Abstract
Glaucoma is a complex neurodegenerative disease with many clinical subtypes. Some of its rare forms include pigmentary glaucoma, uveitic glaucoma and congenital glaucoma. While they all share common features of progressive retinal ganglion cell (RGC) loss, optic nerve damage and corresponding visual field loss, the exact mechanisms underlying glaucomatous neuron loss are not clear. This has largely hindered the development of a real cure for this disease. Elevated intraocular pressure (IOP) is a known major risk factor of glaucoma; however, progressive degeneration of RGCs and axons can also be found in patients with a normal IOP, i.e. normal tension glaucoma (NTG). Interestingly, patients who carry the gain-of-function mutation of the pro-inflammatory gene TBK1 – tumor necrosis factor (TNF) receptor associated factor NF-κB activator (TANK) binding kinase 1 – are at increased risk to develop NTG. This finding suggests a causal link between neuroinflammatory processes and glaucoma. Various studies have reported the presence of neuroinflammatory responses by microglia, astrocytes and other blood-born immune cells in the optic nerve head (ONH) at early stages of experimental glaucoma. Inhibition of certain pro-inflammatory pathways, particularly those associated with microglial activation, appears to be neuroprotective. In this review, we will focus on the inflammatory responses, in particular the proposed roles of microglia, in the pathogenesis of glaucoma.
Keywords: Neurodegeneration, inflammation, microglia, glaucoma
Introduction
Clinically, glaucoma is characterized by cupping of the optic nerve head (ONH) and irreversible visual field loss due to optic nerve damage and retinal ganglion cell (RGC) loss (Quigley, Dunkelberger, & Green, 1988). It is generally accepted that elevated intraocular pressure (IOP) is a major risk factor (Quigley & Broman, 2006). However, glaucoma has many subtypes, and patients with normal-tension glaucoma (NTG) often have an IOP within the normal range or between 15 and 20 mmHg (Kosior-Jarecka et al., 2016). One can conclude that elevation of IOP is not necessary for the development of glaucomatous damage and loss of visual field. On the other hand, people with ocular hypertension, for example, may display a completely normal optic nerve without impaired visual function (Anderson, 2011). Interestingly, the gain-of-function mutation or duplication of an inflammatory mediator—tumor necrosis factor (TNF) receptor associated factor NF-κB activator (TANK) binding kinase 1 (TBK1)—was reported to be linked a rare condition of NTG (Fingert et al., 2011) (Ahmad, Zhang, Casanova, & Sancho-Shimizu, 2016). The above mentioned observations point to a mechanism beyond pressure-mediated damage, likely one that potentiates neuroinflammation as a contributing factor to glaucomatous neurodegeneration (Vu, Jager, & Chen, 2012).
Microglia are immunocompetent cells that act as neuropathological sensors and a first line of defense to injury in the central nervous system (CNS), including the brain and retina (Streit, Conde, Fendrick, Flanary, & Mariani, 2005). They react to neural damage with morphological changes, proliferation, migration and production of inflammatory cytokines that further propagate neuroinflammation (Glass, Saijo, Winner, Marchetto, & Gage, 2010). This reaction also includes the release of reactive oxygen species, nitric oxide (NO) and TNF-α by the activated microglia, leading to neurotoxic effects and aggravated neuronal loss (Magni et al., 2012; (Wei et al., 2011). It has been reported that microglial activation is one of the first events in glaucomatous neural damage occurring prior to RGC loss (Bosco et al., 2015; Ramirez et al., 2017). In a mouse model of inherited glaucoma, DBA/2J mice, the extent of neurodegeneration correlated with early microglial alterations in vivo (Bosco, et al., 2015). Treatments with minocycline, which inhibits microglial activation, also lowered RGC death in the same mouse model of inherited glaucoma (Bosco et al., 2008). Together, the aforementioned data suggest that activated microglia are critical contributors to the pathogenesis of glaucomatous neural damage.
