Abstract
Background and Purpose
The endocannabinoid (EC) system has been implicated in the pathogenesis of diabetic nephropathy (DN). We investigated the effects of peripheral blockade of the cannabinoid CB1 receptor as an add‐on treatment to ACE‐inhibition in type 1 diabetic mice (DM) with established albuminuria.
Experimental Approach
Renal functional parameters (albumin excretion rate, creatinine clearance), tubular injury, renal structure, both EC and CB receptor levels and markers of podocyte dysfunction, fibrosis and inflammation were studied in streptozotocin‐induced DM treated for 14 weeks with vehicle, the ACE‐inhibitor perindopril (2 mg·kg−1·day−1), peripherally‐restricted CB1 receptor antagonist AM6545 (10 mg·kg−1·day−1) or both. Treatments began at 8 weeks after diabetes onset, when early DN is established.
Key Results
CB1 receptors were overexpressed in DM and neither perindopril nor AM6545 altered this effect, while both drugs abolished diabetes‐induced overexpression of angiotensin AT1 receptors. Single treatment with either AM6545 or perindopril significantly reduced progression of albuminuria, down‐regulation of nephrin and podocin, inflammation and expression of markers of fibrosis. However, reversal of albuminuria was only observed in mice administered both treatments. The ability of the combination therapy to completely abolish slit diaphragm protein loss, monocyte infiltration, overexpression of inflammatory markers and favour macrophage polarization towards an M2 phenotype may explain this greater efficacy. In vitro experiments confirmed that CB1 receptor activation directly inhibits retinoic acid‐induced nephrin expression in podocytes and IL‐4‐induced M2 polarization in macrophages.
Conclusion and Implications
Peripheral CB1 receptor blockade used as add‐on treatment to ACE‐inhibition reverses albuminuria, nephrin loss and inflammation in DM.
Abbreviations
- 2‐AG
2‐arachidonoylglycerol
- ACE‐I
ACE inhibitors
- AEA
anandamide
- DM
Diabetic
- DN
diabetic nephropathy
- EC
endocannabinoid
- ECS
endocannabinoid system
- MCP‐1
monocyte chemoattractant protein‐1
- RAS
renin angiotensin system
- STZ
streptozotocin
Introduction
Diabetic nephropathy (DN) is the leading cause of end‐stage renal failure in the Western World and increases the risk of cardiovascular morbidity and mortality in subjects with diabetes (IDF Diabetes Atlas, 2017). Both glycaemic control and inhibition of the renin angiotensin system (RAS) with either ACE inhibitors (ACE‐I) or http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=34 blockers are established treatments for diabetic kidney disease (Fineberg et al., 2013). Despite proven efficacy of these therapies, they are insufficient to completely arrest the progression of the disease and there is, thus, the need to identify additional therapeutic tools.
DN is characterized by increased glomerular permeability to proteins and the accumulation of extracellular matrix components in the mesangium, resulting in albuminuria and a progressive decline in renal function (Forbes and Cooper, 2013; Tuttle et al., 2014). Podocytes are components of the glomerular filtration barrier and the slit diaphragm, a junction connecting neighbouring podocytes, is considered the major restriction site to protein filtration. Loss of slit diaphragm proteins, such as nephrin and podocin, plays a key role in the pathogenesis of albuminuria in diabetes (Huh et al., 2002; Nagata, 2016). In addition, low‐grade inflammation occurs in the diabetic glomeruli and is believed to amplify the glomerular injury (Barutta et al., 2015; Wada and Makino, 2016).
Recent studies in experimental diabetes have demonstrated that a full endocannabinoid system (ECS) is present in the kidney and that, beyond worsening metabolism, a malfunction of the ECS plays a direct role in the pathogenesis of DN (Janiak et al., 2007; Barutta et al., 2010, 2011, 2014; Russell et al., 2010; Tam et al., 2010; Lim et al., 2011; Nam et al., 2012; Jourdan et al., 2014; Lecru et al., 2015; Gruden et al., 2016; Zoja et al., 2016). The ECS comprises two endogenous cannabinoids, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=729, the enzymes that regulate their production and degradation, and two GPCRs, cannabinoid type 1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57 receptors, through which they signal. In both animals and humans with diabetes, CB1 receptors are overexpressed in the diabetic glomeruli, predominantly by podocytes (Barutta et al., 2010; Jourdan et al., 2014; Lecru et al., 2015). Enhanced CB1 receptor signalling has both prosclerotic and inflammatory effects, and studies in various animal models of diabetes have demonstrated that both genetic and pharmacological deletion of the CB1 receptor can ameliorate proteinuria and glomerulosclerosis (Janiak et al., 2007; Barutta et al., 2010; Nam et al., 2012; Jourdan et al., 2014).
Although the use of pharmacological compounds that induce global CB1 receptor blockade is not a viable therapeutic option in humans because of their unwanted central side effects, peripherally‐restricted CB1 receptor antagonists, which poorly cross the blood–brain barrier, have recently been developed. These compounds are devoid of psychoactive effects, but they appear to retain the beneficial effects of peripheral CB1 receptor antagonism. Indeed, two peripherally‐restricted CB1 receptor blockers, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10029 and JD5037, have recently been shown to ameliorate DN in animals, raising the hope that CB1 receptor antagonism may represent a potential new tool for the treatment of DN (Jourdan et al., 2014; Barutta et al., 2017).
Multiple levels of interaction between the ECS and RAS have been reported in various cell types. Angiotensin II (Ang‐II) can induce both CB1 receptor expression and the release of EC. Moreover, heterodimers can form between the AT1 and CB1 receptor (Rozenfeld et al., 2011; Jourdan et al., 2014). Whether the combination of peripheral CB1 receptor blockade and RAS inhibition would result in additional benefit in DN is as yet unclear, and this is a highly relevant question in view of potential future studies of CB1 receptor antagonists in humans.
Therefore, in the present study we investigated the effects of the peripherally‐restricted CB1 receptor antagonist AM6545 as an add‐on treatment to the ACE‐I http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6367 in type 1 diabetic mice (DM) with established albuminuria to assess if the combined treatment has an enhanced efficacy.
Methods
Animals and induction of diabetes
Male C57BL6/J mice from Jackson Laboratories (Bar Harbor, ME, USA) were maintained on a normal diet under standard animal housing conditions. Both housing and care of laboratory animals were in accordance with Italian law (D.L.116/1992), and the study was approved by the Ethical Committee of the University of Turin. Diabetes was induced in mice, aged 8 weeks, by an i.p. injection of streptozotocin (STZ) dissolved in citrate buffer pH 4.5 (55 mg·kg−1 body wt·day−1) and delivered in five consecutive daily doses on sedated animals. Mice sham‐injected with sodium citrate buffer were used as controls. Diabetes onset was confirmed by blood glucose levels >2.50 mg·mL−1 4 weeks after the first dose of STZ (success rate 95%).
Experimental protocol
Treatments began at 8 weeks after diabetes onset, when early DN is established, and continued for 14 weeks. Diabetic (DM) animals were allocated to treatment with vehicle, perindopril, AM6545 or both (six mice per group). Control [non‐diabetic (ND)] animals were treated with vehicle or perindopril plus AM6545 (six mice per group). Perindopril was dissolved in distilled water and given by gavage at dose of 2 mg·kg−1. AM6545 was administered daily at the dosage of 10 mg·kg−1. Although AM6545 can be given p.o., i.p. administration was employed to ensure that mice were receiving the established drug dose. Doses were chosen on the basis of previous studies that have proven both their efficacy and selectivity in mice (Barutta et al., 2017). All animals underwent both oral gavages and i.p. injections of either vehicles (water – mixture 18:1:1 of saline/emulphor‐620/DMSO) or active drugs dissolved in vehicle. After 14 weeks of treatment, mice were anesthetized using flurane and then killed by decapitation. The kidneys were rapidly dissected and weighed. The right kidney was frozen in N2 and stored at −80°C for both mRNA and protein analysis. Half of the left kidney was fixed in 10% PBS‐formalin then paraffin‐embedded for light microscopy; the remaining tissue was embedded in optimal cutting temperature compound and snap‐frozen in N2.
