Classical Myeloproliferative Neoplasms (MPNs) include Essential Thrombocythaemia (ET) and Polycythaemia Vera (PV) (Arber et al, 2016). These diseases are frequently due to a mutation in the pseudokinase domain of the Janus Kinase 2 (JAK2) gene, JAK2 V617F (Baxter et al, 2005; Levine et al, 2005). The World Health Organization (WHO) recently revised its diagnostic criteria for MPNs (Arber et al, 2016; Swerdlow et al, 2017), including laboratory values, clonal driver mutations and bone marrow morphology to define specific MPN types (Table I).
Table I.
Current WHO Criteria and Proposed Paediatric Criteria for PV and ET
| PV* | Proposed Criteria for Paediatric PV | ET* | Proposed Criteria for Paediatric ET |
|---|---|---|---|
|
| |||
|
Major Criteria 1. Hb > 165 g/l in men Hb > 160 g/l in women or Hct > 49% in men Hct > 48% in women or Increased red cell mass (>25% above mean normal predicted value) 2. BM biopsy with hypercellularity for age, trilineage growth, and pleiomorphic megakaryocytic proliferation with mature megakaryocytes 3. Presence of JAK2 V617F or JAK2 exon 12 mutation Minor Criteria 1. Subnormal erythropoietin Must meet all 3 major criteria, or first 2 major and minor criterion |
Major Criteria 1. Hb or Hct > 97.5th percentile for age/gender or RBC count > 97.5th percentile for age/gender without evidence of thalassaemia trait 2. BM biopsy that is hypercellular or normocellular for age, trilineage growth, and pleiomorphic megakaryocytic proliferation with mature megakaryocytes 3. Presence of JAK2 V617F or JAK2 exon 12 mutation Minor Criteria 1. Subnormal erythropoietin Must meet all 3 major criteria, or first 2 major and minor criterion |
Major Criteria 1. Platelet count ≥ 450 × 109/l 2. BM biopsy showing primarily proliferation of megakaryocytes with increased, mature, hyperlobulated megakaryocytes, with no significant increase in granulopoiesis or erythropoiesis, and rarely minor increase in reticulin fibrosis (MF-1) 3. Not meeting WHO criteria for BCR-ABL1+ CML or other MPN 4. JAK2, MPL or CALR mutation Minor Criteria 1. Presence of a clonal marker or absence of a reactive thrombocytosis Must meet all 4 major criteria or the first 3 major and minor criterion |
Major Criteria 1. Platelet count ≥ 450 × 109/l for at least 3 months 2. BM biopsy showing primarily proliferation of megakaryocytes with increased, mature, hyperlobulated megakaryocytes, with no significant increase in granulopoiesis or erythropoiesis, and rarely minor increase in reticulin fibrosis (MF-1) 3. Not meeting WHO criteria for BCR-ABL1+ CML or other MPN 4. Presence of a clonal marker including JAK2, MPL or CALR mutation or absence of a reactive thrombocytosis Must meet all 4 major criterion |
BM=bone marrow, CML=chronic myeloid leukemia, ET= essential thrombocythaemia, Hb=haemoglobin, Hct=haematocrit, MPN=myeloproliferative neoplasm, PV=polycythaemia vera, RBC=red blood cell, WHO=World Health Organization.
adapted with permission from Swerdlow, SH, Campo, E, Harris, NL, Jaffe, ES, Pileri, SA, Stein, H, Thiele, J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised 4th edition. IARC, Lyon, 2017
The WHO criteria utilized adult reference ranges and experience with adult disease, therefore a number of criteria are not generally applicable to children. The normal ranges of haemoglobin for children vary by age and are generally lower than adult values (Cembrowski et al, 2005). Applying the WHO haemoglobin cut-offs for PV would miss identifying children with haemoglobin levels clearly above their age-specific range.
Increased red cell mass is included in the criteria but is virtually never tested in paediatrics. Normal bone marrow cellularity is higher in children (Hartsock et al, 1965), making determination of “hypercellularity” difficult. Erythropoietin levels are infrequently performed in children with thrombocytosis. Given these issues, we reviewed a cohort of eight children diagnosed with JAK2 V617F-postive MPN to explore diagnostic considerations.
