Abstract
Objective:
Studies show that stimulant users have varied substance use patterns and that polysubstance use is associated with poorer past or concurrent medical, mental health, and substance use outcomes. This study examined outcomes of substance use patterns prospectively.
Method:
A latent class analysis was conducted to examine substance use patterns among adults using stimulants (n = 710; 38.6% women) at baseline, and the health and treatment utilization outcomes of different use patterns over the subsequent 3 years. To examine associations between latent class membership and outcomes, generalized estimating equation modeling was conducted.
Results:
Four classes of substance use patterns at baseline were identified, involving high use of (a) methamphetamine and marijuana (23%); (b) crack cocaine and alcohol (25%); (c) powder cocaine, alcohol, and marijuana (23%); and (d) nonprescribed opioids, alcohol, marijuana, crack cocaine, and powder cocaine (i.e., polysubstance [29%]). Polysubstance class members had poorer physical health and mental health status, and more severe substance use, over the subsequent 3-year period, than other class members. Regarding treatment utilization, polysubstance class members had more medical care utilization than crack cocaine class members, and more substance use treatment utilization than powder cocaine class members. The methamphetamine, crack cocaine, and powder cocaine classes did not differ from each other on any health or treatment utilization outcome.
Conclusions:
People using stimulants commonly use other substances, and those whose polysubstance use includes nonprescribed opioids have especially poor health outcomes.
Polysubstance use is the consumption of more than one substance over a defined period, simultaneously or at different times (Connor et al., 2014). It is common and associated with psychiatric and medical problems, engagement in health risk behaviors, and cognitive functioning deficits that increase the chances of poor treatment adherence and outcomes (Booth et al., 2010; Brecht et al., 2008; Connor et al., 2014). Operationalizations of polysubstance use have typically been based on lifetime prevalence, 1–12 month prevalence, or simultaneous substance use, which has been assessed mainly in small studies of high-risk populations because it is impractical to routinely ask about all combinations of substance pairs (Connor et al., 2014). Polysubstance use is associated with the demographic characteristics of being male, young adult, African American, not married or partnered, having less education, living in an urban area, and being employed (Connor et al., 2014; Kedia et al., 2007). The purpose of this study was to examine substance use patterns, that is, the use of alcohol and drugs in the past month, and the outcomes of different patterns over time, among adults using stimulants at baseline.
Although some studies have assessed polysubstance use as a count of substances used during the observation period (Booth et al., 2010), more recently the approach to describing polysubstance use has employed latent class analysis (LCA; Connor et al., 2014). LCA is a person-centered approach to identifying latent classes or common profiles of substance use that reflect relatively distinct subgroups (Chung et al., 2013). For example, LCA was used to determine patterns of past-year illegal drug use in a U.S. national sample of alcohol-dependent respondents (Hedden et al., 2010). Results demonstrated a five-class solution, with the largest class being an almost-zero probability of illegal drug use (65% of the sample), and the second largest being high marijuana plus medium cocaine use (21% of the sample). Alcohol-marijuana-cocaine class members were more likely to have a history of sexually transmitted infection, incarceration, and behaviors consistent with conduct disorder than those in the alcohol-only class (Hedden et al., 2010). Another LCA conducted on past-month drug use among treatment-referred cannabis users found a three-class solution: cannabis plus tobacco (22%); cannabis, tobacco, and alcohol (62%); and wide-ranging substance use (16%) (Connor et al., 2013). Wide-ranging substance users had more severe substance use and mental health symptoms (Connor et al., 2013). Findings of Hedden et al. (2010) and Connor et al. (2013) suggest that people engaged in wider-ranging substance use need more intensive service resources.
Substance use patterns and outcomes among stimulant users
It is important to examine polysubstance use among stimulant users because there are additional risks associated with using stimulants in combination with other substances. According to the Substance Abuse and Mental Health Services Administration (SAMHSA; see Hedden et al., 2015), 2,069,000 Americans were past-month users of stimulants (cocaine and/or methamphetamine). Emergency department visits related to use of stimulants have risen recently, and about two thirds (62%) of these visits involved the use of stimulants with at least one other substance (SAMHSA, 2014). To compare, among all emergency department visits involving the use of any substance, less than one half (49%) of visits involved multiple substances (SAMHSA, 2014). Increasing the risk of harm, current methods are producing purer and more potent formulations of methamphetamine and cocaine as their prices are decreasing (SAMHSA, 2014; Werb et al., 2013).
In addition to being harmful, polysubstance use is common among stimulant users. Among 350 adults treated for methamphetamine use disorders, a majority reported using alcohol, marijuana, and cocaine, and a substantial minority reported use of heroin and tranquilizers. Even when substances used by all or almost all participants were excluded (i.e., methamphetamine, alcohol, marijuana), the mean number of substances used was 5.0 of 10 types (Brecht et al., 2004). Among 1,016 methamphetamine-dependent outpatients, concurrent use of other substances was 63% for alcohol, 59% for marijuana, 10% for prescription opioids, 9% for tranquilizers, and 6% for heroin (Christian et al., 2007). Use of other substances in addition to methamphetamine significantly increased the odds of a history of physical and/or sexual abuse, suicidal ideation and other mental health symptoms, and suicide attempt. Because of the crosssectional design, these results could be interpreted as either a need for more substances to self-medicate psychiatric symptoms or as clinical pathology stemming from polysubstance use (Christian et al., 2007). A subsequent study of adults with previous, long histories of methamphetamine use found varied patterns of past-year substance use: methamphetamine only (7%), marijuana only (16%), methamphetamine plus marijuana (19%), heroin plus cocaine (20%), or had not used any substances (38%). Polysubstance users, compared with single-substance or non-users, had more symptoms of mental health disorders (Herbeck et al., 2013).
More recent studies of stimulant users employed LCA. In examining patterns of past-year substance use disorders in a U.S. national sample of adults reporting past-year nonmedical use of attention-deficit/hyperactivity disorder stimulants (Chen et al., 2014), a four-class model identified low probability of any substance use disorder (53%), high probability of alcohol and marijuana use disorders (29%), use of prescription drugs (13%), and high probability of multiple drugs and alcohol use (5%). Those in the multiple drugs plus alcohol class were more likely to report mental health and behavioral problems as well as past-year use of mental health treatment and of addiction treatment. Among treated patients who were past-year injectors of amphetamine, LCA identified three substance use patterns: low polydrug (low use of all substances; 59%), opiates polydrug (heroin or other opioids plus alcohol and cannabis; 15%), and alcohol polydrug (only alcohol plus cannabis; 26%). The low-polydrug class demonstrated somewhat better functioning and safer injecting practices than the other classes (Kelly et al., 2017).
