Abstract
Background:
Fetal endocrine signals are generally considered to contribute to the timing of birth and the initiation of labor. Fetal tissues under oxidative stress release inflammatory mediators that lead to sterile inflammation within the maternal-fetal interface. Importantly, these inflammatory mediators are packaged into exosomes, bioactive cell-derived extra cellular vesicles that function as vectors and transport them from the fetal side to the uterine tissues where they deposit their cargo into target cells enhancing uterine inflammatory load. This exosome-mediated signaling is a novel mechanism for fetal-maternal communication.
Objective:
This report tested the hypothesis that oxidative stress can induce fetal amnion cells to produce exosomes, which function as a paracrine intermediary between the fetus and mother and biochemically signal readiness for parturition.
Study Design:
Primary amnion epithelial cells (AEC) were grown in normal cell culture (control) or exposed to oxidative stress conditions (induced by cigarette smoke extract). Exosomes were isolated from cell supernatant by sequential ultracentrifugation. Exosomes were quantified and characterized based on size, shape, and biochemical markers. Myometrial, decidual and placental cells (BeWo) were treated with 2×105, 2×107 and 2×109 control or oxidative stress derived AEC exosomes for 24 hours. Entry of AEC exosomes into cells was confirmed by confocal microscopy of fluorescent-labelled exosomes. The effect of AEC exosomes on target cell inflammatory status was determined by measuring production of IL-6, IL-8, IL-1β, TNF-α and PGE2 by ELISA and inflammatory gene transcription factor (NF-κβ) activation status by immunoblotting for phosphorylated RelA/p65. Localization of NANOG in term human myometrium and decidua obtained from women before labor and during labor was performed using immunohistochemistry. Data were analyzed by Wilcoxon-Mann-Whitney test to compare effects of exosomes from control and oxidative stress -treated AEC cells on inflammatory status of target cells.
Results:
AECs released ~125 nm, cup shaped exosomes with ~ 899 and 1211 exosomes released per cell from control and oxidative stress induced cells respectively. AEC exosomes were detected in each target cell type after treatment using confocal microscopy. Treatment with AEC exosomes increased secretion of IL-6, IL-8 and PGE2 and activation of NF-κβ (each p<0.05) in myometrial and decidual cells. Exosome treatments had no effect on IL-6 and PGE2 production in BeWo cells. NANOG staining was higher in term labor myometrium and decidua compared to tissues not in labor.
Conclusion:
In vitro, AEC exosomes lead to an increased inflammatory response in maternal uterine cells whereas placental cells showed refractoriness. Fetal cell exosomes may function to signal parturition by increasing maternal gestational cell inflammation.
Keywords: Microvesicles, exosomes, signal, labor, uterine cells, fetal membranes, senescence, placenta
Condensation:
Amnion epithelial cells produce exosomes which function in a paracrine fashion to exert pro-inflammatory effects on myometrial and decidual cells and may be an important component of parturition.
INTRODUCTION
A substantial body of evidence supports the hypothesis that parturition is sustained by an inflammatory process. Labor in humans and other mammals is associated with infiltration and activation of leukocytes, mainly neutrophils and macrophages, into the fetal (amniochorion) and uterine tissues (decidua myometrium and cervix).1–4 Clinical and animal (mainly mouse) studies have identified key roles of specific cytokines, chemokines and immune cell types in the parturition process. 5–15 Endocrine signals arising from the fetus, such as corticotropin-releasing hormone and adrenocorticotropic hormone, are postulated to function as a biologic clock translating organ maturation and triggering labor at term. These hormones are known to have pro-inflammatory effects on various tissues in vitro.16–18 However, the precise mechanisms by which signals from the fetus initiate human parturition remain a mystery.
Our recent findings support the core hypothesis that oxidative stress and cellular senescence of the fetal (amniochorionic) membranes trigger human parturition by activating intrauterine inflammation. We have shown that human fetal membranes undergo a telomere-dependent process of progressive senescence throughout gestation, which is correlated with fetal growth.19, 20 Studies of senescence using human fetal membranes and cell culture have been corroborated in murine pregnancy models indicating that in utero cell senescence is driven by a p38mitogen activated protein kinase (MAPK) pathway.21–23 Senescence of the fetal membranes peaks at term resulting in dysfunctional fetal membranes. We postulate that signals arising from senescent fetal membranes are a proxy for completion of fetal growth and may trigger parturition. Premature senescence activation in the amniochorion is associated with preterm parturition.24, 25
Examination of signals arising from senescent fetal membranes at term has identified two key classes of inflammatory factors: senescence associated secretory phenotype (SASP) and damage associated molecular pattern markers (DAMPs) arising due to cell and cellular organelle injury.19, 23 SASPs and DAMPs mediate sterile (non-infectious) inflammation in fetal compartments at term during normal gestation. Many of the SASPs (inflammatory cytokines, chemokines, matrix degrading enzymes and growth factors) are activated in parturition.26–28 Two DAMPs released from senescent fetal cells, high mobility group box (HMGB) 1 and cell free fetal telomere fragments (cffTF), induce an inflammatory response in decidua and myometrium suggesting a paracrine communication from the senescing fetal membrane to uterine effector tissues of labor.22, 23, 26, 29, 30 Furthermore, in animal models injection of these DAMPs cause preterm birth.31 Based on these data, we hypothesize that sterile inflammatory signals from senescent fetal membranes are propagated from fetal to maternal compartments in a paracrine fashion to initiate labor.
Exosomes are bioactive, spherical, cell-derived vesicles which are 30–150 nm in size and are secreted via exocytosis.32–34 Exosomes are comprised of bi-layered plasma membranes and contain molecular constituents of their cell of origin, including proteins, DNA, and RNA that reflect the physiological state of their parent cell. In addition to common membrane and cytosolic molecules, exosomes harbor unique, cell specific subsets of proteins. They contain high concentrations of cholesterol and detergent resistant lipid membranes, making them extremely stable and efficient carriers of molecules across tissue layers.33 Exosomes mostly act as transporters of paracrine signals between tissues, but can regulate intracellular pathways by sequestering signaling molecules from the cytoplasm, reducing their bioavailability.32,33,35–37It has recently been shown that senescent amnion epithelial cells (AECs) at term produce exosomes containing pro-inflammatory factors.38, 39 This finding supports the hypothesis that pro-inflammatory signals are transmitted from fetal to maternal tissues via AEC-derived exosomes. Importantly, animal model studies have shown that exosomes injected into the amniotic fluid cavity access the maternal tissues by local and systemic routes.40 There are several studies which have reported exosome trafficking between tissues41, 42 and that indicate exosomes are released from cells from both the apical and basolateral compartments.43–47 Although these data support the core hypothesis that exosomes from the fetus access maternal tissues, the capacity for fetal exosomes in induce inflammatory changes in the maternal tissues remains unknown.
The objectives of this study were to: 1) determine whether exosomes derived from AECs grown under normal cell culture conditions (control exosomes) and under oxidative stress conditions (oxidative stress (OS) exosomes) enter maternal uterine cells (decidua and myometrium) and fetal (syncytiotrophoblast) cells, and 2) determine whether oxidative stress affects the capacity for AEC-derived exosomes to induce an inflammatory response in decidual, myometrial and syncytiotrophoblast cells. In this study, we define exosomes as extracellular vesicles of size between 30-150 nm isolated from AECs using differential centrifugation. We report that AEC derived exosomes produce proinflammatory changes in uterine myometrial and decidual cells.
MATERIALS AND METHODS
This study is basic science study utilizing fetal membrane derived cells, primary decidual cells, and myometrial and trophoblast cell lines. The University of Texas Medical Branch (UTMB) in Galveston, TX, USA, under an approved Investigational Review Board protocol, allowed the use of discarded placentas after delivery. Placentae were collected from women (18–40 years old) undergoing an elective repeat cesarean delivery at term (37-41 weeks gestation) prior to onset of labor. Exclusion criteria included: a history of preterm labor and delivery, premature rupture of the membranes, preeclampsia, placental abruption, intrauterine growth restriction, gestational diabetes, Group B streptococcus carrier status, history of treatment for urinary tract infection, sexually transmitted diseases during pregnancy, chronic infections like HIV and hepatitis, and history of cigarette smoking or reported drug and alcohol abuse.
