Abstract
The role of NOTCH signaling in adipogenesis is highly controversial, with data indicating null, positive or negative effects on this differentiation process. We hypothesize that these contradictory results could be due to the different global NOTCH signaling levels obtained in different experimental settings, because of a specific modulation of NOTCH receptors’ activity by their ligands. We have previously demonstrated that DLK1 and DLK2, two non-canonical NOTCH1 ligands that inhibit NOTCH1 signaling in a dose-dependent manner, modulate the adipogenesis process of 3T3-L1 preadipocytes. In this work, we show that over-expression of any of the four NOTCH receptors enhanced adipogenesis of 3T3-L1 preadipocytes. We also determine that DLK proteins inhibit not only the activity of NOTCH1, but also the activity of NOTCH2, 3 and 4 receptors to different degrees. Interestingly, we have observed, by different approaches, that NOTCH1 over-expression seems to stimulate the differentiation of 3T3-L1 cells towards a brown-like adipocyte phenotype, whereas cells over-expressing NOTCH2, 3 or 4 receptors or DLK proteins would rather differentiate towards a white-like adipocyte phenotype. Finally, our data also demonstrate a complex feed-back mechanism involving Notch and Dlk genes in the regulation of their expression, which suggest that a precise level of global NOTCH expression and NOTCH-dependent transcriptional activity of specific targets could be necessary to determine the final phenotype of 3T3-L1 adipocytes.
Introduction
NOTCH signaling is a key molecular pathway involved in several biological processes, including adipogenesis. It participates in a complex network of several signaling pathways that modulate the conversion of preadipocytes or mesenchymal stem cells into mature adipocytes. However, the role of NOTCH signaling in adipogenesis is highly controversial, and while some authors have claimed it to be dispensable for adipocyte differentiation1, others have shown positive or negative roles for NOTCH signaling in the adipogenesis process2–7. In addition, other works have reported a role for NOTCH signaling in energy metabolism and adipocyte browning8–11. The modulation of NOTCH signaling aimed at inhibiting the generation of white fat cells and stimulating the generation of brown adipocytes may constitute a promising strategy to regulate fat mass in humans.
Mammals possess four NOTCH receptors and five canonical ligands (JAGGED1 and 2, and DLL1, 3 and 4). The activation of NOTCH receptors is achieved by the interaction of the DSL (Delta-Serrate-Lag-2) N-terminal domain of a canonical ligand with specific extracellular EGF repeats of NOTCH receptors. Additional proteolytic events release the intracellular active domain of NOTCH receptors (NICD), which is relocated to the nucleus, and binds to a protein called CSL/RBP-Jk/CBF1 and to other co-activators to modulate the expression of several transcription factors, including those belonging to the HES/HEY family12–15.
The NOTCH protein family also includes some non-canonical ligands that inhibit NOTCH signaling, such as DLK1(Delta-like 1 homolog) and DLK2 (Delta-like 2 homolog)16,17, EGFL7 (Epidermal growth factor-like protein 7)18, or DNER (Delta/Notch Like EGF Repeat Containing)19. DLK1 and DLK2 are two homologous transmembrane proteins with six extracellular EGF-like repeats that interact with the NOTCH1 receptor and function as NOTCH signaling inhibitors20–22. DLK1 and DLK2 lack a DSL domain, although both possess a DOS domain that is postulated to function by competing with the canonical NOTCH receptor ligands23.
Accumulated evidence indicates that Dlk1 is involved in several cell differentiation processes, including adipogenesis. Thus, several works, some of them performed in Dlk1-deficient and transgenic mice, point to Dlk1 as an inhibitor of adipogenesis22,24–28. Several research groups, including ours, have demonstrated that DLK1 over-expression inhibits 3T3-L1 adipogenesis, whereas enforced decrease in Dlk1 expression enhanced this differentiation process. Our research group furthermore demonstrated that Dlk2 also modulates adipogenesis of 3T3-L1 cells17. On the other hand, recent studies implicate Dlk1 in the control of whole body metabolism29,30, the onset of diabetes in humans31,32, and adipocyte browning33,34.
Several mechanisms have been proposed to explain the action of DLK proteins on 3T3-L1 adipogenesis35–41. However, probably the most important fact revealed by our and other research groups is that both proteins interact with the NOTCH1 receptor and function as inhibitory non-canonical ligands of NOTCH1 signaling in a dose-dependent manner7,21,35–43. We then hypothesized that these proteins could regulate adipogenesis and adipocyte phenotype by generating defined levels of NOTCH1 signaling, leading or not to the progression of this differentiation process. However, in our view, to achieve this precise level of NOTCH signaling, DLK proteins should not only modulate NOTCH1 activity, but also the activity of the other three NOTCH receptors.
In this work, we show that over-expression of any of the four NOTCH receptors enhanced the adipogenic potential of 3T3-L1 preadipocytes, and that DLK proteins can inhibit the activity of NOTCH1, 2, 3 and 4 receptors to different degrees. Interestingly, we have demonstrated by performing different assays that NOTCH1 over-expression drives differentiation of 3T3-L1 cells towards a brown-like adipocyte phenotype, whereas preadipocytes over-expressing NOTCH2, 3 or 4 receptors or DLK proteins develop a gene expression profile and phenotype more similar to white adipocytes. Finally, we also observed that over-expression of any of the four NOTCH receptors and their signaling and the over-expression of any DLK protein affect the expression levels of the others to different degrees. To sum up, all these data suggest the existence of a complex feedback regulation mechanism involving the expression of all Notch and Dlk genes that may lead to a precise level of NOTCH signaling to allow 3T3-L1 preadipocytes to differentiate or not to a particular adipocyte phenotype.
Results
Over-expression of each one of the NOTCH receptors enhances adipogenesis of 3T3-L1 preadipocytes
The role of NOTCH signaling in the adipogenesis process is a highly controversial topic. Rather than attributing the contradictory results to technical problems or cell line differences, we hypothesized that they could be related to the possibility that adipogenesis in response to extracellular signals may proceed or not given a precise level of global NOTCH receptor activation22.
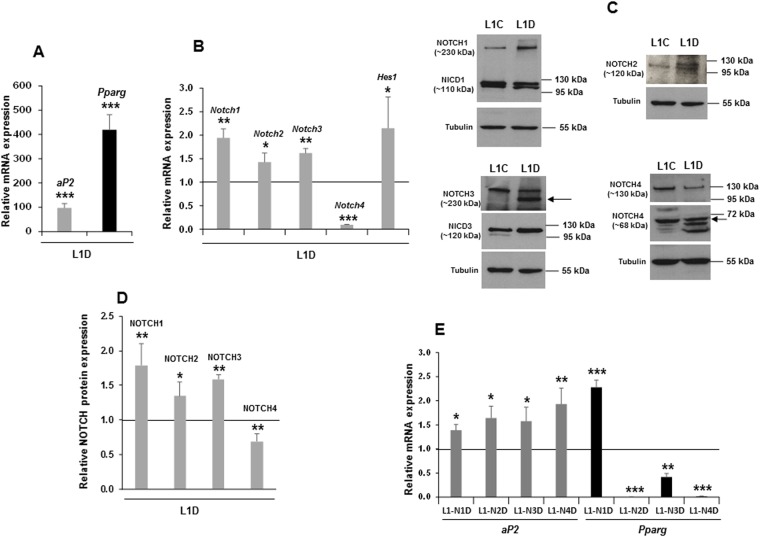
We first analyzed here the basal mRNA expression levels of Notch genes in 3T3-L1 cells and found that these cells express similar levels of the four Notch genes (Supplementary Figure 1A). We observed that the induction of adipogenesis in 3T3-L1 cells increased aP2 and Pparg mRNA expression (Fig. 1A). In addition, whereas the expression of Notch1, Notch2, Notch3, and Hes1 mRNAs increased with the adipogenic treatment, the expression of Notch4 mRNA decreased (Fig. 1B). Western blot analysis also demonstrated that NOTCH4 protein expression decrease at the end of the adipogenic process, whereas the expression of NOTCH1, 2 and 3 increases (Fig. 1C,D).
Figure 1.
The stable over-expression of each one of the Notch genes on 3T3-L1 cells enhances adipogenesis. (A) qRT-PCR analysis of the relative mRNA expression levels of the adipocyte markers aP2 and Pparg in differentiated 3T3-L1 cells. (B) qRT-PCR analysis of the relative Notch and Hes1 mRNA expression levels in differentiated 3T3-L1 cells. Representative Western blots (C) and densitometric analysis (D) of each NOTCH receptor expression in 3T3-L1 adipocytes compared to non-differentiated cells. In the case of the Notch1 gene transfectant intracellular NOTCH1 (NICD1) and complete NOTCH1 protein signals are shown. In the case of the Notch2 gene transfectant, the intracellular NOTCH2 (NICD2) protein signal is shown. For the Notch3 gene transfectant, the complete and the intracellular NOTCH3 (NICD3) protein signals are shown. Finally, for the Notch4 gene transfectant, the complete and the intracellular NOTCH4 (NICD4) protein signals are shown. (E) qRT-PCR analysis of the relative aP2 and Pparg mRNA expression levels in differentiated stable Notch1 gene transfectant (L1-N1D), stable Notch2 gene transfectant (L1-N2D), stable Notch3 gene transfectant (L1-N3D), and stable gene Notch4 transfectant (L1-N4D). Data from qRT-PCR assays were previously normalized to P0 mRNA expression levels. The expression of alpha-tubulin was used as a loading control in all Western blots to normalize expression data. Blot signals from empty vector and over-expressing cells were cropped from original blots and delineated with horizontal white spaces (original blots for each protein signal are shown in Supplementary Figure 4). The fold activation or inhibition was calculated relative to the seven-day differentiated non-transfected or empty-vector-transfected cells, which was set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance calculated by Student’s t-tests is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Next, pools of 3T3-L1 preadipocytes stably over-expressing each one of the Notch genes were used to investigate the effects of each NOTCH receptor on 3T3-L1 adipogenesis. The over-expression of Notch genes and their proteins was confirmed by qRT-PCR and Western blot analysis (Supplementary Figure 1(B–I)). We have observed that over-expression of any Notch gene enhanced the adipogenic potential of these cells, as indicated by an increase in aP2 expression (Fig. 1E). Pparg expression also increased in cells over-expressing Notch1 gene, but its levels drastically decreased in differentiated cells that over-expressed Notch2, Notch3 and Notch4 genes (Fig. 1E).
