Abstract
Recently, many clinical trials have challenged the efficacy of current therapeutics for neuropathic pain after spinal cord injury (SCI) due to their life-threatening side-effects including addictions. Growing evidence suggests that persistent inflammatory responses after primary SCI lead to an imbalance between anti-inflammation and pro-inflammation, resulting in pathogenesis and maintenance of neuropathic pain. Conversely, a variety of data suggest that inflammation contributes to regeneration. Herein, we investigated long-term local immunomodulation using anti-inflammatory cytokine IL-10 or IL-4-encoding lentivirus delivered from multichannel bridges. Multichannel bridges provide guidance for axonal outgrowth and act as delivery vehicles. Anti-inflammatory cytokines were hypothesized to modulate the pronociceptive inflammatory niche and promote axonal regeneration, leading to neuropathic pain attenuation. Gene expression analyses demonstrated that IL-10 and IL-4 decreased pronociceptive genes expression versus control. Moreover, these factors resulted in an increased number of pro-regenerative macrophages and restoration of normal nociceptors expression pattern. Furthermore, the combination of bridges with anti-inflammatory cytokines significantly alleviated both mechanical and thermal hypersensitivity relative to control and promoted axonal regeneration. Collectively, these studies highlight that immunomodulatory strategies target multiple barriers to decrease secondary inflammation and attenuate neuropathic pain after SCI.
Keywords: Spinal cord injury, neuropathic pain, gene delivery, immunoengineering, anti-inflammatory cytokine
1. Introduction
Chronic neuropathic pain after spinal cord injury (SCI) severely impairs quality of life for patients with paralysis, and remains resistant to traditional pharmacologic approaches [1]. Several studies have suggested that opioids may be useful to treat neuropathic pain, yet insufficient data is available to establish guidelines [2, 3]. Furthermore, opioid use is associated with addiction concerns, and clinical reports have suggested that opioid prescriptions could be harmful to the physical recovery of patients [4, 5]. Glucocorticoids such as methylprednisolone and dexamethasone were the standard of care for years after clinical trials demonstrated improved outcomes for neuropathic pain, suggesting a link to anti-inflammatory properties [6, 7]. However, these agents are also associated with increased risk of sepsis, gastrointestinal bleeding, and thromboembolism [7]. Furthermore, the side effects of glucocorticoid administration accumulate over time, attenuating the benefit to be gained for chronic neuropathic pain patients [7]. While glucocorticoid administration for treating neuropathic pain is controversial, reducing inflammatory responses remains a target for therapeutic intervention.
Neuroinflammatory responses by resident microglia and recruited-hematogenous macrophages after SCI lead to a rapid production of pro-inflammatory cytokines, initiating the host defense to cellular damage and pathogens, which are actively controlled by complex regulatory mechanisms [8-10], Anti-inflammatory cytokines can regulate inflammatory processes to limit tissue damage, yet after SCI, these cytokines are insufficiently expressed to modulate the neuro inflam matory milieu by pro-inflammatory cytokines, resulting in excessive transmission of nociceptive signals at almost every level of the somatosensory system [11-13]. In addition, neighboring intact axons that are exposed to the inflammatory milieu also contribute to the initiation and ongoing neuronal hyperexcitability. However, global suppression of inflammation is unlikely to provide long term benefits, since its initial function is to clear damaged tissues and trigger wound healing processes [9]. Attempts to interrupt inflammation by ablation of macrophages and pro-inflammatory cytokines lead to severely impaired axon growth [14], Therefore, an imbalance between pro-inflammation and anti-inflammation in the neural microenvironment may contribute to the transition from the acute to chronic neuropathic pain after SCI [11, 15].
Localized sustained delivery and expression of anti-inflammatory cytokines using lentiviral vectors at SCI has been reported to modulate the pro-inflammatory niche and promote axonal regrowth and remyelination leading to functional recovery after SCI [16, 17]. Furthermore, the presence of anti-inflammatory factors in SCI influences the numbers and phenotypes of multiple immune cell types, such as neutrophils, microglia, and macrophages [16, 17]. In particular, macrophages have been a focus at SCI due to their role in clearance of debris and nerve regeneration [8]. While macrophage phenotypes are not binary, their phenotypes have been described as being plastic and able to vary from pro-inflammatory (M1) to pro-regenerative (M2), with plasticity depending on the microenvironment [18]. Given the central roles in inflammatory responses and neuronal excitability after injury [11-13]. inducing long-term local pro-regenerative microenvironments at the injury may influence neuropathic pain.
Herein, we investigated the impacts of long-term expression of anti-inflammatory cytokines interleukin (IL)-10 or IL-4 on the attenuation of neuropathic pain following SCI. IL-10 and IL-4 are reported to have anti-nociceptive functions [19-21], and localized expression of these cytokines at the injury was achieved through implantation of a poly(lactide co-glycolide) (PLG) multichannel bridge loaded with lentiviral vectors encoding the cytokines into a lateral hemisection. The bridge allows cell infiltration resulting in apposition with the host tissue that stabilizes the injury, with the infiltrating cells supporting axon growth that can be guided across the injury by the channels [16, 22, 23]. We hypothesized that sustained expression of antiinflammatory cytokines would shift the immune responses towards pro-regenerative leading to suppressed neuropathic pain. The growth and structure of sensory axons were analyzed histologically, in addition to the distribution and phenotype of immune cells. A transcriptome analysis of the injury was employed to analyze the expression of genes associated with neuropathic pain. Sensory functional recovery was also investigated via mechanical allodynia (pain in response to previously innocuous stimuli) and thermal hyperalgesia (increased pain with noxious stimulations) tests [12]. Collectively, these studies determine the potential of local immunomodulation with IL-10 and IL-4 as a means to suppress neuropathic pain, and the associated mechanisms of action.
2. Materials and Methods
2.1. Multichannel bridges fabrication
Initially, to create microspheres (z-average diameter ~1μm), PLG (75:25 lactide:glycolide; i.v. 0.76 dL/g; Lakeshore Biomaterials, Birmingham, AL, USA) was dissolved in dichloromethane (6 % w/w) and emulsified in 1% poly(ethylene-alt-maleic anhydride) using a homogenizer (PolyTron 3100; Kinematica AG, Littau, Switzerland). Using D-sucrose (Sigma Aldrich), D-glucose (Sigma Aldrich), and dextran MW 100,000 (Sigma Aldrich), we made a sugar fiber. Initially, those were mixed at a ratio of 5.3:2.5:1 respectively by mass then were caramelized, cooled, and drawn from solution using a Pasteur pipette. These fibers were coated mixture of PLG microspheres and salt (with a 1:1 ratio), then pressed into a salt-lined aluminum mold. Next, it was equilibrated, and gas formed with high pressure CO2 gas (800 psi) for 16 h in a custom-made pressure vessel. The pressure was released over a period of 40 min, which fused adjacent microspheres to produce a continuous polymer structure. The bridges were cut into 1.2 mm sections, and the porogen was leached in water for 2 hours. The bridges were dried over-night and stored in a desiccator (Supplementary Fig. 1a).
2.2. Virus production and Virus loading into the multichannel bridges
Briefly, HEK293FT cells were co-transfected using lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G, with the gene of interest (pLenti-CMV-Luciferase or pLenti-CMV-Human IL-10 or pLenti-CMV-Human IL-4) using Lipofectamine 2000 (Life Technologies, Grand Island, NY) for 48 hours. All lentiviral vectors were designed through VectorBuilder (Santa Clara, CA, USA) (accession number NM_000589.3 for human IL-4, NM_000572.2 for human IL-10, and Firefly Luciferase as a chemiluminescent reporter [24]). Using PEG-it (System Biosciences, Mountain View, CA), supernatant was concentrated for 24 hours. Then it was precipitated using ultracentrifugation, resuspended in PBS and stored at −80 °C until use. We determined virus titers using Lentivirus qPCR Titer Kit (Applied Biological Materials, Richmond, Canada) and 2E9 IU/mL were used throughout the study. Before loading virus into the bridges, they were disinfected in 70% of ethanol and washed with distilled water then dried in room temperature. Initially, 2 μL of virus was added onto the bridge and incubated for 2 min for absorption into the pores. We performed these procedures 3 additional times then, bridges were stored at −80 °C deep freezer until in use.
2.3. Spinal cord hemisection injury model and animal care
All animal surgery procedures and animal care were conducted according to the Animal Care and use Committee guideline and University of Michigan. C57/BL6 female mice (6-8 weeks old, 20-25g; The Jackson Laboratory, Bar Harbor, ME, USA) were used to create hemisection SCI model. To assess the effects of anti-inflammatory cytokines on inflammatory responses and neuropathic pain after SCI, we investigated six treatment groups; Sham (laminectomy only), SCI only (without bridge implantation), Bridge alone, Bridge with control virus (firefly-luciferase encoding lentivirus, vCtrl), Bridge with IL-10 lentivirus (vIL-10), and Bridge with IL-4 lentivirus (vIL-4). Animals were anesthetized using isoflurane (2 %). The spinous process and the vertebral lamina were removed to expose the dorsal surface of T9-T10 level then a 1.2 mm long lateral of the midline spinal cord segment was removed to create a hemisection spinal cord injury model. Then, multichannel bridges were implanted in the injury site and covered using Gelfoam (Pfizer, New York, NY USA) (Fig. 1a). After surgery the animals were allowed to recover on a heating pad. For the postoperative animal care, Baytril (enrofloxacin 2.5 mg/kg SC, once a day for 2 weeks), buprenorphine (0.1 mg/kg SC, twice a day for 3 days), and lactate ringer solution (5 mL/100 g, once a day for 5 days) were administrated via subcutaneously. Bladder was manually expressed until bladder reflexive function was observed.
