Abstract
Context
Multiple endocrine neoplasia type 2B (MEN2B) is characterized by early-onset medullary thyroid cancer in virtually all cases and a 50% lifetime risk of pheochromocytoma (PHEO) development. The literature on PHEO in patients with MEN2B is limited with most data being reported from adult studies that primarily address MEN2A.
Objective
The aim of the current study is to describe PHEO development in a cohort of pediatric patients with MEN2B.
Design
Retrospective chart review of patients with MEN2B evaluated at the National Institutes of Health in the period between July 2007 and February 2018.
Results
A total of 38 patients were identified (21 males and 17 females). Mean age at MEN2B diagnosis was 10.6 ± 3.9 years. Eight patients (21%) developed PHEO in the course of follow-up to date, all of whom were sporadic cases with the classic M918T RET mutation. PHEO was diagnosed based on biochemical and/or imaging screening studies in five patients, whereas three patients presented with symptoms of excess catecholamines. PHEO was diagnosed at a mean age 15.2 ± 4.6 (range, 10 to 25) years and 4.0 ± 3.3 years after MEN2B diagnosis. Only one patient was diagnosed with PHEO as the initial manifestation of MEN2B after she presented with hypertension and secondary amenorrhea.
Conclusion
Undiagnosed PHEO can be associated with substantial morbidity. Current American Thyroid Association guidelines recommend PHEO screening starting at age 11 for the high-/highest risk group. The youngest patient diagnosed with PHEO in our cohort was an asymptomatic 10-year-old, suggesting that PHEO development may begin before the screening-recommended age of 11, though remains clinically undetectable and thus the current screening guidelines seem appropriate.
We described the development of PHEO in 8 pediatric MEN2B patients and found that it may develop earlier than the screening-recommended age of 11; however, it is likely clinically nonsignificant.
Multiple endocrine neoplasia type 2 (MEN2) is a rare hereditary autosomal dominant cancer syndrome, caused by activating germline mutations in the RET proto-oncogene (1–3). MEN2B, which only compromises 5% of all MEN2 cases, is characterized by medullary thyroid cancer (MTC) and typical extraendocrine features, with a 50% risk of pheochromocytoma (PHEO) development. The most common mutation, present in ~95% of patients with MEN2B, is a methionine-to-threonine substitution at codon 918 in the tyrosine-kinase domain (M918T), with the remaining patients carrying a RET A833F codon mutation (2, 4, 5). It is estimated that over 90% of patients with MEN2B have de novo mutations (3, 6).
MTC develops very early in patients with MEN2B and remains the leading cause of death, prompting the American Thyroid Association (ATA) to recommend early prophylactic thyroidectomy before the age of 1 year in patients carrying the M918T mutation (7). Although MTC is well-characterized in the MEN2B literature, this does not hold true for PHEO. The largest series on PHEO in the context of MEN2B was published in 2013 by Thosani et al. (8) and included 15 patients with MEN2B, as part of a larger MEN2 cohort of 85 patients. The youngest that a patient with MEN2B has been reported to develop a PHEO is 12 years old (9). This patient was part of a larger MEN2 cohort that included 84 patients with MEN2A and only three patients with MEN2B.
The current ATA guidelines recommend routine PHEO screening with either free plasma metanephrines and normetanephrines or 24-hour urine metanephrines to begin by the age of 11 for patients with the high/highest risk mutations and by the age of 16 for patients in the moderate risk category (7). However, data from our cohort indicate that PHEO can develop at an earlier age than the screening-recommended age of 11 years. The aim of the current study is to present our experience with PHEO in pediatric patients with MEN2B.
Patients and Methods
We performed a retrospective chart review of all pediatric patients (<18 years) with a diagnosis of MEN2B who were seen at the National Institutes of Health (NIH) between July 2007 and February 2018. Approval by the Institutional Board Review of the National Cancer Institute was obtained and all patients and/or their parents gave written assent/consent. These patients were enrolled on the National Cancer Institute Pediatric Oncology Branch MTC natural history study (NCT01660984) or one of the therapeutic trials of MTC. The NIH Clinical Research Information System was used to obtain medical records and clinical data. MEN2B diagnosis was confirmed by genetic analysis of the RET proto-oncogene. Many patients were referred to the NIH after they had undergone thyroidectomy and/or adrenalectomy at their home institutions. Relevant prior medical records were translated in English as needed and reviewed when available.
