Abstract
The neural substrates of working memory are spread across prefrontal, parietal and cingulate cortices and are thought to be coordinated through low frequency cortical oscillations in the theta (3 – 8 Hz) and alpha (8 – 12 Hz) frequency bands. While the functional role of many subregions have been elucidated using neuroimaging studies, the role of superior frontal gyrus (SFG) is not yet clear. Here, we combined electrocorticography and direct cortical stimulation in three patients implanted with subdural electrodes to assess if superior frontal gyrus is indeed involved in working memory. We found left SFG exhibited task-related modulation of oscillations in the theta and alpha frequency bands specifically during the encoding epoch. Stimulation at the frequency matched to the endogenous oscillations resulted in reduced reaction times in all three participants. Our results provide evidence for SFG playing a functional role in working memory and suggest that SFG may coordinate working memory through low-frequency oscillations thus bolstering the feasibility of using intracranial electric stimulation for restoring cognitive function.
1. Introduction
Working memory (WM), the ability to flexibly maintain and manipulate information for a short period of time, forms an important component of cognition. It supports other higher-order cognitive functions and has been tightly linked to fluid intelligence (Ackerman et al., 2005; Unsworth et al., 2014). Impairment in WM accompanies many neurological and psychiatric disorders and significantly reduces the quality of life of affected patients (Campo et al., 2013; Forbes et al., 2009; Lee and Park, 2005; Snyder, 2013; Uhlhaas and Singer, 2012). A mechanistic understanding of the causal role of circuit dynamics in WM will open new therapeutic avenues.
Functional imaging studies have revealed that the neural substrate of WM is spread across multiple cortical regions including dorsolateral prefrontal cortex, posterior parietal cortex and anterior cingulate cortex. While early studies have suggested superior frontal gyrus (SFG) to be involved in working memory (Awh et al., 1995; Braver et al., 1997; Cornette et al., 2001), subsequent studies have often found the middle frontal gyrus (MFG) to be the key node in working memory (Curtis, 2006; Curtis and D’Esposito, 2003; D’Esposito, 2007; Ranganath et al., 2004; Wager and Smith, 2003). However, lesions in SFG have been shown to result in working memory deficits (du Boisgueheneuc et al., 2006). In addition, electroencephalography (EEG) and magnetoencephalography (MEG) studies have shown that oscillations in the theta frequency band (4 – 8 Hz) observed on fronto-central regions (Gevins et al., 1997; Hsieh and Ranganath, 2014; Jensen et al., 2002; Krause et al., 2000; Tesche and Karhu, 2000) coordinate working memory. The source of these oscillations is thought to be medial prefrontal cortex which includes SFG. Modulations in WM performance by non-invasive brain stimulation like repetitive transcranial magnetic stimulation (rTMS) (Mottaghy et al., 2002; Oliveri et al., 2001) and transcranial alternating current stimulation (tACS) (Jausovec et al., 2014; Polania et al., 2012; Violante et al., 2017; Vosskuhl et al., 2015) targeting prefrontal cortex also provide indirect evidence for the role of SFG in WM performance.
Electrocorticography (ECoG) allows identification of activity signatures at temporal scale of a few milliseconds with a spatial resolution of a few centimeters is an ideal tool to map functions of cortical regions. Direct cortical stimulation, in which stimulation is applied through ECoGelectrodes .allows for focal probing of cortex providing additional information through reversible microlesions (Borchers et al., 2012). Combined recording and stimulation with implanted electrodes have greatly contributed to revealing the substrate of long-term memory (Kim et al., 2016; Kucewicz et al., 2018; Suthana and Fried, 2014; Suthana et al., 2012). Low amplitude periodic stimulation at 10 Hz has been demonstrated to engage ongoing cortical oscillations in a state-dependent manner and enhance oscillation strength measured by signal power (Alagapan et al., 2016). In this study, we employed a similar experimental paradigm to delineate the role of SFG on working memory. We present results from three participants with subdural electrodes over left and right SFG in whom we assessed the electrophysiological signatures of SFG and applied periodic stimulation during a verbal working memory task. We found that left SFG exhibited a task- related modulation in oscillation power and stimulation matched to the frequency of oscillation resulted in an improvement in working memory performance.
2. Material and Methods
2.1. ECoG Data Collection and Direct Cortical Stimulation
All experimental procedures were approved by the Institutional Review Board of University of North Carolina at Chapel Hill (IRB Number 13–2710) and written informed consent was obtained from the participant. The participants underwent implantation of intracranial EEG electrodes followed by long-term monitoring at the Epilepsy Monitoring Unit in UNC Neuroscience hospital for surgical resection planning.
Strips of electrodes were implanted over bilateral frontal, temporal and parietal lobes as shown in Figure 1A. Depth electrodes were implanted in bilateral parahippocampal gyri in P1 and strip electrodes were implanted over bilateral occipital lobe in P2 (not shown in figure). The locations of the electrodes were completely dictated by the clinical needs of the participant. The electrodes, 4 mm in diameter (2.5 mm exposed), were made of platinum-iridium alloy and embedded in silicone (Ad-Tech Medical, Racine, Wisconsin, United States). The electrodes in each strip were separated by 10 mm. Signals from electrodes that were over seizure foci (Table 1) were excluded from analysis.
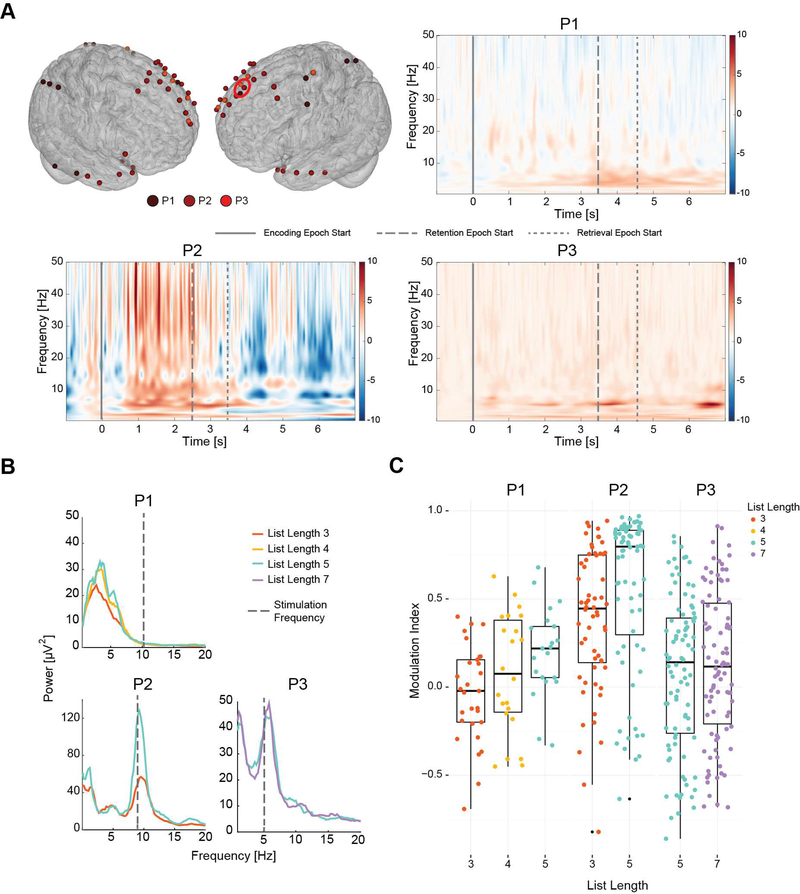
Figure 1.
