Abstract
There are still ongoing debates as to which cut-off percentage of tall cell (TC) should be used to define tall cell variant (TCV) papillary thyroid carcinoma (PTC). In this meta-analysis, we aimed to investigate the clinicopathological significance of PTC with tall cell features (PTC-TCF, PTC with 10–50% of TCs) in comparison with classical PTC and TCVPTC (PTC with more than 50% of TCs) to clarify the controversial issue. Four electronic databases including PubMed, Web of Science, Scopus and Virtual Health Library were accessed to search for relevant articles. We extracted data from published studies and pooled into odds ratio (OR) and its corresponding 95% confidence intervals (CIs) using random-effect modeling. Nine studies comprising 403 TCVPTCs, 325 PTC-TCFs and 3552 classical PTCs were included for meta-analyses. Overall, the clinicopathological profiles of PTC-TCF including multifocality, extrathyroidal extension, lymph node metastasis, distant metastasis and patient mortality were not statistically different from those of TCVPTC. Additionally, PTC-TCF and TCVPTC were both associated with an increased risk for aggressive clinical courses as compared to classical PTC. The prevalence of BRAF mutation in PTC-TCF and TCVPTC was comparable and both were significantly higher than that in classical PTC. The present meta-analysis demonstrated that even a PTC comprising only 10% of TCs might be associated with a poor clinical outcome. Therefore, the proportions of PTC in PTC should be carefully estimated and reported even when the TC component is as little as 10%.
Keywords: papillary thyroid carcinoma, tall cell feature, tall cell, meta-analysis, review, BRAF, TERT, prognosis, survival, aggressive
Introduction
Thyroid cancer is the most common endocrine cancer, and its incidence has been steadily increasing over the last few decades (1). There are several subtypes of thyroid carcinoma: papillary, follicular, poorly differentiated, undifferentiated and medullary carcinoma (2). Papillary thyroid carcinoma (PTC) is the most common subtype, which comprises more than 85% of thyroid cancers. Morphologically, PTCs are subdivided into variants, of which tall cell variant (TCV) is recognized as aggressive and associated with a poor prognosis (2).
Criteria for TCVPTC was first proposed in 1976 by Hawk and Hazard (3). In the previous WHO 2004 classification, however, the morphological criterion for the diagnosis of TCVPTC was vague: ‘The TCV is composed predominantly of cells whose heights are at least three times their width’ (4). In the latest WHO 2017 classification, tall cells (TCs) were modified as tumor cells that are two to three times taller than wide. These cells were required to be present in at least 30% of all tumors cells to fulfill the diagnosis of TCVPTC (2).
However, there are still ongoing debates with regard to the percentage of TCs in a tumor required for the diagnosis of TCVPTC (5, 6, 7, 8). Additionally, recent studies have found that the presence of as few as 10% TCs within a PTC significantly influences patient prognosis, and there was no statistical difference between PTCs with 10–50% and more than 50% TCs (5, 6, 9). The term PTC-TCF was used to define those PTCs having the features of TCs but do not reach the cut-off percentage criteria (6, 8). Because of these continuing debates, we need to identify an appropriate threshold of TCs to use in the diagnosis of TCVPTC. The purpose of this meta-analysis was to investigate the clinicopathological features of PTC-TCF in comparison with TCVPTC and classical PTC to clarify the controversial issue.
Methods
Search strategy and study identification
We searched four main electronic databases (PubMed, Scopus, ISI Web of Science and Virtual Health Library) to identify relevant articles using the following search terms: (tall cell OR tall-cell) AND (papillary thyroid) AND (carcinoma OR cancer OR tumor OR tumour OR neoplasm). We included publications published before July 2018. Our study generally followed the recommendations of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement (10).
