Abstract
Background & Aims:
Little information is available on the effectiveness of organized colorectal cancer (CRC) screening on screening uptake, incidence, and mortality in community-based populations.
Methods:
We contrasted screening rates, age-adjusted annual CRC incidence, and incidence-based mortality rates before (baseline year 2000) and after (through 2015) implementation of organized screening outreach, from 2007 through 2008 (primarily annual fecal immunochemical testing and colonoscopy), in a large, community-based population. Among screening-eligible individuals 51–75 years old, we calculated annual up to date status for cancer screening (by fecal test, sigmoidoscopy, or colonoscopy), CRC incidence, cancer stage distributions, and incidence-based mortality.
Results:
Initiation of organized CRC screening significantly increased the up to date status of screening, from 38.9% in 2000 to 82.7% in 2015 (P<.01). Higher rates of screening were associated with a 25.5% reduction in annual CRC incidence between 2000 and 2015, from 95.8 to 71.4 cases/100,000 (P<.01), and a 52.4% reduction in cancer mortality, from 30.9 to 14.7 deaths/100,000 (P<.01). Increased screening was initially associated with increased CRC incidence, largely due to greater detection of early-stage cancers, followed by decreases in cancer incidence. Advanced-stage CRC incidence rates decreased 36.2%, from 45.9 to 29.3 cases/100,000 (P<.01), and early-stage CRC incidence rates decreased 14.5%, from 48.2 to 41.2 cases/100,000 (P<.04).
Conclusions:
Implementing an organized CRC screening program in a large, community-based population rapidly increased screening participation to the ≥80% target set by national organizations. Screening rates were sustainable and associated with substantial decreases in CRC incidence and mortality within short time intervals, consistent with early detection and cancer prevention.
Keywords: colon cancer, neoplasm, FIT, early detection
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in the United States (US).1 Screening can prevent colorectal cancer through the removal of precancerous adenomatous polyps, and reduce deaths through early detection and treatment of cancer.2,3 The US Preventive Services Task Force recommends several screening tests, including high-sensitivity guaiac-based fecal occult blood testing (gFOBT), fecal immunochemical testing (FIT), multi-targeted stool DNA testing, colonoscopy, computed tomography colonography, and flexible sigmoidoscopy with or without FIT.4 The National Colorectal Cancer Roundtable set a goal of increasing the screening rate from 58% in 2013 to ≥80% of the eligible US population by 2018, and estimated that achieving this goal would result in 19% fewer colorectal cancer deaths.5,6 However, more recent data indicate only 63% of eligible US residents, and fewer than 50% of some race/ethnicity groups, are up to date with screening,7 leading to concern that rates may be plateauing,8 and making it unclear if the 80% target is achievable or sustainable.
Colonoscopy and FIT are commonly-used screening tests worldwide, but the population level impact of screening programs is largely unknown.5,9 Modeling studies suggest these two screening strategies have comparable effectiveness for reducing colorectal cancer-associated mortality.10 However, the strongest evidence to date of screening benefit comes from randomized controlled trials that demonstrated reduced mortality for both gFOBT and sigmoidoscopy,11 tests that are no longer widely used in the US.12 The evidence for colonoscopy’s effectiveness comes indirectly from sigmoidoscopy trials13-19 and observational studies.2,20-24 The evidence for FIT effectiveness comes indirectly from gFOBT trials,25-35 given that FIT operates by a similar mechanism and has a higher sensitivity for colorectal cancer and advanced adenomas than gFOBT.36-38 Using multiple screening options may help increase screening uptake,39,40 but little data exist regarding the influence of population-based organized screening programs on colorectal cancer screening rates, incidence, and mortality.
The present study, in a large community-based integrated healthcare delivery system, evaluated whether an organized colorectal cancer screening program could achieve and sustain the ≥80% screening target proposed by national organizations, and whether changes in screening were associated with changes in colorectal cancer incidence and mortality.
METHODS
Study Population and Oversight
The study was performed using a dynamic cohort of Kaiser Permanente Northern California (KPNC) health plan members for the years 2000-2015. KPNC is an integrated health care delivery organization which serves approximately 4.0 million members in urban, suburban, and semi-rural regions throughout California; membership is similar in demographic and socioeconomic characteristics to the region’s census demographics.41
The study was approved by the KPNC institutional review board, which waived the requirement for individual informed consent. The listed authors had sole responsibility for the study design, data collection, decision to submit the manuscript for publication, and drafting of the manuscript. This study was conducted within the National Cancer Institute-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium (U54 CA163262) which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes.