Present study
The studies reviewed suggest that stimulant users have varied substance use patterns and that polysubstance use is associated with poorer past or concurrent medical, mental health, and substance use outcomes. However, all of the reviewed studies were retrospective or cross-sectional, such that associations of substance use patterns with subsequent outcomes could not be examined. The present study used LCA to characterize patterns of substance use at baseline among adults who were using stimulants, and outcomes of the identified classes over the subsequent 3 years. Research on the identification and consequences of polysubstance use may be beneficial for informing the targeting of specific profiles in treatment settings (Connor et al., 2014). That is, a better understanding of outcomes associated with substance use profiles could shed light on recovery processes and ensure that services address the needs of each identified group (Hagen et al., 2017).
Method
Participants
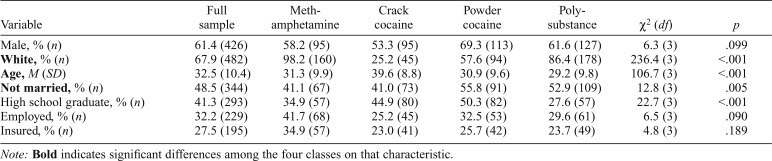
Study participants were 710 stimulant users living in Arkansas, Kentucky, and Ohio counties. The study was approved by the institutional review boards at the investigators’ universities, and a Certificate of Confidentiality was obtained from the National Institute on Drug Abuse. Participant eligibility criteria were use of stimulants (methamphetamine, cocaine) within the past 30 days, no treatment or mutual-help group attendance within the past 30 days, being at least 18 years old, and having a verifiable address within one of the study counties. Participants’ baseline sociodemographic characteristics are provided in Table 1.
Table 1.
Baseline sociodemographic characteristics of the sample (n = 710) and comparison of substance use pattern classes on baseline sociodemographic characteristics
| Variable | Full sample | Methamphetamine | Crack cocaine | Powder cocaine | Polysubstance | χ2 (df) | p |
| Male, % (n) | 61.4 (426) | 58.2 (95) | 53.3 (95) | 69.3 (113) | 61.6 (127) | 6.3 (3) | .099 |
| White, % (n) | 67.9 (482) | 98.2 (160) | 25.2 (45) | 57.6 (94) | 86.4 (178) | 236.4 (3) | <.001 |
| Age, M (SD) | 32.5 (10.4) | 31.3 (9.9) | 39.6 (8.8) | 30.9 (9.6) | 29.2 (9.8) | 106.7 (3) | <.001 |
| Not married, % (n) | 48.5 (344) | 41.1 (67) | 41.0 (73) | 55.8 (91) | 52.9 (109) | 12.8 (3) | .005 |
| High school graduate, % (n) | 41.3 (293) | 34.9 (57) | 44.9 (80) | 50.3 (82) | 27.6 (57) | 22.7 (3) | <.001 |
| Employed, % (n) | 32.2 (229) | 41.7 (68) | 25.2 (45) | 32.5 (53) | 29.6 (61) | 6.5 (3) | .090 |
| Insured, % (n) | 27.5 (195) | 34.9 (57) | 23.0 (41) | 25.7 (42) | 23.7 (49) | 4.8 (3) | .189 |
Note: Bold indicates significant differences among the four classes on that characteristic.
Procedure
Participants were recruited using respondent-driven sampling, a type of snowball sampling (Heckathorn, 1997; Wang et al., 2005). Study seeds completed a baseline interview and handed out information about the study to up to three people they knew who used drugs. Each seed received $10 for up to three referred individuals who contacted the study coordinator and were eligible for, and enrolled in, the study. Participants received $50 for each completed study session.
Participants completed informed consent before the baseline interview. Follow-up interviews were conducted every 6 months for a total of 36 months. Interviews were conducted in person by trained study staff using computer-assisted interview software on laptop computers. Retention rates were 85%, 82%, 81%, 79%, 79%, and 73% for the 6-, 12-, 18-, 24-, 30-, and 36-month interviews, respectively; 73% of the initial sample completed all interviews.
Measures
Baseline substance use.
At baseline, participants stated how many days in the past 30 days they had used each of the following 10 substances: alcohol, amphetamines, crack cocaine, heroin, marijuana, methamphetamine, nonprescribed opioid pain medications, nonprescribed tranquilizers, powder cocaine, and prescribed opioid pain medications.
Health outcomes.
To assess medical status at baseline and each follow-up, four variables were combined to create an index, as follows: (a) the number (0–29) of medical conditions (past 6 months) that participants had been told by a doctor that they had; (b) the number (0–5) of diagnosed physical ailments (past 6 months); (c) the seriousness of the diagnosed physical ailments (past 6 months; 0 = not serious at all to 4 = extremely serious); and (d) the medical severity score from the Addiction Severity Index (ASI; McLellan et al., 1980), which ranged on a continuous scale from 0 to 1, with higher scores indicating greater severity. Mental health status was the composite of the following four variables: (a) the Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001) score (0–27, with higher scores indicating greater depression symptom severity); (b) the number (0–5) of emotional problems (past 6 months); (c) the seriousness of the emotional problems (past 6 months; 0 = not at all to 4 = extremely); and (d) the psychiatric subscale of the ASI (McLellan et al. 1980), which ranged on a continuous scale from 0 to 1 with higher scores indicating greater severity. Substance use severity combined four variables: how many days in the past 30 days the participant had (a) used alcohol and (b) drugs; (c) whether the participant met criteria for an alcohol use disorder (yes/no); and (d) the count of how many other substances the participant met criteria for disorders. Each outcome was computed by converting the variables it included to z scores and then taking the mean of the z scores (Blonigen et al., 2005, 2016; for additional detail, see Woodhead et al., 2018).
Treatment utilization.
Three types of treatment utilization with regard to the past 6 months were examined. The medical care index combined three variables: (a) number of hospitalizations (0–5); (b) number of days for each hospitalization; and (c) number of outpatient visits (0–6); again, this dependent variable was computed by converting the variables it included to z scores and then taking the mean of the z scores (Woodhead et al., 2018). Substance use treatment was assessed with one question (yes/no) that asked participants whether they had been in a substance use treatment program (residential, intensive outpatient, outpatient, methadone, or other). Mental health treatment was assessed with one question that asked participants whether they had been treated for psychological or emotional problems by a professional such as a psychologist, social worker, psychiatrist, or therapist.