(A) Surface model showing the coverage of electrodes for the three participants. (B) Schematic of a single trial of the working memory task used. The task consisted of 3 epochs - Encoding, Retention and Retrieval. Stimulation was applied through the entire trial. (C) Schematic of the periodic pulse stimulation. Stimulation consisted of train of biphasic pulses 400 μs in duration every 100 ms (P1 and P2) or 200 ms (P3) for 5s.
Table 1.
Clinical Information of Participants
| Participant | Age | Sex | Handedness | Clinical Seizure Focus |
|---|---|---|---|---|
| P1 | 23 | F | R | Bilateral parahippocampal gyri |
| P2 | 57 | M | R | Bilateral inferior occipital, posterior temporal |
| P3 | 26 | M | R | Unknown Seizure Focus |
ECoG data from participant P1 was recorded using a 128-channel acquisition system (Aura LTM 64, Grass Technologies, Warwick, Rhode Island, United States) at 800 Hz sampling rate.
Electrical stimulation consisted of 5 second train of biphasic pulses, 2 mA in amplitude, 400 μs in duration and 10 Hz in frequency. The pulses were generated by a cortical stimulator (S12x cortical stimulator, Grass Technologies, Warwick, Rhode Island, United States) and applied between pairs of adjacent electrodes (blue electrodes in Figure 1A).
ECoG data from participants P2 and P3 were recorded using a different 128-channel EEG system (NetAmps 410, Electrical Geodesics Inc, Eugene, Oregon, United States) at 1000 Hz sampling rate. Stimulation was delivered using Cerestim M96 cortical stimulator (Blackrock Microsystems, Salt Lake City, Utah, United States). Stimulation parameters (except frequency) remained the same as in P1 except for the duration which was adjusted to encompass the encoding and retention epochs.
Stimulation frequencies were determined based on the peaks in power spectra during the baseline session in P2 and P3. In P1, we chose the stimulation frequency a priori without any knowledge of the endogenous frequency as baseline stimulation session was not possible. The stimulation frequencies did not match the endogenous frequency exactly as power spectral density was determined with a frequency resolution of 1 Hz. Figure 2B illustrates the power spectral density for the different list lengths and the stimulation frequency.
Figure 2.
(A) Cortical model showing electrodes that exhibited task-related modulation. Red circle denotes the three electrodes in lSFG whose event related spectral perturbation are plotted. observed in left superior frontal gyrus electrodes during sham trials for P1 and baseline session trials for P2 and P3 indicating the modulation of signal in the band 3 – 12 Hz. Hot (red) colors indicate an increase and cold (blue) colors indicate a decrease in signal power relative to baseline. (B) Power spectral density of lSFG electrodes during baseline session in encoding epoch showing peaks that were used to determine stimulation frequency (dotted gray lines) in P2 and P3. (C) Modulation indices during encoding epoch across all lSFG electrodes that exhibited significant task related modulation of signal power. In P1 and P2 there was a significant difference between modulation indices for list length 3 and list length 5.
2.2. Working Memory Task
We adopted a classical Sternberg working memory task previously used in other ECoG studies (Meltzer et al., 2008; Raghavachari et al., 2001; Raghavachari et al., 2006) (Figure 1C). The task consisted of 3 epochs. In the first epoch, lists of 3 to 5 pseudo-randomly chosen letters from the English alphabet were presented sequentially. This was termed the encoding epoch and each alphabet was displayed for 500 ms with 200 ms between each alphabet (the inter-alphabet interval was not present for P2 and P3). The task was initially designed based on Raghavachari et al. (Raghavachari et al., 2001) in which the inter-alphabet interval was present. However, to reduce the overall duration of the task, we decided to remove the inter-alphabet interval in subsequent participants. This modification did not significantly deviate from other implementations of the Sternberg task. Following this, was a retention epoch where a blank screen was presented for 1 second. The final epoch was the retrieval epoch where a single probe (another English alphabet) was shown for 5 seconds and the participants had to indicate if they thought that the probe was present in the list by pressing a specified key on the keyboard. If they did not think the probe was present in the list, they did not have to press any key. The different list lengths correspond to different levels of cognitive load that enabled us to identify task-related modulation in terms of electrode locations as well as frequency band. We adjusted the list lengths for each participant according to their performance in a shortened practice version of the task. We chose the list length at which the participant’s performance was at or below 80 percent. The task was programmed in Matlab using Psychtoolbox (Brainard, 1997) and presented in a laptop. For the experiment in which P1 participated, triggers from the cortical stimulator were detected by an ethernet DAQ (National instruments, Austin, TX, USA) connected to the task computer and used to initiate trials. Sham trials, in which no electrical pulses were delivered, were initiated using a pulse generator and were randomly interleaved with stimulation trials. For the experimental session in which P2 and P3 participated, triggers were generated within the Psychtoolbox task code and sent to Cerestim through the ethernet DAQ. Sham trials were trials during which no triggers were sent to Cerestim and hence no stimulation was applied. Only flags were sent to the recording system to denote the trial epochs.. Stimulation was applied for 5 seconds in P1 and the duration of encoding and retention epochs in P2 and P3. In P1 electrodes over right SFG and bilateral temporal cortices were stimulated as well. However, the low number of stimulation trials did not allow any meaningful analysis to be performed and hence was not included in the study here. In P2, a pair of electrode over right SFG was stimulated and the results are not included here.
Participants P2 and P3 completed 2 sessions - a baseline session and a stimulation session. The baseline session did not include any stimulation and consisted of 40 trials of two different list lengths to assess the baseline performance level as well as determine the parameters for the stimulation session. In contrast to P2 and P3 where baseline sessions were possible, P1 had a time constraint at the epilepsy monitoring unit due to which it was not possible to acquire baseline data before the stimulation experiment. Therefore, stimulation was applied to two electrode pairs over lSFG, as the exact electrodes which exhibited task-related modulation were not known. To ensure targeting of potential regions, the stimulation electrodes were randomly changed over each trial. In P2 and P3, stimulation was applied to the task-modulated electrodes determined from baseline session.