Human amnion epithelial cell isolation and culture
Amniotic membrane was processed as described previously to produce AEC monolayer cultures.19–21 Briefly, amnion membrane was cut into 2 cm × 2 cm pieces and digested twice in 0.25% trypsin and 0.125% Collagenase A (Sigma–Aldrich, St. Louis, MO) in Hanks Balanced Salt Solution (HBSS; Mediatech Inc., Manassas, VA) for 35 minutes at 37°C. The tissue was filtered through a 70 μm cell strainer (Thermo Fisher Scientific, Waltham, MA) after each digestion and the trypsin was inactivated using complete Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 media (DMEM/F12; Mediatech Inc.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 10% Penicillin/Streptomycin (Mediatech Inc.) and 100 μg/mL epidermal growth factor (EGF; Sigma-Aldrich). After filtration, the collected cell filtrate was centrifuged for 10 minutes at 3000 RPM and the pellet was re-suspended in 3.0 mL of complete DMEM/F12. 3–5 million cells were placed per T75 flask and cultured in media containing complete DMEM/F12 media at 37°C, 5 in humidified 5% CO2 to 70–80% confluence.
Primary amnion epithelial cells under normal (control) and oxidative stress cell culture conditions
Cigarette smoke extract (CSE) was used to induce oxidative stress in amnion cells as detailed in prior studies21, 48, 49 with modifications. A single commercial cigarette (unfiltered Camel™, R.J. Reynolds Tobacco Co, Winston Salem, NC) was lit and the smoke was infused into 25 mL of exosome-free media, which consisted of DMEM/F12 supplemented with 10% exosome-free FBS made by ultracentrifuging FBS overnight at 100,000 rpm and filter sterilized. This full strength CSE stock was sterilized by passing through a 0.22 μm Steriflip filter unit (Millipore, Billerica, MA). The stock CSE was diluted 1:50 in exosome-free media prior to use. When the AECs reached 70–80% confluence, their flask was rinsed with sterile 1x PBS followed by treatment with the exosome-free cell media (control conditions) or with exosome-free CSE containing cell media (oxidative stress conditions) at a 1:50 dilution and incubated at 37°C, 5% CO2, and 95% air humidity for a 48 hour treatment. Total cell numbers/flask were counted by hemocytometer at the end of the 48 hour treatment. The culture media, from both control and oxidative stress treatments, were collected after 48 hours of treatment and stored at −80°C.
Exosome isolation
Prior to exosome isolation, cell supernatant media were thawed overnight and exosomes were isolated using differential ultracentrifugation as described previously, with modifications.38, 50, 51 Exosomes isolated from normal cell culture condition media are referred to as “control exosomes” and those isolated from CSE treated (oxidative stress induced) media are referred to as “oxidative stress (OS) exosomes.” Briefly, the media was sequentially centrifuged at 4°C for 10 minutes at 300g and for 20 minutes at 2,000g using a Sorvall Legend X1R and TX-400 swinging bucket rotor (Thermo Fisher Scientific), followed by 30 minutes at 10,000g and 2 hours at 100,000g using a Beckman Optima LX-80 ultracentrifuge with 50.1Ti and 70.1Ti rotors (Beckman Coulter). The resulting pellet after the 2 hour ultracentrifugation was re-suspended in 1x PBS and then centrifuged again at 100,000g for 1 hour. The pellet was re-suspended in 1x PBS and stored at −80°C.
Transmission electron microscopy
Exosome shape was determined using a JEOL transmission electron microscope (TEM). The protocol for this experiment can be seen in prior publications.38–40 Briefly, we treated formvar/carbon-coated 300-mesh copper grids with 10 seconds of hydrogen-oxygen plasma in a Gatan Solarus 950 plasma cleaning system (Gatan, Inc., Pleasanton, CA). The cleaned grid was covered in exosomes and left to dry at room temperature for 10 minutes. After three washes in Millipore water, the exosome-covered grids were negatively stained using phosphotungstic acid (PTA) and dried at room temperature. Exosomes were viewed in a 120 keV JEM 1400 electron microscope (Jeol, Peabody, MA) with a minimum of 15 frames were viewed per sample.
Nanoparticle tracking analysis with ZetaView
Nanoparticle tracking analysis was performed using the ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and its corresponding software (ZetaView 8.02.28).52, 53 Frozen exosomes in 1x PBS were thawed on ice. A 1:500 dilution of the exosome sample was made with MilliQ water. Samples of control or oxidative stress exosomes were loaded in the ZetaView Nanoparticle Tracking Analyzer and number of particles/ml and size distribution were counted for each sample. The machine was cleaned between samples using filtered water. The results of the ZetaView were used to calculate the number of exosomes produced per amnion cell for the two treatment types (control or oxidative stress).
Myometrial cell culture
Myometrial cells were obtained from the hTERT-HMA/B myometrial cell line (a gift from Dr. Sam Mesiano, Case Western Reserve University, Cleveland, OH). The hTERT-HMA/B is a clonal sub-line of hTERT-HM, a telomerase immortalized myometrial cell line produced from uterine fundus obtained from a premenopausal woman.54 The cells express the smooth muscle cell-specific genes calponin, h-caldesmon and smoothelin. They also express the oxytocin receptor and respond to oxytocin with increased intracellular calcium, which is typical of the myometrial cell phenotype. Myometrial cells were plated in a T75 flask and cultured in media containing DMEM, 1X (Corning Cellgro, Manassas, VA) supplemented with 10% charcoal stripped-FBS (Sigma-Aldrich), 10% Penicillin/Streptomycin plus L-glutamine (Sigma-Aldrich), gentamicin (Mediatech), hygromycin B (Life technologies, Carlsbad, CA), blastocidin (Invitrogen, Carlsbad, CA) at 37°C, and 5% CO2, and grown to 80% confluence.
Decidual cell culture
Decidua cells were isolated from placentas collected from women undergoing elective cesarean delivery at term who were not in labor. The method for isolation was adapted from a protocol described by Mills et al. 2006.55 Briefly, fetal membrane was cut from placenta and amnion was removed. The tissue was washed with in pre-warmed 0.9%NaCl to remove blood and then cut into 2 inch squares. Blunt dissection of the decidua from chorion was performed using forceps and scalpel. The tissue was minced into small pieces and incubated in a digestion buffer (Hanks BSS with trypsin and DNAse I) and at 37° C for 30 minutes. The tissue was then centrifuged at 2,000 rpm for 10 minutes at room temperature (RT). The supernatant was removed and pellet was re-suspended in a digestion buffer (Hank’s BSS with trypsin, DNAse I and collagenase type IA) and incubated for 1 hour at 37° C. The digestion was neutralized and filtered through four layers of sterile gauze. The collected cells were centrifuged at 2,000 rpm x 10 minutes at RT and the pellet was re-suspended in DMEM. Next a pre-prepared Optiprep “column” was used with steps ranging from 4%-40% of 4 mL each. The processed decidua cells were added to the top of the gradient, then centrifuged at 1,000g × 30 minutes at RT. Decidual cells were collected between densities of 1.027 – 1.038g/mL (between 4-6%). The decidual cells were collected and washed with DMEM/F-12 50/50, 1X and then centrifuged at 2,000 rpm × 10 minutes at RT. The pellet was re-suspended in DMEM/F12 and placed in T25 flasks. The primary cells were grown in media containing complete DMEM/F12 media plus 10% heat inactivated (HI) FBS (Sigma-Aldrich), penicillin/streptomycin, and endothelial growth factor at 37°C, 5% CO2, and 95% air humidity to 70–80% confluence. The purity of the cells was tested using antibodies to vimentin and cytokeratin. We found that the cultured decidual cells were vimentin positive and cytokeratin negative.
BeWo cell culture
BeWo cells are a human choriocarcinoma cell line (provided by Dr. Robert N Taylor, Wake Forest University, Winston-Salem, NC). Despite being a cell line BeWo cells continue to reveal physiological characteristics of the villous trophoblast.56, 57 Cells were plated in a T75 flask and cultured in media containing Roswell Park Memorial Institute (RPMI) 1640, 1X (Corning Cellgro) media with Penicillin/Streptomycin and 10% HI-FBS at 37°C, 5% CO2, and 95% air humidity and grown to 70–80% confluence.