Selection criteria and abstract screening
We imported search results from all libraries into Endnote (Thompson Reuters) and deleted duplicates. Two reviewers independently screened the abstracts using the predetermined selection criteria. We used the following inclusion criteria: (i) studies containing data of at least two of the following groups: TCVPTC (PTC with more than 50% of TC), PTC-TCF (PTC with less than 50% of TC) and control group (classical PTC) and (ii) studies with clear criteria for TC and TCVPTC/PTC-TCF. The exclusion criteria were (i) studies lack of data of TCVPTC and PTC-TCF, (ii) studies including different variants (e.g., follicular variant) other than classical variant in the control group, (iii) datasets considered as overlapping or duplicated, (iv) reviews, (v) case reports and (vi) proceedings papers, posters, theses and books. The two reviewers solved any disagreements in their analyses through discussion and consensus.
Full-text screening and data extraction
The two reviewers screened the full texts of potential articles and extracted data into a predefined data extraction form. Any disagreements, if present, were solved again by discussion and consensus. The reviewers extracted the following details pertaining to TCVPTC, PTC-TCF and classical PTC: institution, city, country, year of publication, time of operation, criteria for TCVPTC/PTC-TCF, multifocality, extrathyroidal extension, lymph node metastasis, pathological T factor of the TNM staging system (pT factor), distant metastasis at time of presentation, tumor recurrence or cancer-related mortality.
Risk of bias assessment
We assessed the methodologic quality of the studies included in our meta-analyses based on the Newcastle-Ottawa Scale (NOS) for the quality of cohort and case–control studies (11). Each cohort and case–control study received a star (maximum nine stars) based on a developed checklist (11). We considered studies awarded at least six stars as moderate-to-high-quality studies and those with a NOS value of fewer than six stars as low-quality studies.
Data analysis
The Review Manager 5.3 software (Cochrane Collaborative, Oxford, UK) was used for statistical analysis. We calculated pooled estimates of ORs and corresponding 95% CIs using a random-effect model because this model takes into account within-study heterogeneity and its result is similar to the fixed-effect model when heterogeneity is absent.
We quantified heterogeneity between studies by the I 2 statistic (12) and classified inconsistency across studies as low (25% < I 2 < 50%) and high degrees (I 2 ≥ 50%). I 2 ≤ 25% was considered insignificant and interpreted as meaning that the effect size is comparable across studies. Egger’s regression test and funnel plot were used to evaluate the presence of publication bias. We used the MAVIS, version 1.1.2-a R package to analyze the Egger’s regression test and funnel plot. A P value of less than 0.05 was considered statistically significant publication bias.
Results
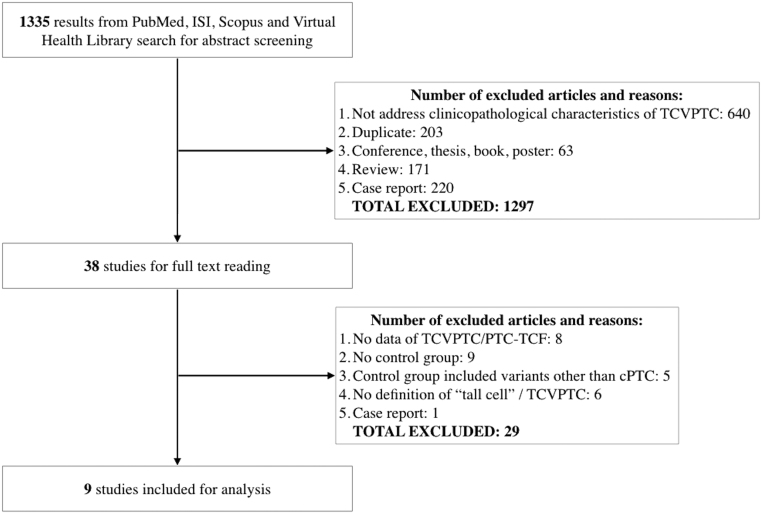
We identified 1335 records after searching four electronic databases using the search terms described earlier. Among them, 38 studies met our selection criteria following the title and abstract screening step. After carefully reading full texts of these studies, we excluded 29 studies. Finally, nine studies comprising 3552 classical PTCs, 325 PTC-TCFs and 403 TCVPTCs were included for final analysis (5, 6, 7, 9, 13, 14, 15, 16, 17) (Fig. 1). The quality of included studies ranged from six to seven stars using the NOS (Table 1).