2.3. Data Analysis
Data analysis was performed using custom written Matlab scripts (The MathWorks Inc., Natick, MA, United States). The recording setup consisted of switching circuits designed to protect the amplifier during stimulation which prevented recording of data from stimulating electrodes. Hence, data from stimulating electrodes were not included in the analysis.
Stimulation artifacts, present in channels adjacent to stimulated electrodes, were removed using an independent component analysis (ICA) based approach (Figure S1). Since artifacts were observed as stereotypical waveforms, ICA resulted in components that contained only artifact waveforms which were then rejected, and the remaining components were used to reconstruct artifact free signals. We used the infomax algorithm (Lee et al., 2000) available as a part of EEGLab toolbox (Delorme and Makeig, 2004) for computing independent components. Following artifact suppression, the signals were low pass filtered with an FIR filter (cutoff frequency 50 Hz) and re-referenced to common average. Signal power spectra was computed with a multi-taper fft based approach using Chronux toolbox (Bokil et al., 2010). To quantify the change induced by stimulation, modulation index was computed as
Where and are average power in specified frequency band in specific epoch (task, encoding or retention) and baseline epoch respectively. The baseline epoch was defined as 5 second interval before the beginning of encoding epoch.
Time-frequency representations were computed by convolving Morlet wavelets with the time series of each trial. Event related spectral perturbation was calculated as
Where Se is the spectra at each time point within an epoch and is the average power in the baseline epoch.
2.4. Statistics
All statistical analyses were performed using R. Linear mixed effects models were fitted using the Imertest package (Kuznetsova et al., 2017) which uses Satterthwaite’s approximation to degrees of freedom to determine the F statistics of the fixed effects.
For the effect of list length on reaction times, we fitted a linear model with reaction time as dependent variable and list length as the factor for each participant separately. For the effect of list length on modulation indices, we fitted linear mixed model with modulation index as the dependent variable and list length as the fixed factor and participant and electrodes as nested random factors. To study the effect of stimulation on reaction time, we fitted a linear mixed model with reaction time as dependent variable and stimulation as fixed factors and participant as the random factor. As post hoc analysis we performed a two-sample t-test to compare the difference between reaction times during sham and stimulation trials for each participant. To study the effect of stimulation on modulation index, we fitted linear mixed models with modulation index as dependent variable, stimulation as fixed factor and electrodes and participants as nested random factors and also with modulation index as dependent variable and list length (3 levels) and stimulation regions (3 levels - sham, frontal region, temporal region) as fixed factors and electrodes as a random factor. As post-hoc analysis, we performed paired t-tests.
2.5. Extraction of Electrode Location from Neuroimaging Data
3D Slicer (Fedorov et al., 2012) was used to analyze and extract electrode locations from CT images obtained after implantation of subdural electrodes. The post-operative MRI was coregistered to post-operative CT in Slicer followed by registering to standard MNI atlas (Fonov et al., 2009). Skull stripping was performed using ROBEX (Iglesias et al., 2011), and the gray matter and white matter were then segmented using ITK-Snap (Yushkevich et al., 2006). The surface model of the MNI atlas brain was generated using Slicer and used for visualization purposes. The anatomical locations of the electrodes were determined by co-registering the MRI Image to the MNI Atlas (Fonov et al., 2011), recomputing electrode locations in the MNI space, transforming these locations to Talairach space, and using the Talairach Client (Lancaster et al., 2000) to obtain the label of the gray matter nearest to the coordinate representing electrode location.
3. Results
We leveraged the access to ECoG signals in three patients with epilepsy undergoing long term monitoring in the Epilepsy monitoring unit at the N.C. Neurosciences Hospital, UNC Medical Center, Chapel Hill. The participants (P1, P2 and P3) had electrodes over frontal, temporal and parietal regions on both hemispheres (Figure 1A). The participants performed a Sternberg verbal working memory task that has been previously used in ECoG research (Meltzer et al., 2008; Raghavachari et al., 2001) (Figure 1B). The cognitive load, measured by the number of items (English letters) in a list to be held in memory (3, 4, or 5 for P1 and 3 or 5 for P2 and 5 or 7 for P3), was varied randomly for each trial. In participant P1, we observed an increase in reaction times with increasing cognitive load (list length 3: 824 ± 31 ms, list length 4: 1119 ± 105 ms, list length 5: 1140 ± 78 ms) in the sham trials (Linear model factor list length: F(2,34) = 4.864; p = 0.014). In participant P2, who performed a separate baseline session of the task without stimulation, there was no significant difference between reaction times for different cognitive loads (F(1,20) = 0.060; p = 0.809). In participant P3, who also performed a separate baseline session without stimulation, there was a significant effect of cognitive load (Linear mixed model factor list length: F(1,45) = 4.646; p = 0.036). The reaction time for trials with 5 items in the list was lower than trials with 7 items in the list (785 ± 26 ms vs 902 ± 48 ms).
Oscillations in the theta (3 – 8 Hz) and alpha (8 – 12 Hz) bands have been shown to be modulated during working memory tasks (Jensen et al., 2002; Jensen and Tesche, 2002; Raghavachari et al., 2001). Spectral analysis revealed oscillations with a peak frequency around 5 Hz in P1 and P3 and 9.5 Hz in P2. To assess if these observed oscillations were modulated by the task, we computed power spectra for baseline, encoding and retention epochs when no stimulation was being delivered (sham trials in P1 and baseline session trials in P2 and P3). Modulation indices were computed relative to baseline epoch. The retrieval epoch was not included in analysis as the epoch may be confounded with action planning and action. We found that electrodes in frontal, temporal and parietal regions exhibited an enhancement of power relative to baseline during sham trials in the theta band (3 – 8 Hz) in P1 and P3 and in alpha band (8 – 10 Hz) in P2 (one sample t- test with FDR correction; p < 0.05). Specifically, electrodes over the left superior frontal gyrus (lSFG) exhibited the task relevant enhancement of oscillation across all three participants. Spectrograms of sample electrodes over SFG illustrating task-related modulation are depicted in Figure 2A. Further analysis of data from electrodes over lSFG revealed that power modulation during the encoding epoch was influenced by list length (Figure 2B; Linear mixed model factor list length: F(3,370) = 3.417; p = 0.017). Post-hoc analysis revealed a significant difference between modulation indices from list lengths 3 and 5 in participants P1 and P2 (Pairwise t-test, p < 0.05). In contrast, power modulation during the retention epoch was not influence by list length (Linear mixed model factor list length: F(3,228) = 1.029; p = 0.38). Taken together, these results suggest that the oscillations may reflect task relevant processing and specifically contribute to encoding.
