Abstract
PURPOSE:
To study if peripheral vascular leakage (PVL) on ultra-widefield fluorescein angiography (UWFFA) prognosticates complications of uveitis or necessitates treatment augmentation.
METHODS:
Retrospective cohort study of uveitis patients imaged with UWFFA and ≥1 year of follow-up.
RESULTS:
We included 73 eyes of 42 patients with uveitis. There was no difference in baseline, intermediate, final VA (p=0.47–0.95) or rates of cystoid macular edema (CME) (p=0.37–0.87) in eyes with PVL vs. those without. Eyes with PVL receiving baseline treatment augmentation were more likely to have baseline CME but were not more likely to have impaired visual acuity at final follow-up. PVL was independently associated with treatment augmentation on generalized estimating equation analysis with multivariable linear regression (OR: 4.39, p=0.015).
CONCLUSIONS:
PVL did not confer an increased risk of impaired VA or CME at ≥1 year follow up but was possibly an independent driver of treatment augmentation.
Keywords: Fluorescein angiography, macular edema, peripheral vascular leakage, uveitis, widefield imaging
INTRODUCTION
Fluorescein angiography (FA) is an important diagnostic tool in the management of uveitis. Important clinical markers of disease activity including cystoid macular edema (CME), retinal vascular leakage and non-perfusion, and choroidal and retinal neovascularization, are often more readily evident on FA than on clinical examination. Traditional fundus viewing cameras have a field of view ranging from 30–50 degrees which does not allow for adequate examination of the retinal periphery. Use of the 7 standard fields or 9-field protocol can allow for a montage providing up to 100 degrees of imaging but is technically challenging and omits the far retinal periphery.1 The Optos fundus camera allows for ultra-widefield fluorescein angiography (UWFFA) providing a 200 degree field of view in a single image.2 Peripheral retinal findings on UWFFA have since been described in a variety of conditions including diabetic retinopathy,3–7 uveitis,8–14 retinal vein occlusion,15,16 retinopathy of prematurity17 and white without pressure18 as well as in normal eyes.18
Peripheral vascular leakage (PVL), which describes angiographic leakage occurring outside the ETDRS 7 standard fields, has been described on UWFFA in diabetics,3 uveitics,8–12 involved and fellow eyes with vein occlusions15,16 and in eyes without clinical disease.18 There is growing evidence that the presence of PVL in uveitic patients may influence the grading of disease activity and the decision to augment therapy. Campbell et al previously reported that among a cohort of patients with non-infectious posterior uveitis, management was altered in 7 of 43 patients (16%) based on examination and limited FA, whereas 21 of 43 patients (48%) had management change with the use of the ultra-widefield imaging and angiography (P < .001).9 This implies that peripheral retinal vascular features, including PVL, impact treatment decisions in uveitis patients. Our group recently described, in a single cross-sectional retrospective consecutive case series, that PVL was more likely to be present among uveitis patients felt to have clinically-active disease compared to those with well-controlled disease, and may be associated with other signs of activity such as CME.12
There is, however, a paucity of data as to whether the presence of PVL among uveitis patients affects visual outcomes or necessitates treatment. In this single-center retrospective cohort series, we examine the visual outcomes on extended follow-up among uveitic patients imaged with UWFFA, stratified based on the presence or absence of PVL. We further compare and analyze the PVL and non-PVL groups for association with disease activity, treatment augmentation, and the development of CME on optical coherence tomography (OCT) over the study period.
MATERIALS AND METHODS
Chart Review
We performed a retrospective cohort study of all uveitis patients imaged with Optos P200 UWFFA at our institution between November 2012 and January 2014. Chart review was performed and clinical data were reviewed for all patients. Patients were only included in this study if they had at least one year of follow-up from the baseline visit and at least one follow-up (intermediate) visit 3–11 months from the baseline visit. The baseline visit was defined as the first visit at which UWFFA was performed. Both eyes were included in cases of bilateral uveitis. Early and late UWFFA images from the baseline visit were evaluated in a standardized, masked fashion by two graders (N.V.P. & J.T.B.) for PVL, optic nerve leakage (ONL), diffuse vascular leakage (DVL) and CME, grading each as present (1) or absent (0). Discordant grades were adjudicated by a third masked grader (J.P.C.).