Metabolic and physiological parameters
Systolic BP was assessed by tail‐cuff plethysmography (failure rate <5%) in pre‐warmed un‐anaesthetized animals, which were acclimatized to the procedure for at least five consecutive days prior to measurements. Glucose levels were measured using a glucometer (Accu‐chek; Roche Applied Science) on a drop of blood taken from 4 h fasted animals the day prior to them being killed. At the time of death, blood samples were taken via a saphenous vein puncture on sedated mice for glycated haemoglobin and serum creatinine measurements by quantitative immunoturbidimetric latex determination (Sentinel Diagnostic, Milan, Italy) and HPLC respectively. Urine was collected over 18 h, with each mouse individually housed in a metabolic cage and provided with food and water ad libitum. Urine collections were performed before treatment and at the end of the study. Urinary albumin concentration was measured by a mouse albumin elisa kit (Bethyl Laboratories, USA). Urinary glucose was measured by the glucose‐oxidase method using a CS‐400 auto‐chemistry analyser. Creatinine clearance was calculated from serum and urine creatinine concentrations, as determined by HPLC according to ADMCC guidelines (Dunn et al., 2004).
Histological analysis
Paraffin‐embedded tissue sections were stained using Periodic acid–Schiff (PAS) staining. The mesangial area was analysed (percentage of glomerular area) from digital pictures of 15 glomeruli per kidney per animal using Axiovision 4.7 software. Glomerular collagen was assessed by picrosirius red staining and the % area of staining quantified by a computer‐aided image analysis system (Axiovision‐software), whereby 15 glomeruli for each section were analysed. Evaluations were performed in a blinded fashion by two investigators.
mRNA analysis
Total RNA was extracted from the renal cortex using the TRIzol reagent (Invitrogen, Milan, Italy). One microgram of total RNA was reverse transcribed into cDNA using the high‐capacity reverse transcription kit from Applied Biosystems (Monza, Italy). Expression of nephrin (Mm00497828), podocin (Mm00499929), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6754 (Mm01256744), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4898 (Mm00483937), CB1 receptors (Mm01212171), CB2 receptors (Mm00438286), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6757 (Mm00516023), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 (Mm00441242), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=59 (Mm00438270), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 (Mm00443258), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1244 (Mm00475988), AT1 receptors (Mm00616371), CD68 (Mm03047340), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4408 (Mm00441258), CD206 (Mm01329362), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250 (Mm00440502) and CD163 (Mm00474091) was measured by real‐time PCR using pre‐developed TaqMan reagents (Applied Biosystems, Monza, Italy). Raw Ct values were calculated using the SDS software and standardized to the expression of hypoxanthine‐guanine phosphoribosyltransferase. Relative expression was calculated using the comparative Ct method (2‐ΔΔCt). Because housekeeping genes ubiquitously expressed in the renal cortex do not control for variations in the glomerular number per specimen or changes in podocyte number, WT‐1, a podocyte‐specific gene, was used as endogenous reference for the evaluation of nephrin and podocin expression.
Immunofluorescence
Sections were fixed in cold acetone for 5 min and blocked in 3% BSA. Subsequently, sections were incubated for an hour with primary antibodies directed against Anti‐Nephrin (AB_1542487), Anti‐Podocin (AB_261982) and Anti‐Fibronectin (AB_476976). Following washing, FITC‐conjugated donkey anti‐guinea pig (Santa Cruz, Glostrup, Denmark) and swine‐anti rabbit (DAKO, Glostrup, Denmark) secondary antibodies were added. Sections were examined using an Olympus epifluorescence microscope (Olympus Bx4I), digitized with a high‐resolution camera (Carl Zeiss, Oberkochen, Germany) and quantitated using image analysis software (Axiovision 4.7, Zeiss). Results were calculated as percentage positively stained tissue within the glomerular tuft. On average, 20 randomly selected hilar glomerular tuft cross sections were assessed per mouse.
Double immunofluorescence
After being blocked in avidin–biotin, sections were incubated overnight with an anti‐CB1 receptor antibody, followed by incubation with a biotinylated swine anti‐rabbit IgG antibody (DAKO, Glostrup, Denmark) and fluorescein isothiocyanate‐conjugated streptavidin (Invitrogen, Milan, Italy). Sections were then exposed to an anti‐http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6619 antibody for 1 h, followed by incubation with Alexa 555‐conjugated donkey anti‐rat antibody (Invitrogen, Milan, Italy).
Immunohistochemistry
Staining for F4/80 was performed on frozen sections. CB1 and CB2 receptor, MAC‐2 and p57 expression was detected on 4 μm paraffin sections of formalin‐fixed tissue. After antigen retrieval in citrate buffer or fixation in acetone for 10 min, sections were exposed to H2O2 to eliminate endogenous peroxidase activity and blocked with avidin‐biotin and BSA. The kidney sections were then incubated overnight at 4°C with primary antibodies against Anti‐CB1‐receptors (AB_10098690), Anti‐CB2‐receptors (AB_10079370), Anti‐MAC2 (AB_10060357), Anti‐P57 (AB_2078155) and Anti‐F4/80 (AB_2098196). The sections were washed with PBS and exposed to secondary antibody, HRP‐labelled donkey anti‐rat IgG (Jackson ImmunoResearch Laboratories, West Grove, USA) or HRP‐labelled swine anti‐rabbit IgG (DAKO, Glostrup, Denmark) for 1 h, followed by streptavidin–HRP for 1 h. Diaminobenzidine was used as a chromogen. Results are expressed either as the number of positive cells or as percentage area of positive staining per glomerulus. Evaluations were performed by two investigators in a blinded fashion.
Immunoblotting
Renal cortex specimens were lysed in RIPA buffer, containing 0.5% NP40 (v.v‐1), 0.5% sodium deoxycholate (w.v‐1), 0.1% SDS (w.v‐1), 10 mmol·L−1 EDTA and proteases inhibitors. Protein extracts were obtained by centrifugation at 14 000× g for 20 min at 4°C, preceded by a 45 min incubation period on ice. Total protein concentration was determined using the DC Protein Assay Kit (Bio‐Rad, Milan, Italy). Proteins were separated by SDS‐PAGE and electrotransferred to nitrocellulose membrane. Following blocking in 5% non‐fat milk in TBS (pH 7.6), membranes were incubated overnight with primary antibodies directed against nephrin, podocin and Anti‐AT1‐receptors (AB_2305402) overnight at 4°C. After being washed, secondary HRP‐linked antibodies (Santa Cruz) were added for 1 h. Detection was performed using either the ECL chemiluminescence substrate (GE Healthcare, Milan, Italy) or SuperSignal west pico (Euroclone, Milan, Italy) and visualized on a Gel‐Doc system (Bio‐Rad). Band intensities were quantified by densitometry. Tubulin was used as internal control.
elisa
Levels of TNF‐α, ICAM‐1 and MCP‐1 were measured in renal tissue homogenates by commercially available elisa following the manufacturer's instructions: mouse TNF‐α elisa kit (MyBiosource, San Diego, CA, USA), mouse MCP‐1/CCL2 elisa kit (R&D System, Oxon, UK) and mouse ICAM‐1/CD54 Quantikine elisa kit (R&D System). Levels of cytokines in renal tissue were normalized to total protein concentrations.
Podocyte apoptosis
Apoptosis was assessed by the TUNEL method using the ApopTag In Situ Apoptosis Detection Kit (Millipore, Billerica, MA). Results are expressed as the number of positive cells per glomerulus counted in at least 20 randomly selected glomeruli. Slides pretreated with 20 000 units·mL−1 of DNase were used as a positive control.
Extraction and quantification of endocannabinoids and EC‐related molecules
Frozen kidney tissue samples from ND (n = 5) and DM treated with either AM6545, ACE‐I or both (n = 4 per group) were homogenized in chloroform/methanol/Tris–HCl 50 mM pH 7.4 (2:1:1, v.v‐1) containing 10 pmol of [2H]‐8‐AEA, [2H]‐4‐palmitoylethanolamine (PEA) and [2H]‐4‐oleoylethanolamide (OEA), and 50 pmol of [2H]‐5‐2AG as internal deuterated standards (Cayman Chemicals, Ann Arbor, MI, USA). The extract was purified by silica gel mini‐columns and the eluted fraction analysed by liquid chromatography atmospheric pressure MS (LC‐APCI‐MS). Analyses were carried out in the selected ion‐monitoring mode using m/z values of 356 and 348 (molecular ions +1 for deuterated and undeuterated AEA), 304 and 300 (molecular ions +1 for deuterated and undeuterated http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3622), 330 and 326 (molecular ions +1 for deuterated and undeuterated http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2661) and 384.35 and 379.35 (molecular ions +1 for deuterated and undeuterated 2‐AG). AEA, OEA, PEA and 2‐AG concentrations were calculated by isotope dilution and expressed as pmol·mg−1 (2‐AG, PEA and OEA) or g (AEA) of wet tissue weight. The concentrations of 2‐AG were obtained by adding the amounts of the 2‐isomer to those of the 1(3)‐isomer, which mostly originates from the isomerization of the former during work‐up.