To test if targeting oscillations that exhibited task-related modulation in SFG, periodic pulse stimulation was applied between pairs of electrodes over the left SFG. Stimulation consisted of pulse trains 2 mA in amplitude and 5 seconds in duration (Figure 1C) and the electrode pair being stimulated was randomly changed for each trial. Stimulation and sham trials were randomly interleaved and trial initiation was time-locked to stimulation initiation. Stimulation frequency was 10 Hz for P1 (chosen a priori), 9 Hz for P2 and 5 Hz for P3. A total of two different pairs of electrodes in left SFG were stimulated in P1, one pair of electrodes in P2 and P3 (blue electrodes in Figure 1A). During the stimulation session, participants P1, P2 and P3 performed the task in which trials consisted of 3, 4 or 5 items, 3 or 5 items, 5 items respectively. As a first step, the effect of stimulation on reaction times of participants P1 and P2 was analyzed using a linear mixed model with fixed factors list length and stimulation condition and participant as random factor as these participants had trials with 3 and 5 items. While there was no significant effect of stimulation (F(2,108) = 1.042; p = 0.356), there was a significant effect of list length ( F(1,108) = 9.072; p = 0.003) and interaction between list length and stimulation condition ( F(2,108) = 6.536; p = 0.002). Next analysis was restricted to only trials with 5 items and the reaction times of all 3 participants in sham trials were compared with that in stimulation trials. The effect of stimulation was statistically significant (Linear Mixed Model F(1,97) = 13.414; p <0.001) with all participants showing a significant decrease in reaction times (P1: 1140 ± 78 ms vs 852 ± 111 ms; P2: 1188 ± 93 ms vs 954 ± 54 ms; P3: 841 ± 48 ms vs 727 ± 27ms; Figure 3A) confirmed by post-hoc analysis (Pairwise t-test, p < 0.05). Analysis of accuracy using chi-squared tests did not reveal any significant interactions (Figure 3B) suggesting stimulation served to reduce reaction times without affecting accuracy.
Figure 3.
(A) Reaction times in trials with 5 items showing a decrease with stimulation. (B) Accuracy was not affected by stimulation (C) Stimulation did not result in any changes in modulation indices in electrodes over lSFG. (D) Differential effect of stimulation on modulation indices in electrodes that exhibited taskrelevant modulation of low frequency oscillations.
In most studies involving electrical stimulation, artifacts caused by stimulation prevent the analysis of electrophysiological signals during stimulation. To overcome this, we developed an independent component analysis (ICA) based method (see Methods and Experimental Procedures).
Stimulation artifacts were sufficiently suppressed (Figure S1) allowing us to study the signals in the frequency band of interest. Power spectra and modulation indices in the endogenous oscillation frequency band (3 – 8 Hz in P1 and P3 and 8 – 12 Hz in P2) were computed as described before. Analysis of modulation indices of the electrodes over lSFG (restricted to trials with 5 items in the list) across all participants did not reveal any significant effect of stimulation (Figure 3C; Linear mixed model factor condition F(1,459) = 0.612; p = 0.434). To explore the effects of stimulation on other regions that exhibited modulation of task-relevant oscillations, we ran analysis on individual participant data including list length as a factor. In P1, stimulation induced a differential change in modulation indices (Linear mixed model factor condition F(1,672)= 20.827; p <0.001, factor list length F(1,672) = 15.793; p = 0.001, interaction F(1,672) = 10.536; p = 0.004). Further analysis revealed that there was a significant effect of stimulation in trials with 5 items in list, with stimulation inducing a decrease in modulation indices (Linear mixed model factor condition F(1,305 = 27.742; p< 0.001). Similarly in P2, stimulation induced a difference change in modulation indices (Linear mixed model factor condition F(1,1738) = 0.495; p = 0.482, factor list length F(1, 1738) = 33.190; p < 0.001, interaction F(1,1738) = 11.134; p < 0.001). Stimulation caused significant decrease in modulation indices in trials with 3 items (Factor condition F(1,908) = 9.04; p = 0.003) while stimulation caused a trend-level significant increase in modulation indices in trials with 5 items (Factor condition F(1,830) = 3.13; p = 0.077). There was no significant effect of stimulation in P3 (Factor condition F(1,215) = 0.005; p = 0.946).
4. Discussion
In this study, we show evidence for the role of superior frontal gyrus (SFG) in working memory using a combination of ECoG and DCS. Electrodes over left SFG exhibited modulation of cortical oscillations in the canonical theta and alpha frequency bands. The degree of modulation, measured using modulation index, depended on the cognitive load, specifically in the encoding epoch. Stimulation of lSFG at frequency close to the peak frequency of the endogenous oscillations led to an enhancement in working memory performance in 2 of the 3 participants. However, analysis of data obtained during stimulation did not provide any conclusive evidence for modulation of task-relevant oscillations. Taken together, the results suggest SFG may be an important node in brain network that coordinates working memory.
We were able to perturb lSFG with enhanced spatial resolution compared to other brain stimulation techniques resulting in improvement of working memory performance. To the best of our knowledge, we are the first to demonstrate the effect of stimulation of SFG on working memory performance. While direct cortical stimulation has been successfully used to map brain function from the days of Wilder Penfield, the strength of our approach lies in the ability to sufficiently recover ECoG activity during stimulation with post-hoc processing, allowing us to study the effect of stimulation on neurophysiology. Although our results on the effect of stimulation on oscillation strength have been inconclusive due to many factors, this approach can provide valuable information in studies with sufficient sample sizes.