Results
Patient demographics
A total of 38 patients with MEN2B were identified, 21 males and 17 females (Table 1). Mean age at MEN2B diagnosis was 10.6 ± 3.9 years, ranging from prenatal diagnosis to 17 years old. First evaluation at NIH was at an average 13.6 ± 4.2 years. RET mutational analysis revealed the M918T mutation in all 38 patients. Thirty-three cases were sporadic and three cases were familial. The three familial cases included one patient who inherited the mutation from his father and was diagnosed prenatally and two patients who inherited the mutation from their mothers and were diagnosed at the age of 8 and 9 years, respectively. Out of the 38 patients, 8 (21%) developed PHEO in the course of their follow-up to date. All individuals who developed PHEO were sporadic cases of MEN2B. Mean age at PHEO diagnosis was 15.2 ± 4.6 years, ranging from 10 to 25 years.
Table 1.
Baseline Characteristics of Pediatric Patients With MEN2B and PHEO as Compared With the Remaining Patients With MEN2B Who Did Not Develop PHEO
| MEN2B With PHEO | MEN2B Without PHEO | Total MEN2B | |
|---|---|---|---|
| No. of patients | 8 (21%) | 30 (79%) | 38 |
| Sex (F/M) | 5/3 | 12/18 | 17/21 |
| Mean age at MEN2B diagnosisa | 10.5 ± 3.5 (5–17) | 10.1 ± 3.9 (prenatal to 18 y) | 10.6 ± 3.9 (prenatal to 17 y) |
| Mean age at PHEO diagnosisa | 15.2 ± 4.6 (10–25) | N/A | N/A |
| Follow-up, mob | 121.4 ± 82.9 (17–263) | 77.8 ± 45.2 (11–180) | 85.4 ± 55.5 (11–263) |
Abbreviation: N/A, not available.
Mean age in years ± SD (range minimum to maximum).
Mean period in months ± SD (range minimum to maximum).
The chronology of PHEO and MEN2B diagnosis is presented in Table 2. Only one patient in our cohort was diagnosed with PHEO as the initial manifestation of MEN2B after she presented with hypertension (HTN) and secondary amenorrhea (patient 4). She initially presented at the age of 16 with intermittent episodes of nausea/vomiting, headaches, and diaphoresis. She was diagnosed with HTN by her primary care provider 1 year later, at which time she also developed amenorrhea. Abdominal ultrasound was obtained to visualize the ovaries as part of the secondary amenorrhea workup and revealed bilateral adrenal masses. Plasma metanephrine and/or normetanephrine levels were markedly elevated. The masses were surgically excised revealing a histopathologic diagnosis of multiple bilateral PHEOs. This led to diagnosis of MEN2B and the patient underwent total thyroidectomy a few months later. The remaining seven patients had an established MEN2B diagnosis prior to being diagnosed with PHEO. PHEO was diagnosed at a mean period of 4.0 ± 3.3 years after MEN2B diagnosis, including three patients who were diagnosed with PHEO within 1 year of MEN2B diagnosis (patients 5, 6, and 8).
Table 2.