The study population was divided into two groups based on presence or absence of PVL at the baseline visit regardless of whether DVL was present. Baseline, intermediate and final visits were analyzed for several covariates and potential outcomes including uveitis classification utilizing the SUN criteria,19 best corrected visual acuity (VA), presence of CME on OCT, and central macular thickness (CMT) on OCT. The assessment and plan sections of clinic notes were evaluated to determine whether the overall clinical assessment was that the patient was stable on current therapy, which was recorded as “stable,” or had clinical evidence of disease activity that required treatment augmentation, which was recorded as “clinically active.” Treatment augmentation included the use of topical, periocular, intraocular or oral steroids/antibiotics, surgical intervention or other immunosuppressive therapy. The media status of study eyes (corneal clarity, cataract grade) and clinical grading of uveitis activity (anterior chamber cell/flare, vitreous cell, vitreous haze) were noted for each visit.
Imaging Protocol
All patients underwent UWFFA using the Optos P200Tx system (Optos, Marlborough, MA). Early frames of the FA were acquired from the transit eye and late arteriovenous phase or laminar venous phase images as well as recirculation phase images were obtained from both eyes. Spectral-domain OCT images of the retina were obtained in both eyes of all patients with horizontal line scans through the macula using the Spectralis system (Heidelberg Engineering, Carlsbad, CA).
Statistical Analysis
Each of the above variables were compared between the PVL and non-PVL groups at baseline, intermediate, and final follow-up visits. As both eyes of some patients were included in comparisons, generalized estimating equations (GEE) were used to account for possible correlation between eyes. Comparisons between the PVL and non-PVL groups were performed using GEE with bivariate analyses followed by multivariate logistic regression for dichotomous outcomes and multivariate linear regression for continuous outcomes. All multivariable regression models assessed potential confounding from age, uveitis classification, time elapsed since baseline visit, and changes in media status. Excel 2011 (Microsoft, Redmond, WA) was used for data management, and all statistical analysis was performed using Stata MP 13 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics
A total of 115 patients with uveitis were imaged with the Optos P200 system at our institution during the study period. Twelve were excluded due to poor image quality. Forty-two of the remaining patients (73 eyes with uveitis) had adequate follow-up and were included in our analysis. Forty-six of 73 eyes from 27 patients (63.0%) had posterior or panuveitis, 19/73 eyes from 11 patients (26.0%) had intermediate uveitis and 8/73 eyes from 4 patients (11.0%) had isolated retinal vasculitis. The mean time from the baseline to the intermediate visit was 7 months (Range 6–11 months). The mean time from the baseline to the final visit was 36 months (Range 12–55 months). The mean age at the baseline visit was 47 years (range 10–90 years). Leading up to the baseline visit, 17 eyes (23%) were on treatment for uveitis and had been stable, 11 eyes (15%) were on treatment and had been worsening, 21 eyes (29%) were off treatment and had been stable and 24 eyes (33 %) were new patients to our institution.
PVL vs. non-PVL group results
Forty-one eyes of 25 patients (56.2% of eyes) were found to have PVL on UWFFA at the baseline visit whereas 32 eyes of 19 patients did not have PVL (Figure 1). Twenty-three of 41 eyes (56.1%) with PVL also had DVL. Among patients with PVL compared to those without PVL, there was no difference in the proportion with VA better than 20/40 (p=0.47–0.95) or CME (p=0.37–0.87) at baseline, intermediate or final follow up (Table 1). The mean logMAR VA in the PVL group was 0.44 at baseline, 0.27 at the intermediate visit, and 0.21 at the final visit. In comparison, among those without PVL baseline mean logMAR VA was 0.41, 0.39 at the intermediate visit, and 0.23 at the final visit. GEE analysis with multivariable linear regression of the change in mean logMAR VA over time, accounting for age, type of uveitis, change in media status, and clinical grading of uveitis activity showed no difference between those with PVL vs. those without (p=0.34–0.92). A significantly greater proportion of eyes with PVL were felt to have clinically active disease at the baseline visit compared to those without PVL (27/41 eyes, 65.9% vs. 12/32 eyes, 38%, p=0.02) and received treatment augmentation (Table 1). This association did not persist during future visits. PVL eyes were more than four times as likely to receive treatment augmentation at the baseline visit than non-PVL eyes on GEE analysis after adjusting for baseline CME, DVL and clinical grading of uveitis activity (OR: 4.39, p=0.015).