Analysis plan
SAS PROC LCA was used for the LCA analysis, that is, to identify use patterns of the 10 substances (alcohol, amphetamines, crack cocaine, heroin, marijuana, methamphetamine, nonprescribed opioid pain medications, nonprescribed tranquilizers, powder cocaine, and prescribed opioid pain medications) during the past 30 days. We used the timeframe of the past 30 days to more accurately examine recent substance use patterns (Connor et al., 2013; Kelly et al., 2017); zero days was coded as no use, and one or more days as use. To examine the assumption of conditional independence that must be met in LCA, we conducted bivariate correlations among the substance use variables and examined the tolerances or VIFs among variables (i.e., we regressed each variable assessing substance use on all of the other substance use variables). Results of these analyses indicated that the assumption of conditional independence was met; that is, correlations were small to moderate, and tolerances and VIFs did not raise concerns about non-independence.
Model fit was assessed using three indices including the Akaike information criterion (AIC), the Bayesian information criterion (BIC), and G2 (likelihood-ratio statistic). The optimal number of classes was selected based on these indices. Model fitting started with a one-class solution and proceeded up to a five-class solution. Comparisons were made among the latent classes with Bonferroni adjusted confidence intervals.
To examine associations between latent class membership and outcomes, because of the repeated structure of the data, we used generalized estimating equation (GEE) models for all outcomes. For each dependent variable, we first checked the distribution. If the normal assumption was satisfied, a normal distribution with an identity link was specified; this held for the outcomes of medical status, mental health status, and substance use severity. For skewed dependent variables, a gamma distribution with log link was specified; this held for the outcome of medical care utilization. For dichotomized outcomes, a binominal distribution with logit link was specified; this held for mental health and substance use treatment. Robust standard errors were used in all models with an AR(1) correlation matrix specified. Demographic covariates were included in all models, that is, gender (male vs. female), race (White vs. not White), age, marital status (not married vs. married), education (high school education or more vs. less), employment status (employed vs. unemployed), health insurance status (insured vs. uninsured), and state of residence (Arkansas and Kentucky vs. Ohio), as well as time indicating each interview.
Results
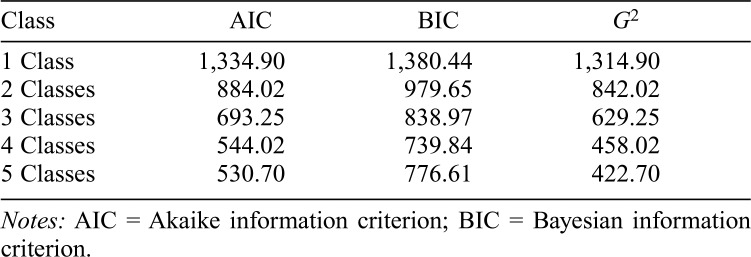
Model fit statistics for 1 to 5 class solutions are presented in Table 2. The value of the information criteria decreased as the number of classes increased, indicating successively better-fitting models. The 4-class solution was selected as the most parsimonious representing the data.
Table 2.
Fit statistics of the latent class analysis
| Class | AIC | BIC | G2 |
| 1 Class | 1,334.90 | 1,380.44 | 1,314.90 |
| 2 Classes | 884.02 | 979.65 | 842.02 |
| 3 Classes | 693.25 | 838.97 | 629.25 |
| 4 Classes | 544.02 | 739.84 | 458.02 |
| 5 Classes | 530.70 | 776.61 | 422.70 |
Notes: AIC = Akaike information criterion; BIC = Bayesian information criterion.
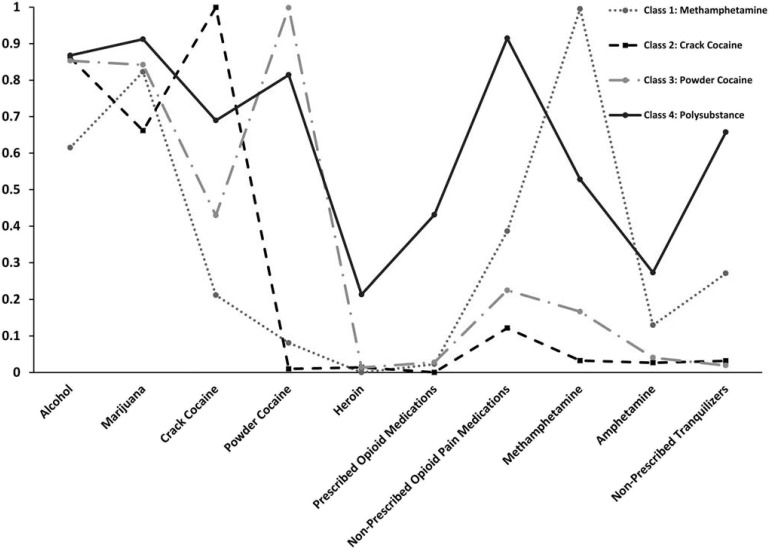
Figure 1 shows the probability of past-30-day use for each substance by class. For brevity, we refer to the high, moderate, or low probability of having used a substance at least once in the past 30 days as “high, moderate, or low use,” respectively. Class 1 (23%, n = 163) was characterized by high use of methamphetamine and marijuana, moderate use of alcohol and nonprescribed opioid pain medications, and low use of crack cocaine, powder cocaine, heroin, prescribed opioid pain medications, amphetamines, and nonprescribed tranquilizers. Class 2 (25%, n = 178) was characterized by high use of crack cocaine and alcohol, moderate use of marijuana, and low use of powder cocaine, heroin, prescribed opioids, nonprescribed opioids, methamphetamine, amphetamine, and nonprescribed tranquilizers. Class 3 (23%, n = 163) was characterized by high use of powder cocaine, alcohol, and marijuana; moderate use of crack cocaine; and low use of heroin, prescribed opioids, nonprescribed opioids, methamphetamines, amphetamines, and nonprescribed tranquilizers. Class 4 (29%, n = 206) was characterized by high use of nonprescribed opioids, alcohol, marijuana, crack cocaine, and powder cocaine; moderate use of prescribed opioids, methamphetamine, and nonprescribed tranquilizers; and low use of heroin and amphetamine. While recognizing that each class characterizes the use of multiple substances, for conciseness, we refer to Class 1 as the Methamphetamine class, Class 2 as the Crack Cocaine class, Class 3 as the Powder Cocaine class, and Class 4 as the Polysubstance class.