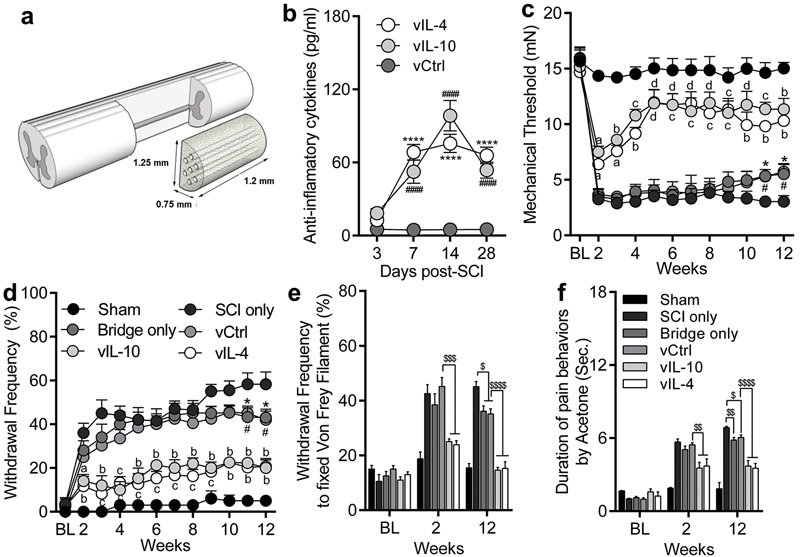
Fig. 1.
Delivered anti-inflammatory cytokines-encoding lentivirus from multichannel bridges attenuate neuropathic pain behaviors after SCI. (a) Hemisection SCI at T9-T10 and implantation of multichannel bridge. (b) IL-10 and IL-4 expression profiles over time in the spinal cord by lentiviral vectors delivery. (c) Mechanical allodynia by von Frey filament. (d) Thermal hyperalgesia by 100% acetone. Both mechanical and thermal hypersensitivities were downregulated by anti-inflammatory cytokines as a function of time. (e and f) The frequency of pain behaviors such as lifting, licking, shaking, or biting to fixed stimulus by von Frey filament (e) and the duration of pain behaviors by 100% acetone (f). A two-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where n=7/group. ####P<0.0001, and ****P<0.0001 compared to vCtrl (b), and n=18/group, aP<0.05, bP<0.01, cP<0.001, and dP<0.0001 compared to vCtrl, *P<0.05 bridge only vs. SCI only, and #P<0.05 vCtrl vs. SCI only (c and d), and $P<0.05, $$P<0.01, $$$P<0.001, $$$$P<0.0001, and n=18/group (eandf), mean±SEM.
2.4. Enzyme Linked Immunosorbent Assay (ELISA)
A 3 mm long section of spinal cord including bridge area was removed to measure the recombinant human IL-10 and IL-4 protein levels from transfected host cells and tissues, then homogenized in RIPA lysis and extraction buffer (Thermo Fisher Scientific, Waltham, MA, USA) with Halt Inhibitor Cocktail (Thermo Fisher Scientific). Next, the homogenized tissue samples were at 14,000 rpm for 30min at 4°C to remove tissue debris. Pierce BCA Protein Assay (Thermo Fisher Scientific) was used to estimate the protein concentration of each sample, and then samples were diluted accordingly. Human IL-10 and IL-4 levels were assayed by Human IL-10 Quantikine ELISA Kit (R&D system, Minneapolis, MN, USA) and Human IL-4 ELISA Kit (LSbio, Seattle, WA, USA) based on the manufacturer’s instructions. We measured the human IL-10 and IL-4 protein levels to eliminate any confounding results with the expression of murine IL-10 and IL-4 protein by delivered lentivirus. Both IL-10 and IL-4 ELISA kits allow for distinguishing human IL-10 and IL-4 from murine IL-10 and IL-4, since the antibodies were not cross reactive as reported by the manufacturer.
2.5. RNA isolation and cDNA microarray
To isolate RNA, the spinal cord tissues and dorsal root ganglia (DRGs) (L2-L6) were removed from all conditions. The spinal cord tissues were removed and cut into about 3 mm segments centered on 1.2 mm of bridge region; samples were not pooled. In briefly, samples were homogenized using 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) with a tissue grinder. RNA isolation was followed by chloroform extraction and isopropanol precipitation. The concentration of extracted RNA was measured using NanoDrop 2000C (Thermo Scientific, Newark, DE). For the cDNA microarray from spinal cord, gene analysis was conducted using the Mouse Gene ST 2.1 microarray platform (Affymetrix). Data was processed using the oligo R package. Raw output from the multi-array was converted to expression values through robust microarray averaging. Data quality was assessed by principle components analysis. Sample in each group was normalized to expression values from a vCtrl or sham group and log transformed. Probe set annotation was downloaded from BioConductor. Gene ontology (GO) accession number and analysis was performed using PANTHER [16].
2.6. Quantitative Reverse-Transcriptase PCR
We performed the quantitative Reverse-Transcriptase PCR (qRT-PCR) using spinal cord and DRGs for the gene expression analysis over time. cDNA was synthesized using iScript™ cDNA Synthesis kit (Bio-Rad, Hercules, CA). Primers were designed for qRT-PCR (Supplementary table 1). 18s-rRNA was used as an internal control [25]. The qRT-PCR products were measured using the accumulation level of iQ™ SYBR Green Supermix (Bio-Rad) fluorescence following a manufacturer’s protocol on CFX Connect™ Real-Time PCR Detection System (Bio-Rad). The gene expression level was normalized by the expression of 18s-rRNA and differences in gene expression were presented as fold ratios from control spinal cord or DRG samples. Relative quantification was calculated as X = 2 ™ΔΔCt, where ΔΔCt = ΔE−ΔCt and ΔE = Ct,exp − Ct,18s-rRNA, ΔCt = Ct, control − Ct,18s-rRNA [26].
2.7. Behavioral analyses
Mechanical hypersensitivity
The foot withdrawal threshold to mechanical stimuli by von Frey filaments was assessed as an indicator of mechanical sensitivity weekly for 12 weeks after SCI. The tests were conducted when the SCI animals support their body weight via their hindlimb (from 2 weeks post-SCI). The SCI mice were placed on top of metal mesh table and covered with a plastic box and acclimated to the test environment for at least 15 minutes. For mechanical stimulation, a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA) were applied perpendicular to the both sides of plantar surface of the hindlimb with sufficient bending force for 3-5 sec. then removed. The gauge of von Frey filaments was 0.04, 0.07, 0.16, 0.4, 0.6, 1, 1.4, and 2 grams. Mechanical stimuli were presented at 3-minute intervals. A brisk hindlimb withdrawal movement with or without licking and biting was considered as a positive response. In the event of positive response, the filament force immediately below the response was applied, and in the absence of a response, the filament of the next greater stimulus was presented. The mechanical threshold was calculated as described previously and was obtained by taking an average of both sides of the hindlimb [27]. The force was converted into millinewtons (mN). Withdrawal frequency to von Frey filaments was investigated using fixed-value (1 gram) of von Frey filament. The filament was applied five times to both sides of the hindlimb plantar surface at intervals of 3 minutes, then an average of frequency of positive responses on both sides was expressed as a percentage.
Thermal hypersensitivity
Hypersensitivity to cold stimulus was tested using acetone-mediated evaporative cooling effect based on previous study [28]. Under the same setting of mechanical sensitivity test, 50 μL of 100% acetone was applied to the both sides of hindlimb plantar surface. The acetone was applied five times to each plantar surface at intervals of 5 minutes and an average of positive responses to acetone was expressed as a percentage. Mechanical stimulation of the plantar surface was avoided during acetone application. In addition, the total pain behaviors such as lifting, shaking, licking, and biting time was recorded with maximum cutoff time of 30 sec. in response to acetone. To minimize the stress to the SCI animals, the order of the sensitivity tests was mechanical von Frey filament test then followed by acetone application assay. The behavioral tests order was kept the same throughout the experiment periods, and animals were allowed to rest at least 20 minutes in between mechanical and cold sensitivity tests. To establish the baseline (BL) for mechanical allodynia and thermal hyperalgesia, all animals were tested using von Frey filaments and 100% acetone at day 7 and 1 prior to the hemisection SCI.
2.8. Tissue processing and immunofluorescence
Spinal cord tissues (bridge implanted area and L2-L6) were collected at 4 and 12, and DRGs (L2-L6) were collected at 2 and 12 weeks after SCI at a time point when neuropathic pain behaviors had been established. Those tissues were then snap frozen in isopentane and embedded in Tissue Tek O.C.T compound (Sakura Finetek, Torrance, CA, USA) with 30% sucrose. Samples were cryosectioned in 18 μm transversely or in 13 μm longitudinally. Primary and secondary antibodies used are listed in Supplementary Table 2. The number of immunepositive cells were manually counted and done under blinded conditions. Multiple markers by co-staining was examined by evaluating pixel overlap of different channels in NIH ImageJ (Bethesda, MD, USA). Nonadjacent nine spinal cord tissues were selected randomly from each three distinct locations at caudal (0-400 μm), central (400-800 μm), and rostral (800-1200 μm) from the caudal edge of the bridge/tissue interface (total 27 tissues were assessed per animal) (Supplementary Fig. 1b). The total number of macrophages and pro-regenerative M2 macrophages within a bridge area were evaluated by determining Hoechst+/F4/80+ cells and Hoechst+/F4/80+/CD206+ cells. Macrophages were identified by localizing Hoechst+ staining to F4/80+ immunoreactive and labeling them in ImageJ. Then cell numbers were normalized to the counted area in each tissue section. To determine the percentage of neurons expressing nociceptor markers, twelve to fifteen nonadjacent DRG (L2-L6) sections per animal were labelled with nociceptive makers, then the numbers of nociceptor markers-positive cells in the lumbar levels of DRGs were compared by calculating the percentage of Hoechst+ cells that expressed specific nociceptive markers. For the quantitative assessment of immunostaining, we measured the immunofluorescence intensity within the medial superficial dorsal horn from twelve to fifteen nonadjacent randomly selected L2-L6 spinal cord sections per animal. To measure and quantify immunolabeled for CGRP, IB4, synaptophysin, TRPA1, TRPV1 and GFAP, immunofluorescence images were converted to black and white and applied a standardized optical density threshold to each image, then the amount of stained area measured using ImageJ and were normalized to the stained area in each tissue section. Spinal laminae within spinal dorsal horn and their boundaries were determined as described previously [29, 30] (Figs. 6 and 7). The area of the specific spinal dorsal horn (laminae II-IV; Fig. 7) was measured from twelve nonadjacent randomly selected L2-L6 spinal cord sections per animal. The distribution area of synaptic clusters relative to naive condition was investigated using a fixed box of 100 × 250 μm within the spinal dorsal horn (total twelve sampling boxes were investigated per animal), then were normalized to the stained area in naive condition. To evaluate regenerated ascending sensory fibers via bridge, three nonadjacent sagittal sections were selected randomly at between lamina I and out layer of lamina II [29, 30] from each subject and the area of regenerated ascending sensory axons within bridge was investigated at three distinct locations at caudal (0-400 μm), central (400-800 μm), and rostral (800-1200 μm) from the caudal edge of the bridge/tissue interface. Within this interval, CGRP+ area within a fixed box of 150 × 500 μm was measured from each location on sections from each animal of each condition, then were divided by the sampling box size to obtain mean axonal density (total nine sampling boxes were investigated per animal). All Tissues were imaged on an Axio Observer Z1 (Zesis, Oberkochen, Germany) using a 10x or 20x/0.45 M27 apochromatic objective and an ORCA-Flash 4.0 V2 Digital CMOS camera (C11440-22CU, Hamamatsu Photonics, Hamamatsu City, Shizuoka, Japan).