In vitro experiments
Podocytes
Conditionally immortalized human podocytes were cultured as previously described (Saleem et al., 2002). After differentiation and serum deprivation for 24 h, podocytes were exposed to retinoic acid (RA 1 μmol·L−1) for 24 h either in the presence or absence of the selective CB1 receptor agonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=738 (1 μmol·L−1). Appropriate dilutions of DMSO were used as controls.
Raw264.7 macrophage cell line
Cells were cultured in DMEM, supplemented with 10% FBS. After 24 h of serum‐starvation, cells were treated with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=733/vehicle (1 μmol·L−1) for 18 h either in the presence or in the absence of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10029 (1 μmol·L−1) or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 (1 μmol·L−1), followed by incubation with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4996 (5 ng·mL−1; Thermo Scientific) for 6 h. At the end of the experimental period, total RNA was extracted and arginase‐1 mRNA levels measured by real‐time PCR. GAPDH was used as an internal control.
Data presentation and statistical analysis
Data are presented as mean ± SEM, geometric mean (25th–75th percentile), or fold change over control as appropriate. Results were analysed by ANOVA. Data with a non‐parametric distribution [albumin excretion rate (AER), percentages] were log‐transformed prior to analyses. The LDS test was used for post hoc comparisons. Values for P < 0.05 were considered statistically significant.
Materials
All materials were purchased from Sigma‐Aldrich (St Louis, USA) unless otherwise stated. Details of the antibodies used in the study are reported in the Supporting Information Table S1.
AM6545 5‐(4‐[4‐cyanobut‐1‐ynyl]phenyl)‐1‐(2,4‐dichlorophenyl)‐4‐methyl‐N‐(1,1‐dioxo‐thiomorpholino)‐1H‐pyrazole‐3‐carboxamide, a selective CB1 receptor antagonist (Cluny et al., 2010), was kindly provided by V.K. Vemuri. The compound was dissolved in DMSO, stored at −20°C and diluted in 18:1:1 ratio of saline/emulphor‐620/DMSO immediately prior to use. WIN55,212‐2, ACEA and AM630 were purchased from Cayman (Ann Arbor, MI, USA) and dissolved in DMSO.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b).
Results
Metabolic and physiological parameters
Table 1 shows both metabolic and physiological parameters measured at the end of the treatment period in all animal groups. As expected, blood glucose, glycated haemoglobin and kidney weight/body weight ratio were significantly higher and body weight significantly lower in DM compared to ND mice. Treatment with perindopril, AM6545 or both did not alter these parameters. Changes in body weight over time are reported in the Supporting Information Figure S1. Systolic BP was in the normal range and did not differ between DM and ND mice, although measurements using a non‐invasive tail‐cuff method can miss small differences in BP. A significant reduction in systolic BP was observed in mice treated with perindopril, and this effect was not modified by AM6545.
Table 1.
Metabolic and physiological parameters after 14 weeks of treatment
| Body weight (g) | BG (mg·dL−1) | Glycated Hb (%) | sBP (mmHg) | KW/BW ratio | |
|---|---|---|---|---|---|
| ND | 29.3 ± 0.42 | 135 ± 3.21 | 4.1 ± 0.19 | 113 ± 4.28 | 6.2 ± 0.26 |
| ND Combo | 29.2 ± 1.47 | 140 ± 9.85 | 3.6 ± 0.32 | 92 ± 3.74* | 6.2 ± 054 |
| DM | 23.5 ± 0.66# | 390 ± 7.33# | 12.3 ± 0.42# | 109 ± 4.54 | 7.6 ± 0.50* |
| DM AM6545 | 22.0 ± 0.79# | 396 ± 31.7# | 12.7 ± 0.64# | 112 ± 7.16 | 7.9 ± 0.22* |
| DM ACE‐I | 21.7 ± 2.16# | 397 ± 25.1# | 12.9 ± 0.91# | 92 ± 6.77* | 7.5 ± 0.54* |
| DM Combo | 22.6 ± 1.39# | 346 ± 41.7# | 12.4 ± 0.95# | 94 ± 6.89* | 7.5 ± 0.50* |
Data are shown as mean ± SEM or geometric mean (25th–75th percentile); ACE‐I, treatment with perindopril; AM6545, treatment with AM6545; BG, blood glucose; Combo, treatment with AM6545 plus perindopril; glycated Hb, glycated haemoglobin; KW/BW, kidney weight/body weight; sBP, systolic BP.
P < 0.05 DM versus ND and ND Combo, DM ACE‐I and DM Combo versus others.
P < 0.001 DM versus ND.
ECS system and AT1 receptors
In DM, glomerular immunostaining for CB1 receptors was enhanced, while staining for CB2 receptors was unchanged. Consistent with this, diabetes induced a significant increase in glomerular CB1 receptor mRNA levels without changing the CB2 receptor expression (Figure 1A–F), resulting in a threefold increase in glomerular CB1/CB2 receptor mRNA ratio (Table 2). Moreover, measurement of both EC and EC‐related molecules by mass‐spectrometry showed a diabetes‐induced reduction in the levels of 2‐AG, the predominant CB2 receptor ligand (Table 2). Overall, these data indicate a predominant CB1 receptor‐mediated EC tone in experimental DN.
Figure 1.
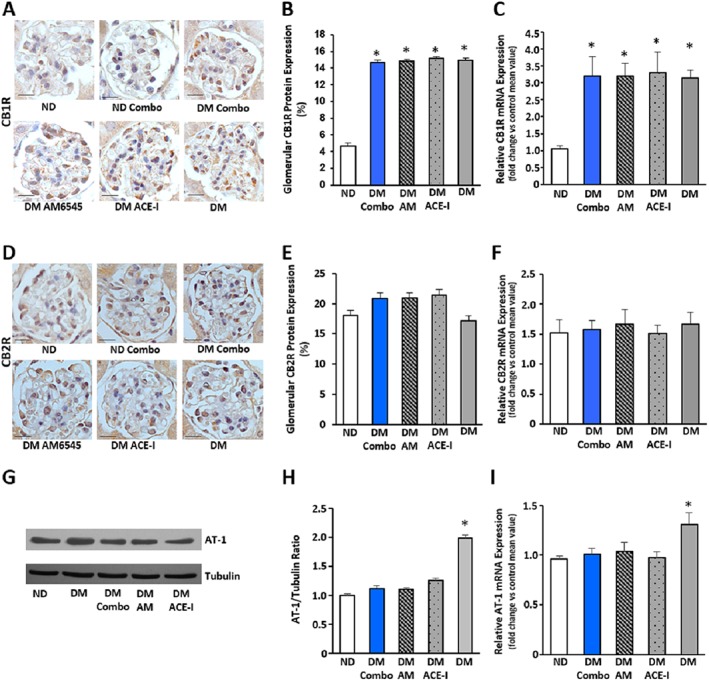
Effect of treatment with AM6545 and/or perindopril on CB1, CB2 and AT1 receptors in DM. Control ND and DM were treated with vehicle, AM6545 (AM), perindopril (ACE‐I) or AM6545 plus perindopril (Combo) (n = 6 mice per group). Representative immunohistochemistry analysis of CB1 receptors (A) and CB2 receptors (D) are shown (original magnification ×400) and quantification of glomerular positive staining reported in the graphs (B–E). CB1, CB2 and AT1 receptor mRNA levels were measured by real‐time PCR on total RNA extracted from the renal cortex. Results were corrected for the expression of the housekeeping gene HPRT and shown in the graphs (C, F, I). Representative immunoblot (G) and densitometry analysis (H) of AT1 receptor protein expression in total protein extracts from the total renal cortex are shown. Tubulin was used as internal control (*P < 0.05, vs. ND).
Table 2.
