Abstract
Background and Purpose
IL‐1β is a cytokine of critical importance in inflammatory, infectious and autoimmune diseases. Zinc finger protein 91 (ZFP91) has been reported to be involved in multiple biological processes. Here, we identified a previously unknown role for ZFP91 in the production of biologically active IL‐1β and investigated the underlying mechanisms of its effects.
Experimental Approach
In vitro, the underlying mechanisms of ZFP91 at inhibiting the expression of IL‐1β were investigated by ELISA, RT‐PCR, Western blotting, immunoprecipitation and immunofluorescence assays. In vivo, colitis was induced by giving 4% dextran sulfate sodium (DSS) p.o. in drinking water for 5 days. Peritonitis was induced by injecting 700 μg alum i.p. for 12 h.
Key Results
ZFP91 activated the non‐canonical caspase‐8 inflammasome, which resulted in robust IL‐1β secretion. Using an immunoprecipitation assay and immunofluorescence assay, we found that ZFP91 promoted the assembly of the non‐canonical caspase‐8 inflammasome complex. Moreover, ZFP91 enhanced the activation of ERK, p38 MAPK and JNK in macrophages. In addition, our data demonstrate that the synthesis of pro‐IL‐1β is dependent on activation of these MAPK signalling pathways. In vivo experiments, the symptoms and colonic inflammation associated with DSS‐induced colitis were ameliorated in mice deficient in ZFP91. Furthermore, the inflammation in alum‐induced peritonitis was also attenuated in mice deficient in ZFP91.
Conclusions and Implications
Our research describes a mechanism by which ZFP91 promotes production of IL‐1β under physiological conditions and suggests that ZFP91 may be a promising therapeutic target for intervention in inflammatory, infectious and autoimmune‐related diseases.
Abbreviations
- ARF
alternative reading frame
- ASC
apoptosis associated speck‐like protein containing CARD
- BMDM
bone marrow‐derived macrophage
- DAI
disease activity index
- FACS
fluorescence‐activated cell sorting
- IP
immunoprecipitation
- MOI
multiplicities of infection
- NLRs
nucleotide‐binding oligomerization domain‐like receptors
- PECs
peritoneal exudate cells
- TLR3
Toll‐like receptor 3
- ZFP91
zinc finger protein 91
Introduction
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 is a cytokine of critical importance in inflammatory, infectious and autoimmune diseases. The release of IL‐1β consists of two steps: the first signal is mediated by activation of the NF‐κB transcription factor or the stress‐activated protein kinases, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=518. This activation results in the production of the 35 kDa pro‐IL‐1β, a biologically inactive precursor of IL‐1β. In the second step, pro‐IL‐1β is cleaved to bioactive IL‐1β by several enzymatic complexes, the most important of which are the inflammasomes (van de Veerdonk et al., 2011; Netea et al., 2015). Inflammasome complex assembly can be triggered by specific stimuli from invading pathogens and endogenous ‘danger signals’, known as pathogen‐associated molecular patterns and danger‐associated molecular patterns respectively. Once activated, the cysteine protease caspase can be cleaved to its activated form and facilitates the cleavage of pro‐IL‐1β to mature IL‐1β.
Canonical inflammasomes contain apoptosis associated speck‐like protein containing CARD (ASC) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1617 (cysteine‐requiring aspartate protease‐1) and assemble upon stimulation of cytosolic pattern recognition receptor such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=317 (Latz et al., 2013). Recent studies indicate that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1624 can substitute for caspase‐1 and can function as an efficient ‘IL‐1‐converting enzyme’ for proteolytic maturation of IL‐1β (Maelfait et al., 2008; Chen et al., 2015). Gurung et al. and Monie et al. have recently reviewed the growing literature describing diverse roles for caspase‐8 in the regulation of different inflammatory signalling platforms, including inflammasomes and IL‐1β production (Gurung and Kanneganti, 2015; Monie and Bryant, 2015). Antonopoulos et al. (2015) demonstrated that activated http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770–ASC inflammasomes recruit caspase‐8 to drive IL‐1β processing in murine bone marrow‐derived dendritic cells independent of caspase‐1 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1620. These studies indicate that caspase‐8 is recruited to diverse signalling platforms that regulate the proteolytic maturation of IL‐1β.
Zinc finger protein 91 (ZFP91), a conserved 63.5 kDa nuclear protein with structural motifs characteristic of transcription factors, contains five zinc‐finger domains, one leucine‐zipper pattern, one coiled‐coil structure and several nuclear localization signals (Saotome et al., 1995). It has been reported that ZFP91 interacts with the alternative reading frame tumour suppressor (cyclin‐dependent kinase inhibitor 2A), which is known for its induction of p53‐dependent cell death or growth arrest in response to activated oncogenes (Tompkins et al., 2006). Furthermore, ZFP91 plays a critical role in acute myelogenous leukaemia and prostate cancer pathology (Unoki et al., 2003; Paschke et al., 2014). Our previous study indicated that ZFP91 is an atypical E3 ligase activating NF‐κB‐inducing kinase via Lys63‐linked ubiquitination in the non‐canonical NF‐κB signalling pathway, and that ZFP91 activates HIF‐1α via NF‐κB/p65 to promote proliferation and tumourigenesis of colon cancer (Jin et al., 2010a; Jin et al., 2010b; Ma et al., 2016). However, the underlying mechanism of ZFP91 activation in promoting pro‐IL‐1β production and IL‐1β secretion is unclear.
In this study, we aimed to elucidate the physiological function of ZFP91 with regard to IL‐1β production in macrophages. First, an in vitro study showed that ZFP91 promoted inflammatory cytokine IL‐1β production in macrophages. Furthermore, we found that endogenous inflammasomes were crucial for the mature IL‐1β production in ZFP91‐overexpressing macrophages. Then, we detected the signalling pathways involved in ZFP91 overexpressing pro‐IL‐1β expression and showed that ZFP91 stimulation activated the MAPKs’ pathway. To confirm these results, we blocked MAPKs function with ERK, JNK or p38 inhibitors in ZFP91‐overexpressing macrophages, and we also found impaired pro‐IL‐1β and IL‐1β expression. Thus, we concluded that ZFP91 induces the production of IL‐1β by activation of MAPKs and non‐canonical caspase‐8 inflammasome in macrophages, suggesting that ZFP91 might be a potential target for ameliorating inflammation.
Methods
Cell culture and inhibitors
Human THP‐1 cells were obtained from American Type Culture Collection (Manassas, VA, USA). PMA (Sigma, St. Louis, MO, USA)‐activated THP‐1 cells were used as human macrophages. The cells were cultured at 37°C under 5% CO2 in DMEM supplemented with 10% heat‐inactivated FBS (Invitrogen, Carlsbad, CA, USA), 100 U·mL−1 penicillin and 100 mg·mL−1 streptomycin. Bone marrow‐derived macrophages (BMDMs) were generated by culturing bone marrow cells in RPMI containing 10% heat‐inactivated FBS, 30% L cell‐conditioned medium, 100 U·mL−1 penicillin and 100 mg·mL−1 streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were untreated or treated with 1 μg·mL−1 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019 (Sigma) for 6 h. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5296 (caspase‐8 inhibitor) was bought from Santa Cruz (Dallas, Texas, USA). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5302 (caspase‐1 inhibitor) was bought from Millipore (Bedford, MA, USA). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5241 (ERK inhibitor) was bought from Sigma. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5269 (p38 inhibitor) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5273 (JNK inhibitor) were bought from MedChemExpress (Monmouth Junction, NJ, USA).
Lentivirus transduction
ZFP91 expression plasmid was constructed by PCR‐based amplification of cDNA from human gastric cancer cell line SNU‐638 and then cloned into pSuper‐retro‐puro vector. Human ZFP91‐specific siRNA (5′‐CCAGGTGGCATTAGTAGTGAA‐3′), NLRP3‐specific siRNA (5′‐GUCAUCGGGUGGAGUCACU‐3′), caspase 8‐specific siRNA (5′‐CAGAUCAGAAUUGAGGUCU′), ASC‐specific siRNA (5′‐CACCAAAUCAUCCUGAAUC′) and a scrambled control siRNA (5′‐AAGGAGACGAGCAAGAGAA‐3′) oligonucleotides were cloned into pSuper‐retro‐puro vector. Retroviral production and infection were conducted as previously described (Ma et al., 2016). In brief, THP‐1 cells were stimulated by PMA (100 ng·mL−1) to differentiate into macrophages. After 24 h, all cells were counted and plated, and then, 1 × 105 THP‐1 cells were incubated with lentiviral particles in growth medium that contained 5 μg·mL−1 polybrene for 24 h.