While there is an abundance of evidence for the role of middle frontal gyrus (MFG; Brodmann Area 9/46) in working memory from neuroimaging studies (Curtis and D’Esposito, 2003;D’Esposito and Postle, 2015; Owen et al., 2005; Wager and Smith, 2003), the role of SFG is not clear. There have been a few neuroimaging studies that suggest SFG may be involved in working memory (Awh et al., 1995; Braver et al., 1997; Cornette et al., 2001; Rypma et al., 1999). SFG gray matter volume has been linked to working memory activation in intra-parietal sulcus (Harms et al., 2013). The strongest evidence for the role of SFG in working memory has come from a lesion study (du Boisgueheneuc et al., 2006) in which patients with lesions in lSFG exhibited deficits in working memory involving verbal, spatial and face stimuli. Our results strengthen the evidence for SFG’s role in working memory. However, the proximal location our stimulation targets to MFG may confound our interpretation of the results. Diffusion tensor tractography has revealed that SFG can be divided into subregions with strong connectivity to ACC, a key node in cognitive control network and MFG, a key node in executive control network (Li et al., 2013). As both networks are essential to working memory processes (Cole and Schneider, 2007; Engle and Kane, 2004; Harding et al., 2015), stimulation of SFG may have distributed effects across multiple regions including MFG. The lack of sufficient coverage of these areas in these three patients limited our ability to examine this idea. Previous studies have observed oscillations in the range 3 – 15 Hz to be modulated during working memory tasks (Jensen and Lisman, 1998; Raghavachari et al., 2001; Sauseng et al., 2009) and the strength of oscillations to reflect working memory load (Jensen et al., 2002; Jensen and Tesche, 2002; Meltzer et al., 2008). Frontal midline theta (FMT) is a commonly observed oscillatory signature in EEG studies of working memory (Hsieh and Ranganath, 2014) typically in Fz and neighboring electrodes in the 10–20 electrode system. The sources of FMT are thought to include lateral PFC and ACC (Mitchell et al., 2008). The theta oscillations we observed in our study may be related to FMT although we did not have any scalp electrodes to confirm this. We found task-related modulation specifically in the encoding period. Analysis of oscillation strength in the retention epoch did not reveal any significant difference between the cognitive loads. This suggests that SFG may play a role that is different from that of MFG/IFG which is known to predominantly be active during the retention epoch (Curtis and D’Esposito, 2003).
To the best of our knowledge, this is the first study where effects of intracranial stimulation on working memory and on oscillation strength were investigated. Periodic pulse stimulation of entorhinal region has been shown to improve performance in a spatial learning task (Suthana et al., 2012). Concurrently there was an increase in theta-phase resetting. In another study, stimulation with very weak sinusoidal currents (0.01mA) produced trend level effects in memory performance although no improvement compared to sham was seen (Fell et al., 2013).
Impairment of performance has been more commonly reported than improvement especially for hippocampal stimulation. One study showed that single pulse stimulation of hippocampus impaired episodic memory (Lacruz et al., 2010). In another study, stimulation at 50 Hz impaired recognition of specific stimuli depending on whether left or right hippocampus was stimulated (Coleshill et al., 2004). More recently, stimulation of entorhinal/ hippocampal and medial temporal regions was shown to affect both verbal and spatial memory (Jacobs et al., 2016; Kucewicz et al., 2018). One key difference between the studies described above and our current study is the frequency of stimulation used. Often, 50 Hz was chosen as the stimulation frequency as opposed to the low frequency used in our study. A study that utilized low frequency stimulation showed that stimulation at 5 Hz resulted in improvement of delayed recall (Koubeissi et al., 2013). Another study in which theta burst stimulation (100 ms trains of 0.1 ms pulses at 200 Hz repeated 5 times per second) of fornix resulted in improvement of visual-spatial memory (Miller et al., 2015). These results suggest that frequency of stimulation might be crucial to the effects observed. Intracranial stimulation studies have often focused on episodic memory and stimulation of hippocampus. In contrast, non-invasive stimulation studies have focused on working memory specifically and target cortical regions such as dlPFC, PPC, inferior frontal gyrus. Transcranial magnetic stimulation, which produces local suprathreshold effects, i.e., evoking action potentials like those expected in intracranial stimulation, has been shown to enhance working memory performance based on the stimulation frequency, location and specific epoch within the task or before the task (Bagherzadeh et al., 2016; Blumenfeld et al., 2014; Esslinger et al., 2014; Guse et al., 2013; Hoy et al., 2016; Luber et al., 2007; Yamanaka et al., 2014; Yamanaka et al., 2010). It must also be noted that many studies report impairments of working memory and episodic memory by TMS as well (Gagnon et al., 2010; Mottaghy, 2006; Osaka et al., 2007; Postle et al., 2006). Transcranial alternating current stimulation, which likely produces more global subthreshold effects, has been shown to increase performance by targeting dlPFC and PPC (Polania et al., 2012; Vosskuhl et al., 2015). The neurophysiological underpinnings of the effects in these studies are often unclear (Violante et al., 2017; Yamanaka et al., 2010). Recently, rTMS applied at theta frequency to left intraparietal sulcus was shown to entrain theta oscillations with a concurrent improvement in auditory working memory (Albouy et al., 2017).
One of the advances we put forth in this study is the artifact removal process that allowed us to examine the effect of stimulation on oscillations during stimulation. The use of ICA for artifact removal while already established in EEG studies (Albouy et al., 2017) has not been, to our knowledge, used in studies with intracranial electrical pulse stimulation. The effectiveness of this approach is aided by the experimental setup and stimulation modality used. The setups used in the study had switching (Grass) and buffer circuits (Cerestim96) which ensured the current applied was routed to the electrodes and not to the amplifier. The amplifiers had sufficient input range that no saturation occurred. Also, the current flow in the case of direct cortical stimulation is between two local electrodes with little leakage. Hence the amplitudes of the artifacts are much less than observed in EEG/MEG studies. In addition, we ran ICA on each individual trial in contrast to the whole dataset which allowed better decomposition of artifacts into separate components. All of these factors contributed to the successful artifact removal. However, the approach may also lead to overcorrection of the data leading to underestimation of the effect of stimulation on neurophysiology. It is hard to estimate the extent to which such overcorrection may occur as ground truth is not available. Further experiments using phantoms and analyses of waveform shape in time domain and spectral analysis in frequency domain are needed to fully explain the performance of the algorithm.
We initially hypothesized that stimulation matched in frequency to the frequency of ongoing oscillations should strengthen the oscillations, i.e., increase the spectral power of the oscillations. Previous studies using computational models and noninvasive brain stimulation have suggested the effect of stimulation is maximized when frequency of imposed perturbation is matched to the endogenous frequency (Ali et al., 2013; Vossen et al., 2015). However, we did not find any consistent effect of stimulation modulation index across the three participants. In participant P1, stimulation of lSFG resulted in a decrease of modulation index while in P2, stimulation of lSFG resulted in an increase of modulation index in those electrodes that exhibited task-related modulation. This apparent contradictory result could be partially due to the differences in the anatomical location of the electrodes that are included in the analysis. In P1, electrodes that were modulated (apart from SFG) were mostly over posterior regions like motor cortex, posterior temporal lobe, and posterior parietal lobe while in P2, electrodes that were modulated were over anterior regions like middle frontal gyrus and anterior temporal cortex. In P3 electrodes that were modulated were mostly over SFG except for one electrode which was over motor area. While we observed an increase in mean modulation index in 8 out of the 10 electrodes over lSFG across the 3 participants, statistical analysis did not yield conclusive results, suggesting that the effect of stimulation may be subtler and may require larger samples. An alternate explanation could be that stimulation resulted in activation of regions that are functionally connected to SFG like the middle frontal gyrus that lead to the observed behavioral effect. However, the lack of sufficient coverage over these adjacent regions did not allow us to verify the effect.