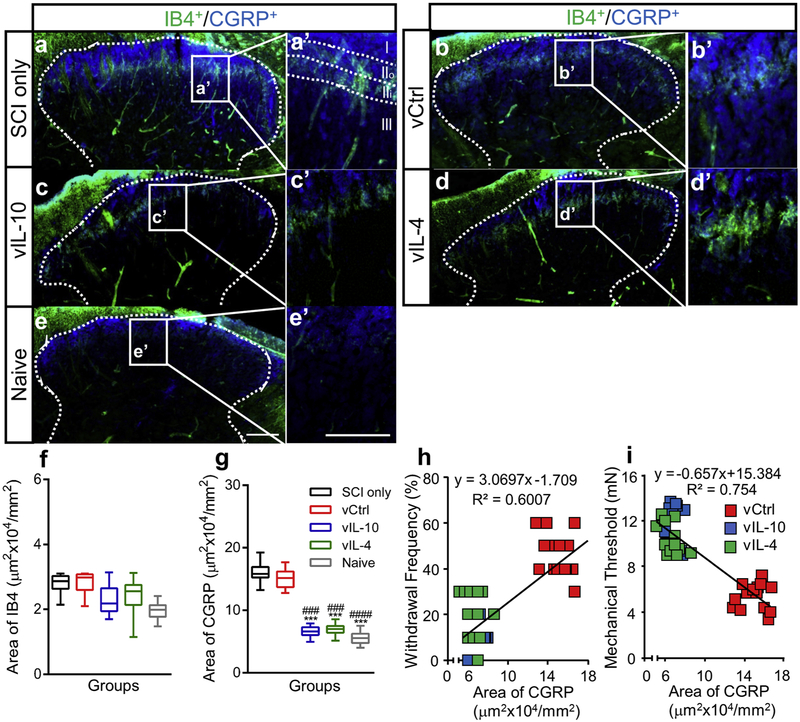
Fig. 6.
Anti-inflammatory cytokines limit abnormal projection of CGRP+ fibers into spinal dorsal horn. (a-e) Lumbar spinal cord sections from (a) SCI only, (b) vCtrl, (c) vIL-10, (d) vIL-4, and (e) naïve groups were labelled to show CRGP+ or IB4+ axons. (scale bar 100 μm), (a’-e’) High-magnification images of spinal dorsal horn laminae I-II (IIo; outer layer of lamina II, IIi; inner layer of lamina II, scale bar 100 μm) from figures a-e, indicating a normal-like lamina specific expression patterns in anti-inflammatory cytokines groups, (f and g) The area of (f) IB4+ and (g) CGRP+ axons were quantified within the spinal dorsal horn. A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where ***P<0.001 compared to SCI only and ###P<0.001, ####P<0.0001 compared to vCtrl, n=5/group. (h and i) The area of CGRP+ axons within the spinal dorsal horn was associated with the severity of thermal (h, Pearson Coefficient=0.775, P<0.0001) and mechanical (i, Pearson Coefficient=−0.8683, P<0.0001) hypersensitivities.
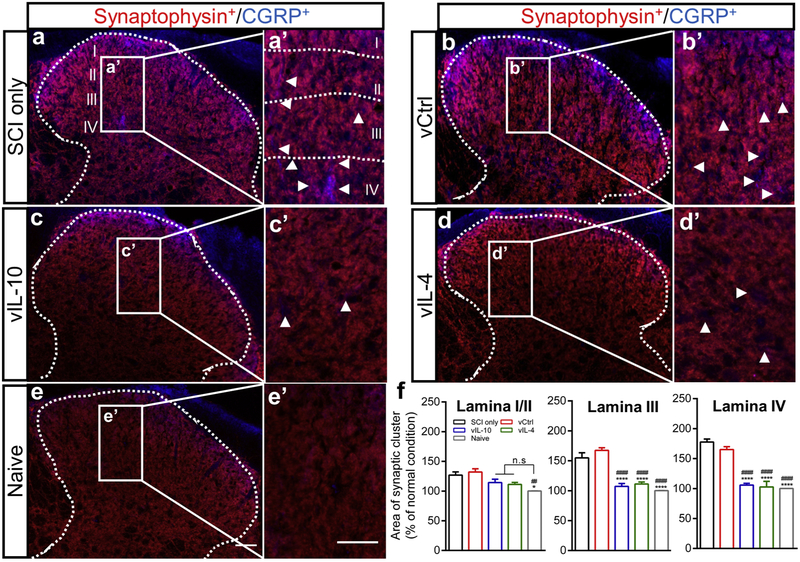
Fig. 7.
Restored-CGRP+ sensory fibers by anti-inflammatory cytokines form synaptic connections within spinal dorsal horn. (a-e) The spinal cord was stained using CGRP and synaptophysin from the chronic phase of SCI. Co-labelling for CGRP (Blue) and synaptophysin (Red) indicated the presence of synaptic clusters (Violet) associating with synaptic formations within dorsal horn in (a) SCI only, (b) vCtrl, (c) vIL-10, (d) vIL-4, and (e) naïve (scale bar 50 μm), (a’-e’) The insets indicated higher magnification within laminae I-IV from (a-e). The lower density of synaptic clusters (white arrowheads) was confirmed in vIL-10 and vIL-4 groups compared to vCtrl and SCI only groups scale bar=50 μm), (f) Graph showing the alterations of distribution of synaptic clusters within laminae I/II, lamina III, and lamina IV by anti-inflammatory cytokines. A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where *P<0.05 and ***P<0.001 compared to SCI only and #P<0.05 and ###P<0.001 compared to vCtrl, mean±SEM, n=5/group.
2.9. Statistical analysis
One or two-way ANOVA was used for multiple comparisons. For multiple comparisons, we performed Tukey’s post hoc testing. Type II errors were controlled at 0.2 level with following parameters: α=0.05, a conservative effect size of 0.25, to achieve reasonable statistical power analyses. Pearson’s correlation coefficient was used for determining the statistical significance of regression data. Equal variance (ANOVA Model) was validated and assumed for each study. Given the above parameters, power calculations, sample size and all statistical analysis were performed using G* Power Software, OriginPro (OriginLab Corporation, Northampton, MA, USA), and Prism 6 (GraphPad Software, La Jolla, CA, USA). P<0.05 was considered as statistically significant and the all values were expressed in mean±SEM.
3. Results
3.1. Sustained expression of anti-inflammatory cytokines alleviate neuropathic pain after SCI
Local transgene expression of IL-10 and IL-4 by delivery of lentiviral vectors from multichannel bridges in spinal cord tissues was assessed over time (Figs. 1a-1b). Delivered lentiviral vectors transduced host cells and enhanced a sustained expression of both IL-10 and IL-4 at the injury over time compared to vCtrl. Next, we performed mechanical and thermal hypersensitivity tests on both sides of the hindlimb plantar surface in order to determine whether expression of anti-inflammatory cytokines influences behavioral hypersensitivities following SCI. SCI-induced mechanical and thermal hypersensitivities were well established at 2 weeks after SCI and maintained until 12 weeks (Figs. 1c-1f). No statistical difference was observed for painful behaviors between bridge only and vCtrl conditions over time. However, the vIL-10 and vIL-4 groups exhibited a significant decrease in both mechanical and thermal hypersensitivities relative to vCtrl over time. Interestingly, neuropathic pain behaviors in both bridge only and vCtrl groups were substantially decreased compared to SCI only at week 11 to 12 post-SCI, indicating that the implanted-multichannel bridge itself also has a positive effect on amelioration of neuronal hypersensitivity. These data demonstrate that multichannel bridge implantation attenuates aberrant neural sensations following SCI, and that localized overexpression of antiinflammatory factors in combination with bridge implantation produces a synergistic effect.