Organized Colorectal Cancer Screening Program
Prior to 2006, colorectal cancer screening within the cohort was performed by physician request, predominantly using sigmoidoscopy and gFOBT. FIT was pilot tested in 2006. Starting in 2007, screening transitioned region-wide to direct-to-patient annual FIT outreach for members 60-69 years of age who were not screening up-to-date by other methods and, in 2008, it was expanded to those 51-75 years of age; colonoscopy was a screening option throughout this period, by request. As described previously,42-44 each year FIT kits are mailed to health plan members without a record of a colonoscopy within 10 years or a sigmoidoscopy within 5 years. The program’s goal is, primarily through FIT or colonoscopy, to screen all screening-eligible members by December 31 of each calendar year, starting the year they turn 51 through 75 years of age, in accordance with the Healthcare Effectiveness Data and Information Set (HEDIS) measurement approach.45,46 Screening outreach includes mail, secure email, and telephone reminders as needed. In-reach includes in-person reminders for members attending office or preventive health visits with any health care provider through prompts in the electronic medical record. FIT is analyzed by an automated OC-Sensor Diana (Polymedco Inc) with a cutoff level of ≥20 micrograms hemoglobin per gram of stool for a positive result. Patients with a positive FIT were contacted by their primary care physician or local gastroenterology department staff to schedule a follow-up colonoscopy through a combination of telephone calls, secure messaging, and mail.
Cohort Eligibility Criteria
The study cohort was comprised of KPNC members 51-75 years of age in 2000-2015, who were continuously enrolled (allowing a coverage gap of ≤45-day) in the calendar year before cohort entry to allow time to document screening history, including outside of KPNC, and enrolled in the measurement year (any year in which screening status was ascertained).
Censoring
Cohort members were censored at the first of the following: end of the study interval (December 31, 2015), December 31 in the year in which they reached 75 years of age or ended continuous health plan membership (defined as any gap of >45 days in a calendar year), or their date of death.
Study Outcomes
We evaluated the influence of organized screening on three primary outcomes: screening up-to-date status, colorectal cancer incidence, and colorectal cancer-specific mortality; and two secondary outcomes: FIT/gFOBT positivity and the percentage of fecal test-positive patients who received a follow-up colonoscopy within 6 months of their positive test. An individual was considered up-to-date with screening if they completed fecal testing (FIT or gFOBT) in a given year, or had a sigmoidoscopy (for any indication) within 5 years or colonoscopy (for any indication) within 10 years (including the measurement year). To avoid double counting of screening tests, the first test performed on a patient in a given year was counted as the screening method. For example, if a colonoscopy was performed following a positive FIT, the patient was counted as screened by FIT.
New colorectal cancer diagnoses (first primary only) among cohort members were used to generate annual incidence rates and incidence-based mortality rates; the latter was defined as a colorectal cancer-related death in any individual ages 51-75 who had a colorectal cancer diagnosis in the prior 10 years. Incidence-based mortality (derived from cancer registry data rather than death certificates) is less subject to bias from migration than non-incidence-based colorectal cancer mortality.47 A 10-year interval between diagnosis and death was selected to allow sufficient time for disease progression to result in death.
Data Sources and Definitions
Data regarding colorectal cancer screening, diagnoses and deaths, demographics, and other covariates were obtained from validated electronic laboratory, cancer registry, medical visit, demographic, and membership databases.48,49 Colonoscopy procedures were identified using Current Procedural Terminology codes (44388-44394, 44397, 44398, 44401-44403, 44405, 45355, 45378-45393), International Classification of Disease procedure codes (45.21-45.23, 45.25, 45.42, 45.43, 98.04, as well as codes 48.24 and 48.36 [rectal biopsy] when there was no corresponding sigmoidoscopy procedure on or near the procedure date), Healthcare Common Procedure Coding System codes (G0105, G0121), and internal codes for tracking colonoscopies performed prior to joining KPNC (12142332, 204456, 230847, and 235525). Sigmoidoscopy procedures were identified using Current Procedural Terminology codes (45300, 45303, 45305, 45307-45309, 45315, 45317, 45320, 45321, 45327, 45330-45335, 45337-45342, and 45345), International Classification of Disease procedure codes (45.24 and 48.21-48.23), and internal codes for tracking sigmoidoscopies performed prior to joining (224770 and 230854).