The eight children with clinically-diagnosed JAK2 V617F-positive ET had white blood cell (WBC) counts, red blood cell (RBC) counts, haemoglobin level and platelet counts assessed. Bone marrows were re-evaluated for cellularity, fibrosis and megakaryocyte morphology. Pre-treatment erythropoietin and von Willebrand levels, and peak JAK2 allele burden were reviewed. The Weill Cornell Medical College Institutional Review Board (IRB) approved this study. Consents and assents were obtained from 7 of 8 families; consent could not be obtained from one family. Since the data was pooled and fully de-identified, approval to include this 8th subject was given by the IRB.
Ages ranged from 3 to 17 years old, with a median of 10 years; five out of eight were female. Five experienced extreme thrombocytosis; three had peak counts over 2000 × 109/l. Seven had peak WBC counts over 11 × 109/l and RBC counts above the 97.5th percentile for age; none had thalassemia trait. Three had peak haemoglobin levels above the 97.5th percentile for age. Serum erythropoietin levels were subnormal in five of six children (median 3 iu/l). Peak JAK2 V617F allele burden ranged from 19 to 54%, with a median of 39%. Three of four children had acquired von Willebrand disease; of which two had extreme thrombocytosis. No severe bleeding was reported. Headache was observed in all subjects. Six children presented with splenomegaly, three children of whom had Budd-Chiari syndrome.
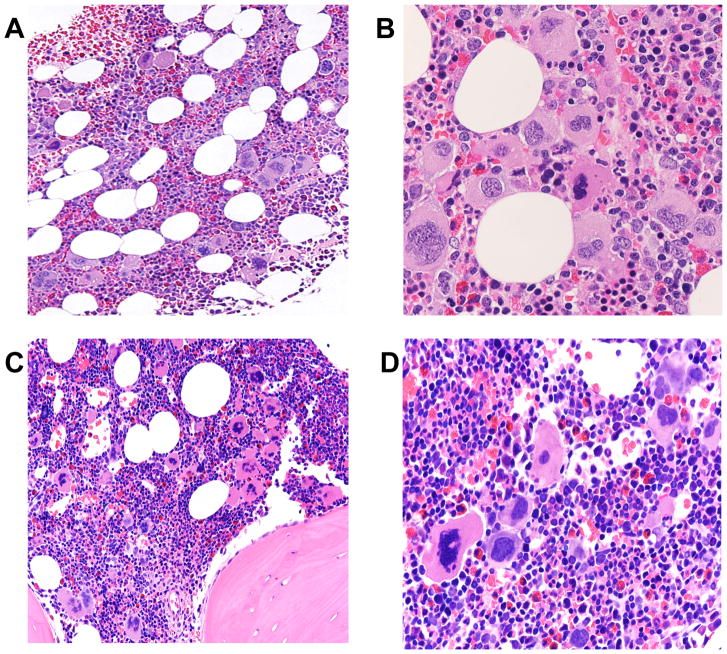
Bone marrow cellularity pre-treatment was normal in two children; six had hypercellular marrows. Megakaryocyte morphology revealed more pleiomorphism than typically reported with ET (Figure 1). Reticulin fibrosis, MF-1, was noted in three children.
Figure 1. Representative Bone Marrow Morphology in Paediatric Patients With JAK2 V617F-positive MPN.
(A, B) a patient under 10 years old showing mildly hypercellular bone marrow (average cellularity 80%) with pleomorphic megakaryocytes (A, 200X). The megakaryocytes are surrounded by maturing erythroid and granulocytic precursors (B, 400X).
(C, D) a patient under 5 years old showing mildly hypercellular bone marrow (average cellularity 90%) (C, 200X). Note megakaryocytes displaying pleomorphic features surrounded by a preponderance of maturing erythroid precursors (D, 400X).
The presence of a JAK2 V617F mutation in thrombocytosis may imply ET, but PV cannot be excluded. The WHO criteria for ET (Table I) requires that the subject does not meet other MPN criteria, which can be complicated. Low serum erythropoietin and pleiomorphic megakaryocytes in some children favour PV. Using age-specific normal levels, five children had polycythaemia, defined by elevated haemoglobin level or RBC count. However, these children did not meet WHO PV criteria, creating uncertainty between two diagnoses.