ELISA
The concentrations of human IL‐1β, mouse IL‐1β, mouse http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 and mouse IL‐6 were measured using ELISA kits (BD Biosciences, San Diego, CA, USA) according to the manufacturer's instructions.
RT‐PCR analysis
Total RNA from THP‐1 cells were obtained using RNA Mini kit (Qiagen, Valencia, CA, USA). cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Schweiz). To analyse mRNA levels, RT‐PCR was performed. The cycling conditions were 95°C for 5 min followed by 45 cycles of 95°C for 10 s, 60°C for 15 s and 72°C for 20 s. Gene expression levels were normalized to GAPDH. The primers are as follows: 5′‐AGCTACCATTTGCCTACA A‐3′ (forward) and 5′‐GGGAAACGGCTGAGATAGTTT‐3′ (reverse) for ZFP91; 5′‐AGGCTGCTCTGGGATTC‐3′ (forward) and 5′‐GCCACAACAACTGACGC‐3′ (reverse) for pro‐IL‐1β; 5′‐GATCTTCGCTGCGATCAACAG‐3′ (forward) and 5′‐CGTGCATTATCTGAACCCCAC‐3′ (reverse) for NLRP3; 5′‐TGGGCCTGCAGGAGATG‐3′ (forward) and 5′‐ATTTGGTGGGATTGCCAG‐3′ (reverse) for ASC; 5′‐ACGCCTTGCCCTCATAAT‐3′ (forward) and 5′‐TCTAATACATCTGGGACTTCTT‐3′ (reverse) for caspase‐1; 5′‐TACTACCGAAA CTTGGACC‐3′ (forward) and 5′‐GTGAAAGTAGGTTGTGGC‐3′ (reverse) for caspase‐8; and 5′‐ACCACAGTCCATGCCATCAC‐3′ (forward) and 5′‐TCCACCACCCTGTTGCTGTA‐3′ (reverse) for GAPDH.
Mice
Wild‐type (WT) C57BL/6 mice and ZFP91 knockout (ZFP91−/−) mice on a C57BL/6 background were generated by Shanghai Biomodel Organism Science & Technology Development Co., Ltd (Shanghai, China). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Yanbian University (Yanji, Jilin Province, China). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010).
Immunoprecipitation (IP) and immunoblot analysis
For IP, whole‐cell extracts were lysed in IP buffer containing 1% Nonidet P‐40, 50 mM Tris–HCl, pH 7.4, 50 mM EDTA, 150 mM NaCl and a protease inhibitor cocktail (BD Biosciences). After centrifugation for 10 min at 14 000× g, supernatants were collected and incubated with protein G PLUS‐agarose IP reagent together with specific antibody. After 6 h of incubation, beads were washed five times with IP buffer. Immunoprecipitates were eluted by boiling with 1% SDS sample buffer. For immunoblot analysis, whole‐cell extracts were obtained by lysing cells in ice‐cold lysis buffer (50 mM Tris–HCl, pH 7.5, 1% Nonidet P‐40, 1 mM EDTA, 150 mM NaCl and 1 mM PMSF) supplemented with the protease inhibitor cocktail. In certain experiments, the nuclear extracts were prepared using NE‐PER nuclear and cytoplasmic extraction reagent. An aliquot of protein extracts was used to determine protein concentration by the Bradford method. Equal amounts of extracts per lane was separated by SDS‐PAGE, followed by transferring to a PVDF membrane (Millipore). The membrane was blocked with 5% skimmed milk and then incubated overnight with primary antibodies against cleaved caspase‐8 (cat# 9496 and 9429, Cell Signaling Technology, Beverly, MA, USA), caspase‐8 (cat# 9746, Cell Signaling Technology), cleaved caspase‐1 (cat# 4199, Cell Signaling Technology), NLRP3 (cat# 15101, Cell Signaling Technology), ASC (cat# SC‐514414, Santa Cruz), IL‐1β (cat# NB600‐633, Novus Biologicals, Littleton, CO, USA), phospho‐ERK1/2 (cat #4370, Cell Signaling Technology), ERK1/2 (Santa Cruz), phospho‐SAPK/JNK (cat# 9251, Cell Signaling Technology), SAPK/JNK (Santa Cruz), phospho‐p38 (cat# 9215, Cell Signaling Technology), p38 (Santa Cruz), p65 (Santa Cruz), Topo‐I (Santa Cruz), α‐tubulin (cat# T5168, Sigma) and ZFP91 (Jin et al., 2010b). After being bound to an appropriate secondary antibody coupled to HRP, proteins were visualized by enhanced chemiluminescence (Millipore).
Immunofluorescence
Cells were rinsed once in PBS, fixed in fresh 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.2% Triton X‐100. Cells were blocked with 5% BSA in PBS for 30 min and incubated overnight with the primary antibodies at 4°C, followed by incubation with the secondary antibodies for 30 min at room temperature. The slides were stained with DAPI for 30 min before observation. Images were acquired by confocal laser‐scanning microscope (Nikon, Tokyo, Japan).
Dextran sulfate sodium (DSS)‐induced colitis
Colitis was induced in WT or ZFP91 knockout (ZFP91−/−) C57BL/6 mice (females, 6‐weeks‐old) with 4% DSS dissolved in drinking water for 5 days, followed by regular drinking water. Mice were monitored for body weight, stool consistency and the presence of occult blood every day. Scoring for weight loss, stool consistency and occult blood was done. No weight loss was denoted as 0 points, 1–5% as 1 point, 5–10% as 2 points, 10–20% as 3 points and >20% as 4 points. For stool consistency, 0 points were given for well‐formed pellets, 2 points were given for pasty and semiformed stools that did not stick to the anus and 4 points were given for liquid stools that stuck to the anus. Bleeding was scored as 0 points for no blood, 2 points for positive finding and 4 points for gross bleeding. The sum of these scores formed the clinical score, ranging from 0 (healthy) to 12 (maximal activity of colitis). After day 6, the entire colon was excised and the length of the colon was measured and the weight of caecum recorded. Then, mice colon tissues close to the rectum were collected for histological analysis and protein extraction.
Histopathology
Colon tissues were collected and fixed in 4% formaldehyde overnight. The fixed portion of the colon tissue was embedded in paraffin, cut into 6 μm samples and put onto microscopic slides. Slides were either stained with haematoxylin–eosin (H&E) for histological analysis by optical microscopy or stained by immunohistochemistry for IL‐1β and counterstained using 3,3′‐diaminobenzidine followed by haematoxylin counterstain.
In vivo peritonitis
WT or ZFP91 knockout (ZFP91−/−) C57BL/6 mice (females, 6‐weeks‐old) were i.p. injected with 700 μg alum for 12 h. Peritoneal cavities were washed with 6 mL of PBS. The peritoneal fluids were harvested and concentrated for ELISA analysis with Amicon Ultra 10 K from Millipore. Peritoneal exudate cells (PECs) were collected and analysed by flow cytometry.
Statistical analysis
All experiments were independently performed five times. Data are presented as means ± SD of five experiments. Analysis was performed using Student's t‐test or ANOVA. A P value of less than 0.05 was considered statistically significant in all cases.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b).
Results
ZFP91 up‐regulates the production and activation of pro‐IL‐1β in THP‐1 cells and BMDMs
To investigate the potential functions of ZFP91 in the innate immune response, the effects of ZFP91 on the production of pro‐IL‐1β and IL‐1β in THP‐1 cells and BMDMs were evaluated. ELISA analysis showed that ZFP91 overexpression significantly enhanced the secretion of IL‐1β in a dose‐dependent manner in LPS‐stimulated THP‐1 cells (Figure 1A), whereas the knockdown of ZFP91 decreased the LPS‐stimulated secretion of IL‐1β (Figure 1B). Western blot and RT‐PCR results showed that ZFP91 overexpression significantly enhanced the IL‐1β, pro‐IL‐1β protein and mRNA levels in a dose‐dependent manner in LPS‐stimulated THP‐1 cells (Figures S1A and 1C,E), whereas the knockdown of ZFP91 decreased the LPS‐stimulated expression of pro‐IL‐1β protein and mRNA level (Figure 1D,F). Overexpressing ZFP91 also enhanced the IL‐1β and pro‐IL‐1β proteins in unstimulated THP‐1 cells (Figure S1B). To confirm the role of ZFP91 in pro‐IL‐1β production and activation, the effects of ZFP91 deficiency on pro‐IL‐1β and IL‐1β expression in BMDMs were observed. Results showed that the production of pro‐IL‐1β and the secretion of IL‐1β were significantly reduced in ZFP91‐deficient BMDMs (Figure 1G,H).