Colorectal cancer was defined as an adenocarcinoma within the colon or rectum using Surveillance Epidemiology and End Results (SEER) cancer site group codes 21040 and 21050, and International Classification of Disease oncology codes C18.0-C18.9, C19.9, and C20.9. The KPNC cancer registry reports to the SEER registry and maintains a >97% population-based completeness standard as verified by random audits by the cancer registry and SEER. Additional retrospective audits and death clearance processes have historically captured approximately 1-2% additional cases.
Colorectal cancer staging definitions:
Advanced-stage cancers were defined as stage III (regional disease with spread to the regional lymph nodes only) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system. For members without such staging information, advanced-stage cancers were defined as SEER stage 3 (disease in the regional lymph nodes), 4 (regional disease with direct extension and spread to the regional lymph nodes), or 7 (distant metastasis) according to the SEER Program Coding and Staging Manual 2013.50
Colon location definitions:
Proximal cancers were those located in the cecum, ascending colon, hepatic flexure, and transverse colon; distal cancers were those in the splenic flexure, descending colon, sigmoid colon, and rectum.
FIT/gFOBT positivity was defined as the percentage of individuals who completed a FIT or gFOBT in a given year and had a positive result. Colonoscopy follow-up was defined as, among those with a positive FIT or gFOBT in a given year, the percentage that received a follow-up colonoscopy within 6 months after the positive test.
Statistical Analyses
Comparisons of proportions were evaluated using chi-square tests. Annual colorectal cancer incidence rates and incidence-based mortality rates for the years 2000-2015 were adjusted to the 2000 US census population using single-year age intervals (e.g., 51, 52, 53… 74 or 75 years) as provided by SEER.51 Single-year age-adjusted incidence rates were also stratified by age categories (51-64, 65-75 years), sex, stage (early, advanced), and colon location (proximal, distal). Statistical comparisons of incidence and mortality rates utilized 95% confidence intervals (CI) for age-adjusted rates and the z-test. Hypothesis testing was two-sided with an alpha of 0.05, and analyses used SAS statistical software, version 9.3 (Cary, NC).
Role of the Funding Source
The study was funded by the National Cancer Institute. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit for publication.
RESULTS
Characteristics of the Screening-Eligible Population
Patient cohort characteristics at 3 points during the study interval (years 2000, 2008, and 2015) are provided in Table 1. At each point, the cohort was predominately 51-64 years of age (68.5% to 74.0%), female (52.5% to 53.0%), and non-Hispanic white (58.4% to 64.4%). The overall size of the screening-eligible cohort increased 49.7% during the study interval, from 651,675 in 2000, to 975,637 in 2015, although membership duration was stable. In 2000, the average (± standard deviation) length of membership was 11.2±4.6 years and 17% were members for ≤5 years, 18% for 6-10 years, and 64% for ≥11 years. In 2008, the average length of membership was 11.3±4.6 years and 17% were members for ≤5 years, 18% for 6-10 years, and 65% for ≥11 years. In 2015, the average length of membership was 11.4±4.6 years and 17% were members for ≤5 years, 17% for 6-10 years, and 66% for ≥11 years.
Table 1.
Cohort characteristics in 2000, 2008, and 2015.
