Abstract
Objective
To develop a prediction model to confirm or exclude primary aldosteronism (PA) in patients with an inconclusive salt loading test (SLT).
Context
Diagnosis in patients with a suspicion of PA can be confirmed using an SLT. In case of inconclusive test results the decision about how to manage the patient is usually based on contextual clinical data.
Design
We included a retrospective cohort of 276 patients in the final analysis.
Methods
All patients underwent an SLT between 2005 and 2016 in our university medical center. The SLT was inconclusive (post-infusion aldosterone levels 140–280 pmol/L) in 115 patients. An expert panel then used contextual clinical data to diagnose PA in 45 of them. Together with 101 patients with a positive SLT this resulted in a total of 146 patients with PA. A total of 11 variables were used in a multivariable logistic regression analysis. We assessed internal validity by bootstrapping techniques.
Results
The following variables were independently associated with PA: more intense potassium supplementation, lower plasma potassium concentration, lower plasma renin concentration before SLT and higher plasma aldosterone concentration after SLT. The resulting prediction model had a sensitivity of 84.4% and a specificity of 94.3% in patients with an inconclusive SLT. The positive and negative predictive values were 90.5 and 90.4%, respectively.
Conclusions
We developed a prediction model for the diagnosis of PA in patients with an inconclusive SLT that results in a diagnosis that was in high agreement with that of an expert panel.
Keywords: adrenal, primary aldosteronism, inconclusive salt loading test, logistic regression, prediction model
Introduction
For the detection of primary aldosteronism (PA), hypertensive patients are screened using the aldosterone-to-renin ratio (ARR). An elevated ratio requires confirmatory testing. One commonly used confirmation test as recommended by the Endocrine Society guideline is the salt loading test (SLT) (1). Suppression of plasma aldosterone concentration (PAC) after saline infusion is assumed to exclude PA. The optimal cut-off level of post-infusion PAC is a matter of debate and varies from 139 to 194 pmol/L with corresponding sensitivity of 73–88% and specificity of 76–100% (2, 3, 4). The Endocrine Society guideline suggests the use of two cut-off values: a post-infusion PAC >280 pmol/L confirms the diagnosis of PA, whereas a post-infusion <140 pmol/L excludes the diagnosis (1). A post-infusion PAC from 140 to 280 pmol/L is referred to as inconclusive. In such cases, a diagnosis of PA is arbitrarily established or rejected based on contextual clinical and biochemical parameters. The objective of this study was to develop a tool for rendering the diagnosis in patients with suspected PA and an inconclusive SLT more objective and transparent.
Subjects and methods
Methods were based on the TRIPOD Statement for development and validation of prediction models (5).
Patients
Our cohort consisted of adult patients who underwent an SLT for clinically suspected PA at the Radboud Adrenal Center, a tertiary center of expertise for patients with adrenal disorders in the Netherlands. Dutch law allows to analyze data from patient records without specific informed consent, provided that anonymity is guaranteed.
We included the most recently referred consecutive patients (n = 290) from January 2016 back to January 2005 in order to attain a number of approximately 140 patients with and without PA based on the assumption that ~10 patients per variable are needed (6).
Salt loading test
To prepare patients for the SLT, all antihypertensive agents interfering with aldosterone and/or renin levels were stopped and replaced by non-interfering agents for 4–6 weeks (1). In case of hypokalemia, potassium supplementation was prescribed. Plasma renin concentration (PRC), PAC and potassium concentration were assessed before and after the intravenous infusion of 2 L of sodium chloride 0.9% over 4 hours, starting between 08:00 and 09:30 h in the morning. Patients stayed in the semi-recumbent position during the infusion (1). Blood was sampled from an intravenous cannula inserted prior to the procedure according to standard operating procedures by trained personnel. Blood pressure was measured every 30 min.
