Abstract
Galectin-3 is a beta-galactoside-binding lectin that plays a role in the regulation of several conditions that are associated with atherosclerosis. The goal of this cross-sectional study was to assess the association of plasma galectin-3 concentrations with sonographic measures of carotid atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) study. Linear regression was used to determine the difference and 95% confidence intervals (CI) for carotid intima-media thickness (cIMT) by categorical and continuous representations of galectin-3. Logistic regression was used to determine the odds ratio and 95% CI, separately, for dichotomized cIMT (75th percentile = 0.9 mm) and carotid plaque and/or shadowing. Compared with those in the first quintile of galectin-3, those in the 5th quintile of galectin-3 level had higher cIMT (mean difference: 0.020 mm after multivariable adjustment; p-trend = 0.04). Moreover, compared with those in the lowest galectin-3 quintile, those in the highest galectin-3 quintile had higher odds of carotid plaque/and or shadowing (OR: 1.13 after multivariable adjustment; p-trend 0.014). Higher levels of galectin-3 are associated with greater carotid atherosclerosis. Our findings provide support for the role of inflammatory biomarkers in the pathogenesis of atherosclerosis and suggest galectin-3 as a possible target for intervention in the prevention or management of atherosclerotic disease.
Keywords: galectin-3, carotid intima-media thickness, plaque, shadowing, atherosclerosis, ARIC
Introduction
Atherosclerosis, a disease of the intima and media of large and medium arteries, is involved in the pathogenesis of coronary artery disease, cerebrovascular disease, peripheral artery disease and renovascular disease. These conditions lead to significant morbidity and mortality and secondarily further accelerate the atherosclerotic process, leading to other complications. The broad effects of atherosclerosis on multiple organ systems – directly and indirectly–make it a major cause of morbidity and mortality in developed countries.1, 2
Many of the identified risk factors for atherosclerosis damage the endothelial as a first step in the atherosclerotic process. Risk factors additionally serve as a stimulus for the production of pro-inflammatory cytokines that oxidize lipids, recruit circulating monocytes, and serve as growth factors for smooth muscle cells.3–5 Monocytes contribute to atherosclerosis by producing pro-inflammatory mediators, one of which is galectin-3.
Galectin-3 is a beta-galactoside-binding lectin expressed by activated macrophages. It has several biological functions, some of which include: intracellular signaling, regulation of gene-expression, cell-to-cell interaction, short-distance signaling, and exchanges between cells and the extracellular matrix.6, 7 Recently, epidemiological studies have shown that circulating galectin-3 concentrations are positively associated with risk of heart failure, atrial fibrillation and coronary artery disease.8, 9 [Aguilar D et al. ARIC unpublished data] Galectin-3 also plays a role in the regulation of immunological, inflammatory, and nutritional conditions, many of which are associated with atherosclerosis.10 Some experimental studies in mice have shown that galectin-3 activity is modifiable through the use of inhibitors.11, 12 Understanding whether galectin-3 is associated with atherosclerosis, in humans, might help in the development of new methods to detect early atherosclerosis, in the assessment of atherosclerotic burden, or in the development new treatments aimed at slowing down the progression of atherosclerosis.13, 14
B-mode ultrasound to measure carotid artery intima-media thickness (cIMT) or plaque and/or shadowing is a non-invasive and relatively inexpensive way to assess subclinical atherosclerosis. These carotid measures are predictive of future cardiovascular events.9, 15, 16
The aim of this study was to assess cross-sectionally the association of plasma galectin-3 concentrations with sonographic measures of carotid atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) study.
Methods
The ARIC study is a population based cohort comprising 45–64 year old men and women (n = 15, 792) recruited between 1987 and 1989 from Washington County, MD; the northwest suburbs of Minneapolis, MN; Jackson, MS (Blacks only); and Forsyth County, NC.17 Participants have been re-examined 5 additional times since study onset and are also being followed by semi-annual telephone interviews and active surveillance of ARIC community hospitals. ARIC was approved by the institutional review board of each participating center, and all participants provided written informed consent for each study visit. We used data from ARIC visit 4 (1996 – 1998) for this cross-sectional analysis.
As depicted in Figure 1, among 11,656 study participants present at ARIC visit 4, we excluded those who were not black or white (n = 31), those with missing galectin-3 (n = 934), those who did not undergo carotid ultrasound measurements (n = 5,449) and those with missing covariates (n = 181). This left a total of 5,061 study participants for analysis. There were statistically significant, albeit mostly clinically minor, differences in the characteristics of the participants included in this analysis (n = 5,061) and the participants who were excluded (n = 6,595) (Supplement Table 1). The most striking differences were a 13.2% prevalence of diabetes and 43.7% prevalence of hypertension in the included participants versus 19.4% and 50.7%, respectively, for the excluded.
Figure 1.
Eligibility Determination for Galectin-3 and Atherosclerosis, ARIC 1996–1998.