Our study suffers from the low sample size limitation that is inherent in single-center studies involving invasive recordings in human. The sample size of three participants limits the ability to generalize across a wider population. In addition, we had to restrict the duration of the experiment. With longer durations of experiment, participants may get tired introducing additional confounds that we sought to avoid. This resulted in fewer trials per condition than that is typically the norm in cognitive tasks used in studies with healthy volunteers. This drawback is to be noted when the results of the study are interpreted. Moreover, owing to the unique nature of the participant sample, there were differences in the task design and stimulation frequencies. The differences in working memory load in baseline session, calibrated according to individual performance, across three participants makes direct comparison difficult. However, the loads for the results of stimulation presented here were all maintained at 5 items across the three participants thereby reducing the uncertainty. The differences in stimulation frequencies preclude any interpretation of the exact process being modulated that resulted in working memory performance. Our study was motivated with the hypothesis that stimulation matched to the frequency of endogenous oscillations would be the most effective in enhancing oscillations with potential benefits in behavior. Therefore, we targeted functionally relevant oscillations irrespective of frequency. Alpha oscillations are thought to represent inhibition of task-irrelevant regions (Jensen and Mazaheri, 2010) while theta oscillations are thought to represent memory processes including maintenance of serial order (Hsieh and Ranganath, 2014; Roux and Uhlhaas, 2014). Given the evidence for individual differences in memorization strategies used in Sternberg task (Corbin and Marquer, 2009), the oscillations may also represent different strategies the participants used to perform the task. When participants use a rehearsal procedure during encoding and retention, theta oscillation may have been modulated as in participants P1 and P3 in this study. In contrast, participants who use an active maintenance of the verbal information may exhibit an increased alpha oscillation (Khader et al., 2010) as was observed in participant P2. Regardless of the strategy used, stimulation may have served to reinforce these processes resulting in an improvement in performance. Future studies where the strategy used by the participant is controlled for in experimental design will enable answering these questions at a deeper mechanistic level. Apart from this, the specificity of the effects to parameters of stimulation such as frequency and location cannot be determined from the current study as appropriate control frequency or control location was not included in the study design.
The experimental paradigm reported here was limited to applying stimulation during the entire trial due to technical limitations of the FDA-approved cortical stimulator used in the study. This limitation precluded us from identifying if stimulation during an epoch within a trial, i.e. encoding or retention, is more effective than stimulation during the entire trial. Moreover, the frequency of stimulation was restricted to a few discrete frequencies that did not allow matching of the stimulation frequency to frequency of endogenous oscillations in P1. Another limitation of the current study design is that it used only a single stimulation amplitude and stimulation frequency. Given the large parameter space, it is prohibitively difficult to try all possible parameters in studies with limited participant pools as the current study. For P1, we chose stimulation regions based on previous literature due to technical limitations. A more effective strategy was followed for P2 and P3 where we identified electrodes that exhibited task-related modulation in low frequency bands and applied stimulation accordingly. Also, the stimulation used in our study was restricted to a single site. However, memory processes are distributed across different brain regions and the most effective strategy would likely involve stimulation of multiple regions to produce more of a network effect (Kim et al., 2016; Kim et al., 2018) or an adaptive approach using closed-loop stimulation based on the state of the network (Ezzyat et al., 2017; Ezzyat et al., 2018).
In P1, although the stimulation frequency was 10 Hz, oscillations in the frequency band 3 – 8 Hz were significantly modulated concurrently with changes in WM performance. This discrepancy is hard to reconcile if entrainment is thought to be the underlying mechanism of interaction between stimulation and oscillation (Helfrich et al., 2014; Thut et al., 2011). However, the interaction between stimulation and an ongoing oscillation has been found to be nonlinear and the effects depend on the strength of the prevailing oscillations (Alagapan et al., 2016). When there is a strong ongoing oscillation, stimulation does not alter the strength of the endogenous oscillation and only in cases where the strength of the oscillation is low, entrainment is possible. This state- dependent effect of stimulation is likely the underlying mechanism in the current study as well.
Alternatively, 10 Hz stimulation may have engaged with the strong 5 Hz oscillation through subharmonic entrainment as predicted in computational models (Li et al., 2017).
The results presented for P1 is from the sham trials as time constraints did not allow a separate baseline session. It is possible the behavior and neurophysiology may be influenced by stimulation trials that happened along with the sham trials. However, the effects of DCS are typically shortlived on the order of a few seconds (Alagapan et al., 2016) and longer stimulation durations are needed to observe longer-lasting effects (Keller et al., 2018). Therefore, we do not expect significant differences between the baseline and sham session.
In conclusion, we show that periodic pulse stimulation of cortex through subdural electrodes at low frequency can enhance working memory. Despite the limitations, the study provides valuable insights into the feasibility of using oscillations as brain stimulation targets. The importance is highlighted by the emerging interest in using invasive recordings and electrical stimulation to understand and alter pathological signatures of brain activity, whether it be neurological disorders, like epilepsy and Parkinson’s disease, or psychiatric disorders, like depression and obsessive- compulsive disorder. Our results suggest that the same technology could be leveraged to also address cognitive impairment.
Supplementary Material
Table 2:
Experimental Parameters for the three participants
| Participant | Duration (ms) | List Lengths | Response | Stimulation Frequency | Number of Trials | lSFG Electrode Pairs Stimulated | |||
|---|---|---|---|---|---|---|---|---|---|
| Encoding | Inter-item | Retention | Sham | Stim | |||||
| P1 | 500 | 200 | 1000 | 3, 4, 5 | Only probe present | 10 Hz | 24 | 13 | 2 |
| P2 | 500 | 0 | 1000 | 3, 5 | Both probe present and probe absent | 9 Hz | 27 | 26 | 1 |
| P3 | 500 | 0 | 1000 | 5, 7 | Both probe present and probe absent | 5 Hz | 30 | 30 | 1 |
Acknowledgments
The authors thank the members of the Frohlich Lab for their valuable input, with special thanks to Sangtae Ahn for verifying the accuracy of the codes used for analysis and providing valuable feedback on the manuscript. The authors also thank the EEG technicians at the UNC Epilepsy monitoring unit for their generous help with the ECoG recordings.