Figure 1.
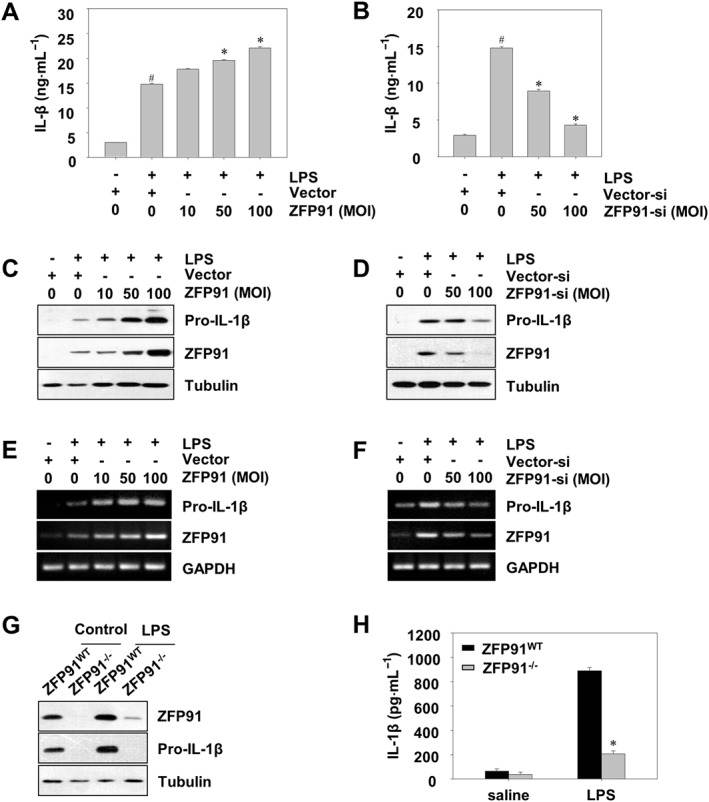
ZFP91 up‐regulates the production and activation of pro‐IL‐1β in LPS‐stimulated THP‐1 cells and BMDMs. (A, C) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using multiplicities of infection (MOI) of 10, 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments. # P < 0.05 versus control group (cultured in medium alone); *P < 0.05 versus LPS‐induced group. The protein levels of pro‐IL‐1β were measured by Western blot analysis. (B, D) PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying siRNA against ZFP91 using MOI of 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments. # P < 0.05 versus control group (cultured in medium alone); *P < 0.05 versus LPS‐induced group. The protein levels of pro‐IL‐1β were measured by Western blot analysis. (E) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using MOI of 10, 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The mRNA level of pro‐IL‐1β was measured by RT‐PCR analysis. (F) PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying siRNA against ZFP91 using MOI of 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The mRNA level of pro‐IL‐1β was measured by RT‐PCR analysis. (G) BMDMs from wild type (WT) or ZFP91 knockout (ZFP91−/−) C57BL/6 mice stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of pro‐IL‐1β were measured by Western blot analysis. (H) BMDMs from WT or ZFP91 knockout (ZFP91−/−) C57BL/6 mice stimulated with 1 μg·mL−1 LPS for 6 h. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments. *P < 0.05 compared with LPS‐treated WT mice.
ZFP91 enhances the expression of non‐canonical caspase‐8 inflammasome components in THP‐1 cells and BMDMs
It has been documented that the canonical caspase‐1 and non‐canonical caspase‐8 inflammasomes may play crucial roles in the activation of IL‐1β (Latz et al., 2013; Chen et al., 2015). We further examined whether ZFP91 promotes the activation of inflammasomes. Intriguingly, overexpression of ZFP91 increased the LPS‐induced expression of NLRP3, ASC, cleaved caspase‐8 proteins and NLRP3, ASC, caspase‐8 mRNA levels dose‐dependently in THP‐1 cells (Figures 2A,C and S2A). However, no apparent cleaved caspase‐1 protein or change of caspase‐1 mRNA levels were observed after overexpression of ZFP91 (Figure 2A,C). Overexpressing ZFP91 also enhanced the NLRP3, ASC and cleaved caspase‐8 proteins in unstimulated THP‐1 cells (Figure S2B). Meanwhile, silencing of ZFP91 reduced the LPS‐induced expression of NLRP3, ASC, cleaved caspase‐8 protein and NLRP3, ASC and caspase‐8 mRNA levels dose‐dependently in THP‐1 cells (Figure 2B,D). To confirm the role of ZFP91 in non‐canonical caspase‐8 inflammasome activation, the effects of ZFP91 deficiency on non‐canonical caspase‐8 inflammasome expression in BMDMs were observed. Notably, the expression of NLRP3, ASC and cleaved caspase‐8 protein was significantly reduced in ZFP91‐deficient BMDMs (Figure 2E). However, no apparent change in cleaved caspase‐1 was observed in ZFP91‐deficient BMDMs (Figure 2E). We next investigate whether caspase‐1 mediates the expression of the caspase‐8 inflammasome component in ZFP91‐deficient BMDMs. The results showed that adding caspase‐1 inhibitor had no effect on NLRP3, ASC and cleaved caspase‐8 protein expression (Figure 2F).
Figure 2.
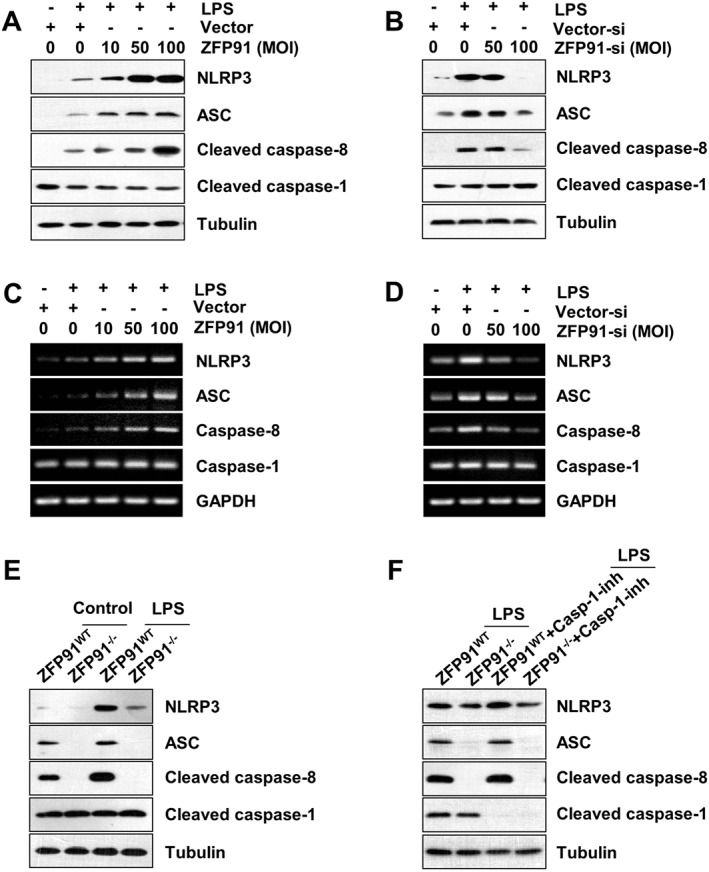
ZFP91 enhances the expression of non‐canonical caspase‐8 inflammasome components in LPS‐stimulated THP‐1 cells and BMDMs. (A, B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using multiplicities of infection (MOI) of 10, 50 and 100 or lentiviruses carrying ZFP91 siRNA using MOI of 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of NLRP3, ASC, cleaved caspase‐8 and cleaved caspase‐1 were measured by Western blot analysis. (C, D) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using MOI of 10, 50 and 100 or lentiviruses carrying ZFP91 siRNA using MOI of 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The mRNA levels of NLRP3, ASC, caspase‐8 and caspase‐1 were measured by RT‐PCR. (E) BMDMs from wild type (WT) or ZFP91 knockout (ZFP91−/−) C57BL/6 mice stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of NLRP3, ASC, cleaved caspase‐8 and cleaved caspase‐1 were measured by Western blot analysis. (F) BMDMs from WT or ZFP91 knockout (ZFP91−/−) C57BL/6 mice stimulated with 1 μg·mL−1 LPS alone or with caspase‐1 inhibitor for 2 h. The protein levels of NLRP3, ASC, cleaved caspase‐8 and cleaved caspase‐1 were measured by Western blot analysis.