Neuroinflammation in glaucoma
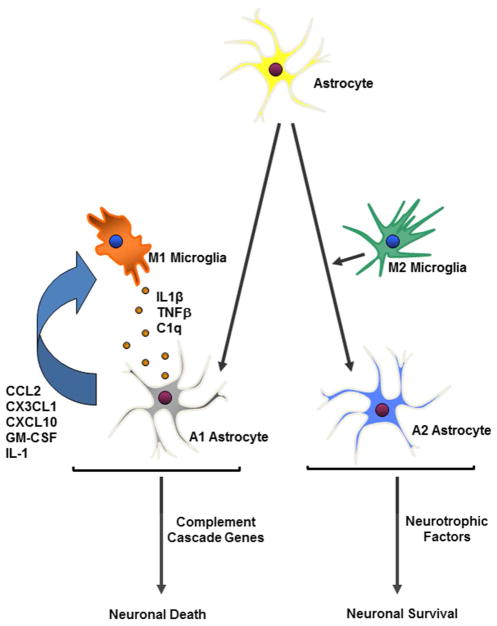
While inflammation as a cause for glaucoma peaks the interest of many, it is important to consider that the retina in fact is an immune privileged site. The impact of the systemic immune system to the retina is strictly regulated. Microglia and astroglia (astrocytes and Müller cells) are the primary retinal cell types that perform immune surveillance and mediate inflammatory responses to infection, disease, or injury (Fig. 1) (Soto & Howell, 2014). Müller cells and astrocytes are in close contact with neurons and blood vessels and provide an array of functions including metabolic support, modulation of synaptic activity, and regulation of extracellular ion concentrations (Iadecola & Nedergaard, 2007; Rouach, Koulakoff, Abudara, Willecke, & Giaume, 2008; Ullian, Sapperstein, Christopherson, & Barres, 2001).
Fig. 1.
Schematics of microglia and astrocytes reactions. Retinal neural injury transforms microglia into an M1- and M2-like phenotype and astrocytes into an A1- and A2-type, correspondingly. Astrocytes and microglia work together to initiate either a neuroinflammatory or neuroprotective response after injury through the release of cytokines or neurotrophic factors that can lead to neuronal death or survival.
Astroglia and microglia have been shown to act as neuropathological sensors and exhibit phagocytosis and antigen-presenting capacities (Guerrero-Garcia, 2017; Karlstetter et al., 2015; Kettenmann, Hanisch, Noda, & Verkhratsky, 2011; X. G. Luo, Ding, & Chen, 2010; Ransohoff & El Khoury, 2015). Microglia are cells of a monocytic lineage which are derived from monocytes that enter the retina from the blood stream during development. They maintain numerous cellular antigens that characterize macrophages and monocytes, such as CD11b/c and chemokine fractalkine receptor (CX3CR1) (Dudvarski Stankovic, Teodorczyk, Ploen, Zipp, & Schmidt, 2016). Fate mapping analysis revealed that adult microglia (derived from primitive erythromyeloid progenitors that leave the yolk sac in the early embryonic stage) enter the CNS via the primitive blood stream while they gain expression of new lineage specific genes and differentiate into mature microglia (Ginhoux et al., 2010). The development of microglia is primarily regulated by colony-stimulating factor 1 receptor (CSF-1R) (Erblich, Zhu, Etgen, Dobrenis, & Pollard, 2011) and its alternate ligand interleukin-34 (IL-34) (Greter et al., 2012). Transforming growth factor-β (TGF-β) has recently been identified as a critical differentiation factor for microglia (Butovsky et al., 2014).
Under normal conditions, microglia survey the retina to detect any damage and respond by combining defensive reactions with neuroprotective activities (Ramirez, et al., 2017). When a pro-inflammatory stimulus arises during injury or disease, astrocytes, Müller cells, and microglia become activated and produce cytokines and chemokines. This recruits blood-derived immune cells to the retina, which cause amplification of the inflammatory response in the retina (Callahan & Ransohoff, 2004; Ransohoff, Kivisakk, & Kidd, 2003). Thus, glaucomatous insult or elevation of IOP triggers the responses of microglia, astrocytes and Müller cells to participate in the process of neuroinflammation.