Funding
Research reported in this publication was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH101547 and R21MH105557, National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21NS094988–01A1, Translational Team Science Award (TTSA) with funding provided by the UNC School of Medicine and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111, Helen Lyng White Postdoctoral Fellowship (S.A.) and Swiss National Science Foundation (grant P300PA_164693; C.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: FF is the lead inventor of IP filed on the topics of noninvasive brain stimulation by UNC. FF is the founder, CSO and majority owner of Pulvinar Neuro LLC. The other authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ackerman PL, Beier ME, Boyle MO, 2005. Working memory and intelligence: the same or different constructs? Psychol Bull 131, 30–60. [DOI] [PubMed] [Google Scholar]
- Alagapan S, Schmidt SL, Lefebvre J, Hadar E, Shin HW, Frhlich F, 2016. Modulation of Cortical Oscillations by Low-Frequency Direct Cortical Stimulation Is State- Dependent. PLoS Biol 14, e1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy P, Weiss A, Baillet S, Zatorre RJ, 2017. Selective Entrainment of Theta Oscillations in the Dorsal Stream Causally Enhances Auditory Working Memory Performance. Neuron. [DOI] [PubMed] [Google Scholar]
- Ali MM, Sellers KK, Frohlich F, 2013. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci 33, 11262–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Smith EE, Jonides J, 1995. Human rehearsal processes and the frontal lobes: PET evidence. Structure and Functions of the Human Prefrontal Cortex 769, 97–117. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh Y, Khorrami A, Zarrindast MR, Shariat SV, Pantazis D, 2016. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory. Exp Brain Res 234, 1807–1818. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Lee TG, D’Esposito M, 2014. The effects of lateral prefrontal transcranial magnetic stimulation on item memory encoding. Neuropsychologia 53, 197202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP, 2010. Chronux: a platform for analyzing neural signals. J Neurosci Methods 192, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers S, Himmelbach M, Logothetis N, Karnath HO, 2012. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat Rev Neurosci 13, 63–70. [DOI] [PubMed] [Google Scholar]
- Brainard DH, 1997. The Psychophysics Toolbox. Spat Vis 10, 433–436. [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC, 1997. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5, 49–62. [DOI] [PubMed] [Google Scholar]
- Campo P, Garrido MI, Moran RJ, Garcia-Morales I, Poch C, Toledano R, Gil- Nagel A, Dolan RJ, Friston KJ, 2013. Network reconfiguration and working memory impairment in mesial temporal lobe epilepsy. Neuroimage 72, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W, 2007. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37, 343–360. [DOI] [PubMed] [Google Scholar]
- Coleshill SG, Binnie CD, Morris RG, Alarcon G, van Emde Boas W, Velis DN, Simmons A, Polkey CE, van Veelen CW, van Rijen PC, 2004. Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. J Neurosci 24, 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin L, Marquer J, 2009. Individual differences in Sternberg’s memory scanning task. Acta Psychol (Amst) 131, 153–162. [DOI] [PubMed] [Google Scholar]
- Cornette L, Dupont P, Salmon E, Orban GA, 2001. The neural substrate of orientation working memory. J Cogn Neurosci 13, 813–828. [DOI] [PubMed] [Google Scholar]
- Curtis CE, 2006. Prefrontal and parietal contributions to spatial working memory. Neuroscience 139, 173–180. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M, 2003. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7, 415–423. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, 2007. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 362, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, 2015. The cognitive neuroscience of working memory. Annu Rev Psychol 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of singletrial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B, 2006. Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129, 3315–3328. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, 2004. Executive attention, working memory capacity, and a two- factor theory of cognitive control. Psychology of Learning and Motivation: Advances in Research and Theory, Vol 44 44, 145–199. [Google Scholar]
- Esslinger C, Schuler N, Sauer C, Gass D, Mier D, Braun U, Ochs E, Schulze TG, Rietschel M, Kirsch P, Meyer-Lindenberg A, 2014. Induction and Quantification of Prefrontal Cortical Network Plasticity Using 5 Hz rTMS and fMRI. Hum Brain Mapp 35, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Kragel JE, Burke JF, Levy DF, Lyalenko A, Wanda P, O’Sullivan L, Hurley KB, Busygin S, Pedisich I, Sperling MR, Worrell GA, Kucewicz MT, Davis KA, Lucas TH, Inman CS, Lega BC, Jobst BC, Sheth SA, Zaghloul K, Jutras MJ, Stein JM, Das SR, Gorniak R, Rizzuto DS, Kahana MJ, 2017. Direct Brain Stimulation Modulates Encoding States and Memory Performance in Humans. Curr Biol 27, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, Sperling MR, Sharan AD, Lega BC, Burks A, Gross RE, Inman CS, Jobst BC, Gorenstein MA, Davis KA, Worrell GA, Kucewicz MT, Stein JM, Gorniak R, Das SR, Rizzuto DS, Kahana MJ, 2018. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun 9, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R, 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30, 1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Staresina BP, Do Lam AT, Widman G, Helmstaedter C, Elger CE, Axmacher N, 2013. Memory modulation by weak synchronous deep brain stimulation: a pilot study. Brain Stimul 6, 270–273. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative G, 2011. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL, 2009. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47, Supplement 1, S102. [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM, 2009. Working memory in schizophrenia: a meta-analysis. Psychol Med 39, 889–905. [DOI] [PubMed] [Google Scholar]
- Gagnon G, Blanchet S, Grondin S, Schneider C, 2010. Paired-pulse transcranial magnetic stimulation over the dorsolateral prefrontal cortex interferes with episodic encoding and retrieval for both verbal and non-verbal materials. Brain Res 1344, 148–158. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D, 1997. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7, 374–385. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Gruber O, Whalley H, Gibson L, Hasan A, Obst K, Dechent P, McIntosh A, Suchan B, Wobrock T, 2013. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls--a randomized placebo-controlled, double-blind fMRI study. Behav Brain Res 237, 300–307. [DOI] [PubMed] [Google Scholar]
- Harding IH, Yucel M, Harrison BJ, Pantelis C, Breakspear M, 2015. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. Neuroimage 106, 144–153. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Csernansky JG, Barch DM, 2013. Structure-function relationship of working memory activity with hippocampal and prefrontal cortex volumes. Brain Struct Funct 218, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS, 2014. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24, 333–339. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Bailey N, Michael M, Fitzgibbon B, Rogasch NC, Saeki T, Fitzgerald PB, 2016. Enhancement of Working Memory and Task-Related Oscillatory Activity Following Intermittent Theta Burst Stimulation in Healthy Controls. Cereb Cortex 26, 4563–4573. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Ranganath C, 2014. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 85 Pt 2, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Liu CY, Thompson PM, Tu Z, 2011. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging 30, 1617–1634. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, Sharan A, Worrell G, Berry B, Lega B, Jobst BC, Davis K, Gross RE, Sheth SA, Ezzyat Y, Das SR, Stein J, Gorniak R, Kahana MJ, Rizzuto DS, 2016. Direct Electrical Stimulation of the Human Entorhinal Region and Hippocampus Impairs Memory. Neuron 92, 983–990. [DOI] [PubMed] [Google Scholar]