ZFP91 promotes the production of IL‐1β in LPS‐stimulated THP‐1 cells via non‐canonical caspase‐8 inflammasome
To determine whether the impact of ZFP91 on IL‐1β production resulted from activation of the non‐canonical caspase‐8 inflammasome, we transduced THP‐1 cells with lentiviruses carrying siRNA against NLRP3, ASC or caspase‐8. The results showed that silencing of NLRP3 did not affect the expression of pro‐IL‐1β in ZFP91‐overexpressing THP‐1 cells after LPS stimulation (Figure 3A), whereas the production of IL‐1β was impaired after NLRP3 was silenced in THP‐1 cells (Figure 3C). Similarly, silencing of ASC reduced the production of IL‐1β in ZFP91‐overexpressing THP‐1 cells after LPS stimulation (Figure 3D) but did not affect the expression of pro‐IL‐1β (Figure 3B). Silencing of caspase‐8 or adding a caspase‐8 inhibitor blocked the production of IL‐1β in ZFP91‐overexpressing THP‐1 cells after LPS stimulation (Figure 3G,H) but did not affect the expression of pro‐IL‐1β (Figure 3E,F). Meanwhile, silencing of NLRP3, ASC or caspase‐8 slightly reduced the production of IL‐1β in THP‐1 cells after LPS stimulation (Figure S3). Thus, we concluded that ZFP91 promotes the production of IL‐1β via the non‐canonical caspase‐8 inflammasome.
Figure 3.
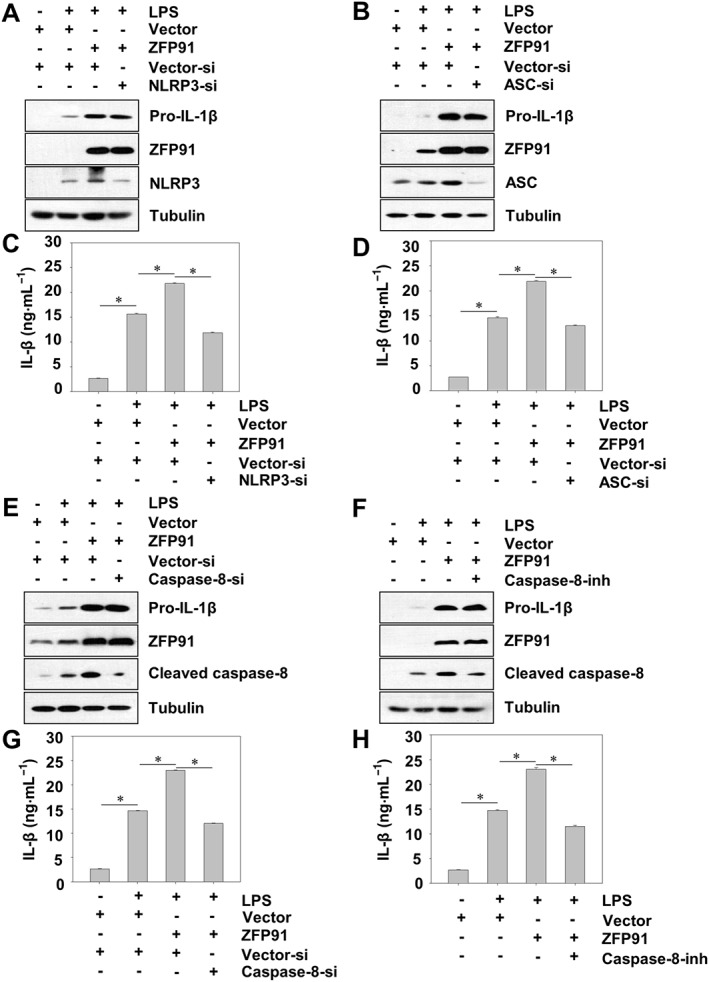
ZFP91 promotes the production of IL‐1β in LPS‐stimulated THP‐1 cells via non‐canonical caspase‐8 inflammasome. (A, C) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) and lentiviruses carrying NLRP3 siRNA (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of pro‐IL‐1β, NLRP3 and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05. (B, D) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) and lentiviruses carrying ASC siRNA (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of pro‐IL‐1β, ASC and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05. (E, G) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) and lentiviruses carrying caspase‐8 siRNA (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of pro‐IL‐1β, cleaved caspase‐8 and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05. (F, H) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS alone (6 h) or with caspase‐8 inhibitor for 2 h. The protein levels of pro‐IL‐1β, cleaved caspase‐8 and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05.
ZFP91 promotes non‐canonical caspase‐8 inflammasome complex assembly in LPS‐stimulated THP‐1 cells
Next, we sought to elucidate the mechanism by which ZFP91 overexpression promoted non‐canonical caspase‐8 inflammasome complex assembly. Here, we examined if ZFP91 could affect the formation of the NLRP3–ASC–caspase‐8 inflammasome complex. Indeed, both NLRP3 and caspase‐8 were detected in the ASC immunoprecipitates from cells stimulated or unstimulated with LPS, and the NLRP3–ASC–caspase‐8 interaction was enhanced by ZFP91 overexpression (Figure 4A), whereas the knockdown of ZFP91 inhibited the association of NLRP3–ASC–caspase‐8 in LPS‐stimulated THP‐1 cells (Figure 4B). Immunofluorescence colocalization revealed that ZFP91 overexpression promoted non‐canonical caspase‐8 inflammasome complex formation in LPS‐stimulated THP‐1 cells (Figure 4C). Taken together, we conclude that ZFP91 promotes association and colocalization of the non‐canonical caspase‐8 inflammasome complex.
Figure 4.
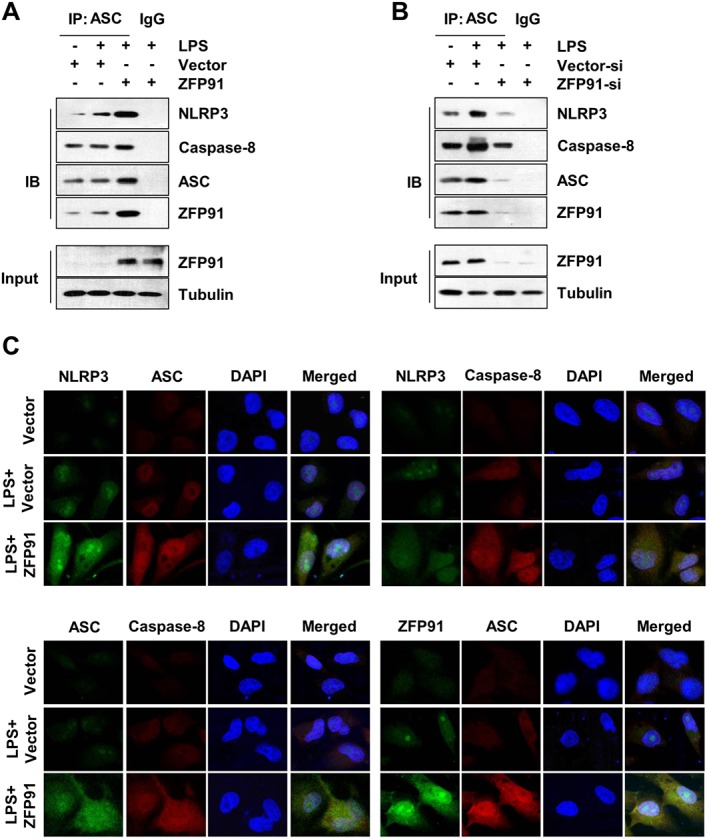
ZFP91 promotes non‐canonical caspase‐8 inflammasome complex assembly in LPS‐stimulated THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. Immunoblot analysis of NLRP3, caspase‐8, ZFP91 and ASC after immunoprecipitation of proteins with antibody to ASC. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying siRNA against ZFP91 (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. Immunoblot analysis of NLRP3, caspase‐8, ZFP91 and ASC after IP of proteins with antibody to ASC. (C) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. Immunofluorescence colocalization was analysed by immunofluorescence cytochemistry. Nuclei were stained with DAPI. Original magnification: ×600.