Immunofluorescence staining of exosomes and confocal microscopy to localize exosomes in recipient cells
Isolated control and oxidative stress AEC exosomes were labeled with carboxyfluorescein succinimidyl ester (CFSE) by re-suspending the final exosome pellet in 7.5 μM CFSE. Exosomes were incubated at 37°C for 30 minutes then diluted with media containing 10% exosome-depleted FBS. Exosomes were utlracentrifuged overnight (>16 hours) at 4°C and pellets were re-suspended in cold PBS. Myometrial, decidual and BeWo cells were plated on glass coverslips at a density of 20-50,000 cells per slip and incubated overnight prior to treatment with CSFE labeled control or oxidative stress exosomes. After a 4 hour incubation with the labeled exosomes, cells were fixed with 4% paraformaldehyde (PFA), permeablized with 0.5% Triton X and blocked with 3% BSA in PBS. To counter stain and to visualize cell morphology, cells were incubated with primary antibodies to α-smooth muscle actin (Affymetrix, Santa Clara, CA) (for myometrial and decidua) or anti-β actin (Sigma-Aldrich) (for BeWo cells) overnight at 4°C 3% BSA in PBS. After washing the slides several times with PBS, slides were incubated with secondary antibody Alexa Fluor 488 or 594 (Life Technologies) diluted 1:400 in PBS for 1 hour in the dark. Slides were then washed with PBS and treated with 4’, 6-diamidino-2-phenylindole (DAPI) (Invitrogen by Thermo Scientific) then washed and then mounted using MOWIOL 4–88 (Sigma-Aldrich) mounting medium. Slides were allowed to dry overnight and then the cells were imaged using the LSM 510 Meta UV confocal microscope (63x) (Zeiss, Germany). Multiple (at least 5) cells on each slide were imaged with the confocal microscope. Images were obtained and analyzed using Image J (open source) to visualize z-stacks and confirm the location of the exosomes in regards to the cells. 3D reconstructions of the cells were created to further confirm the location of the exosomes in relation to the target cell.
Exosome treatments of cells
Myometrial, decidual and BeWo cells were placed in 6 well plates and grown overnight. The next day the cell media was removed, cells were washed with PBS and media was replaced with exosome free cell media. Cell treatments with control and oxidative stress exosomes were performed by adding them to the wells. Exosomes from either control or oxidative stress conditions were added in 3 titrations of 2×105, 2×107, and 2×109 exosomes/well. The cells were allowed to incubate with the exosomes for 24 hours. A negative control well was included that consisted of exosome free media only and a positive control well was included which was treated with LPS (100 ng/mL). At the completion of the treatment, media was collected from each well and stored at −80° C. The cells were collected from the wells after being washed with PBS. To collect the cells, wells were treated with radio immunoprecipitation assay buffer including phenylmethanesulfonyl fluoride (Fluka), protease inhibitor cocktail (Sigma-Aldrich) and Halt phosphatase inhibitor cocktail (Thermo-Scientific) and cells were manually scraped from the well using a cell scraper. The cells were then placed on ice for 10 minutes, vortexed for 10 seconds, sonicated for 30 seconds, vortexed an additional 10 seconds, and placed on ice for 10 minutes. The lysed cells were then flash frozen using liquid nitrogen and stored at −80° C. This experiment was repeated a total of 7 times.
Exosome blocking experiments
To determine if the effects in recipient cells were mediated by exosomes, several control experiments were performed. These included cold incubation of recipient cells and treatment with heat inactivated and sonicated exosomes. For the cold incubation treatment, the exact treatments as explained in the last section were performed, with the following changes: cells treated with exosomes were incubated at 4° C for 6 hours. For heat inactivation and sonication treatments, the above described treatments were performed with the following changes: the exosomes (both control and oxidative stress) were either heated in a 65° C water bath for 30 minutes or sonicated for 30 minutes prior to being added for the exosome treatment.58 A total of 4×107 exosomes were added per well and treatment type. The cell media and cells were collected at the end of the 24 hour treatment as described above.
Enzyme Linked Immunosorbent Assay for determining inflammatory marker response
All of the media collected from exosome treatments and exosome blocking treatments were analyzed using an enzyme-linked immunosorbent assay (ELISA) for 5 common inflammatory cytokines/mediators: IL-1β, TNF-α, IL-6, IL-8, and PGE2. These inflammatory cytokines were chosen based on the results of a systematic review, performed by our lab, which indicated that these cytokines/mediators are present at the time of labor in all the gestational tissues included in this report.59 The ELISA was performed after media was thawed and spun to remove cellular and other debris. The media was pipetted into the ELISA plate wells as per kit instructions (R&D Systems- Quantikine ELISA). The results of the ELISA were obtained by using the Synergy H4 microplate reader (BIO-TEK).
Western Blot
Western blot was performed to determine total and phosphorylated NF-κβ (Rel-A) from the myometrial, decidual and BeWo cells, which had been treated with 2×109 exosomes from control or oxidative stress induced cells. Cell samples, which had previously been suspended in RIPA, were thawed and then centrifuged at 10,000 rpm for 20 minutes. The supernatant was collected and then a bicinchoninic acid assay (BCA) (Pierce, Rockford, IL) was performed to determine protein concentrations of the samples. Then SDS-PAGE on a gradient (4–15%) Mini-PROTEAN1TGX™ Precast Gels (Bio-Rad, Hercules, CA) was used to separate protein samples. The samples were then transferred to a membrane using iBlot1Gel Transfer Device (Thermo Fisher Scientific). The membrane was blocked in 5% nonfat milk in 1x Tris buffered saline- Tween 20 (TBS-T) buffer for 1 hour at room temperature. The membrane was probed with a primary antibody for either Phospho Rel-A, total Rel-A, or total actin in either 5% nonfat milk or 5% BSA in 1x Tris buffered saline- Tween 20 rocking overnight at 4°C. The next day, the membrane was washed with Tween 20 three times and then incubated with a secondary antibody for 1 hour. The immunoreactive proteins were visualized using Luminata Forte Western horse radish peroxidase substrate (Millipore, Billerica, MA). The stripping protocol used between blots followed the instructions of Restore Western Blot Stripping Buffer (Thermo Fisher).
Immunohistochemical analysis of amnion exosomes in maternal gestational tissues
To determine that fetal cell derived material can reach maternal gestational tissue during parturition, we collected myometrial tissues and decidual tissues from pregnant women undergoing cesarean delivery (not in labor) or vaginal delivery (term labor) and looked for the presence of stem cell marker (NANOG), which is also expressed in amnion derived exosomes. Dual staining was performed for NANOG and CD9 (background marker). Tissues were fixed in 10% Neutral Buffered Formalin (NBF) for 24 hours at room temperature before embedding them in paraffin blocks and sectioning 4μm slices. Formalin-fixed paraffin-embedded (FFPE) were baked overnight at 50°C and tissue slides were re-hydrated the next day, by immersing in Xylene three times for 10 minutes each followed by 100% EtOH, 95% EtOH, 70% EtOH, 50% EtOH, distilled water; each step performed twice for 5 minutes. Antigen retrieval was then carried out in 2100 Antigen Retriever (Electron Microscopy Sciences, USA) with citrate buffer, pH 6.0 for 20 minutes followed by cooling for approximately 2 hours and rinsed in TBS buffer.
Endogenous peroxidase activity was quenched by incubation with 0.3% hydrogen peroxide for 10 minutes. The tissue was then blocked for non-specific signals using Protein Block buffer (Abcam) in a moist chamber for 1 hour at room temperature. Sequential dual staining was performed with the polyclonal primary antibody NANOG (Rabbit, 1:400, Cell signaling #3580, Danvers, MA) followed by second primary antibody CD9 (Rabbit, 1:100, Novus Biologicals, Littleton, CO). Secondary antibody incubation was carried out for 30 minutes at room temperature with each antibody. NANOG was stained using DAB substrate (Abcam, Cambridge, United Kingdom) for 5 minutes. Slides were then washed in TBS-tween20, antigen retrieved and re-blocked prior to second primary antibody staining. CD9 staining was developed using AP substrate (Vector Blue) for 10 minutes. The Olympus light microscope BX43 (OLYMPUS) was used to image the slide and images were captured using software Q Capture Pro.
To quantify NANOG expression in each tissue, images were loaded onto Image J (open source). After color deconvolution, regions of interest (9 regions/image) were randomly selected based on grid overlay and analyzed for NANOG staining intensity.