Effect of treatment with AM6545 and/or perindopril on EC/EC‐related molecule levels and CB1/CB2 receptor mRNA ratio
| 2‐AG (pmol·mg−1) | AEA (pmol·g−1) | PEA (pmol·mg−1) | OEA (pmol·mg−1) | CB1/CB2 mRNA ratio | |
|---|---|---|---|---|---|
| ND | 7.05 ± 0.46 | 2.44 ± 0.21 | 0.07 ± 0.01 | 0.21 ± 0.02 | 0.77 ± 0.14 |
| DM | 4.76 ± 0.55* | 2.28 ± 0.71 | 0.09 ± 0.03 | 0.16 ± 0.02 | 2.12 ± 0.42* |
| DM‐ACE‐I | 4.15 ± 1.36* | 2.01 ± 0.16 | 0.10 ± 0.03 | 0.24 ± 0.05 | 1.98 ± 0.13* |
| DM‐AM6545 | 4.61 ± 0.36* | 2.81 ± 1.46 | 0.18 ± 0.07 | 0.20 ± 0.05 | 2.14 ± 0.28* |
| DM‐Combo | 4.55 ± 0.41* | 2.82 ± 0.44 | 0.12 ± 0.06 | 0.16 ± 0.03 | 2.13 ± 0.44* |
Data are shown as mean ± SEM; ACE‐I, treatment with perindopril; AM6545, treatment with AM6545; Combo, treatment with AM6545 plus perindopril.
P < 0.01 versus ND.
Neither EC (Table 2) nor CB receptor levels (Figure 1A–F) were changed by any treatment. On the contrary, the rise in AT1 receptor mRNA and protein expression induced by diabetes was suppressed by all treatments (Figure 1G–I), indicating that both RAS and CB1 receptor blockade can abolish local increased responsiveness to Ang‐II.
Albuminuria and renal functional parameters
As shown in Table 3, prior to treatment levels of albuminuria were significantly greater in DM compared with ND mice, indicating the development of early DN. Animals allocated to the different treatment groups had comparable urinary albumin levels. At the end of the treatment period, DM showed at least a threefold increase in albuminuria compared to controls. Single treatments with either AM6545 or perindopril reduced albuminuria by 50%. In DM under dual therapy, albuminuria was comparable to that in ND animals, indicating a reversal of early DN. In DM, neither glycosuria nor the rise in N‐acetyl‐β(D)‐glucosaminidase (NAG) activity/creatinine ratio was ameliorated by treatment with AM6545, alone or in combination with therapy, suggesting that AM6545 acted predominantly at the glomerular levels (Table 3). There was only a modest and non‐significant decline in renal function in vehicle‐treated DM, which did not allow this functional parameter to be used to assess efficacy of treatments on.
Table 3.
Albuminuria and renal functional parameters
| Prior to treatments (8 weeks of diabetes) | At the end of the study (14 weeks of treatments) | ||||
|---|---|---|---|---|---|
| AER (μg.18 h−1) | AER (μg.18 h−1) | Cr Cl (mL·min−1) | NAG activity (U·g−1) | Glycosuria (mg.18 h−1) | |
| ND | 30.6 (26–36.3) | 118.8 (109.5–130.1) | 0.47 ± 0.06 | 65.3 ± 5.47 | 0.01 ± 0.002 |
| ND Combo | 30.9 (25.8–36.1) | 111.5 (93.1–127.4) | 0.50 ± 0.08 | 61.2 ± 5.32 | 0.01 ± 0.005 |
| DM | 53.1 (40.4–78.9)* | 429.7 (382.8–513.7)* | 0.40 ± 0.04 | 255.7 ± 11.97* | 3.83 ± 0.66* |
| DM AM6545 | 52.5 (39–83.6)* | 242.9 (224.5–275.4)* , † | 0.45 ± 0.13 | 240.6 ± 13.71* | 3.18 ± 0.10* |
| DM ACE‐I | 53.6 (40.8–79.1)* | 235.1 (228.6–260.4)* , † | 0.42 ± 0.05 | 256.7 ± 43.13* | 3.15 ± 0.24* |
| DM Combo | 51.3 (34.3–82.7)* | 144.8 (132.1–173.4)† , ‡ | 0.48 ± 0.05 | 249.8 ± 44.24* | 3.24 ± 0.37* |
Data are shown as mean ± SEM or geometric mean (25th–75th percentile); ACE‐I, treatment with perindopril; AM6545, treatment with AM6545; Combo, treatment with AM6545 plus perindopril; Cr Cl, creatinine clearance.
P < 0.05, versus ND.
P < 0.05, versus DM.
P < 0.05 DM Combo versus single treatments.
Effect of treatments on podocytes
Diabetes induced a significant reduction in both nephrin and podocin immunostaining. In keeping with the results on albuminuria, this decrease in both nephrin and podocin was reduced by a single treatment with either AM6545 or perindopril, but only the combined treatment abolished the reduction seen in DM. These semi‐quantitative data were confirmed by measuring both nephrin and podocin expression in total renal cortex by real‐time PCR and Western blotting (Figure 2A–I). Podocyte number did not differ among groups as assessed by counting the p57‐positive cells (Figure 2J,K). Furthermore, diabetes‐induced apoptosis was modest and did not vary significantly in treated and untreated animals (ND: 0.27 ± 0.06; DM: 0.75 ± 0.07; DM‐AM6545: 0.83 ± 0.06; DM‐ACE‐I: 0.79 ± 0.05; DM Combo: 0.79 ± 0.06; P = NS podocyte number/glomerular area). Therefore, drugs were likely to act directly on nephrin/podocin expression rather than indirectly through prevention of podocyte apoptosis/loss.
Figure 2.
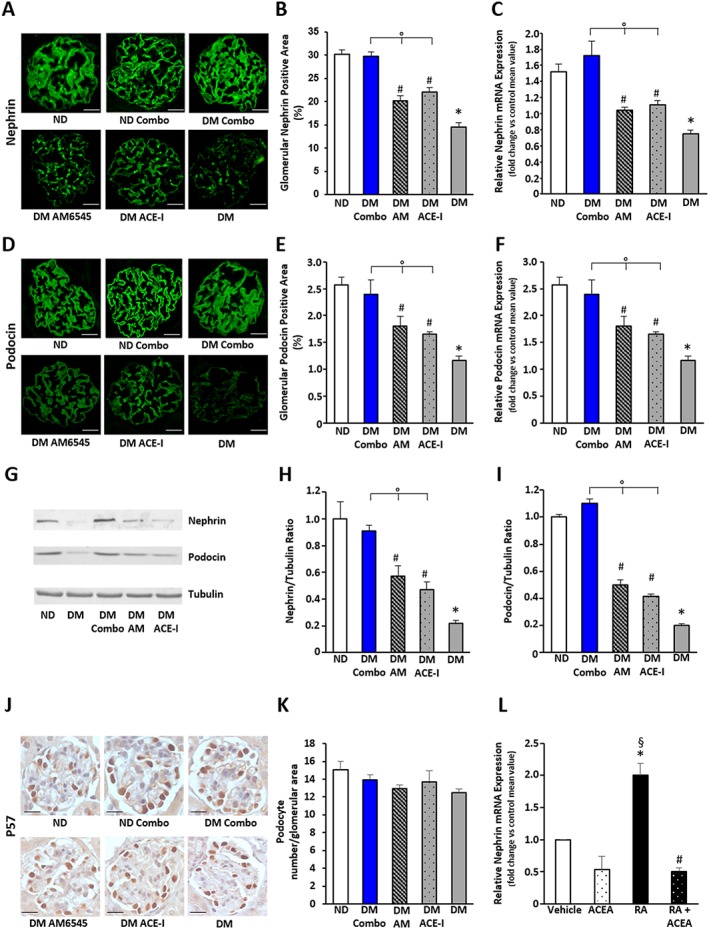
Effect of treatment with AM6545 and/or perindopril on nephrin and podocin expression and podocyte number in DM. Control ND and DM were treated with vehicle, AM6545 (AM), perindopril (ACE‐I) or AM6545 plus perindopril (Combo) (n = 6 mice per group). Both nephrin (A–C) and podocin (D–F) mRNA and protein expression was assessed. Representative immunofluorescence images of nephrin (A) and podocin (D) are shown (original magnification ×400). Quantification of both glomerular staining (B, E) and mRNA levels (C, F) is reported in the graphs. Representative immunoblots (G) and results of densitometry analysis (H, I) of nephrin and podocin protein expression in total protein extracts from the total renal cortex. Tubulin was used as an internal control. Representative immunohistochemistry images (J original magnification ×400) and quantification of glomerular positive staining of p57 (K) are shown (*P < 0.05 vs. ND; # P < 0.05 vs. DM; °P < 0.05 dual treatment vs. single treatment). (L) Nephrin mRNA levels were measured by real‐time PCR on total RNA extracts from podocytes exposed to RA (1 μmol·L−1)/vehicle for 24 h in the presence and in the absence of ACEA (1 μmol·L−1) (*P < 0.05 RA vs. vehicle; § P < 0.05 RA vs. ACEA and RA + ACEA; # P < 0.05 RA + ACEA vs. vehicle).