Figure 1.
Flowchart of the study selection process.
Table 1.
Characteristics of included studies.
| Study | Study design | Criterion of TC (H:W ratio) | Threshold of TC (%) | Number of patients | Newcastle–Ottawa Scale | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCVPTC | PTC-TCF | TCVPTC | PTC-TCF | cPTC | S | C | O | |||
| Beninato 2013 | Retro. cohort | At least 2 | >50 | 10–50 | 26 | 33 | 58 | 3 | 0 | 3 |
| Dettmer 2015 | Retro. cohort | At least 3 | >50 | 10–50 | 21 | 27 | 77* | 3 | 0 | 3 |
| Ganly 2014 | Retro. cohort | At least 2 | >50 | 30–50 | 134 | 31 | 288 | 3 | 0 | 3 |
| Ghossein 2007 | Retro. cohort | At least 3 | >50 | NA | 62 | NA | 83 | 3 | 1 | 3 |
| Ito 2017 | Retro. cohort** | At least 3 | >50 | 30–50 | 19 | 51 | 210* | 3 | 0 | 3 |
| Lee 2014 | Retro. Cohort | At least 2 | >50 | 10–50 | 13 | 16 | 202 | 3 | 0 | 3 |
| Oh 2014 | Retro. Cohort | At least 3 | >50 | 10–50 | 95 | 149 | 203 | 3 | 0 | 3 |
| Park 2009 | Retro. Cohort | At least 3 | >50 | NA | 11 | NA | 2366 | 3 | 0 | 3 |
| Sampathkumar 2018 | Retro. Cohort | At least 3 | >50 | 10–50 | 22 | 18 | 352 | 3 | 0 | 3 |
*These data were not included for analysis because the control group contained different variants (e.g., follicular variant) other than classical variant; **in this study, the control group was matched with TCVPTC group by age and gender. However, the control group was not included for analysis because it comprised of different variants other than classical variant. There were no matched factors between TCVPTC and PTC-TCF.
C, comparability; cPTC, classical PTC; H:W, height to width ratio; NA, not available; O, outcome; PTC-TCF, papillary thyroid carcinoma with tall cell features; S, selection; TC, tall cell; TCVPTC, tall cell variant papillary thyroid carcinoma.
Among the included studies, the thresholds of TCs for PTC-TCF were inconsistent: two studies selected the cut-off of 30–50% (7, 13) and the remaining five studies used the threshold of 10–50% to define PTC-TCF (5, 6, 9, 14, 17). The main characteristics of included studies are shown in Table 1. We performed comparisons for the following groups: TCVPTC vs PTC-TCF, PTC-TCF vs classical PTC and TCVPTC vs classical PTC.
TCVPTC vs PTC-TCF PTC
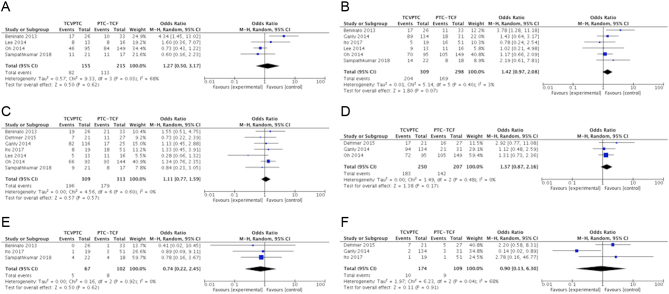
The risks for multifocality, extrathyroidal extension, nodal involvement, pT3–T4, distant metastasis and patient mortality were not statistically different between the two groups (Fig. 2 and Table 2). Data for tumor relapse were insufficient for meta-analysis. Among-study heterogeneity was not present in most of the analyses. In the meta-analysis of multifocality, the significant amount of heterogeneity was completely removed after omitting the Beninato et al. study (5) and the insignificant effect remained unaffected (OR = 0.76; 95% CI = 0.48–1.21; I 2 = 0%). A high amount of heterogeneity was also present in the meta-analysis of disease mortality (I 2 = 68%). This amount of heterogeneity was completely disappeared after excluding the Ganly et al. study (13) and the overall estimate increased but still insignificant (OR = 2.30; 95% CI = 0.69–7.64; I 2 = 0%).