- Jausovec N, Jausovec K, Pahor A, 2014. The influence of theta transcranial alternating current stimulation (tACS) on working memory storage and processing functions. Acta Psychol (Amst) 146, 1–6. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE, 2002. Oscillations in the alpha band (912 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 12, 877–882. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE, 1998. An oscillatory short-term memory buffer model can account for data on the Sternberg task. J Neurosci 18, 10688–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A, 2010. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD, 2002. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci 15, 1395–1399. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Huang YH, Herrero JL, Fini ME, Du V, Lado FA, Honey CJ, Mehta AD, 2018. Induction and Quantification of Excitability Changes in Human Cortical Networks. Journal of Neuroscience 38, S384–S398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader PH, Jost K, Ranganath C, Rosler F, 2010. Theta and alpha oscillations during working-memory maintenance predict successful long-term memory encoding. Neurosci Lett 468, 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Ekstrom AD, Tandon N, 2016. A network approach for modulating memory processes via direct and indirect brain stimulation: Toward a causal approach for the neural basis of memory. Neurobiol Learn Mem. [DOI] [PubMed] [Google Scholar]
- Kim K, Schedlbauer A, Rollo M, Karunakaran S, Ekstrom AD, Tandon N, 2018. Network-based brain stimulation selectively impairs spatial retrieval. Brain Stimul 11,213221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM, 2013. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol 74, 223–231. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, Laine M, Hamalainen H, 2000. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol 111,2071–2078. [DOI] [PubMed] [Google Scholar]
- Kucewicz MT, Berry BM, Miller LR, Khadjevand F, Ezzyat Y, Stein JM, Kremen V, Brinkmann BH, Wanda P, Sperling MR, Gorniak R, Davis KA, Jobst BC, Gross RE, Lega B, Van Gompel J, Stead SM, Rizzuto DS, Kahana MJ, Worrell GA, 2018. Evidence for verbal memory enhancement with electrical brain stimulation in the lateral temporal cortex. Brain. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 82, 1–26. [Google Scholar]
- Lacruz ME, Valentin A, Seoane JJ, Morris RG, Selway RP, Alarcon G, 2010. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience 170, 623–632. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT, 2000. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S, 2005. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114, 599–611. [DOI] [PubMed] [Google Scholar]
- Lee TW, Girolami M, Bell AJ, Sejnowski TJ, 2000. A unifying information-theoretic framework for independent component analysis. Computers & Mathematics with Applications 39, 1–21. [Google Scholar]
- Li G, Henriquez CS, Frohlich F, 2017. Unified thalamic model generates multiple distinct oscillations with state-dependent entrainment by stimulation. PLoS Comput Biol 13, e1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Qin W, Liu HG, Fan LZ, Wang JJ, Jiang TZ, Yu CS, 2013. Subregions of the human superior frontal gyrus and their connections. Neuroimage 78, 46–58. [DOI] [PubMed] [Google Scholar]
- Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH, 2007. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: Frequency- and time-dependent effects. Brain Res 1128, 120–129. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT, 2008. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex 18, 1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Sweet JA, Bailey CM, Munyon CN, Luders HO, Fastenau PS, 2015. Visual-spatial memory may be enhanced with theta burst deep brain stimulation of the fornix: a preliminary investigation with four cases. Brain 138, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ, 2008. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol 86, 156–185. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, 2006. Interfering with working memory in humans. Neuroscience 139, 8590. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A, 2002. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex 12, 369–375. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Turriziani P, Carlesimo GA, Koch G, Tomaiuolo F, Panella M, Caltagirone C, 2001. Parieto-frontal interactions in visual-object and visual-spatial working memory: evidence from transcranial magnetic stimulation. Cereb Cortex 11,606618. [DOI] [PubMed] [Google Scholar]
- Osaka N, Otsuka Y, Hirose N, Ikeda T, Mima T, Fukuyama H, Osaka M, 2007. Transcranial magnetic stimulation (TMS) applied to left dorsolateral prefrontal cortex disrupts verbal working memory performance in humans. Neurosci Lett 418, 232–235. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E, 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polania R, Nitsche MA, Korman C, Batsikadze G, Paulus W, 2012. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol 22, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G, 2006. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci 18, 1712–1722. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE, 2001. Gating of human theta oscillations by a working memory task. J Neurosci 21,3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ, 2006. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol 95, 1630–1638. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D’Esposito M, 2004. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 24, 3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ, 2014. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn Sci 18, 16–25. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD, 1999. Load- dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 9, 216–226. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC, 2009. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol 19, 1846–1852. [DOI] [PubMed] [Google Scholar]
- Snyder HR, 2013. Major Depressive Disorder Is Associated With Broad Impairments on Neuropsychological Measures of Executive Function: A Meta-Analysis and Review. Psychol Bull 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana N, Fried I, 2014. Deep brain stimulation for enhancement of learning and memory. Neuroimage 85 Pt 3, 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I, 2012. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med 366, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD, Karhu J, 2000. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci U S A 97, 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schyns PG, Gross J, 2011. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol 2, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2012. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 75, 963980. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Fukuda K, Awh E, Vogel EK, 2014. Working memory and fluid intelligence: capacity, attention control, and secondary memory retrieval. Cogn Psychol 71, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Li LM, Carmichael DW, Lorenz R, Leech R, Hampshire A, Rothwell JC, Sharp DJ, 2017. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen A, Gross J, Thut G, 2015. Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (alpha-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimul 8, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosskuhl J, Huster RJ, Herrmann CS, 2015. Increase in short-term memory capacity induced by down-regulating individual theta frequency via transcranial alternating current stimulation. Front Hum Neurosci 9, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE, 2003. Neuroimaging studies of working memory: a metaanalysis. Cogn Affect Behav Neurosci 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Tomioka H, Kawasaki S, Noda Y, Yamagata B, Iwanami A, Mimura M, 2014. Effect of parietal transcranial magnetic stimulation on spatial working memory in healthy elderly persons--comparison of near infrared spectroscopy for young and elderly. PLoS One 9, e102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Yamagata B, Tomioka H, Kawasaki S, Mimura M, 2010. Transcranial magnetic stimulation of the parietal cortex facilitates spatial working memory: nearinfrared spectroscopy study. Cereb Cortex 20, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G, 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.