NOTCH receptors and DLK proteins modulate 3T3-L1 adipocyte browning
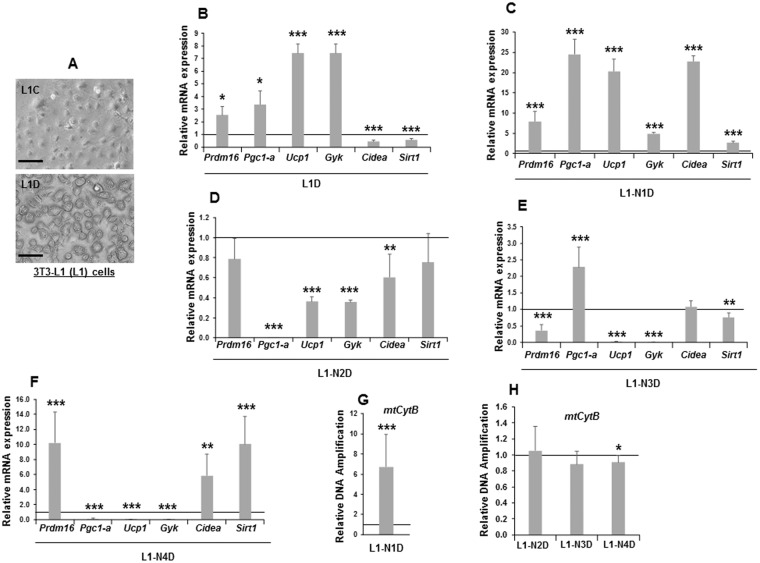
3T3-L1 preadipocytes have been always referred to as a typical cellular model of white adipogenesis. However, some authors described that 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages44. In this work, we have observed that the induction of adipogenesis in 3T3-L1 cells generates adipocytes (L1D) with multilocular lipid droplets (Fig. 2A), and increases the expression of the brown adipocyte and mitochondrial biogenesis markers Pgc1a, Ucp1, Gyk and Prdm16 (Fig. 2B). We also observed a decrease in Cidea expression, which might be associated with the multilocular droplets observed45. However, the expression of the mitochondrial biogenesis marker Sirt1 decreased in 3T3-L1 differentiated cells (Fig. 2B).
Figure 2.
Effects of stable over-expression of each one of the Notch genes in 3T3-L1 adipocyte browning. (A) Representative microscopy images (400X magnification) of 3T3-L1 adipocytes (L1D) seven days after standard adipogenic induction (48 hours with IBMX and dexamethasone, and 5 days with insulin, see Methods) and non-treated 3T3-L1 cells (L1C). Scale bar (250 μm) is shown. (B) qRT-PCR mRNA expression analysis of the brown adipocyte markers Ucp1, Pgc1a, Gyk, Prdm16, Cidea and Sirt1 in seven-day-differentiated 3T3-L1 cells. qRT-PCR analysis of the relative mRNA expression levels of Ucp1, Pgc1a, Gyk, Prdm16, Cidea and Sirt1 markers in seven-day differentiated Notch1 gene transfectant (L1-N1D) (C), Notch2 gene transfectant (L1-N2D) (D), Notch3 gene transfectant (L1-N3D) (E), Notch4 gene transfectant (L1-N4D) (F). Data from qRT-PCR assays were previously normalized to P0 mRNA expression levels. qPCR analysis of mitochondrial CytB DNA amplification (related to genomic ApoB DNA amplification, see Methods) in seven-day differentiated 3T3-L1 cells over-expressing Notch1 gene (G) and Notch2, Notch3 or Notch4 genes (H). The fold activation or inhibition was calculated relative to the seven-day differentiated non-transfected or empty-vector-transfected cells, which was set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance of the Student’s t-tests performed is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Taking these results into consideration, we were interested in exploring whether the stable over-expression of each one of the four NOTCH receptors could also affect the expression levels of the brown adipocyte genes after induction of adipogenesis (Fig. 2). Compared with differentiated control cells, Notch1 over-expressing adipocytes showed higher expression levels of the brown adipocyte and the mitochondrial biogenesis markers Pgc1a, Ucp1, Gyk, Prdm16, Cidea and Sirt1 (Fig. 2C). Likewise, Notch2 over-expression diminished Pgc1a, Ucp1, Gyk, and Cidea expression, although no significant differences were observed in Prdm16 and Sirt1 expression (Fig. 2D). Notch3 over-expression diminished the expression of Ucp1, Gyk, Prdm16 and Sirt1 in differentiated cells, although Pgc1a levels were increased and no significant differences were observed in Cidea expression (Fig. 2E). Finally, Notch4 over-expression diminished the expression levels of Pgc1a, Ucp and Gyk in differentiated cells, but it increased the expression of Prdm16, Cidea and Sirt1 (Fig. 2F). We also analyzed the amplification levels of mitochondrial CytB gene in these differentiated stable transfectants (see Methods), which indicates the rate of mitochondrial biogenesis. The over-expression of Notch1 increases the amplification level of mitochondrial CytB gene in differentiated cells (Fig. 2G), whereas the over-expression of Notch4 decreases the amplification of this gene (Fig. 2H). No significant differences in mitochondrial CytB gene amplification were found when cells over-express Notch2 or Notch3 genes (Fig. 2H).
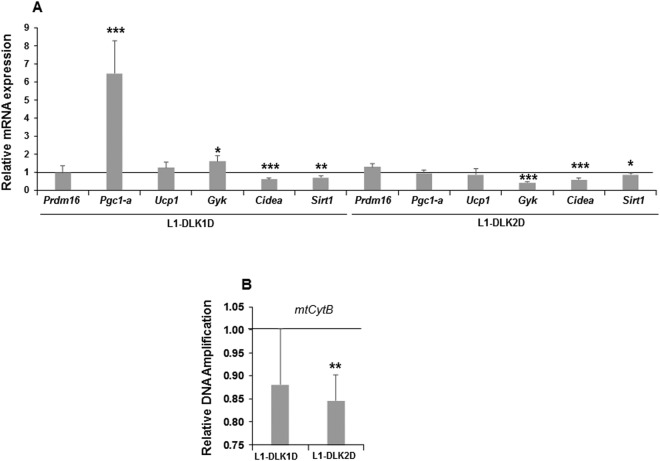
Being inhibitors of NOTCH signaling, we expected that stable over-expression of Dlk1 or Dlk2 would affect also the expression of these markers (Fig. 3). Thus, we generated 3T3-L1 cells over-expressing Dlk1 or Dlk2 (Supplementary Figure 2) to study this hypothesis. We observed that Dlk1 over-expression significantly increased the expression of Pgc1a and Gyk, decreased that of Cidea and Sirt1, and left that of Ucp1 and Prdm16 unaffected (Fig. 3A). On the other hand, cells stably over-expressing Dlk2 showed a decrease in Gyk, Cidea, Sirt1 expression, although no significant differences were observed in Pgc1a, Prdm16 and Ucp1 expression (Fig. 3A). We also analyzed the amplification levels of mitochondrial CytB gene amplification in these stable transfectants (see Methods). The over-expression of Dlk2 decreases the amplification levels of this gene (Fig. 3B). No significant differences in mitochondrial CytB gene amplification were found when cells over-express Dlk1 gene (Fig. 3B).
Figure 3.