Galectin-3 and Covariate Ascertainment
During July 2015 through February 2016, galectin-3 was measured on ARIC participants’ samples of EDTA-plasma stored at −80°C since visit 4 (1996–1998), using a chemiluminescent micro particle immunoassay on an Architect i 2000sr instrument (Abbott, Abbott Park, IL). The Architect galectin-3 assay has a limit of detection of 1.1 ng/ml and a limit of quantitation of 4.0 ng/ml. Interassay coefficients of variation (CV) were 5.2, 3.3 and 2.3% at mean galectin-3 levels of 8.8, 19.2 and 72.0 ng/ml, respectively. At the time of blood processing, 402 participants’ plasma specimens were split, masked, and sent to the laboratory to assess galectin-3 laboratory reliability. The reliability coefficient was r = 0.95 and CV = 5.7 %.
All covariates were measured at visit 4. Participants reported demographic information, use of antihypertensive and cholesterol medications within the previous 2 weeks, and smoking status. Blood pressure was measured with the use of a random-zero sphygmomanometer with 2 readings taken after the participant had rested for 5 min and these readings were averaged. Height and weight were measured using a standardized protocol, and BMI was calculated in kg/m2. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl (7.0 mmol/L), non-fasting glucose ≥ 200 mg/dl (11.1 mmol/L), treatment for diabetes mellitus, or self-reported physician diagnosis of diabetes mellitus. Plasma total cholesterol was measured18 and high density lipoprotein cholesterol (HDL-C) was measured after dextran-magnesium precipitation of non-HDL lipoproteins. High-sensitivity C-reactive protein (hsCRP) was measured by an immunoturbidimetric assay using a Siemens (Dade Behring) BNII analyzer (Dade Behring, Deerfield, I11).19 The reliability coefficient for blinded replicate measurements of hsCRP was 0.99 (n = 421 pairs). Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine and cystatin-C using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.20
Carotid Ultrasound Measurement
The ultrasound procedure in the ARIC study has been previously described.21–24 Briefly, a Biosound 2000IISA system was used and images recorded on VHS tape. cIMT was measured centrally by trained readers at the ARIC Ultrasound Reading Center. The cIMT was assessed in 3 segments: the distal common carotid (1 cm proximal to dilation of the carotid bulb), the carotid artery bifurcation (1 cm proximal to the flow divider), and the proximal internal carotid arteries (1 cm section of the internal carotid artery immediately distal to the flow divider). At each of these segments, 11 measurements of the far wall (in 1 mm increments) were attempted. The overall mean of the mean measurements across these segments of both right and the left sides were estimated. Trained readers adjudicated plaque presence or absence if 2 of the following 3 criteria were met: abnormal wall thickness (defined as cIMT >1.5 mm), abnormal shape (protrusion into the lumen, loss of alignment with adjacent arterial wall boundary), and abnormal wall texture (brighter echoes than adjacent boundaries).22, 25 Readers were not clinically trained, nor did their training as readers prepare them to recognize special qualitative features of artery plaque lesions visualized by B-mode ultrasound such as heterogeneity or intraplaque hemorrhage. The recognition of acoustic shadowing, defined as the reduction in amplitude of echoes caused by intervening structures with high attenuation, was an element of the reading protocol included in the readers’ training, and was independently recorded. Acoustic shadowing is indicative of mineral deposits within the plaque,26 and clinically it is assumed to be suggestive of prior intraplaque hemorrhage.22
The reproducibility and variation of cIMT and plaque measurements in the ARIC study have been previously published.22, 27 The site-specific reliability coefficients were estimated as 0.77, 0.73, and 0.70 for the mean carotid far wall IMT at the carotid bifurcation, internal carotid arteries, and common carotid arteries, respectively. For the presence or absence of plaque in any of the 6 artery segments, the intra-reader agreement was associated with a Kappa statistic of 0.76, while the inter-reader agreement was 0.56; the corresponding Kappa statistics for plaque with shadowing in any of the 6 segments were 0.76 and 0.45, respectively28 which suggests good agreement beyond chance.
Statistical Analysis
Baseline characteristics of participants were described by galectin-3 quintiles. Categorical variables were presented as percentages. Normally distributed continuous variables are presented as means and standard deviations (SD). Non-normally distributed variables are presented as medians with their interquartile range (25th to 75th percentiles).
We performed a linear regression to test the relationship between galectin-3 quintiles and cIMT and between continuous galectin-3 and cIMT. We also performed logistic regression with the same galectin-3 measures in relation to using categorical cIMT - using a cut-off of 0.9 mm.29–31 We tested for linear trends in cIMT and the presence of plaque/shadowing across galectin-3 quintiles by using an ordinal variable incorporating the mean galectin-3 within quintiles.