Characteristics of Eight Patients With Sporadic MEN2B Due to M918T Mutation and PHEO
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | F | F | M | F | F |
| Age at MEN2B diagnosis, y | 5 | 14 | 12 | 17 | 11 | 15 | 7 | 14 |
| Age at PHEO diagnosis, y | 13 | 25 | 17 | 17 | 11 | 15 | 10 | 14 |
| PHEO relative to MEN2B diagnosis | Metachronous- 6 y | Metachronous- 9 y | Metachronous- 6 y | Synchronous | Metachronous- 8 mo | Metachronous- 3 mo | Metachronous- 3.5 y | Metachronous- 11 mo |
| PHEO-mode of diagnosis | Screening | Screening | Clinical symptoms: HTN crisis requiring ECMO | Clinical symptoms: HTN, diaphoresis, secondary amenorrhea | Screening | Screening | Screening | Clinical symptoms: anxiety, palpitations |
| Biochemical studies at PHEO diagnosis (aupper normal limits are listed below) | n/a | Plasma: Rising f mn from 55 pg/mL to 161 pg/mL within 1 year | n/a | Plasma: high f mn 4029.5 pg/mL and free nmn 3393.5 pg/mL | Plasma: Rising f mn from 159 pg/mL to 202 pg/mL within 4 mo | Plasma: normal 24-hurine: high mn 877 µg/24 h | n/a | Plasma: high total mn 230.87 pg/mL 24-h urine: high mn 313 µg/24 h and nmn 323 µg/24 h |
| Imaging studies (CT) at PHEO diagnosis | L adrenal mass (info on size n/a) | L adrenal nodule on CT 1.2 × 0.9 cm | n/a | n/a | Slight prominence of L adrenal gland | L adrenal mass 2.7 × 2.4 cm | L adrenal mass (info on size n/a) | Nodule in the R adrenal gland (info on size n/a) |
| PHEO tumor size | n/a | 1.8 × 1.4 × 1.2 cm | n/a | R PHEOs 5 × 4 × 2.5 cm, 4 × 3 × 2.5 cm. No info available for L PHEO | 4 × 2.5 × 0.6 cm | 4.2 × 3 × 2.5 cm | n/a | n/a |
| PHEO localization | Unilateral (L) | Unilateral (L) | Bilateral | Bilateral | Unilateral (L) | Unilateral (L) | Unilateral (L) | Unilateral (R) |
| Type of adrenalectomy (date) | Total L (8/2004) | Adrenal sparing L (1/2017) | Total bilateral (4/2012) | Adrenal sparing (11/2014, 1/2015) | Total L (1/2010) | Total L (12/2013) | Total L (7/2002) | Total R (11/2016) |
| Follow-up, mo | 263 | 147 | 123 | 34 | 82 | 50 | Expired 12.5 y after PHEO diagnosis | 17 |
Plasma nmn: normetanephrine (normal < 18 pg/mL); plasma total mn: total metanephrine (normal < 176 pg/mL); 24-h urine mn: 24-h urine metanephrine (normal 25 to 312 µg/24 h); 24-h urine nmn: 24-h urine normetanephrine (normal < 314/24 h).
Abbreviations: ECMO, extracorporeal membrane oxygenation; HTN, hypertension; L, left; n/a, not available; R, right.
Plasma f mn: fractionated metanephrine (normal < 61 pg/mL).
PHEO mode of diagnosis
PHEO was diagnosed using both biochemical and imaging screening studies in three patients (patients 2, 5, and 6) and imaging alone in two patients (patients 1 and 7). The remaining three patients (patients 3, 4, and 8) presented with symptoms of excess catecholamines, including the only patient who presented with PHEO as the initial manifestation of her MEN2B (patient 4).
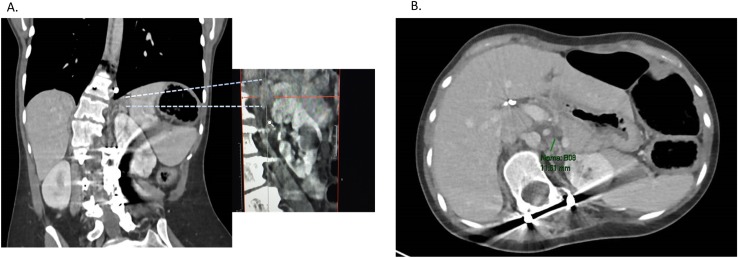
All five patients who were diagnosed with PHEO based on screening tests were asymptomatic at the time of their diagnosis. Biochemical and imaging studies were available for three out of the five patients; in two patients there was a documented rise from their baseline fractionated metanephrine level (patients 2 and 5). Both patients had evidence of adrenal abnormalities on CT imaging (Fig. 1). Patient 6 had a greater than twofold elevation of urine metanephrine level with a left adrenal mass measuring >2 cm on CT. There were two patients for whom biochemical and imaging data were not available for review (patients 1 and 7). Both had been diagnosed with MEN2B and PHEO prior to their initial evaluation at our institution. Medical records from patient 1 report normal plasma metanephrines with a left adrenal mass visualized on imaging; however, the actual laboratory reports were not available. Patient 7 was initially evaluated at our institution 5 years after she underwent adrenalectomy at her local hospital and 8 years after her MEN2B diagnosis. Outside medical records were retrieved and report an adrenal mass identified on screening CT but biochemical studies from the time of her PHEO diagnosis were not available.