Effects of stable over-expression of Dlk1 or Dlk2 genes in 3T3-L1 adipocyte browning. (A) qRT-PCR analysis of the relative mRNA expression levels of Ucp1, Pgc1a, Gyk, Prdm16, Cidea and Sirt1 markers in seven-day differentiated Dlk1 gene transfectant (L1-DLK1D) and Dlk2 gene transfectant (L1-DLK2D). Data from qRT-PCR assays were previously normalized to P0 mRNA expression levels. (B) qPCR analysis of mitochondrial CytB DNA amplification (related to genomic ApoB DNA amplification, see Methods) in seven-day differentiated 3T3-L1 cells over-expressing Dlk1 or Dlk2 genes. The fold activation or inhibition was calculated relative to the seven-day differentiated non-transfected or empty-vector-transfected cells, which was set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance of the Student’s t-tests performed is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
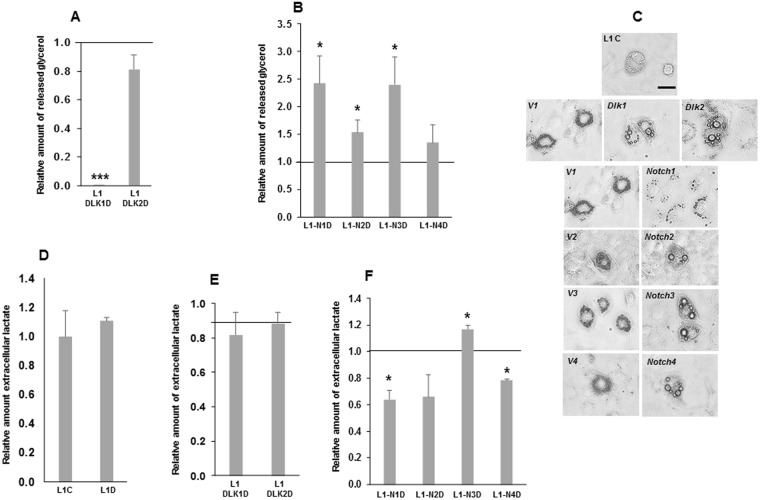
To estimate the cytoplasmic lipid accumulation, we measured the amount of glycerol released from the different transfected adipocytes by inducing lipolysis with the β-adrenergic agonist isoproterenol (Fig. 4). 3T3-L1 transfectants over-expressing Dlk1 released lower glycerol levels into the medium than control cells (Fig. 4A), whereas the transfectants over-expressing Notch1, Notch2 or Notch3 showed higher levels of glycerol release when compared with their respective control cells (Fig. 4B). No significant differences were observed in cells over-expressing Dlk2 or Notch4 (Fig. 4A,B). We also observed that 80–90% of 3T3-L1 adipocytes over-expressing Dlk1, Dlk2, Notch2, Notch3 or Notch4 genes contained larger lipid droplets than their corresponding controls or non-transfected 3T3-L1 differentiated cells (L1C) (Fig. 4C). However, differentiated Notch1 over-expressing cells, which show an increase in the number and size of adipocytes compared with their controls, exhibited a reduction in the multilocular lipid droplet size (Fig. 4C).
Figure 4.
Release of glycerol and lactate to the extracellular medium in 3T3-L1 adipocytes over-expressing Dlk or Notch genes. Relative levels of glycerol released to the extracellular medium in response to isoproterenol from Dlk1 or Dlk2 genes (A), and Notch1, 2, 3 or 4 genes (B) over-expressing adipocytes. (C) Representative microscopy images (400X magnification) of non-transfected (L1C) and transfected 3T3-L1 adipocytes (Empty vectors V1, V2, V3 and V4, and their corresponding over-expressing transfectant) under study. The size of their lipid droplets is showed. Scale bar (80 μm) is shown. Relative levels of lactate in the culture supernatant of differentiated non-transfected 3T3-L1 cells (D), Dlk1 or Dlk2 genes over-expressing adipocytes (E), and each of the Notch genes over-expressing adipocytes (F). The fold activation or inhibition was calculated relative to the seven-day differentiated non-transfected or empty-vector-transfected cells, which was set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance calculated by Student’s t-tests is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
To further characterize at a functional level the different types of adipocytes derived from each transfectant, we also analysed the lactate released into the extracellular medium, which indirectly provides the extra-cellular acidification rate (ECAR) (Fig. 4). Compared to their corresponding controls, differentiated 3T3-L1 transfectants over-expressing Notch1 or Notch4 showed lower levels of lactate released to the extracellular medium, whereas the differentiated transfectants over-expressing Notch3 released higher levels of lactate (Fig. 4F). No significant differences were observed in cells over-expressing Notch2, Dlk1 or Dlk2, or in non-transfected 3T3-L1 differentiated cells (Fig. 4D,E,F).
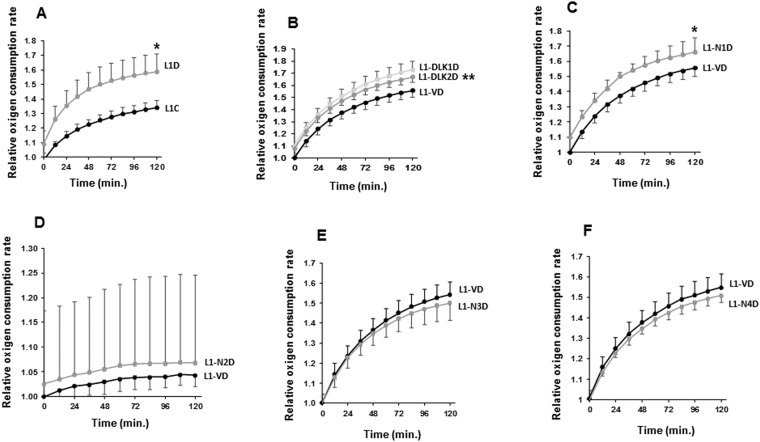
Finally, we measured the rate of oxygen consumption (OCR), which is a measure of cellular respiration and mitochondrial function, by using a phosphorescent oxygen probe (Fig. 5). As shown, differentiation of 3T3-L1 cells into adipocytes increased the oxygen consumption rate (OCR) (Fig. 5A). Besides, we observed higher levels of OCR in Dlk1, Dlk2, or Notch1 transfectants when compared with their respective controls (Fig. 5B,C). On the other hand, the OCR change in 3T3-L1 transfectants over-expressing Notch2, Notch3 and Notch4 was not significant (Fig. 5D,E,F).
Figure 5.
Oxygen consumption rate (OCR) in 3T3-L1 adipocytes over-expressing Dlk and Notch genes. Analysis of the relative oxygen consumption rate (OCR) in non-transfected 3T3-L1 cells (A) and 3T3-L1 cells over-expressing Dlk1 or Dlk2 genes (B), and Notch1 (C), Notch2 (D), Notch3 (E) or Notch4 genes (F). The fold activation or inhibition was calculated relative to the time 0 of seven-day differentiated non-transfected or empty-vector-transfected cells, which was set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance calculated by Student’s t-tests is indicated at 120 minutes (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
The proteins DLK1 and DLK2 inhibit the activation and signaling of each one of the four mammalian NOTCH receptors
Since both DLK proteins inhibit adipogenesis of 3T3-L1 cells (Supplementary Figure 2) and inhibit NOTCH1 activation and signaling36, we considered important to study here whether DLK1 or DLK2 could block the observed proadipogenic effects of the over-expression of NOTCH1 in 3T3-L1 cells. For this purpose, we performed standard adipogenic assays with 3T3-L1 cells stably over-expressing the NOTCH1 receptor, cultured with control conditioned media or conditioned media containing sDLK1 or sDLK2 proteins during the entire adipogenic treatment. Soluble DLK proteins in the media were able to diminish the increment in the adipogenic potential of 3T3-L1 cells generated by the over-expression of Notch1, as indicated by a decrease in aP2 and Pparg expression (Supplementary Figure 3B). These results indicate that soluble DLK proteins can inhibit the 3T3-L1 adipogenesis process through the inhibition of NOTCH1 signaling.
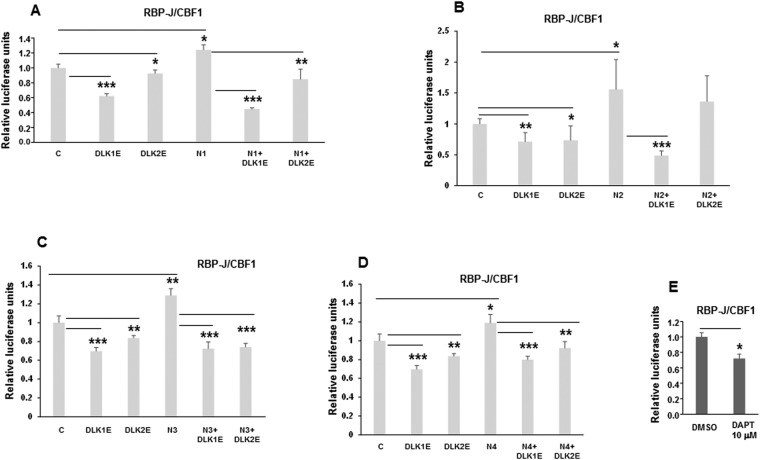
Following this observation, we decided to study whether DLK proteins could also affect the activation of the other three NOTCH receptors. We analyzed NOTCH-dependent transcriptional activity by luciferase assays in Balb/c14 cells transiently expressing the extracellular regions of DLK1 (DLK1E) or DLK2 (DLK2E), alone or in combination with one of the four full length NOTCH receptor genes (Fig. 6). The results indicated that transient over-expression of DLK1 or DLK2 extracellular regions inhibited NOTCH1, 3 and 4 signaling to different extents (Fig. 6A,C,D). DLK1 also seemed to strongly inhibit NOTCH2 signaling, whereas no significant differences were found in NOTCH2 signaling when DLK2 was transiently over-expressed (Fig. 6B). We could observe that the grade of inhibition caused by DAPT, a gamma-secretase complex inhibitor, was similar to that observed when each DLK protein was over-expressed (Fig. 6E). These results indicate that DLK proteins are able to inhibit the activity of the four mammalian NOTCH receptors to different extents, and suggest that the effects of NOTCH receptors on adipogenesis and adipocyte browning can be modulated by membrane and soluble DLK proteins.
Figure 6.