Proposed modifications of paediatric MPN diagnostic criteria are shown in Table I. Given different normal ranges, children may not demonstrate polycythaemia according to the WHO PV criteria, but may still have PV; comparison of haemoglobin and/or RBC counts to age-specific norms is more appropriate (in the absence of thalassemia trait). Given how common reactive thrombocytosis is in children, our proposed criteria suggest a minimum duration of thrombocytosis. As many children with MPN are negative for known mutations, we gave absence of a reactive cause equal weight to mutation status. Erythropoietin is often not tested in children with thrombocytosis and should be part of their work-up. Furthermore, marrow pathology should be carefully assessed by haematopathologists experienced in reviewing paediatric specimens to optimally distinguish among MPN.
A recent study compared adults with JAK2 V617F-mutant ET and PV (Rumi et al, 2014). Patients with PV had higher WBC counts than those with ET. While some ET patients showed subnormal erythropoietin levels, the median erythropoietin level was significantly lower in PV (2.7 iu/l), close to that found in our group (3 iu/l). Similarly, some PV patients exhibited thrombocytosis with counts over 1000 × 109/l. The median JAK2 mutant allele burden was 18% in ET compared to 42% in PV, in line with the 39% seen in our cohort. JAK2-mutated MPN exist on a continuum and can progress from ET to PV (Campbell et al, 2005; Rumi et al, 2014). Given the spectrum of JAK2-mutated MPN described in adults and varying laboratory findings in our cohort, it seems likely that some children may actually have PV.
When diagnosing MPN in a paediatric patient, assigning the diagnosis of MPN unclassified may currently be best. It is likely that knowing the MPN type will affect management and prognosis in children, as in adults. The development of paediatric-specific diagnostic criteria should allow more accurate identification of specific MPN type; our proposed alternative criteria for children take into account key differences between children and adults. Long-term follow-up and large patient numbers are required to validate these proposed criteria. Given the rarity of these diseases, this would require international, multi-centre, collaborative discussion and study.
Acknowledgments
This work was supported by a grant from the NIH/NCATS (USA), Grant # KL2TR000458 (to NK) and from the National Heart, Lung, And Blood Institute of the National Institutes of Health (USA), under award #K23HL127223 (to NK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the families and colleagues who made this study possible.
Footnotes
Author Contributions
Study designed by NK, JB, AO. Research performed by NK, MA, DH, AO. Data analysed by NK, MA, DH, AO. Paper written by NK, MA, DH, JB, AO. Paper edited by NK, JB, AO. All authors reviewed the manuscript and agreed with its submission. The authors declare no conflicts of interest.
References
- Arber DA, Orazi A, Hasserjian R, Jürgen T, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, Vassiliou GS, Milligan DW, Smith SR, Erber WN, Bareford D, Wilkins BS, Reilly JT, Harrison CN, Green AR United Kingdom Myeloproliferative Disorders Study Group; Medical Research Council Adult Leukemia Working Party; Australasian Leukemia Lymphoma Group. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- Cembrowski GS, Chan J, Cheng F, Bamforth J. NHANES 1999–2000 Data used to Create Comprehensive Health-Associated Race-, Sex-, and Age-Stratified Pediatric Reference Intervals for the Coulter MAXM. Laboratory Hematology. 2005;10:245–246. [Google Scholar]
- Hartsock RJ, Smith EB, Petty CS. Normal variations with aging of the amount of hematopoietic tissue in bone marrow from the anterior iliac crest. A study made from 177 cases of sudden death examined by necropsy. American Journal of Clinical Pathology. 1965;43:326–331. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, Them NC, Berg T, Elena C, Casetti IC, Milanesi C, Sant’antonio E, Bellini M, Fugazza E, Renna MC, Boveri E, Astori C, Pascutto C, Kralovics R, Cazzola M Associazione Italiana per la Ricerca sul Cancr Gruppo Italiano Malattie Mieloprolifertive Investigators. JAK2 or CALR mutation status defines subtypes of essential thrombycythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–1551. doi: 10.1182/blood-2013-11-539098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised 4th edition. IARC; Lyon: 2017. pp. 39–50. [Google Scholar]