CB1 receptor and nephrin expression in cultured podocytes
To clarify the mechanism whereby the CB1 receptor affects nephrin, we tested, in vitro, the hypothesis that CB1 receptor signalling may interfere with retinoic acid (RA)‐induced nephrin expression. As expected, exposure of cultured podocytes to RA induced a twofold increase in nephrin mRNA levels. CB1 receptor activation with ACEA not only prevented the increase in nephrin in response to RA, but even lowered the nephrin expression below control levels (Figure 2L). This suggests that an increased CB1 receptor‐mediated EC tone within the diabetic glomeruli may inhibit the RA‐receptor pathway controlling the expression of nephrin.
Renal hypertrophy, mesangial expansion and extracellular matrix components
PAS staining revealed a mild mesangial expansion in DM that was reduced to a similar extent by all treatments. DM‐induced overexpression of both collagen type 1 and fibronectin mRNA was reduced by all treatments. Although the effect of dual therapy was slightly greater with a rise in fibronectin and collagen, which were then no longer significant as compared to controls, the difference between dual and single treatments was modest and not statistically significant. Positive glomerular immunostaining for both fibronectin and collagen was increased by DM and lowered by all treatments, although values returned to control levels only in the combination therapy group (Figure 3).
Figure 3.
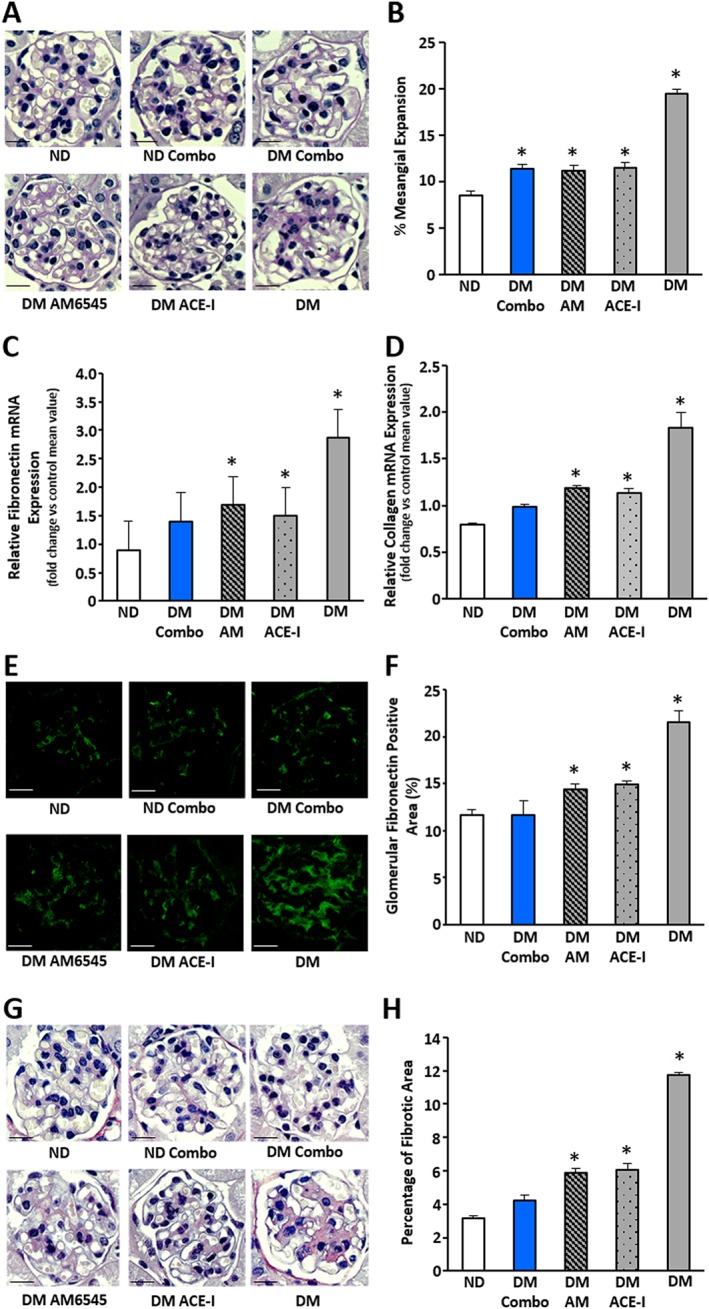
Effect of AM6545 and/or perindopril on markers of fibrosis in DM. ND and DM were treated with vehicle, AM6545 (AM), perindopril (ACE‐I) or AM6545 plus perindopril (Combo) (n = 6 mice per group) for 14 weeks. PAS staining was assessed in renal cortex sections. Representative images (scale bar: 50 μm) and percentage PAS glomerular‐positive areas are shown (A, B). Both fibronectin (C) and collagen type I (D) mRNA expression was measured by real‐time PCR on total RNA extracted from the renal cortex and results are presented in the graphs. Representative images of glomerular fibronectin protein expression (E), picrosirius red staining (G) and quantitation of glomerular staining are shown (F, H) (*P < 0.05 vs. ND).
Monocyte/macrophage accumulation
Immunohistochemical staining revealed that the number of monocytes/macrophages infiltrating the glomeruli, assessed by counting the number of both F4/80 and MAC‐2 positive cells, was significantly greater in DM compared to ND mice. The combined therapy abolished DM‐induced increase in number of accumulated monocytes/macrophages, while the number of infiltrating monocytes/macrophages fell only by half in mice under single treatments and it was still significantly greater than in control animals (Figure 4A–D). However, the absolute number of monocytes/macrophages was quite low, and thus, results should be treated with caution. A similar trend was observed in the gene expression of the macrophage marker CD68 (Figure 4E) and in both mRNA and protein expression of the adhesion molecule ICAM‐1 (Figure 4F,G). The combined therapy even reduced ICAM‐1 protein levels below control values.
Figure 4.
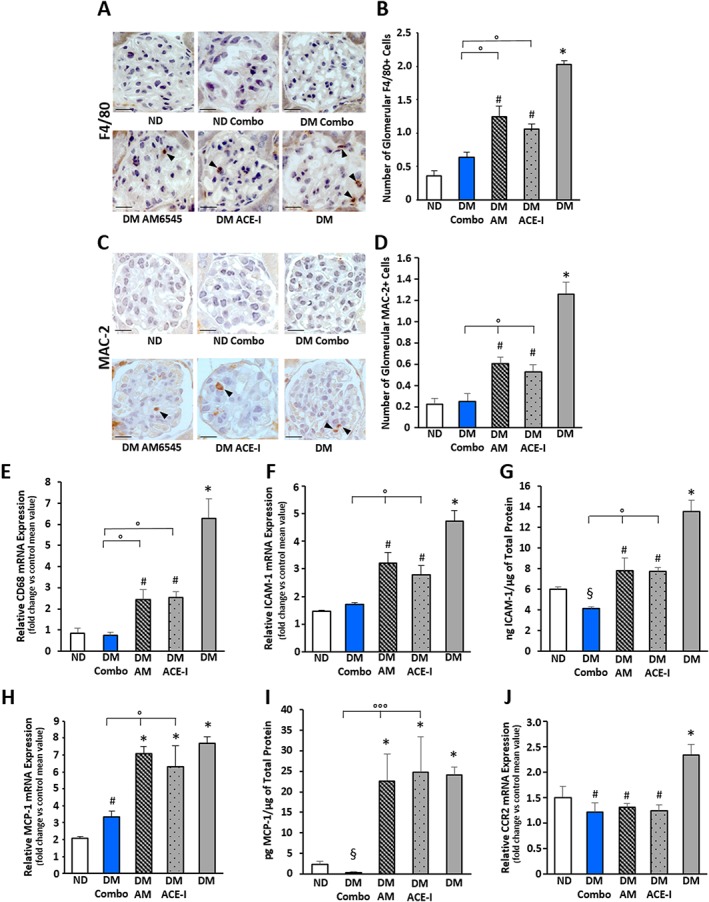
Effect of AM6545 and/or perindopril on diabetes‐induced inflammation. ND and DM were treated with vehicle, AM6545 (AM), perindopril (ACE‐I) or AM6545 plus perindopril (Combo) (n = 6 mice per group) for 14 weeks. Representative images of glomerular F4/80 (A) and MAC‐2 (C) positive immunostaining are shown and quantitation reported in the graph (B, D). Total RNA was extracted from the renal cortex and levels of mRNA encoding for CD68 (E), ICAM‐1 (F), MCP‐1 (H) and CCR2 (J), measured by real‐time PCR. Protein levels of ICAM‐1 (G) and MCP‐1 (I) in renal tissue were determined by elisa and normalized to total protein (*P < 0.05 vs. ND; # P < 0.05 vs. DM group; °P < 0.05 dual vs. single treatment; § P < 0.05 combo vs. ND).