Statistical analysis
Each cell type (BeWo, Myometrial and Decidua) was either untreated (negative controls) or treated with exosomes at 2 × 105, 2 × 107 and 2 × 109 from either normal or CSE conditions to examine the distribution of inflammatory markers between untreated (negative controls) and exosome treated cells (from both conditions). For each cell type, there were a total of 4 negative controls which served as the reference group and for each exosome treatment (2 × 105, 2 × 107 and 2 × 109) there were 5 observations under normal conditions and 5 under CSE. Normality for each inflammatory marker (IL-6, IL-8 and PGE2) was tested using the Kolmogorov-Smirnov test, with a p-value of <0.05 indicating that the distribution was non-normal. No markers had a normal distribution. The distribution of inflammatory markers was compared between controls (untreated cells) and exosome treated cells (from both normal and oxidative stress conditions) using non-parametric Wilcoxon-Mann-Whitney test (non-parametric analog to the independent samples t-test). These analyses were conducted for each cell type. A p-value <0.05 was considered statistically significant. All analyses were conducted using SAS V9.2 (Cary, NC). IHC intensity values were analyzed using a t-test in Graphpad Prism (GraphPad, San Diego, CA). P < 0.05 was considered significant.
RESULTS
Exosome Quantification and Characterization
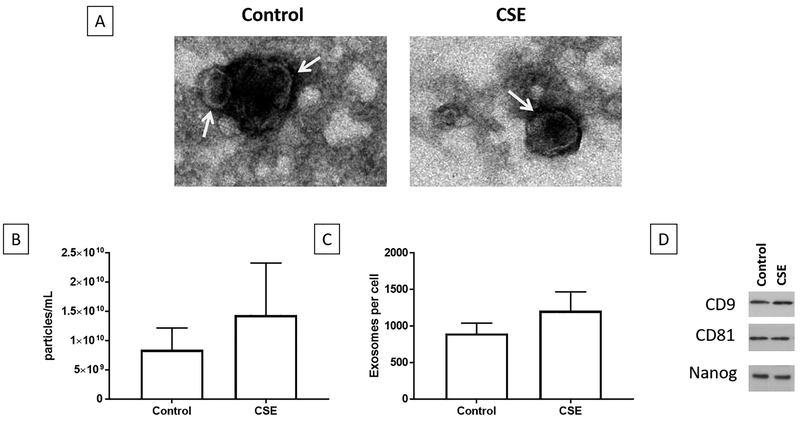
The size and quantity of exosomes were determined using Zetaview analysis (Fig 1). Electronic microscopy of exosomes isolated from conditioned media samples showed round, cup-shaped exosomes with a size range between 50–150 nm (Fig 1A). AECs under normal cell culture (control) conditions produced an average of 9.4×109 particles/ml which correlates with 899 exosomes/cell, while AECs under oxidative stress conditions produced 1.5×1010/ml which correlated with 1211 exosomes/cell (Fig 1B and C). The average size of exosomes from control and oxidative stress treatments were 112 nm and 101 nm respectively. AEC exosomes were shown in a previous experiment to contain exosome markers CD9, CD81 along with AEC marker NANOG (Fig 1D).38
Figure 1: Characterization of control and oxidative stress exosomes.
1A – Transition electron micrograph of control and oxidative stress exosomes show round/cup shaped exosomes
1B – Total number of particles/ml of media show no difference in exosomes between treatments.
1C – Number of exosomes/AEC from both control and oxidative stress treatments were not different.
1D – Both control and oxidative stress derived AEC exosomes showed exosome markers CD9, CD81 and stem cell marker NANOG.
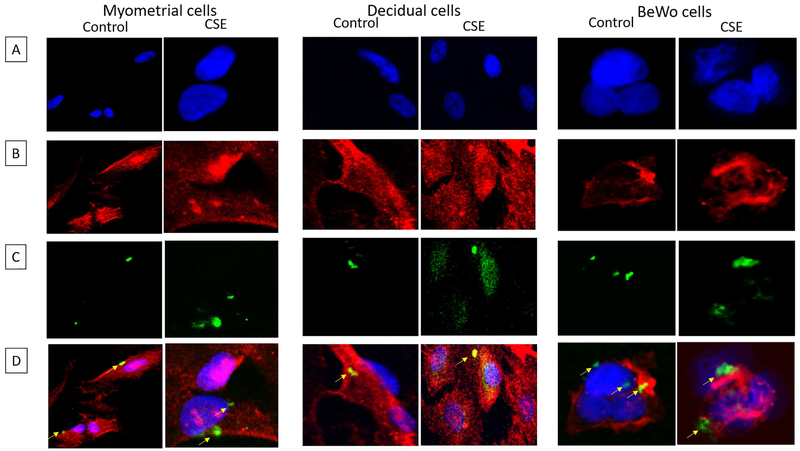
Exosomes were localized in recipient cells
Confocal microscopy and z-stack analysis was used to localize exosomes in recipient myometrial, decidual and placental cells. As shown in figure 2, CFSE labelled control and oxidative stress exosomes were detected within myometrial, decidual and BeWo cells. The location of the exosomes within cells, as opposed to adjacent to the cells, was confirmed using z-stack analysis and 3D reconstructions as shown in supplemental figure 2.
Figure 2: Localization of AEC derived exosomes (from control and oxidative stress treatments) inside gestational cells.
Carboxyfluorescein succinimidyl ester (CFSE) labelled exosome localization inside myometrial, decidual and BeWo cells. Left panel – Myometrial cells; Middle panel – Decidual cells; and Right panel – BeWo cells. A – DAPI; B – Cell specific marker – α-smooth muscle actin (myometrium and decidua) or β-actin (BeWo); C – CFSE labelled exosomes; D: merged images.
AEC exosomes induce a pro-inflammatory response in myometrial and decidual cells
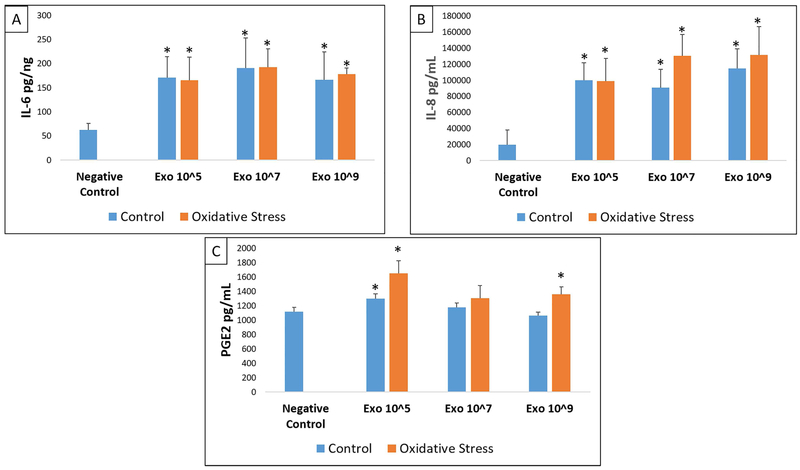
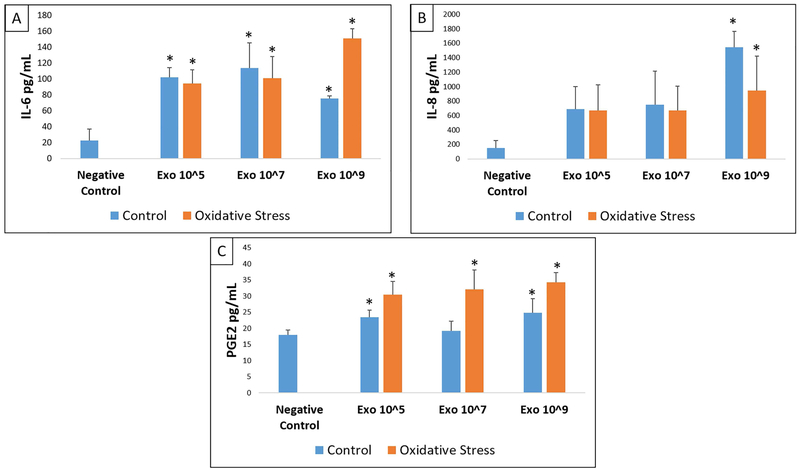
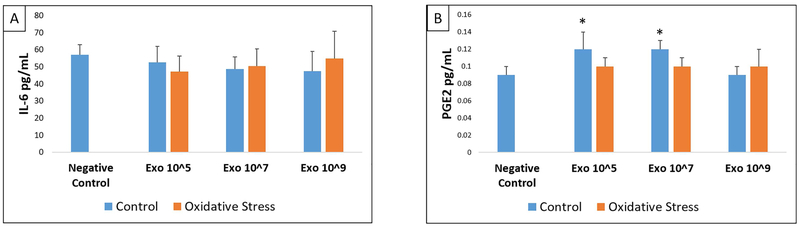
To determine the effect of AEC derived exosomes to cause functional changes, we determined inflammatory cytokines and prostaglandin levels in cell culture supernatants after treatment with various doses of control and oxidative stress exosomes and compared them to the analytes from normal, untreated, cell cultures. The markers studied were shown to be associated with human parturition in each of these cell types.59 Control and oxidative stress AEC exosomes significantly increased the concentration of IL-6, IL-8 and PGE2 but not IL-1β or TNF-α in the media of myometrial and decidual cells compared to normal (untreated) cells in culture (Figures 3-4, Supplemental Tables 1-2). The capacity for oxidative stress exosomes to increase myometrial and decidual cell media IL-6, IL-8 and PGE2 levels appeared to be slightly higher compared to control AECs. A dose dependent effect of exosomes (control or oxidative stress) to stimulate inflammatory response was not observed in our experiments. BeWo cells only produced detectable levels of IL-6 and PGE2 and none of the other cytokines studied. Control and oxidative stress exosomes had no effect on BeWo cell media IL-6, IL-8, PGE2, IL-1β and TNF-α levels (Figure 5, Supplemental Table 3).