Figure 1.
Latent classes of substance use patterns among adults using stimulants
Table 1 compares the four classes on baseline sociodemographic characteristics. Most members of the Methamphetamine, Polysubstance, and Powder Cocaine classes were White, compared with only 25% of Crack Cocaine class members; Crack Cocaine class members were also older than other class members. Most Polysubstance and Powder Cocaine class members were not married, whereas most Methamphetamine and Crack Cocaine class members were married. About one half of Crack and of Powder Cocaine class participants had at least a high school education, compared to roughly one third of Methamphetamine and of Polysubstance class members. Classes did not differ on gender or employment or insurance status.
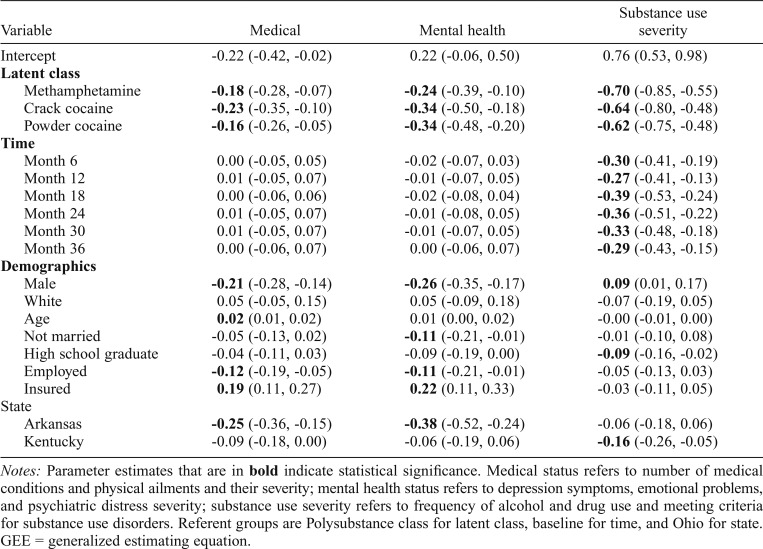
Analyses initially used GEE to compare each class to each other class, considering time and demographics, on each outcome. These analyses found differences only between the Polysubstance class and other classes. Therefore, for parsimony in reporting, Table 3 shows results of GEE models comparing the Methamphetamine, Crack Cocaine, and Powder Cocaine classes to the Polysubstance class on physical and mental health status, and substance use severity. Polysubstance class members had poorer physical health and mental health status, and more severe substance use, than other class members.
Table 3.
Parameter estimates from GEE models for medical, mental health, and substance use status
| Variable | Medical | Mental health | Substance use severity |
| Intercept | -0.22 (-0.42, -0.02) | 0.22 (-0.06, 0.50) | 0.76 (0.53, 0.98) |
| Latent class | |||
| Methamphetamine | -0.18 (-0.28, -0.07) | -0.24 (-0.39, -0.10) | -0.70 (-0.85, -0.55) |
| Crack cocaine | -0.23 (-0.35, -0.10) | -0.34 (-0.50, -0.18) | -0.64 (-0.80, -0.48) |
| Powder cocaine | -0.16 (-0.26, -0.05) | -0.34 (-0.48, -0.20) | -0.62 (-0.75, -0.48) |
| Time | |||
| Month 6 | 0.00 (-0.05, 0.05) | -0.02 (-0.07, 0.03) | -0.30 (-0.41, -0.19) |
| Month 12 | 0.01 (-0.05, 0.07) | -0.01 (-0.07, 0.05) | -0.27 (-0.41, -0.13) |
| Month 18 | 0.00 (-0.06, 0.06) | -0.02 (-0.08, 0.04) | -0.39 (-0.53, -0.24) |
| Month 24 | 0.01 (-0.05, 0.07) | -0.01 (-0.08, 0.05) | -0.36 (-0.51, -0.22) |
| Month 30 | 0.01 (-0.05, 0.07) | -0.01 (-0.07, 0.05) | -0.33 (-0.48, -0.18) |
| Month 36 | 0.00 (-0.06, 0.07) | 0.00 (-0.06, 0.07) | -0.29 (-0.43, -0.15) |
| Demographics | |||
| Male | -0.21 (-0.28, -0.14) | -0.26 (-0.35, -0.17) | 0.09 (0.01, 0.17) |
| White | 0.05 (-0.05, 0.15) | 0.05 (-0.09, 0.18) | -0.07 (-0.19, 0.05) |
| Age | 0.02 (0.01, 0.02) | 0.01 (0.00, 0.02) | -0.00 (-0.01, 0.00) |
| Not married | -0.05 (-0.13, 0.02) | -0.11 (-0.21, -0.01) | -0.01 (-0.10, 0.08) |
| High school graduate | -0.04 (-0.11, 0.03) | -0.09 (-0.19, 0.00) | -0.09 (-0.16, -0.02) |
| Employed | -0.12 (-0.19, -0.05) | -0.11 (-0.21, -0.01) | -0.05 (-0.13, 0.03) |
| Insured | 0.19 (0.11, 0.27) | 0.22 (0.11, 0.33) | -0.03 (-0.11, 0.05) |
| State | |||
| Arkansas | -0.25 (-0.36, -0.15) | -0.38 (-0.52, -0.24) | -0.06 (-0.18, 0.06) |
| Kentucky | -0.09 (-0.18, 0.00) | -0.06 (-0.19, 0.06) | -0.16 (-0.26, -0.05) |
Notes: Parameter estimates that are in bold indicate statistical significance. Medical status refers to number of medical conditions and physical ailments and their severity; mental health status refers to depression symptoms, emotional problems, and psychiatric distress severity; substance use severity refers to frequency of alcohol and drug use and meeting criteria for substance use disorders. Referent groups are Polysubstance class for latent class, baseline for time, and Ohio for state.
GEE = generalized estimating equation.