Microglial activation
To date, the molecular signals associated with stress or neural injury that trigger the inflammatory responses in glaucoma remain unclear. Damage-associated molecular patterns (DAMPs) released by stressed RGCs or astroglial cells in the optic nerve head (ONH) are speculated to trigger these inflammatory responses (Rifkin, Leadbetter, Busconi, Viglianti, & Marshak-Rothstein, 2005; Yu, Wang, & Chen, 2010; Zhu et al., 2011). Heat shock proteins (HSPs) belong to the DAMP family and are up-regulated in RGCs in response to elevated IOP, as shown in experimental models of glaucoma and in the retinas of human patients with glaucoma (C. Luo et al., 2010). Astroglial and/or microglial cells also produce DAMPs independent of or before the damage occurred in RGCs. Tenascin-C, an endogenous activator of Toll-Like Receptor 4 (TLR4), is another DMAP that has been found to be upregulated in astrocytes of the glaucomatous ONH (Howell et al., 2011; Johnson et al., 2011; Midwood et al., 2009). Both HSPs and tenascin-C can induce TLR activation in microglia.
Microglial activation is detected in early stages of experimental glaucoma. In fact, a short-term elevation of IOP (30 mmHg for one hour) was sufficient to induce rapid changes in microglia, including retraction of cellular processes, display of enlarged cell soma and increased expression of myeloid cell markers and phagocytosis-related protein CD68 (Sun, Qu, & Jakobs, 2013). When highly activated, microglia acquire an amoeboid morphology and start acting like macrophages. This is usually accompanied by their production of pro-inflammatory and cytotoxic molecules, such as complement factors, NO, TNF-α, interleukin 6 (IL-6), and an increased expression of major histocompatibility complex class I (MHC I) and II antigens. (Bosco et al., 2012; Ebneter, Casson, Wood, & Chidlow, 2010; Howell, Macalinao, et al., 2011). Release of pro-inflammatory cytokines by activated microglia is thought to aggravate and propagate the innate and adaptive immune responses. It is suspected that this leads to an exacerbation of neuronal damage in glaucoma.
The extent of microglial activation is related to the extent of glaucomatous axonal degeneration in the ONH. Howell et al found that radiation treatment to the eye or mouse head significantly reduced the number of activated microglia and axonal degeneration without affecting the IOP in glaucomatous animals (Bosco, et al., 2012; Howell et al., 2012). Administration of minocycline also inhibits microglial activation and attenuates RGC loss in glaucoma models of mouse, rat and rabbit (Bosco, et al., 2012). Quantitative correlations between microglial activation and axon loss have been documented using DBA/2J mice (Bosco et al., 2016). On the other hand, neural damage is thought to directly induce an inflammatory response after injury through releasing nucleotides and up-regulating purinergic receptors on microglia, which also activates the phagocytic ability and motility of microglia (Koizumi et al., 2007; Wu, Vadakkan, & Zhuo, 2007). Wang et al. showed CX3CR1 deficiency increased microglial activation and RGC death in an experimental glaucoma model (Wang, Peng, & Lin, 2014). Damaged neurons also release HMGB1 which can bind to the CD11b receptor in the microglia and induce production of inflammatory and neurotoxic factors. In contrast, absence of CD11b receptor prevents microglia activation and has a neuroprotective role in an experimental mouse model of glaucoma (Nakazawa et al., 2006).
Classification of activated microglia: M1 VS M2
It is now recognized that activation of retinal or brain microglia indices a variety of cellular phenotypes. Two distinct phenotypes are defined M1-like and M2-like, corresponding to the M1 and M2 phenotypes of activated macrophages (Fig. 1). Microglia respond to pro-inflammatory molecules, such as bacterial lipopolysaccharide (LPS) or interferon-γ (IFN-γ), to adopt a “classical” M1-like phenotype, which produces high levels of pro-inflammatory cytokines (IL-1β, IL-12, and TNF-α) (Varnum & Ikezu, 2012). M1-like microglia exhibit amoeboid morphology and are associated with a high phagocytic capacity and motility. In many cases, the M1-like response is protective and is down-regulated after the original insult is removed. However, uncontrolled M1-like activation can induce neurotoxicity due to excessive release of pro-inflammatory cytokines and neurotoxic agents (Burguillos et al., 2011; Glezer, Simard, & Rivest, 2007; Gonzalez, Elgueta, Montoya, & Pacheco, 2014; Gordon, Anantharam, Kanthasamy, & Kanthasamy, 2012). Microglia also respond to TH2 cytokines, such as IL-4, to induce an “alternative” M2-like phenotype that is characterized by displaying a thin cell body with ramified cellular processes. M2-like microglia produce anti-inflammatory mediators (e.g. IL-10 and TGF-β) and neurotrophic factors (IGF-1) and are thought to associate with tissue repair and growth stimulation (Suh, Zhao, Derico, Choi, & Lee, 2013; Tang & Le, 2016). Because of the different ontogeny, one should not presume that the phenotypes and functions of peripheral macrophages can translate directly into M1-like and M2-like microglia.
