Altered thyroid hormone levels as caused by environmental stress, developmental temperature and their interaction reduce the potential for physiological acclimation during metamorphosis in Xenopus laevis.
Keywords: Climate change, metabolic costs, metamorphosis, standard metabolic rate (SMR), thermal tolerance
Abstract
Environmental variation induced by natural and anthropogenic processes including climate change may threaten species by causing environmental stress. Anuran larvae experiencing environmental stress may display altered thyroid hormone (TH) status with potential implications for physiological traits. Therefore, any capacity to adapt to environmental changes through plastic responses provides a key to determining species vulnerability to environmental variation. We investigated whether developmental temperature (Tdev), altered TH levels and whether the interactive effect of both affect standard metabolic rate (SMR), body condition (BC), survival and thermal tolerance in larvae of the African clawed frog (Xenopus laevis) reared at five temperatures with experimentally altered TH levels. At metamorphosis, SMR, BC and survival were significantly affected by Tdev, TH status and their interaction with the latter often intensified impacts. Larvae developing at warmer temperatures exhibited significantly higher SMRs and BC was reduced at warm Tdev and high TH levels suggesting decreased ability to acclimate to variation in temperature. Accordingly, tadpoles that developed at warm temperatures had higher maximum thermal limits but more narrow thermal tolerance windows. High and low TH levels decreased and increased upper thermal limits, respectively. Thus, when experiencing both warmer temperatures and environmental stress, larvae may be less able to compensate for changes in Tdev. Our results demonstrate that physiological traits in larvae of X. laevis are strongly affected by increased TH levels and warmer temperatures. Altered TH levels and increasing Tdev due to global change may result in a reduced capacity for physiological plasticity. This has far reaching consequences since the energetic requirement at the onset of metamorphosis is known to determine metamorphic success and thus, is indirectly linked to individual fitness in later life stages.
Introduction
Environmental variation exposes wildlife to multiple chemical, physical and biological stressors that arise partly from anthropogenic activity (e.g. climate change, pollution), but also from natural sources (Noyes et al., 2009). Many environmental stressors have the ability to impair endocrine function in wildlife (Carr and Patiño, 2011). Stressors which alter or disturb endocrine systems are characterized as endocrine disruptors (EDs) (Kloas and Lutz, 2006; Kloas et al., 2009). The impact of EDs in the environment is of special concern in amphibians, which are the most threatened class of vertebrates on the planet (Stuart et al., 2004; Hayes et al., 2006; Gabor et al., 2018). Particularly vulnerable are larval stages of amphibians due to the inability of this life stage to select or avoid habitats (Sanzo and Hecnar, 2006; Yu et al., 2013) and their increased risk of exposure to chemical contaminants due to their highly permeable skin (Hayes et al., 2006; Strong et al., 2017). Furthermore, amphibian larvae are particularly sensitive to EDs since metamorphosis is linked to a reorganization of several organ systems, and this complex change underlies complicated and tight hormonal control (Hayes et al., 2010; Searcy et al., 2015).
Amphibian metamorphosis is a crucial event in amphibian life history due to the complex reorganization of larval to juvenile structures which is mainly regulated by thyroid hormones (TH) (i.e. T3 and T4) (Tata, 2006; Furlow and Neff, 2006). THs increase in concentration during metamorphosis and determine the developmental rate (Brown and Cai, 2007; Shi, 2000). Many EDs influence the hypothalamus–pituitary–thyroid axis, which is responsible for production of THs (Carr and Patiño, 2011). A large number of aquatic contaminants such as pesticides and herbicides, road salt, fertilizers, heavy metals and active pharmaceutical ingredients have been shown to disrupt and inhibit the normal action of THs in amphibians, leading to changes in growth, development and metabolism (Kashiwag et al., 2009; Carr and Patiño, 2011). Inhibition or a decrease of TH production pathways slows the rate of development (Carr et al., 2003; Bulaeva et al., 2015) and decreases metabolic rates (Carr and Patiño, 2011; Ortiz-Santaliestra and Sparling, 2007) causing tadpoles to metamorphose at a larger size and older age (Shi, 2000). An environmentally relevant ED is perchlorate (ClO4−) which is a goitrogen that inhibits TH synthesis (Ortiz-Santaliestra and Sparling, 2007). Concentrations of perchlorate measured in the field are often high enough to inhibit amphibian metamorphosis (Goleman et al., 2002; Ortiz-Santaliestra and Sparling, 2007; Tietge et al., 2005).
Whereas most environmental contaminants inhibit TH activity or production pathways, some contaminants and other abiotic and biotic environmental factors appear to enhance TH activity or increase TH levels by the activation of the neuroendocrine stress axis (Mann et al., 2009; Dantzer et al., 2014) and increase of stress hormone levels (Denver, 1997). These stress hormones may lead to a synergistic increase in TH production (Glennemeier and Denver, 2002; Laudet, 2011; Kulkarni and Buchholz, 2012). The presence of predators (Relyea, 2002; Capellán and Nicieza, 2007), crowding (Morey and Reznick, 2001), desiccation risk (Gervasi and Foufopoulos, 2008), food scarcity (Kupferberg, 1997) and extreme temperature (Smith-Gill and Berven, 1979) may increase TH production by activating the neuroendocrine stress axis. Anuran larvae with high TH levels display increased developmental and metabolic rates and decreased growth rates (Rowe et al., 1998; Brown and Cai, 2007), which results in shorter larval periods, smaller size at the onset of metamorphosis and higher energetic maintenance costs in addition to energetic developmental costs (Denver et al., 1998; Denver, 2009; Orlofske and Hopkins, 2009). In this study, we simulated ecological stress by exposing larvae to L-thyroxin for an increase in TH levels and by inhibiting TH production by the environmental relevant ED sodium perchlorate.
In all vertebrates, THs are not only critical for regulating growth and development, but also for regulating energy metabolism (Sheridan, 1994; Choi et al., 2017). If the TH concentration changes due to environmental stress, a whole suite of physiological processes may be affected. (Steyermark et al., 2005; Hulbert and Else, 2004). Even if the effect of THs on metabolic heat production in ectotherms such as amphibians is negligible (John-Alder, 1983), THs increase the standard metabolic rate (SMR) which is estimated by measuring rates of O2 consumption at rest and represents the energy required to cover basic physiological functions (Rowe et al., 1998; Beck and Congdon, 2003). In amphibians, elevated SMR due to increased TH level manifests in increased activities of enzymes and densities of mitochondria in metabolic relevant tissues such as liver and red skeletal muscle (Chiu and Woo, 1988; Rowe et al., 1998; Steyermark et al., 2005). In individuals with a low SMR but not reduced metabolic scope (the difference between active metabolic rate and SMR), more energy is available for physical performance or development (Steyermark et al., 2005; Orlofske and Hopkins, 2009). As metamorphosis is an energy-consuming process (Sheridan and Kao, 1998; Beck and Congdon, 2003), it may be advantageous to maintain a low SMR. Tadpoles which have larger energy reserves at the onset of metamorphosis are more likely to successfully complete metamorphosis and become juvenile froglets with larger energy stores and higher rates of survival (Orlofske and Hopkins, 2009). Therefore, the SMR and body condition at the onset and after completion of metamorphosis are important fitness variables (Steyermark et al., 2005; Muir et al., 2014; Ruthsatz et al., 2018).
Through its impact on physiology, temperature is considered to be the ‘abiotic master factor’ for ectotherms (Dalvi et al., 2009; Turriago et al., 2015; Sunday et al., 2014; Berg et al., 2017; Theisinger et al., 2017). The tolerable thermal window of ectotherms is bracketed by critical temperatures (CTmin and CTmax) beyond which survival is not possible and these limits occur where aerobic scope is either zero or negative (Holzman and McManus, 1973). Therefore, environmental factors that either load (increase) or unload (decrease) SMR will impact aerobic scope and, thus, the width of tolerable thermal windows (Burraco and Gomez-Mestre, 2016). Climate change is expected to not only result in long-term warming of aquatic habitats but also increased variability in temperature leading to new thermal challenges for tadpoles in their larval habitats (Gutiérrez‐Pesquera et al., 2016) with likely impacts on growth, development and survival (Pörtner et al., 2001; Dalvi et al., 2009). Altered TH levels, through their impact on SMR, may exacerbate these thermal challenges experienced prior to and at the onset of metamorphosis (Formicki et al., 2003). As THs have recently been shown to play a key regulatory role in thermal acclimation in fish (Little and Seebacher, 2014; 2016) and several studies provide an indication on thyroid-regulated acclimation in amphibians and reptiles (Locker and Weish, 1966; Packard and Packard, 1975; Little and Seebacher, 2016).