Figure 2.
Forest plots examined the clinicopathological significance between TCVPTC and PTC-TCF in multifocality (A), extrathyroidal extension (B), lymph node metastasis (C), pT3 – T4 (D), distant metastasis (E) and patient mortality (F).
Table 2.
Comparison of clinicopathological features between PTC-TCF, TCVPTC and classical PTC.
| Clinical features | TCVPTC vs PTC-TCF | PTC-TCF vs classical PTC | TCVPTC vs classical PTC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | OR | 95% CI | P Value | I2 (%) | No. of studies | OR | 95% CI | P Value | I2 (%) | No. of studies | OR | 95% CI | P Value | I2 (%) | |
| MF | 4 | 1.27 | 0.50–3.17 | 0.62 | 68 | 4 | 1.44 | 1.02–2.02 | 0.04 | 0 | 6 | 1.67 | 1.16–2.39 | 0.006 | 16 |
| ETE | 6 | 1.42 | 0.97–2.08 | 0.07 | 3 | 5 | 2.93 | 1.99–4.32 | <0.001 | 21 | 6 | 3.91 | 2.79–5.50 | <0.001 | 17 |
| LNM | 7 | 1.11 | 0.77–1.59 | 0.57 | 0 | 5 | 2.20 | 1.44–3.36 | <0.001 | 29 | 6 | 2.23 | 1.26–3.94 | 0.006 | 67 |
| pT3 – T4 | 3 | 1.37 | 0.87–2.16 | 0.17 | 0 | 2 | 3.79 | 2.56–5.61 | <0.001 | 0 | 2 | 4.07 | 2.41–6.85 | <0.001 | 55 |
| DM | 3 | 0.74 | 0.22–2.45 | 0.62 | 0 | 2 | 4.23 | 1.38–12.89 | 0.01 | 0 | 3 | 3.36 | 1.29–8.72 | 0.01 | 0 |
| TR | Lack of data | Lack of data | 3 | 5.12 | 1.70–15.44 | 0.004 | 14 | ||||||||
| CRM | 3 | 0.90 | 0.13–6.30 | 0.91 | 68 | Lack of data | 2 | 13.03 | 1.38–123.11 | 0.03 | 60 | ||||
Bold interface indicates significant results.
CI, confidence interval; CRM, cancer-related mortality; DM, distant metastasis; ETE, extrathyroidal extension; LNM, lymph node metastasis; MF, multifocality; OR, odds ratio; PTC-TCF, papillary thyroid carcinoma with tall cell features; TCVPTC, tall cell variant papillary thyroid carcinoma; TR, tumor recurrence.
PTC-TCF vs classical PTC
In the studies by Ito et al. (7) and Dettmer et al. (9), the control group contained other PTC variants which could bias the analyses. Therefore, we did not include these two studies in the meta-analyses concerning the comparison of classical PTC with PTC-TCF and TCVPTC. Our results demonstrated that PTC-TCF was associated with a significantly increased risk for all adverse prognostic factors when compared to classical PTC, except tumor recurrence and patient mortality due to lack of data (Fig. 3 and Table 2). The meta-analyses were consistent among all included studies. Only a small amount of heterogeneity was found in the effect of lymph node metastasis (I 2 = 29%). After omitting the study by Ganly et al. (13), the overall effect still remained significant and the among-study heterogeneity was no longer present (OR = 2.38; 95% CI = 1.68–3.37; I 2 = 0%).
Figure 3.
Forest plots examined the clinicopathological significance between PTC-TCF and classical PTC in multifocality (A), extrathyroidal extension (B), lymph node metastasis (C), pT3 – T4 (D), distant metastasis (E) and BRAF mutation (F).