3.2. Anti-inflammatory cytokines modulate pro-nociceptive microenvironment after SCI
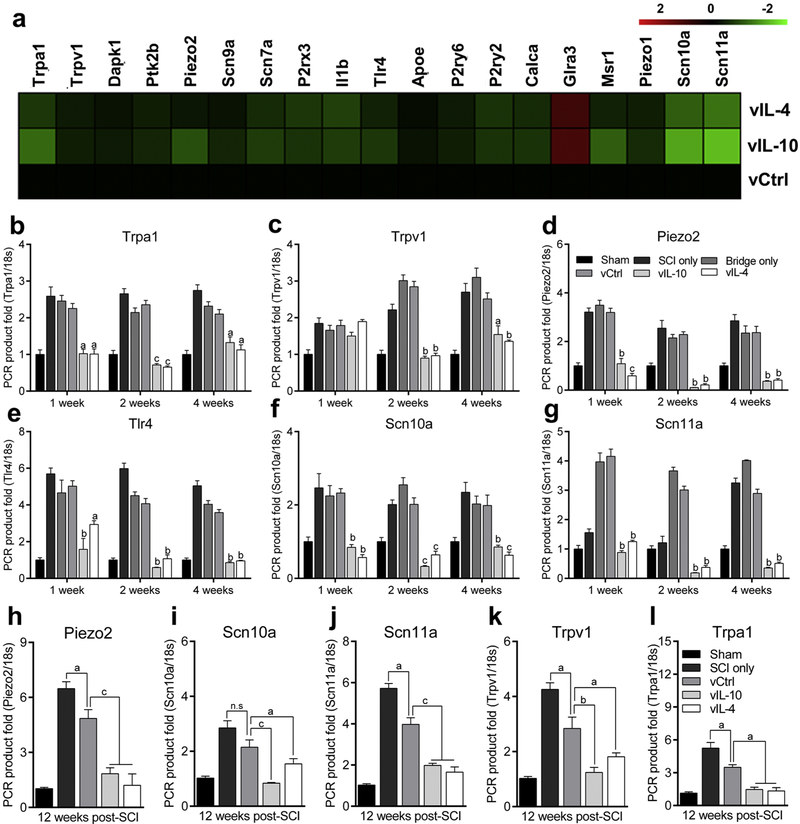
We subsequently investigated alteration in the transcriptome of Sensory Perception of Pain-associated gene ontology (GO:0019233) by anti-inflammatory cytokines through cDNA microarray at 1 week post-SCI in spinal cord. The expression levels in the spinal cord of pain-associated genes were decreased in anti-inflammatory groups relative to vCtrl (Fig. 2a). Subsets of genes within the microarray were validated using qRT-PCR at multiple time points (Figs. 2b-2g). We analyzed expression for Transient receptor potential ankyrin 1 (Trpa1) and vanilloid 1 (Trpv1), which are activated by pro-inflammatory factors and lead to neuronal hyperexcitability [31]. Pro-Inflammatory factors potentiate the Piezo2-mediated mechanical hypersensitivity after injury [32]. Toll-like receptor 4 (Tlr4) is expressed primarily by microglia and mediate inflammation leading to neuropathic pain [33]. Finally, a sodium ion channel subtype Nav 1.8 and Nav 1.9 are encoded by the Scn10a and Scn11a genes respectively and are activated by the pro-inflammatory factors resulting in neuronal excitability [34, 35]. The qRT-PCR results were consistent with the microarray data at 1 week, and their expressions were downregulated by vIL-10 and vIL-4 over time. We also characterized the long-term alteration in the expression of pro-nociceptive factors in the presence of anti-inflammatory cytokines (Figs. 2h-2l). Since no differences were observed between bridge only and vCtrl groups (Figs. 1c-1f, and 2b-2g), vCtrl was used for the comparisons. The expression of pro-nociceptive factors was significantly downregulated in response to vIL-10 and vIL-4 relative to vCtrl at 12 weeks post-SCI, while those levels were also decreased in vCtrl compared to SCI only groups. Collectively, these data indicate that anti-inflammatory cytokines with bridge synergistically reduce the expression of multiple pro-nociceptive genes for extended times in the spinal cord after SCI.
Fig. 2.
Anti-inflammatory cytokines modulate neuropathic pain-associated genes expression in the both acute and chronic phase of SCI. (a) cDNA microarray heat map for Sensory Perception of Pain-associated gene ontology (accession number: GO:0019233) in the spinal cord at a week post-SCI. vIL-10 and vIL-4 downregulated the levels of Sensory Perception of Pain-associated genes more than two-fold relative to vCtrl. (b-g) Selected genes from cDNA microarray (b) Trpa1, (c) Trpv1, (d) Piezo2, (e) Tlr4, (f) Scn10a, and (g) Scn11a were validated via qRT-PCR as a function of time from spinal cord, (h-l) Gene expression levels of (h) Piezo2, (i) Scn10a, (j) Scn11a, (k) Trpv1, and (l) Trpa1 were assessed using qRT-PCR at the chronic SCI phase. A two-way (b-g) or one-way (h-l) ANOVA with Tukey’s post hoc test for the multiple comparisons, where aP<0.05, bP<0.01, and cP<0.001 compared to vCtrl, mean±SEM, n=5/group and time point.
3.3. Anti-inflammatory cytokines influence macrophage polarization.
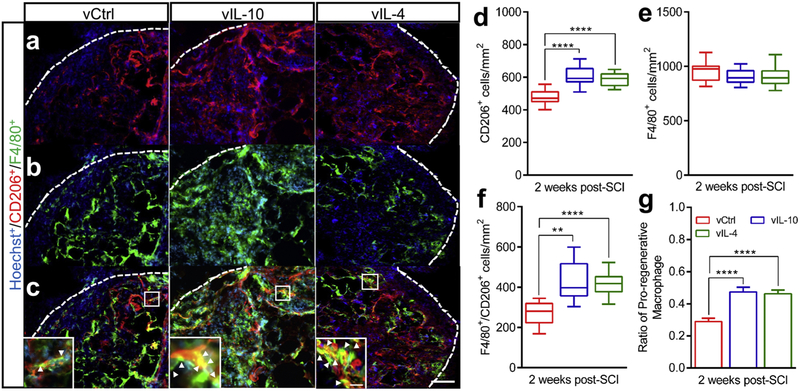
We next assessed macrophage polarization in the presence of IL-4 and IL-10, as macrophages play pivotal roles in inflammatory responses and neuronal sensitivities after SCI. Immunofluorescence staining was performed to evaluate the densities and phenotypes of total macrophages (Hoechst+/F4/80+), CD206 (mannose receptor)-expressing cells (Hoechst+/CD206+) and CD206-expressing pro-regenerative macrophages (M2, Hoechst+/F4/80+/CD206+) at 2 weeks post-SCI (Fig. 3). The number of CD206+ cells was significantly increased in the vIL-10 and vIL-4 groups compared to vCtrl (Figs. 3a and 3d). Furthermore, the number of pro-regenerative M2 macrophages was significantly increased in the anti-inflammatory groups relative to the vCtrl (Figs.3c and 3f). Importantly, no statistical differences were observed in the number of macrophages within the bridge for the all tested conditions (Figs. 3b and 3e). Therefore, the ratio of M2 to the total number of macrophages was significantly increased in anti-inflammatory groups compared to vCtrl (Fig. 3g). In addition, the expression of IL-10 and IL-4 was associated with the upregulation of pro-regenerative macrophages at the injury (Pearson coefficients; 0.8758 and 0.8278, and R2; 0.7671 and 0.6853 respectively, Supplementary Fig. 2a and 2b). Furthermore, qRT-PCR analysis results revealed that the expression of pro-regenerative markers were substantially upregulated in antiinflammatory cytokine groups compared to vCtrl in the chronic SCI phase (at 12 weeks post-bridge implantation) (Supplementary Figs. 2c-2f), suggesting that overexpression of antiinflammatory cytokines modulates macrophage differentiation and polarization at the injury.
Fig. 3.
The effects of anti-inflammatory cytokines on macrophage polarization after SCI. (a-c) The line indicates implanted bridge area for quantification (scale bar 300 μm). (a) CD206+/Hoechst+, (b) F4/80+/Hoechst+ and (c) Merge at 2 weeks after SCI. Insets: high-magnification image of bridge area within spinal cord shows pro-regenerative M2 macrophages (yellow, white arrow heads, scale bar 100 μm). (d) The quantitative analysis of CD206+ cells within the bridge. (e) The density of F4/80+ macrophages. (f) The intensity of pro-regenerative M2 and (g) the ratio of M2 to the total number of macrophages. A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where **P<0.01, and ****P<0.0001, mean±SEM, n=7/group, mean±SEM.
3.4. Anti-inflammatory cytokines restore expression of nociceptors in DRG
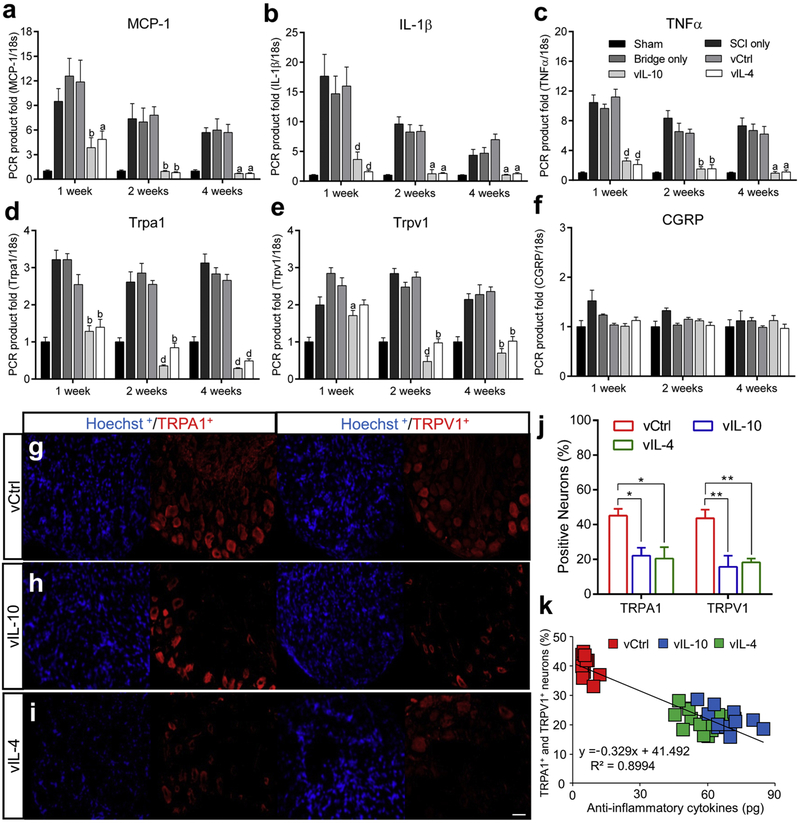
We next analyzed selected pro-nociceptive genes from Sensory Perception of Pain gene ontology (accession number; GO:0019233) in DRGs via qRT-PCR (Figs. 4a-4f). Both sides of the lumbar level of DRGs (L2-L6) were collected as the majority of sensory information from the hindlimb is transmitted to the spinal cord through the ipsilateral lumbar DRGs (L2-L6) [36]. The data revealed that neuropathic pain-associated genes levels with expression of antiinflammatory groups were significantly downregulated compared to vCtrl. Pro-nociceptive factors such as Monocyte chemoattractant protein-1 (MCP-1) [37], IL-1β [38]. Tumor necrosis factors (TNF)α [39], and Trpa1 and Trpv1 [28] were significantly decreased in vIL-10 and vIL-4 groups relative to control. However, no alterations were observed in the nociceptor calcitonin gene-related protein (CGRP) [36] gene level in all conditions. These data suggest that antiinflammatory cytokines also modulate the expression of pro-nociceptive factors in the DRGs after SCI. Next, we analyzed L2-L6 DRGs by immunofluorescence for TRPA1 and TRPV1 ion channels, which serve as key signal transduction integrators for pro-nociceptive factors (Figs. 4g-4i) [31, 40, 41]. Various pro-inflammatory mediators can directly modulate excitatory synaptic transmission through TRPA1 and TRPV1, for which activation and expression levels are significantly potentiated after SCI [27, 28]. We initially analyzed the alteration of TRPA1 and TRPV1 expression by vIL-10 or vIL-4 at 2 weeks post-SCI, a time that coincided with the emergence of sensory hypersensitivity (Figs. 1c-1f). The immunofluorescence demonstrated that overexpression of anti-inflammatory cytokines decreased the percentage of both TRPA1 + and TRPV1+ neurons in the DRGs (Fig. 4j). Furthermore, this expression correlated to the reduction of both TRPA1 and TRPV1 channels expression (Fig. 4k).
