI] [PubMed] [Google Scholar]
- 29.Walker K, Hjelmeland A (2014) Method for efficient transduction of cancer stem cells. J Cancer Stem Cell Res 2 pii: e1008 Epub 2014 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studebaker AW, Hutzen B, Pierson CR, Russell SJ, Galanis E, Raffel C (2012) Oncolytic measles virus prolongs survival in a murine model of cerebral spinal fluid-disseminated medulloblastoma. Neuro Oncol 14:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudas LJ, Wagner JA (2011) Retinoids regulate stem cell differentiation. J Cell Physiol 226:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson JA, Sidell N (1985) Ultrastructural features of a human neuroblastoma cell line treated with retinoic acid. Neuroscience 14:1149–1162. [DOI] [PubMed] [Google Scholar]
- 33.Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, Tredici G (2004) Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res 75:241–252. [DOI] [PubMed] [Google Scholar]
- 34.Qiao J, Paul P, Lee S, Qiao L, Josifi E, Tiao JR, Chung DH (2012) PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Comm 424:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annabi B, Rojas-Sutterlin S, Laflamme C, Lachambre MP, Rolland Y, Sartelet H, Béliveau R (2008) Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res 6:907–916. [DOI] [PubMed] [Google Scholar]
- 36.Di Martino D, Avignolo C, Marsano B, Di Vinci A, Cara A, Giaretti W, Tonini GP (1990) Neurite outgrowth and cell cycle kinetic changes induced by cisdiamminedichloroplatinum II and retinoic acid in a human neuroblastoma cell line. Cancer Lett 52:101–106. [DOI] [PubMed] [Google Scholar]
- 37.Chang Q, Chen Z, You J, McNutt MA, Zhang T, Han Z, Zhang X, Gong E, Gu J (2007) All-trans-retinoic acid induces cell growth arrest in a human medulloblastoma cell line. J Neurooncol 84:263–267. [DOI] [PubMed] [Google Scholar]
- 38.Clark O, Daga S, Stoker AW (2013) Tyrosine phosphatase inhibitors combined with retinoic acid can enhance differentiation of neuroblastoma cells and trigger ERK- and AKT-dependent, p53-independent senescence. Cancer Lett 328:44–54. [DOI] [PubMed] [Google Scholar]
- 39.Taylor RE, Bailey CC, Robinson KJ, Weston CL, Walker DA, Ellison D, Ironside J, Pizer BL, Lashford LS (2005) Outcome for patients with metastatic (M2–3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer 41:727–734. [DOI] [PubMed] [Google Scholar]
- 40.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG (2009) Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol 27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ (2003) Retinoid therapy of high-risk neuroblastoma. Cancer Lett 197:185–192. [DOI] [PubMed] [Google Scholar]
- 42.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 341:1165–1173. [DOI] [PubMed] [Google Scholar]
- 43.Muccio DD, Atigadda VR, Brouillette WJ, Bland KI, Krontiras H, Grubbs CJ (2017) Translation of a tissue-selective rexinoid, UAB30, to the clinic for breast cancer prevention. Curr Top Med Chem 17:676–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambon P (1994) The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol 5:115–125. [DOI] [PubMed] [Google Scholar]
- 45.Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner T, Grippo JF, Chambon P, Levin AA (1993) Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc Natl Acad Sci USA 90:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumireddy K, Sutton LN, Phillips PC, Reddy CD (2003) All-trans-retinoic acidinduced apoptosis in human medulloblastoma: activation of caspase-3/poly(ADPribose) polymerase 1 pathway. Clin Cancer Res 9:4052–4059. [PubMed] [Google Scholar]
- 47.Hallahan AR, Pritchard JI, Chandraratna RA, Ellenbogen RG, Geyer JR, Overland RP, Strand AD, Tapscott SJ, Olson JM (2003) BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 9:1033–1038. [DOI] [PubMed] [Google Scholar]
- 48.Fu YS, Wang Q, Ma JX, Yang XH, Wu ML, Zhang KL, Kong QY, Chen XY, Sun Y, Chen NN, Shu XH, Li H, Liu J (2012) CRABP-II methylation: a critical determinant of retinoic acid resistance of medulloblastoma cells. Mol Oncol 6:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celay J, Blanco I, Lazcoz P, Rotinen M, Castresana JS, Encio I (2013) Changes in gene expression profiling of apoptotic genes in neuroblastoma cell lines upon retinoic acid treatment. PloS One 8:e62771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imran M, Park TJ, Lim IK (2012) TIS21/BTG2/PC3 enhances downregulation of c-Myc during differentiation of HL-60 cells by activating Erk1/2 and inhibiting Akt in response to all-trans-retinoic acid. Eur J Cancer 48:2474–2485. [DOI] [PubMed] [Google Scholar]
- 51.Cheepala SB, Yin W, Syed Z, Gill JN, McMillian A, Kleiner HE, Lynch M, Loganantharaj R, Trutschl M, Cvek U, Clifford JL (2009) Identification of the B-Raf/Mek/Erk MAP kinase pathway as a target for all-trans retinoic acid during skin cancer promotion. Mol Cancer 8:27. doi: 10.1186/1476-4598-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassani B, Bartolini D, Pagani A, Principi E, Zollo M, Noonan DM, Albini A, Bruno A (2016) Fenretinide (4-HPR) targets caspase-9, ERK 1/2 and the Wnt3a/βCatenin pathway in medulloblastoma cells and medulloblastoma cell spheroids. PLoS One 11(7):e0154111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, Warnatz HJ, Sidiropoulos N, Phillips AH, Schumacher S, Kleinheinz K, Waszak SM, Erkek S, Jones DTW, Worst BC, Kool M, Zapatka M, Jäger N, Chavez L, Hutter B, Bieg M, Paramasivam N, Heinold M, Gu Z, Ishaque N, Jäger-Schmidt C, Imbusch CD, Jugold A, Hübschmann D, Risch T, Amstislavskiy V, Gonzalez FGR, Weber UD, Wolf S, Robinson GW, Zhou X, Wu G, Finkelstein D, Liu Y, Cavalli FMG, Luu B, Ramaswamy V, Wu X, Koster J, Ryzhova M, Cho YJ, Pomeroy SL, HeroldMende C, Schuhmann M, Ebinger M, Liau LM, Mora J, McLendon RE, Jabado N, Kumabe T, Chuah E, Ma Y, Moore RA, Mungall AJ, Mungall KL, Thiessen N, Tse K, Wong T, Jones SJM, Witt O, Milde T, Von Deimling A, Capper D, Korshunov A, Yaspo ML, Kriwacki R, Gajjar A, Zhang J, Beroukhim R, Fraenkel E, Korbel JO, Brors B, Schlesner M, Eils R, Marra MA, Pfister SM, Taylor MD, Lichter P (2017) The whole-genome landscape of medulloblastoma subtypes. Nature 547:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