Figure 1.
Abdomen CT scan of patient 2 showing indeterminate left adrenal nodules, largest measuring 1.16 cm; (A) coronal view; (B) axial view.
Three patients were diagnosed with PHEO based on clinical symptoms of catecholamine excess (patients 3, 4, and 8). Patient 3 was diagnosed with MTC and MEN2B at the age of 12. Despite the fact that he was followed by endocrinology on a biannual basis, as per family’s report, his excess catecholamine status was not identified until the age of 17, when he presented emergently with hypertensive crisis complicated by pulmonary edema and deteriorating cardiopulmonary status, requiring extracorporeal membrane oxygenation (ECMO) support for 2 weeks. Imaging revealed bilateral PHEOs. Patient 8 was diagnosed with PHEO a few months after her MEN2B diagnosis, at which time screening for PHEO was performed and revealed normal 24-hour urine metanephrine at 1118 nmol/24 hours (ref range < 1231) and normal 24-hour urine normetanephrine at 60 nmol/24 hours (ref range < 473). Eleven months after her MEN2B diagnosis and subsequent total thyroidectomy for MTC, she reported increasing anxiety and intermittent palpitations. Screening laboratories at that time revealed elevated 24-hour urine metanephrine at 1583 nmol/24 hours (ref range < 1231) and elevated 24-hour urine normetanephrine at 1634 nmol/24 hours (ref range < 473), showing an increase of 29% and almost 90%, respectively. CT of the abdomen showed a nodule in the right adrenal gland.
PHEO characteristics and management
Bilateral PHEO was found in two patients; both presented with severe symptoms of excess catecholamines (patients 3 and 4). The remaining six patients had unilateral PHEO; five patients on the left adrenal gland and one patient on the right adrenal. Information on tumor size was available on four patients, median PHEO tumor size was 4 cm, ranging from 1.8 to 5 cm. Only one patient in our cohort underwent total bilateral adrenalectomy with subsequent adrenal insufficiency requiring glucocorticoid and mineralocorticoid replacement therapy. Of the remaining patients, five underwent total unilateral adrenalectomy and two patients who were more recently operated (patients 2 and 4) had adrenal sparing surgeries (subtotal adrenalectomies) with preservation of the adrenal cortical function.
Mortality and follow-up
Follow-up in the MEN2B/PHEO group was a mean of 121 ± 82.9 months from diagnosis of PHEO. No patients in this group have developed a recurrent or new primary PHEO. One patient died of metastatic MTC with ongoing follow-up in the remaining patients.
Discussion
As MTC remains the most common and aggressive manifestation of MEN2B, most of the current literature focuses on MTC. Thus, the timing of PHEO development in MEN2B and clinical outcome in these patients is not well-characterized. Most of the literature comprises adult studies that mainly address patients with MEN2A. The goal of this study is to describe the characteristics of PHEO development in a large cohort of pediatric patients with MEN2B. Our findings have potential implications on the current guidelines for PHEO screening in patients with MEN2.
The largest series of patients with MEN2B published by Brauckhoff et al. (6) includes 44 patients from Germany and Norway, all with the M918T mutation. Of these, 41 patients had de novo mutations. Seven patients in this cohort developed PHEO after puberty; however, the specific age at PHEO diagnosis or the mode of PHEO diagnosis (based on screening as opposed to clinical symptoms) was not reported. Symptomatic PHEO was the presenting sign of MEN2B in one patient in this cohort. This study emphasized the importance of early recognition of the unique nonendocrine phenotypic features of MEN2B, as the authors showed that MTC was curable in all the patients with de novo mutations who underwent thyroidectomy before the age of 4.