Analytical methods
Vacutainer blood collection tubes from Beckton Dickinson were used. PRC was measured in EDTA plasma by an immunoradiometric assay (RENIN III generation, CIS Bio International) with within- and between-day imprecision of 6.9 and 10.1% at 11.2 mU/L and 2.6 and 5.1% at 168 mU/L. PAC was measured in serum after extraction and paper chromatography with recovery correction, as described earlier (7), and with an within- and between-day imprecision of 8.2 and 7.5% at 360 pmol/L. Potassium and sodium concentration was measured by Architect c16000 (Abbott) or Cobas 6000 (Roche) random access analyzers. Bicarbonate was calculated from measured pH and pCO2 on a Rapidpoint 500 blood gas analyzer (Siemens).
Collected data
In addition to age and gender, we collected the following clinical characteristics that were candidates for inclusion in our model, based on literature and suggested by our expert panel: plasma sodium and bicarbonate concentration, office systolic and diastolic blood pressure and intensity of antihypertensive therapy (expressed as defined daily dose, DDD, http://www.whocc.no/ddd/definition) at first outpatient clinic visit, plasma potassium concentration and amount of potassium supplementation before SLT, and pre- and post-infusion PRC and PAC values (8, 9, 10, 11). All data were anonymized. When the laboratory reported a PRC <3 mU/L, the PRC was considered as equal to 3 mU/L.
Diagnostic criteria
The diagnosis of PA was based on a positive SLT result (post-infusion PAC >280 pmol/L) or, in case of an inconclusive SLT result (PAC after salt loading ≥140 to ≤280 pmol/L), based on consensus reached during our regular clinical Adrenal Center meetings attended by clinicians with expertise in the field of PA: a minimum of three endocrinologists and a specialist in vascular medicine. This consensus was based on available clinical and demographic data regarded as relevant by all experts (i.e. result of SLT, potassium, medication, blood pressure, age etc.) and reached by discussion. We used no other confirmatory tests.
Exclusion of the diagnosis of PA was based on a negative SLT result (PAC after salt loading <140 pmol/L) or, in case of an inconclusive SLT result (PAC after salt loading ≥140 to ≤280 pmol/L), based on consensus reached during the same expert meetings.
Furthermore, all SLT results were discussed in these meetings. When SLT was conclusive for the diagnosis of PA but other characteristics were contradictory to the diagnosis of PA the experts could decide to reject the diagnosis. This was done in two patients with borderline positive SLT results (PAC after salt loading 288 and 290 pmol/L respectively) because PRC concentrations before SLT appeared to be high (both 23 mU/L), and PAC dropped steeply during SLT, suggestive of secondary aldosteronism or even normal renin–angiotensin–aldosterone regulation. For subtyping, we used primarily adrenal vein sampling (AVS), with continuous ACTH infusion (50 µg/h), applying a selectivity index of >3 and a lateralization ratio of >4 as cut-off values. Alternatively we used a CT scan for this purpose.
Statistical analysis
In order to identify factors that could predict the presence of PA, we performed multivariable binary logistic regression analysis with a diagnosis of PA as dependent variable. All candidate predictors were included in the model as continuous variables, except for gender which was included as a categorical variable. We used backward stepwise Wald regression in which each step consisted of removing the variable with the smallest contribution to the model until only variables with a corresponding P value <0.05 remained. The candidate predictors were available in 261 of 290 patients. To test for possible bias caused by missing data, the backward selection procedure was performed using complete cases (n = 261) and using five datasets in which the missing values had been imputed by multiple imputation using fully conditional specification and predictive mean matching (each data set n = 290) (12, 13).
We used the pooled results from the imputed datasets to decide if possible predictors should be dropped from the model. All analyses resulted in the same four predictors, which were retained in the model. The coefficients that were found for these predictors appeared to be comparable for both approaches (Supplementary Table 1, see section on supplementary data given at the end of this article). We developed the final model with the four identified predictors in the 276 patients for whom complete data were available for these four variables. This analysis gave very similar results to the preceding two approaches (Table 1 and Supplementary Table 1). Additionally, we tested in this model all clinically plausible first-order interactions. All analyses were performed using the statistical program SPSS statistics 22.0 for Windows (SPSS Inc.).
Table 1.