TCVPTC vs classical PTC
The presence of more than 50% TC in a given PTC was a prognostic indicator for all adverse factors, including tumor recurrence and patient mortality, as compared with classical PTC (Table 2). A considerable amount of between-study heterogeneity was present in only a few analyses (lymph node metastasis, pT factor and patient death).
The difference in genetic backgrounds between TCVPTC, PTC-TCF and classical PTC
Data for BRAF mutation were reported in three studies (6, 9, 17). The prevalence of BRAF mutation was comparable between TCVPTC and PTC-TCF PTC (OR = 0.84; 95% CI = 0.18–3.85; I 2 = 0%). However, the prevalence of this mutation in TCVPTC and PTC-TCF was significantly higher than that in classical PTC (OR = 5.43; 95% CI = 1.78–16.53; I 2 = 0% and OR = 6.47; 95% CI = 2.39–17.48; I 2 = 0%, respectively). Data of other genetic alterations were insufficient for analysis.
Subgroup analysis
When we removed the two studies using criterion of 30–50% TC to define PTC-TCF (7, 13), the overall pooled results of PTC-TCFs (10–50% TCs) did not alter: they showed more aggressive behaviors when compared to classical PTC and did not differ from TCVPTC (Table 3).
Table 3.
Comparison of clinicopathological features of PTC-TCF (only limited to studies using cut-off percentage of 10–50% TCs) with TCVPTC and classical PTC.
| Clinical features | TCVPTC vs PTC-TCF | PTC-TCF vs classical PTC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | OR | 95% CI | P Value | I2 (%) | No. of studies | OR | 95% CI | P Value | I2 (%) | |
| MF | 4 | 1.27 | 0.50–3.17 | 0.62 | 68 | 4 | 1.44 | 1.02–2.02 | 0.04 | 0 |
| ETE | 4 | 1.67 | 0.93–2.99 | 0.09 | 26 | 4 | 3.46 | 2.42–4.95 | <0.001 | 0 |
| LNM | 5 | 1.04 | 0.65–1.65 | 0.87 | 10 | 4 | 2.38 | 1.68–3.37 | <0.001 | 0 |
| pT3 – T4 | 2 | 1.55 | 0.82–2.94 | 0.18 | 14 | 2 | 4.16 | 2.64–6.55 | <0.001 | 0 |
| DM | 2 | 0.69 | 0.17–2.80 | 0.6 | 0 | 2 | 4.16 | 1.38–12.89 | 0.01 | 0 |
| TR | Lack of data | Lack of data | ||||||||
| CRM | 2 | 2.30 | 0.69–7.64 | 0.18 | 0 | Lack of data | ||||
Bold interface indicates significant results.
CI, confidence interval; CRM, cancer-related mortality; DM, distant metastasis; ETE, extrathyroidal extension; LNM, lymph node metastasis; MF, multifocality; OR, odds ratio; PTC-TCF, papillary thyroid carcinoma with tall cell features; TCVPTC, tall cell variant papillary thyroid carcinoma; TR, tumor recurrence.
Publication bias
To investigate the publication bias, we calculated funnel plots of effects from individual studies. The funnel plots did not indicate a strong publication bias among the set of included studies. Furthermore, tests of asymmetry did not suggest any evidence of publication bias (data not shown).
Discussion
PTCs are classified into a number of variants and some of them have been considered aggressive variants (e.g., tall cell, diffuse sclerosing, solid, columnar cell, hobnail variant) (2, 18, 19, 20). TCVPTC is a rare variant, but it is of great interest to surgeons and pathologists because it is associated with aggressive behaviors and unfavorable outcomes (21). In the former WHO 2004 classification, the morphological criterion for the diagnosis of a TC was a height at least thrice its width (4). This criterion, however, was modified in the current WHO 2017 classification to a height at least two to three times the cell’s width and these cells were required to be present in at least 30% of all tumor cells to satisfy the diagnosis of TCVPTC (2).