Figure 3: ELISA data showing IL-6 (A), IL-8 (B) and PGE2 (C) in myometrial cells.
Comparisons were made between IL-6, IL-8, or PGE2 analyte concentrations in negative control cell media and concentrations in media after treatment of myometrial cells with each dose (Exo 10^5, 10^7, 10^9) of either control (blue) or oxidative stress (orange) AEC derived exosomes. All experiments include n=5. Significant results (p<0.05) between specific treatment compared to untreated control cell media are marked with an asterisk (*).
Figure 4: ELISA data showing IL-6 (A), IL-8 (B) and PGE2 (C) in decidual cells.
Comparisons were made between IL-6, IL-8, or PGE2 analyte concentrations in negative control cell media and concentration in media after treatment of decidual cells with each dose (Exo 10^5, 10^7, 10^9) of either control (blue) or oxidative stress (orange) AEC derived exosomes. All experiments include n=5. Significant results (p<0.05) between specific treatment compared to untreated control cell media are marked with an asterisk (*).
Figure 5: ELISA data showing IL-6 (A) and PGE2 (B) in BeWo cells.
Comparisons were made between IL-6 or PGE2 analyte concentrations in negative control cell media and concentration in media after treatment of BeWo cells with each dose (Exo 10^5, 10^7, 10^9) of either control (blue) or oxidative stress (orange) AEC derived exosomes. All experiments include n=5. Significant results (p<0.05) between specific treatment compared to untreated control cell media are marked with an asterisk (*).
Positive control experiments show exosome-mediated effect
To confirm that cells are responding to the treatments and that the effects are truly mediated by exosomes, multiple control experiments were performed. LPS treatment (100 ng/ml) was used as a positive control to confirm inflammatory responses from each cell type. LPS produced significant increase in cytokine production from all cell types compared to untreated cells. A sample of these data are shown in supplemental Figure 1. IL-6 levels after LPS treatment were higher in all cell types compared to control. However, IL-6 concentrations after LPS treatment was similar to that observed after exosome treatment. In BeWo cells, LPS significantly increased only IL-6, but not IL-8 or PGE2. Control data from LPS treatments are also graphically represented in supplemental table 4.
Determination of exosome mediated cytokine response
To confirm exosome specificity of stimulation, media samples from the exosome blocking experiments were subjected to ELISA. Incubation of cells in cold lead to decreased IL-6, IL-8 and PGE2 production by all three cell types. This suggests that exosome entry into these cells were blocked due to reduced endocytosis at 4°C. Two other experiments were performed to disrupt exosomes and cargo. Media samples from cells treated with a single dose of exosomes (107) whose cargo was inactivated by either heat inactivation or sonication were compared to control and control or oxidative stress exosome treatments. Heating and sonicating the exosomes prior to treatment lead to no change in IL-6, IL-8, and PGE2 levels which were similar to negative control treatments. This suggests that the exosome’s cargo, either destroyed or disrupted, were not sufficient to cause inflammatory mediator response from these cells (supplemental Figure 1 and supplemental table 4). These data partly confirmed exosome mediated effects.
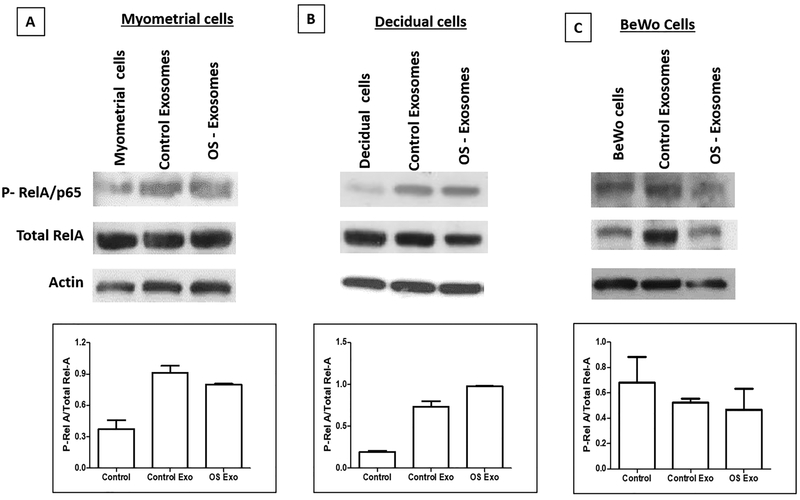
Exosomes increase NF-κβ activation in myometrial and decidual cells
Exosomes, regardless of source (control or oxidative stress) produced inflammatory response by increasing IL-6, IL-8 and PGE2 release suggesting activation of NF-κβ, a key transcription activator by exosomes. To test this, we performed western blot analysis for p-RelA/p65, total RelA/p65, and actin on cells collected from myometrial, decidual, and BeWo cells. Myometrial and decidual cells increased p-RelA in response to exosomes (regardless of control or oxidative stress) and BeWo cells increased less. Densitometry (based on the ratio of active/total [mean arbitrary units]) (Figure 6 bar graphs) corroborated that myometrial and decidual cells had higher p-RelA than controls after treatment with both control and oxidative stress exosomes (Figure 6); however, RelA baseline activation was similar between normal BeWo cells compared to cells exposed to exosomes (see bar graphs). This further verifies the previous cytokine data presented which indicates that increased cytokine and PGE2 levels induced by both control and oxidative stress exosomes are likely mediated by increased phosphorylation of NF-κβ by exosomal cargo. BeWo cells are refractory to NF-κβ activation by AEC exosomes.
Figure 6: Activation of NF-κB as determined by RelA/p65 Phosphorylation.Top panel RelA/p65; Middle panel – Total RelA/p65; Bottom Panel – Actin.
A – Myometrial cells – Myometrial cells= normal myometrial cells in culture; Control exosomes= myometrial cells treated with exosomes (dose 2×109) from AEC grown under normal cell culture conditions; OS exosomes= myometrial cells treated with oxidative stress exosomes (dose 2×109).
B – Decidual cells – Decidual cells= normal decidual cells in culture; Control exosomes= decidual cells treated with exosomes (dose 2×109) from AEC grown under normal cell culture conditions; OS exosomes= decidual cells treated with oxidative stress exosomes (dose 2×109).
C – BeWo cells – BeWo cells= normal BeWo cells in culture; Control exosomes= BeWo cells treated with exosomes (dose 2×109) from AEC grown under normal cell culture conditions; OS exosomes= BeWo cells treated with oxidative stress exosomes (dose 2×109).