The proteins DLK1 and DLK2 inhibit the activation of each one of the four NOTCH receptors. NOTCH transcriptional activity, as measured by gene reporter luciferase assays, was determined in Balb/c14 cells transiently co-transfected with a plasmid driving the expression of each NOTCH receptor: N1 (A), N2 (B), N3 (C) and N4 (D); and/or a plasmid driving the expression of DLK1 or DLK2 extracellular regions (A–D). (E) Analysis of NOTCH transcriptional activity in Balb/c14 cells treated with DMSO or with the inhibitor DAPT (10 micromolar). The fold-activation or inhibition in these luciferase assays is measured relative to the control (C), set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance of Student’s t-tests results is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Feedback modulation of gene expression among NOTCH receptors and DLK proteins
The inhibitory effect of DLK proteins on all NOTCH receptors’ activity, and additional published data confirming the inhibition of Dlk1 expression by NOTCH signaling4,5, suggested to us the existence of a complex feedback mechanism that would modulate the overall expression levels of DLK proteins and NOTCH receptors, as well as NOTCH activation and signaling. This feedback modulation could influence the adipogenic potential and the final adipocyte phenotype of 3T3-L1 cells. To analyze this potential feedback mechanism, we used our 3T3-L1 transfectant pools over-expressing each one of the four NOTCH receptors, the NOTCH target gene Hes1 or each one of the two DLK proteins (Figs 7 and 8).
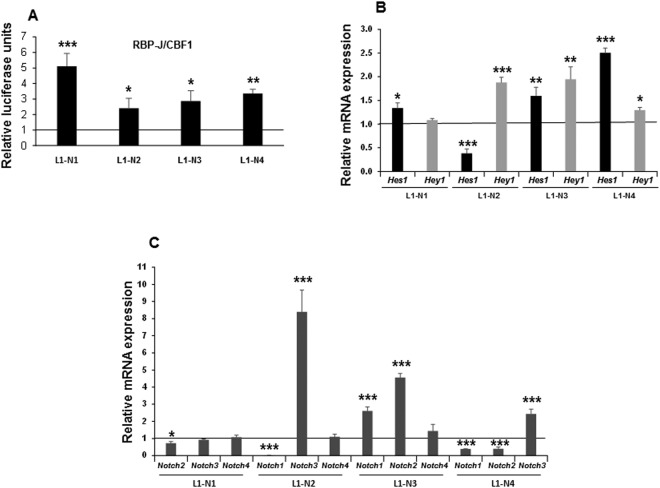
Figure 7.
NOTCH activation and signaling in 3T3-L1 cells stably over-expressing each one of the four NOTCH receptors. (A) qRT-PCR analysis of the relative Hes1 and Hey1 mRNA expression levels in the stable Notch1 gene transfectant (L1-N1), the stable Notch2 gene transfectant (L1-N2), the stable Notch3 gene transfectant (L1-N3), and the stable Notch4 gene transfectant (L1-N4). (B) NOTCH transcriptional activity, as measured by gene reporter luciferase assays, in these four Notch genes stable transfectants. (C) qRT-PCR analysis of the relative individual Notch mRNA expression levels in stable Notch1 gene transfectant (L1-N1), the stable Notch2 gene transfectant (L1-N2), the stable Notch3 gene transfectant (L1-N3), and the stable Notch4 gene transfectant (L1-N4). The relative luciferase activities were always normalized with renilla values and referred to those of control cells. Data in all qRT-PCR assays were normalized to P0 mRNA expression levels. The fold activation or inhibition in all assays is measured relative to the empty vector control, set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance of Student’s t-tests results is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
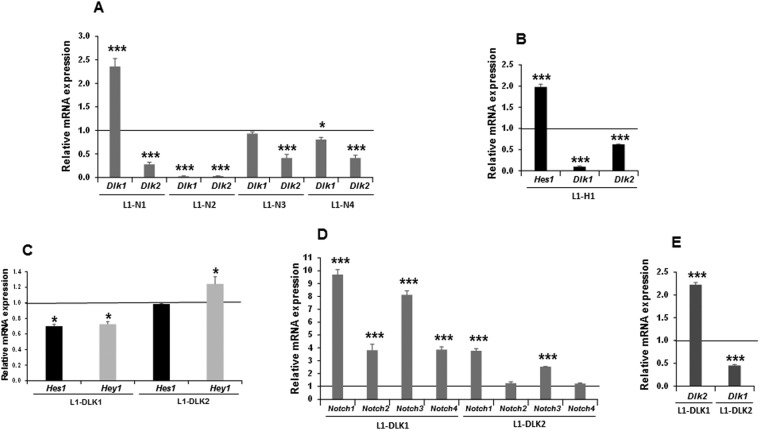
Figure 8.
Feedback modulation among Notch and Dlk gene expression in 3T3-L1 preadipocytes. (A) qRT-PCR analysis of the relative individual Dlk (B) mRNA expression levels in the stable Notch1 gene transfectant (L1-N1), the stable Notch2 gene transfectant (L1-N2), the stable Notch3 gene transfectant (L1-N3), and the stable Notch4 gene transfectant (L1-N4). (B) qRT-PCR analysis of the relative Hes1 and Dlk mRNA expression levels in the stable Hes1 gene transfectant (L1-H1). (C) qRT-PCR analysis of the relative Hes1 and Hey1 mRNA expression levels in the stable Dlk1 gene transfectant (L1-DLK1) and the stable Dlk2 gene transfectant (L1-DLK2). qRT-PCR analysis of the relative Notch (D) and Dlk (E) mRNA expression levels in the stable Dlk1 gene transfectant (L1-DLK1), and the stable Dlk2 gene transfectant (L1-DLK2). In all qRT-PCR assays, data were normalized to P0 mRNA expression levels. The fold activation or inhibition in all assays was measured relative to the empty vector, set arbitrarily at 1. Data are shown as the mean ± SD of at least three biological assays performed in triplicate. The statistical significance of Student’s t-tests results is indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
We first observed that the over-expression of any of the four NOTCH receptors resulted in an increase in NOTCH-dependent transcriptional activity (Fig. 7A), which indicated that all NOTCH receptors were transcriptionally active in our cells. As expected, stable over-expression of NOTCH1, NOTCH3 and NOTCH4 in 3T3-L1 cells increased the expression of the NOTCH signaling target genes Hes1 and Hey1. However, NOTCH2 over-expression increased Hey1, but surprisingly inhibited Hes1 expression (Fig. 7B). This fact suggested to us that changes in Notch2 expression levels might influence the expression of the other three Notch genes, which, in turn, may affect the global NOTCH signaling and, ultimately, Hes1 expression. We show in Fig. 7C that over-expression of Notch1 exerted no significant effect on the expression of Notch3 and Notch4, but caused a small but significant decrease in Notch2 expression. Notch2 over-expression caused no significant changes in Notch4 mRNA levels, but dramatically increased the expression of Notch3 and decreased that of Notch1. On the other hand, over-expression of Notch3 up-regulated the expression of Notch1 and Notch2, but did not cause significant changes in Notch4 expression. Finally, Notch4 over-expression increased the expression of Notch3, but down-regulated that of Notch1 and Notch2. Thus, a variation in the expression of only one of the Notch genes seems to modulate the expression of the others in a way that could allow cells to reach a particular stoichiometry in the levels of global NOTCH activity.
We also studied here whether the alteration of Notch expression could affect the expression of Dlk1 and Dlk2. We observed that over-expression of each Notch gene decreased Dlk2 expression in different amounts (Fig. 8A). Notch2 and Notch4 also decreased Dlk1 expression, but no significant changes were observed in Dlk1 expression levels following Notch3 over-expression. Surprisingly, Notch1 over-expression in these cells was accompanied by a significant increase in Dlk1 levels (Fig. 6A). NOTCH target genes, such as Hes1, whose expression has previously been inversely correlated with Dlk1 gene expression levels5, could mediate some of the observed effects of every NOTCH receptor signaling on Dlk1 and Dlk2 gene expression. Thus, we have observed that Hes1 over-expression in 3T3-L1 cells strongly decreased the expression of Dlk1, as published previously, and, to a lower extent, that of Dlk2 (Fig. 8B).
We show here that the over-expression of Dlk1 led to a significant decrease in Hes1 and Hey1 expression. However, despite DLK2 also inhibiting NOTCH1, 3 and 4 receptors’ transcriptional activity, no significant changes in Hes1 expression and even a slight increase in Hey1 expression were observed when Dlk2 was over-expressed in these cells (Fig. 8C). The slight effects of Dlk2 over-expression on Hes1 and Hey1 expression suggested to us that the response of 3T3-L1 cells to the inhibition of NOTCH signaling by DLK proteins could lead also to a change in the expression pattern of Notch1–4. Indeed, Dlk1 and Dlk2 over-expression led to variations in each Notch gene expression to different extents (Fig. 8D). Finally, we observed here that the over-expression of one Dlk gene affects the expression of the other (Fig. 8E), as described before21,36.
All these data suggest that the expression of Notch and Dlk genes seems to be coordinated in the cells by a mechanism that globally regulates the NOTCH transcriptional activity of each NOTCH receptor and signaling to permit or not a particular biological process, such as white or brown adipogenesis, to proceed.
Discussion
The role of NOTCH signaling in adipogenesis is still unclear. Different authors have claimed either null, positive, or negative effects on the adipogenesis of different cell lines, among them 3T3-L1 preadipocytes. Thus, some works suggested that the NOTCH signaling pathway is dispensable for adipocyte specification and differentiation from either mesenchymal or epithelial progenitors1. However, Garcés and coworkers showed that Notch1 expression and function were necessary for adipogenesis of 3T3-L1 cells2. A more recent work described that the intracellular active region of NOTCH4 also enhanced 3T3-L1 cell proliferation and adipogenesis, and decreased Dlk1 expression4. On the contrary, blocking NOTCH signalling with DAPT, an inhibitor of the gamma-secretase complex, enhances adipogenesis of differentiated mASCs at an early stage46. This effect may be due to depression of Dlk1/Pref-1 and promotion of PPAR-gamma activation, which works through the inhibition of NOTCH2-HES1 pathway by DAPT. Other authors demonstrated that the expression of the gamma-secretase complex, which activates NOTCH signaling, decreases during adipocyte differentiation, and ectopic expression of bovine PSENEN protein reduced the adipogenesis process in 3T3-L1 cells47. Urs and collaborators proposed an initial requirement of NOTCH signaling inactivation for preadipocyte cell commitment6. The adipocyte differentiation of 3T3-L1 cells was significantly reduced when cells constitutively expressed JAGGED1 or HES15. Finally, recent work indicate that increased NOTCH signaling in mouse adipocytes results in the blockage of the expansion of white adipose tissue48. We hypothesize that all these contradictory results could be attributed to the need of the cells to reach a precise level of each NOTCH receptor activation leading to a stoichiometry of global NOTCH signaling permissive or not for adipogenesis.