In experimental models of glaucoma, IOP elevation induces most microglia to become MHC-II+/M1-like cells, while only a few CD86+ M2-like microglia are seen in the ganglion cell and never fiber layers. Presumably, T-cell activation is suppressed due to the omission of co-stimulation, leading to a downregulation of the immune response. After M1 activation, microglia adopt a transitory M2 state and upregulate CD68, CD206 and Ym1 before returning to the rest state (Menzies, Henriquez, Alexander, & Roberts, 2010; Ramirez, et al., 2017; Zhou, He, & Ren, 2014). In an experimental model of unilateral glaucoma, only microglia expressing Ym1 and Iba-1 with an amoeboid morphology were detected in the NFL and GCL of glaucomatous mice. Thus, most microglia in this model exhibit an M1 phenotype. Interestingly, microglia in the mouse retina contralateral to the eye with an elevated IOP also exhibit multiple signs of activation (Rojas et al., 2014). Obviously, the M1/M2 paradigm is an oversimplified model describing microglial activation. Confronted with injury, microglia often undergo a dynamic process and can present mixed characters or switch between the M1 and M2 phenotypes (Cherry, Olschowka, & O’Banion, 2014).
Interaction between microglia and astroglia
Under pathological conditions, astrocytes and Müller cells also undergo a pronounced transformation termed “reactive gliosis” (Liddelow et al., 2017). Functions of reactive astroglial cells (including astrocytes and Müller cells) have for a long time been a subject of debate. Previous studies revealed that reactive astroglial cells can have both protective and detrimental influences on neuronal survival in glaucoma and other neurodegenerative conditions (da Fonseca et al., 2014). Until recently, it has been shown that neuroinflammation induces two types of reactive astrocytes, termed “A1” and “A2”, respectively (Fig. 1). This parallels the “M1” and “M2” macrophage/microglia nomenclature (Heppner, Ransohoff, & Becher, 2015; Liddelow, et al., 2017; Martinez & Gordon, 2014). Gene transcriptome analyses of reactive astrocytes show that A1-reactive astrocytes are neuroinflammatory and upregulate many genes (e.g. complement cascade genes) which have previously been shown to be destructive to synapses, suggesting a detrimental effect (Bush et al., 1999; Sofroniew & Vinters, 2010). By contrast, A2 reactive astrocytes upregulate neurotrophic factors that promote the survival and growth of neurons and synaptic repair, consistent with their neuroprotective/supportive functions (Bush, et al., 1999; Zador, Stiver, Wang, & Manley, 2009).
Astroglial cells and microglia work together to fine-tune the regulation and resolution of the inflammatory response in retinal disease (Fig. 1) (da Fonseca, et al., 2014). Recently, it has been reported that activated M1- and M2-like microglia secret different cytokines to drive the reactive responses of astrocytes (Liddelow, et al., 2017). M1-like microglia releases IL-1α, C1q and TNF-α that induce astrocytes to the A1 phenotype and contribute to the death of neurons in neurodegenerative disorders. By contrast, reactive astrocytes can also secret cytokines and chemokines, such as CCL2, CXCL1, CXCL10, GM-CSF, and IL-6 that activate microglia, infiltrating dendritic cells, monocytes/macrophages, and T-cells in the inflamed tissue (Liddelow, et al., 2017). As reactive astrocytes and microglia accompany every acute injury and chronic neurological diseases, it has been difficult to distinguish between the contributions of astrocytes and those of microglia on the neurodegenerative process. Indeed, the activations of these micro- and macro-glia occur so close in time that a temporal sequence of events has not be defined. It is currently unknown if microglial activation occurs prior to, at the same time as, or after astroglial activation. Improved methods of separating and purifying microglia and astrocytes may allow future dissection of their relative contributions (Sousa, Biber, & Michelucci, 2017).