Some published meta-analyses have confirmed the increased aggressiveness of TCVPTCs compared with cPTCs (22, 23). However, the definition of TCs and the threshold of these cells for diagnosing TCVPTC were not clearly summarized in these studies. Additionally, a large amount of heterogeneity existed among the analyses in these studies, which may stem from the differences in the diagnostic threshold criteria for TCVPTC between studies. The pooled effect in a meta-analysis is a result of the average effect across all included studies, and if the included studies are too heterogeneous, that average effect is probably not saying anything very useful about what might be observed in a different patient population in another study or another place (12). Recently, a few studies have reported that a PTC with as little as 10% of TCs behaves aggressively (5, 6, 9). Because of these reasons, we performed this meta-analysis to answer the question whether the clinical manifestations of PTC-TCF are different from those of TCVPTC and classical PTC.
Our meta-analysis results demonstrated that all clinicopathological features of PTC-TCF were statistically analogous to TCVPTC, except data of disease recurrence due to lack of data. In a previous study, the recurrence rate between these two groups was not statistically different (5). On the other hand, both these two groups showed more aggressive behaviors when compared to classical PTC. Additionally, when we limited PTC-TCFs to the group of tumors only comprising of 10–50% of TCs, these results did not differ from the primary analyses. These findings highlight that even a PTC with as little as 10% of TCs might have a worse clinical course than classical PTC but does not differ from PTC with more than 50% of TCs. As a result, we recommend that reporting the percentage of TCs in every case of PTC is important for predicting the aggressiveness of the tumor. Pathologists should carefully examine all tissue blocks of a tumor because the percentage of TCs may not be uniformly present within the sample. Many, if not most, pathologists only examine representative sections. An inaccurate determination of TCs could lower the rate of detection and diagnosis of PTC-TCF/TCVPTC. Currently, there are no guidelines addressing the minimal amount of tissue blocks required to be submitted for histological examination. With the rapid development of computer-assisted techniques and machine learning, there are promising tools that can help clinicians better identify TCs and quantify the number of TCs in a given tumor. However, these tools need to be validated in further studies (24).
In our meta-analysis, it should be noted that no demographic factors (e.g., age, gender) were matched between the TCVPTC, PTC-TCF or classical PTC groups among the included studies. Several studies have pointed out that TCVPTC not only has a worse clinical outcome but is also associated with an older age at presentation (16, 25, 26). Johnson et al. (27) reported that when adjusted for age, there was no significant difference in the incidence of nodal involvement, extrathyroidal extension and distant metastasis between TCVPTC and classical PTC. In another case–control study, however, TCVPTC was still associated with an aggressive behavior and a high recurrence rate when controlled for age and gender (28). Therefore, it has to be acknowledged that the clinical significance of TCVPTC is not reached a definitive consensus, and future studies using strict selection/diagnostic criteria and standardized reporting are necessary.
Although there are still ongoing questions as to why TCVPTC exhibit aggressive clinical behaviors, recent progress in the molecular pathogenesis of thyroid cancer may help clarify the biological aggressiveness of this variant. Although the data are limited, our results showed that PTC-TCF has a similar BRAF mutation prevalence to TCVPTC but significantly higher as compared to classical PTC. The more recently discovered TERT promoter mutations were also more prevalent in TCVPTCs than in classical PTCs (9, 29). The association of BRAF and TERT promoter mutations with a poor outcome in PTC has been well confirmed in recent meta-analyses (30, 31).
This meta-analysis has a few limitations that need to be addressed. All included studies were retrospective cohort studies in nature, and it could lead to selection bias. Another limitation is that follow-up data (tumor recurrence and cancer mortality) were rarely reported among the included studies, especially in the comparison of classical PTC vs PTC-TCF. It could be due to the overall good prognosis of PTC patients. Additional prospective large series with appropriately long follow-up are needed to confirm the prognostic outcomes of the PTC-TCF in comparison with TCVPTC and classical PTC.