Variables included in final analysis (n = 276) and their contribution to the model.
| Variable | Coefficient | P-Value | OR (95% CI) |
|---|---|---|---|
| PRC before saline infusion (mU/L) | −0.256 | <0.001 | 0.73 (0.63–0.86) |
| PAC after saline infusion (pmol/L) | 0.052 | <0.001 | 1.05 (1.03–1.07) |
| Potassium supplementation (mmol/day) | 0.074 | <0.001 | 1.07 (1.04–1.12) |
| Plasma potassium concentration (mmol/L) | −2.922 | 0.012 | 0.05 (0.01–0.53) |
| Constant | −0.582 |
Coefficients are unadjusted for the shrinkage factor.
CI, confidence interval; OR, odds ratio (for 1 unit difference); PAC, plasma aldosterone concentration; PRC, plasma renin concentration.
Internal validation of the model
We applied bootstrapping techniques to estimate overoptimism and to assess internal validity (14, 15). Random bootstrap samples with replacement were drawn from the whole database. This was repeated 200 times. Logistic regression with the selected predictors was repeated within every bootstrap sample, leading to a logistic function for every bootstrap sample. We then calculated the area under the curve (AUC) for all 200 logistic functions, when applied to their own bootstrap sample. The 200 logistic functions were then applied to our original database. For each logistic function, but now applied to the original data, an AUC was calculated. Overoptimism was defined as the mean AUC of the bootstrap samples minus the mean AUC of the original database. We derived a shrinkage factor from this validity analysis to adjust for overoptimism. We multiplied all coefficients with this shrinkage factor to recalibrate the final model (16).
Model performance
To assess model performance, we used the computed AUCs resulting from the receiver operator characteristic (ROC) analyses. This was done for the total cohort and for the patients with an inconclusive SLT separately. The cut-off value with the highest sum of sensitivity and specificity was considered as the optimal cut-off value. Additionally, we calculated the AUC for the ARR.
Results
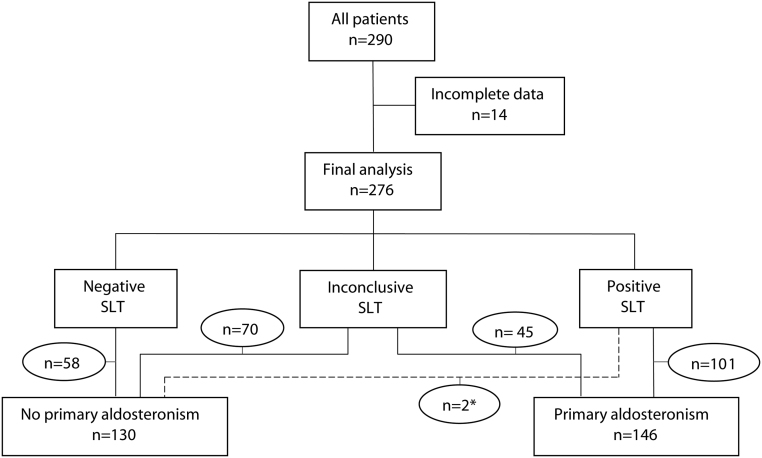
Patient characteristics are shown in Table 2. For the characteristics of the patients with an inconclusive SLT specifically we refer to Supplementary Table 2. After exclusion of cases (n = 14) with missing data in the final predictors, 276 patients remained of which 103 patients had a positive, 115 an inconclusive and 58 a negative SLT. Of all patients with an inconclusive SLT, 45 were diagnosed with PA (Fig. 1). Two patients with borderline positive SLT results (PAC after salt loading 288 and 290 pmol/L respectively) were considered by the expert team as having no PA because PRC concentrations before SLT were high and PAC dropped steeply during SLT. In none of the patients, an adverse event occurred which led to discontinuation of the test. All reported blood pressures during SLT remained <200/120 mmHg.
Table 2.