Model 1 of our regression analyses adjusted for demographic variables – age (years), sex (male, female), and race (black, white). Model 2, our main model, additionally adjusted for eGFR and several established atherosclerosis risk factors considered to be possible confounding factors - diabetes, systolic blood pressure (mmHg), total cholesterol, HDL-C (mg/dl), triglycerides, BMI (kg/m2), use of antihypertensive medications (yes, no), use of anti-inflammatory medications (yes, no), use of anti-ulcer medications (yes, no), and use of nutritional supplements (yes, no). The variable nutritional supplements was a composite variable made up of several supplements the participants indicated they were taking at visit 4, these included: “multivitamin” pills (the most common) as well as individual vitamins B1-B6, B12, C, A, D, E, K, Folic acid, bioflavonoids, Mg, Zn, amino acid mixtures, etc. Model 3 was further adjusted for hsCRP. Differences in the association between galectin-3 and cIMT by race and sex were tested multiplicative interaction terms for logistic regression. Statistical significance was based on a 2-sided type 1 error p = 0.05.
We performed a sensitivity analysis to estimate the effect of prevalent cardiovascular disease (CVD) on the association between galectin-3 and cIMT. Prevalent CVD was a composite variable made up of prevalent peripheral artery disease, prevalent coronary heart disease and prevalent stroke. For this analysis, we included only participants without any prevalent CVD (n= 3975).
All statistical analyses were performed using SAS 9.4 (SAS institute Inc., Cary, NC).
Results
Among the 5,061 participants eligible for this analysis, the median (25th - 75th percentile) galectin-3 concentration was 14.1 (11.9 – 16.6) ng/ml. As shown in Table 1, higher plasma galectin-3 levels were associated with higher mean age, systolic blood pressure, total cholesterol, HDL-C, BMI, and lower mean eGFR. Higher plasma galectin-3 levels were also associated with higher median hsCRP and higher median plasma triglycerides. The proportions of participants who were females, blacks, diabetic, hypertensive, and on nutritional supplements, anti-hypertensive, anti-ulcer, anti-inflammatory medication were associated positively with galectin-3 quintiles. However, there was no statistically significant difference in the proportions of participants who were current smokers or who were on statins among the galectin-3 quintiles.
Table 1.
Participant Characteristics According to Plasma Galectin-3 Quintiles, Atherosclerosis Risk in Communities (ARIC) Study, 1996 −1998.
| Characteristics* | Galectin-3 Quintiles (ng/ml) | p-trend | ||||
|---|---|---|---|---|---|---|
| Q1 (4.4 −11.4) |
Q2 (11.5 – 13.2) |
Q3 (13.3 – 15.0) |
Q4 (15.1 – 17.3) |
Q5 (17.4 – 96.9) |
||
| N | 1027 | 1012 | 1016 | 981 | 1025 | |
| Age (years) | 61.7 (5.4) | 62.1 (5.6) | 62.8 (5.5) | 63.1 (5.5) | 64.5 (5.6) | <0.0001 |
| Sex (% female) | 34.9 | 47.3 | 54.7 | 64.9 | 70.5 | <0.0001 |
| Race (% Black) | 13.5 | 13.8 | 14.9 | 17.9 | 19.2 | < 0.0001 |
| Diabetes (%) | 11.9 | 10.9 | 12.7 | 13.4 | 17.0 | 0.0001 |
| Current cigarette smoking (%) | 14.3 | 15.7 | 15.3 | 14.7 | 16.1 | 0.14 |
| Systolic blood pressure (mmHg) | 125 (18) | 126 (19) | 126 (18) | 126 (18) | 128 (20) | 0.0001 |
| Hypertension (%) | 36.1 | 40.0 | 38.3 | 46.9 | 57.3 | <0.0001 |
| Antihypertensive medication (%) | 32.1 | 32.8 | 33.9 | 45.0 | 58.8 | <0.0001 |
| Use of statins (%) | 10.5 | 10.4 | 11.1 | 11.2 | 12.2 | 0.18 |
| Anti-inflammatory medication (%) | 70.1 | 70.1 | 68.8 | 72.6 | 76.7 | 0.0004 |
| Anti-ulcer medications (%) | 18.3 | 20.9 | 21.9 | 24.2 | 24.8 | <.0001 |
| Nutritional supplements (%) | 48.8 | 55.0 | 53.1 | 53.6 | 56.1 | 0.0071 |
| Total cholesterol (mg/dl) | 197 (34) | 199 (33) | 201 (36) | 201 (36) | 202 (37) | 0.0002 |
| HDL-C (mg/dl) | 50 (17) | 51 (16) | 51 (17) | 52 (17) | 51 (17) | 0.01 |
| Triglycerides (mg/dl)† | 111 (80 – 157) |
117 (87 – 165) |
119 (86 – 165) |
119 (90 – 173) |
134 (95 – 183) |
< 0.0001 |
| BMI (kg/m2) | 27.4 (4.2) | 27.7 (4.8) | 27.8 (5) | 28.3 (5.3) | 29.4 (6.2) | <0.0001 |
| eGFR (ml/min/1.73m2) | 92 (14) | 89 (14) | 87 (14) | 85 (15) | 75 (19) | <0.0001 |
| hsCRP (mg/L)† | 1.52 (0.78 – 3.29) |
1.88 (0.96 – 4.41) |
2.19 (1 – 4.75) |
2.69 (1.15 – 5.60) |
3.64 (1.51 – 7.62) |
<0.0001 |
BMI = Body mass index; eGFR = Estimated glomerular filtration rate; HDL-C = High density lipoprotein cholesterol; hsCRP = High-sensitivity C-reactive protein.