Although previous studies have examined the impact of stress-induced alteration of TH levels on metamorphic and physiological traits of anuran larvae, studies have rarely examined the interaction of different stressors which is known to affect amphibian metamorphosis under natural conditions (Rowe et al., 1998; Freitag et al., 2017). This study examined the interactive effects of temperature and altered TH levels on the capacity for physiological acclimation (SMR and thermal tolerance) at the onset of metamorphosis in larvae of Xenopus laevis. For larvae acclimated to five different temperatures and experimentally enhanced or lowered TH levels, we tested the following hypotheses: (i) High and low levels of TH, as caused by the thyroid altering effect of several environmental stressors, increase and decrease SMR of tadpoles, respectively. (ii) Changes in TH will be reflected in changes in body condition and survival. (iii) Developmental temperature (Tdev) correlates positively with CTmin, CTmax, and negatively with the thermal range of tolerance. (iv) Tdev will interact with altered TH levels to intensify the effect on larval physiological traits.
Material and methods
Animal husbandry and experimental design
Three, unrelated clutches of X. laevis were obtained from the captive breeding facility of the Universitätsklinikum Hamburg Eppendorf (Martinistr. 52, 20 246 Hamburg, Germany). Larvae were allowed to develop to Gosner stage 25 (free-swimming larvae; Gosner, 1960). The experiment consisted of three replicate aquaria at each of three treatments and five temperatures. From these larvae, 675 individuals originating from different families were intermixed before allocating them randomly to 45 aquaria. Fifteen larvae of X. laevis were kept each in a standard 9.5-L aquarium filled with 8 L of water. The experiment was conducted in two controlled climate chambers (Weiss Umwelttechnik GmbH, 35 447 Reiskirchen, Germany) with a light regime of 12:12 L:D. The mean (±SD) water temperatures were 16 (±0.4), 19 (±0.5), 22 (±0.1), 25 (±0.5) and 28 (±0.3)°C. Temperature was maintained using ambient chamber temperature or via heaters and waterbaths. The experiments were conducted over 4 weeks, until all surviving larvae had reached the onset of metamorphosis (Gosner stage 42; Gosner, 1960). Amphibian larvae were fed high-protein flaked food (Sera, 52 518 Heinsberg, Germany) and spirulina algae ad libitum. The flakes were free of perchlorate according to the manufacturer. Dead or abnormal tadpoles were removed each day. All temperature measurements were made using a digital thermometer (Amarell, Maxi-Pen, −50 to 200°C: 0.1°C). All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The experiments were approved by the Amt für Verbraucherschutz, Lebensmittelsicherheit und Veterinärwesen in Hamburg, Germany (Billstraße 80, 20 539 Hamburg, Germany; Gz. V1305/591–00.33, Nr. 03/16).
Thyroxine and sodium perchlorate exposures
We used a concentration of 250 μg/L SP (Sodium perchlorate hydrate 99.99% trace metals basis, 381 225 Aldrich, Sigma-Aldrich, St. Louis, MO, USA) to achieve a decrease in TH levels (Tietge et al., 2005). This concentration of SP is within environmental ranges measured in the surface and ground waters of many industrial nations (Motzer, 2001; Tietge et al., 2005; Carr & Theodorakis, 2006; Mukhi and Patiño, 2007) and in bodies of water in which amphibians breed (Smith et al., 2001; Ortiz-Santaliestra & Sparling, 2007).
We achieved increased TH levels by exposing tadpoles to 10 μg/L exogenous T4 (Thyroxine T4, IRMM468 Sigma-Aldrich, Sigma-Aldrich, St. Louis, MO, USA), a concentration which is known to influence amphibian metamorphosis (Lucas & Reynolds, 1967; Mann et al., 2009) and is related to increases in T4 observed in tadpoles responding to stress (Denver, 1997; Denver et al., 1998). Tadpoles absorb exogenous T4 directly through their permeable skin (Shi, 2000; Tata, 2006; Coady et al., 2010).
T4 and SP treatments were prepared in 0.1 N sodium hydroxide solutions (Sodium hydroxide solution 0.1 N, S2770 SIGMA, Sigma-Aldrich, St. Louis, MO, USA) buffered with 0.1 N muriatic acid solutions as solvents. A clean solution of 0.1 M sodium hydroxide solution buffered with 0.1 M muriatic acid solution was added to the control aquaria to control for any effect of solvents addition. Each treatment and the control set-up was replicated three times (i.e. 45 larvae, 15 larvae per aquarium, per treatment and control in total). Water was changed every second day and fresh SP and T4 were added, which is frequent enough to maintain a constant hormone and perchlorate level under given experimental temperatures, in accordance with the standard procedure for chemical and hormonal addition (Miwa & Inui, 1987; Goleman et al., 2002; Iwamuro et al., 2003; Rot-Nikcevic & Wassersug, 2004; Tietge et al. 2005; Ortiz-Santaliestra & Sparling, 2007; Bulaeva et al., 2015).
Processing of specimens
Developmental stage was determined by evaluating the status of key morphological features typical of specific developmental stages, as detailed in Gosner (1960). The developmental stage of each tadpole was recorded according to the procedure of Ortiz-Santaliestra & Sparling (2007): Gosner stage groups 1–5: 1. pre-limb (absence of hind limbs, Gosner stages 24–26); 2. limb bud (hind limb visible, but no clear joint formed, Gosner stages 27–34); 3. middle hind limb (knee joint apparent, but toes not completely separated, Gosner stages 35–37); 4. late hind limbs (hind limb tubercles and subarticular patches formed, Gosner stages 38–41); and 5. metamorph (at least one forelimb present, Gosner stage 42 and above) (Gosner, 1960; Ortiz-Santaliestra & Sparling, 2007). The onset of metamorphosis was defined by the emergence of at least one forelimb (Gosner stage 42). The snout vent length (SVL) and total length of each larva was measured with a caliper to the nearest 0.5 mm. Larvae were blotted and weighed to the nearest 0.001 g with an electronic balance (digital gold scale, Smart Weigh). At the end of the experiment tadpoles were euthanized with 200 mg/L of tricaine methanesulfonate ([MS-222], Ethyl 3-aminobenzoate methanesulfonate, E10521 ALDRICH, Sigma-Aldrich, St. Louis, MO, USA) buffered with 200 mg/L of sodium bicarbonate (Sodium bicarbonate, S5761 SIGMA, Sigma-Aldrich, St. Louis, MO, USA) (Stuart et al., 2007) and transferred into ethanol (70%).
Body condition
We estimated the body condition (i.e. energy stores) at the onset of metamorphosis by calculating the scaled mass index (SMI). This is a measure of the entire body condition of an individual as it accounts for the allometric relationship between mass and a body structure measure. It standardizes each measure so that direct comparisons among individuals can be made (Peig & Green, 2009; 2010; MacCracken & Stebbings, 2012). The SMI was considered as an accurate condition index in anuran larvae (MacCracken & Stebbings, 2012; Dittrich et al., 2016; Ruthsatz et al., 2018). A high BC suggests larger energy storages and thus, a good body condition. We followed the procedure outlined by Peig and Green (2009) to calculate the SMI for each individual.
Respiration measurements
Respiration measurements were made at the onset of metamorphosis on 45 individuals, three randomly chosen tadpoles from each aquarium. Animals were not fed for 48 h prior to and during the measurement of SMR such that tadpoles were in a post-absorptive state (Orlofske et al., 2017). Oxygen consumption was measured by closed respirometry conducted between 0900 and 2100 h to control for the influence of natural circadian rhythms on respiration (Orlofske et al., 2017). Larvae were placed in respirometers consisting of 30-mL beakers containing 30 mL (minus the volume of the animals) of autoclaved tap water to exclude microbial oxygen consumption. The water was at 100% O2 saturation at the start. Each respirometer was equipped with a fiber optic sensor (Oxygen Dipping Probe DP-PSt7; PreSens Precision Sensing GmbH, Regensburg, Germany) connected to a multichannel oxygen measuring system (Oxy 4 mini; PreSens Precision Sensing GmbH, Regensburg, Germany) and sealed with an airtight rubber plug. O2 concentration was recorded every 15 s and measured as ml O2 × L−1. Measurements were conducted at the developmental temperature of the individual. Prior to each trial, fiber optic O2 sensors were calibrated using air-saturated water and a factory-set zero oxygen calibration point at the respective measurement temperature. Water temperature was maintained by the continuous mixing of the waterbath. Oxygen consumption was measured for every tadpole for 20 min at each of five temperatures. Empty (control) chambers were run simultaneously in every trial and values were adjusted accordingly. We took care that <10% of total O2 was removed during the measurements to avoid impediment of respiration at low saturation levels. At the end of the measurements, each larva was removed and its TL, SVL and blotted wet body mass was determined.