Characteristics of the study population.
| No primary aldosteronism (n = 136) | Primary aldosteronism (n = 154) | |
|---|---|---|
| Age (years) | 52 (44–60) | 51 (46–58) |
| Gender (male:female) – n (%) | 49:87 (36:64) | 107:47 (69:31) |
| Plasma sodium concentration (mmol/L)a | 141 (140–143) | 141 (140–142) |
| PRC before saline infusion (mU/L)b | 9.8 (6.5–16.3) | 5.4 (3.0–8.3) |
| PRC after saline infusion (mU/L)c | 7.2 (4.8–10.2) | 4.2 (3.0–7.3) |
| PAC before saline infusion (pmol/L)d | 300 (240–438) | 585 (443–1055) |
| PAC after saline infusion (pmol/L) | 150 (110–188) | 410 (270–683) |
| Office systolic blood pressure (mmHg) | 158 (144–170) | 157 (142–172) |
| Office diastolic blood pressure (mmHg) | 93 (86–100) | 92 (84–100) |
| Antihypertensive medication (DDD) | 0.83 (0.00–2.00) | 3.00 (1.33–5.04) |
| Plasma bicarbonate concentration (mmol/L)e | 26.8 (25.4–28.7) | 26.6 (25.2–28.4) |
| Potassium supplementation (mmol/day)f | 0 (0–0) | 32 (0–72) |
| Plasma potassium concentration (mmol/L)f | 3.8 (3.6–4.0) | 3.6 (3.3–3.8) |
| Hypokalemia prior to SLT – n (%)f | 20 (15.5) | 61 (41.2) |
| Screening ARR (pmol/mU)g | 53.2 (40.0–72.3) | 127.0 (87.0–273.3) |
Data are expressed as median (interquartile range) unless otherwise specified. Hypokalemia is defined as plasma potassium concentration <3.5 mmol/L.
aNo PA n = 130; PA n = 152; bno PA n = 133; PA n = 151; cno PA n = 132; PA n = 148; dPA n = 152; eno PA n = 40; PA n = 53; fno PA n = 133; PA n = 148; gPA n = 153.
ARR, aldosterone-to-renin ratio; DDD, defined daily dose; PA, primary aldosteronism; PAC, plasma aldosterone concentration; PRC, plasma renin concentration; SLT, salt loading test.
Figure 1.
Flow-chart of the study participants. SLT, salt loading test. *These patients with borderline positive SLT results (PAC after salt loading 288 and 290 pmol/L respectively) were considered as having no PA because PRC concentrations before SLT were high, and PAC dropped steeply during SLT.
Of all patients with PA (n = 146), 44 patients (28.8%) had a screenings ARR <91 (aldosterone in pmol/L, renin in mU/L; one of the recommended diagnostic cut-offs for the screening of PA according to the Endocrine Society guideline (1); when ARR screening was not performed we used ARR from SLT). For the patients without PA this number was 110 (80.9%). Since there were 197 missing values (68%) for bicarbonate concentration, we omitted this variable from further analyses. All other candidate predictors and gender (n = 11) were explored in our model for their predictive value. We identified four variables predictive of PA: plasma potassium and PRC before saline infusion, PAC after saline infusion and the required amount of potassium supplementation. We found no interaction between PRC before saline infusion and PAC after saline infusion, nor between potassium supplementation and plasma potassium concentration.
Coefficients of the regression equation, P values and odds ratios are shown in Table 1. Bootstrapping estimated the overoptimism at <0.0001. The shrinkage factor was 0.94, resulting in the following logistic function:
Prediction score (p) = ebx/(1+ebx)
Where bx = 0.547–0.241 (0.434 for PRC in ng/L) * PRC before saline infusion (mU/L) + 0.049 (1.364 for PAC in ng/dL) * PAC after saline infusion (pmol/L) + 0.070 * potassium supplementation prior to SLT (mmol/day) − 2.746 * plasma potassium concentration prior to SLT (mmol/L).