Signaling events of neuroinflammation in glaucoma
Despite the recognized important roles of inflammatory responses in glaucoma, to date, we do not yet know the key inflammatory signals that lead to the polarized induction of microglia and astrocytes in disease progression. Transcriptional profiling showed that many genes which may be involved in the inflammatory pathways are upregulated in the retina and ONH in the early stage of glaucoma (Ahmed et al., 2004); (Howell, et al., 2012; Z. Yang et al., 2007). More specifically, up-regulation of the inflammatory inducers/sensors such as TLRs, transducers Trif and MyD88, and amplifiers such as IL-1 and IL-6 has been reported (C. Luo, et al., 2010; Tezel, Yang, Luo, Cai, & Powell, 2012). In DBA/2J mice, a model of glaucoma as a result of iris pigment dispersion, genes associated with “immune response”, “leukocyte active”, and “chemotaxis” are among the earliest up-regulated, occurring well-before RGC and axon losses are detected (Howell, Macalinao, et al., 2011). The data suggest that aforementioned inflammatory factors may, at least in part, mediate the immune responses in glaucoma. (Soto & Howell, 2014)
TLR signaling pathways are among the first to be up-regulated in the retinas of patients with glaucoma: Luo et al. reported that HSPs and oxidative stress upregulate glial expression of MHC II and cytokine production through TLR signaling that in turn stimulate the proliferation of T cells (C. Luo, et al., 2010). Expression of TLR2, TLR3, and TLR4 was detected in microglia and astrocytes of the glaucomatous retinas. In the DBA/2J mice, 11 of 13 TLRs were up-regulated in the ONH (Howell, Macalinao, et al., 2011; Howell, Walton, King, Libby, & John, 2011). The TLRs adaptor protein MyD88 and members of the MAPK pathway were also up-regulated in the retinas of patients with primary open-angle glaucoma and in the ONH of DBA/2J mice (Howell, Macalinao, et al., 2011) (Howell, Walton, et al., 2011).
The second factor involves NF-κB. Many kinases that are involved in the activation of the NF-κB pathways, such as RIPK, NIK, and IκK, were seen up-regulated in humans and rats following induction of glaucoma (Tezel, et al., 2012; X. Yang et al., 2011). Activation of the NF-κB pathway leads to the increased expression of the IL-1 cytokine family that subsequently promotes the production of a secondary cascade of inflammatory cytokines in microglia (e.g., TNF-α) and astrocytes (e.g., IL-6). These cytokines amplify the immune responses by recruiting other cells such as T cells to the damaged area (Howell, Macalinao, et al., 2011; Howell, Walton, et al., 2011).
Other critical factors that have been reported to play a role in the immune responses in glaucoma are the TNF family members. The level of TNF-α is increased in the aqueous humor, retina, and optic nerve in glaucoma patients, especially associated with microglia and optic nerve astrocytes (Yan, Tezel, Wax, & Edward, 2000) (Sawada, Fukuchi, Tanaka, & Abe, 2010; Tezel, Li, Patil, & Wax, 2001). Pharmaceutical inhibition or genetic deletion of TNF-α attenuates microglia activation, axonal degeneration, and RGC loss (Nakazawa, et al., 2006; Roh et al., 2012). Fas ligand, another member of the TNF family and pro-apoptotic protein, has also been implicated in glaucoma pathogenesis, contributing to RGC damage in glaucoma mice. Inhibition of Fas ligand on the other hand reduces TNF-α cytotoxicity and protected against glaucomatous RGC loss (Gregory et al., 2011). To date, much remains to be learned about how cross-talks of the cell signaling mediate the activity and functions of different cell types in the retina at different stages of glaucoma.
Conclusion
Evidence suggests a crucial role for microglia in propagating inflammatory processes and the development of neurodegeneration in glaucoma. It is general accepted now that activated microglia exhibit diverse phenotypes and contribute to both the initiation and resolution stages of inflammation and tissue regeneration via switching functions. For simplicity, activated microglia are divided into inflammatory M1-like versus a resolution phase M2-like microglia. Understanding how microglia integrate injury signals to propagate tissue responses in the retina provides an opportunity for controlling excessive inflammation and developing novel therapeutic strategies. It is important to keep in mind that no cells function in isolation. Besides microglia, other cell types including neurons, astroglia, and infiltrating leukocytes such as T cells participate in the neuroinflammatory process triggered by the disease. Uncovering the molecular pathways that define inflammatory versus resolution phenotypes of the retinal cells may allow better targets to modulate these cellular processes. Such studies may lead to novel diagnostic and therapeutic strategies.