| Characteristics | 2000 | 2008 | 2015 | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Total cohort members | 651675 | 100.0 | 821710 | 100.0 | 975637 | 100.0 |
| Age: | ||||||
| 50-64 years | 463325 | 71.1 | 608138 | 74.0 | 668658 | 68.5 |
| 65-75 years | 188350 | 28.9 | 213572 | 26.0 | 306979 | 31.5 |
| Sex: | ||||||
| Male | 309394 | 47.5 | 386751 | 47.1 | 458263 | 47.0 |
| Female | 342281 | 52.5 | 434959 | 52.9 | 517374 | 53.0 |
| Race/ethnicity: | ||||||
| Non-Hispanic White | 419850 | 64.4 | 498576 | 60.7 | 569317 | 58.4 |
| Black | 48248 | 7.4 | 59820 | 7.3 | 70225 | 7.2 |
| Asian or Pacific | 73458 | 11.3 | 121092 | 14.7 | 163516 | 16.8 |
| Islander | ||||||
| Hispanic | 60412 | 9.3 | 91723 | 11.2 | 124155 | 12.7 |
| Other | 4755 | 0.7 | 6949 | 0.8 | 9039 | 0.9 |
| Unknown | 44952 | 6.9 | 43550 | 5.3 | 39385 | 4.0 |
| KPNC membership duration: | ||||||
| ≤5 years | 111939 | 17.2 | 141794 | 17.3 | 162945 | 16.7 |
| 6-10 years | 119773 | 18.4 | 146977 | 17.9 | 169290 | 17.4 |
| ≥11 years | 419963 | 64.4 | 532939 | 64.9 | 643402 | 66.0 |
| Mean±SD, years | 11.2±4.6 | 11.3±4.6 | 11.4±4.6 | |||
KPNC, Kaiser Permanente Northern California; SD, standard deviation
During follow-up, 1,768 colorectal cancer cases were diagnosed; 141 (1.2%) had unknown stage and 382 (3.2%) had unknown location; these latter cases were not included in analyses stratified by stage or location, respectively.
Screening Participation
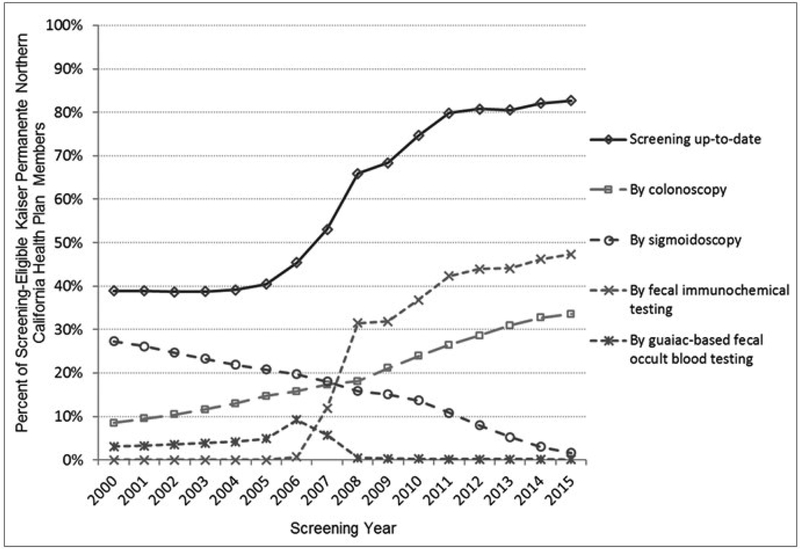
Screening participation was stable in years 2000-2005, between 39.7% to 40.5%. Participation began to rise after the initiation of FIT pilot testing in 2006 and organized screening in 2007 and 2008 (Figure 1 and eTable 1). The percentage of the cohort up-to-date with screening significantly increased from 38.9% in 2000, to 82.7% in 2015 (P<.01); the increase was primarily due to increased uptake of FIT and colonoscopy.
Figure 1.
Percentage of Eligible Cohort Members Screening Up-to-Date: Overall and by Modality.
FIT/gFOBT Positivity and Colonoscopy Follow-up
FIT/gFOBT positivity across the study interval ranged between 3.1% and 5.3%, and the percentage of individuals with colonoscopy follow-up within 6 months after a positive fecal test increased from 41.1% in 2000 to 83.1% in 2015 (eTable 1).