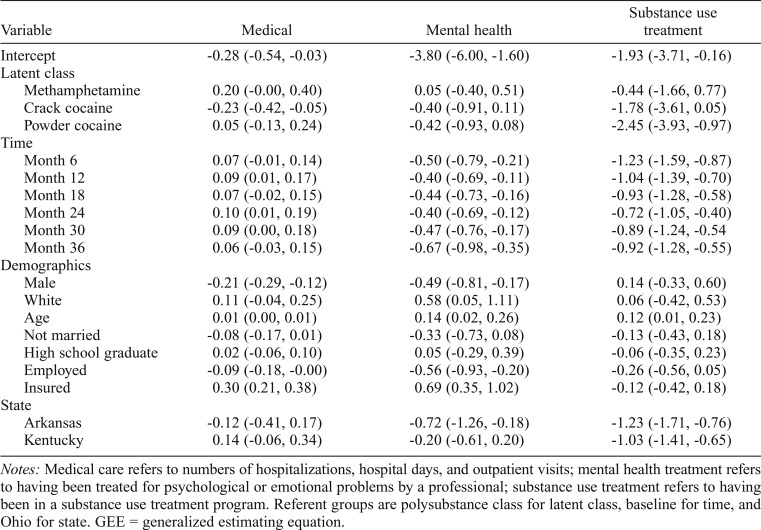
Table 4 shows results of GEE models comparing the Methamphetamine, Crack Cocaine, and Powder Cocaine classes to the Polysubstance class on medical, mental health, and substance use treatment use. Regarding medical care, Polysubstance class members had more utilization than Crack Cocaine class members. Classes did not differ on mental health care utilization. Regarding substance use treatment, Polysubstance class members were more likely to have received treatment than Powder Cocaine class members.
Table 4.
Parameter estimates from GEE models for medical, mental health, and substance use treatment utilization
| Variable | Medical | Mental health | Substance use treatment |
| Intercept | -0.28 (-0.54, -0.03) | -3.80 (-6.00, -1.60) | -1.93 (-3.71, -0.16) |
| Latent class | |||
| Methamphetamine | 0.20 (-0.00, 0.40) | 0.05 (-0.40, 0.51) | -0.44 (-1.66, 0.77) |
| Crack cocaine | -0.23 (-0.42, -0.05) | -0.40 (-0.91, 0.11) | -1.78 (-3.61, 0.05) |
| Powder cocaine | 0.05 (-0.13, 0.24) | -0.42 (-0.93, 0.08) | -2.45 (-3.93, -0.97) |
| Time | |||
| Month 6 | 0.07 (-0.01, 0.14) | -0.50 (-0.79, -0.21) | -1.23 (-1.59, -0.87) |
| Month 12 | 0.09 (0.01, 0.17) | -0.40 (-0.69, -0.11) | -1.04 (-1.39, -0.70) |
| Month 18 | 0.07 (-0.02, 0.15) | -0.44 (-0.73, -0.16) | -0.93 (-1.28, -0.58) |
| Month 24 | 0.10 (0.01, 0.19) | -0.40 (-0.69, -0.12) | -0.72 (-1.05, -0.40) |
| Month 30 | 0.09 (0.00, 0.18) | -0.47 (-0.76, -0.17) | -0.89 (-1.24, -0.54 |
| Month 36 | 0.06 (-0.03, 0.15) | -0.67 (-0.98, -0.35) | -0.92 (-1.28, -0.55) |
| Demographics | |||
| Male | -0.21 (-0.29, -0.12) | -0.49 (-0.81, -0.17) | 0.14 (-0.33, 0.60) |
| White | 0.11 (-0.04, 0.25) | 0.58 (0.05, 1.11) | 0.06 (-0.42, 0.53) |
| Age | 0.01 (0.00, 0.01) | 0.14 (0.02, 0.26) | 0.12 (0.01, 0.23) |
| Not married | -0.08 (-0.17, 0.01) | -0.33 (-0.73, 0.08) | -0.13 (-0.43, 0.18) |
| High school graduate | 0.02 (-0.06, 0.10) | 0.05 (-0.29, 0.39) | -0.06 (-0.35, 0.23) |
| Employed | -0.09 (-0.18, -0.00) | -0.56 (-0.93, -0.20) | -0.26 (-0.56, 0.05) |
| Insured | 0.30 (0.21, 0.38) | 0.69 (0.35, 1.02) | -0.12 (-0.42, 0.18) |
| State | |||
| Arkansas | -0.12 (-0.41, 0.17) | -0.72 (-1.26, -0.18) | -1.23 (-1.71, -0.76) |
| Kentucky | 0.14 (-0.06, 0.34) | -0.20 (-0.61, 0.20) | -1.03 (-1.41, -0.65) |
Notes: Medical care refers to numbers of hospitalizations, hospital days, and outpatient visits; mental health treatment refers to having been treated for psychological or emotional problems by a professional; substance use treatment refers to having been in a substance use treatment program. Referent groups are polysubstance class for latent class, baseline for time, and Ohio for state. GEE = generalized estimating equation.
Discussion
This study accomplished two aims. The first was to identify classes of substance use patterns among people using stimulants by conducting LCA. The second was to extend previous retrospective and cross-sectional studies of the outcomes of different substance use patterns by prospectively examining outcomes over the 3-year postbaseline period. The findings are useful to substance use treatment practitioners and researchers because they highlight the particularly poor health associated with polysubstance use over time, despite more use of medical and substance use treatment by Polysubstance class members, and thus the need for more effective interventions for people using particular combinations of substances.
The identified four classes indicate that substance use patterns are diverse among people using stimulants. Classes were differentiated by (a) high use of methamphetamine and marijuana, and moderate use of alcohol and nonprescribed opioid pain medications (Methamphetamine class); (b) high use of crack cocaine and alcohol, and moderate use of marijuana (Crack Cocaine class); (c) high use of powder cocaine, alcohol, and marijuana, and moderate use of crack cocaine (Powder Cocaine class); and high use of nonprescribed opioids, alcohol, marijuana, crack cocaine, and powder cocaine, and moderate use of prescribed opioids, methamphetamine, and nonprescribed tranquilizers (Polysubstance class). Each class represented about one quarter of the sample of stimulant users at baseline.