Fig. 4.
Anti-inflammatory cytokines downregulate the expression of nociceptors in the DRGs. (a-f) The gene expression level of pro-nociceptive factors (a) MCP-1, (b) IL-1β, (c) TNFα, (d) Trpa1, (e) Trpv1, and (f) CRGP were investigated from the DRGs (L2-L6) at multiple time points using qRT-PCR. A two-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where aP<0.05, bP<0.01, and dP<0.0001 compared to vCtrl, mean±SEM, n=5/group and time point, (g-i) DRGs sections (L2-L6) from (g) vCtrl, (h) vIL-10, and (i) vIL-4 were stained with anti-TRPA1, anti-TRPV1, and Hoechst at the acute SCI phase. (j) The proportion of TRPA1 or TRPV1 expressing neurons in all conditions. (k) Overexpression of anti-inflammatory cytokines was associated with the reduction of TRP channels expression in DRGs (P<0.0001, Pearson Coefficient=−0.9353). A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where *P<0.05and **P<0.01, n=7/group, mean±SEM, scale bar; 50 μm.
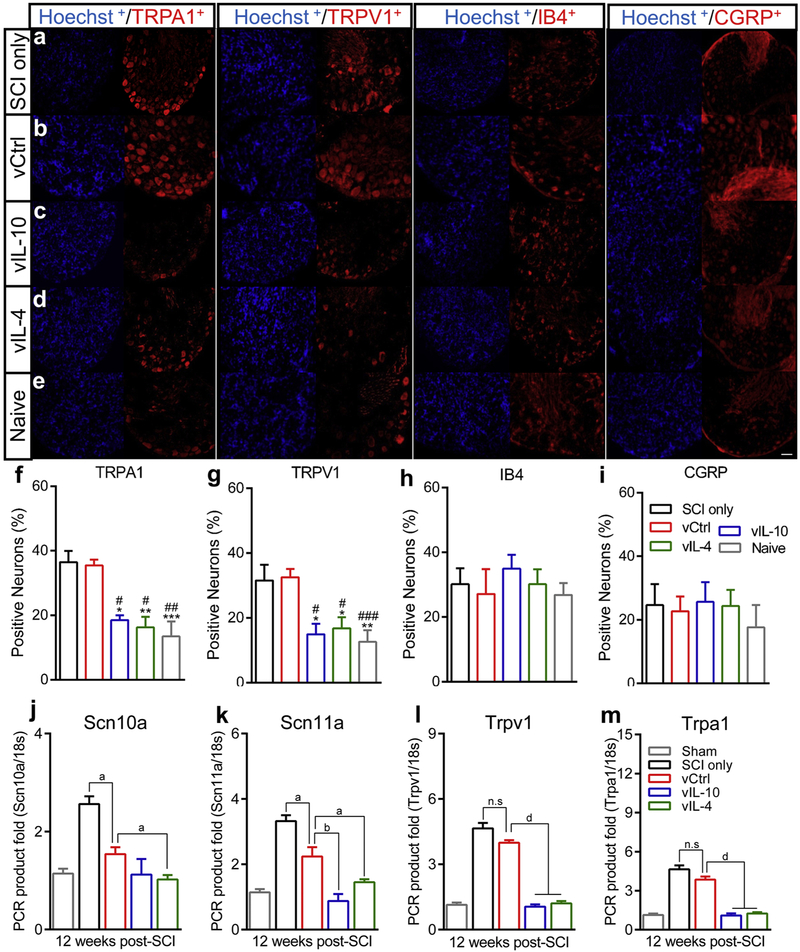
Analysis of immunofluorescence in the chronic phase of SCI indicated that the number of isolectin B4+ (IB4) nonpeptidergic and CGRP+ peptidergic nociceptors were similar between groups (Figs. 5a-5e and Figs. 5h and 5i), while the relative number of TRPA1+ and TRPV1+ neurons were decreased in anti-inflammatory cytokine groups compared to SCI only and vCtrl groups (Figs. 5f and 5g, and Supplementary Fig. 3a). No statistical differences were observed for the nociceptors expression between SCI only and vCtrl groups. The level of pro-nociceptive factors in the DRGs was next investigated through qRT-PCR in the chronic SCI phase (Figs. 5j-5m). The data demonstrated that the expression levels were substantially decreased in response to anti-inflammatory cytokines compared to vCtrl. However, gene expressions of nociceptors in vCtrl were downregulated relative to SCI only group. In agreement with immunofluorescence results, no differences were observed for Trpa1 and Trpv1 gene expression levels between SCI only and vCtrl from DRGs. Subsequently, we examined the colocalization of the nociceptor markers (TRPA1, TRPV1, CGRP, and IB4) in the chronic phase (Supplementary Figs. 3b-3f). The immunofluorescence revealed that both TRPA1+ and TRPV1+ neurons were colocalized with both peptidergic and nonpeptidergic nociceptive neurons in vCtrl group. In contrast, fewer TRPA1+ and TRPV1+ neurons were colocalized with CGRP+ or IB4+ neurons in vIL-10 and vIL-4 groups. Collectively, these data indicated that anti-inflammatory cytokines may downregulate nociceptors expression including TRPA1 and TRPV1 rather than IB4 and CGRP in DRGs, leading to amelioration of neuronal hypersensitivity.
Fig. 5.
Expression of nociceptors on DRGs in the chronic phase of SCI (12 weeks after SCI). (a-e) Lumbar level of DRGs (L2-L6) sections from (a) SCI only, (b) vCtrl, (c) vIL-10, (d) vIL-4, and (e) naïve groups were labelled with anti-TRPA1, anti-TRPV1, anti-lectin IB4, or anti-CGRP. (f and g) The proportion of TRPA1+ (f) and TRPV1+ neurons (g) were significantly decreased in response to vIL-10 and vIL-4 groups compared to SCI only and vCtrl groups. (h and i) The proportion of IB4+ (h) and CGRP+ neurons on DRGs (i). No alterations were observed between groups indicating that both IB4+ and CGRP+ neurons were unaffected by both SCI and anti-inflammatory cytokines. A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons, where *P<0.05, **P<0.01, ***P<0.001 compared to SCI only and #P<0.05, ##P<0.01, ###P<0.001 compared to vCtrl, mean±SEM, n=5/group, scale bar 50μm. (j-m) The expression of pro-nociceptive factors (j) Scn10a, (k) Scn11a, (I) Trpv1, and (m) Trpa1 levels were investigated via qRT-PCR from the chronic phase of SCI. A one-way ANOVA with Tukey’s post hoc test for the multiple comparisons. aP<0.05, bP<0.01, cP<0.001, and dP<0.0001, mean±SEM, n=5/group.
Neurons at an injury can release chemokines that contribute to sensitizing and stimulating nociceptors [12, 13, 37], and we thus investigated the expression of MCP-1, which has been implicated in enhancing nociceptive transmission after injury by attracting monocytes and T cells to the central nervous system (CNS) [37] (Supplementary Fig. 4). The gene expression levels of MCP-1 and its receptor Ccr2 were decreased in vIL-10 and vIL-4 relative to vCtrl over time (Fig. 4a, and Supplementary Fig. 4a). The immunofluorescence showed lower MCP-1+neurons in the DRGs for vIL-10 and vIL-4 conditions relative to vCtrl (Supplementary Figs. 4b-4e) and less MCP-1+ neurons were observed on CGRP+ nociceptors, yet many of them were colocalized with nonpeptidergic nociceptors (Supplementary Figs. 4f and 4g). In addition, MCP-1 and Ccr2 gene expression levels were decreased by anti-inflammatory cytokines in the chronic SCI phase. Furthermore, fewer MCP-1+ neurons were observed for anti-inflammatory cytokine groups compared to vCtrl (Supplementary Figs. 5a-5g).
3.5. Anti-inflammatory factors restore expression patterns of nociceptors within spinal dorsal horn.
We next investigated the distribution of CGRP+ nociceptive axons, as their collateral sprouting into deeper spinal dorsal horn is associated with pathogenesis of neuropathic pain [36, 42, 43]. Initially, the distribution pattern of CGRP+ and IB4+ axons was investigated in the chronic phase (Fig. 6). In SCI only and vCtrl groups, CGRP+ axons projected throughout the dorsal horn, while vIL-10 and vIL-4 restored a normal-like lamina specific expression pattern which was more confined to laminae I and II, resulting in a decreased area of CGRP+ axons within the spinal dorsal horn (Figs. 6a-6e and Fig. 6g). Consistent with a previous report [36], the distribution area of IB4+ nociceptive axons was unaffected by either SCI or anti-inflammatory cytokines (Fig. 6f). The relationship between the area of CGRP+ axons and neuropathic pain behaviors was investigated, and an increased area of CGRP+ axons correlated with greater thermal and mechanical hypersensitivities developed after SCI (Figs. 6h and 6i).