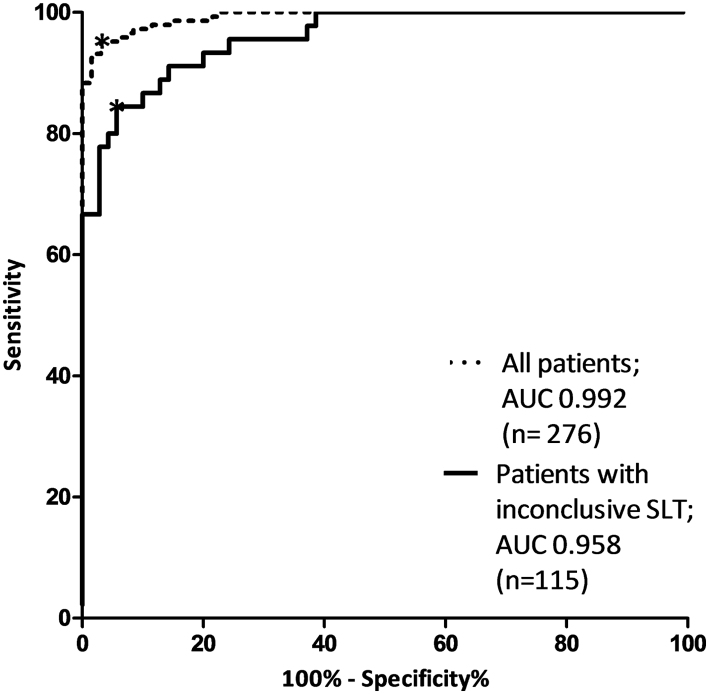
The ranges of the predictors are presented in Supplementary Table 3. The ROC curves of the model are displayed in Fig. 2. When applied to all patients the AUC was 0.992 (95% CI 0.986–0.998). For the patients with an inconclusive SLT only, our model showed an AUC of 0.958 (95% CI 0.926–0.990). Sensitivity, specificity, positive and negative predictive value of various cut-off values are shown in Supplementary Table 4. The optimal prediction score cut-off value was 0.59. A prediction score <0.59 indicates the absence and >0.59 the presence of PA. This is associated with a sensitivity of 95.2% (95% CI 90.4–98.1%), a specificity of 96.9% (95% CI 92.3–99.2%), a positive predictive value of 97.2 (95% CI 93.0–98.9%) and a negative predictive value of 94.7% (95% CI 89.5–97.4%) for the total cohort. When applied to patients with an inconclusive SLT only, sensitivity and specificity were 84.4 (95% CI 71.2–92.3%) and 94.3% (95% CI 86.2–97.8%) respectively. The positive and negative predictive values were 90.5% (95% CI 77.9–96.2%) and 90.4% (95% CI 81.5–95.3%) in our population. Hence, in patients with an inconclusive SLT, a positive score increased the probability of PA from 39.1% (45/115) to 90.5%. Likewise, a negative score decreased this probability for PA from 39.1 to 9.6%. A correct diagnosis was reached in 90% of the patients (Supplementary Table 5). In patients with a conclusive SLT, the model always predicted the diagnosis correctly.
Figure 2.
ROC curves of the model. ROC curves of the prediction model after adjustment for shrinkage factor applied to all patients and to patients with inconclusive SLT only. SLT, salt loading test. *Optimal cut-off value of prediction score (0.59).
For the ARR, calculated from the renin and aldosterone concentration prior to salt loading, the AUC for all patients was 0.851 (95% CI 0.807–0.896) and for the patients with an inconclusive SLT 0.774 (95% CI 0.686–0.863), both statistically lower than the AUCs of our model.
Of the 146 patients with PA 75 underwent adrenalectomy (based on AVS n = 55/75, based on CT scan n = 20/75). According to the consensus criteria on surgery outcomes (17) 3 patients (4.0%) had absent biochemical success, 58 patients (77.3%) had complete biochemical success and for 14 patients (18.7%) follow-up data were incomplete for full assessment of outcome. Of the three patients with biochemical failure, one had an inconclusive SLT result prior to surgery. According to the model this patients also had PA.
Discussion
Major findings
In this retrospective study, we have developed and internally validated a model with adequate test characteristics to diagnose PA similarly to experts in patients with an inconclusive SLT. The identified predictors are in line with previous research on characteristics of patients with PA (1, 8, 10, 11). Our model should be viewed as a means to make implicit reasoning by experts in the field, based on their expert knowledge and experience, more explicit and accountable.