Increased localization of NANOG in myometrial and decidual tissues at term labor
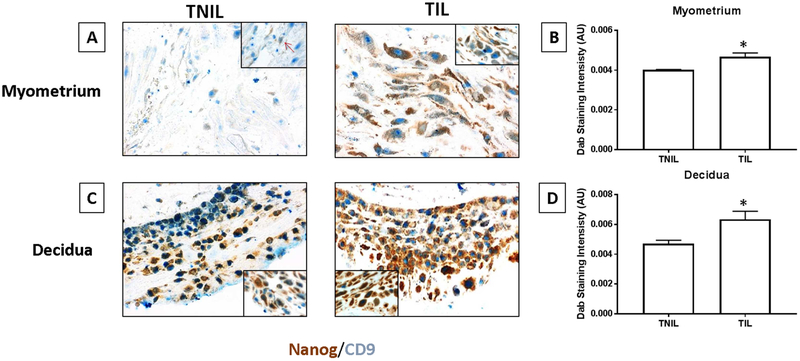
Immunohistochemical analysis and dual staining of NANOG showed increased staining of NANOG (brown) in myometrial tissues and decidual tissues from term delivery samples compared to not in labor deliveries (Fig 7). Semi quantitative estimation of this data (as shown in bar graphs) showed significantly high staining in both term labor tissues.
Figure 7: Immunohistochemical localization of NANOG (amnion stem cell marker constitutively expressed in AEC derived exosomes) in term labor and term not in labor gestational tissues.
A – Term not in labor (TNIL) and term in labor (TIL) myometrium – Brown staining indicates NANOG (fetal amnion stem cell marker) and blue staining indicates CD9 (background marker). NANOG expression was higher in term labor myometrium than term not in labor myometrium.
B – Quantitation of NANOG staining expression indicating significantly higher NANOG in TIL compared to TNIL myometrial tissue.
C - Term not in labor (TNIL) and Term in labor (TIL) decidua (attached to chorion layer of fetal membranes) – Brown staining indicates NANOG (fetal amnion stem cell marker) and blue staining indicates CD9 (background marker). NANOG expression was higher in term labor decidua than term not in labor decidua.
D – Quantitation of NANOG staining expression indicating significantly higher NANOG in TIL compared to TNIL decidua.
COMMENT
Principal findings of the study
This study tested if senescent fetal amnion epithelial cell derived exosomes can cause inflammatory changes in maternal and placental tissues. Our main findings are: 1. AECs produce exosomes that are quantitatively the same regardless of cell culture conditions (Figure 1). 2. AEC exosomes are taken up by myometrial, decidual and BeWo cells (Figure 2 and supplemental figure 2). 3. Treatment with control and oxidative stress AEC exosomes increase production of pro-labor inflammatory mediators (IL-6, IL-8 and PGE2) and cause activation of NF-κβ in maternal myometrial and decidual cells (Figure 3 and 4 and 6). 4. Production of pro-inflammatory mediators was reduced when exosome uptake was blocked (Supplemental figure 1 and table 4).
Although feto-maternal endocrine mediators have been reported to be associated with initiation of labor,60–64 the exact pathway of labor initiation remains a mystery.65 Inflammatory activation is one of the functional facilitators of parturition in all gestational tissues, as an imbalanced inflammatory state transitions quiescent gestational tissues to an active state.66–70 Thus, factors that increase inflammatory load, directed either by endocrine signals or paracrine signals, can cause mechanistic activation of the labor process.30,71,72 This process ideally occurs when fetal growth and maturation are sufficient to ensure newborn survival. Based on recent findings of senescence in various gestational tissues that coincide with fetal growth, and our findings in fetal membrane models showing that membrane senescence and damage are associated with parturition, we hypothesized that senescent fetal membranes generate inflammatory mediators to signal fetal readiness for parturition.25,73,74We propose that these signals are propagated from fetal tissues to the uterine parturition effector tissues (decidua and myometrium) via fetal cell derived exosomes.
We believe that AEC exosomes as well as other cell membrane derived vesicles can reach maternal tissues in multiple ways; 1. Basolateral secretion of AEC derived exosomes than can traverse through layers and reach the uterine tissues 2. Apical secretion of exosomes into amniotic fluid, taken up by fetus and reaching maternal systemic circulation 3. Exosomes reaching maternal circulation and thus reaching maternal reproductive tissues by crossing placental barriers, specifically those exosomes released from membrane cells overlaying the placenta. Our lab has shown in animal models that exosomes in the fetal compartment can reach the maternal compartment either via systemic spread or by diffusion through tissue layers. Fluorescently labeled AEC exosomes injected into the amniotic cavity of pregnant mice were identified in maternal gestational tissues and blood stream, indicating that exosomes are able to traverse the maternal fetal barrier.40 Several studies in other labs have reported exosome trafficking between tissues.41, 42 There is also evidence in multiple studies to indicate that exosomes are released from cells from both the apical and basolateral compartments.43–47
Fetal exosomes, irrespective of the physiologic status of cell of origin, cause inflammatory activation in maternal cells
The number of exosomes released from cells under normal culture conditions or after CSE treatments were similar. This can partly be explained by the fact that the same number of cells were treated for each treatment type. Additionally, CSE treatment alone is not sufficient to cause an increase in exosome quantity but does lead to a change in exosome cargo content reflecting the physiologic state of cells.38 A key finding to highlight is that regardless of the source of exosomes (from cells grown under normal or oxidative stress conditions), exosome treatments produced inflammation in recipient maternal cells (myometrial and decidual cells).
It is not totally unexpected that exosomes from control environments would cause an inflammatory response as we have reported in a proteomic analysis of AEC exosomes derived under control conditions that they contain markers suggestive of NF-κβ signaling.38 Oxidative stress treatment with CSE also resulted in exosome cargo with inflammatory signals but mostly contained inflammation mediated by transforming growth factor (TGF)β pathway.38 TGFβ is produced in fetal membrane cells in response to CSE treatment and oxidative stress and it is a well-known activator of epithelial mesenchymal transition (EMT).75–77 EMT is an inflammatory state78 and Chaudhuri et al. has shown fetal membrane EMT occurring at term.79 Similar findings were reported by Mogami H et al in fetal membranes rupture models.80 Ongoing data from our laboratory suggest exosomes can alter the fetal membrane microenvironment at term enhancing senescence, EMT and inflammation.
Exosomes are generated by cells and propagated throughout gestation. It is plausible that minimal levels of inflammation generated by normal cell exosomes during gestation are used for tissue remodeling and their quantity and cargo are insufficient to cause labor related inflammation. We speculate that oxidative stress builds up at term produces an exclusive group of exosomes that can induce unique inflammatory conditions resulting in parturition. As shown in Fig. 6, oxidative stress derived exosomes induced NF-κβ activation in myometrial cells, which is known to be associated with inflammation and functional progesterone withdrawal. Our previous work has also shown that CSE induced oxidative stress leads to packaging of p38MAPK, an activated form of stress signaler, into AEC exosomes.38 p38MAPK has been shown to be a potential mediator of functional progesterone withdrawal.81, 82
In this study we used primary decidual cells and myometrial and placental cell lines. It can be argued that primary vs cell line differences may impact our observed outcome. However, similarities in response to exosomes between decidual primary cells and myometrial cell line cells’ suggest that comparable outcomes can be expected irrespective of cell types. We acknowledge that more studies are needed using primary cells as well as intact tissues to verify our data.
Placental cells are refractory to immune response by amnion exosomes
Placental cells were found to be refractory to stimulation by AEC exosomes. Regardless of concentration or exosome origin (control vs oxidative stress), placental (BeWo) cells did not respond to exosomes or show any inflammatory change. It is possible that AEC derived exosomes are not capable of generating an inflammatory response from placenta. It is also possible that the inflammatory response may be different in primary cells as compared to the BeWo cell line. A study by Koh et al. found while BeWo cells will produce IL-6 after stimulation with OS, they will not produce IL-8 or IL-1β.83 Our results indicated no increase in IL-6 production by placental (BeWo) cells after treatment with exosomes which makes the conclusion that placental cells may be refractory to AEC exosomes more plausible. We speculate that exosomes show tropism and they are capable of causing functional impact in specific target tissues and likely at specific times. The mechanism of exosomal tropism and its selection of target tissues are yet to be determined. Specific surface proteins acquired by exosomes under distinct physiologic state of a cell may determine tissue tropism and the functional role of exosomes. Proximity of placenta and fetal membranes makes placenta less likely to respond to inflammatory challenges produced by membranes because any inflammatory response by membranes spread via exosomes can be detrimental to the survival of placenta and thus the fetus. We do not rule out that refractoriness of BeWo cells may be attributed to transitioned state of trophoblast cells and primary cytotrophoblast cells may have yield different results.