SMR calculations
Prior to statistical analysis, we plotted the O2 consumption rate of each tadpole over time and visually assessed activity peaks to exclude them for the determination of SMR (Orlofske and Hopkins, 2009). The SMR was expressed in ml O2 × h−1 × mg−1 wet body mass and was determined from the slope of linear least squares regressions of O2 concentration vs. time (Hastings and Burggren, 1995; Rowe and Funck, 2017).
Values for SMR and mass were log transformed because metabolism is a power function of mass (Orlofske and Hopkins, 2009; Orlofske et al., 2017). To exclude the mass-specific effect (Hulbert and Else, 2004) on SMR we did a linear regression of log transformed mass and log transformed SMR to calculate residuals. Residuals obtained from this regression (SMRresiduals) were entered into the analyses instead of SMR. We performed a multiple linear regression of log transformed SMR (dependent variable), log transformed mass (independent variable) and developmental temperature (independent) to describe the mass and temperature dependence of SMR.
Thermal tolerance
Thermal tolerance of X. laevis was evaluated when tadpoles reached the onset of metamorphosis (Gosner stage 42) using the critical thermal methodology (CTM) (Holzman and McManus, 1973). Both critical thermal maximum (CTmax) and minimum (CTmin) endpoints are defined as the thermal point at which locomotor activity becomes disorganized and the animal loses the ability to right itself (Lutterschmidt and Hutchison, 1997; Turriago et al., 2015). A total of 90 tadpoles were used for determination of thermal tolerance. From each aquarium six tadpoles (n = 3, CTmax; and n = 3, CTmin) were tested at set time intervals. CTmin and CTmax were determined by using the dynamic method according to Cowles and Bogert (1944) and Hutchison (1961) except for the end point (Wu and Kam, 2005). This method involves increasing (for CTmax) or decreasing (for CTmin) test temperatures by a specific rate until an appropriate end point is reached (Lutterschmidt and Hutchison, 1997). Tadpoles were placed individually in a 250-ml flask with 200 ml of water which was then placed in a temperature-controlled waterbath. The heating and cooling rates were ±0.1°C × min−1, and the water temperature served as a proxy of body temperature (Hutchison, 1961). The initial temperature in the waterbath was set at the respective developmental temperature. In tadpoles, the occurrence of spasms is difficult to determine, and thus we decided to use the loss of the righting response after being flipped on its back in the water with a probe as our criterion for the end point (Lutterschmidt and Hutchison, 1997; Wu and Kam, 2005) for both, CTmin and CTmax determinations (Turriago et al., 2015). A time limit of 30 s between flipping the animal and righting was adopted (Layne and Claussen, 1982). All thermal tolerance tests were performed between 1100 and 1500 h. After the experiments, we euthanized the tadpoles with MS-222, weighed them and measured SVL and TL, and finally transferred them into ethanol (70%).
We adopted the method of Dalvi et al. (2009) used in fish to generate a thermal tolerance window (TW) for X. laevis by calculating the difference between CTmax and CTmin estimates obtained at various acclimation temperatures. To simulate long-term changes in environmental temperature, X. laevis were reared at a range of different temperatures. The thermal tolerance polygon was generated by plotting the five developmental temperatures for each treatment (T4, SP and Control) on the X-axis and the mean CTmin and CTmax values on the Y-axis. The TW was calculated from the polygon and expressed as °C2 (Dalvi et al., 2009). We performed a linear regression for developmental temperature and thermal tolerance (as measured by CTmin, CTmax and thermal range of tolerance). The slope of the regression for CTmax and CTmin defined the effect of developmental temperature on critical thermal limits of X. laevis.
Statistical analyses
For all statistical tests R 3.4.1 (R Development Core Team, 2007) for Windows was used. All plots were constructed using ggplot2 (Wickham 2009) and Adobe Illustrator CS6. Data were analyzed using linear mixed-effect models [lme, Type III model, covariance type: variance components, REML (restricted maximum likelihood) method for parameter estimation, 100 iterations (Bates and Sarkar, 2007)], using the covariate ‘Tdev’, and ‘treatment’ (T4, SP and Control) and the interactions of ‘treatment’ and ‘Tdev’ as fixed factors. ‘SMRresiduals’, ‘body condition’, ‘survival’ and ‘thermal tolerance’ (as measured by CTmin, CTmax and the thermal range of tolerance) were used as dependent variables in separate models. P-values were obtained from likelihood-ratio tests (Crawley, 2012). To address dependencies in the data, the variable ‘aquarium’ was included as a random factor. Residuals of each model were visually checked for normal distribution. N refers to the total number of analyzed tadpoles. Linear mixed-effect models were followed by post hoc comparisons (Tukey’s test; Tukey HSP function, multcomp package, vers. 1.2–13) to compare all possible pairwise combinations of treatments when overall tests were significant.
‘Thermal tolerance’ (as measured by CTmin, CTmax and the thermal range of tolerance) was correlated with SMR and Tdev using Spearman’s rank correlation (Table 1). Correlations were performed on subgroups according to TH-treatment and Control. The slope of the linear regression for CTmax and CTmin with Tdev defined the effect of temperature during development on the thermal tolerance of X. laevis (Table 1) (Dalvi et al., 2009).
Table 1:
Effects of altered TH levels and temperature during development on SMR, body condition, survival and thermal tolerance (as measured by CTmin, CTmax and thermal range of tolerance) in tadpoles of the African clawed frog (X. laevis) at the onset of metamorphosis (Gosner stage 42) (Gosner, 1960). Chi2 and P for linear mixed-effects models, using the covariates ‘Treatment’ (T4, SP and Control), ‘Tdev’ and the interactions of ‘Treatment*Tdev’ as fixed factors; ‘aquarium’ as random factor. T4 = increased TH concentration; SP = decreased TH concentration. Significance was set at P < 0.05. SMR was taken as the residuals from the regression of log transformed mass on log transformed SMR. Body condition was determined by the scaled mass index (SMI). Bold for significant values
| Dependent variable | Fixed effects | Xenopus laevis | |||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | Chi2 | Df | P | N (n) | ||
| SMRresiduals | Treatment [Control] | −0.08 | 0.04 | 39.63 | 2 | <0.001 | 119 (9) |
| Treatment [SP] | −0.10 | 0.08 | 39.63 | 2 | <0.001 | 119 (9) | |
| Treatment [T4] | 0.13 | 0.06 | 39.63 | 2 | <0.001 | 119 (9) | |
| Tdev | −0.08 | 0.06 | 39.63 | 1 | <0.001 | 119 (9) | |
| Tdev* Treatment [SP] | −0.15 | 0.09 | 39.63 | 2 | <0.001 | 119 (9) | |