In our cohort, 8 out of 38 (or 21%) patients with MEN2B developed PHEO, a percentage similar to the study by Brauckhoff et al. (6). It is generally estimated that the lifelong risk of developing PHEO in patients with MEN2B is ~50%. Although in our cohort, this percentage was approximately half, this discrepancy can be explained by the fact that our study included only pediatric patients with a mean age at PHEO diagnosis of 15.2 ± 4.6 years. Furthermore, when comparing the duration of follow-up in the PHEO vs non-PHEO group, the former was followed for a longer period of time (121.4 ± 82.9 vs 77.8 ± 45.2 months), indicating that the longer we follow these patients, the more likely it is that more cases of PHEO will be diagnosed.
The largest series of patients with MEN2B with PHEO was published in 2013 by Thosani et al. (8). It included 70 patients with MEN2A and 15 patients with MEN2B. The median age at MTC diagnosis for the MEN2B group was 22 years ranging from 8 to 39, whereas median age of PHEO diagnosis for the same group was 25 years ranging from 18 to 40. The median size of the PHEO tumor was 2.5 cm, ranging from 1.0 to 6.4 cm. Sixty percent of patients were diagnosed with PHEO based on screening studies and 67% were found to have bilateral PHEO. Our data are similar with a median PHEO tumor size of 4 cm (1.8 to 5 cm) and 62% diagnosed based on screening studies. Twenty-five percent (two out of eight patients) had bilateral tumors and correlated to a more severe presentation, with both patients presenting with HTN-related comorbidities. The series reported by Thosani et al. (8) included two patients identified with PHEO at autopsy after death due to PHEO-related complications (hypertensive crises during anesthesia and contrast administration). The only death in our PHEO/MEN2B cohort was attributed to metastatic MTC. However, one patient with established MEN2B syndrome, presented 6 years after his initial diagnosis of HTN-related comorbidities, leading to intensive care unit admission and requirement for ECMO. These reports underscore the substantial morbidity and/or mortality associated with undiagnosed PHEO.
The youngest patient with MEN2B with PHEO reported in the literature is a 12-year-old (9). This study included 84 patients with MEN2A and three patients with MEN2B. Fourteen patients developed PHEO, 12 from the MEN2A group and two from the MEN2B group. The mean age at PHEO diagnosis for all the patients with MEN2 was 23.2 ± 6 years (range, 12 to 30). The 12-year-old patient presented with symptoms of sweating and HTN.
The youngest patient with MEN2 with PHEO is reported as a case report by Rowland et al. (10): an 8-year-old female with MEN2A (C634Y RET mutation), with strong family history of MEN2A and PHEO, presented with occasional headaches and elevated plasma metanephrines and was found to have a 6-cm unilateral PHEO.
PHEO in MEN2 syndromes are histologically benign tumors with an exceedingly low chance of metastasis. The development of PHEO is codon-specific with mutations on RET codons 634 and 918 being the most commonly associated mutations (11). Not many studies have compared PHEO presentation in patients with MEN2B vs patients with MEN2A. In the study by Thosani et al. (8), median age of PHEO diagnosis, tumor size, localization, and mode of diagnosis were reported in a group of 70 patients with MEN2A and 15 patients with MEN2; PHEO was diagnosed earlier in the MEN2B group (median age 25 vs 34 years), median tumor size was 2.5 cm in MEN2B as opposed to 3.8 cm in MEN2A, bilateral PHEO was present in 67% of patients with MEN2B and 73% of patients with MEN2A, and finally PHEO was diagnosed based on screening in 60% of patients with MEN2B and 69% of patients with MEN2A.
Successful adrenal sparing surgery was performed in two of our patients. Given the risk for bilateral disease in patients with MEN2B, adrenal sparing surgery should be considered as the preferred option. A large international study published in 2014 by Castinetti et al. (11) studied 552 patients with MEN2 who were operated for PHEO. Recurrence of PHEO was noted in 4 of 153 operated glands after adrenal-sparing surgery compared with 11 of 717 glands operated by adrenalectomy. Patients with bilateral PHEO who underwent adrenalectomy had a higher risk of developing postoperative adrenal insufficiency or steroid dependency (292 of 339 patients) as compared with patients who underwent adrenal-sparing surgery (47 of 82 patients did not become steroid dependent).