Determining fidelity of exosomal functions
In this current study, multiple experiments were conducted where exosome uptake was blocked. Exosome uptake or functional contribution of exosomes are mostly manifested by the following routes: 1. Endocytosis of exosomes and cytoplasmic delivery of cargo84 2. Specific ligand (markers on exosomes) – receptor (on recipient cell) interaction85, 86 3. Fusion of exosomes directly with plasma membrane and release of cargo87 4. Delivery of cargo into the environment of the target cell after undergoing lysis outside the recipient cell.33, 88 We primarily tested the endocytosis effect, a well reported mechanism of exosome entry. Energy dependent endocytosis was stopped by incubating cells at 4° C, as described in prior studies of exosome uptake and function,88–92 which lead to a reduced production of IL-6, IL-8 and PGE2 by all cell types. This indicates that exosomes are contributing to the increased inflammatory mediator production, predominantly via endocytosis. We also either heated or sonicated the exosomes prior to treatment.58 Heating can denature the surface proteins of the exosome while sonication breaks the exosome open. Heating or sonicating the exosomes prior to treatment reduces the number of routes through which the exosome can be taken up by the target cell, but likely releases the contents of the exosomes into the recipient cell extra-cellular environment. There was not a significant increase in cytokine production after treating with heated or sonicated exosomes (supplemental figure 1). This indicates that the AEC exosomes can exert effects via several different routes in gestational cells.
Exosomes may contain various molecules and it was theoretically possible that the AEC exosomes contained the inflammatory analytes of interest. To test this, as a part of an ongoing study in our laboratory, we verified whether exosomes from control and oxidative stress exosomes carried cytokines contributing to the observed data. For this, a proteomic analysis of the exosomes was conducted by Dr. Salomon’s laboratory using LC/MS-MS approach and we report that none of the analytes measured in this study (IL-6, IL-8 and PGE2) were detectable in our exosomes preparations from control or oxidative stress conditions.
Oxidative stress of amnion epithelial cells lead to production of exosomes with pronounced effect on target cells
Exosomes produced under oxidative stress conditions have a more dominant effect than those produced under normal cell conditions (control exosomes) as almost all treatments using oxidative stress exosomes increased pro-parturient biomarkers in decidua and myometrium. Dose dependent effect was not seen at the end of a 24 hour incubation and it is likely that all doses used are either saturating the response or that additional doses or longer incubation may be necessary to show the true kinetics of cytokine response.
Summary
The results of our study indicate that exosomes produced by AECs are capable of being taken up by other gestational tissue cells and cause inflammatory, labor-promoting changes in maternal gestational cells. This indicates that AEC derived exosomes may be involved in the labor cascade by functioning as messengers carrying specific signals between the fetal and maternal compartments. We conclude that AEC exosomes are a novel paracrine mechanism of fetal-maternal communication.
Supplementary Material
Implications and Contributions:
A. This study was conducted to investigate what role amnion epithelial cell derived exosomes may play in human parturition.
B. Amnion epithelial cell derived exosomes, generated under control and oxidative stress conditions, are taken up by myometrial, decidual and placental (BeWo) cells. The exosomes from both conditions significantly increased inflammatory cytokine load and activated NF-kB in maternal cells.
C. What this study adds to our knowledge: This study indicates that fetal derived exosomes may be an important contributor to the pathogenesis of human parturition.
ACKNOWLEDGEMENTS
Samantha Sheller-Miller is an appointed Pre-doctoral Trainee in the Environmental Toxicology Training Program (T32ES007254), supported by the National Institute of Environmental Health Sciences of the National Institutes of Health of the United States, administered through the University of Texas Medical Branch in Galveston, Texas. We would also like to acknowledge the help we received from our research team including Christopher Luke Dixon, MD, for exosome protocols, Jayshil Trivedi for microscopy images,, Lauren S. Richardson (Graduate student mentored by Dr. R Menon) for photomicroscopy, Talar Kechichian, and Rheanna Urrabaz-Garza for support in the lab.
Conflicts of Interest and Funding: The authors report no conflicts of interest. This study is supported by the Innovative Catalyst Grant from March of Dimes Ohio Center, Cincinnati, OH, and 1R01HD084532-01A1 (NIH/NICHD) to R Menon.
Abbreviations used in this manuscript
- AEC
Amnion epithelial cells
- CD
Cluster differentiation
- cffTF
Cell free fetal telomere fragments
- CFSE
Carboxyfluorescein succinimidyl ester
- CSE
Cigarette smoke extract
- DAPI
4’,6-diamidino-2-phenylindole
- DAMPs
Damage associated molecular pattern markers
- DNA
Deoxy ribonucleic acid
- ELISA
Enzyme Linked Immunosorbent Assay
- EMT
Epithelial mesenchymal transition
- EtOH
Ethyl alcohol
- FBS
Fetal bovine serum
- FFPE
Formalin-fixed paraffin-embedded
- HMGB
High mobility group box
- HI
Heat Inactivated
- HBSS
Hanks Balanced Salt Solution
- IL
Interleukin
- LC/MS-MS
Liquid chromatography/mass spectrometry
- LPS
Lipopolysaccharide
- MAPK
Mitogen activated protein kinase
- NANOG
Transcription factor of self-renewing embryonic stem cells
- NF-κB
Nuclear factor kappa B
- OS
Oxidative stress
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- PGE
Prostaglandin E
- RelA
RELA Proto-Oncogene, NF-κB Subunit
- RNA
Ribonucleic acid
- SASP
Senescence associated secretory phenotype
- TEM
Transmission electron microscope
- TGFβ
Transforming growth factor beta
- TNF-α
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The findings in this manuscript were presented at the Society for Reproductive Investigation 64th Annual Scientific Meeting.
REFERENCES
- 1.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999;14:229–36. [PubMed] [Google Scholar]
- 2.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. Journal of the Society for Gynecologic Investigation 2006;13:97–103. [DOI] [PubMed] [Google Scholar]
- 3.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction 2003;9:41–45. [DOI] [PubMed] [Google Scholar]
- 4.Bokström H, Brännström M, Alexandersson M, Norström A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod 1997;12:586–90. [DOI] [PubMed] [Google Scholar]
- 5.Elliott CL, Slater DM, Dennes W, Poston L, Bennett PR. Interleukin 8 expression in human myometrium: Changes in relation to labor onset and with gestational age. American Journal of Reproductive Immunology 2000;43:272–77. [DOI] [PubMed] [Google Scholar]
- 6.Menon R, Swan KF, Lyden TW, Rote NS, Fortunato SJ. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol 1995;172:493–500. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev 1994;15:684–706. [DOI] [PubMed] [Google Scholar]
- 8.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999;181:1530–6. [DOI] [PubMed] [Google Scholar]
- 9.Dudley DJ, Trautman MS, Edwin SS, Lundin-Schiller S, Mitchell MD. Biosynthesis of interleukin-6 by cultured human chorion laeve cells: regulation by cytokines. J Clin Endocrinol Metab 1992;75:1081–6. [DOI] [PubMed] [Google Scholar]
- 10.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: Regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biology of Reproduction 1997;57:1438–44. [DOI] [PubMed] [Google Scholar]
- 11.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biology of Reproduction 2002;66:445–49. [DOI] [PubMed] [Google Scholar]
- 12.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004;101:4978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migale R, Herbert BR, Lee YS, et al. Specific Lipopolysaccharide Serotypes Induce Differential Maternal and Neonatal Inflammatory Responses in a Murine Model of Preterm Labor. Am J Pathol 2015;185:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salminen A, Paananen R, Vuolteenaho R, et al. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res 2008;63:280–6. [DOI] [PubMed] [Google Scholar]
- 15.Marcellin L, Schmitz T, Messaoudene M, et al. Immune Modifications in Fetal Membranes Overlying the Cervix Precede Parturition in Humans. J Immunol 2017;198:1345–56. [DOI] [PubMed] [Google Scholar]
- 16.Smith R. Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol 1998;39:215–20. [DOI] [PubMed] [Google Scholar]
- 17.You XJ, Liu J, Xu C, et al. Corticotropin-Releasing Hormone (CRH) Promotes Inflammation in Human Pregnant Myometrium: The Evidence of CRH Initiating Parturition? Journal of Clinical Endocrinology & Metabolism 2014;99:E199–E208. [DOI] [PubMed] [Google Scholar]
- 18.Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol 2011;335:52–9. [DOI] [PubMed] [Google Scholar]
- 19.Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol 2015;213:359.e1–16. [DOI] [PubMed] [Google Scholar]
- 20.Menon R, Boldogh I, Hawkins HK, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 2014;184:1740–51. [DOI] [PubMed] [Google Scholar]