Figure 1.
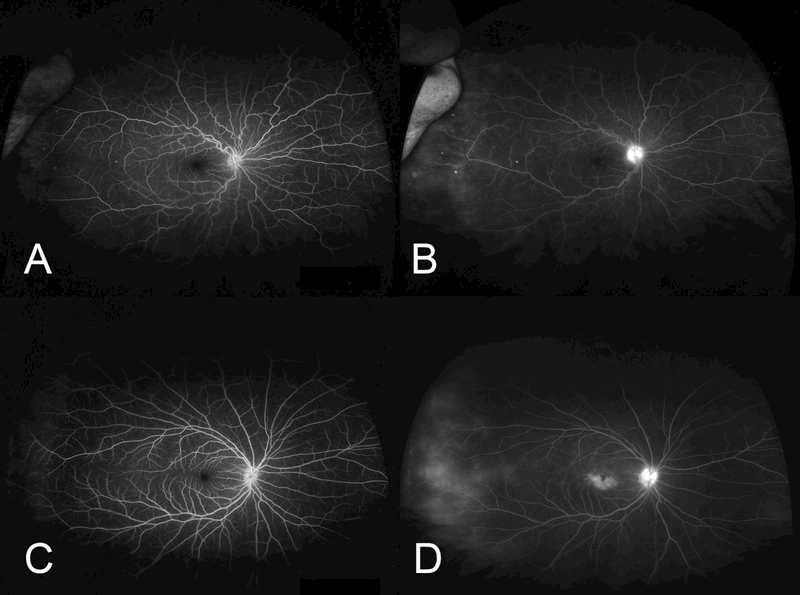
Representative ultra-widefield fluorescein angiograms (UWFFA) of patients with peripheral vascular leakage (PVL) included in this study. Early (A, C) and late UWFFA frames (B, D) of two patients with PVL and intermediate uveitis. Both patients showed additional, at least mild, angiographic findings such as optic nerve leakage and/or cystoid macular edema.
Table 1.
Comparison of visual acuity, central macular thickness and treatment augmentation among uveitic eyes with peripheral vascular leakage (PVL) vs. those without PVL
| OUTCOMES | Initial visit | OR | p | Intermediate visit | OR | p | Final visit | OR | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time elapsed since initial visit [months; mean (range)] |
7 (6–11) | 36 (12–55) | ||||||||||
| Visual Acuity | 20/40 or better | PVL | 24/41(59%) | 0.73a | 31/41(76%) | 0.95a | 33/41(80%) | 0.47a | ||||
| No PVL | 20/32(63%) | 24/32(75%) | 24/32(75%) | |||||||||
| Comparison of change in logMAR VA from baseline (PVL vs. no PVL) | Ref | -0.096b | 0.34 | +0.014b | 0.92 | |||||||
| Central Macular thickness | Proportion with CME on OCT | PVL | 15/41(37%) | 1.27c | 0.63 | 7/41(17%) | 1.11c | 0.87 | 2/41(5%) | 0.61c | 0.37 | |
| No PVL | 10/32(31%) | 5/32(16%) | 4/32(13%) | |||||||||
| Comparison of change in CMT from baseline (PVL vs. no PVL) | Ref | +10.49d | 0.80 | -12.14d | 0.82 | |||||||
| Treatment Augmentation | PVL | 27/41(66%) | 0.02a,e | 8/41(20%) | 0.94a | 3/41(7%) | 0.67a | |||||
| No PVL | 12/32(38%) | 6/32(19%) | 2/32(6%) | |||||||||
PVL = Peripheral Vascular Leakage; OCT = Optical Coherence Tomography; CME = Cystoid Macular Edema; CMT = Central Macular Thickness; VA = Visual Acuity; GEE = Generalize Estimating Equation
GEE with Chi2 statistics
GEE with multivariable linear regression coefficient comparing patients with and without PVL. “+” indicates a greater increase in logMAR VA in the PVL group, i.e. poorer visual acuity than those without PVL
GEE with multivariable logistic regression. OR>1 indicates higher likelihood of CME among patients with PVL compared to those without PVL
GEE with multivariable linear regression coefficient comparing patients with and without PVL. “+” indicates a greater increase in CMT in the PVL group
Statistically significant at alpha=0.05
All multivariable regression models assessed potential confounding from age, uveitis classification, time elapsed since baseline visit, and changes in media status
PVL subgroup analysis
Among eyes with PVL, those which were determined to be clinically active at baseline (and received additional therapy) had a higher prevalence of CME (13/27 eyes of 16 patients, 48%) than those who were deemed stable (2/14 eyes of 9 patients, 14%, p=0.03). This difference, however, did not persist at the intermediate or final follow up visit (after adjusting for baseline presence of CME) as the prevalence of CME decreased in the treated group (Table 2). Those with PVL plus DVL had identical baseline logMAR VA to those with PVL alone (0.43).