As the chemokine MCP‐1 plays a key role in glomerular monocyte accumulation, we also assessed the expression of MCP‐1 and its cognate receptor CCR2. DM‐induced MCP‐1 expression was not altered by single treatments, but it was abolished by dual therapy, and MCP‐1 protein levels were significantly lower than in control mice. By contrast, DM‐induced CCR2 overexpression was attenuated by all therapies (Figure 4H–J). Therefore, all therapies can normalize responsiveness to MCP‐1, but only the combination of both ACE and CB1 receptor inhibition abolished MCP‐1 overexpression, possibly explaining the greater efficacy of dual therapy in lowering the glomerular accumulation of monocytes/macrophages.
M1 and M2 macrophage polarization
Diabetes induced a rise in markers of M1 macrophages (TNF‐α, CCL3 and NOS2) that was reduced by single treatments and abolished by dual therapy (Figure 5A–D). Markers of M2 anti‐inflammatory macrophages, CD163 and CD206, were down‐regulated by diabetes, normalized by single treatments and significantly increased above control levels by the combined therapy (Figure 5E, F). Moreover, perindopril failed to reverse the DM‐induced down‐regulation of Arg‐1, whereas AM6545, either alone or in combination therapy, not only normalized but also up‐regulated Arg‐1 mRNA levels (Figure 5G). Because monocyte/macrophages infiltrating the diabetic glomeruli expressed the CB1 receptor in vivo, they were potential direct targets of AM6545 (Supporting Information Figure S2A).
Figure 5.
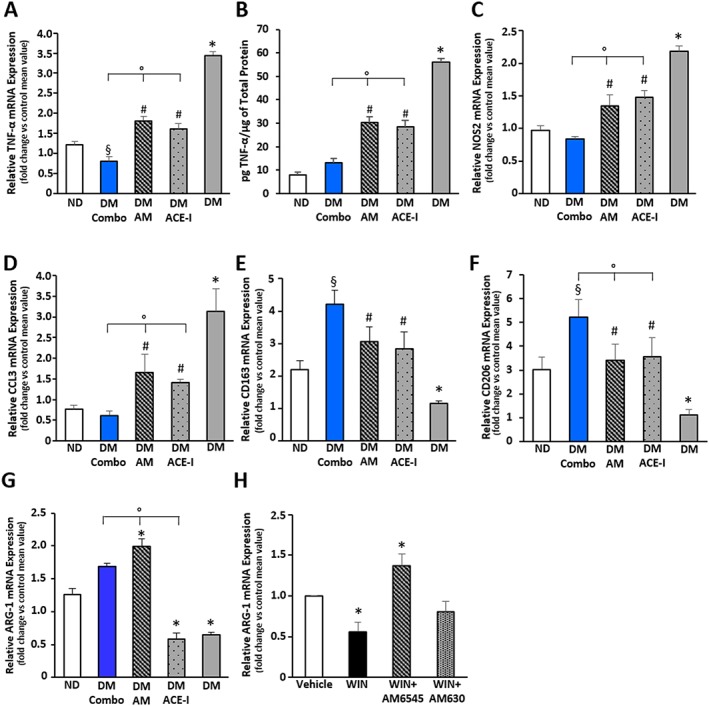
Effect of AM6545 and/or perindopril on macrophage phenotype. ND and DM were treated with vehicle (DM), AM6545 (AM), perindopril (ACE‐I) or AM6545 plus perindopril (Combo) (n = 6 mice per group) for 14 weeks. Total RNA was extracted from the renal cortex and levels of mRNA encoding for TNF‐α (A), NOS2 (C), CCL3 (D), CD163 (E), CD206 (F), arginase‐1 (G), measured by real time‐PCR. (B) Levels of TNF‐α in kidney tissue were determined by elisa and normalized to total protein (*P < 0.05 vs. ND; # P < 0.05 vs. DM; °P < 0.05 dual vs. single treatment; § P < 0.05 combo vs. ND). (H) Arginase‐1 mRNA levels were measured by real‐time PCR on total RNA extracts from RAW 264.7 cells exposed to WIN55,212‐2 (1 μmol·L−1)/vehicle for 18 h in the presence and in the absence of AM6545 (1 μmol·L−1) and AM630 (1 μmol·L−1), followed by incubation with IL‐4 (5 ng·mL−1) for 6 h (*P < 0.05 WIN and AM6545 vs. vehicle).
In vitro M2 polarization
To assess the hypothesis that CB1 receptor activation can directly affect M2 polarization, we performed in vitro experiments with Raw264.7 macrophages expressing both CB receptors (Supporting Information Figure S2B), and investigated the effect of CB receptor activation on IL‐4‐induced M2 polarization. The non‐selective agonist WIN55,212‐2, which activates both CB1 and CB2 receptors, markedly reduced the expression of Arg‐1 in macrophage exposed to IL‐4, indicating that the ECS has an inhibitory effect on M2 macrophage polarization. Moreover, the effect of WIN55,212‐2 was abolished by AM6545 and left unchanged by the CB2 receptor antagonist AM630, indicating a specific CB1 receptor‐mediated effect (Figure 5H).
Discussion
The present study has provided evidence that in an animal model of type 1 diabetes with early DN, peripheral CB1 receptor blockade used as add‐on treatment to ACE‐inhibition can reverse albuminuria, nephrin loss and reduce inflammation. This is the first study to investigate the renal effect of combined inhibition of CB1 receptors and ACE.
A delayed intervention was chosen to mimic the clinical scenario of introducing therapy in patients with established microalbuminuria. Prior to treatment albuminuria was 1.7‐fold higher in DM than in ND mice. Nephropathy progressed over time, and after a further 14 weeks of diabetes, albuminuria was 3.6‐fold greater in untreated DM than in controls. Treatment with either AM6545 or perindopril slowed the rate of progression, but albuminuria was still significantly greater than in controls, indicating that delayed treatment is less effective than treatment delivered from the onset of diabetes, reported in previous studies (Trojacanec et al., 2013; Barutta et al., 2017). On the contrary, in mice under dual therapy albumin excretion rates were similar to control values, proving reversal of increased glomerular permeability to albumin. Renal function was, however, preserved in this animal model, precluding assessment of treatment efficacy on this factor.
Blood glucose and glycated haemoglobin levels were similar in untreated and treated DM, showing dissociation of albuminuria from hyperglycaemia, the former being reversed and the latter remaining unaffected by dual treatment. Although CB1 receptor blockade has been reported to modestly ameliorate hyperglycaemia in mice with type 1 diabetes by reducing tubular glucose re‐adsorption and thus glycosuria (Hinden et al., 2017), this was not observed in our AM6545‐treated mice. Treatment with perindopril significantly lowered BP levels, providing evidence of perindopril efficacy, although angiotensin levels were not directly measured. No changes in BP were observed in AM6545‐treated mice, and thus, the anti‐proteinuric effect of AM6545 appears independent of BP. This is important in the light of recent results showing that systemic CB1 receptor blockade can improve both BP and metabolic profile in hypertensive and insulin‐resistant rats (Schaich et al., 2014). A previous study has reported reversal of albuminuria in obese and hypertensive Zucker Diabetic Fatty (ZDF) rats treated with JD5037 (Jourdan et al., 2014). However, the effect of treatment on BP was not assessed, and the BP‐lowering properties of JD5037 in this model may have contributed substantially to efficacy.
The anti‐albuminuric effect of dual therapy was likely to be due to the rescue of both nephrin and podocin down‐regulation as no changes in podocyte apoptosis/loss were observed. Both AT1 and CB1 receptor activation are known to down‐regulate nephrin (Jourdan et al., 2014), and the two pathways may overlap as AM6545 abolished AT1 overexpression consistent with previous findings in experimental type 2 diabetes (Jourdan et al., 2014). However, the greater efficacy of the combined therapy suggests that the mechanisms were partially independent. Both PKC and Akt are involved in Ang‐II‐mediated nephrin down‐regulation. Moreover, Ang‐II increases nephrin binding to β‐arrestin2, resulting in nephrin endocytosis and loss (Bussolati et al., 2005; Takano et al., 2007; Xiao et al., 2009; Königshausen et al., 2016). The mechanism of CB1 receptor‐induced nephrin down‐regulation is less established, but our in vitro data, showing that ACEA prevents RA‐induced nephrin expression, suggest that CB1 receptors may act via inhibition of the cAMP‐RA receptor pathway. In keeping with this, CB1 receptor activation reduced cAMP levels in cultured podocytes, and a similar mechanism of nephrin down‐regulation was described in response to TNF‐a (Saito et al., 2010; Jourdan et al., 2014). This is also of relevance given the growing evidence of a key role of RA in podocytes for both differentiation and regeneration (Lazzeri et al., 2014).