Statement about the significance.
This review focuses on the potential involvement of inflammation as a contributing factor to the pathogenesis of neural damage and vision loss in glaucoma. Activation of residential immune-competent cells of the retina—microglia—and their interaction with astroglial cells are notably associated with axonal degeneration in glaucoma. It is emerging that microglia and astroglial cells work together in glaucoma to fine-tune the regulation and resolution of inflammatory responses in the neuroretina and the optic nerve. Further elucidation of their roles in glaucoma may offer new insights into the disease pathophysiology and lead to novel diagnostic and therapeutic strategies.
Acknowledgments
Grant information: This research was supported by funding from the National Institutes of Health (EY025259, EY025913, R21EY027067 and EY027067), Lion’s Foundation Grants, and the core grant to the Schepens Eye Research Institute (P30 EY03790-33)
Footnotes
Conflict Of Interest
The authors have no conflict of interest to declare.
Author Contributions
All authors had full access to all the contents of this paper and take responsibility for the integrity and the accuracy of it. Writing – Original Draft, X.W.; Writing – Review & Editing, K.S.C., E.T., M.J.J. and D.F.C.; Supervision, D.F.C.; Funding Acquisition, D.F.C., M.J.J.
References
- Ahmad L, Zhang SY, Casanova JL, Sancho-Shimizu V. Human TBK1: A Gatekeeper of Neuroinflammation. Trends in molecular medicine. 2016;22(6):511–527. doi: 10.1016/j.molmed.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Investigative ophthalmology & visual science. 2004;45(4):1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Normal-tension glaucoma (Low-tension glaucoma) Indian journal of ophthalmology. 2011;59(Suppl):S97–101. doi: 10.4103/0301-4738.73695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Breen KT, Anderson SR, Steele MR, Calkins DJ, Vetter ML. Glial coverage in the optic nerve expands in proportion to optic axon loss in chronic mouse glaucoma. Experimental eye research. 2016;150:34–43. doi: 10.1016/j.exer.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, … Vetter ML. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PloS one. 2012;7(8):e43602. doi: 10.1371/journal.pone.0043602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, … Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investigative ophthalmology & visual science. 2008;49(4):1437–1446. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Disease models & mechanisms. 2015;8(5):443–455. doi: 10.1242/dmm.018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, … Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, … Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, … Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Ransohoff RM. Analysis of leukocyte extravasation across the blood-brain barrier: conceptual and technical aspects. Current allergy and asthma reports. 2004;4(1):65–73. doi: 10.1007/s11882-004-0046-9. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. Journal of neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR. The impact of microglial activation on blood-brain barrier in brain diseases. Frontiers in cellular neuroscience. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta neuropathologica. 2016;131(3):347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Investigative ophthalmology & visual science. 2010;51(12):6448–6460. doi: 10.1167/iovs.10-5284. [DOI] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PloS one. 2011;6(10):e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, … Stone EM. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Human molecular genetics. 2011;20(12):2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, … Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. Journal of neuroimmunology. 2014;274(1–2):1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. Journal of neuroinflammation. 2012;9:82. doi: 10.1186/1742-2094-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, … Ksander BR. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PloS one. 2011;6(3):e17659. doi: 10.1371/journal.pone.0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, … Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37(6):1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Garcia JJ. The role of astrocytes in multiple sclerosis pathogenesis. Neurologia. 2017;(17):30281–5. doi: 10.1016/j.nrl.2017.07.021. S0213-4853. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature reviews Neuroscience. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, … John SW. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. The Journal of clinical investigation. 2011;121(4):1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, … John SW. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. The Journal of clinical investigation. 2012;122(4):1246–1261. doi: 10.1172/JCI61135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Walton DO, King BL, Libby RT, John SW. Datgan, a reusable software system for facile interrogation and visualization of complex transcription profiling data. BMC genomics. 2011;12:429. doi: 10.1186/1471-2164-12-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature neuroscience. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Doser TA, Cepurna WO, Dyck JA, Jia L, Guo Y, … Morrison JC. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Investigative ophthalmology & visual science. 