Table 2.
Prevalence of cystoid macular edema (CME) in eyes with peripheral vascular leakage as a function of whether treatment augmentation occurred.
| CME | ||||
|---|---|---|---|---|
| n | Initial visit | Intermediate visit | Final visit | |
| Treatment augmentation | 27 | 13 (48%) | 5 (19%) | 2 (7%) |
| No Treatment Augmentation | 14 | 2 (14%) | 2 (14%) | 1 (7%) |
| p-value | 0.03a | 0.79b | 0.92b | |
Statistically significant at alpha=0.05
P-values calculated after adjusting for baseline presence of CME
Among eyes with PVL, those with treatment augmentation at the baseline visit had similar baseline VA compared to those without treatment augmentation, though there was a trend towards those with treatment augmentation having better baseline acuity (18/27 eyes of 16 patients, 67% vs. 6/14 eyes of 9 patients, 43% with baseline acuity of 20/40 or better, p=0.16). Additionally, VA was similar at intermediate visit (p=0.14) and final follow-up (p=0.20) on GEE analysis after adjusting for baseline acuity and change in media status (Table 3). Eyes with PVL which received treatment augmentation at baseline had an improvement in mean logMAR VA from 0.39 to 0.14, while those without treatment augmentation improved from 0.54 to 0.45. The difference in this change in logMAR over time between the two groups was not statistically significant (p=0.24).
Table 3.
Comparison of visual acuity in eyes with peripheral vascular leakage receiving vs. not receiving treatment augmentation
| Visual acuity 20/40 or better | ||||
|---|---|---|---|---|
| n | Initial visit | Intermediate visit | Final visit | |
| Treatment augmentation | 27 | 18 (67%) | 23 (85%) | 24 (89%) |
| No Treatment Augmentation | 14 | 6 (43%) | 8 (57%) | 9 (64%) |
| p-value | 0.16 | 0.14a | 0.20a | |
GEE = Generalized Estimating Equation
p-values calculated with GEE analysis after adjusting for baseline acuity and change in media status (corneal clarity, lens clarity)
DISCUSSION
We conducted a retrospective cohort study of patients with uveitis imaged with UWFFA to assess how PVL affects treatment decisions and clinical outcomes. The key findings of this study were: 1) The presence of PVL in uveitic patients at the baseline visit was associated with the decision to augment clinical treatment of uveitis even after adjusting for CME, DVL and clinical grading of uveitis activity; 2) The presence of PVL was not associated with greater impairment of VA, presence of CME on OCT, or CMT at baseline or follow up visits, regardless of the type of uveitis; and 3) Whether PVL necessitates treatment remains unclear.
Based on our finding that among patients with PVL, those undergoing treatment augmentation were more likely to have CME on OCT, it is tempting to conclude that CME was the driver of treatment augmentation. However, patients with PVL were four-times more likely to have treatment augmented after adjusting for baseline presence of CME, DVL and clinical grading of uveitis activity. This suggests that PVL may have, to some extent, influenced the decision to augment therapy in our patients.