Values are mean (standard deviation) for continuous variables and percentages for categorical variables unless otherwise specified.
Values are expressed as median (25th – 75th percentile).
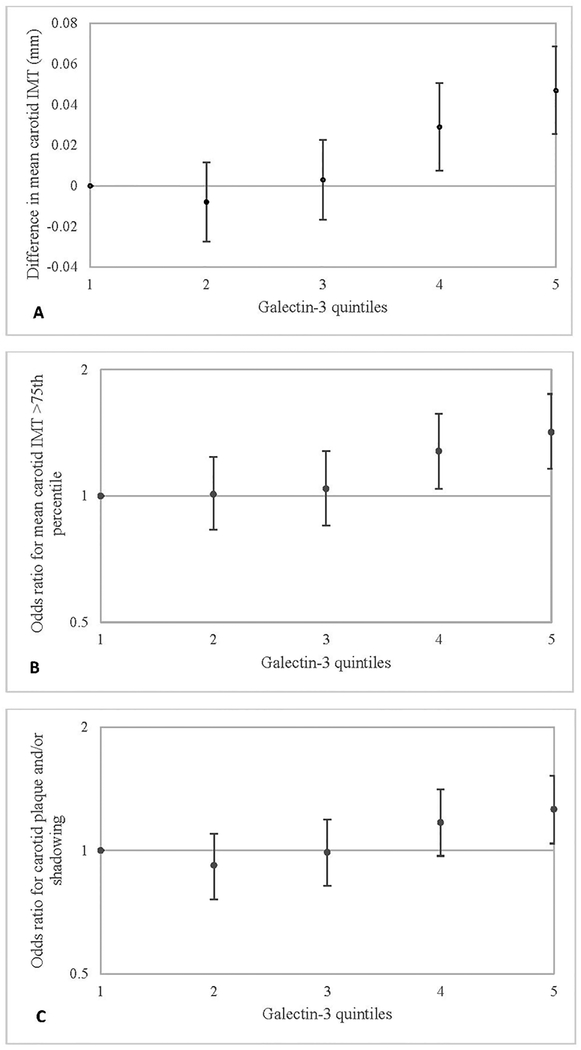
The relation between the galectin-3 quintiles and sonographic measures of carotid atherosclerosis - cIMT and plaque/shadowing - was positive and curvilinear (Table 2 and Figure 2). There was little difference in mean cIMT (mm) across the first 3 galectin-3 quintiles, but cIMT was 0.047 mm higher in quintile 5 compared with quintile 1 in our minimally adjusted model (p-trend = 0.009); this difference was 0.025 mm in Model 2, after adjusting for atherosclerotic risk factors, medications, and eGFR (p-trend = 0.04) and 0.020 mm in Model 3, after further adjustment for hsCRP (p-trend = 0.04).
Table 2.
Associations of Plasma Galectin-3 Quintiles with Carotid Atherosclerosis Measures, Atherosclerosis Risk in Communities (ARIC) Study, 1996–1998.
| Difference (SE) in Mean Carotid IMT (mm) per Galectin-3 Quintile (ng/ml) | p-trend | |||||
| Q1 (4.4 −11.4) |
Q2 (11.5 −13.2) |
Q3 (13.3 −15.0) |
Q4 (15.1 −17.3) |
Q5 (17.4 −96.9) |
||
| Model 1 | (Ref.) | −0.008 (0.010) | 0.003 (0.010) | 0.029 (0.011) | 0.047 (0.011) | 0.009 |
| Model 2 | −0.009 (0.010) | −0.001 (0.010) | 0.018 (0.011) | 0.025 (0.011) | 0.04 | |
| Model 3 | −0.012 (0.010) | −0.004 (0.010) | 0.014 (0.011) | 0.020 (0.011) | 0.04 | |
| Odds Ratio for Mean Carotid IMT >75th Percentile per Galectin-3 Quintile (ng/ml) | ||||||
| Q1 (4.4 −11.4) |
Q2 (11.5 −13.2) |
Q3 (13.3 −15.0) |
Q4 (15.1 −17.3) |
Q5 (17.4 −96.9) |
||
| Model 1 | (Ref.) | 1.01 (0.83–1.24) | 1.04 (0.85–1.28) | 1.28 (1.04–1.57) | 1.42 (1.16–1.75) | 0.0011 |
| Model 2 | 1.00 (0.81–1.23) | 1.01 (0.82–1.24) | 1.17 (0.95–1.45) | 1.21 (0.97–1.52) | 0.06 | |
| Model 3 | 0.97 (0.79–1.20) | 0.98 (0.80–1.21) | 1.13 (0.91–1.40) | 1.15 (0.92–1.44) | 0.10 | |
| Odds Ratio for Carotid Plaque and/or Shadowing per Galectin-3 Quintile (ng/ml) | ||||||
| Q1 (4.4 −11.4) |
Q2 (11.5 −13.2) |
Q3 (13.3 −15.0) |
Q4 (15.1 −17.3) |
Q5 (17.4 −96.9) |
||
| Model 1 | (Ref.) | 0.92 (0.76–1.10) | 0.99 (0.82–1.19) | 1.17 (0.97–1.41) | 1.26 (1.04–1.52) | 0.0006 |
| Model 2 | 0.92 (0.76–1.11) | 0.98 (0.81–1.19) | 1.12 (0.92–1.36) | 1.19 (0.97–1.46) | 0.007 | |
| Model 3 | 0.90 (0.74–1.08) | 0.96 (0.79–1.16) | 1.09 (0.89–1.32) | 1.13 (0.92–1.39) | 0.014 | |
CI = confidence interval; IMT = Intima-media thickness; SE = Standard Error
Model 1: Adjusted for age, race-center, and gender.