| Tdev* Treatment [T4] | 0.33 | 0.09 | 39.63 | 2 | <0.001 | 119 (9) | |
| Body condition (SMI) | Treatment [Control] | 275.56 | 36.11 | 11.42 | 2 | 0.003 | 475 (45) |
| Treatment [SP] | 69.48 | 53.23 | 11.42 | 2 | 0.003 | 475 (45) | |
| Treatment [T4] | −156.08 | 55.72 | 11.42 | 2 | 0.003 | 475 (45) | |
| Tdev | −3.98 | 1.61 | 11.42 | 1 | 0.003 | 475 (45) | |
| Tdev* Treatment [SP] | −3.09 | 2.36 | 11.42 | 2 | 0.003 | 475 (45) | |
| Tdev* Treatment [T4] | −5.52 | 2.49 | 11.42 | 2 | 0.003 | 475 (45) | |
| Survival (%) | Treatment [Control] | −0.23 | 0.11 | 11.05 | 2 | 0.003 | (45) |
| Treatment [SP] | 0.32 | 0.16 | 11.05 | 2 | 0.003 | (45) | |
| Treatment [T4] | 0.61 | 0.16 | 11.05 | 2 | 0.003 | (45) | |
| Tdev | 0.01 | 0.01 | 11.05 | 1 | 0.003 | (45) | |
| Tdev* Treatment [SP] | −0.01 | 0.01 | 11.05 | 2 | 0.003 | (45) | |
| Tdev* Treatment [T4] | −0.02 | 0.01 | 11.05 | 2 | 0.003 | (45) | |
| CTmin (°C) | Treatment [Control] | −11.26 | 0.96 | 37.02 | 2 | <0.001 | 135 (45) |
| Treatment [SP] | 0.76 | 1.35 | 37.02 | 2 | <0.001 | 135 (45) | |
| Treatment [T4] | 0.39 | 1.35 | 37.02 | 2 | <0.001 | 135 (45) | |
| Tdev | 0.99 | 0.04 | 343.16 | 1 | <0.001 | 135 (45) | |
| Tdev* Treatment [SP] | −0.01 | 0.06 | 1.41 | 2 | 0.49 | 135 (45) | |
| Tdev* Treatment [T4] | −0.01 | 0.06 | 1.41 | 2 | 0.49 | 135 (45) | |
| CTmax (°C) | Treatment [Control] | 28.15 | 2.23 | 8.59 | 2 | 0.01 | 135 (45) |
| Treatment [SP] | 1.64 | 3.16 | 8.59 | 2 | 0.01 | 135 (45) | |
| Treatment [T4] | −0.7 | 3.16 | 8.59 | 2 | 0.01 | 135 (45) | |
| Tdev | −0.28 | 0.09 | 24.02 | 1 | <0.001 | 135 (45) | |
| Tdev* Treatment [SP] | −0.02 | 0.14 | 0.05 | 2 | 0.97 | 135 (45) | |
| Tdev* Treatment [T4] | −0.00 | 0.14 | 0.05 | 2 | 0.97 | 135 (45) | |
| Thermal range of tolerance | Treatment [Control] | 39.41 | 1.73 | 0.89 | 2 | 0.63 | 135 (45) |
| Treatment [SP] | 0.87 | 2.44 | 0.89 | 2 | 0.63 | 135 (45) | |
| Treatment [T4] | −1.09 | 2.44 | 0.89 | 2 | 0.63 | 135 (45) | |
| Tdev | −0.69 | 0.07 | 138.08 | 1 | <0.001 | 135 (45) | |
| Tdev* Treatment [SP] | −0.02 | 0.1 | 0.68 | 2 | 0.71 | 135 (45) | |
| Tdev* Treatment [T4] | 0.06 | 0.1 | 0.68 | 2 | 0.71 | 135 (45) | |
Results
Standard metabolic rate
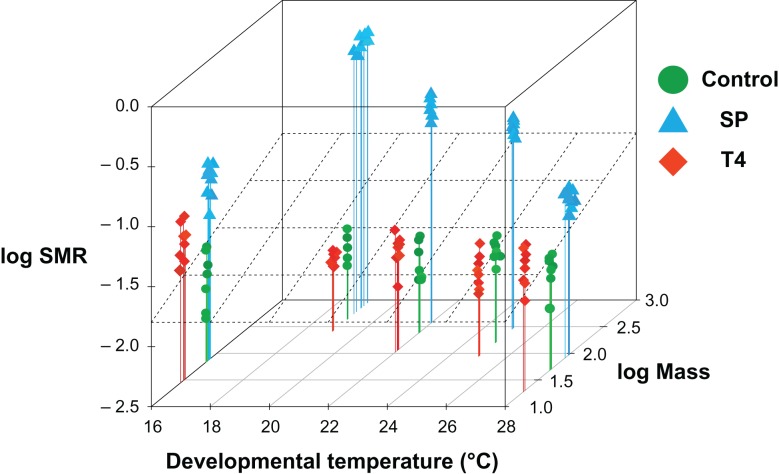
SMR at Tdev of tadpoles was significantly influenced by the hormone treatment, by Tdev and the interactive effect of both (Table 1). There was no consistent effect of time of day on SMR tested during this experiment. At all developmental temperatures tadpoles from the SP treatment were the largest at the onset of metamorphosis followed by control animals and tadpoles from the T4 treatment being the smallest (Table A1 and Fig. 1). Mass-specific SMR decreased with increasing mass (Fig. 1) with the highest SMR observed in tadpoles from the T4 treatment followed by individuals in the SP treatment. The lowest SMR was observed in the largest tadpoles (SP treatment), followed by control group animals, and T4 treated animals (Fig. 1). At all developmental temperatures, tadpoles exposed to T4 had the highest SMR.
Figure 1:
Mass and temperature dependence of standard metabolic rate (SMR) in Xenopus laevis. Multiple linear regression of SMR (ml O2 × h−1 × mg−1) on developmental temperature and mass (mg; log transformed) at in various experimental treatments in X. laevis in a total of 119 animals. Gray lines and dots: control animals. Orange lines and triangle: Low thyroid hormone levels (SP treatment). Blue lines and squares: High thyroid hormone levels (T4 treatment). Dotted plane: Average regression plane for multiple linear regressions including all treatments: log SMR(Control) =−1.62488 + (−0.21295) × log Mass + (0.01217) × Tdev; log SMR(T4) = −0.2795 + (−0.4918) × log Mass + (−0.0201) × Tdev; log SMR(SP) =−2.06228 + (0.74817) × log Mass + (−0.02252) × Tdev.
Body condition and survival
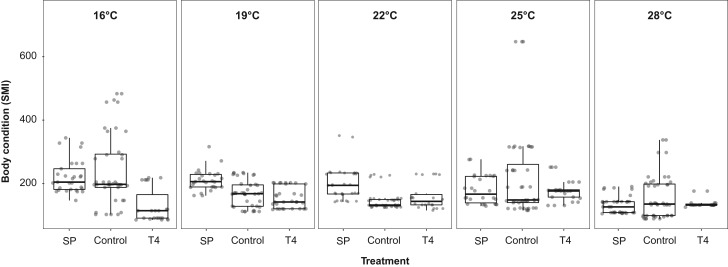
Body condition of X. laevis was significantly affected by treatment, temperature during development, as well as by the interactive effect of both (Table 1 and Fig. 2). High TH levels, Tdev and the interactive effect of altered TH levels and Tdev lead to a reduced body condition and thus, energy stores in tadpoles of X. laevis whereas low TH levels increased body condition.
Figure 2:
Interactive effect of altered thyroid hormone levels and five developmental temperatures on body condition in tadpoles of the African clawed frog (X. laevis) at the onset of metamorphosis (Gosner stage 42) (Gosner, 1960). Body condition was determined by the scaled mass index (SMI).
Survival of X. laevis was significantly affected by hormone treatment, developmental temperature, as well as by the interactive effect of both (Table 1). Mean (±SD) survival from the start of the experiment (Gosner stage 25) to the onset of metamorphosis (Gosner stage 42) in the Control, SP and T4 treatment groups was 92.0 (±10.0), 65.7 (±13.3) and 53.3 (±13.3) %, respectively. High levels of TH reduced survival to nearly half of that observed in the Control. This effect was intensified by the interactive effect of TH level and temperature. Larvae from 19°C—Control, 25°C—SP and 28°C—T4 treatments/groups revealed the lowest survival among all X. laevis tadpoles (Table A2). Most larvae survived in 25°C—Control, 28°C—SP and 19°C—T4 treatments/groups (Table A2).
Thermal tolerance and thermal window
There were significant effects of Tdev on CTmin, CTmax and the thermal range of tolerance within all three treatment groups (Table 1). There was no interactive effect of altered TH status and temperature during development on CTmin, CTmax and the thermal range of tolerance. Whereas TH status affected CTmin and CTmax, there was no effect on the thermal range of tolerance. CTmax was reduced at high TH levels and increased at low TH levels, whereas low TH levels increased both CTmin and CTmax. The thermal range of tolerance was only affected by temperature during development.
The CTmin and CTmax increased significantly with increasing developmental temperature, whereas the thermal range of tolerance decreased with increasing developmental temperature (Table A3). The SMR did not correlate with CTmin, CTmax or the thermal range of tolerance (Table A3).
The regression slope for CTmin and CTmax of X. laevis shows that for every 1°C increase in Tdev the CTmin and CTmax increased by 0.98 and 0.31°C, respectively, in the control group, by 0.96 and 0.28°C in the T4 treatment, and by 0.95 and 0.27°C in the SP treatment (Table A4). In all treatments and control group developmental temperature had a greater effect on CTmin than on CTmax (Table A4).
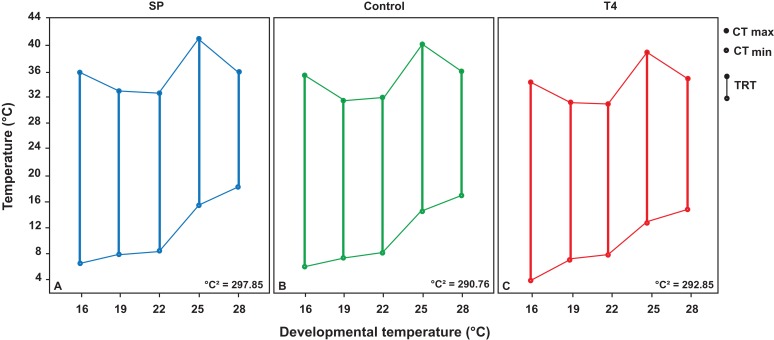
The thermal window polygon area (TW) for X. laevis reared at five different temperatures between 16 and 28°C was calculated as 290.76°C2 for the control group, 297.86°C2 for the SP treatment and 292.87°C2 for the T4 treatment (Fig. 3 and Table 1).