We observed no difference in visual acuity outcomes in those with PVL compared to those without PVL. We previously reported that presence of PVL was associated with ONL and angiographic CME on multivariate analysis and CME on OCT on univariate analysis.12 That study included a number of patients included in our current analysis. In our present analysis, we similarly found an association between PVL, angiographic CME and ONL on multivariate analysis. In our cohort, however, the presence of PVL at the baseline visit was not associated with baseline CME on OCT on GEE with univariate or multivariate analysis. As we did not have follow-up UWFFA in most of our patients, it is unclear if an association exists with PVL and persistence of angiographic CME and whether this is affected by treatment augmentation.
There was not a statistically significant difference in visual acuity outcomes among those with PVL having treatment augmentation compared to those without treatment augmentation. There was additionally no difference in change in logMAR acuity between the two groups over the course of the study. It is still unclear, however, whether PVL necessitates treatment. Our data showed that treatment of patients with PVL resulted in improvement in vision with 89% of treated eyes (vs 64% of untreated eyes) achieving 20/40 or better vision at final follow-up. While prevalence of CME was higher at baseline in the treatment group, prevalence was similar at subsequent visits in the treatment and no treatment groups. Though this suggests that the improvement in vision was likely from reduction in CME (Table 2, 3), we cannot conclusively state that treatment of PVL had no impact on the improvement in vision. The impact of PVL on peripheral visual field loss was not documented in this study, and may be a marker of the importance of treating PVL that was unable to be captured in this retrospective study.
With PVL having been described among patients with and without clear ocular disease in the literature, the etiology and impact of this finding remains debatable. Among uveitis patients and especially in those with DVL, PVL could certainly be a result of active retinal vascular inflammation. Another possible etiology of PVL in eyes with significant capillary non-perfusion could be altered retinal vascular hemodynamics in which an increased volume of blood flows through the remaining perfused capillary beds causing leakage. In healthy eyes, the presence of PVL may perhaps reflect traction on the peripheral retinal vasculature by the vitreous base.
This study has several limitations inherent to a single-center retrospective analysis including a relatively small sample size. An important limitation is that follow-up UWFFA were not analyzed in this cohort mainly because the majority of patients did not have such imaging performed. To help better understand the etiology of PVL in uveitis patients, it would be informative to see if treatment augmentation was associated with resolution of PVL on subsequent angiography, and future studies to address this question are certainly warranted. The fact that follow-up UWFFA was not obtained in the majority of our patients suggest that we rely on other morphologic and functional findings to assess for disease activity. In this study, we observed that patients with PVL were more likely to have treatment augmentation than those without PVL. There was a high degree of collinearity between PVL, angiographic CME and other angiographic and clinical signs of active disease. It was thus impossible to adjust for the effects of each individual variable. It is therefore unclear how much the presence of PVL alone, factors into the clinical decision to augment therapy. In this study, we included data from both eyes of several patients who were followed for bilateral uveitis. This could lead to issues with independence when studying the effects of treatment augmentation. We performed statistical analysis with GEE to account for the potential correlation. Additionally, in our study all patients with bilateral disease who had systemic treatment augmentation at any visit had bilateral clinically active disease at that visit. Those patients with bilateral uveitis who were only felt to have clinically active disease in one eye only had local treatment augmentation.
This is the first study, to our knowledge, which looks at the visual outcomes on extended follow-up among uveitis patients with PVL. While it is unclear whether PVL in isolation warrants treatment augmentation, we have shown that PVL can be associated with vision-threatening conditions like CME which does warrant therapy. Additionally, it is important to note that merely the presence or absence of retinal vascular leakage in uveitis patients may not impact the decision to augment therapy as much as the change in degree of retinal vascular leakage. This highlights the need to develop a grading system for retinal vasculitis based on long-term visual outcomes as a function of clinical and angiographic findings such as PVL, DVL, peripheral non-perfusion, angiographic CME, CME on OCT, vascular sheathing, and retinal neovascularization. Development of such a grading scheme could help us better understand when treatment is indicated for PVL in uveitis.