Model 2: Additional adjustment for diabetes, systolic blood pressure, total cholesterol, High density lipoprotein cholesterol, triglycerides, body mass index, use of antihypertensive medication, use of anti-inflammatory medication, use of anti-ulcer medication, use of nutritional supplements, and estimated glomerular filtration rate.
Model 3: Additional adjustment for High-sensitivity C-reactive protein (log transformed).
Figure 2.
A. Difference (SE) in Mean Carotid IMT (mm). B. Odds Ratios (95% CI) for Mean Carotid IMT > 75th Percentile. C. Odds Ratios (95% CI) for Carotid Plaque and/or shadowing, in Relation to Plasma Galectin-3 Quintiles, ARIC 1996–1998.
*Graphs Shown are for Model 1.
As also shown in Table 2 and Figure 2, the odds of having cIMT >75th percentile (0.9 mm) was 42% higher (95% CI: 16 – 75%) for participants in quintile 5 of galectin-3 compared with quintile 1 (ref.) (p-trend = 0.001) in Model 1. However, the trend was not statistically significant in Models 2 and 3.
The odds of having carotid plaque and/or shadowing was 26% (95% CI: 4 – 52%) higher for participants in quintile 5 vs quintile 1 (Table 2, Figure 2). Although this odds ratio was attenuated after adjustment, the positive association between galectin-3 quintiles and plaque/shadowing remained statistically significant after adjusting for atherosclerotic risk factors, medications, and eGFR in Model 2 (OR 95% CI: 1.19 (0.97–1.46)), and hsCRP in Models 3 (OR 95% CI: 1.13 (0.92–1.39)) (p-trend = 0.014).
When galectin-3 was analyzed as a continuous variable (Table 3), the associations with mean cIMT, with cIMT >75th percentile, and with carotid plaque/shadowing in Models 1–3 were all positive and statistically significant (Table 3). These findings did not differ by gender (p-interaction = 0.87) or race-center (p-interaction = 0.90).
Table 3.
Associations of 1 Standard Deviation Increment of Plasma Galectin-3 with Carotid Atherosclerosis Measures, Atherosclerosis Risk in Communities (ARIC) Study, 1996–1998.
| Difference in Overall Mean IMT per 1 SD Galectin-3 Increment | ||||
| Diff (mm) | SE | p | ||
| Model 1 | 0.022 | 0.004 | <.0001 | |
| Model 2 | 0.015 | 0.004 | <.0001 | |
| Model 3 | 0.013 | 0.004 | 0.0005 | |
| Odds Ratio for Mean Carotid IMT >75th Percentile per 1 SD Galectin-3 Increment | ||||
| OR (95% CI) | p | |||
| Model 1 | 1.15 (1.08–1.23) | <.0001 | ||
| Model 2 | 1.09 (1.02–1.17) | 0.02 | ||
| Model 3 | 1.08 (1.00–1.16) | 0.04 | ||
| Odds Ratio for Carotid Plaque and/or Shadowing per 1 SD Galectin-3 Increment | ||||
| OR (95% CI) | p | |||
| Model 1 | 1.12 (1.05–1.19) | 0.0004 | ||
| Model 2 | 1.09 (1.02 – 1.17) | 0.01 | ||
| Model 3 | 1.08 (1.01 – 1.16) | 0.03 | ||
1 galectin-3 Standard Deviation = 4.5 ng/ml.
CI = confidence interval; IMT = Intima-media thickness; SD = Standard Deviation; SE = Standard Error.
Model 1: Adjusted for age, race-center, and gender.
Model 2: Additional adjustment for diabetes, systolic blood pressure, total cholesterol, High density lipoprotein cholesterol, triglycerides, body mass index, use of antihypertensive medication, use of anti-inflammatory medication, use of anti-ulcer medication, use of nutritional supplements, and estimated glomerular filtration rate.
Model 3: Additional adjustment for High-sensitivity C-reactive protein (log transformed).