Figure 3:
Developmental thermal windows of X. laevis. Thermal tolerance polygons generated from the critical thermal limits (CTmin and CTmax) at five developmental temperatures in a total of 45 animals. (A) Control animals. (B) Low thyroid hormone levels (SP treatment). (C) High thyroid hormone levels (T4 treatment).
Discussion
Aquatic organisms such as anuran larvae are limited in their ability to search for favorable microhabitats (Gutiérrez‐Pesquera et al., 2016). Therefore, knowing the capacity of species to react to environmental change through plastic responses is a key to determining how vulnerable species with limited mobility will be to environmental variation and global climate change (Martinez et al., 2016; Gutiérrez‐Pesquera et al., 2016; Berg et al., 2017). Species that cannot compensate for long-term (e.g. average warming) or short-term (e.g. increased variability) changes in abiotic factors by buffering metamorphic and physiological traits will be most affected (Kern et al., 2015). Therefore, we investigated whether Tdev, altered TH levels, and the interactive effect of both affect physiological traits in larvae of X. laevis as a model system and determined their capacity for physiological plasticity. Our results demonstrate that physiological traits in larvae of X. laevis are strongly affected by increased TH levels and warmer developmental temperatures. Our results for X. laevis suggest that altered TH levels, which can result from various environmental stressors, and warmer developmental temperatures will result in a reduced capacity for physiological plasticity in anuran larvae.
Altered TH levels and warmer temperatures increase SMR and affect body condition
Amphibians are ectothermic animals and ambient temperature regulates the rates of all physiological and biochemical processes (Smith-Gill and Berven, 1979; Tata, 2006; Little and Seebacher, 2016) impacting growth, development and metabolism. An increase in ambient temperature may, therefore, increase the SMR but animals may compensate for those thermal changes through acclimation (Angilletta et al., 2006; Berg et al., 2017). In this study, the SMR of larval X. laevis markedly increased at warmer temperatures suggesting limited capacity to compensate for warming through physiological acclimation.
As climate change is increasing mean environmental temperatures and the frequency of extreme thermal events (Pachauri et al., 2014; Gutiérrez‐Pesquera et al., 2016; Theisinger et al., 2017), species with a limited capacity for acclimation will suffer from high maintenance costs as caused by the high SMRs. Especially in anuran larvae, having a low energy expenditure before and during metamorphosis is favorable since metamorphosis is a highly energy-consuming process (Orlofske and Hopkins, 2009). A high SMR before and at the onset of metamorphosis may reduce the ability of larvae to store energy (Sheridan and Kao, 1998; Orlofske and Hopkins, 2009; own unpublished data). Energy stores are used during metamorphic climax when larvae stop feeding due to the rebuilding of the gastro-intestinal tract and changes in oral morphology (Beck and Congdon, 2003; Orlofske and Hopkins, 2009). Depending on how much of the accumulated energy is needed for covering maintenance costs (i.e. high or low SMR), more or less of this stored energy is available for covering the costs of development (Steyermark et al., 2005; Beck and Congdon, 2003; Orlofske and Hopkins, 2009) and, consequently, the larvae will have a higher or lower probability of successfully completing metamorphosis.
Anuran larvae which show limited capacity for an acclimation in SMR, such as X. laevis in the present study but especially tropical amphibians in general (Janzen, 1967), may be least able to tolerate additional, climate-driven warming of their habitat. Under natural conditions, any trait, such as increased SMR, that increases metamorphic rates and reduces the transition time through this vulnerable life history stage, would be preferentially selected. However, we observed reduced body condition (lower energy stores) in tadpoles reared at warmer temperatures and experiencing high levels of THs as caused by environmental stress. Environmental stress and global warming, thus, may result in increased SMRs which, in turn, impair the capacity to store energy needed for the complex reorganization during metamorphic climax. Metabolic acclimation (decreasing SMR) may be advantageous when the risk of desiccation and predation is low as it allows more energy to be accumulated and subsequently allocated to development during metamorphic climax. In addition to increases in mean environmental temperatures, altered TH levels frequently accompanies the thermal effects on developmental and metabolic rate in anuran larvae. In this study, larvae of X. laevis revealed a higher SMR when exposed to T4 and a reduced SMR when exposed to SP. This effect was intensified at warmer temperatures. Hence, our results confirmed that the level of TH determines the metabolic rate in X. laevis as shown for lizards (Dipsosaurus dorsalis and Sceloporus occidentalis; John-Alder, 1983; 1990), snakes (Thamnophis sirtalis; Etheridge, 1993) and the leopard frog (Rana pipiens; Steyermark et al., 2005), but had been negated for juvenile X. laevis (Dupré et al., 1986). Therefore, larvae exposed to warmer temperatures are likely to be affected more by environmental stressors than larvae at colder temperatures before and during metamorphic climax.
Long-term temperature changes: developmental temperatures determine critical thermal limits , thermal range of tolerance and the thermal window
Apart from metabolic acclimation concomitant with a low sensitivity of SMR to short-term temperature variation, changes in thermal tolerance may provide a key mechanism to help amphibian larvae cope with the longer-term impacts of climate-driven warming and increased frequency of extreme environmental? events (Seebacher et al., 2015; Gutiérrez‐Pesquera et al., 2016). In this study, tadpoles from warm developmental temperatures had higher thermal limits, but a narrower thermal range of tolerance than tadpoles from colder treatments. Consequently, larvae of X. laevis have the ability to compensate for changes in developmental temperature as they increased their thermal limits at warmer developmental temperatures (Schaefer and Ryan, 2006; Gunderson and Stillman, 2015; Little and Seebacher, 2016). The driver for this adjustment are changes in the thermal reaction norm and hence of physiological nature (Little and Seebacher, 2016; Theisinger et al., 2017).
Although our results demonstrate the ability of X. laevis tadpoles to acclimate to different temperatures by changing their critical thermal limits and thermal range of tolerance, the latter was narrower when tadpoles were raised at warmer temperatures. Those warm-acclimated larvae may be more vulnerable to the impacts of climate change in terms of lacking the capacity for an acclimation in other physiological traits (Gunderson and Stillman, 2015) as larvae in the present study were not able to acclimate their SMR to warmer temperatures.
We found a significanteffect of altered TH levels on the thermal limits in X. laevis indicating that the thyroid systems affects the capacity to acclimate to temperature variation. Therefore, altered TH levels as caused by environmental stress affect the capacity for an acclimation in thermal limits but not in the thermal range of tolerance. Accordingly, larvae under environmental stress are constrained in their ability to compensate for changes in developmental temperature. Furthermore, they might suffer from the consequences of high SMR on body condition and thus, energy stores.
Conclusions
Considering the current worldwide decline of amphibians (Alroy, 2015; Stuart et al., 2004) it is of major interest to investigate whether and how anuran larvae adjust their physiological traits to new thermal challenges and to altered TH status, as caused by natural or anthropogenic stressors in their larval habitat (Strong et al., 2017). Increased metabolic rates are the expected future responses of ectotherms (Seebacher et al., 2015; Berg et al., 2017), especially in species with reduced physiological plasticity due to extreme but stable environments as common in tropical species (Janzen, 1967; Huey et al., 2012; Oyamaguchi et al., 2017). Even though X. laevis is often used for laboratory experiments and, thus, cultured under constant thermal conditions, this is a tropical (Sub-Saharan Africa) species adapted to warm temperatures. Our findings emphasize how environmental stress and climate-driven warming may be detrimental to tadpoles of X. laevis under natural conditions by causing limits to the acclimation of physiological traits leading to reduced body condition. The lack of comparative data on SMR at different temperatures and critical thermal limits necessitates future work to explore how environmental stress may influence physiological traits at the onset of metamorphosis in other species of amphibians. Comparative studies across populations and species would help to identify the potential for local adaptation or inter-specific differences, respectively, on physiological traits affecting the age and size at metamorphosis. Furthermore, as TH levels alter metabolism and body condition in both larval and adult amphibians (Tata et al., 1962), environmental stress may affect froglets and frogs alike, having long-lasting effects on amphibian populations. Long-term studies are needed to understand the consequences of various stressors during larval stages on the phenotype and on the fitness of juveniles and the adults.
Acknowledgements
The authors thank B. Walter for methodical advice and M. Wichmann for providing autoclaved tap water during the experiment. We also thank J. Bank for his experimental advice and technical assistance.
Appendices
Table A1.