ACKNOWLEDGMENTS:
None
Footnotes
DECLARATION OF INTEREST:
TR, JC, ES, JR, PL are supported by an unrestricted institutional grant from Research to Prevent Blindness (New York, NY) and core grant P30 EY010572 from the National Institute of Health (Bethesda, MD).
PL receives grant support from the National Eye Institute (K08EY022948) and an Individual Career Development Award from Research to Prevent Blindness (New York, NY)
JTR is a consultant for Abbvie, UCB, Gilead, Genentech, Janssen, Santen, Novartis, Cavtherx, Portage, Mitotech, Digital Theraeutics, Xoma, Theravance
EBS is a consultant for Abbvie, Mallinckrodt, Santen, and XOMA and currently receives or has received research support from Abbvie, Bristol Meyers Squibb, Clearside, EyeGate, Genentech, pSivida,and the National Institute of Health.
AT, NP and JB have no financial disclosures to report
REFERENCES
- 1.Manivannan A, Plskova J, Farrow A, Mckay S, Sharp PF, Forrester JV. Ultra-wide-field fluorescein angiography of the ocular fundus. Am J Ophthalmol. 2005;140(3):525–7 [DOI] [PubMed] [Google Scholar]
- 2.Nicholson BP, Nigam D, Miller D, et al. Comparison of wide-field fluorescein angiography and 9-field montage angiography in uveitis. Am J Ophthalmol. 2014;157(3):673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25(1–2):27–33. [DOI] [PubMed] [Google Scholar]
- 4.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessel MM, Aaker GD, Parlitsis G, Cho M, D’amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina (Philadelphia, Pa). 2012;32(4):785–91. [DOI] [PubMed] [Google Scholar]
- 6.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587–95. [DOI] [PubMed] [Google Scholar]
- 7.Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology. 2015;122(5):949–56. [DOI] [PubMed] [Google Scholar]
- 8.Kaines A, Tsui I, Sarraf D, Schwartz S. The use of ultra wide field fluorescein angiography in evaluation and management of uveitis. Semin Ophthalmol. 2009;24(1):19–24. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JP, Leder HA, Sepah YJ, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. Am J Ophthalmol. 2012;154(5):908–911.e2. [DOI] [PubMed] [Google Scholar]
- 10.Leder HA, Campbell JP, Sepah YJ, et al. Ultra-wide-field retinal imaging in the management of non-infectious retinal vasculitis. J Ophthalmic Inflamm Infect. 2013;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesquida M, Llorenç V, Fontenla JR, Navarro MJ, Adán A. Use of ultra-wide-field retinal imaging in the management of active Behçet retinal vasculitis. Retina. 2014;34(10):2121–7. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JP, Beardsley RM, Palejwala NV, et al. Peripheral vascular leakage in uveitis: clinical and angiographic findings. Ophthalmology. 2015;122(6):1269–70. [DOI] [PubMed] [Google Scholar]
- 13.Karampelas M, Sim DA, Chu C, et al. Quantitative analysis of peripheral vasculitis, ischemia, and vascular leakage in uveitis using ultra-widefield fluorescein angiography. Am J Ophthalmol. 2015;159(6):1161–1168.e1. [DOI] [PubMed] [Google Scholar]
- 14.Chi Y, Guo C, Peng Y, Qiao L, Yang L. A prospective, observational study on the application of ultra-wide-field angiography in the evaluation and management of patients with anterior uveitis. PLoS ONE. 2015;10(3):e0122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117(4):780–4. [DOI] [PubMed] [Google Scholar]
- 16.Tsui I, Bajwa A, Franco-cardenas V, Pan CK, Kim HY, Schwartz SD. Peripheral fluorescein angiographic findings in fellow eyes of patients with branch retinal vein occlusion. Int J Inflam. 2013;2013:464127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung TH, Muqit MM, Mordant DJ, Smith LM, Patel CK. Noncontact high-resolution ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014;132(1):108–10. [DOI] [PubMed] [Google Scholar]
- 18.Orlin A, Fatoo A, Ehrlich J, D’amico DJ, Chan RP, Kiss S. Ultra-widefield fluorescein angiography of white without pressure. Clin Ophthalmol. 2013;7:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]