Two commonalities across the first three classes may help to explain why they did not differ on outcomes related to health and health care utilization. One was that in each of those classes a high probability of use occurred for only two or three substances, in contrast to the polysubstance use class, which had a high probability of use of five substances. The number as well as the type of multiple substances used may be associated with health (Bhalla et al., 2017). Another commonality across the first three classes was the use of stimulants with alcohol and marijuana, despite known dangers of such use. Marijuana is often used to decrease side effects of “coming down” from stimulants (Compton & Volkow, 2006; Olière et al., 2013), but its use increases risk of severe paranoia and psychosis (Hanna et al., 2017). Alcohol is often used with stimulants because its sedative properties counteract some of the stimulant’s effects, such as sleep disturbances (Compton & Volkow, 2006). However, whereas methamphetamine use elevates the risk of cardiac pathology, mixing alcohol with methamphetamine increases this risk (Kaye et al., 2007). Further, methamphetamine use masks the effects of drinking such that drinking-impaired persons do not perceive themselves as so and are more likely to engage in dangerous behaviors such as drinking and driving (Compton & Volkow, 2006). In addition, the specific combination of cocaine and alcohol produces cocaethylene, a metabolite that is more toxic than either cocaine or alcohol alone, in the liver (Compton & Volkow, 2006). Finally, whereas methamphetamine use increases the likelihood of psychotic symptoms, additional frequent cannabis or marijuana use increases the likelihood even more (McKetin et al., 2013).
Our findings on the common use of stimulants with other substances, combined with the dangers of this practice, serve to underscore the importance of screening for and targeting treatment to ongoing multiple substances use among stimulant users presenting for treatment, as do findings from Wang et al. (2017). Wang et al. (2017) found that secondary substance use consistently moderated associations between treatment and primary drug use. During months without any secondary substance use, treatment was strongly associated with decreased odds of primary drug use, but during months of secondary use, the association was weaker. However, clinical guidelines provide minimal guidance on the management of polydrug use (Wang et al., 2017); they suggest only that multiple substance use disorders be managed according to recommendations made for individual disorders (e.g., National Institute on Drug Abuse, 2012).
In our examination of demographic characteristics, Crack Cocaine class members were more likely to be Black and older than other class members. Similarly, in U.S. national samples, older and Black respondents were at increased risk for crack cocaine use in unconditional models (Palamar et al., 2015). However, because the association with race no longer held when socioeconomic variables were controlled, the higher risk for crack cocaine use among racial minorities may be driven by socioeconomic factors (Palamar et al., 2015). More generally, our sample was notably uneducated (40% with <12 years of schooling), uninsured (73%), and unemployed (50%), supporting the notion that socioeconomic factors are contributing determinants of substance use. We found that Crack and Powder Cocaine class members were more likely to have completed high school than Methamphetamine and Polysubstance class members, whereas previous studies found noncompletion of high school associated with more use of tobacco, alcohol, marijuana, and illicit drugs (Tice et al., 2013).
Our prospective findings that Polysubstance class members had poorer physical, mental, and substance-related health status, and a greater likelihood of use of medical and substance use care, than other stimulant users over the follow-up period are consistent with and extend previous studies relying on retrospective or cross-sectional data; these studies also found polysubstance use to be associated with poorer functioning (Kelly et al., 2017). In our study, only the Polysubstance class had members with high use of nonprescribed opioids, which may help to explain members’ poor outcomes. Opioid use is associated with poorer health and quality of life and more health care use among noncancer pain patients (Eriksen et al., 2006). Specifically, opioids cause adverse events in several organ systems through a variety of mechanisms, such that longer-term opioid use is associated with gastrointestinal problems (constipation and nausea), hyperalgesia, sleep-related breathing problems, increased risk of fractures (due to dizziness and reduced alertness causing falls), cardiovascular problems (myocardial infarction and heart failure), hormone problems, and depression (Baldini et al., 2012; Benyamin et al., 2008). Accordingly, adults who used opioids or multiple drugs had higher health care utilization than adults who used only stimulants (Calcaterra et al., 2015).
Despite this study’s strengths, including successfully following a large sample of stimulant users over a relatively long time, it also had limitations. We relied on participants’ self-reports, which may have been influenced by social desirability and other response biases. Although studies have found substance-using individuals to provide accurate self-reports of use (Napper et al., 2010; SAMHSA, 2010), biological tests and/or collateral reports would provide corroboration. In this study, we used the time frame of the past 30 days (zero or any days of use) to characterize substance use patterns. This fits with the current definition of polysubstance use as use of at least two different substances (no or yes) either simultaneously or separately in a defined period of time, including lifetime or the past year, 6 months, month, or 2 weeks (Tomczyk et al., 2016). However, we did not differentiate simultaneous (co-ingestion of different substances at the same time) from concurrent (use of different substances on the same or separate occasions within the same period) polysubstance use. Studies examining both simultaneous and concurrent use in the same samples have found that most polysubstance use is simultaneous (Baggio et al., 2014; McCabe et al., 2006; Quek et al., 2013; Subbaraman & Kerr, 2015). Reliable and valid measures are essential for describing substance use patterns, evaluating effectiveness of interventions, and enacting harm-prevention policies. Another limitation was that substance use and mental health treatment were each analyzed as only one dichotomous item. A better understanding of substance use patterns and treatment would be achieved with more comprehensive differentiation of types and amounts of help obtained.
Conclusion
This study’s findings that people using stimulants commonly also use other substances, and that people whose polysubstance use includes nonprescribed opioids have especially poor health outcomes, suggest that intervention is needed at multiple levels. This includes increasing public awareness of the dangers of polysubstance use through effective educational efforts that provide clear communication about harms of mixing substances (SAMHSA, 2014). Raising awareness among first responders, such as emergency medical technicians and emergency department staff, about the likelihood and negative effects of polysubstance use can also help reduce the harmful consequences of mixing stimulants and other substances on patients’ health and well-being.
Treatment providers are typically trained to diagnose and treat disorders separately even though there is growing awareness that multimorbidity, such as polysubstance use with co-occurring mental health conditions, is the norm in clinical practice and represents more than a simple occurrence of simultaneous problems. However, treating each substance use problem and its medical and mental health correlates in isolation may fail to adequately address these challenging clinical situations in their full complexity. Studies support the adoption of gradually stepped-up treatments, such that polysubstance use patients are first stabilized to achieve abstinence, and then interventions for comorbid problems are offered later (Hagen et al., 2017). These same studies found that patients treated for polysubstance use showed improved life satisfaction, executive functioning, and psychological distress following 1 year of abstinence (Hagan et al., 2017). This knowledge may provide hope for patients (thereby reducing treatment dropout) and treatment providers in that it highlights the benefits of sustained abstinence.