Descriptive statistics of mass (mg) at the onset of metamorphosis in tadpoles of the African clawed frog Xenopus laevis at five different developmental temperatures exposed at different TH levels. T4 = high TH levels; SP = low TH levels
| Temperature | Treatment | Maximum | Minimum | Mean | SE | N | |
|---|---|---|---|---|---|---|---|
| Mass (mg) | 16 | Control | 68 | 74 | 71.27 | 0.312 | 40 |
| SP | 73 | 84 | 77.76 | 0.574 | 29 | ||
| T4 | 28 | 33 | 31.26 | 0.399 | 23 | ||
| 19 | Control | 410 | 452 | 442.64 | 1.190 | 39 | |
| SP | 551 | 897 | 695.07 | 18.186 | 27 | ||
| T4 | 259 | 275 | 267.87 | 0.698 | 31 | ||
| 22 | Control | 234 | 254 | 243.37 | 0.930 | 41 | |
| SP | 369 | 381 | 375.45 | 0.681 | 31 | ||
| T4 | 100 | 119 | 110.45 | 1.309 | 22 | ||
| 25 | Control | 152 | 164 | 159.89 | 0.455 | 44 | |
| SP | 284 | 303 | 296.12 | 1.144 | 26 | ||
| T4 | 85 | 95 | 89.88 | 0.441 | 25 | ||
| 28 | Control | 45 | 54 | 48.81 | 0.357 | 43 | |
| SP | 82 | 96 | 93.14 | 0.600 | 35 | ||
| T4 | 19 | 21 | 20.11 | 0.105 | 19 |
Table A2.
Survival (%)at the onset of metamorphosis in tadpoles of Xenopus laevis exposed to different combinations of developmental temperature (Tdev) and altered TH levels. T4 = increased TH levels; SP = decreased TH levels
| Group | Survival (%) ± SD | |
|---|---|---|
| T dev | Treatment | Xenopus laevis |
| 16 | Control | 91.11 ± 8.89 |
| T4 | 51.11 ± 2.22 | |
| SP | 66.67 ± 0 | |
| 19 | Control | 86.67 ± 6.67 |
| T4 | 68.89 ± 4.44 | |
| SP | 60.6 ± 6.67 | |
| 22 | Control | 91.11 ± 8.89 |
| T4 | 48.89 ± 4.44 | |
| SP | 68.89 ± 4.41 | |
| 25 | Control | 97.78 ± 2.22 |
| T4 | 55.56 ± 4.44 | |
| SP | 57.78 ± 8.89 | |
| 28 | Control | 93.33 ± 0 |
| T4 | 42.22 ± 4.45 | |
| SP | 75.56 ± 4.44 | |
Table A3.
Correlation of critical thermal limits (as measured by CTmin and CTmax) and thermal range of tolerance with temperature during development and standard metabolic rate in X. laevis tadpoles. ρ (correlation coefficient) and P for Spearman’s rank correlation. Significance was set at P < 0.05. T4 = high TH levels; SP = low TH levels
| Dependent variable | Treatment | Temperature during development (°C) | SMR (ml O2 × h−1 × mg−1) | ||
|---|---|---|---|---|---|
| ρ | P | ρ | P | ||
| CTmin | Control | 0.96 | <0.001 | 0.09 | 0.54 |
| T4 | 0.96 | <0.001 | 0.08 | 0.57 | |
| SP | 0.97 | <0.001 | 0.03 | 0.84 | |
| CTmax | Control | 0.36 | 0.014 | 0.07 | 0.61 |
| T4 | 0.43 | 0.003 | 0.42 | 0.40 | |
| SP | 0.38 | 0.009 | 0.07 | 0.61 | |
| Thermal range of tolerance | Control | −0.66 | <0.001 | −0.14 | 0.34 |
| T4 | −0.68 | <0.001 | 0.08 | 0.57 | |
| SP | −0.67 | <0.001 | −0.12 | 0.41 | |
Bold indicates significant P-values.
Table A4.
Critical thermal minima (CTmin), critical thermal maxima (CTmax), thermal range of tolerance and thermal window (TW) (±SD) of tadpoles of the African clawed frog Xenopus laevis at five different temperatures during development exposed at different TH levels. T4 = high TH levels; SP = low TH levels. Regression slopes show the increase of CTmin and CTmax for every 1°C increase in Tdev. Bold for mean values
| Developmental temperature (°C) | Treatment | CTmin (°C) ±SD | CTmax (°C) ±SD | Thermal range of tolerance ±SD | TW (°C2) | Regression slope CTmin (R2) | Regression slope CTmax (R2) |
|---|---|---|---|---|---|---|---|
| 16 | Control | 5.5 ± 0.4 | 35.1 ± 0.8 | 29.6 ± 0.4 | 290.76 | Y = 0.98x−11.28 (0.891) | Y = 0.31x+28.04 (0.161) |
| 19 | 7.3 ± 0.1 | 31.5 ± 0.5 | 24.1 ± 0.3 | ||||
| 22 | 8.4 ± 0.1 | 31.6 ± 0.3 | 23.1 ± 0.8 | ||||
| 25 | 14.2 ± 0.2 | 39.7 ± 0.2 | 25.5 ± 0.5 | ||||
| 28 | 16.9 ± 0.1 | 35.5 ± 0.5 | 18.5 ± 0.4 | ||||
| 16 | SP | 4.2 ± 0.7 | 34.3 ± 0.6 | 30.1 ± 0.3 | 297.85 | Y = 0.96x−10.66 (0.893) | Y = 0.28x+28.58 (0.122) |
| 19 | 7.1 ± 0.9 | 30.9 ± 0.1 | 23.8 ± 0.6 | ||||
| 22 | 7.8 ± 0.1 | 30.8 ± 0.1 | 23 ± 0.5 | ||||
| 25 | 13.1 ± 0.8 | 39.0 ± 0.5 | 25.8 ± 0.6 | ||||
| 28 | 15.1 ± 0.4 | 34.8 ± 0.1 | 19.7 ± 0.7 | ||||
| 16 | T4 | 6.5 ± 0.5 | 35.9 ± 1.1 | 29.4 ± 1.1 | 292.875 | Y = 0.95x−10.68 (0.907) | Y = 0.27x+28.77 (0.124) |
| 19 | 7.9 ± 0.5 | 32.7 ± 0.2 | 24.8 ± 0.1 | ||||
| 22 | 8.5 ± 0.5 | 33.1 ± 0.9 | 24.5 ± 0.4 | ||||
| 25 | 15 ± 0.5 | 41.1 ± 0.3 | 26.1 ± 0.3 | ||||
| 28 | 17.7 ± 0.7 | 35.8 ± 0.1 | 18.1 ± 1.9 |
Funding
Not funded.
Author contributions
K.R., K.D., M.P. and J.G. conceived and designed the study. K.R., N.S., L.B., J.R. and L.H. conducted the experiments. K.R. and C.D. performed the statistical analysis. All authors participated in article editing and final approval.
References
- Alroy J. (2015) Current extinction rates of reptiles and amphibians. Proc Natl Acad Sci USA 112: 13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta MJ Jr, Bennett AF, Guderley H, Navas CA, Seebacher F, Wilson RS (2006) Coadaptation: a unifying principle in evolutionary thermal biology. Physiol Biochem Zool 79: 282–294. [DOI] [PubMed] [Google Scholar]
- Bates DM, Sarkar D (2007) lme 4: Linear mixed-effects models using s4 classes (version 0999375-39) [Computer software and manual].