Colorectal Cancer Incidence Rates
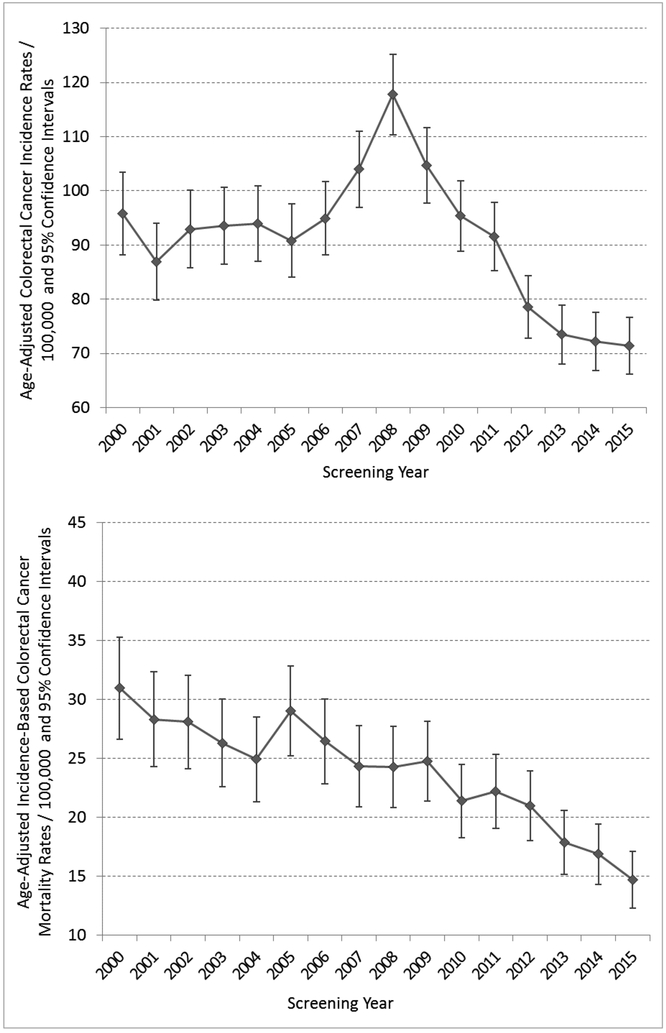
Age-adjusted colorectal cancer incidence rates increased significantly from 95.8 cases/100,000 (95% CI 88.1, 103.4) in 2000, to a peak of 117.8/100,000 (95% CI 110.4, 125.2; P<.01) in 2008, which coincided with rapidly rising screening rates following implementation of organized screening (Figure 2 and eTable 2), before declining to significantly below baseline (year 2000) in years 2012-2015 (P<.01 for all years). Overall, age-adjusted cancer incidence rates decreased 25.5% between 2000 and 2015, from 95.8 cases/100,000 to 71.4/100,000 (95% CI 66.1, 76.7; P<.01) (Figure 2 and eTable 2).
Figure 2.
Cohort Age-Adjusted Colorectal Cancer (CRC) Incidence Rates and Incidence-Based Mortality Rates: Age-Adjusted to the United States 2000 Census Population.
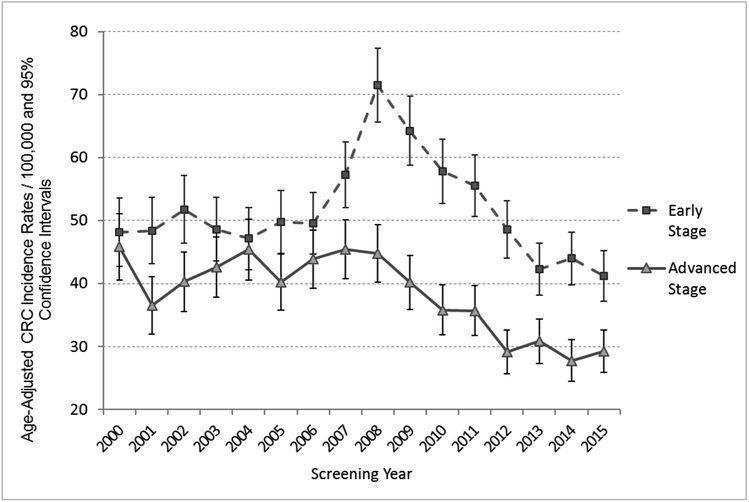
The initial increase in colorectal cancer incidence associated with the rapid rise in screening rates was largely due to greater detection of early-stage cancers, which peaked in 2008 (Figure 3 and eTable 3). Age-adjusted early-stage cancer incidence rates subsequently decreased despite enhanced early detection; across the full study period rates declined 14.5%, from 48.2 cases/100,000 (95% CI 42.8, 53.6) in 2000, to 41.2/100,000 (95% CI 37.2, 45.2) in 2015 (P<.04). Age-adjusted advanced-stage incidence rates decreased 36.2%, from 45.9 cases/100,000 (95% CI 40.6, 51.1) to 29.3/100,000 (95% CI 25.9, 32.6; P<.01).
Figure 3:
Cohort Colorectal Cancer (CRC) Incidence Rates by Stage: Age-Adjusted to the United States 2000 Census Population.