Our findings on harms of using nonprescribed opioids in combination with stimulants and other substances are consistent with findings that more than 90% of opioid overdose deaths also involve non-opioid drugs. The near ubiquity of multiple drugs in opioid overdose deaths is also true of deaths caused by drugs other than opioids (DuPont, 2018). DuPont observes that sustained recovery from polysubstance use can be achieved in treatments such as state physician health programs, which involve comprehensive evaluation, high-quality initial treatment, and extensive recovery support, such as 12-step groups, including Al-Anon for family members (McLellan et al., 2008; Merlo et al., 2016). Clinical research is critically needed to further inform targeted treatments for polysubstance use to prevent harm, promote recovery, and lessen incurred treatment burdens and costs for individuals and society.
Footnotes
This research was supported by the National Institute on Drug Abuse (R01 DA15363 and R01 DA14340) and a Veterans Affairs Health Services Research and Development grant (RCS 00-001).
References
- Baggio S., Deline S., Studer J., N’Goran A., Mohler-Kuo M., Daeppen J. B., Gmel G. Concurrent versus simultaneous use of alcohol and non-medical use of prescription drugs: Is simultaneous use worse for mental, social, and health issues? Journal of Psychoactive Drugs. 2014;46:334–339. doi: 10.1080/02791072.2014.921747. doi:10.1080/02791072.2014.921747. [DOI] [PubMed] [Google Scholar]
- Baldini A., Von Korff M., Lin E. H. B. A review of potential adverse effects of long-term opioid therapy: A practitioner’s guide. The Primary Care Companion for CNS Disorders. 2012;14(3) doi: 10.4088/PCC.11m01326. PCC.11m01326. doi:10.4088/PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin R., Trescot A. M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11(Supplement 2):S105–S120. [PubMed] [Google Scholar]
- Bhalla I. P., Stefanovics E. A., Rosenheck R. A. Clinical epidemiology of single versus multiple substance use disorders: Polysubstance use disorder. Medical Care. 2017;55(Supplement):S24–S32. doi: 10.1097/MLR.0000000000000731. doi:10.1097/MLR.0000000000000731. [DOI] [PubMed] [Google Scholar]
- Blonigen D. M., Bui L., Britt J. Y., Thomas K. M., Timko C. Internalizing and externalizing personality subtypes predict differences in functioning and outcomes among veterans in residential substance use disorder treatment. Psychological Assessment. 2016;28:1186–1197. doi: 10.1037/pas0000250. doi:10.1037/pas0000250. [DOI] [PubMed] [Google Scholar]
- Blonigen D. M., Hicks B. M., Krueger R. F., Patrick C. J., Iacono W. G. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35:637–648. doi: 10.1017/S0033291704004180. doi:10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. M., Curran G., Han X., Wright P., Frith S., Leukefeld C., Carlson R. G. Longitudinal relationship between psychological distress and multiple substance use: Results from a three-year multisite natural-history study of rural stimulant users. Journal of Studies on Alcohol and Drugs. 2010;71:258–267. doi: 10.15288/jsad.2010.71.258. doi:10.15288/jsad.2010.71.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. L., Huang D., Evans E., Hser Y. I. Polydrug use and implications for longitudinal research: Ten-year trajectories for heroin, cocaine, and methamphetamine users. Drug and Alcohol Dependence. 2008;96:193–201. doi: 10.1016/j.drugalcdep.2008.01.021. doi:10.1016/j.drugalcdep.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. L., O’Brien A., von Mayrhauser C., Anglin M. D. Methamphetamine use behaviors and gender differences. Addictive Behaviors. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. doi:10.1016/S0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Calcaterra S. L., Keniston A., Blum J., Crume T., Binswanger I. A. The association between stimulant, opioid, and multiple drug use on behavioral health care utilization in a safety-net health system. Substance Abuse. 2015;36:407–412. doi: 10.1080/08897077.2014.996697. doi:10.1080/08897077.2014.996697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Y., Crum R. M., Martins S. S., Kaufmann C. N., Strain E. C., Mojtabai R. Patterns of concurrent substance use among nonmedical ADHD stimulant users: Results from the National Survey on Drug Use and Health. Drug and Alcohol Dependence. 2014;142:86–90. doi: 10.1016/j.drugalcdep.2014.05.022. doi:10.1016/j.drugalcdep.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian D. R., Huber A., Brecht M.-L., McCann M. J., Marinelli-Casey P., Lord R. H.. . .Lu T.-H. & the Methamphetamine Treatment Project. Methamphetamine users entering treatment: Characteristics of the methamphetamine treatment project sample. Substance Use & Misuse. 2007;42:2207–2222. doi: 10.1080/10826080701209341. doi:10.1080/10826080701209341. [DOI] [PubMed] [Google Scholar]
- Chung T., Kim K. H., Hipwell A. E., Stepp S. D. White and black adolescent females differ in profiles and longitudinal patterns of alcohol, cigarette, and marijuana use. Psychology of Addictive Behaviors. 2013;27:1110–1121. doi: 10.1037/a0031173. doi:10.1037/a0031173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton W. M., Volkow N. D. Abuse of prescription drugs and the risk of addiction. Drug and Alcohol Dependence. 2006;83(Supplement 1):S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. doi:10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Connor J. P., Gullo M. J., Chan G., Young R. M., Hall W. D., Feeney G. F. Polysubstance use in cannabis users referred for treatment: Drug use profiles, psychiatric comorbidity and cannabis-related beliefs. Frontiers in Psychiatry. 2013;4:79. doi: 10.3389/fpsyt.2013.00079. doi:10.3389/fpsyt.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. P., Gullo M. J., White A., Kelly A. B. Polysubstance use: diagnostic challenges, patterns of use and health. Current Opinion in Psychiatry. 2014;27:269–275. doi: 10.1097/YCO.0000000000000069. doi:10.1097/YCO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- DuPont R. L. The opioid epidemic is an historic opportunity to improve both prevention and treatment. Brain Research Bulletin. 2018;138:112–114. doi: 10.1016/j.brainresbull.2017.06.008. doi:10.1016/j.brainresbull.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Eriksen J., Sjøgren P., Bruera E., Ekholm O., Rasmussen N. K. Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain. 2006;125:172–179. doi: 10.1016/j.pain.2006.06.009. doi:10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hagen E., Erga A. H., Hagen K. P., Nesvåg S. M., McKay J. R., Lundervold A. J., Walderhaug E. One-year sobriety improves satisfaction with life, executive functions and psychological distress among patients with polysubstance use disorder. Journal of Substance Abuse Treatment. 2017;76:81–87. doi: 10.1016/j.jsat.2017.01.016. doi:10.1016/j.jsat.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Hanna R. C., Perez J. M., Ghose S. Cannabis and development of dual diagnoses: A literature review. American Journal of Drug and Alcohol Abuse. 2017;43:442–455. doi: 10.1080/00952990.2016.1213273. doi:10.1080/00952990.2016.1213273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn D. D. Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems. 1997;44:174–199. doi:10.2307/3096941. [Google Scholar]