- Beck CW, Congdon JD (2003) Energetics of metamorphic climax in the southern toad (Bufo terrestris). Oecologia 137: 344–351. [DOI] [PubMed] [Google Scholar]
- Berg W, Theisinger O, Dausmann KH (2017) Acclimatization patterns in tropical reptiles: uncoupling temperature and energetics. Sci Nat 104: 91. [DOI] [PubMed] [Google Scholar]
- Brown DD, Cai L (2007) Amphibian metamorphosis. Dev Biol 306: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulaeva E, Lanctôt C, Reynolds L, Trudeau VL, Navarro-Martín L (2015) Sodium perchlorate disrupts development and affects metamorphosis-and growSPrelated gene expression in tadpoles of the wood frog (Lithobates sylvaticus). Gen Comp Endocrinol 22: 33–43. [DOI] [PubMed] [Google Scholar]
- Burraco P, Gomez-Mestre I (2016) Physiological stress responses in amphibian larvae to multiple stressors reveal marked anthropogenic effects even below lethal levels. Physiol Biochem Zool 89: 462–472. [DOI] [PubMed] [Google Scholar]
- Capellán E, Nicieza AG (2007) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21: 445–458. [Google Scholar]
- Carr JA, Patiño R (2011) The hypothalamus–pituitary–thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen Comp Endocrinol 170: 299–312. [DOI] [PubMed] [Google Scholar]
- Carr JA, Gentles A, Smith EE, Goleman WL, Urquidi LJ, Thuett K, Kendall RJ, Giesy JP, Gross TS, Solomon KR (2003) Response of larval Xenopus laevis to atrazine: assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ Toxicol Chem 22: 396–405. [PubMed] [Google Scholar]
- Carr JA, Theodorakis CW (2006) Perchlorate effects in amphibians In: Kendall RJ, Smith PN (eds.). The ecotoxicology of perchlorate. SETAC Press, Pensacola FL, pp 127–154. [Google Scholar]
- Chiu KW, Woo NYS (1988) Metabolic effects of thyroid hormones at a low temperature in the snake. J Therm Biol 13: 179–184. [Google Scholar]
- Choi J, Ishizuya-Oka A, Buchholz DR (2017) Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor α in tadpoles of Xenopus tropicali.s. Endocrinology 158: 1623–1633. [DOI] [PubMed] [Google Scholar]
- Coady K, Marino T, Thomas J, Currie R, Hancock G, Crofoo J, McNalley L, McFadden L, Geter D, Klecka G (2010) Evaluation of the amphibian metamorphosis assay: exposure to the goitrogen methimazole and the endogenous thyroid hormone L‐thyroxine. Environ Toxicol Chem 29: 869–880. [DOI] [PubMed] [Google Scholar]
- Cowles RB, Bogert CM (1944) A preliminary study of the thermal requirements of desert reptiles. Bull AMNH 83: article 5. [Google Scholar]
- Crawley MJ. (2012) The R book. Chichester, UK, John Wiley and Sons. [Google Scholar]
- Dalvi RS, Pal AK, Tiwari LR, Das T, Baruah K (2009) Thermal tolerance and oxygen consumption rates of the catfish Horabagrus brachysoma (Günther) acclimated to different temperatures. Aquaculture 295: 116–119. [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2: cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver RJ. (1997) Proximate mechanisms of phenotypic plasticity in amphibian metamorphosis. Am Zool 37: 172–184. [DOI] [PubMed] [Google Scholar]
- Denver RJ. (2009) Stress hormones mediate environment-genotype interactions during amphibian development. Gen Comp Endocrinol 164: 20–31. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Mirhadi N, Phillips M (1998) Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus Hammondii tadpoles to habitat desiccation. Ecology 79: 1859–1872. [Google Scholar]
- Dittrich C, Drakulić S, Schellenberg M, Thein J, Rödel MO (2016) Some like it hot? Developmental differences in Yellow-bellied Toad (Bombina variegata) tadpoles from geographically close but different habitats. Can J Zool 94(2): 69–77. [Google Scholar]
- Dupré RK, Just JJ, Ritchart JP (1986) Thyroid hormones and behavioral thermoregulation by Xenopus laevis: differential effects of thyroxine and triiodothyronine. Can J Zool 64: 1076–1079. [Google Scholar]
- Etheridge K. (1993) Thyroxine-induced changes in metabolic rate and cytochrome oxidase activity in Thamnophis sirtalis: effects of nutritional status. Gen Comp Endocrinol 91: 66–73. [DOI] [PubMed] [Google Scholar]
- Formicki G, Zamachowski W, Stawarz R (2003) Effects of UV-A and UV-B on oxygen consumption in common toad (Bufo bufo) tadpoles. J Zool 259: 317–326. [Google Scholar]
- Freitag MB, Brown CT, Karaso WH (2017) Warmer temperature modifies effects of polybrominated diphenyl ethers on hormone profiles in leopard frog tadpoles (Lithobates pipiens). Environ Toxicol Chem 36: 120–127. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Neff ES (2006) A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol Metab 17: 40–47. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Knutie SA, Roznik EA, Rohr JR (2018) Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? Oecologia 186: 393–404. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Foufopoulos J (2008) Costs of plasticity: responses to desiccation decrease post‐metamorphic immune function in a pond‐breeding amphibian. Funct Ecol 22: 100–108. [Google Scholar]
- Glennemeier KA, Denver RJ (2002) Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. Gen Comp Endocrinol 127: 16–25. [DOI] [PubMed] [Google Scholar]
- Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall R, Carr JA (2002) Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem 21: 424–430. [PubMed] [Google Scholar]
- Gosner KL. (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190. [Google Scholar]
- Gunderson AR, Stillman JH (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming, 1808. In Proc Biol Sci, Vol 282, No 1808, The Royal Society, p. 20150401. [DOI] [PMC free article] [PubMed]
- Gutiérrez‐Pesquera LM, Tejedo M, Olalla‐Tárraga MÁ, Duarte H, Nicieza A, Solé M (2016) Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. J Biogeogr 43: 1166–1178. [Google Scholar]
- Hasting D, Burggren W (1995) Developmental changes in oxygen consumption regulation in larvae of the South African clawed frog Xenopus laevis. J Exp Biol 198: 2465–2475. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J (2006) Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Persp 114: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Falso P, Gallipeau S, Stice M (2010) The cause of global amphibian declines: a developmental endocrinologist’s perspective. J Exp Biol 213: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman N, McManus JJ (1973) Effects of acclimation on metabolic rate and thermal tolerance in the carpenter frog, Rana vergatipes. Comp Biochem Physiol A Physiol 45: 833–842. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Am Holtum J, Jess M, Williams SE (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil Trans R Soc Lond B Biol Sci 367: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL (2004) Basal metabolic rate: history, composition, regulation, and usefulness. Physiol Biochem Zool 77: 869–876. [DOI] [PubMed] [Google Scholar]
- Hutchison VH. (1961) Critical thermal maxima in salamanders. Physiol Zool 34: 92–125. [Google Scholar]
- Iwamuro S, Sakakibara M, Terao M, Ozawa A, Kurobe C, Shigeura T, Kato M, Kikuyama S (2003) Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis. Gen Comp Endocrinol 133: 189–198. [DOI] [PubMed] [Google Scholar]
- Janzen DH. (1967) Why mountain passes are higher in the tropics. Am Nat 101: 233–249. [Google Scholar]
- John-Alder HB. (1983) Effects of thyroxine supplementation on metabolic rate and aerobic capacity in a lizard. Am J Physiol Regul Integr Comp Physiol 244: R659–R666. [DOI] [PubMed] [Google Scholar]
- John-Alder HB. (1990) Thyroid regulation of resting metabolic rate and intermediary metabolic enzymes in a lizard (Sceloporus occidentalis). Gen Comp Endocrinol 77: 52–62. [DOI] [PubMed] [Google Scholar]
- Kashiwag K, Furuno N, Kitamura S, Ohta S, Sugihara K, Utsumi K, Hanada H, Taniguchi K, Suzuki K-I, Kashiwagi A (2009) Disruption of thyroid hormone function by environmental pollutants. J Health Sci 55: 147–160. [Google Scholar]
- Kern P, Cramp RL, Franklin CE (2015) Physiological responses of ectotherms to daily temperature variation. J Exp Biol 218: 3068–76. [DOI] [PubMed] [Google Scholar]
- Kloas W, Lutz I (2006) Amphibians as model to study endocrine disrupters. J Chromatogr A 1130: 16–27. [DOI] [PubMed] [Google Scholar]
- Kloas W, Urbatzka R, Opitz R, Würtz S, Behrends T, Hermelink B, Hofmann F, Jagnytsch O, Kroupova H, Lorenz C (2009) Endocrine disruption in aquatic vertebrates. Ann N Y Acad Sci 1163: 187–200. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Buchholz DR (2012) Beyond synergy: corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 153: 5309–5324. [DOI] [PubMed] [Google Scholar]
- Kupferberg SJ. (1997) The role of larval diet in anuran metamorphosis. Am Zool 37: 146–159. [Google Scholar]
- Laudet V. (2011) The origins and evolution of vertebrate metamorphosis. Curr Biol 21: R726–R737. [DOI] [PubMed] [Google Scholar]