In conclusion, PTC-TCF and TCVPTC have similar clinicopathological outcomes and both are associated with a worse prognosis as compared to classical PTC. Our study demonstrated that even a PTC comprising only 10% of TCs might be associated with a poor clinical outcome. Therefore, it is important to estimate and report the proportions of TC components in PTC even when the proportion is as little as 10%. These findings help clinician better assess patient outcomes, identify the high-risk tumors and tailor appropriate therapeutic decisions.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngology: Head and Neck Surgery 2014. 140 317–322. ( 10.1001/jamaoto.2014.1) [DOI] [PubMed] [Google Scholar]
- 2.Lloyd RV, Osamura RY, Kloppel G, Rosai J. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press, 2017. [Google Scholar]
- 3.Hawk WA, Hazard JB. The many appearances of papillary carcinoma of the thyroid. Cleveland Clinic Quarterly 1976. 43 207–216. ( 10.3949/ccjm.43.4.207) [DOI] [PubMed] [Google Scholar]
- 4.De Lellis R, Lloyd R, Heitz P, Eng C. World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press, 2004. [Google Scholar]
- 5.Beninato T, Scognamiglio T, Kleiman DA, Uccelli A, Vaca D, Fahey Iii TJ, Zarnegar R. Ten percent tall cells confer the aggressive features of the tall cell variant of papillary thyroid carcinoma. Surgery 2013. 154 1331–1336. ( 10.1016/j.surg.2013.05.009) [DOI] [PubMed] [Google Scholar]
- 6.Oh WJ, Lee YS, Cho U, Bae JS, Lee S, Kim MH, Lim DJ, Park GS, Lee YS, Jung CK. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean Journal of Pathology 2014. 48 201–208. ( 10.4132/KoreanJPathol.2014.48.3.201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Hirokawa M, Miyauchi A, Higashiyama T, Kihara M, Miya A. Prognostic significance of the proportion of tall cell components in papillary thyroid carcinoma. World Journal of Surgery 2017. 41 742–747. ( 10.1007/s00268-016-3784-7) [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Prera JC, Machado RA, Asa SL, Baloch Z, Faquin WC, Ghossein R, LiVolsi VA, Lloyd RV, Mete O, Nikiforov YE, et al Pathologic reporting of tall-cell variant of papillary thyroid cancer: have we reached a consensus? Thyroid 2017. 27 1498–1504. ( 10.1089/thy.2017.0280) [DOI] [PubMed] [Google Scholar]
- 9.Dettmer MS, Schmitt A, Steinert H, Capper D, Moch H, Komminoth P, Perren A. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocrine-Related Cancer 2015. 22 419–429. ( 10.1530/ERC-15-0057) [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. & PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009. 6 e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O'connell O, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute 2011.
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002. 21 1539–1558. ( 10.1002/sim.1186) [DOI] [PubMed] [Google Scholar]
- 13.Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, Tuttle RM, Shah JP, Ghossein R. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid 2014. 24 662–670. ( 10.1089/thy.2013.0503) [DOI] [PubMed] [Google Scholar]
- 14.Sampathkumar G, Nair V, Menon U, Smitha N, Sundaram S, Kumar H, Pavithran P, Bhavani N, Menon A, Abraham N, et al A Comparison of clinicopathological characteristics and short-term outcome of papillary thyroid carcinoma with tall cell histology and classic papillary thyroid carcinoma: a single-institution experience. Indian Journal of Endocrinology and Metabolism 2018. 22 405–409. ( 10.4103/ijem.IJEM_65_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghossein RA, Leboeuf R, Patel KN, Rivera M, Katabi N, Carlson DL, Tallini G, Shaha A, Singh B, Tuttle RM. Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: biologic behavior and clinical implications. Thyroid 2007. 17 655–661. ( 10.1089/thy.2007.0061) [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Lee JI, Tan AHK, Jang HW, Shin HW, Oh YL, Shin JH, Kim JH, Kim JS, Son YI, et al Clinical differences between classic papillary thyroid carcinoma and variants. Endocrinology and Metabolism 2009. 24 165–173. ( 10.3803/jkes.2009.24.3.165) [DOI] [Google Scholar]