ZFP91 enhances the activation of MAPKs in THP‐1 cells and BMDMs
Since activation of MAPKs are upstream events leading to de novo synthesis of pro‐IL‐1β (Kim et al., 2004), we tested whether ZFP91 regulates the phosphorylation of MAPKs. Western blot results showed that ZFP91 overexpression significantly increased the LPS‐induced expression of p‐ERK, p‐p38 and p‐JNK dose‐dependently in THP‐1 cells (Figure 5A), whereas the knockdown of ZFP91 decreased the expression of p‐ERK, p‐p38 and p‐JNK dose‐dependently in THP‐1 cells (Figure 5B). Overexpressing ZFP91 also enhanced the p‐ERK, p‐p38 and p‐JNK proteins in unstimulated THP‐1 cells (Figure S4A). To confirm the role of ZFP91 in MAPKs activation, the effects of ZFP91 deficiency on p‐ERK, p‐p38 and p‐JNK expression in BMDMs were observed. The production of p‐ERK, p‐p38 and p‐JNK was significantly reduced in ZFP91‐deficient BMDMs (Figure 5C). The canonical transcriptional factor NF‐κB also mediates regulation of pro‐IL‐1β gene expression (Krishnan et al., 2014), so we examined whether ZFP91 regulates the nuclear translocation of p65. However, we were unable to observe any promotion effects of ZFP91 on p65 nuclear translocation (Figure S4B). Based on these findings, it is concluded that ZFP91 enhances the activation of MAPKs in THP‐1 cells and BMDMs.
Figure 5.
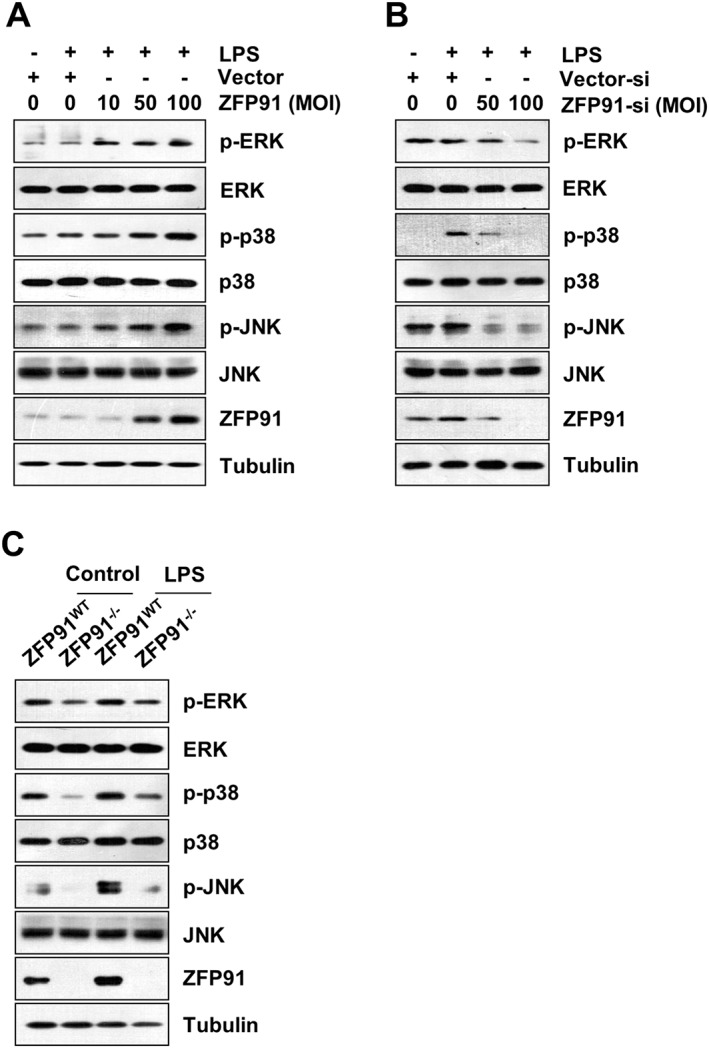
ZFP91 enhances the activation of MAPKs in LPS‐stimulated THP‐1 cells and BMDMs. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using MOI of 10, 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of p‐ERK, ERK, p‐p38, p38, p‐JNK and JNK were measured by Western blot analysis. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying ZFP91 siRNA using MOI of 50 and 100 for 24 h and then stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of p‐ERK, ERK, p‐p38, p38, p‐JNK and JNK were measured by Western blot analysis. (C) BMDMs from wild type (WT) or ZFP91 knockout (ZFP91−/−) C57BL/6 mice stimulated with 1 μg·mL−1 LPS for 6 h. The protein levels of p‐ERK, ERK, p‐p38, p38, p‐JNK and JNK were measured by Western blot analysis.
ZFP91 induces synthesis of pro‐IL‐1β by activation of MAPKs in LPS‐stimulated THP‐1 cells
Next, we investigated the relationship of p‐ERK, p‐p38 and p‐JNK in the synthesis of pro‐IL‐1β in response to ZFP91. Inhibitors of ERK (PD98059), p38 (SB203580) and JNK (SP600125) were added to the medium for 6 h, and expression of pro‐IL‐1β was analysed by western blot. Consistent with previous reports (Kim et al., 2004), adding of ERK, p38 or JNK inhibitor reduced the production of pro‐IL‐1β in THP‐1 cells after LPS stimulation (Figure S5). Meanwhile, it was revealed that treatment of macrophages with an inhibitor of p38 (SB203580), ERK (PD98059) or JNK (SP600125) inhibited the synthesis of pro‐IL‐1β in ZFP91‐overexpressing THP‐1 cells (Figure 6A,C and E). Furthermore, ELISA results revealed that treatment of macrophages with inhibitors of p38 (SB203580), ERK (PD98059) or JNK (SP600125) also inhibited the secretion of IL‐1β (Figure 6B,D and F), supporting the hypothesis that ZFP91 promotes the synthesis of pro‐IL‐1β in THP‐1 cells depending on activation of ERK, p38 and JNK.
Figure 6.
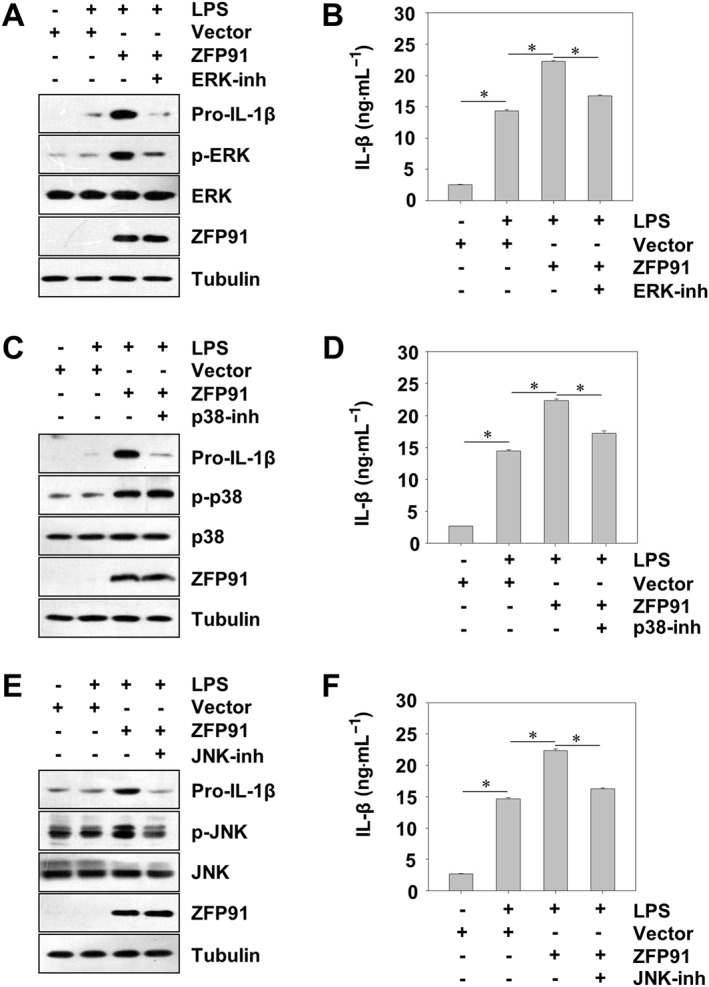
ZFP91 induces synthesis of pro‐IL‐1β by activation of MAPKs in LPS‐stimulated THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS alone or with 50 μM ERK inhibitor for 6 h. The protein levels of pro‐IL‐1β, p‐ERK, ERK and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS alone or with 25 μM p38 inhibitor for 6 h. The protein levels of pro‐IL‐1β, p‐p38, p38 and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05. (C) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then stimulated with 1 μg·mL−1 LPS alone or with 50 μM JNK inhibitor for 6 h. The protein levels of pro‐IL‐1β, p‐JNK, JNK and ZFP91 were measured by Western blot analysis. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments, *P < 0.05.