- Layne JR Jr, Claussen DL (1982) Seasonal variation in the thermal acclimation of critical thermal maxima (CTMax) and minima (CTMin) in the salamander Eurycea bislineata. J Therm Biol 7: 29–33. [Google Scholar]
- Little AG, Seebacher F (2014) Thyroid hormone regulates cardiac performance during cold acclimation in zebrafish (Danio rerio). J Exp Biol 217: 718–725. [DOI] [PubMed] [Google Scholar]
- Little AG, Seebacher F (2016) Acclimation, acclimatization, and seasonal variation in amphibians and reptiles In de Andrade DV, Bevier CR, de Carvalho JE, eds. Amphibian and Reptile Adaptations to the Environment: Interplay Between Physiology and Behavior. CRC Press, Boca Raton, pp 41–62. [Google Scholar]
- Locker A, Weish P (1966) Quantitative aspects of cold-adaptation and its thyroxine model in cold-and warm-blooded animals. Helgol Wiss Meeres 14: 503. [Google Scholar]
- Lucas EA, Reynolds WA (1967) Temperature selection by amphibian larvae. Physiol Zool 40: 159–171. [Google Scholar]
- Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can J Zool 75: 1553–1560. [Google Scholar]
- MacCracken JG, Stebbings JL (2012) Test of a body condition index with amphibians. J Herpetol 46(3): 346–350. [Google Scholar]
- Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Poll 157: 2903–2927. [DOI] [PubMed] [Google Scholar]
- Martinez E, Porreca AP, Colombo RE, Menze MA (2016) Tradeoffs of warm adaptation in aquatic ectotherms: live fast, die young? Comp Biochem Physiol A Mol Integr Physiol 191: 209–215. [DOI] [PubMed] [Google Scholar]
- Miwa S, Inui Y (1987) Effects of various doses of thyroxine and triiodothyronine on the metamorphosis of flounder (Paralichthys olivaceus). Gen Comp Endocrinol 67: 356–363. [DOI] [PubMed] [Google Scholar]
- Morey S, Reznick D (2001) Effects of larval density on postmetamorphic spadefoot toads (Spea hammondii). Ecology 82: 510–522. [Google Scholar]
- Motzer WE. (2001) Perchlorate: problems, detection, and solutions. Environ For 2: 301–311. [Google Scholar]
- Muir AP, Biek R, Thomas R, Mable BK (2014) Local adaptation with high gene flow: temperature parameters drive adaptation to altitude in the common frog (Rana temporaria). Mol Ecol 23: 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhi S, Patiño R (2007) Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol Sci 96: 246–254. [DOI] [PubMed] [Google Scholar]
- Noyes PD, McElwee MK, Miller HD, Clark BW, van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Internat 35: 971–986. [DOI] [PubMed] [Google Scholar]
- Orlofske SA, Belden LK, Hopkins WA (2017) Effects of Echinostoma trivolvis metacercariae infection during development and metamorphosis of the wood frog (Lithobates sylvaticus). Comp Biochem Physiol A Mol Integr Physiol 203: 40–48. [DOI] [PubMed] [Google Scholar]
- Orlofske SA, Hopkins WA (2009) Energetics of metamorphic climax in the pickerel frog (Lithobates palustris). Comp Biochem Physiol A Mol Integr Physiol 154: 191–196. [DOI] [PubMed] [Google Scholar]
- Ortiz-Santaliestra ME, Sparling DW (2007) Alteration of larval development and metamorphosis by nitrate and perchlorate in southern leopard frogs (Rana sphenocephala). Arch Environ Contam Toxicol 53: 639–646. [DOI] [PubMed] [Google Scholar]
- Oyamaguchi HM, Vo P, Grewal K, Do R, Erwin E, Jeong N, Tse K, Chen C, Miyake M, Lin A (2017) Thermal sensitivity of a Neotropical amphibian (Engystomops pustulosus) and its vulnerability to climate change. Biotropica 50: 326–337. [Google Scholar]
- Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, Church JA, Clarke L, Dahe Q, Dasgupta P (2014) Climate change 2014: synthesis report; Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change: IPCC.
- Packard GC, Packard MJ (1975) The influence of acclimation temperature on the metabolic response of frog tissue to thyroxine administered in vivo. Gen Comp Endocrinol 27: 162–168. [DOI] [PubMed] [Google Scholar]
- Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118(12): 1883–1891. [Google Scholar]
- Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24(6): 1323–1332. [Google Scholar]
- Pörtner H-O, Berdal B, Blust R, Brix O, Colosimo A, Wachter B, de, Giuliani A, Johansen T, Fischer T, Knust R (2001) Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont Shelf Res 21: 1975–1997. [Google Scholar]
- R Development Core Team (2007).
- Relyea RA. (2002) Competitor‐induced plasticity in tadpoles: consequences, cues, and connections to predator‐induced plasticity. Ecol Monogr 72: 523–540. [Google Scholar]
- Rot-Nikcevic I, Wassersug RJ (2004) Arrested development in Xenopus laevis tadpoles: how size constrains metamorphosis. J Exp Biol 207: 2133–2145. [DOI] [PubMed] [Google Scholar]
- Rowe CL, Funck SA (2017) Respiration rates of larval Cope’s Gray Tree Frogs (Hyla chrysoscelis) across a range in temperatures. J Herpetol 51: 130–133. [Google Scholar]
- Rowe CL, Kinney OM, Nagle RD, Congdon JD (1998) Elevated maintenance costs in an anuran (Rana catesbeiana) exposed to a mixture of trace elements during the embryonic and early larval periods. Physiol Zool 71: 27–35. [DOI] [PubMed] [Google Scholar]
- Ruthsatz K, Dausmann KH, Drees C, Becker LI, Hartmann L, Reese J, Sabatino NM, Peck MA, Glos J (2018) Altered thyroid hormone levels affect body condition at metamorphosis in larvae of Xenopus laevis. J Appl Toxicol 38: 1416–1425. In press. [DOI] [PubMed] [Google Scholar]
- Sanzo D, Hecnar SJ (2006) Effects of road de-icing salt (NaCl) on larval wood frogs (Rana sylvatica). Environ Poll 140: 247–256. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Ryan A (2006) Developmental plasticity in the thermal tolerance of zebrafish. Danio rerio J Fish Biol 69: 722–734. [Google Scholar]
- Searcy CA, Snaas H, Shaffer HB (2015) Determinants of size at metamorphosis in an endangered amphibian and their projected effects on population stability. Oikos 124: 724–731. [Google Scholar]
- Seebacher F, White CR, Franklin CE (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Change 5: 61. [Google Scholar]
- Sheridan MA. (1994) Regulation of lipid metabolism in poikilothermic vertebrates. Comp Biochem Physiol B 107: 495–508. [Google Scholar]
- Sheridan MA, Kao Y-h (1998) Regulation of metamorphosis-associated changes in the lipid metabolism of selected vertebrates. Am Zool 38: 350–368. [Google Scholar]
- Shi YB. (2000) Amphibian Metamorphosis. Wiley-Liss, New York. [Google Scholar]
- Smith PN, Theodorakis CW, Anderson TA, Kendall RJ (2001) Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology 10: 305–313. [DOI] [PubMed] [Google Scholar]
- Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113: 563–585. [Google Scholar]
- Steyermark AC, Miamen AG, Feghahati HS, Lewno AW (2005) Physiological and morphological correlates of among-individual variation in standard metabolic rate in the leopard frog Rana pipiens. J Exp Biol 208: 1201–1208. [DOI] [PubMed] [Google Scholar]
- Strong R, Martin FL, Jones KC, Shore RF, Halsall CJ (2017) Subtle effects of environmental stress observed in the early life stages of the Common frog, Rana temporaria. Sci Rep 7: 44438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL (2007) A comparison of the effectiveness of recommended doses of MS-222 (tricaine methanesulfonate) and Orajel® (benzocaine) for amphibian anesthesia. Herpetol Rev 38: 63–66. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Nat Acad Sci USA 111: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tat JR, Ernster L, Lindberg O (1962) Control of basal metabolic rate by thyroid hormones and cellular function. Nature 193: 058–1060. [DOI] [PubMed] [Google Scholar]
- Tata JR. (2006) Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol 246: 10–20. [DOI] [PubMed] [Google Scholar]
- Theisinger O, Berg W, Dausmann KH (2017) Compensation of thermal constraints along a natural environmental gradient in a Malagasy iguanid lizard (Oplurus quadrimaculatus). J Therm Biol 68: 21–26. [DOI] [PubMed] [Google Scholar]
- Tietge JE, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Anderson LE, Wolf DC, Degitz SJ (2005) Metamorphic inhibition of Xenopus laevis by sodium perchlorate: effects on development and thyroid histology. Environ Toxicol Chem 24: 926–933. [DOI] [PubMed] [Google Scholar]
- Turriago JL, Parra CA, Bernal MH (2015) Upper thermal tolerance in anuran embryos and tadpoles at constant and variable peak temperatures. Can J Zool 93: 267–272. [Google Scholar]
- Wickham H. (2009) ggplot2: elegant graphics for data analysis. Springer, New York. [Google Scholar]
- Wu CS, Kam YC (2005) Thermal tolerance and thermoregulation by Taiwanese rhacophorid tadpoles (Buergeria japonica) living in geothermal hot springs and streams. Herpetologica 61: 35–46. [Google Scholar]
- Yu S, Wages MR, Cai Q, Maul JD, Cobb GP (2013) Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem 32: 2056–2064. [DOI] [PubMed] [Google Scholar]