In this work, we studied the role of the NOTCH receptors and their non-canonical ligands, DLK1 and DLK2, in 3T3-L1 cells adipogenesis and adipocyte browning. We selected 3T3-L1 cells for these studies because they are one of the main cellular models of adipogenesis in which other authors have observed a high variability in the effects of NOTCH receptors and ligands. Our results show that stable over-expression of any of the four NOTCH receptors in 3T3-L1 preadipocytes enhances the adipogenic response of these cells. The lipolysis assays performed indicated that over-expression of the four NOTCH proteins increases the release of glycerol into the culture medium in response to isoproterenol, compared with their respective controls. Moreover, adipocytes showed different phenotypes depending on the Notch gene over-expressed. Thus, the over-expression of Notch2, Notch3 or Notch4 generated adipocytes with larger lipid droplets than controls, but Notch1-overexpressing adipocytes contained smaler multilocular lipid droplets as compared with its control cells. Thus, Notch1 overexpression, but not Notch 2–4 overexpression, seems to induce a brown-like adipocyte phenotype in 3T3-L1 cells.
An increasing number of works by us and others have confirmed that DLK1 causes the inhibition of NOTCH signaling in different cellular processes7,35,38–43. In previous works, we have demonstrated that DLK1 and DLK2 can inhibit the adipogenic process of 3T3-L1 cells17,49, and that one of the molecular partners that could mediate the function of DLK proteins in adipogenesis was the NOTCH1 receptor, whose activation and signaling was inhibited by both DLK proteins in a dose-dependent manner21,36,39,43. We hypothesized here that DLK proteins could inhibit not only the activation and signaling of NOTCH1, but that of the four mammalian NOTCH receptors to different extents, thus generating a global level of NOTCH signaling permissive or not of the adipogenesis process. In agreement with this hypothesis, in this work we have demonstrated that soluble DLK1 and DLK2 proteins, as it is also the case with the membrane variants, reduce the augmented adipogenic potential of 3T3-L1 cells that over-express the NOTCH1 receptor. These results are in agreement with recent work from other authors demonstrating that blockage of the NOTCH1 receptor by DLK1 modulates the size of adipocytes in vivo7. As expected, we found that the over-expression of Dlk1 in these cells decreases the amount of glycerol released from adipocytes into the culture medium in response to isoproterenol, which indicates that these adipocytes foster a lower lipolytic activity despite showing larger lipid droplets than control cells. Alternately, these adipocytes may have accumulated lower lipid levels throughout their adipogenic process. However, Dlk2-over expressing adipocytes also show large lipid droplets, although the release levels of glycerol were not significantly different. Moreover, we have also demonstrated that the DLK1 and DLK2 proteins inhibit the activity of each one of the four mammalian NOTCH receptors to different degrees, with the exception of NOTCH2, which was only inhibited by DLK1 but not by DLK2.
Even though 3T3-L1 preadipocytes have always been considered a well-known cellular model of white adipogenesis, Morrison and McGee observed that 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages, express Pgc1a and increase oxygen consumption and the expression of brown adipocyte genes in response to catecholamines44. In agreement with these authors, we have observed that the expression of several brown adipogenic markers is increased in 3T3-L1 adipocytes and the size and distribution of their lipid droplets is more coherent with that of brown or beige adipocytes. Despite a decreased expression of Cidea and Sirt1, differentiated 3T3-L1 cells show increased levels of Ucp1, Pgc1a, Gyk and Prdm16 markers. These results, together with the multilocular small lipid droplets observed in these cells, are more consistent with a brown-like phenotype, as other authors have described50. The Pparg transcription factor is considered both as an adipocyte marker in 3T3-L1 cells and as a factor that favors adipocyte browning by activating Ucp expression51. Therefore, the induction of Pparg expression after 3T3-L1 differentiation also supports the idea that 3T3-L1 adipocytes show a brown-like phenotype. As expected, the level of glycerol release and the respiration rate were generally higher in differentiated adipocytes than in undifferentiated 3T3-L1 cells; however, not significant differences were observed for lactate output in 3T3-L1 adipocytes44,52.
The brown adipose tissue (BAT) functions by generating heat through mitochondrial uncoupling proteins, in particular UCP1 and UCP353. The role of NOTCH signaling on brown adipogenesis is also controversial. Thus, some authors showed that inhibition of NOTCH signaling promotes browning and alleviates obesity8,9. On the contrary, Pasut and co-workers demonstrated that, subsequent to the deletion of the transcription factor PAX7 (Paired Box 7) and following acute muscle injury, NOTCH signaling promoted the differentiation of satellite cells into brown adipocytes rather than into a skeletal muscle cell phenotype10. Some works have also shown a role for DLK1 in adipocyte browning and suggest a possible involvement of DLK2 in this process. Thus, the expression of Dlk1 is high in fetal BAT and declines after birth, although Dlk1-null mice showed unaltered BAT fetal development, with an over-activation of thermogenesis in the postnatal period. As it occurs in 3T3-L1 preadipocytes, Dlk1 expression decreases after the induction of the differentiation in brown preadipocyte cell lines33. Other authors described that Dlk1 inhibits thermogenesis in brown adipocyte models34. Young women with cold-activated brown adipose tissue have lower serum DLK1 than women lacking brown adipose tissue54.
It is believed that an increase in the expression of brown-fat signature genes and in the number of mitochondria or in mitochondrial biogenesis and function are key features of BAT. Interestingly, we have observed that Notch1 over-expression in differentiated 3T3-L1 cells upregulates the expression of Ucp1, Pgc1a, Gyk, Prdm16, Cidea, Pparg and Sirt1, and there is more amplification of CytB than in control cells, whereas their expression or amplification is down-regulated or it does not change when Notch2 or Notch3 are over-expressed in these cells. In the case of Notch4 transfectants, the expression of Ucp1, Pgc1a, Gyk, and Pparg markers is down-regulated in differentiated cells, and the amplification of CytB is also lower than control. However, unexpectedely, the expression of Prdm16, Cidea and Sirt1 is increased in Notch4 transfectants. CIDEA is a multifunctional protein, highly expressed in brown adipose tisusue, with a clearly defined role in the promotion of lipid droplets in brown and white adipocytes50. These data suggest that Notch1 may promote differentiation into 3T3-L1 brown adipocytes, whereas Notch2, Notch3 and Notch4 may promote 3T3-L1 cells to differentiate toward the white phenotype. It has been described that brown adipocytes showed higher levels of OCR and ECAR when compared with control cells55. We observed that only Notch1 adipocytes showed significantly higher levels of OCR and diminished levels of lactate released into the culture medium. Our findings may imply that lactate could be transported to mitochondria and used as a fuel substrate. Interestingly, a recent study described that lactate was a major substrate for the TCA cycle in several tissues, including adipocytes and glucose feeds the TCA cycle via circulating lactate56. Besides,the increased number of mitochondria in Notch1-over-expressing cells would lead to a greater use of lactate as fuel in these cells. This suggests that only Notch1-overexpressing adipocytes could undergo functional lineage transformation toward a brown adipocyte phenotype. All these data suggest that Notch1-over-expressing transfectants could have developed smaller lipid droplets than their empty vector-transfected control adipocytes because of a higher expression of UCP1 and increased mitochondrial biogenesis, which would lead to a higher OCR and lipolysis rate.
On the other hand, we have observed that differentiated 3T3-L1 cells over-expressing Dlk1 show decreased levels of Cidea and Sirt1 and increased expression levels of Pgc1a and Gyk, although no significant changes in Ucp1 and Prdm16 expression were observed. The expression of Gyk, Cidea and Sirt1 decreases in stable Dlk2-over-expressing transfectants, but no significant changes were observed in Pgc1a, Ucp1 or Prdm16 expression. Therefore, these transfected cells, when differentiated, do not show evidence of a gene expression profile typical of brown adipose cells, despite the increase in Pgc1a and Gyk expression in Dlk1-stably transfected cells. Besides, the levels of mitochondrial CytB in Dlk2 transfectants, but not in Dlk1 transfectants, are lower than in control cells, although, as it occurs with Notch1 transfectants, both Dlk1 and Dlk2 transfectants show higher OCR than control cells. It has been described that DLK1-overexpressing mice showed increased oxygen consumption, indicating an increase in energy expenditure29. These slight and contradictory effects could be probably explained by a differential inhibition of each NOTCH receptor´activity, since each NOTCH receptor exerts different effects on the expression of these markers. We think that qualitative and quantitative differences in overall NOTCH signaling may place the cells in different states allowing them to interpret the same extracellular signals so that they differentiate or not to at least two different adipocyte phenotypes.