- 17.Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagnostic Cytopathology 2014. 42 11–17. ( 10.1002/dc.23007) [DOI] [PubMed] [Google Scholar]
- 18.Vuong HG, Kondo T, Pham TQ, Oishi N, Mochizuki K, Nakazawa T, Hassell L, Katoh R. Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: a systematic review and meta-analysis. European Journal of Endocrinology 2017. 176 431–439. ( 10.1530/EJE-16-0863) [DOI] [PubMed] [Google Scholar]
- 19.Vuong HG, Odate T, Duong UNP, Mochizuki K, Nakazawa T, Katoh R, Kondo T. Prognostic importance of solid variant papillary thyroid carcinoma: a systematic review and meta-analysis. Head and Neck 2018. 40 1588–1597. ( 10.1002/hed.25123) [DOI] [PubMed] [Google Scholar]
- 20.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016. 26 1–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LiVolsi VA. Papillary carcinoma tall cell variant (TCV): a review. Endocrine Pathology 2010. 21 12–15. ( 10.1007/s12022-010-9106-y) [DOI] [PubMed] [Google Scholar]
- 22.Jalisi S, Ainsworth T, La Valley M. Prognostic outcomes of tall cell variant papillary thyroid cancer: a meta-analysis. Journal of Thyroid Research 2010. 2010 325602 ( 10.4061/2010/325602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Zeng W, Chen T, Guo Y, Zhang C, Liu C, Huang T. A comparison of the clinicopathological features and prognoses of the classical and the tall cell variant of papillary thyroid cancer: a meta-analysis. Oncotarget 2016. 8 6222–6232. ( 10.18632/oncotarget.14055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim E, Baloch Z, Kim C. Computer assisted detection and analysis of tall cell variant papillary thyroid carcinoma in histological images. Medical Imaging 2015: Digital Pathology. Proceedings SPIE, 9420 2015. 94200A. ( 10.1117/12.2082156) [DOI] [Google Scholar]
- 25.Okuyucu K, Alagoz E, Arslan N, Emer O, Ince S, Deveci S, Ayan A, Taslpnar A, Gunalp B, Azal O. Clinicopathologic features and prognostic factors of tall cell variant of papillary thyroid carcinoma: comparison with classic variant of papillary thyroid carcinoma. Nuclear Medicine Communications 2015. 36 1021–1025. ( 10.1097/MNM.0000000000000360) [DOI] [PubMed] [Google Scholar]
- 26.Moreno Egea A, Rodriguez Gonzalez JM, Sola Perez J, Soria Cogollos T, Parrilla Paricio P. Prognostic value of the tall cell variety of papillary cancer of the thyroid. European Journal of Surgical Oncology 1993. 19 517–521. [PubMed] [Google Scholar]
- 27.Johnson TL, Lloyd RV, Thompson NW, Beierwaltes WH, Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. American Journal of Surgical Pathology 1988. 12 22–27. ( 10.1097/00000478-198801000-00003) [DOI] [PubMed] [Google Scholar]
- 28.Ruter A, Dreifus J, Jones M, Nishiyama R, Lennquist S, Hay ID, Shaha AR, Thompson NW, Clark OH. Overexpression of p53 in tall cell variants of papillary thyroid carcinoma. Surgery 1996. 120 1046–1050. ( 10.1016/S0039-6060(96)80053-5) [DOI] [PubMed] [Google Scholar]
- 29.Gandolfi G, Ragazzi M, Frasoldati A, Piana S, Ciarrocchi A, Sancisi V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. European Journal of Endocrinology 2015. 172 403–413. ( 10.1530/EJE-14-0837) [DOI] [PubMed] [Google Scholar]
- 30.Vuong HG, Duong UNP, Altibi AMA, Ngo HTT, Pham TQ, Tran HM, Gandolfi G, Hassell L. A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocrine Connections 2017. 6 R8–R17. ( 10.1530/EC-17-0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clinical Endocrinology 2017. 87 411–417. ( 10.1111/cen.13413) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a