ZFP91 deficiency ameliorates symptoms and colon inflammation in mice with DSS‐induced colitis
The DSS model is one of the most extensively used animal models to investigate the innate immune mechanisms of colitis (Wirtz et al., 2007). Mice with DSS‐induced colitis exhibited profound weight loss, whereas ZFP91 deficiency could significantly attenuate the loss of body weight (Figure 7A). DSS‐induced colon shortening, a marker of inflammation, was markedly improved by ZFP91 deficiency (Figure 7B,C). Simultaneously, disease activity index (DAI) scores (weight loss, rectal bleeding and stool consistency scores) of ZFP91‐deficient mice were significantly attenuated compared with those of the DSS‐treated WT mice (Figure 7D). The severity of colonicinflammation and ulceration was further evaluated by histopathological analysis using H&E staining. In comparison to DSS‐induced WT mice, ZFP91‐deficient mice exhibited less inflammatory cell infiltration and intact colonic architecture with no apparent ulceration (Figure 7F, line 1). As increased pro‐inflammatory cytokine secretion is a key feature of DSS‐induced colitis (Bauer et al., 2010; Esposito et al., 2014), we also detected inflammatory cytokines secretion in this study. The results showed that the secretion of IL‐1β in the serum was markedly increased after DSS challenge, which was suppressed by ZFP91 deficiency (Figure 7E). Moreover, immunohistochemistry analysis demonstrated that ZFP91‐deficient significantly reduced the number of IL‐1β‐positive cells (brown stained) in colonic mucosa of DSS‐induced mice (Figure 7F, line 2). In ZFP91‐deficient mice, the expressions of pro‐IL‐1β, NLRP3, ASC, cleaved caspase‐8, p‐ERK1/2, p‐JNK and p‐p38 in colon tissues were reduced (Figure 7G–I). Collectively, these data indicated that when there is a deficiency in ZFP91 the non‐canonical caspase‐8 inflammasome expression and phosphorylation of MAPKs are limited and thus DSS‐induced colitis is ameliorated.
Figure 7.
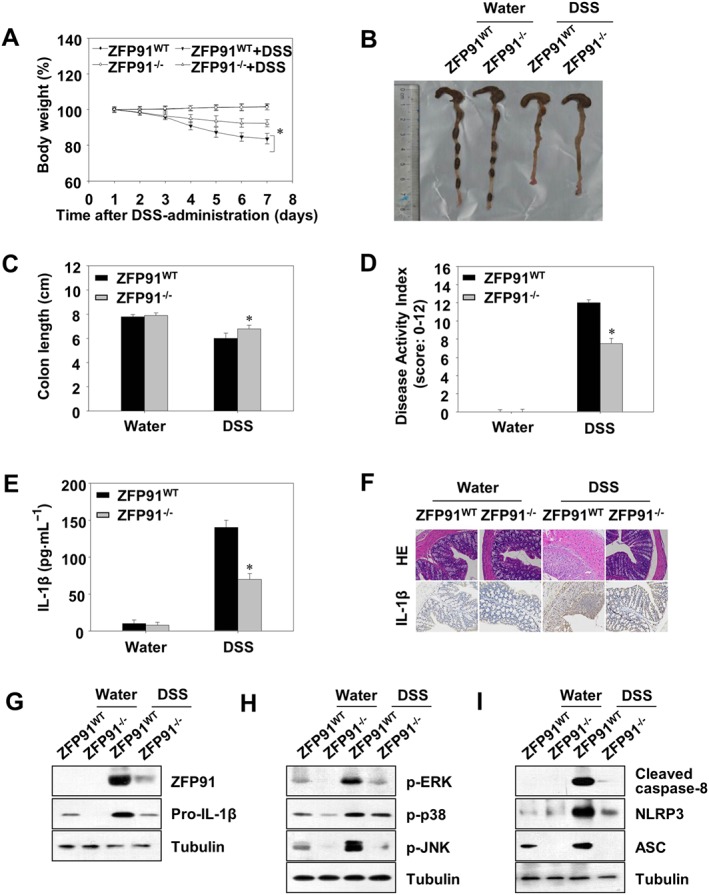
The symptoms and inflammation of DSS‐induced colitis were ameliorated in ZFP91‐deficient mice. Wild type (WT) or ZFP91 knockout (ZFP91−/−) C57BL/6 mice were fed 4% DSS dissolved in drinking water for 5 days. (A) Basal body weight changes of each group (n = 8 per group) after DSS induction of colitis. (B) Macroscopic appearances and (C) colon lengths of the mice were measured. *P < 0.05 compared with DSS‐treated WT mice. (D) Disease activity index. *P < 0.05 compared with DSS‐treated WT mice. (E) The production of inflammation‐related cytokine IL‐1β in serum was determined by ELISA. *P < 0.05 compared with DSS‐treated WT mice. (F) Histopathological changes in colon tissue were examined by H&E staining. The protein expression of IL‐1β in colon tissues was detected by immunohistochemical analysis. Original magnification: ×200. (G–I) Immunoblot analysis of lysates from colon tissue.
ZFP91 deficiency reduces alum‐induced peritonitis
We further investigated whether the biological effects of ZFP91 on IL‐1β production were similar in vivo using a mouse peritonitis model. Peritonitis was induced in mice by i.p. injection of alum (Guarda et al., 2011; Huai et al., 2014). PECs were collected, and alum‐induced recruitment of inflammatory cells was analysed by flow cytometry. As IL‐1β, IL‐6 and TNF‐α are associated with peritonitis (Guarda et al., 2011; Huai et al., 2014), we investigated the effects of ZFP91 on the expression of these cytokines in the mouse peritonitis model. Alum challenge greatly upregulated the secretion of IL‐1β, IL‐6 and TNF‐α in the lavage fluid, which was significantly reduced in ZFP91 deficient mice (Figure 8A–C), consistent with the in vitro observations. In addition, the expression of pro‐IL‐1β, NLRP3, ASC, cleaved caspase‐8, p‐ERK1/2, p‐JNK and p‐p38 was also reduced in the PECs lysates from ZFP91‐deficient mice (Figure 8D–F). We consequently analysed alum‐induced recruitment of inflammatory cells in the lavage fluid. Alum‐induced recruitment of neutrophils and Ly6C+ monocytes was strongly reduced in the ZFP91‐deficient mice (Figure 8G,H). Collectively, these findings further demonstrated that in ZFP91‐deficient mice non‐canonical caspase‐8 inflammasome activity, phosphorylation of MAPKs and subsequent immune cell accumulation are inhibited in an in vivo model of peritonitis.
Figure 8.
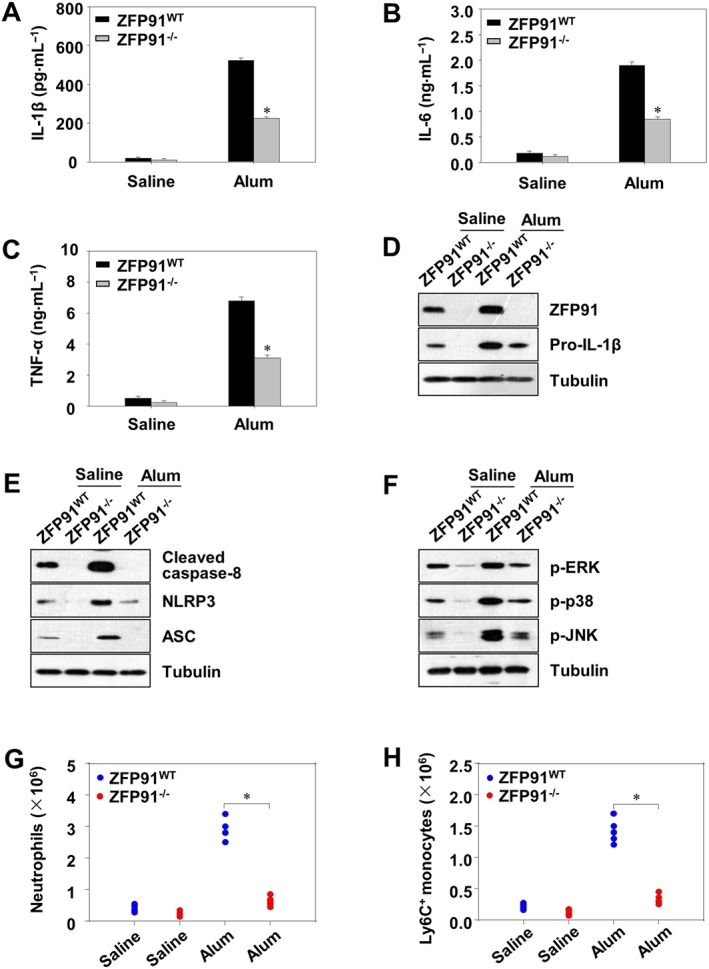
Alum‐induced peritonitis as reduced in ZFP91‐deficient mice. Wild type (WT) or ZFP91 knockout (ZFP91−/−) C57BL/6 mice were i.p. injected with alum (700 μg per mouse) for 12 h. (A–C) The levels of IL‐1β, TNF‐α and IL‐6 in peritoneal lavage fluid was measured by ELISA after the i.p. injection of alum. *P < 0.01 compared with alum‐treated WT mice. (D–F) PECs were lysed and analysed for the expression of proteins by immunoblot. (G, H) Absolute numbers of neutrophils or Ly6C+ monocytes recruited to the peritoneum were evaluated by fluorescence‐activated cell sorting (FACS) analysis. *P < 0.05 compared with alum‐treated WT mice.