Finally, in this work we have also revealed the existence of a complex feedback mechanism that modulates the overall expression levels of the Notch and Dlk genes and the global NOTCH activation and signaling. Each NOTCH receptor increases the level of global NOTCH signaling and induces the expression of the NOTCH signaling targets Hes1 and Hey1, except for Notch2, which unexpectedly reduces Hes1 mRNA levels. Furthermore, stable over-expression of each one of the NOTCH receptors in 3T3-L1 cells influences the expression levels of the other Notch genes to different extents. This interplay among Notch members, specifically between Notch1 and Notch3, has been shown by other authors57,58. We have also shown here that DLK proteins inhibit each one of the four NOTCH receptors’ activity to different extents and that the over-expression of Dlk1 inhibits the global NOTCH signaling by reducing the level of expression of Hes1 and Hey1. The observed unexpected effects of stable Dlk2 over-expression on Hes1 and Hey1 suggest that the potential inhibitory effect of the stable over-expression of Dlk2 on NOTCH signaling could be achieved through other factors different from HES1 and HEY1.
NOTCH signaling can both positively and negatively regulate canonical ligand expression, such that defects in NOTCH signaling are associated with increased expression of DLL159 or DLL460. As other authors have indicated5, non-canonical ligands such as DLK1 are also regulated by NOTCH signaling. In this work we have observed that NOTCH receptors’ activity modulate Dlk1 and Dlk2 expression levels to different extents. Besides, the results presented here demonstrate that Hes1 over-expression in 3T3-L1 cells inhibits the expression of both Dlk2 and Dlk1. These data are in agreement with those of Ross and coworkers, who demonstrated that HES1 seemed to down-regulate Dlk1 expression, which would permit adipogenesis of 3T3-L1 cells to complete5.
Importantly, it has been reported that the expression of Dlk1 and Dlk2 is coordinated in 3T3-L1 cells and that DLK1 and DLK2 proteins interact and may inhibit each other’s activities21,36,39,43. Indeed, in this work, we have observed that the over-expression of one Dlk gene affects to the expression of the other. Therefore, it is also possible that, depending upon the different expression levels of DLK1 and DLK2 in these cells, both proteins may lead either to a decrease or to an increase in the global NOTCH signaling levels, depending on their stoichiometry and their interaction affinities. This kind of coordination of the expression and competition between NOTCH ligands has been also described by other authors61. Furthermore, we have observed that the over-expression of Dlk1 and Dlk2 increases the expression of endogenous Notch genes to different extents, suggesting a response of the cells aimed at re-equilibrating global NOTCH signaling. Our data reveal that the interplay among the NOTCH receptors and DLK proteins in 3T3-L1 cells affect the expression of each one of these genes, and suggest that the expression of the NOTCH canonical and non-canonical ligands participates in these complex feed-back mechanisms. Further analysis, beyond the scope of this work, will be needed to study in detail the coordination in the expression of these genes in 3T3-L1 preadipocytes.
In summary, the data presented here deepen into the understanding of the role of NOTCH and DLK proteins on the control of 3T3-L1 adipogenesis and adipocyte fate. In Fig. 9, we schematically summarize the potential effects of NOTCH receptors and DLK proteins on 3T3-L1 adipogenesis and preadipocyte whitening/browning. We believe that continuing with the study of the molecular pathways involved in this cell differentiation process and the coordination among the four NOTCH signaling activities and their modulation by canonical and non-canonical ligands would permit to advance in the development of novel and promising anti-obesity therapies.
Figure 9.
Outline of the role of Notch and Dlk gene over-expression on 3T3-L1 adipogenesis and adipocyte browning. The effects of adipogenic inductors on control 3T3-L1 preadipocytes is compared with the effects of the same adipogenic inductors on 3T3-L1 cells stably over-expressing each one of the Notch or Dlk genes. The direction (up or down) of the variations in the expression of aP2, Pparg, and several brown adipocyte and mitochondrial biogenesis markers, are shown by arrows.
Methods
Plasmids
Bacterial cultures, plasmid DNA isolation and purification, and bacterial transformation and amplification of TOP10 Escherichia coli competent cells was performed as previously described36. Plasmids pCDLK1 (DLK1) and pCDLK2 (DLK2) derivate from pCD2 vector (V1) and contain the complete cDNA sequence of either Dlk1 or Dlk2 in sense orientation, respectively17. Plasmid pC-N1 (N1) derivates from pCD2 (V1) and contains the complete mouse Notch1 cDNA (ATCC clone: MBA-105) in sense orientation36. Plasmid pCN-N2 (N2) (A gift from Dr. Anna Bigas, IMIM, Barcelona, Spain) contains the complete mouse Notch2 cDNA cloned into the EcoRI-NotI restriction sites of the pCDNA3 expression vector (V2). Plasmid pEntry-N3 (N3) contains the complete mouse Notch3 cDNA sequence in pCMV6-Entry vector (Origene) (V3). pG-N4 (N4) contains the complete mouse Notch4 cDNA sequence cloned into the HindIII-XbaI restriction sites of the pEGFP-N1 vector (Clontech) (V4). Plasmid pN-HES1 (H1) expresses the complete HES1 protein36. Plasmids pL-DLK1 (DLK1E) and pL-DLK2 (DLK2E) contain de cDNA of the extracellular regions of DLK1 and DLK236. All plasmids were confirmed by restriction analysis and sequenced by Macrogen. The oligonucleotides used for sequencing were obtained from Bonsai Technologies.
Cell culture and cell transfections
Mammalian cell lines were cultured as describe before17,36. The cell lines used were: 3T3-L1 (L1; ATCC CCL-92.1); HEK 293 T/17 (ATCC CRL-11268); and Balb/c14 (Balb/c 3T3 sub-clones negative for Dlk1 expression62. Stable and transient transfections were performed as previously described36.
Quantitative RT-PCR expression analysis
Confluent cell monolayers were processed as previously described to obtain total RNA and cDNAs36. Gene expression assays by qRT-PCR were performed as previously described36. P0 expression was used as the control to compare the CT from the different samples in all qPCR experiments63. The qRT-PCR primers to determine Dlk1, Dlk2 and Hes1 expression levels were previously described17,21. The expression of Notch genes and Hey1 gene was analyzed with the oligonucleotides showed in Table 1 (Bonsai Technologies).
Table 1.
Oligonucleotides used in qRT-PCR assays for the expression of the four Notch genes and the Hey1 gene.
| Notch1 E: 1,068 | mNotch1-up: 5′-GCTGAGCATGTACCCGAGC-3′ |
| mNotch1-down: 5′-ATCACGCTTGAAGACCACGTT-3′ | |
| Notch2 E: 1,0464 | mNotch2-up: 5′-TTGGGCAGGTTACATCCAGTTCCT-3′ |
| mNotch2-down: 5′-AGCCAGGACCATGCCAAACATTTC-3′ | |
| Notch3 E: 0.9796 | mNotch3-up: 5′-TGCAGTCAGCTGAGAATGACCACT-3′ |
| mNotch3-down: 5′-ACATCCCGAAGTGGGTATGGGAAA-3′ | |
| Notch4 E: 1,0174 | mNotch4-up: 5′-AAGGCCAAAATAACCGTTAAGCT-3′ |
| mNotch4-down: 5′-ACCGGACATCCTAAACCCTCTT-3′ | |
| Hey1 | mHey1-up: 5′-ATGTGGCCTACTTCAGCTCCATGT-3′ |
| mHey1-down: 5′-TCTCCAGGCAGGTAAACAATGGGA-3′ |
Oligonucleotide amplification efficiencies of Notch primers (E) are shown.
Conditioned media
HEK 293T/17 cells were transiently transfected with plasmids pL-DLK1 (DLK1E) and pL-DLK2 (DLK2E), which express the soluble forms of DLK1 (sDLK1) and DLK2 (sDLK2), respectively. Protein expression and secretion of these proteins were analyzed as previously described36 (Supplementary Figure 3A).
Protein sample preparation and Western blotting
Protein samples were obtained, quantified and electrophoresed as previously described36. Western blot was performed by using an appropriated dilution of the primary and the secondary antibodies (usually, 1: 2,000) (Table 2). Detection of alpha-tubulin with a specific antibody (Sigma) was used as a protein loading control.
Table 2.
Primary and secondary antibodies used in Western blot analysis.
| Protein | Dilution of primary and secondary antibodies | Company |
|---|---|---|
| NOTCH1 | Rabbit anti-NOTCH1 C20R (1:1000) | Santa Cruz Biotechnology |
| Active NICD1 | Rabbit anti-Cleaved NOTCH1 (Val 1744) (1:000) | Cell signaling |
| NOTCH2 | Goat anti-NOTCH2 M20 (1:500) | Santa Cruz Biotechnology |
| NOTCH3 | Rabbit anti-NOTCH3 (1:1000) | Abcam |
| NOTCH4 | Rabbit anti-NOTCH4 (1:1000) | Upstate Millipore |
| HA | Mouse anti-HA 16B12 (1:5000) | Covance |
| DLK1 | Rabbit anti-DLK1 (1:1000) | Nueda et al.49 |
| DLK2 | Rabbit anti-DLK2 (1:500) | Proteintech |
| α-tubulin | Mouse anti-alpha-Tubulin (1:5000) | Sigma |
Luciferase assays
NOTCH transcriptional activity was analyzed by luciferase assays in Balb/c14 cells and stably transfected 3T3-L1 pools as previously described36. When cells were transiently co-transfected with DLK expression plasmids, the assays were performed in Balb/c14 cells because these cells do not express DLK1 and express very low levels of DLK2, and have higher transient transfection efficiency compared to 3T3-L1 cells. To analyze the effect of DLK proteins on NOTCH activity, we performed these assays by co-transfecting Balb/c14 cells with a Notch expression plasmid and with plasmids expressing sDLK1 or sDLK2 proteins. We also treated Balb/c14 cells with the gamma-secretase inhibitor DAPT (10 μM) as a NOTCH signaling inhibition control. Transfected cells were processed as previously described36.