Limitations
A limitation of this study is the lack of a gold standard as a confirmation test is not available. A recent study reported that patients with a conclusively negative SLT result may still have an aldosterone-producing adenoma according to adrenal vein sampling (18). If true this would nullify the value of SLTs but this observation should first be reproduced in a prospective study. Another limitation is the retrospective single-center design of our study. Nevertheless, all consecutive patients who fulfilled the inclusion criteria were included. A third limitation is that our model has not been validated in another data set, although our method of internal validation is also accepted (5). Various renin and aldosterone assays with different units and reference values exist. The regression coefficients of our model should therefore be adjusted when other assays are used. Plasma renin activity is not easily converted into plasma renin concentration (19, 20) and may therefore not be used for our model.
Perspectives
It must be acknowledged that recent literature questions the dichotomous approach to PA (21). For example, in patients with normal blood pressure levels aldosterone-producing cell clusters (APCCs) have been demonstrated (22). Some APCCs harbor mutations associated with autonomous aldosterone production in aldosterone-producing adenomas, suggesting that an APCC might be considered as a precursor for an aldosterone-producing adenoma (23). Furthermore, Ito et al. found a prevalence of PA of 6.8% in patients with prehypertension and normal potassium level. So, it might well be that we should consider it as continuum from low-renin hypertension and subclinical aldosteronism to overt PA.
A matter of debate in our study is our choice not to use an elevated ARR as an inclusion criterion. In daily practice, sometimes clinicians choose to omit ARR testing to save time in case blood pressure is uncontrolled or potassium difficult to normalize. In addition sensitivity of the ARR for PA is, although depending on the cut-off value, limited (24, 25, 26). Consequently we observed a considerable number of patients with PA (n = 44) that had a normal ARR but an abnormal or inconclusive result of the SLT in whom a final diagnosis of PA was made.
Regarding this moderate sensitivity of the ARR and the high percentage of patients with an inconclusive SLT result, an alternative strategy would be replacement of these two diagnostic steps by an SLT combined with our model. Prospective studies are needed to validate our model and explore this approach.
Conclusion
A decision model containing the predictors PRC before and PAC after saline infusion, the quantum of potassium supplementation and plasma potassium concentration, seems a well-performing tool in diagnosing PA in patients with an inconclusive SLT. Prospective research and external validation have to be performed before implementing this model in clinical practice.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgement
Bianca Schalk, PhD, is gratefully acknowledged for her contribution to the study.
References
- 1.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism 2016. 101 1889–1916. ( 10.1210/jc.2015-4061) [DOI] [PubMed] [Google Scholar]
- 2.Giacchetti G, Ronconi V, Lucarelli G, Boscaro M, Mantero F. Analysis of screening and confirmatory tests in the diagnosis of primary aldosteronism: need for a standardized protocol. Journal of Hypertension 2006. 24 737–745. ( 10.1097/01.hjh.0000217857.20241.0f) [DOI] [PubMed] [Google Scholar]
- 3.Rossi GP, Belfiore A, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, et al Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone-producing adenoma. Journal of Hypertension 2007. 25 1433–1442. ( 10.1097/HJH.0b013e328126856e) [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Milan A, Fallo F, Regolisti G, Pizzolo F, Fardella C, Mosso L, Marafetti L, Veglio F, Maccario M. Comparison of confirmatory tests for the diagnosis of primary aldosteronism. Journal of Clinical Endocrinology and Metabolism 2006. 91 2618–2623. ( 10.1210/jc.2006-0078) [DOI] [PubMed] [Google Scholar]
- 5.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Annals of Internal Medicine 2015. 162 W1–W73. ( 10.7326/M14-0698) [DOI] [PubMed] [Google Scholar]
- 6.Wynants L, Bouwmeester W, Moons KG, Moerbeek M, Timmerman D, Van Huffel S, Van Calster B, Vergouwe Y. A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. Journal of Clinical Epidemiology 2015. 68 1406–1414. ( 10.1016/j.jclinepi.2015.02.002) [DOI] [PubMed] [Google Scholar]
- 7.De Man AJ, Hofman JA, Hendriks T, Rosmalen FM, Ross HA, Benraad TJ. A direct radio-immunoassay for aldosterone: significance of endogenous cortisol. Netherlands Journal of Medicine 1980. 23 79–83. [PubMed] [Google Scholar]
- 8.Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K, Zamboulis C. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008. 371 1921–1926. ( 10.1016/S0140-6736(08)60834-X) [DOI] [PubMed] [Google Scholar]
- 9.Khanna A, Kurtzman NA. Metabolic alkalosis. Journal of Nephrology 2006. 19 (Supplement 9) S86–S96. [PubMed] [Google Scholar]
- 10.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF., Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. Journal of Clinical Endocrinology and Metabolism 2004. 89 1045–1050. ( 10.1210/jc.2003-031337) [DOI] [PubMed] [Google Scholar]
- 11.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, et al A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. Journal of the American College of Cardiology 2006. 48 2293–2300. ( 10.1016/j.jacc.2006.07.059) [DOI] [PubMed] [Google Scholar]
- 12.Janssen KJ, Vergouwe Y, Donders AR, Harrell FE, Jr, Chen Q, Grobbee DE, Moons KG. Dealing with missing predictor values when applying clinical prediction models. Clinical Chemistry 2009. 55 994–1001. ( 10.1373/clinchem.2008.115345) [DOI] [PubMed] [Google Scholar]
- 13.https://www.ibm.com/support/knowledgecenter/SSLVMB_21.0.0/com.ibm.spss.statistics.help/mi_analysis.htm.