- 21.Menon R, Boldogh I, Urrabaz-Garza R, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 2013;8:e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredeson S, Papaconstantinou J, Deford JH, et al. HMGB1 Promotes a p38MAPK Associated Non-Infectious Inflammatory Response Pathway in Human Fetal Membranes. Plos One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS One 2015;10:e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta EH, Behnia F, Boldogh I, et al. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod 2016;22:143–57. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol 2017;217:592.e1–92.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillippe M. Cell-Free Fetal DNA, Telomeres, and the Spontaneous Onset of Parturition. Reproductive Sciences 2015;22:1186–201. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell MD, Peiris HN, Kobayashi M, et al. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol 2015;213:S173–81. [DOI] [PubMed] [Google Scholar]
- 33.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eldh M, Ekström K, Valadi H, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 2010;5:e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta 2014;35 Suppl:S69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi M, Salomon C, Tapia J, Illanes SE, Mitchell MD, Rice GE. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J Transl Med 2014;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verweij FJ, Middeldorp JM, Pegtel DM. Intracellular signaling controlled by the endosomal-exosomal pathway. Commun Integr Biol 2012;5:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheller S, Papaconstantinou J, Urrabaz-Garza R, et al. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS One 2016;11:e0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-Associated molecular pattern markers HMGB1 and cell-Free fetal telomere fragments in oxidative-Stressed amnion epithelial cell-Derived exosomes. J Reprod Immunol 2017;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-Maternal Trafficking of Exosomes in Murine Pregnancy Models. Front Pharmacol 2016;7:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi T, Hoffman MP. Exosomal microRNA communication between tissues during organogenesis. RNA Biol 2017;14:1683–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krause M, Rak-Raszewska A, Naillat F, et al. Exosomes as secondary inductive signals involved in kidney organogenesis. J Extracell Vesicles 2018;7:1422675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Niel G, Heyman M. The epithelial cell cytoskeleton and intracellular trafficking. II. Intestinal epithelial cell exosomes: perspectives on their structure and function. Am J Physiol Gastrointest Liver Physiol 2002;283:G251–5. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Boulan E, Kreitzer G, Müsch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 2005;6:233–47. [DOI] [PubMed] [Google Scholar]
- 45.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol 2008;18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mallegol J, van Niel G, Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis 2005;35:11–6. [DOI] [PubMed] [Google Scholar]
- 47.Lin XP, Almqvist N, Telemo E. Human small intestinal epithelial cells constitutively express the key elements for antigen processing and the production of exosomes. Blood Cells Mol Dis 2005;35:122–8. [DOI] [PubMed] [Google Scholar]
- 48.Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade G, Menon R. Expression profiles of fetal membrane nicotinamide adenine dinucleotide phosphate oxidases (NOX) 2 and 3 differentiates spontaneous preterm birth and pPROM pathophysiologies. Placenta 2014;35:188–94. [DOI] [PubMed] [Google Scholar]
- 49.Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade GR, Menon R. Screening of lysyl oxidase (LOX) and lysyl oxidase like (LOXL) enzyme expression and activity in preterm prelabor rupture of fetal membranes. J Perinat Med 2016;44:99–109. [DOI] [PubMed] [Google Scholar]
- 50.Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp 2012:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bullerdiek J, Flor I. Exosome-delivered microRNAs of “chromosome 19 microRNA cluster” as immunomodulators in pregnancy and tumorigenesis. Mol Cytogenet 2012;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehdiani A, Maier A, Pinto A, Barth M, Akhyari P, Lichtenberg A. An innovative method for exosome quantification and size measurement. J Vis Exp 2015:50974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helwa I, Cai J, Drewry MD, et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One 2017;12:e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod 2002;67:506–14. [DOI] [PubMed] [Google Scholar]
- 55.Mills AA, Yonish B, Feng L, Schomberg DW, Heine RP, Murtha AP. Characterization of progesterone receptor isoform expression in fetal membranes. Am J Obstet Gynecol 2006;195:998–1003. [DOI] [PubMed] [Google Scholar]
- 56.Gauster M, Huppertz B. The paradox of caspase 8 in human villous trophoblast fusion. Placenta 2010;31:82–8. [DOI] [PubMed] [Google Scholar]
- 57.Kudo Y, Boyd CA, Kimura H, Cook PR, Redman CW, Sargent IL. Quantifying the syncytialisation of human placental trophoblast BeWo cells grown in vitro. Biochim Biophys Acta 2003;1640:25–31. [DOI] [PubMed] [Google Scholar]
- 58.Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 2013;8:e68451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadley EE, Richardson LS, Torloni MR, Menon R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am J Reprod Immunol 2018;79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh SY, Kim CJ, Park I, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol 2005;193:1156–60. [DOI] [PubMed] [Google Scholar]
- 61.Myatt L, Sun K. Role of fetal membranes in signaling of fetal maturation and parturition. International Journal of Developmental Biology 2010;54:545–53. [DOI] [PubMed] [Google Scholar]
- 62.Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011;18:6–19. [DOI] [PubMed] [Google Scholar]
- 63.Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 2009;144 Suppl 1:S2–10. [DOI] [PubMed] [Google Scholar]
- 64.Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular Regulation of Parturition: The Role of the Decidual Clock. Cold Spring Harb Perspect Med 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menon R, Mesiano S, Taylor RN. Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front Endocrinol (Lausanne) 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephen GL, Lui S, Hamilton SA, et al. Transcriptomic Profiling of Human Choriodecidua During Term Labor: Inflammation as a Key Driver of Labor. American Journal of Reproductive Immunology 2015;73:36–55. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Xu Y, Plazyo O, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez-Lopez N, Romero R, Xu Y, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol 2011;204:223.e1–5. [DOI] [PubMed] [Google Scholar]
- 70.El-Azzamy H, Balogh A, Romero R, et al. Characteristic Changes in Decidual Gene Expression Signature in Spontaneous Term Parturition. J Pathol Transl Med 2017;51:264–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iliodromiti Z, Antonakopoulos N, Sifakis S, et al. Endocrine, paracrine, and autocrine placental mediators in labor. Hormones 2012;11:397–409. [DOI] [PubMed] [Google Scholar]
- 72.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 2016;8:216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menon R. Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta 2016;44:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonney EA, Krebs K, Saade G, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta 2016;43:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alcaraz A, Mrowiec A, Insausti CL, et al. Autocrine TGF-β induces epithelial to mesenchymal transition in human amniotic epithelial cells. Cell Transplant 2013;22:1351–67. [DOI] [PubMed] [Google Scholar]
- 76.Epstein Shochet G, Tartakover-Matalon S, Drucker L, et al. Placenta-breast cancer cell interactions promote cancer cell epithelial mesenchymal transition via TGFβ/JNK pathway. Clin Exp Metastasis 2014;31:961–75. [DOI] [PubMed] [Google Scholar]
- 77.Fuxe J, Karlsson MC. TGF-βinduced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol 2012;22:455–61. [DOI] [PubMed] [Google Scholar]
- 78.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci 2005;118:4325–6. [DOI] [PubMed] [Google Scholar]
- 79.Janzen C, Sen S, Lei MY, Gagliardi de Assumpcao M, Challis J, Chaudhuri G. The Role of Epithelial to Mesenchymal Transition in Human Amniotic Membrane Rupture. J Clin Endocrinol Metab 2017;102:1261–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mogami H, Hari Kishore A, Akgul Y, Word RA. Healing of Preterm Ruptured Fetal Membranes. Sci Rep 2017;7:13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombés M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol 2011;25:1710–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update 2016;22:535–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koh YQ, Chan HW, Nitert MD, Vaswani K, Mitchell MD, Rice GE. Differential response to lipopolysaccharide by JEG-3 and BeWo human choriocarcinoma cell lines. Eur J Obstet Gynecol Reprod Biol 2014;175:129–33. [DOI] [PubMed] [Google Scholar]
- 84.Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 2014;289:22258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A 2013;110:17380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem 2016;291:1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prada I, Meldolesi J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004;104:3257–66. [DOI] [PubMed] [Google Scholar]
- 91.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol 2013;228:1487–95. [DOI] [PubMed] [Google Scholar]
- 92.Zech D, Rana S, Büchler MW, Zöller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal 2012;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.