Reactive astrocytes upregulate long-term expression of pro-nociceptive factors, suggesting that these cells play a critical role in the maintenance of chronic neuropathic pain rather than in its pathogenesis [11, 12, 21]. We therefore analyzed the astrocytic response by immunofluorescence staining for glial fibrillary acidic protein (GFAP). GFAP-occupying area within the spinal dorsal horn was significantly reduced in anti-inflammatory factor groups compared to SCI only and vCtrl (Supplementary Fig. 6). Analysis of co-localization with TRPA1+ axons indicated a substantial overlap between TRPV1+ and CGRP+-axons (Supplementary Figs. 7a-7e). Both TRPA1- and TRPV1-occupying area were upregulated within the spinal dorsal horn in control groups, while expression was decreased in anti-inflammatory groups. However, in agreement with gene expression results (Figs. 2k and 2I), expression of TRPA1+ and TRPV1+ neurons in vCtrl was significantly downregulated compared to SCI only (Supplementary Figs. 7f and 7g). Collectively, anti-inflammatory cytokines influenced expression patterns of nociceptors within spinal dorsal horn, resulting in attenuation of abnormal neuronal sensitivity after SCI.
3.6. Restricted-CGRP+ axons by anti-inflammatory cytokines form synaptic connections in the spinal cord
Recovery from neuropathic pain behaviors by anti-inflammatory factors suggested that CGRP+-nociceptive axons may have been restricted and formed synapses with secondary neurons within the spinal dorsal horn [36]. To assess synaptic formation and distribution, CGRP and a presynaptic terminal marker synaptophysin were co-labelled, indicating the presence of synaptic clusters. In the SCI only and vCtrl groups, synaptic networks projected deep into the dorsal horn, with localization observed at lamina IV. However, anti-inflammatory groups displayed a synaptic distribution of CGRP+ axons sprouting that was similar to naive condition (i.e., localized to the superficial dorsal horn) (Figs. 7a-7e and Supplementary Fig. 8a). High magnification images indicated fewer identifiable synaptic clusters within deeper laminae (Figs. 7a’-7e’ and 7f).
Semaphorin-3A (Sema3A) is a repulsive guidance factor which contributes to preventing aberrant sprouting of primary sensory axons during development, resulting in lamina-specific projection of primary sensory axons in the spinal cord [36, 43]. Therefore, we investigated alteration in the Sema3A expression caused by anti-inflammatory cytokines via qRT-PCR. The gene expression results indicated that Sema3A expression was substantially upregulated within spinal dorsal horn for anti-inflammatory groups compared to vCtrl group in the chronic SCI phase (Supplementary Fig. 8b). These data indicated that anti-inflammatory cytokines limit aberrant CGRP+ axons sprouting into the spinal dorsal horn, and enhance formation of synaptic connections, reducing neuronal hypersensitivities after SCI.
3.7. Regeneration of ascending sensory axons into multichannel bridge with antiinflammatory cytokines
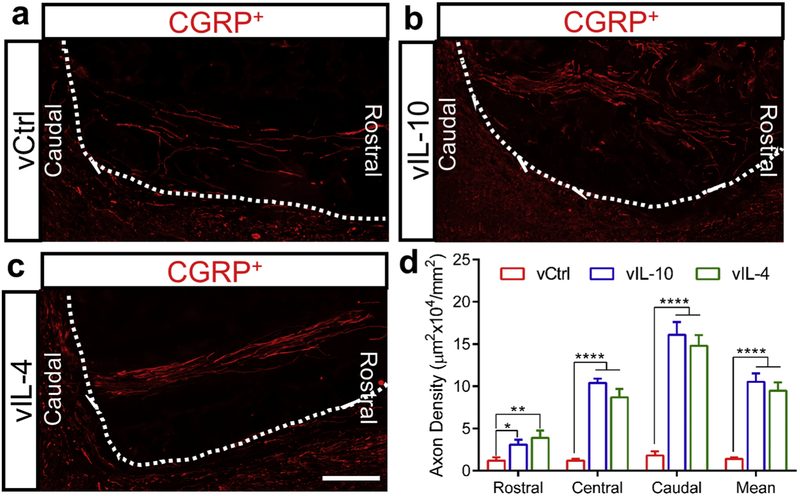
Sensory axonal systems in the spinal cord ascend into distinct functional fascicles, and we thus stained ascending CGRP+ sensory axons 4 weeks post-SCI to evaluate the effects of bridges with anti-inflammatory cytokines on axonal regeneration. Coronal sections were grouped based on 3 locations of the bridge; rostral, central, and caudal (Figs. 8a-8c, and Supplementary Fig. 1b). In vIL-10 and vIL-4 groups, sensory axons regenerated into channels and extended into the lesion, with large numbers of sensory axons present in all 3 regions of the bridge relative to vCtrl (Fig. 8d). No difference was observed between vIL-10 and vIL-4 groups. These results indicated that the multichannel bridge may synergize with the immunomodulation by anti-inflammatory cytokines to support axonal regeneration and organize the regenerating sensory axons.
Fig. 8.
Regeneration of ascending sensory fibers throughout multichannel bridges. (a-c) Regenerated sensory fibers (stained red with anti-CGRP) was investigated using longitudinally sectioned multichannel bridges from (a) vCtrl, (b) vIL-10, and (c) vIL-4 at caudal location (scale bar 200 μm), (d) Quantification of area of CGRP+ sensory axon was assessed at 3 distinct locations at 0-400 μm (Caudal), 400-800 μm (Central), and 800-1200 μm (Rostral) from the caudal edge of the bridge/tissue interface at 4 weeks post-bridge implantation. A Two-way ANOVA and Tukey’s post hoc test, *P<0.05, **P<0.01 and ****P<0.0001, mean±SEM, n=5/group.
4. Discussion
We investigated the potential for long-term local immunomodulation using lentiviral vectors delivery following SCI to attenuate neuropathic pain. The immune responses have numerous beneficial effects on wound healing and tissue regeneration, thus systemic immune suppression may have adverse effects yet in the context of SCI, it is one of the major contributors to neuropathic pain [12, 13]. After SCI, infiltrated-immune and glia cells begin to release pronociceptive factors such as pro-inflammatory cytokines (e.g. TNFα and IL-1β), chemokines (e.g. MCP-1), and reactive oxygen species (ROS) at every somatosensory level [11]. These factors activate N-methyl-D-aspartate (NMDA) receptors, and TRPA1 and TRPV1 channels to modulate synaptic transmission [44, 45], leading to release of excitatory neurotransmitters and transmission of excessive nociceptive information [41, 45]. In addition, the inflammatory milieu affects neighboring intact axons, involved in neuronal hypersensitivities. Furthermore, pronociceptive factors decrease inhibitory synaptic transmission (disinhibition) by reducing the release of inhibitory neurotransmitters such as γ-aminobutyric acid (GABA) and glycine from the interneurons, contributing to neuronal excitability [12, 38]
Sustained expression of IL-10 and IL-4 decreased expression of neuropathic pain-associated genes more than two-fold relative to vCtrl, with the downregulating persisting over time. Localized long-term expression of therapeutics to the injured spinal cord has been unavailable without additional surgery process. Implantation of intrathecal catheters and osmotic pumps have been widely used for continuous delivery of therapeutics. However, implantations inside the intrathecal space have been shown to generate the scar tissue and an additional injury [46, 47]. Recently, alternative methods such as intravenous or intramuscular injections have been employed to avoid additional tissue damage. Although these methods diminish the risk of tissue trauma, the localized delivery of therapeutics has been unavailable [48]. Furthermore, increased dosages can trigger an immune reaction [48, 49]. Due to short half-life and instability in circulation, repeated administrations of cytokines are required to achieve therapeutic effects. Systemic overexpression of IL-10 or IL-4 alone without bridge has been conflicting with some studies suggesting modulation of pro-inflammatory microenvironment with neuroprotection and others indicating impaired motor function with tissue damage, yet these reports have focused on immune responses over short-time periods [50, 51]. Consistent with our previous reports, IL-10 and IL-4 were induced by local delivery of lentiviral vectors to enhance transgene expression with measurable levels at day 3 post-implantation and significant upregulation relative to control at day 7 (Fig. 1b), and persisting for over 12 weeks at the injury [48, 52]. Lentiviral vector delivery is associated with a modest, transient inflammatory response, and has the potential to become infectious. Furthermore, lentiviral vector-infected cells have a risk of becoming oncogenic due to the insertion into the chromosomes, which can lead to activation of an oncogene or downregulation of tumor suppressor genes [53]. We did not observe any negative effects of lentiviral vectors in this report, and vectors are being used in clinical trials, with designs emerging to address the potential risks [54]. Previously, our gene ontologies analysis in IL-10 and IL-4 expression after SCI indicated that inflammation-associated gene ontologies were downregulated more than two-fold relative to control [16]. Herein, both IL-10 and IL-4 downregulate the production of pro-nociceptive factors until 12 weeks post-SCI that was consistent with an attenuation of neuropathic pain behaviors. The control groups demonstrated elevation of pro-inflammatory and neuropathic pain-associated factors after SCI, which coincided with the establishment of neuropathic pain behaviors. However, nociceptors expression levels in vCtrl for spinal cord were decreased compared to SCI only group in the chronic SCI phase (Fig. 2 and Supplementary Fig. 7), which may lead to alleviation of abnormal sensitivities in vCtrl relative to SCI only group (Figs. 1c-1f). Furthermore, in DRGs, the anti-inflammatory cytokines and bridges promoted long-term downregulation of pro-nociceptive factors gene expressions (Figs. 4 and 5), also resulting in attenuation of neural hypersensitivities. Although gene data indicated a trend toward decreased expressions of TRPA1 and TRPV1 in vCtrl compared to SCI only, no significant differences were observed. In agreement with histologic data (Fig. 5), CGRP gene level in DRGs was stable between groups over time. While the functional responses induced by IL-10 and IL-4 are similar, they may employ distinct pathways. IL-10 leads to a STAT3-mediated intracellular pathway resulting in anti-inflammatory cytokines release and suppressing nuclear factor-κB (NF-κB) activation and its binding to DNA to diminish pro-inflammatory factors synthesis including IL-1β and TNFα [55, 56]. IL-4 utilizes a STAT6-medicated pathway to suppress the interferon-γ (IFN-γ) signaling, leading to expression of anti-inflammatory factors [55, 56]. Furthermore, IL-4 attenuates release of inducible enzymes, which prevents inflammatory factors-medicated nitric oxide synthesis, leading to synthesis of anti-inflammatory factors [20, 56]. In addition, glia cells express receptors for both IL-10 and IL-4 in the CNS, where anti-inflammatory cytokines act as antagonists to inhibit release of pro-nociceptive factors from glia cells after injury [19, 57].