When these analyses were repeated on the separate regions of the carotid artery – common carotid, internal carotid, and bifurcation – the association between galectin-3 and cIMT was strongest in the internal carotid artery. The difference in mean internal carotid IMT for every 4.5 ng/ml change in plasma galectin-3 was 0.030 mm in Model 1 (p<0.0001) and 0.021 mm in Model 3 (p<0.0001) (Supplemental Table 2). Also, for every 4.5 ng/ml increment in plasma galectin 3 the odds of having cIMT >75th percentile in the internal carotid was 14% higher (95% CI 7 – 22%); the odds ratio was attenuated to 8% (95% CI: 1 – 17%) after additional adjustment for atherosclerotic risk factors, medications, eGFR and hsCRP (Supplemental Table 3).
In our sensitivity analysis, there was further attenuation of the association of galectin-3 with cIMT and carotid plaque and/or shadowing, after excluding participants with prevalent CVD (Supplemental Tables 4–5). However, overall, the findings were consistent with those of the primary analysis.
Discussion
In this large cross-sectional population-based study, we found that higher levels of circulating galectin-3 were positively associated with higher mean cIMT and greater odds of carotid plaque/shadowing. The associations of galectin-3 with these measures of subclinical atherosclerosis were modest, but were minimally attenuated by adjustment for other measured atherosclerosis risk factors.
Few previous studies have examined the relationship between atherosclerosis and circulating galectin-3. Some cross-sectional clinical studies showed a positive relationship between circulating galectin-3 and coronary atherosclerosis,32–34 and several have shown a positive prospective association with coronary events. A previous cross-sectional study carried out in Spain found a strong correlation between plasma galectin-3 concentration and cIMT and higher plasma galectin-3 in subjects with carotid atherosclerosis compared with subjects without carotid atherosclerosis.35 Another study reported a positive association between galectin-3 and large artery atherosclerotic stroke.36 Another examining galectin-3 expression in human atherosclerotic lesions found a higher occurrence of galectin-3-positive cells in carotid artery atherosclerotic lesions than in lower limb atherosclerotic lesions.37 In addition to these, a few experimental studies have shown a reduction in the progression of atherosclerosis following inhibition of galectin-3 secretion or inactivation of galectin-3 gene in mice.13,14,38
Although most studies have shown a positive association between levels of galectin-3 and atherosclerotic disease, two have shown the opposite. A cross-sectional study of patients undergoing carotid endarterectomy carried out to examine the relationship between galectin-3 and plaque vulnerability in patients with high grade carotid stenosis found lower intra-plaque concentration of galectin-3 in patients in the symptomatic group compared with the asymptomatic group, but found no statistically significant difference in mean serum galectin-3 levels between the 2 groups. The authors also found higher intra-plaque concentrations of galectin-3 among patients on long-term statin therapy (>1 month) compared with patients on short-term therapy (7 – 15 days preoperatively).39 An experimental study showed that galectin-3 knockout mice developed more complex atherosclerotic lesions compared to wild-type mice after been fed a high fat diet.40 These findings might be because the study done on the patients undergoing endarterectomy was small and likely underpowered and the findings in mice might point to species-specific difference in the role of galectin-3.
Although most research supports a role of galectin-3 in inflammation and the development and progression of atherosclerosis,37 it is difficult to ascribe a direct causal relationship between plasma galectin-3 elevation and atherosclerosis.41–43 Differences in cell types, external stimuli, and environmental conditions may alter the expression level of galectin-3 and function of galectin-3.42 Also, understanding of the pathway for extracellular expression, including secretion, of galectin-3 is limited; it has been shown that some stimuli lead to secretion while others favor intracellular action of galectin-3. Not all stimuli that lead to secretion are pro-inflammatory or atherogenic,41,43 although a few of the pro-secretory stimuli for galectin-3 are also associated with atherosclerotic lesion formation.44,45
Limitations in our study warrant consideration. Firstly, due to its cross-sectional nature we cannot ascribe a temporal relationship between high circulating galectin-3 levels and greater cIMT. It has been shown that the higher levels of circulating galectin-3 leads to increased recruitment of monocytes46 and growth of fibroblasts, which could result in increased plaque growth and hence higher cIMT. Alternately, it is also plausible that increased numbers of activated macrophages in atherosclerotic plaques secrete galectin-3 and explain the higher plasma circulating levels meaning galectin-3 might be best thought about as a marker of atherosclerosis, but not a key molecule in the causal pathway. In addition, it may be that galectin-3 is more important in later atherosclerosis, rather than at the earlier stages of atherosclerosis present in most ARIC participants.