- Hedden S. L., Kennet J., Lipari R., Medley G., Tice P. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2015. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. [Google Scholar]
- Hedden S. L., Martins S. S., Malcolm R. J., Floyd L., Cavanaugh C. E., Latimer W. W. Patterns of illegal drug use among an adult alcohol dependent population: Results from the National Survey on Drug Use and Health. Drug and Alcohol Dependence. 2010;106:119–125. doi: 10.1016/j.drugalcdep.2009.08.002. doi:10.1016/j.drugalcdep.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck D. M., Brecht M. L., Lovinger K., Raihan A., Christou D., Sheaff P. Poly-drug and marijuana use among adults who primarily used methamphetamine. Journal of Psychoactive Drugs. 2013;45:132–140. doi: 10.1080/02791072.2013.785824. doi:10.1080/02791072.2013.785824. [DOI] [PubMed] [Google Scholar]
- Kaye S., McKetin R., Duflou J., Darke S. Methamphetamine and cardiovascular pathology: A review of the evidence. Addiction. 2007;102:1204–1211. doi: 10.1111/j.1360-0443.2007.01874.x. doi:10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- Kedia S., Sell M. A., Relyea G. Mono- versus polydrug abuse patterns among publicly funded clients. Substance Abuse Treatment, Prevention, and Policy. 2007;2:33. doi: 10.1186/1747-597X-2-33. doi:10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. J., Robinson L. D., Baker A. L., Deane F. P., McKetin R., Hudson S., Keane C. Polysubstance use in treatment seekers who inject amphetamine: Drug use profiles, injecting practices and quality of life. Addictive Behaviors. 2017;71:25–30. doi: 10.1016/j.addbeh.2017.02.006. doi:10.1016/j.addbeh.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. doi:10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S. E., Cranford J. A., Morales M., Young A. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: Prevalence, correlates, and consequences. Journal of Studies on Alcohol. 2006;67:529–537. doi: 10.15288/jsa.2006.67.529. doi:10.15288/jsa.2006.67.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R., Lubman D. I., Baker A. L., Dawe S., Ali R. L. Dose-related psychotic symptoms in chronic methamphetamine users: Evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70:319–324. doi: 10.1001/jamapsychiatry.2013.283. doi:10.1001/jamapsychiatry.2013.283. [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Luborsky L., Woody G. E., O’Brien C. P. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. doi:10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Merlo L. J., Campbell M. D., Skipper G. E., Shea C. L., DuPont R. L. Outcomes for physicians with opioid dependence treated without agonist pharmacotherapy in physician health programs. Journal of Substance Abuse Treatment. 2016;64:47–54. doi: 10.1016/j.jsat.2016.02.004. doi:10.1016/j.jsat.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Napper L. E., Fisher D. G., Johnson M. E., Wood M. M. The reliability and validity of drug users’ self reports of amphetamine use among primarily heroin and cocaine users. Addictive Behaviors. 2010;35:350–354. doi: 10.1016/j.addbeh.2009.12.006. doi:10.1016/j.addbeh.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Rockville, MD: Author; 2012. Principles of drug addiction treatment: A research-based guide. [Google Scholar]
- Olière S., Joliette-Riopel A., Potvin S., Jutras-Aswad D. Modulation of the endocannabinoid system: Vulnerability factor and new treatment target for stimulant addiction. Frontiers in Psychiatry. 2013;4:109. doi: 10.3389/fpsyt.2013.00109. doi:10.3389/fpsyt.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar J. J., Davies S., Ompad D. C., Cleland C. M., Weitzman M. Powder cocaine and crack use in the United States: An examination of risk for arrest and socioeconomic disparities in use. Drug and Alcohol Dependence. 2015;149:108–116. doi: 10.1016/j.drugalcdep.2015.01.029. doi:10.1016/j.drugalcdep.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek L. H., Chan G. C. K., White A., Connor J. P., Baker P. J., Saunders J. B., Kelly A. B. Concurrent and simultaneous polydrug use: Latent class analysis of an Australian nationally representative sample of young adults. Frontiers in Public Health. 2013;1:61. doi: 10.3389/fpubh.2013.00061. doi:10.3389/fpubh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman M. S., Kerr W. C. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcoholism: Clinical and Experimental Research. 2015;39:872–879. doi: 10.1111/acer.12698. doi:10.1111/acer.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies; 2010. Reliability of key measures in the National Survey on Drug Use and Health. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2014. The DAWN Report: Emergency department visits involving methamphetamine: 2007 to 2011. [PubMed] [Google Scholar]
- Tice P. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Substance use among 12th grade aged youths by dropout status. [PubMed] [Google Scholar]
- Tomczyk S., Isensee B., Hanewinkel R. Latent classes of polysubstance use among adolescents–a systematic review. Drug and Alcohol Dependence. 2016;160:12–29. doi: 10.1016/j.drugalcdep.2015.11.035. doi:10.1016/j.drugalcdep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Wang J., Carlson R. G., Falck R. S., Siegal H. A., Rahman A., Li L. Respondent-driven sampling to recruit MDMA users: A methodological assessment. Drug and Alcohol Dependence. 2005;78:147–157. doi: 10.1016/j.drugalcdep.2004.10.011. doi:10.1016/j.drugalcdep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Wang L., Min J. E., Krebs E., Evans E., Huang D., Liu L., Nosyk B. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. International Journal on Drug Policy. 2017;49:32–40. doi: 10.1016/j.drugpo.2017.07.009. doi:10.1016/j.drugpo.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb D., Kerr T., Nosyk B., Strathdee S., Montaner J., Wood E. The temporal relationship between drug supply indicators: An audit of international government surveillance systems. BMJ Open. 2013;3(9):e003077. doi: 10.1136/bmjopen-2013-003077. doi:10.1136/bmjopen-2013-003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead E. L., Booth B. M., Timko C., Tjemsland A., Han X., Cucciare M. A. Journal of Adult Development. Advance online publication; 2018. Longitudinal health outcomes and treatment utilization among emerging, early-mid, and older rural adults using stimulants. doi:10.1007/s10804-018-9309-x. [Google Scholar]