The long-term local immunomodulation by anti-inflammatory cytokines directs macrophage activation and polarization (Fig. 3). Within the intact spinal cord, the number of M1 and M2 macrophages are similar. However, in the injured spinal cord, the macrophage phenotype becomes largely pro-inflammatory without intervention with a decline in pro-regenerative macrophages and an increase in pro-inflammatory macrophages, the latter of which produce pro-nociceptive mediators. Consequently, this bias towards an inflammatory microenvironment may cause imbalance between pro- and anti-inflammation in the CNS resulting in transition from the acute to chronic neuropathic pain after SCI. Furthermore, the inflammatory microenvironment may enhance sensory neurons to grow abnormally, characterizing as short-distance and arborizing outgrowth [58, 59]. This type of aberrant axonal sprouting is considered to cause neuropathic pain after SCI [43, 59, 60]. In contrast, the neuronal outgrowth stimulated with pro-regenerative macrophages exhibit a unipolar or bipolar phenotype with long distance projecting even under growth-inhibitory conditions [59, 61]. Herein, we demonstrated that antiinflammatory cytokines enhanced the number of CD206+ cells and pro-regenerative macrophages within the bridges relative to vCtrl. Even though immunofluorescence showed a trend toward lower numbers of infiltrated F4/80+ macrophages in vIL-10 and vIL-4 at 2 weeks post-SCI relative to vCtrl, the increased number of F4/80+/CD206+ macrophages may be correlated with induction of a pro-regenerative microenvironment that is achieved by overexpression of anti-inflammatory cytokines, and a time frame after injury that coincides with the alleviation of neuropathic pain behaviors. Previous studies have shown that pro-regenerative macrophages produce both IL-10 and IL-4, potentially leading to a feed forward process when anti-inflammatory cytokines are overexpressed at the injury [17, 18]. In addition, long-term gene expression data suggest that delivered anti-inflammatory cytokines upregulate pro-regenerative factors within the bridges for at least 12 weeks post-SCI (Supplementary Fig. 2). These macrophages enhance clearance of necrotic debris via CD206-mediated way. CD206 binds to late stage apoptotic and necrotic cells and removes these cells without substantial cytotoxic byproducts such as oxidative metabolites [59]. Ym1 (or Chitinase-3-Like Protein 3) is upregulated with a pro-regenerative phenotype and inhibits leukocyte evasion at the injury preventing further inflammatory responses [62]. Found in inflammatory zone 1 (Fizz1) diminishes inflammatory reactions and prevents the extracellular-signal-regulated kinase (ERK) pathway-mediated neuronal apoptosis by caspase-3 and caspase-8 [16]. Arginase1 expression by pro-regenerative macrophages leads to overexpression of polyamines promoting axonal regeneration against a neural inhibitory environment after SCI [59, 63]. Moreover, these macrophages can produce opioid-mediated analgesic effects to ameliorate hypersensitivities [64]. Through these underlying mechanisms, pro-regenerative macrophages may facilitate remodeling of the inflammatory microenvironment and attenuate neuropathic pain after SCI.
Anti-inflammatory cytokines restore expression patterns of nociceptors within DRGs and spinal cord, leading to downregulation of neuronal hypersensitivities. Under pathophysiological conditions, TRPA1 and TRPV1 are upregulated and activated, contributing to release of excitatory neurotransmitters [31, 41, 65]. Endogenous pro-inflammatory mediators during immune responses after SCI activate TRPA1, resulting in neuronal hypersensitivities [25, 66]. TRPV1 is also activated by pro-inflammatory cytokines, which enhances TRPV1 membrane currents through protein kinase C (PKC)-mediated pathway and upregulates channel activation inducing neuronal excitability [41, 65]. Moreover, pro-inflammatory cytokines have been shown to mediate long-term upregulation of both TRPA1 and TRPV1 channels expression within DRGs and spinal dorsal horn. Pro-inflammatory cytokines such as TNFα activate NF-κB signaling, where transcription factor NF-κB binds to TRPA1 promoter region, increasing TRPA1 expression levels [41, 67]. Furthermore, oxidative metabolites through immune responses lead to stimulation of p38 and ERK pathway, resulting in upregulation of TRPV1 expression on sensory neurons [40, 41, 65]. Therefore, hyperactivity of TRPA1 and TRPV1 is a hallmark of persistent pathological states. Previous studies have demonstrated that anti-inflammatory mediators modulate expression level of TRP channels in DRGs and spinal cord resulting in attenuation of hypersensitivities [40, 66]. Herein, induced IL-10 and IL-4 may downregulate downstream signaling pathways, leading to attenuation of both TRPA1 and TRPV1 expression and translocation to the membrane surface of DRGs and spinal dorsal horn. In addition, overexpressed IL-10 and IL-4 persistently downregulate pro-nociceptive factors within spinal cord and DRGs (Figs. 2, 4 and 5). Furthermore, as pro-regenerative macrophages are increased by anti-inflammatory cytokines, they modulate inflammatory microenvironments, decreasing the expression of pro-inflammatory factors. SCI also induces delayed but persistent astrocytic activation, enhancing release of pro-inflammatory factors for an extended period, leading to imbalance between pro- and anti-inflammatory factors in the environment [11, 15]. It is speculated that astrocytic responses are initiated by pro-inflammatory factors, which upregulate ERK signaling pathway to activate astrocytes [68], enhancing secretion of various pro-nociceptive factors. This secretion is consistent with the significant alteration of GFAP expression in vCtrl relative to anti-inflammatory cytokine groups in the chronic SCI phase. Moreover, in addition to TRP channels, the greater expression of MCP-1+ neurons on the DRG were observed at 2 weeks in vCtrl, which also coincided with the emergence of pain behaviors after SCI, while their levels were downregulated for anti-inflammatory cytokine groups in the DRGs. These observations were maintained in the DRGs until 12 weeks. Even though the proportion of IB4+ and CGRP+ neurons were persistent in all conditions over time, the TRP channels and MCP-1+ neurons were less colocalized with IB4+ or CGRP+ nociceptors in vIL-10 and vIL-4 groups relative to vCtrl, indicating peripheral levels of IB4+ and CGRP+ neurons were unaffected by both SCI and anti-inflammatory cytokines.
Anti-inflammatory cytokines direct the termination patterns of nociceptive axons and facilitate appropriate synaptic connections within the spinal cord, resulting in attenuation of neuropathic pain after SCI. Collateral sprouting of CGRP+ nociceptive fibers into the deeper spinal dorsal horn has been associated with the pathogenesis of neuropathic pain [36, 42, 43]. This abnormal growth occurs both rostral and caudal to the injury, leading to the neuronal hypersensitivities. Therefore, restricting their termination to their normal synaptic location within the spinal cord may avoid or reduce neuropathic pain. As a guidance molecule, Sema3A has shown to prevent aberrant outgrowth of the CGRP+ fibers and helps to establish accurate reconstruction of lamina-specific projection within spinal dorsal horn [36, 42, 43]. In addition, Sema3A and its receptors are expressed on macrophages and act as potent immune-regulators during the immune responses, suggesting that Sema3A has a role in modulating inflammatory conditions [69, 70]. A recent study reported an upregulation of Sema3A and its receptors during M2 differentiation, while pro-inflammatory cytokines suppressed expression of Sema3A and its receptors [69]. Herein, we reported that delivery of anti-inflammatory cytokines downregulated expression of pro-inflammatory factors and promoted formation of pro-regenerative macrophages at the injury. These alterations may result in upregulation of Sema3A within the spinal cord, restricting atypical sprouting of CGRP+ nociceptive fibers and leading to suitable synaptic formations within spinal dorsal horn, which matches for normal physiological condition.
Supporting topographical guidance through multichannel bridges with long-term local immunomodulation by anti-inflammatory cytokines creates synergistic effects on axonal regeneration and linear outgrowth of ascending sensory tracts. Previously, we have shown that a naked PLG bridge provides a more permissive environment for infiltration of endogenous supportive cells, leading to release of factors to promote long-term axonal growth [23, 71]. In addition, descending motor tracts regenerated through the bridge and re-entered host tissue at the caudal end of the bridge, leading to locomotor recovery [72]. Furthermore, previous gene expression data indicated that the bridge itself upregulated synaptogenesis- and axonal guidance-related gene expressions over time [16]. These results support that the bridge has the potential to support nerve regeneration through modest modulation of inflammatory responses, leading to downregulation of expression levels of pro-nociceptive factors, thus alleviating neuronal excitabilities after SCI (Figs. 1c-1f). However, secondary inflammatory responses remain and contribute to neuronal cells death and neuronal hypersensitivities that may cause pathogenesis of neuropathic pain. Therefore, reducing inflammatory responses combined with inducing long-term expression of anti-nociceptive mediators may alleviate neuronal excitability after SCI. We have demonstrated that lentiviral delivery of anti-inflammatory cytokines has shown to increase axonal regrowth and remyelination and improve locomotor function after hemisection SCI [16, 17]. Structural neuroplasticity and/or spontaneous collateral sprouting of injured motor fibers lead to rewiring and strengthening the new synaptic connections, causing locomotor functional recovery after SCI [43, 73, 74]. However, maladaptive structural neuroplasticity is a main feature of the negative outcomes of SCI such as neuropathic pain [43]. Providing mechanical cues via aligned multichannel bridge, combined with anti-inflammatory cytokines organize the linear outgrowth of sensory track axons and increase probability to connect to appropriate functional fascicles beyond injury site, leading to attenuation of neuropathic pain after SCI.