Adipogenic assays
The induction of 3T3-L1 adipogenesis and the staining of adipocytes with red oil O was performed according to standard procedures as previously described17. Sometimes, cells were induced to differentiate in the presence of control conditioned media or conditioned media containing sDLK1 or sDLK2 during the entire differentiation process. Assays were repeated at least three times.
We determined the level of differentiation by analyzing the expression of the late adipocyte differentiation marker aP2 and the intermediate marker Pparg, as previously described64, seven days after induction of adipogenesis. To study the phenotype of 3T3-L1 adipocytes, we also analyzed in the same samples the expression of the following brown adipose markers: Ucp1 (coding for uncoupling protein-1); Pgc1a (Peroxisome proliferator-activated receptor-gamma coactivator); Gyk, encoding for a glycerol kinase activated in brown adipocytes and involved in triglyceride and glycerophospholipid synthesis65; Prdm16, thought to function as a key transcriptional co-regulator of brown cell adipogenesis function55; Cidea, highly expressed in lipid droplet membranes and mitocondria of brown adipocytes,involved in the browning process and considered as a BAT differentiation marker45; and Sirt1, a mitochondrial biogenesis marker66. Pre-designed qRT-PCR oligonucleotides of these genes were obtained from Sigma (Table 3).
Table 3.
Oligonucleotides used in qRT-PCR assays to quantify the expression of the genes indicated.
| Gene | Oligonucleotide sequences |
|---|---|
| Ucp1 | mUcp-1up: 5′-CAATTGTACAGAGCTGGTAAC-3′ |
| mUcp1-down: 5′-TGTTTTTACCACATCCACGT-3′ | |
| Gyk | mGyk-up: 5′-ATCTATGGCCTAATGAAAGC-3´ |
| mGyk-down: 5′-GAAAATACACACTTATGGCCC-3′ | |
| Pgc1a | mPgc1-a-up: 5′-TCCTCTTCAAGATCCTGTTAC-3′ |
| mPgc1-a-down: 5′-CACATACAAGGGAGAATTGC-3′ | |
| Prdm16 | mPrdm16-up: 5′-ATCTACAGGGTAGAAAAGCG-3′ |
| mPrdm16-down: 5′-TCTCCGTCATGGTTTCTATG-3′ | |
| Cidea | mCidea-up: 5′-GTGTTAAGGAATCTGCTGAG-3′ |
| mCidea-down: 5′-CTATAACAGAGAGCAGGGTC-3 | |
| Sirt1 | mSirt1-up: 5′-AAACAGTGAGAAAATGCTGG-3′ |
| mSirt1-down: 5′-′GGTATTGATTACCCTCAAGC-3 |
We also analyzed the biogenesis of mitochondria by qPCR analysis of the ratio of the levels of the mitochondrial gene CytB and the genomic gene ApoB in terminal differentiated cells by using oligonucleotides previously described67,68.
The amount of glycerol released into the culture medium was determined using the lipolysis colorimetric assay kit (BioVision). The extracellular lactate was measured using the lactatate colorimetric assay kit (BioVision). Finally, to measure the oxygen consumtion rate (OCR), we used the oxigen consumption rate assay kit (AbCam). In these three last assays, we seeded 15,000 cells per well in 96-well plates, then we proceeded with the adipogenic differentiation protocol and, finally, we performed the assays following the manufacturer’s recommendations. Data were normalized with total protein amount or the number of cells seeded in each well.
Equipment and Settings
RNA and DNA concentration and purity (20 nm/280 nm) were analyzed by using the spectrophotometer NanoDrop One (Thermo Scientific).
Quantitative RT-qPCRS were performed with StepOne Plus RT-qPCR system (Applied Biosystems) by using Fast SYBR green mix. The results were obtained by using the StepOne software 2.3 with the parameters recommended by the company, analyzing always the melting curve for each gene.
Microscopy images were visualized with an objective 40X of Motic AE31 microscopy connected to a Moticam 2300 camera (3.0 M Pixel USB 2.0). Cell images, 400X magnification, were acquired with the software Motic Images Plus 2.0 with the standard parameters (Exposition: 418.9, Gamma: 0.8019).
Western blot images s were obtained by developing exposed films (CP-BU New, Agfa) for 30 seconds (Tubulin), 1 minute (NOTCH1, NICD1, NOTCH2), 5 minutes (NOTCH3) or 10 minutes (NOTCH4), with the Pierce ECL Plus Western Blotting substrate kit (Thermo Scientific) in a Curix 60 developing apparatus (AGFA). Films were scanned with HP Officejet Pro 8600 scanner and signals of the different proteins were quantified by using the QuantityOne 4. 6. 5. (Basic) software. We analyzed the intensity of the signal per mm2 with a Volume Rect Tool. Blots for the Supplementary Figure 1 were converted to a grey color.
Colorimetric determinations to quantify total protein amount, extracellular lactate in the culture medium, and the release of glycerol to the the medium were performed with a plate reader Axis UVM340 (Biochrom). To measure the OCR of the different cultured adipocytes, we used a fluorescence spectrophotometer F-7000 (Hitachi).
Luciferase assays were measured by using a Monolight 3096 Microplate Luminometer (Becton Dickinson) and samples were processed with the Dual-224 Luciferase Reporter Assay System (Promega), following the supplier’s recommendations.
Statistical analysis
Data are presented as the mean ± S.D of at least three different independent assays performed in triplicate. Data were also analyzed with Student’s t test to determine statistical significance. A P value of ≤0.05 was considered statistically significant (*); a P value ≤0.01 was considered highly statistically significant (**); and a P value of ≤0.001 was considered extremely statistically significant (***).
Electronic supplementary material
Acknowledgements
We thank Dr. Anna Bigas from IMIM (Barcelona, Spain) for her gift of the complete NOTCH2 expression plasmid. We thank Dr. Marta Casado, from Instituto de Biomedicina de Valencia (Valencia, Spain), for the information about the oligonucleotides used to amplify the genomic gene ApoB and the mitochondrial gene CytB, and the protocols to obtain total DNA. We also thank the laboratory technician Ms. María-Ángeles Ballesteros for her invaluable help. This work was supported by funds from the Ministry of Economy and Competitiveness of Spain (BFU2010-16433), and by funds PII1I09-0164-00 and PEII11-0062-2456 from the Health Council of the Regional Government of Castilla-La Mancha (Spain), supported by the Fondo Europeo de Desarrollo Regional (FEDER).
Author Contributions
V. Baladrón, M.-L. Nueda and J. Laborda designed experiments and supervised the work. V. Baladrón, M.-L. Nueda, M.-J. Gónzalez-Gómez, and M.-M. Rodríguez-Cano performed experiments and gathered and analyze the data. B. Sánchez-Solana constructed some of the D.L.K. and NOTCH expression plasmids. E.-M. Monsalve and M.-J. M. Díaz-Guerra designed and analyzed the efficiency of Notch and Hes1/Hey1 oligonucleotides. M.-J. M. Díaz-Guerra revised the manuscript. V. Baladrón, M.-L. Nueda, B. Sánchez-Solana and J. Laborda wrote the manuscript in its final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/7/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35252-3.