- 14.Harrel FEJ, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine 1996. 15 361–387. () [DOI] [PubMed] [Google Scholar]
- 15.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. European Heart Journal 2014. 35 1925–1931. ( 10.1093/eurheartj/ehu207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steyerberg E, Eijkemans M, Habbema J. Application of shrinkage techniques in logistic regression analysis: a case study. Statistica Neerlandica 2001. 55 76–88. ( 10.1111/1467-9574.00157) [DOI] [Google Scholar]
- 17.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, et al Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes and Endocrinology 2017. 5 689–699. ( 10.1016/S2213-8587(17)30135-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornu E, Steichen O, Nogueira-Silva L, Kupers E, Pagny JY, Grataloup C, Baron S, Zinzindohoue F, Plouin PF, Amar L. Suppression of aldosterone secretion after recumbent saline infusion does not exclude lateralized primary aldosteronism. Hypertension 2016. 68 989–994. ( 10.1161/HYPERTENSIONAHA.116.07214) [DOI] [PubMed] [Google Scholar]
- 19.Campbell DJ, Nussberger J, Stowasser M, Danser AH, Morganti A, Frandsen E, Menard J. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clinical Chemistry 2009. 55 867–877. ( 10.1373/clinchem.2008.118000) [DOI] [PubMed] [Google Scholar]
- 20.Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clinical Biochemistry 2015. 48 377–387. ( 10.1016/j.clinbiochem.2015.01.003) [DOI] [PubMed] [Google Scholar]
- 21.Buffolo F, Monticone S, Tetti M, Mulatero P. Primary aldosteronism in the primary care setting. Current Opinion in Endocrinology, Diabetes and Obesity 2018. 25 155–159. ( 10.1097/MED.0000000000000408) [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, et al Human adrenocortical remodeling leading to aldosterone-producing cell cluster generation. International Journal of Endocrinology 2016. 2016 7834356 ( 10.1155/2016/7834356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, et al Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. Journal of Clinical Endocrinology and Metabolism 2016. 101 6–9. ( 10.1210/jc.2015-3285) [DOI] [PubMed] [Google Scholar]
- 24.Jansen PM, van den Born BJ, Frenkel WJ, de Bruijne EL, Deinum J, Kerstens MN, Smulders YM, Woittiez AJ, Wijbenga JA, Zietse R, et al Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. Journal of Hypertension 2014. 32 115–126. ( 10.1097/HJH.0b013e3283656b54) [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GL, Chapman AB, Boerwinkle E, Kisabeth RM, Turner ST. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clinical Chemistry 2002. 48 1919–1923. [PubMed] [Google Scholar]
- 26.Nishizaka MK, Pratt-Ubunama M, Zaman MA, Cofield S, Calhoun DA. Validity of plasma aldosterone-to-renin activity ratio in African American and white subjects with resistant hypertension. American Journal of Hypertension 2005. 18 805–812. ( 10.1016/j.amjhyper.2005.01.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a