Age-adjusted colorectal cancer incidence rates were consistently higher for distal compared to proximal cancers (eFigure 1), men compared to women (eFigure 2), and older versus younger cohort members (eFigure 3), and in all groups rates peaked in 2008, following implementation of organized screening. Incidence rates decreased significantly among patients 65-75 years of age (from 148.5 cases/100,000 in 2000 to 90.1/100,000 in 2015; P<.01), but not among patients 50-64 years of age (from 68.0 cases/100,000 in 2000 to 61.5/100,000 in 2015; P=.19).
Incidence-Based Colorectal Cancer Mortality Rates
Age-adjusted incidence-based mortality rates decreased by 52.4%, from 30.9 deaths/100,000 (95% CI 26.6, 35.3) in 2000, to 14.7/100,000 (95% CI 12.3, 17.1) in 2015 (P<.01) (Figure 2 and eTable 2).
DISCUSSION
The replacement of an opportunistic colorectal cancer screening program based primarily on sigmoidoscopy and gFOBT, with an organized screening program of annual FIT combined with opportunistic colonoscopy doubled the percentage of patients screening up-to-date, from almost 40% to over 80%. The increase in screening was associated with an immediate increase in colorectal cancer incidence, particularly early-stage disease, followed by a substantial 25.5% decline in cancer incidence and a remarkable 52.4% decrease in cancer mortality over a relatively short 12-16-year period. FIT positivity fluctuated between 3.1% to 5.3% across the study interval and colonoscopy follow-up within 6 months after a positive test increased from 41.1% in 2000 to 83.1% in 2015.
Our findings indicate that, even in very large community-based settings, the 80% screening target set by the National Colorectal Cancer Roundtable is both feasible and sustainable using organized screening programs.5 These findings underscore the potential for organized screening programs to achieve national target screening rates.
The temporal changes in colorectal cancer outcomes following the implementation of organized screening are consistent with shorter duration community-based colorectal cancer mortality studies which have evaluated programmatic FIT, as well as modeling studies of the ≥80% screening target. An Italian study with staggered initiation of biennial FIT reported that the region starting FIT in 2002-2004 had a 22% greater reduction in subsequent colorectal cancer mortality than the region starting FIT in 2008-2009.52 A Taiwanese study of FIT demonstrated lower CRC mortality rates among a cohort exposed to 1-3 rounds of biennial FIT compared to an unscreened cohort (adjusted relative risk: 0.90; 95% CI: 0.84-0.95).53 A modeling study estimated that increasing US screening rates from 58% in 2013 to the 80% target by 2018 would reduce colorectal cancer incidence and mortality rates by 17% and 19% in the short-term, and 22% and 33% in the long-term, respectively, and avert approximately 280,000 new cancer cases and 200,000 cancer deaths within <20 years.6 In the current study, between 2000 and 2015, the increase in screening coincided with decreases in colorectal cancer incidence and mortality within the cohort of 25.5% and 52.4%, respectively.
Although the observational design precludes confirming a direct causal link between the increases in screening and the decreases in colorectal cancer outcomes, temporal changes in cancer risk factors or treatment are unlikely sole alternative explanations for several reasons. First, colorectal cancer incidence is stable or increasing in many comparable developed countries without substantial screening programs, including Finland, Norway, France and Australia. Substantial declines are reported almost exclusively in countries with at least moderate use of cancer screening tests.54 Second, a sophisticated modeling study suggested that changes in risk factors and treatment have relatively small influences on population-level colorectal cancer mortality statistics.55 For example, between 1975 and 2000, when there was an overall 26% absolute decrease in colorectal cancer mortality in the US, 53% of this reduction was attributed to screening and only 12% to improved treatment.55 Third, colorectal cancer incidence rates in the study cohort were stable in the baseline pre-intervention period, an interval with stable screening rates within the cohort; cancer incidence changes abruptly coincided with increased screening. Fourth, the observed incidence changes, with lower cancer mortality among all age groups but lower cancer incidence mainly among older patients, are concordant with biological knowledge regarding progression of polyps to cancer, and known effects of screening tests on early cancer detection.56,57 Changes from modified risk factors, for example, would be expected to influence both cancer incidence and mortality among all age groups, whereas reduced incidence from CRC screening, such as through polyp removal in the younger age group, would largely be anticipated, as seen in the current data, with cancer incidence until several years later, among older patients.56