Discussion
IL‐1β is a pro‐inflammatory cytokine produced by activated macrophages and monocytes. It functions in the generation of systemic and local responses to infection, injury and immunological challenges by generating fever, activating lymphocytes and promoting migration of leukocytes into the site of injury or infection (Burns et al., 2003; Zaki et al., 2010). It is the primary cause of chronic and acute inflammation, and, as an endogenous pyrogen, it is a key player in the febrile response (Dinarello, 2000; Dreskin et al., 2001). This study advances our knowledge of the mechanism of IL‐1β production in ZFP91‐overexpressing or deleted cells and indicates that ZFP91 plays an important role in inflammation, infection and autoimmunity. We found that overexpression of ZFP91 triggers increased production of pro‐IL‐1β and the secretion of IL‐1β, enhances the expression of non‐canonical caspase‐8 inflammasome components, promotes non‐canonical caspase‐8 inflammasome complex assembly and increases expression of p‐p38, p‐JNK and p‐ERK.
NLR‐mediated activation of caspase‐1 is believed to be responsible for pro‐IL‐1β processing in response to multiple stimuli. The data described in this study demonstrate that caspase‐1 did not seem to be involved in pro‐IL‐1β processing in ZFP91‐overexpressing macrophages. More specifically, we provide evidence that caspase‐8 mediates pro‐IL‐1β processing in ZFP91‐overexpressing macrophages. As an initiating and apical activating caspase, caspase‐8 is well known for its important role in apoptosis (McIlwain et al., 2015). Interestingly, recent studies have found that caspase‐8 is also involved in modulating IL‐1β maturation. Maelfait et al. (2008) observed that activation of the TRIF (TIR domain‐containing adapter‐inducing IFN‐β) signalling pathway by Toll‐like receptor 3 (TLR3) or TLR4 induced a caspase‐8 signalling pathway that, when combined with cyclohexamide‐mediated inhibition of protein translation, was sufficient to drive efficient pro‐IL‐1β processing even in caspase‐1‐knockout macrophages. Moreover, it has been found that caspase‐8 is an apical mediator for the canonical and non‐canonical NLRP3 inflammasome activation induced by LPS or Citrobacter rodentium (Gurung et al., 2014). In addition, caspase‐8 promotes NLRP1/NLRP3 inflammasome activation and TLR4‐mediated IL‐1β production in acute glaucoma (Chi et al., 2014). These studies indicate a very complex role of caspase‐8 in several important signalling pathways.
The function of the non‐canonical caspase‐8 inflammasome has also been explored. Chen et al. (2015) observed that the internalized Cryptococcus neoformans activated the non‐canonical NLRP3–ASC–caspase‐8 inflammasome, which resulted in robust IL‐1β secretion from caspase‐1‐deficient primary dendritic cells. Moreover, it has been found that HMGB1 led to the activation of non‐canonical caspase‐8 inflammasomes and the processing of pro‐IL‐1β in retinal ischaemia reperfusion injury (Chi et al., 2015). Dectin‐1 acted as an extracellular sensor for pathogens that induced both IL‐1β production and maturation through a non‐canonical caspase‐8‐dependent inflammasome in protective immunity (Gringhuis et al., 2012). These studies indicate that non‐canonical caspase‐8 inflammasomes play an important role in pro‐IL‐1β processing. In our study, ZFP91 promotes the production of the non‐canonical caspase‐8 inflammasome. The production of IL‐1β is regulated by ZFP91 via non‐canonical caspase‐8 inflammasome in LPS‐stimulated THP‐1 cells. Moreover, we found that ZFP91 promotes the assembly of the NLRP3–caspase‐8–ASC inflammasome in LPS‐stimulated THP‐1 cells.
Recent studies suggest that MAP kinases including ERK, JNK and p38 modulate the production of inflammatory cytokines, including IL‐1β. Garcia et al. (1998) reported that Mycoplasma fermentans‐derived membrane lipoproteins or M. fermentans‐derived synthetic lipopeptide induces cytokine (IL‐1β and TNF‐α) secretion in macrophages via the activation of p38 and ERK MAPK pathways (Rawadi et al., 1998). Li et al. (2006) reported that hyperosmolarity is a pro‐inflammatory stimulus on the corneal epithelium, increasing the expression and production of pro‐inflammatory cytokines (IL‐1β and TNF‐α), a process that may be mediated through activation of the JNK and ERK MAPK signalling pathways. Moreover, ERK, JNK or p38 inhibition led to statistically significant decreases in both pro‐IL‐1β and mature IL‐1β levels compared with the relevant forms of IL‐1β in cells treated with LPS alone (Kim et al., 2004). In the present study, we sought to determine the role of MAPKs in the production of pro‐IL‐1β and IL‐1β. Our data showed that ERK, JNK and p38 inhibitors reduced the production of pro‐IL‐1β and IL‐1β in ZFP91‐overexpressing THP‐1 macrophages.
In conclusion, ZFP91 could promote the production and activation of pro‐IL‐1β. These effects appear to be produced by promoting activation of MAPKs and non‐canonical caspase‐8 inflammasome pathways. Furthermore, our results suggest that ZFP91 could be a potential target for the therapeutic modulation of inflammatory, infectious and autoimmune diseases.
Author contributions
C.M. is the primary investigator in this study. Z.W. and M.Y.L. performed part of culture cell experiments. Z.H.Z. and H.X.Z. performed part of animal experiments. J.M. and X.J. designed this study and wrote the paper.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
supportingInfo_Item
Figure S1 ZFP91 up‐regulates the production and activation of pro‐IL‐1β in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using multiplicities of infection (MOI) of10, 50, and 100 for 24 hand then stimulated with 1 μg/ml LPS for 6 h. The protein levelsof IL‐1βwere measured by western blot analysis. (B)PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 genefor 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. The protein levels of IL‐1βand pro‐IL‐1β were measured by western blot analysis.
Figure S2 ZFP91 enhances the expression of non‐canonical caspase‐8 inflammasome components in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using MOI of 10, 50, and 100 for 24 h and then stimulated with 1 μg/mlLPS for 6 h. The protein levels of caspase‐8 and cleaved caspase‐8 were measured by western blot analysis. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. The protein levels of NLRP3, ASC, and cleaved caspase‐8 were measured by western blot analysis.
Figure S3 Silencing of NLRP3, ASC or caspase‐8 reduced the production of IL‐1β in LPS‐stimulated THP‐1 cells. PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying NLRP3, ASC or caspase‐8 siRNA (MOI = 100) for 24 h and then stimulated with 1 μg/ml LPS for 6 h. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments. ### P < 0.001 vs. control group (cultured in medium alone); *P < 0.05 vs. LPS‐induced group. The protein levels of pro‐IL‐1β, NLRP3, ASC and cleaved caspase‐8 were measured by western blot analysis.
Figure S4 ZFP91 enhances the activation of MAPKs in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/mlLPS for 6 h. The protein levels of p‐ERK, p‐p38,p‐JNK, ERK, p38 and JNK were measured by western blot analysis. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. Nuclear extracts were analyzed by western blot using indicated antibodies for p65 and Topo‐I.Cytoplasmic extracts were analyzed by western blot using indicated antibodies for p65 and tubulin.
Figure S5 The inhibitor of ERK, p38, or JNK reduced the production of pro‐IL‐1βin LPS‐stimulated THP‐1 cells. PMA‐differentiated THP‐1 cells were stimulated with 1 μg/ml LPS alone or with ERK inhibitor (50 μM), p38 inhibitor (25 μM), JNK inhibitor (50 μM) for 6 h. The protein levels of pro‐IL‐1β, p‐ERK, ERK,p‐p38, p38, p‐JNK and JNK were measured by western blot analysis.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (81760657 and 81660608), the Jilin Province Science and Technology Development Outstanding Young Talents (20180520057JH) and the Yanbian University Youth Research Fund Project (2017.No. 31).