References
- 1.Nichols AM, et al. Notch pathway is dispensable for adipocyte specification. Genesis. 2004;40:40–44. doi: 10.1002/gene.20061. [DOI] [PubMed] [Google Scholar]
- 2.Garcés C, et al. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem. 1997;272:29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 3.Ba K, et al. Jagged-1-mediated activation of notch signalling induces adipogenesis of adipose-derived stem cells. Cell Prolif. 2012;45:538–544. doi: 10.1111/j.1365-2184.2012.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai P-Y, Tsai C-B, Tseng M-J. Active form Notch4 promotes the proliferation and differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013;430:1132–1139. doi: 10.1016/j.bbrc.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol. Cell. Biol. 2004;24:3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urs S, et al. Effect of soluble Jagged1-mediated inhibition of Notch signaling on proliferation and differentiation of an adipocyte progenitor cell model. Adipocyte. 2012;1:46–57. doi: 10.4161/adip.19186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilian TM, Klöting N, Blüher M, Beck-Sickinger AG. Prenatal notch1 receptor blockade by protein delta homolog 1 (DLK1) modulates adipocyte size in vivo. Int J Obes (Lond) 2016;40:698–705. doi: 10.1038/ijo.2015.227. [DOI] [PubMed] [Google Scholar]
- 8.Bi P, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat. Med. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gridley T, Kajimura S. Lightening up a notch: Notch regulation of energy metabolism. Nat. Med. 2014;20:811–812. doi: 10.1038/nm.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasut A, et al. Notch Signaling Rescues Loss of Satellite Cells Lacking Pax7 and Promotes Brown Adipogenic Differentiation. Cell Rep. 2016;16:333–343. doi: 10.1016/j.celrep.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan T, Liu J, Wu W, Xu Z, Wang Y. Roles of Notch Signaling in Adipocyte Progenitor Cells and Mature Adipocytes. J. Cell. Physiol. 2017;232:1258–1261. doi: 10.1002/jcp.25697. [DOI] [PubMed] [Google Scholar]
- 12.Fiúza U-M, Arias AM. Cell and molecular biology of Notch. J. Endocrinol. 2007;194:459–474. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luca VC, et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355:1320–1324. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J. Biol. Chem. 1993;268:3817–3820. [PubMed] [Google Scholar]
- 17.Nueda ML, et al. The novel gene EGFL9/Dlk2, highly homologous to Dlk1, functions as a modulator of adipogenesis. J. Mol. Biol. 2007;367:1270–1280. doi: 10.1016/j.jmb.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt MHH, et al. Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat. Cell Biol. 2009;11:873–880. doi: 10.1038/ncb1896. [DOI] [PubMed] [Google Scholar]
- 19.Eiraku M, Hirata Y, Takeshima H, Hirano T, Kengaku M. Delta/notch-like epidermal growth factor (EGF)-related receptor, a novel EGF-like repeat-containing protein targeted to dendrites of developing and adult central nervous system neurons. J. Biol. Chem. 2002;277:25400–25407. doi: 10.1074/jbc.M110793200. [DOI] [PubMed] [Google Scholar]
- 20.Garcés C, Ruiz-Hidalgo MJ, Bonvini E, Goldstein J, Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation. 1999;64:103–114. doi: 10.1046/j.1432-0436.1999.6420103.x. [DOI] [PubMed] [Google Scholar]
- 21.Baladrón V, et al. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 2005;303:343–359. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Nueda ML, Baladrón V, Sánchez-Solana B, Ballesteros M-A, Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 2007;367:1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr. Top. Dev. Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sul HS, Smas C, Mei B, Zhou L. Function of pref-1 as an inhibitor of adipocyte differentiation. Int. J. Obes. Relat. Metab. Disord. 2000;24(Suppl 4):S15–9. doi: 10.1038/sj.ijo.0801494. [DOI] [PubMed] [Google Scholar]
- 25.Moon YS, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J. Clin. Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortensen SB, et al. Membrane-tethered delta-like 1 homolog (DLK1) restricts adipose tissue size by inhibiting preadipocyte proliferation. Diabetes. 2012;61:2814–2822. doi: 10.2337/db12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traustadottir GA, Kosmina R, Sheikh SP, Jensen CH, Andersen DC. Preadipocytes proliferate and differentiate under the guidance of Delta-like 1 homolog (DLK1) Adipocyte. 2013;2:272–275. doi: 10.4161/adip.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charalambous M, et al. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc. Natl. Acad. Sci. USA. 2014;111:16088–16093. doi: 10.1073/pnas.1406119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdallah BM, Ditzel N, Laborda J, Karsenty G, Kassem M. DLK1 Regulates Whole-Body Glucose Metabolism: A Negative Feedback Regulation of the Osteocalcin-Insulin Loop. Diabetes. 2015;64:3069–3080. doi: 10.2337/db14-1642. [DOI] [PubMed] [Google Scholar]
- 31.Hermida C, et al. The serum levels of the EGF-like homeotic protein dlk1 correlate with different metabolic parameters in two hormonally different children populations in Spain. Clin. Endocrinol. (Oxf) 2008;69:216–224. doi: 10.1111/j.1365-2265.2008.03170.x. [DOI] [PubMed] [Google Scholar]
- 32.Wallace C, et al. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat. Genet. 2010;42:68–71. doi: 10.1038/ng.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armengol J, et al. Pref-1 in brown adipose tissue: specific involvement in brown adipocyte differentiation and regulatory role of C/EBPδ. Biochem. J. 2012;443:799–810. doi: 10.1042/BJ20111714. [DOI] [PubMed] [Google Scholar]
- 34.Rakhshandehroo M, Koppen A, Kalkhoven E. Pref-1 preferentially inhibits heat production in brown adipose tissue. Biochem. J. 2012;443:e3–5. doi: 10.1042/BJ20120382. [DOI] [PubMed] [Google Scholar]
- 35.Bray SJ, Takada S, Harrison E, Shen S-C, Ferguson-Smith AC. The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev. Biol. 2008;8:11. doi: 10.1186/1471-213X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Solana B, et al. The EGF-like proteins DLK1 and DLK2 function as inhibitory non-canonical ligands of NOTCH1 receptor that modulate each other’s activities. Biochim. Biophys. Acta. 2011;1813:1153–1164. doi: 10.1016/j.bbamcr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim. Biophys. Acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 38.García-Gallastegui P, et al. DLK1 regulates branching morphogenesis and parasympathetic innervation of salivary glands through inhibition of NOTCH signalling. Biol. Cell. 2014;106:237–253. doi: 10.1111/boc.201300086. [DOI] [PubMed] [Google Scholar]
- 39.Nueda ML, Naranjo A-I, Baladrón V, Laborda J. The proteins DLK1 and DLK2 modulate NOTCH1-dependent proliferation and oncogenic potential of human SK-MEL-2 melanoma cells. Biochim. Biophys. Acta. 2014;1843:2674–2684. doi: 10.1016/j.bbamcr.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 40.González MJ, et al. DLK1 is a novel inflammatory inhibitor which interferes with NOTCH1 signaling in TLR-activated murine macrophages. Eur. J. Immunol. 2015;45:2615–2627. doi: 10.1002/eji.201545514. [DOI] [PubMed] [Google Scholar]
- 41.Traustadottir GA, et al. Evidence of non-canonical NOTCH signaling: Delta-like 1 homolog (DLK1) directly interacts with the NOTCH1 receptor in mammals. Cell. Signal. 2016;28:246–254. doi: 10.1016/j.cellsig.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez P, et al. The non-canonical NOTCH ligand DLK1 exhibits a novel vascular role as a strong inhibitor of angiogenesis. Cardiovasc. Res. 2012;93:232–241. doi: 10.1093/cvr/cvr296. [DOI] [PubMed] [Google Scholar]
- 43.Nueda ML, Naranjo A-I, Baladrón V, Laborda J. Different expression levels of DLK1 inversely modulate the oncogenic potential of human MDA-MB-231 breast cancer cells through inhibition of NOTCH1 signaling. FASEB J. 2017;31:3484–3496. doi: 10.1096/fj.201601341RRR. [DOI] [PubMed] [Google Scholar]
- 44.Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4:295–302. doi: 10.1080/21623945.2015.1040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calderon-Dominguez M, et al. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5:98–118. doi: 10.1080/21623945.2015.1122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y, et al. gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-gamma. Cell Prolif. 2010;43:147–156. doi: 10.1111/j.1365-2184.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SM, et al. Presenilin enhancer-2 (PSENEN), a component of the gamma-secretase complex, is involved in adipocyte differentiation. Domest. Anim. Endocrinol. 2009;37:170–180. doi: 10.1016/j.domaniend.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Chartoumpekis DV, et al. Notch intracellular domain overexpression in adipocytes confers lipodystrophy in mice. Mol Metab. 2015;4:543–550. doi: 10.1016/j.molmet.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nueda ML, García-Ramírez JJ, Laborda J, Baladrón V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J. Mol. Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 50.Barneda D, Frontini A, Cinti S, Christian M. Dynamic changes in lipid droplet-associated proteins in the ‘browning’ of white adipose tissues. Biochim. Biophys. Acta. 2013;1831:924–933. doi: 10.1016/j.bbalip.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Villarroya F, Iglesias R, Giralt M. PPARs in the Control of Uncoupling Proteins Gene Expression. PPAR Res. 2007;2007:74364–12. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klaus S, Ely M, Encke D, Heldmaier G. Functional assessment of white and brown adipocyte development and energy metabolism in cell culture. Dissociation of terminal differentiation and thermogenesis in brown adipocytes. J. Cell. Sci. 1995;108(Pt 10):3171–3180. doi: 10.1242/jcs.108.10.3171. [DOI] [PubMed] [Google Scholar]
- 53.Nakagami H. The mechanism of white and brown adipocyte differentiation. Diabetes Metab J. 2013;37:85–90. doi: 10.4093/dmj.2013.37.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bredella MA, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J. Clin. Endocrinol. Metab. 2012;97:E584–90. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahfeldt T, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat. Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui S, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohashi S, et al. NOTCH1 and NOTCH3 coordinate esophageal squamous differentiation through a CSL-dependent transcriptional network. Gastroenterology. 2010;139:2113–2123. doi: 10.1053/j.gastro.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Natsuizaka M, et al. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nat Commun. 2017;8:1758. doi: 10.1038/s41467-017-01500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.la Pompa de JL, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 60.Suchting S, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 62.Baladrón V, et al. The EGF-like homeotic protein dlk affects cell growth and interacts with growth-modulating molecules in the yeast two-hybrid system. Biochem. Biophys. Res. Commun. 2002;291:193–204. doi: 10.1006/bbrc.2002.6431. [DOI] [PubMed] [Google Scholar]
- 63.Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19:3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sánchez-Solana B, Laborda J, Baladrón V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol. Endocrinol. 2012;26:110–127. doi: 10.1210/me.2011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Festuccia WTL, et al. Expression of glycerokinase in brown adipose tissue is stimulated by the sympathetic nervous system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1536–41. doi: 10.1152/ajpregu.00764.2002. [DOI] [PubMed] [Google Scholar]
- 66.Imran KM, et al. Cryptotanshinone promotes commitment to the brown adipocyte lineage and mitochondrial biogenesis in C3H10T1/2 mesenchymal stem cells via AMPK and p38-MAPK signaling. Biochim. Biophys. Acta. 2017;1862:1110–1120. doi: 10.1016/j.bbalip.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Ylikallio E, et al. Ribonucleotide reductase is not limiting for mitochondrial DNA copy number in mice. Nucleic Acids Res. 2010;38:8208–8218. doi: 10.1093/nar/gkq735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuke S, Kubota-Sakashita M, Kasahara T, Shigeyoshi Y, Kato T. Regional variation in mitochondrial DNA copy number in mouse brain. Biochim. Biophys. Acta. 2011;1807:270–274. doi: 10.1016/j.bbabio.2010.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.