Also, concordant with randomized trials and observational studies, increased screening in the study cohort coincided with an immediate increase in early-stage cancer diagnoses.13,20,58 In addition, as would be expected for FIT and colonoscopy which evaluate the entire colon, we observed reductions in both right- and left-sided cancer. This is in contrast to sigmoidoscopy, which has been demonstrated to mainly decrease the risk of left-sided colorectal cancer.13,14,21 Colorectal cancer screening rates nationally in the US increased among those 50 years and older during the study period, from about 38% in 2000 to 62% in 2015,59,60 while colorectal cancer incidence and mortality nationally decreased during this period,1,59 largely likely related to increased use of screening.55 The reported results suggest that an organized screening program can achieve higher rates of screening and greater incidence and mortality reductions; the colorectal cancer mortality rate in the study cohort for 2014 (10.2 /100,000) (the most recent year with comparable date), for example, was 28% lower than the rate reported nationally (14.1/100,000).59
The study design has several strengths, including a large, diverse, and stable community-based population and 16 years of data covering the periods before and after implementation of organized screening. There was systematic and comprehensive capture of screening tests using validated methods48 and of colorectal cancer outcomes through a SEER-affiliated cancer registry. The study directly ascertained individual-level screening completion; this differs from US population screening estimates, which largely utilized indirect measures, such as surveys, which overestimate screening prevalence.61 The pre-existing opportunistic screening program provided the study population with stable pre-outreach screening and incidence rates from 2000-2005. This permitted evaluation of background temporal colorectal cancer trends; the stable colorectal cancer incidence during this period provides reassurance that the subsequent changes seen were not solely from background risk factors or cancer treatment. The large cohort size allowed evaluation of important strata by age, sex, and cancer location. The study design also eliminated the healthy volunteer bias associated with screening trials,62 since all health plan members, screened and unscreened, were followed for clinical outcomes. A potential question is whether similar rates can be reached outside of an integrated healthcare system. However, comparable screening rates have been achieved in challenging populations using organized screening,63-65 although the sustainability of the described interventions, in these populations, has not been well evaluated.
In conclusion, these findings suggest that implementing organized colorectal cancer screening, using annual FIT and colonoscopy, can rapidly increase screening participation. They also suggest that the screening target of ≥80%, set by the National Colorectal Cancer Roundtable,5 is achievable, sustainable in a large population, and associated with substantial decreases in colorectal cancer incidence and mortality within short time intervals.
Supplementary Material
Acronyms/abbreviations:
- CI
confidence interval
- FIT
fecal immunochemical testing
- gFOBT
guaiac-based fecal occult blood testing
- HEDIS
Healthcare Effectiveness Data and Information Set
- KPNC
Kaiser Permanente Northern California
- PROSPR
Population-based Research Optimizing Screening through Personalized Regimens
- SEER
Surveillance Epidemiology and End Results
- SD
standard deviation
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest exist for any of the authors.
Disclaimer: Dr. Doubeni is a member of the United States Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
Role of study sponsor: The study sponsor had no role in the study design or in the collection, analysis, and interpretation of data.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 5.About 80% by 2018. http://nccrt.org/wp-content/uploads/80-by-2018-TALKINGPOINTS-Final-2.16.17.pdf. Accessed May 30, 2017.
- 6.Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colorectal Cancer Facts & Figures 2017-2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. Accessed November 9, 2017.
- 8.Screening Rates for Several Cancers Miss Their Targets. https://www.cancer.gov/news-events/cancer-currents-blog/2015/screening-targets. Accessed November 9, 2017.