Changes in renal haemodynamic are unlikely to explain the superior anti‐albuminuric effect of the combination therapy as AEA reduces glomerular capillary pressure (GCP) via CB1 receptors (Koura et al., 2004), and thus, AM6545 may limit the benefit of lowering GCP through ACE‐inhibition. The potential role of the tubuli in the reversal of albuminuria cannot be excluded as CB1R has been implicated in tubular cell apoptosis, trans‐differentiation in myofibroblasts (Jenkin et al., 2012; Lecru et al., 2015), and reduced albumin reuptake through megalin down‐regulation (Jourdan et al., 2018). However, NAG activity, a marker of tubular injury, was not affected by treatments, and the relevance of the tubuli in diabetic albuminuria remains highly controversial (Jarad and Miner, 2009).
The expression of CB1 receptors was increased in DM, and this was not altered by any treatments. On the contrary, in a study performed in ZDF rats, CB1 receptor overexpression was reduced by losartan, supporting a direct link between AT1 receptor activation and CB1 receptor expression (Jourdan et al., 2014). The reason of this discrepancy is unknown; however, the different animal model is a possible explanation. Auto‐induction of CB1 receptors has been consistently demonstrated in models of type 2 diabetes at variance with STZ‐induced diabetes, suggesting a different mechanism of CB1 receptor up‐regulation (Nam et al., 2012; Jourdan et al., 2014). Moreover, obesity and insulin resistance may partially explain the link between RAS activation and CB1 receptor expression in ZDF rats as increasing evidence suggests an interaction between the ECS and the RAS mediated by the metabolic syndrome.
It has been proposed that enhanced CB1 receptor expression/signalling in podocytes may represent a final common pathway whereby both hyperglycaemia/DM and Ang‐II cause podocyte dysfunction. Our data showing an additive effect of CB1 receptor and ACE blockade in reversing both the albuminuria and nephrin down‐regulation, together with the lack of effect of perindopril on glomerular CB1 receptor overexpression and renal EC levels, do not support this hypothesis and, instead, provide a rationale for the use of a combined therapy.
Despite a modest glomerular monocyte/macrophage infiltration, a marked rise in inflammatory cytokines was seen in DM, showing that inflammatory/resident cells can trigger cascades amplifying the inflammatory response. Dual therapy performed better than single treatments on inflammation as it abolished both monocyte infiltration and TNF‐α overexpression, and it was the only effective treatment in lowering MCP‐1. This is of relevance as the chemokine MCP‐1 can also induce extracellular matrix production in mesangial cells and nephrin loss in podocytes and MCP‐1 deletion ameliorates both proteinuria and glomerulosclerosis in experimental diabetes (Chow et al., 2006; Giunti et al., 2006, 2008; Tarabra et al., 2009).
There was a shift towards a pro‐inflammatory M1 macrophage polarization in DM as demonstrated by the overexpression of M1 and down‐regulation of M2 macrophage markers. This was reduced by the monotherapies. Therefore, our data do not confirm that ACE‐inhibition may promote a deleterious M1‐polarization in experimental DN (Cucak et al. 2015). The effect of the dual therapy was greater, particularly on M2 markers. The expression of both CD163 and CD206 rose above control levels in mice under dual treatment. Moreover, the expression of Arg‐1, a key switch in M2‐polarization, was reduced by diabetes, left unchanged by perindopril and up‐regulated by AM6545, either alone or as a combination therapy. The direct effect of CB1 receptors on M2 polarization was further confirmed by our experiments on RAW267 macrophages showing that a cannabinoid agonist inhibited IL‐4‐induced M2 polarization in a CB1 receptor‐dependent manner, while CB2 receptor blockade was not effective. Moreover, neither CB2 receptor expression nor 2‐AG levels were altered in AM6545‐treated mice, making an indirect CB2 receptor‐mediated effect unlikely. This is also in agreement with our previous data showing that treatment with a CB2 receptor agonist does not alter Arg‐1 expression in the renal cortex of DM (Barutta et al., 2017).
Because enhanced M2 polarization occurred in the presence of a normal number of macrophages, this suggests that dual treatment and in particular AM6545 may affect resident macrophages and induce their re‐education towards an M2‐phenotype. This is of pathophysiological relevance as M2‐macrophages express scavenging receptors and produce various anti‐inflammatory mediators that promote the resolution of inflammation and tissue repair (Murray 2017). A beneficial effect of M2‐macrophages has been shown in models of acute kidney injury, although their role in chronic kidney diseases is more uncertain (Guiteras et al. 2016).
Although a low‐grade inflammation is considered important in driving fibrotic processes, glomerular PAS staining was reduced to a similar extent by all treatments. The effect of dual therapy on the expression of ECM was slightly greater than that of single treatments, but differences were modest. Therefore, our data failed to show that dual therapy is superior to standard therapy with an ACE‐I in preventing fibrosis. However, studies in animal models more prone to develop glomerulosclerosis are required to specifically address this question.
Our results may be of relevance for DN in humans. The current standard therapy for DN with RAS‐blockers is insufficient to completely halt disease progression. The potential benefit of CB1 receptor antagonists as an add‐on therapy was assessed in mice with established albuminuria. Therefore, we used a clinically relevant model, reflecting the current indications for treatment. Our results, showing a superior effect of dual therapy on albuminuria, nephrin loss and inflammation, raise hope that CB1 receptor blockade may be a valuable therapeutic option as an additional therapy. Although further studies in more advanced DN are required, reversal of albuminuria is by itself clinically relevant as it has been associated with a slower early decline of renal function and better cardiovascular outcomes in clinical studies in humans (Jansson et al., n.d.; Perkins et al., 2007).
Author contributions
F.B. wrote the manuscript, performed experiments and analysed data; R.M., R.G., S.B., L.A., F.B. and B.C. performed experiments; K.V., A.M., V.D.M. and G.B. edited the manuscript; G.G. wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Changes in body weight over time in the study groups. Body weight was measured in all animal groups prior to streptozotocin (STZ)/vehicle injection (time: 0), at the start of treatment (time: 8 weeks) and at the end of the study (time: 22 weeks). ND, non‐diabetic mice; DM, diabetic mice; ACE‐I, treatment with perindopril; AM6545, treatment with AM6545; Combo, treatment with AM6545 plus perindopril (***P < 0.001 ND vs. DM).
Figure S2 EC receptor expression by macrophages. (A) Double immunofluorescence for CB1R and MAC‐2 performed in renal cortex sections from diabetic mice showed colocalization as shown in the merge image. Nuclei were counterstained with DAPI. The dashed square delimits the area shown at higher magnification in the insert. (B) CB1R and CB2R mRNA expression was assessed by PCR in total RNA extracts from RAW 264.7 cells. A representative 2% agarose gel stained with ethidium bromide is shown MW, molecular weight marker.
Table S1 Antibodies features and concentrations.
Acknowledgement
This work was supported by a grant from the European Foundation for the study of diabetes.