Secondly, the galectin-3 measurements were made on samples stored for years. Although our laboratory internal quality control and blinded external quality control data indicate excellent assay performance, we cannot rule out that sample degradation may have affected galectin-3 measurements. However, if sample integrity were a factor, we would have expected this degradation to be random among samples, which should lead to our associations being underestimated. A previous report has shown that galectin-3 concentrations appear to be stable after storage for at least 6 months.47
Thirdly, cIMT and carotid plaque/shadowing have measurement error,48,49 but this error would be unlikely related to galectin-3 level and so it would also tend to weaken observed associations. Carotid measures, of course, are limited to one segment of the vascular tree and are strongly influenced by age and hypertension.50,51 They therefore are considered to be indirect assessments of atherosclerotic burden.52–54
Lastly, since our measurement of atherosclerotic burden was made using 2D ultrasound, we were not able to assess the volume of the plaques, this might have led to underestimation of the relationship between circulating galectin-3 and carotid plaque burden.55,56
Strengths of our study include it being a population-based cohort and a larger sample size than previous reports on this topic.35,36,39 In addition, we assessed subclinical atherosclerosis in generally healthier35,39 and younger participants than previous studies,36 using plasma galectin-3 as opposed to intra-plaque galectin-3.37 Our study quantifies the association between circulating galectin-3 and carotid atherosclerosis and suggests that galectin-3 might be important in the pathogenesis of subclinical atherosclerosis in healthy individuals.
In conclusion, higher levels of galectin-3 are associated with greater carotid atherosclerosis as measured by ultrasonography. Our findings provide support for the role of inflammatory biomarkers in the pathogenesis of atherosclerosis, as confirmed by the recent CANTOS trial,57 and suggest galectin-3 as a possible target for trials of intervention in the prevention or management of atherosclerotic disease.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Abbott Diagnostics provided funding for the galectin-3 measurements.
Footnotes
Declaration of Conflicting Interests
Financial disclosure for Christie M. Ballantyne, MD:
Grant/research support: Abbott Diagnostics, Roche Diagnostics (to institution, not individual).
Consultant: Abbott Diagnostics, Roche Diagnostics.
Patent pending: Provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine and Roche.
The other co-authors have no conflicts of interest to disclose.
References
- 1.Roquer J, Ois A. Atherosclerotic Burden and Mortality In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer New York; 2010, p. 899–918. [Google Scholar]
- 2.Barquera S, Pedroza-Tobias A, Medina C, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46;328–38. [DOI] [PubMed] [Google Scholar]
- 3.Falk E Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis — An inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 6.Di Lella S, Sundblad V, Cerliani JP, et al. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry. 2011;50:7842–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863:427–37. [DOI] [PubMed] [Google Scholar]
- 8.Fashanu OE, Norby FL, Aguilar D, et al. Galectin-3 and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2017;192:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisowska A, Knapp M, Tycińska A, et al. Predictive value of galectin-3 for the occurrence of coronary artery disease and prognosis after myocardial infarction and its association with carotid IMT values in these patients: A mid-term prospective cohort study. Atherosclerosis. 2016;246:309–17. [DOI] [PubMed] [Google Scholar]
- 10.Shimura T, Shibata M, Gonda K, et al. Association between circulating galectin-3 levels and the immunological, inflammatory and nutritional parameters in patients with colorectal cancer. Biomed Rep. 2016;5:203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacKinnon AC, Liu X, Hadoke PW, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WS, Cao Z, Leffler H, Nilsson UJ, Panjwani N. Galectin-3 inhibition by a small-molecule inhibitor reduces both pathological corneal neovascularization and fibrosis. Invest Ophthalmol Vis Sci. 2017;58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Zhang M, Zhao P, et al. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol Med Rep. 2017;16:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachtigal M, Ghaffar A, Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol. 2008;172:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bots ML, Evans GW, Tegeler CH, Meijer R. Carotid intima-media thickness measurements: Relations with atherosclerosis, risk of cardiovascular disease and application in randomized controlled trials. Chin Med J (Engl). 2016;129:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–104. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009;102:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita K1, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by B-mode ultrasound: the Atherosclerosis Risk in Communities Study. Ultrasound Med Biol. 1996;22:791–9. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–83. [DOI] [PubMed] [Google Scholar]
- 23.The ARIC Study Group. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. J Neuroimaging. 1991;1:168–72. [PubMed] [Google Scholar]