2011;52(1):504–518. doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Progress in retinal and eye research. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, … Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosior-Jarecka E, Lukasik U, Wrobel-Dudzinska D, Kocki J, Bartosinska J, Witczak A, … Zarnowski T. Risk Factors for Normal and High-Tension Glaucoma in Poland in Connection with Polymorphisms of the Endothelial Nitric Oxide Synthase Gene. PloS one. 2016;11(1):e0147540. doi: 10.1371/journal.pone.0147540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Investigative ophthalmology & visual science. 2010;51(11):5697–5707. doi: 10.1167/iovs.10-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Molecular neurodegeneration. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni P, Ruscica M, Dozio E, Rizzi E, Beretta G, Maffei Facino R. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-alpha and NF-kappaB nuclear translocation in BV-2 microglia. Phytotherapy research: PTR. 2012;26(9):1405–1409. doi: 10.1002/ptr.3732. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clinical and experimental immunology. 2010;160(3):369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, … Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature medicine. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, … Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(49):12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95(3):357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- Ramirez AI, de Hoz R, Salobrar-Garcia E, Salazar JJ, Rojas B, Ajoy D, … Ramirez JM. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Frontiers in aging neuroscience. 2017;9:214. doi: 10.3389/fnagi.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, El Khoury J. Microglia in Health and Disease. Cold Spring Harbor perspectives in biology. 2015;8(1):a020560. doi: 10.1101/cshperspect.a020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nature reviews Immunology. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunological reviews. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, … Miller JW. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PloS one. 2012;7(7):e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas B, Gallego BI, Ramirez AI, Salazar JJ, de Hoz R, Valiente-Soriano FJ, … Ramirez JM. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. Journal of neuroinflammation. 2014;11:133. doi: 10.1186/1742-2094-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Investigative ophthalmology & visual science. 2010;51(2):903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harbor perspectives in medicine. 2014;4(8) doi: 10.1101/cshperspect.a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C, Biber K, Michelucci A. Cellular and Molecular Characterization of Microglia: A Unique Immune Cell Population. Frontiers in immunology. 2017;8:198. doi: 10.3389/fimmu.2017.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurological research. 2005;27(7):685–691. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. Journal of neuroinflammation. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Qu J, Jakobs TC. Reversible reactivity by optic nerve astrocytes. Glia. 2013;61(8):1218–1235. doi: 10.1002/glia.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Molecular neurobiology. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Investigative ophthalmology & visual science. 2001;42(8):1787–1794. [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Powell DW. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investigative ophthalmology & visual science. 2012;53(7):4220–4233. doi: 10.1167/iovs.11-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Archivum immunologiae et therapiae experimentalis. 2012;60(4):251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Jager MJ, Chen DF. The Immunology of Glaucoma. Asia-Pacific journal of ophthalmology. 2012;1(5):303–311. doi: 10.1097/APO.0b013e31826f57a3. [DOI] [PubMed] [Google Scholar]
- Wang K, Peng B, Lin B. Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia. 2014;62(12):1943–1954. doi: 10.1002/glia.22715. [DOI] [PubMed] [Google Scholar]
- Wei X, Yu Z, Cho KS, Chen H, Malik MT, Chen X, … Chen DF. Neuroglobin is an endogenous neuroprotectant for retinal ganglion cells against glaucomatous damage. The American journal of pathology. 2011;179(6):2788–2797. doi: 10.1016/j.ajpath.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55(8):810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Archives of ophthalmology. 2000;118(5):666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Investigative ophthalmology & visual science. 2011;52(11):8442–8454. doi: 10.1167/iovs.11-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Investigative ophthalmology & visual science. 2007;48(12):5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. Journal of cellular and molecular medicine. 2010;14(11):2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handbook of experimental pharmacology. 2009;(190):159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural regeneration research. 2014;9(20):1787–1795. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang L, Ruan Y, Zhou L, Zhang D, Min Z, … Gu J. An efficient delivery of DAMPs on the cell surface by the unconventional secretion pathway. Biochemical and biophysical research communications. 2011;404(3):790–795. doi: 10.1016/j.bbrc.2010.12.061. [DOI] [PubMed] [Google Scholar]