Barutta, F. , Bellini, S. , Mastrocola, R. , Gambino, R. , Piscitelli, F. , di Marzo, V. , Corbetta, B. , Vemuri, V. K. , Makriyannis, A. , Annaratone, L. , Bruno, G. , and Gruden, G. (2018) Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. British Journal of Pharmacology, 175: 4371–4385. 10.1111/bph.14495.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Bruno G, Grimaldi S, Gruden G (2015). Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine 48: 730–742. [DOI] [PubMed] [Google Scholar]
- Barutta F, Corbelli A, Mastrocola R Gambino R, Di Marzo V, Pinach S et al (2010). Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 59: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutta F, Grimaldi S, Franco I, Bellini S, Gambino R, Pinach S et al (2014). Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin‐induced diabetic mice. Kidney Int 86: 979–990. [DOI] [PubMed] [Google Scholar]
- Barutta F, Grimaldi S, Gambino R, Vemuri K, Makriyannis A, Annaratone L et al (2017). Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol Dial Transplant 32: 1655–1665. [DOI] [PubMed] [Google Scholar]
- Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP et al (2011). Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60: 2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S et al (2005). Statins prevent oxidized LDL‐induced injury of glomerular podocytes by activating the phosphatidylinositol 3‐kinase/AKT‐signaling pathway. J Am Soc Nephrol 16: 1936–1947. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic‐Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH (2006). Monocyte chemoattractant protein‐1 promotes the development of diabetic renal injury in streptozotocin‐treated mice. Kidney Int 69: 73–80. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT et al (2010). A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161: 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucak H, Nielsen Fink L, Højgaard Pedersen M, Rosendahl A (2015). Enalapril treatment increases T cell number and promotes polarization towards M1‐like macrophages locally in diabetic nephropathy. Int Immunopharmacol 25: 30–41. [DOI] [PubMed] [Google Scholar]
- Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K (2004). Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967. [DOI] [PubMed] [Google Scholar]
- Fineberg D, Jandeleit‐Dahm KA, Cooper ME (2013). Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol 9: 713–723. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Cooper ME (2013). Mechanisms of diabetic complications. Physiol Rev 93: 137–188. [DOI] [PubMed] [Google Scholar]
- Giunti S, Pinach S, Arnaldi L, Viberti G, Perin PC, Camussi G et al (2006). The MCP1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int 69: 856–863. [DOI] [PubMed] [Google Scholar]
- Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, Cavallo‐Perin P et al (2008). Monocyte chemoattractant protein‐1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 51: 198–207. [DOI] [PubMed] [Google Scholar]
- Gruden G, Barutta F, Kunos G, Pacher P (2016). Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol 173: 1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiteras R, Flaquer M, Cruzado JM (2016). Macrophage in chronic kidney disease. Clin Kidney J 9: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinden L, Udi S, Drori A, Gammal A, Nemirovski A, Hadar R et al (2017). Modulation of renal GLUT2 by the cannabinoid‐1 receptor: implications for the treatment of diabetic nephropathy. J Am Soc Nephrol 29: 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W, Kim DJ, Kim MK, Kim YG, Oh HY, Ruotsalainen V et al (2002). Expression of nephrin in acquired human glomerular disease. Nephrol Dial Transplant 17: 478–484. [DOI] [PubMed] [Google Scholar]
- IDF Diabetes Atlas (2017). 7th Edition Available at http://www.diabetesatlas.org (accessed 13/7/2017).
- Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L et al (2007). Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int 72: 1345–1357. [DOI] [PubMed] [Google Scholar]
- Jansson F, Forsblom C, Harjutsalol V, Thorn L, Wadén J, Elonen N et al (2018). Regression of albuminuria and its association with incident cardiovascular outcomesand mortality in type 1 diabetes: the FinnDiane Study. Diabetologia 61: 1203–1211. [DOI] [PubMed] [Google Scholar]
- Jarad G, Miner JH (2009). Albuminuria, wherefore art thou? J Am Soc Nephrol 20: 455–457. [DOI] [PubMed] [Google Scholar]
- Jenkin KA, Verty AN, McAinch AJ, Hryciw DH (2012). Endocannabinoids and the renal proximal tubule: an emerging role in diabetic nephropathy. Int J Biochem Cell Biol 44: 2028–2031. [DOI] [PubMed] [Google Scholar]
- Jourdan T, Szanda G, Rosenberg AZ, Tam J, Earley BJ, Godlewski G et al (2014). Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A 111: E5420–E5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan T, Park JK, Varga ZV, Pálóczi J, Coffey NJ, Rosenberg AZ et al (2018). Cannabinoid‐1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes Metab 20: 698–708. [DOI] [PubMed] [Google Scholar]
- Königshausen E, Zierhut UM, Ruetze M, Potthoff SA, Stegbauer J, Woznowski M et al (2016). Angiotensin II increases glomerular permeability by β‐arrestin mediated nephrin endocytosis. Sci Rep 6: 39513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ et al (2004). Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 15: 1488–1494. [DOI] [PubMed] [Google Scholar]
- Lazzeri E, Peired AJ, Lasagni L, Romagnani P (2014). Retinoids and glomerular regeneration. Semin Nephrol 34: 429–436. [DOI] [PubMed] [Google Scholar]
- Lecru L, Desterke C, Grassin‐Delyle S, Chatziantoniou C, Vandermeersch S, Devocelle A et al (2015). Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 88: 72–84. [DOI] [PubMed] [Google Scholar]
- Lim JC, Lim SK, Park MJ, Kim GY, Han HJ, Park SH (2011). Cannabinoid receptor 1 mediates high glucose‐induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. Am J Physiol Renal Physiol 301: F179–F188. [DOI] [PubMed] [Google Scholar]
- Murray PJ (2017). Macrophage polarization. Annu Rev Physiol 10: 541–566. [DOI] [PubMed] [Google Scholar]
- Nagata M (2016). Podocyte injury and its consequences. Kidney Int 86: 1221–1230. [DOI] [PubMed] [Google Scholar]
- Nam DH, Lee MH, Kim JE, Song HK, Kang YS, Lee JE et al (2012). Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology 153: 1387–1396. [DOI] [PubMed] [Google Scholar]
- Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH et al (2007). Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee‐Ramos D et al (2011). AT1R‐CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBOJ 3: 2350–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JC, Kelly SE, Diane A, Wang Y, Mangat R, Novak S et al (2010). Rimonabant‐mediated changes in intestinal lipid metabolism and improved renal vascular dysfunction in the JCR:LA‐cp rat model of prediabetic metabolic syndrome. Am J Physiol Gastrointest Liver Physiol 299: G507–G516. [DOI] [PubMed] [Google Scholar]
- Saito Y, Okamura M, Nakajima S, Hayakawa K, Huang T, Yao J et al (2010). Suppression of nephrin expression by TNF‐alpha via interfering with the cAMP‐retinoic acid receptor pathway. Am J Physiol Renal Physiol 298: F1436–F1444. [DOI] [PubMed] [Google Scholar]
- Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T et al (2002). A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638. [DOI] [PubMed] [Google Scholar]
- Schaich CL, Shaltout HA, Brosnihan KB, Howlett AC, Diz DI (2014). Acute and chronic systemic CB1 cannabinoid receptor blockade improves blood pressure regulation and metabolic profile in hypertensive (mRen2)27 rats. Physiol Rep 2: e12108 10.14814/phy2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Yamauchi K, Hayakawa K, Hiramatsu N, Kasai A, Okamura M et al (2007). Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett 581: 421–426. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G et al (2010). Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabra E, Giunti S, Barutta F, Salvidio G, Burt D, Deferrari G et al (2009). Effect of the monocyte chemoattractant protein‐1/CC chemokine receptor 2 system on nephrin expression in streptozotocin‐treated mice and human cultured podocytes. Diabetes 58: 2109–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojacanec J, Zafirov D, Labacevski N, Jakjovski K, Zdravkovski P, Trojacanec P et al (2013). Perindopril treatment in streptozotocin induced diabetic nephropathy. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 34: 99–108. [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein‐Fuchs J et al (2014). Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 37: 2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J, Makino H (2016). Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 12: 13–26. [DOI] [PubMed] [Google Scholar]
- Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y et al (2009). 1, 25‐Dihydroxyvitamin D3prevents puromycin aminonucleoside‐induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3‐kinase/Akt‐signaling pathway. Am J Nephrol 30: 34–43. [DOI] [PubMed] [Google Scholar]
- Zoja C, Locatelli M, Corna D, Villa S, Rottoli D, Nava V et al (2016). Therapy with a selective cannabinoid receptor type 2 agonist limits albuminuria and renal injury in mice with type 2 diabetic nephropathy. Nephron 132: 59–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Changes in body weight over time in the study groups. Body weight was measured in all animal groups prior to streptozotocin (STZ)/vehicle injection (time: 0), at the start of treatment (time: 8 weeks) and at the end of the study (time: 22 weeks). ND, non‐diabetic mice; DM, diabetic mice; ACE‐I, treatment with perindopril; AM6545, treatment with AM6545; Combo, treatment with AM6545 plus perindopril (***P < 0.001 ND vs. DM).
Figure S2 EC receptor expression by macrophages. (A) Double immunofluorescence for CB1R and MAC‐2 performed in renal cortex sections from diabetic mice showed colocalization as shown in the merge image. Nuclei were counterstained with DAPI. The dashed square delimits the area shown at higher magnification in the insert. (B) CB1R and CB2R mRNA expression was assessed by PCR in total RNA extracts from RAW 264.7 cells. A representative 2% agarose gel stained with ethidium bromide is shown MW, molecular weight marker.
Table S1 Antibodies features and concentrations.