- 24.National Heart L, and Blood Institute. Atherosclerosis Risk in Communities (ARIC) Study. Operations Manual, No. 7: Blood Collection and Processing: Version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 25.Hunt KJ, Sharrett AR, Chambless LE, Folsom AR, Evans GW, Heiss G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts CHD. Ultrasound Med Biol. 2001;27:357–65. [DOI] [PubMed] [Google Scholar]
- 26.Kremkau FW. Diagnostic Ultrasound: Principles, Instruments, and Exercises. 3rd ed. Philadelphia, PA: WB Saunders Co; 1992. [Google Scholar]
- 27.Chambless LE, Zhong MM, Arnett D, Folsom AR, Riley WA, Heiss G. Variability in B-mode ultrasound measurements in the Atherosclerosis Risk in Communities (ARIC) study. Ultrasound Med Biol. 1996;22:545–54. [DOI] [PubMed] [Google Scholar]
- 28.Duncan BB, Metcalf P, Crouse JR 3rd, et al. , for the Atherosclerosis Risk in Communities Study Investigators. Risk factors differ for carotid artery plaque with and without acoustic shadowing. J Neuroimaging. 1997;7:28–34. [DOI] [PubMed] [Google Scholar]
- 29.Naqvi TZ, Lee MS. carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–38. [DOI] [PubMed] [Google Scholar]
- 30.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation.1997;96:1432–7. [DOI] [PubMed] [Google Scholar]
- 31.Liviakis L, Pogue B, Paramsothy P, Bourne A, Gill EA. Carotid intima-media thickness for the practicing lipidologist. J Clin Lipidol. 2010;4:24–35. [DOI] [PubMed] [Google Scholar]
- 32.Ozturk D, Celik O, Satilmis S, et al. Association between serum galectin-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron Artery Dis. 2015;26:396–401. [DOI] [PubMed] [Google Scholar]
- 33.Falcone C, Lucibello S, Mazzucchelli I, et al. Galectin-3 plasma levels and coronary artery disease: a new possible biomarker of acute coronary syndrome. Int J Immunopathol Pharmacol. 2011;24:905–13. [DOI] [PubMed] [Google Scholar]
- 34.Aksan G, Gedikli Ö, Keskin K, et al. Is galectin-3 a biomarker, a player - or both - in the presence of coronary atherosclerosis? J Investig Med. 2016;64:764–70. [DOI] [PubMed] [Google Scholar]
- 35.Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3:e000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He XW, Li WL, Li C, et al. Serum levels of galectin-1, galectin-3, and galectin-9 are associated with large artery atherosclerotic stroke. Sci Rep. 2017;7:40994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachtigal M, Al-Assaad Z, Mayer EP, Kim K, Monsigny M. Galectin-3 expression in human atherosclerotic lesions. Am J Pathol. 1998;152:1199–208. [PMC free article] [PubMed] [Google Scholar]
- 38.MacKinnon AC, Liu X, Hadoke PW, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadoglou NP, Sfyroeras GS, Spathis A, et al. Galectin-3, carotid plaque vulnerability, and potential effects of statin therapy. Eur J Vasc Endovasc Surg. 2015;49:4–9. [DOI] [PubMed] [Google Scholar]
- 40.Iacobini C, Menini S, Ricci C, et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: role of lipoxidation via receptor-mediated mechanisms. Arterioscler Thromb Vascular Biol. 2009;29:831–6. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Alvarez L, Ortega E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators Inflamm. 2017;2017:9247574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumic J, Dabelic S, Flögel M. Galectin-3: An open-ended story. Biochim Biophys Acta. 2006;1760:616–35. [DOI] [PubMed] [Google Scholar]
- 43.Wan L, Liu F-T. Galectin-3 and inflammation. Glycobiol Insights. 2016;6:1–9. [Google Scholar]
- 44.Cullen P, Rauterberg J, Lorkowski S. The Pathogenesis of Atherosclerosis In: von Eckardstein A, editor. Atherosclerosis: Diet and Drugs. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005. p. 3–70. [DOI] [PubMed] [Google Scholar]
- 45.Subhash VV, Ling SSM, Ho B. Extracellular galectin-3 counteracts adhesion and exhibits chemoattraction in Helicobacter pylori-infected gastric cancer cells. Microbiology. 2016;162:1360–6. [DOI] [PubMed] [Google Scholar]
- 46.Sano H, Hsu DK, Yu L, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–64. [DOI] [PubMed] [Google Scholar]
- 47.Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43:683–90. [DOI] [PubMed] [Google Scholar]
- 48.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–81. [DOI] [PubMed] [Google Scholar]
- 49.Spence JD. Ultrasound measurement of carotid plaque as a surrogate outcome for coronary artery disease. Am J Cardiol. 2002;89(4A):10B–15B; discussion 15B-16B. [DOI] [PubMed] [Google Scholar]
- 50.Qu B, Qu T. Causes of changes in carotid intima-media thickness: a literature review. Cardiovasc Ultrasound. 2015;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen ML, Njølstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke. A 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke. 2011;42:972–8. [DOI] [PubMed] [Google Scholar]
- 52.Kasliwal RR, Bansal M, Desai D, Sharma M. Carotid intima–media thickness: Current evidence, practices, and Indian experience. Indian J Endocrinol Metab. 2014;18:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasterkamp G, Schoneveld AH, Hillen B, Banga JD, Haudenschild CC, Borst C. Is plaque formation in the common carotid artery representative for plaque formation and luminal stenosis in other atherosclerotic peripheral arteries? A post mortem study. Atherosclerosis. 1998;137:205–10. [DOI] [PubMed] [Google Scholar]
- 54.Adams MR, Nakagomi A, Keech A, et al. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995;92:2127–34. [DOI] [PubMed] [Google Scholar]
- 55.López-Melgar B, Fernández-Friera L, Sánchez-González J, et al. Accurate quantification of atherosclerotic plaque volume by 3D vascular ultrasound using the volumetric linear array method. Atherosclerosis. 2016;248:230–7. [DOI] [PubMed] [Google Scholar]
- 56.Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681–9. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.