- 9.Benson VS, Atkin WS, Green J, et al. Toward standardizing and reporting colorectal cancer screening indicators on an international level: The International Colorectal Cancer Screening Network. Int J Cancer. 2012;130(12):2961–2973. [DOI] [PubMed] [Google Scholar]
- 10.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–2594. [DOI] [PubMed] [Google Scholar]
- 12.Lin JS, Piper MA, Perdue LA, et al. In: Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Rockville (MD)2016. [PubMed] [Google Scholar]
- 13.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726): 1624–1633. [DOI] [PubMed] [Google Scholar]
- 14.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–1322. [DOI] [PubMed] [Google Scholar]
- 16.Atkin WS, Cook CF, Cuzick J, et al. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359(9314):1291–1300. [DOI] [PubMed] [Google Scholar]
- 17.Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segnan N, Senore C, Andreoni B, et al. Baseline findings of the Italian multicenter randomized controlled trial of "once-only sigmoidoscopy"--SCORE. J Natl Cancer Inst. 2002;94(23): 1763–1772. [DOI] [PubMed] [Google Scholar]
- 19.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the PLCO cancer screening trial: results from the baseline screening examination of a randomized trial. J Natl Cancer Inst. 2005;97(13):989–997. [DOI] [PubMed] [Google Scholar]
- 20.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6(10):1117–1121; quiz 1064. [DOI] [PubMed] [Google Scholar]
- 23.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128–1137. [DOI] [PubMed] [Google Scholar]
- 25.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126(7):1674–1680. [DOI] [PubMed] [Google Scholar]
- 26.Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39(9):846–851. [DOI] [PubMed] [Google Scholar]
- 27.Malila N, Palva T, Malminiemi O, et al. Coverage and performance of colorectal cancer screening with the faecal occult blood test in Finland. J Med Screen. 2011;18(1):18–23. [DOI] [PubMed] [Google Scholar]
- 28.Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61(7):1036–1040. [DOI] [PubMed] [Google Scholar]
- 29.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114. [DOI] [PubMed] [Google Scholar]
- 30.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. [DOI] [PubMed] [Google Scholar]
- 31.Malila N, Oivanen T, Malminiemi O, Hakama M. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ. 2008;337:a2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. [DOI] [PubMed] [Google Scholar]
- 33.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. [DOI] [PubMed] [Google Scholar]
- 34.Thomas W, White CM, Mah J, Geisser MS, Church TR, Mandel JS. Longitudinal compliance with annual screening for fecal occult blood. Minnesota Colon Cancer Control Study. Am J Epidemiol. 1995;142(2):176–182. [DOI] [PubMed] [Google Scholar]
- 35.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95(8):1029–1036. [DOI] [PubMed] [Google Scholar]
- 36.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008; 135(1):82–90. [DOI] [PubMed] [Google Scholar]
- 37.Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99(19): 1462–1470. [DOI] [PubMed] [Google Scholar]
- 38.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107(9):2152–2159. [DOI] [PubMed] [Google Scholar]
- 39.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Archives of internal medicine. 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? 2006. http://www.dor.kaiser.org/dor/mhsnet/public/kpnc_community.htm. Accessed March 10, 2017. [Google Scholar]
- 42.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011. ;33(1):101–110. [DOI] [PubMed] [Google Scholar]
- 43.Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016;164(7):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta SJ, Jensen CD, Quinn VP, et al. Race/Ethnicity and Adoption of a Population Health Management Approach to Colorectal Cancer Screening in a Community-Based Healthcare System. J Gen Intern Med. 2016;31(11):1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarfaty M, Myers RE. The effect of HEDIS measurement of colorectal cancer screening on insurance plans in Pennsylvania. Am J Manag Care. 2008;14(5):277–282. [PubMed] [Google Scholar]
- 46.HEDIS & Performance Measurement. http://www.ncqa.org/hedis-quality-measurement.
- 47.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461. [DOI] [PubMed] [Google Scholar]
- 48.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England journal of medicine. 2014;370(14):1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointestinal Endoscopy. 2015;81(3):575–582 e574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SEER Program Coding and Staging Manual 2013. 2013; http://seer.cancer.gov/manuals/2013/SPCSM_2013_maindoc.pdf. Accessed March 10, 2017.
- 51.Standard Populations - Single Ages. https://seer.cancer.gov/stdpopulations/stdpop.singleages.html.
- 52.Zorzi M, Fedeli U, Schievano E, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64(5):784–790. [DOI] [PubMed] [Google Scholar]
- 53.Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121(18):3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SEER Stat Fact Sheets: Colon and Rectum Cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed June 8, 2017.
- 55.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21(3):411–416. [DOI] [PubMed] [Google Scholar]
- 56.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–437. [DOI] [PubMed] [Google Scholar]
- 57.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin Gastroenterol Hepatol. 2017;15(6):903–909 e906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 59.Colorectal Cancer Screening. Cancer Trends Progress Report https://progressreport.cancer.gov/detection/colorectal_cancer. Accessed September 27, 2017.
- 60.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancerscreening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748–757. [DOI] [PubMed] [Google Scholar]
- 62.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165(8):874–881. [DOI] [PubMed] [Google Scholar]
- 63.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.