Mi, C. , Wang, Z. , Li, M. Y. , Zhang, Z. H. , Ma, J. , and Jin, X. (2018) Zinc finger protein 91 positively regulates the production of IL‐1β in macrophages by activation of MAPKs and non‐canonical caspase‐8 inflammasome. British Journal of Pharmacology, 175: 4338–4352. 10.1111/bph.14493.
Contributor Information
Juan Ma, Email: majuan@ybu.edu.cn.
Xuejun Jin, Email: xjjin@ybu.edu.cn.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ et al (2015). Caspase‐8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem 290: 20167–20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M et al (2010). Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Burns K, Martinon F, Tschopp J (2003). New insights into the mechanism of IL‐1beta maturation. Curr Opin Immunol 15: 26–30. [DOI] [PubMed] [Google Scholar]
- Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C et al (2015). Internalized Cryptococcus neoformans activates the canonical caspase‐1 and the noncanonical caspase‐8 inflammasomes. J Immunol 195: 4962–4972. [DOI] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y (2015). HMGB1 promotes the activation of NLRP3 and caspase‐8 inflammasomes via NF‐kappaB pathway in acute glaucoma. J Neuroinflammation 12: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X et al (2014). Caspase‐8 promotes NLRP1/NLRP3 inflammasome activation and IL‐1β production in acute glaucoma. Proc Natl Acad Sci U S A 111: 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2000). Proinflammatory cytokines. Chest 118: 503–508. [DOI] [PubMed] [Google Scholar]
- Dreskin SC, Thomas GW, Dale SN, Heasley LE (2001). Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP‐1) cells. J Immunol 166: 5646–5653. [DOI] [PubMed] [Google Scholar]
- Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A et al (2014). Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4‐dependent PPAR‐α activation. Gut 63: 1300–1312. [DOI] [PubMed] [Google Scholar]
- Garcia J, Lemercier B, Roman‐Roman S, Rawadi G (1998). A mycoplasma fermentans‐derived synthetic lipopeptide induces AP‐1 and NF‐kappaB activity and cytokine secretion in macrophages via the activation of mitogen‐activated protein kinase pathways. J Biol Chem 273: 34391–34398. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T et al (2012). Dectin‐1 is an extracellular pathogen sensor for the induction and processing of IL‐1β via a noncanonical caspase‐8 inflammasome. Nat Immunol 13: 246–254. [DOI] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I et al (2011). Type I interferon inhibits interleukin‐1 production and inflammasome activation. Immunity 34: 213–223. [DOI] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP et al (2014). FADD and caspase‐8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192: 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Kanneganti TD (2015). Novel roles for caspase‐8 in IL‐1β and inflammasome regulation. Am J Pathol 185: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai W, Zhao R, Song H, Zhao J, Zhang L, Zhang L et al (2014). Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun 5: 4738. [DOI] [PubMed] [Google Scholar]
- Jin HR, Jin X, Lee JJ (2010a). Zinc‐finger protein 91 plays a key role in LIGHT‐induced activation of non‐canonical NF‐κB pathway. Biochem Biophys Res Commun 400: 581–586. [DOI] [PubMed] [Google Scholar]
- Jin X, Jin HR, Jung HS, Lee SJ, Lee JH, Lee JJ (2010b). An atypical E3 ligase zinc finger protein 91 stabilizes and activates NF‐kappaB‐inducing kinase via Lys63‐linked ubiquitination. J Biol Chem 285: 30539–30547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Smith CJ, Van Eldik LJ (2004). Importance of MAPK pathways for microglial pro‐inflammatory cytokine IL‐1 beta production. Neurobiol Aging 25: 431–439. [DOI] [PubMed] [Google Scholar]
- Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR (2014). IL‐1β and IL‐18: inflammatory markers or mediators of hypertension? Br J Pharmacol 171: 5589–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A (2013). Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC (2006). JNK and ERK MAP kinases mediate induction of IL‐1β, TNF‐α and IL‐8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res 82: 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Mi C, Wang KS, Lee JJ, Jin X (2016). Zinc finger protein 91 (ZFP91) activates HIF‐1α via NF‐κB/p65 to promote proliferation and tumorigenesis of colon cancer. Oncotarget 7: 36551–36562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S et al (2008). Stimulation of Toll‐like receptor 3 and 4 induces interleukin‐1beta maturation by caspase‐8. J Exp Med 205: 1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW (2015). Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 7: a026716. 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monie TP, Bryant CE (2015). Caspase‐8 functions as a key mediator of inflammation and pro‐IL‐1β processing via both canonical and non‐canonical pathways. Immunol Rev 265: 181–193. [DOI] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA (2015). Inflammasome‐independent regulation of IL‐1‐family cytokines. Annu Rev Immunol 33: 49–77. [DOI] [PubMed] [Google Scholar]
- Paschke L, Rucinski M, Ziolkowska A, Zemleduch T, Malendowicz W, Kwias Z et al (2014). ZFP91‐a newly described gene potentially involved in prostate pathology. Pathol Oncol Res: POR 20: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawadi G, Ramez V, Lemercier B, Roman‐Roman S (1998). Activation of mitogen‐activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J Immunol 160: 1330–1339. [PubMed] [Google Scholar]
- Saotome Y, Winter CG, Hirsh D (1995). A widely expressed novel C2H2 zinc‐finger protein with multiple consensus phosphorylation sites is conserved in mouse and man. Gene 152: 233–238. [DOI] [PubMed] [Google Scholar]
- Tompkins V, Hagen J, Zediak VP, Quelle DE (2006). Identification of novel ARF binding proteins by two‐hybrid screening. Cell Cycle 5: 641–646. [PubMed] [Google Scholar]
- Unoki M, Okutsu J, Nakamura Y (2003). Identification of a novel human gene, ZFP91, involved in acute myelogenous leukemia. Int J Oncol 22: 1217–1223. [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA (2011). Inflammasome activation and IL‐1β and IL‐18 processing during infection. Trends Immunol 32: 110–116. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007). Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD (2010). The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supportingInfo_Item
Figure S1 ZFP91 up‐regulates the production and activation of pro‐IL‐1β in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using multiplicities of infection (MOI) of10, 50, and 100 for 24 hand then stimulated with 1 μg/ml LPS for 6 h. The protein levelsof IL‐1βwere measured by western blot analysis. (B)PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 genefor 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. The protein levels of IL‐1βand pro‐IL‐1β were measured by western blot analysis.
Figure S2 ZFP91 enhances the expression of non‐canonical caspase‐8 inflammasome components in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene using MOI of 10, 50, and 100 for 24 h and then stimulated with 1 μg/mlLPS for 6 h. The protein levels of caspase‐8 and cleaved caspase‐8 were measured by western blot analysis. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. The protein levels of NLRP3, ASC, and cleaved caspase‐8 were measured by western blot analysis.
Figure S3 Silencing of NLRP3, ASC or caspase‐8 reduced the production of IL‐1β in LPS‐stimulated THP‐1 cells. PMA‐differentiated THP‐1 cells were transduced with lentiviruses carrying NLRP3, ASC or caspase‐8 siRNA (MOI = 100) for 24 h and then stimulated with 1 μg/ml LPS for 6 h. IL‐1β was detected by ELISA. Data are the mean ± SD of five independent experiments. ### P < 0.001 vs. control group (cultured in medium alone); *P < 0.05 vs. LPS‐induced group. The protein levels of pro‐IL‐1β, NLRP3, ASC and cleaved caspase‐8 were measured by western blot analysis.
Figure S4 ZFP91 enhances the activation of MAPKs in THP‐1 cells. (A) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/mlLPS for 6 h. The protein levels of p‐ERK, p‐p38,p‐JNK, ERK, p38 and JNK were measured by western blot analysis. (B) PMA‐differentiated THP‐1 cells were transduced with lentiviruses containing ZFP91 gene (MOI = 100) for 24 h and then un‐stimulated or stimulated with 1 μg/ml LPS for 6 h. Nuclear extracts were analyzed by western blot using indicated antibodies for p65 and Topo‐I.Cytoplasmic extracts were analyzed by western blot using indicated antibodies for p65 and tubulin.
Figure S5 The inhibitor of ERK, p38, or JNK reduced the production of pro‐IL‐1βin LPS‐stimulated THP‐1 cells. PMA‐differentiated THP‐1 cells were stimulated with 1 μg/ml LPS alone or with ERK inhibitor (50 μM), p38 inhibitor (25 μM), JNK inhibitor (50 μM) for 6 h. The protein levels of pro‐IL‐1β, p‐ERK, ERK,p‐p38, p38, p‐JNK and JNK were measured by western blot analysis.