Although MEN2B is a hereditary autosomal dominant syndrome that is present since infancy, the unfortunate reality is that most patients are diagnosed when MTC, the leading cause of death in MEN2B, has already developed and even metastasized. The main reason for this delay in diagnosis is that most patients carry de novo mutations and thus, the distinct phenotypic features of MEN2B often go unrecognized until late childhood/adolescence. Furthermore, as previous studies have noted, some of the typical MEN2B features, including mucosal neuromas and Marfanoid habitus, may not be clinically evident until several years of age (3, 12). The mean age of MEN2B diagnosis at our cohort was 10.6 ± 3.9 years, in accordance with this observation.
As discussed earlier, the current ATA guidelines recommend routine PHEO screening with either free plasma metanephrines and normetanephrines or 24-hour urine metanephrines to begin by the age of 11 years for patients with the high/highest risk mutations and by the age of 16 years for patients in the moderate risk category (7). The highest category includes patients with RET codon M918T mutation, the high category includes patients with RET codon C634 mutations and the RET codon A883F mutation, and the moderate category includes patients with RET codon mutations other than M918T, C634, and A883F. Our cohort includes a single patient who developed PHEO prior to the screening-recommended age of 11. This patient did not present with clinical symptoms of catecholamine excess but rather he was diagnosed based on surveillance studies. Because PHEO is generally a histologically benign tumor, perhaps the current guidelines are appropriate, with the proviso that clinicians caring for affected children be aware of potential signs and symptoms and screen earlier if there is clinical concern.
Acknowledgments
Financial Support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Funded by the Division of Intramural Research NICHD.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ATA
American Thyroid Association
- ECMO
extracorporeal membrane oxygenation
- HTN
hypertension
- MEN2B
multiple endocrine neoplasia type 2B
- MTC
medullary thyroid cancer
- NIH
National Institutes of Health
- PHEO
pheochromocytoma
References
- 1. Carlson KM, Dou S, Chi D, Scavarda N, Toshima K, Jackson CE, Wells SA Jr, Goodfellow PJ, Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA. 1994;91(4):1579–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hofstra RMW, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Höppener JW, van Amstel HK, Romeo G, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367(6461):375–376. [DOI] [PubMed] [Google Scholar]
- 3. Castinetti F, Moley J, Mulligan L, Waguespack SG. A comprehensive review on MEN2B. Endocr Relat Cancer. 2018;25(2):T29–T39. [DOI] [PubMed] [Google Scholar]
- 4. Eng C, Smith DP, Mulligan LM, Nagai MA, Healey CS, Ponder MA, Gardner E, Scheumann GF, Jackson CE, Tunnacliffe A, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994;3(2):237–241. [DOI] [PubMed] [Google Scholar]
- 5. Gimm O, Marsh DJ, Andrew SD, Frilling A, Dahia PL, Mulligan LM, Zajac JD, Robinson BG, Eng C. Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J Clin Endocrinol Metab. 1997;82(11):3902–3904. [DOI] [PubMed] [Google Scholar]
- 6. Brauckhoff M, Machens A, Lorenz K, Bjøro T, Varhaug JE, Dralle H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg. 2014;259(4):800–806. [DOI] [PubMed] [Google Scholar]
- 7. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thosani S, Ayala-Ramirez M, Palmer L, Hu MI, Rich T, Gagel RF, Cote G, Waguespack SG, Habra MA, Jimenez C. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2013;98(11):E1813–E1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen L, Niccoli-Sire P, Caron P, Bastie D, Maes B, Chabrier G, Chabre O, Rohmer V, Lecomte P, Henry JF, Conte-Devolx B; French Calcitonin Tumors Study Group . Pheochromocytoma in multiple endocrine neoplasia type 2: a prospective study. Eur J Endocrinol. 2001;144(1):37–44. [DOI] [PubMed] [Google Scholar]
- 10. Rowland KJ, Chernock RD, Moley JF. Pheochromocytoma in an 8-year-old patient with multiple endocrine neoplasia type 2A: implications for screening. J Surg Oncol. 2013;108(4):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95(16):1196–1204. [DOI] [PubMed] [Google Scholar]
- 12. Brauckhoff M, Machens A, Hess S, Lorenz K, Gimm O, Brauckhoff K, Sekulla C, Dralle H. Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: an exploratory analysis. Surgery. 2008;144(6):1044–1050, discussion 1050–1053. [DOI] [PubMed] [Google Scholar]