5. Conclusion
Collectively, the data presented herein suggest that the sustained local expression of antiinflammatory cytokines by lentiviral delivery after SCI can dramatically modulate the inflammatory microenvironment, enhance the permissiveness of multichannel bridges, control macrophage polarization, and downregulate expression of nociceptors after SCI. Furthermore, aligned configuration of the multichannel bridge, combined with anti-inflammatory cytokines provide synergistic effects to establish appropriate synaptic connections into functional fascicles, resulting in attenuation of chronic neuropathic pain. The expression of anti-inflammatory cytokines by lentiviral vector delivery in combination with the bridge provides a tool for sustained and long-term localized expression of therapeutics and a guide to direct and orient tissue growth for functional regeneration. The results from this study support the potential for immunomodulation to balance immune responses for neuropathic pain treatment after SCI.
Supplementary Material
Acknowledgements
This study was supported by the National Institute of Health (R01EB005678). Authors thank Unit for laboratory Animal Medicine at University of Michigan for animal care and maintenance and Microarray core at University of Michigan for microarray analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Turner JA, Cardenas DD, Warms CA, McClellan CB, Chronic pain associated with spinal cord injuries: A community survey, Arch Phys Med Rehab, 82 (2001) 501–508. [DOI] [PubMed] [Google Scholar]
- [2].Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D, Oral opioid therapy for chronic peripheral and central neuropathic pain, N Engl J Med, 348 (2003) 1223–1232. [DOI] [PubMed] [Google Scholar]
- [3].O'Connor AB, Dworkin RH, Treatment of neuropathic pain: an overview of recent guidelines, Am J Med, 122 (2009) S22–32. [DOI] [PubMed] [Google Scholar]
- [4].Bostick GP, Toth C, Carr EC, Stitt LW, Morley-Forster P, Clark AJ, Lynch M, Gordon A, Nathan H, Smyth C, Ware MA, Moulin DE, Physical Functioning and Opioid use in Patients with Neuropathic Pain, Pain Med, 16 (2015) 1361–1368. [DOI] [PubMed] [Google Scholar]
- [5].Krashin D, Murinova N, Sullivan M, Challenges to Treatment of Chronic Pain and Addiction During the "Opioid Crisis", Curr Pain Headache Rep, 20 (2016) 65. [DOI] [PubMed] [Google Scholar]
- [6].Takeda K, Sawamura S, Sekiyama H, Tamai H, Hanaoka K, Effect of methylprednisolone on neuropathic pain and spinal glial activation in rats, Anesthesiology, 100 (2004) 1249–1257. [DOI] [PubMed] [Google Scholar]
- [7].Vyvey M, Steroids as pain relief adjuvants, Can Fam Physician, 56 (2010) 1295–1297. [PMC free article] [PubMed] [Google Scholar]
- [8].Donnelly DJ, Popovich PG, Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury, Exp Neurol, 209 (2008) 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].David S, Lopez-Vales R, Wee Yong V, Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications, Handb Clin Neurol, 109 (2012) 485–502. [DOI] [PubMed] [Google Scholar]
- [10].Park J, Lim E, Back S, Na H, Park Y Sun K, Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor, J Biomed Mater Res A, 93 (2010) 1091–1099. [DOI] [PubMed] [Google Scholar]
- [11].Calvo M, Dawes JM, Bennett DL, The role of the immune system in the generation of neuropathic pain, Lancet Neurol, 11 (2012) 629–642. [DOI] [PubMed] [Google Scholar]
- [12].Grace PM, Hutchinson MR, Maier SF, Watkins LR, Pathological pain and the neuroimmune interface, Nat Rev Immunol, 14(2014) 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marchand F, Perretti M, McMahon SB, Role of the immune system in chronic pain, Nat Rev Neurosci, 6 (2005) 521–532. [DOI] [PubMed] [Google Scholar]
- [14].Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S, Requirement of myeloid cells for axon regeneration, J Neurosci, 28 (2008) 9363–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang J, Peripheral and Central Immune Mechanisms in Neuropathic Pain, Neuroinflammation: New Insights into Beneficial and Detrimental Functions, (2015) 107–121. [Google Scholar]
- [16].Park J, Decker JT, Margul DJ, Smith DR, Cummings BJ, Anderson AJ, Shea LD, Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury, Mol Ther, 26 (2018) 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Margul DJ, Park J, Boehler RM, Smith DR, Johnson MA, McCreedy DA, He T, Ataliwala A, Kukushliev TV, Liang J, Sohrabi A, Goodman AG, Walthers CM, Shea LD, Seidlits SK, Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges, Bioeng Transl Med, 1 (2016) 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].David S, Kroner A, Repertoire of microglial and macrophage responses after spinal cord injury, Nat Rev Neurosci, 12 (2011) 388–399. [DOI] [PubMed] [Google Scholar]
- [19].Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A, The therapeutic role of interleukin-10 after spinal cord injury, J Neurotrauma, 30 (2013) 1311–1324. [DOI] [PubMed] [Google Scholar]
- [20].Luzina IG, Keegan AD, Heller NM, Rook GA, Shea-Donohue T, Atamas SP, Regulation of inflammation by interleukin-4: a review of "alternatives", J Leukoc Biol, 92 (2012) 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clark AK, Old EA, Malcangio M, Neuropathic pain and cytokines: current perspectives, J Pain Res, 6 (2013) 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, Cummings BJ, Anderson AJ, Shea LD, Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury, Biomaterials, 33 (2012) 1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD, Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration, Tissue Eng Part A, 20 (2014) 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gould SJ, Subramani S, Firefly Luciferase as a Tool in Molecular and Cell Biology, Anal Biochem, 175 (1988) 5–13. [DOI] [PubMed] [Google Scholar]
- [25].Park J, Zheng L, Marquis A, Walls M, Duerstock B, Pond A, Vega-Alvarez S, Wang H, Ouyang Z, Shi R, Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage, J Neurochem, 129 (2014) 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method, Methods, 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [27].Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, Shi R, Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat, J Neurochem, 128 (2014) 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Park J, Zheng L, Acosta G, Vega-Alvarez S, Chen Z, Muratori B, Cao P, Shi R, Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury, J Neurochem, 135 (2015) 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weng HR, Mansikka H, Winchurch R, Raja SN, Dougherty PM, Sensory processing in the deep spinal dorsal horn of neurokinin-1 receptor knockout mice, Anesthesiology, 94 (2001) 1105–1112. [DOI] [PubMed] [Google Scholar]
- [30].Sengul G, Puchalski RB, Watson C, Cytoarchitecture of the spinal cord of the postnatal (P4) mouse, Anat Rec (Hoboken), 295 (2012) 837–845. [DOI] [PubMed] [Google Scholar]
- [31].Moran MM, Mc Alexander MA, Biro T, Szallasi A, Transient receptor potential channels as therapeutic targets, Nat Rev Drug Discov, 10 (2011) 601–620. [DOI] [PubMed] [Google Scholar]
- [32].Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao BL, Coste B, Patapoutian A, Inflammatory Signals Enhance Piezo2-Mediated Mechanosensitive Currents, Cell Reports, 2 (2012) 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B, Glial TLR4 receptor as new target to treat neuropathic pain: Efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice, Glia, 56 (2008) 1312–1319. [DOI] [PubMed] [Google Scholar]
- [34].Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS, Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain, Eur J Pain, 12 (2008) 564–572. [DOI] [PubMed] [Google Scholar]
- [35].Wu DF, Chandra D, McMahon T, Wang D, Dadgar J, Kharazia VN, Liang YJ, Waxman SG, Dib-Hajj SD, Messing RO, PKCepsilon phosphorylation of the sodium channel NaV1.8 increases channel function and produces mechanical hyperalgesia in mice, J Clin Invest, 122 (2012) 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tang XQ, Heron P, Mashburn C, Smith GM, Targeting sensory axon regeneration in adult spinal cord, J Neurosci, 27 (2007) 6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA, Chemokines and pain mechanisms, Brain Res Rev, 60 (2009) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kawasaki Y, Zhang L, Cheng JK, Ji RR, Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1 beta, interleukin-6, and tumor necrosis factor-beta in regulating synaptic and neuronal activity in the superficial spinal cord, Journal of Neuroscience, 28 (2008) 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ogawa N, Kawai H, Terashima T, Kojima H, Oka K, Chan L, Maegawa H, Gene therapy for neuropathic pain by silencing of TNF-alpha expression with lentiviral vectors targeting the dorsal root ganglion in mice, PLoS One, 9 (2014) e92073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ji RR, Xu ZZ, Gao YJ, Emerging targets in neuroinflammation-driven chronic pain, Nat Rev Drug Discov, 13 (2014) 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patapoutian A, Tate S, Woolf CJ, Transient receptor potential channels: targeting pain at the source, Nat Rev Drug Discov, 8 (2009) 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG, Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia, J Neurosci, 26 (2006) 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brown A, Weaver LC, The dark side of neuroplasticity, Exp Neurol, 235 (2012) 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR, JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain, J Neurosci, 29 (2009) 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nishio N, Taniguchi W, Sugimura YK, Takiguchi N, Yamanaka M, Kiyoyuki Y, Yamada H, Miyazaki N, Yoshida M, Nakatsuka T, Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels, Neuroscience, 247 (2013) 201–212. [DOI] [PubMed] [Google Scholar]
- [46].Jones LL, Tuszynski MH, Chronic intrathecal infusions after spinal cord injury cause scarring and compression, Microsc Res Techniq, 54 (2001) 317–324. [DOI] [PubMed] [Google Scholar]
- [47].Ziemba AM, Gilbert RJ, Biomaterials for Local, Controlled Drug Delivery to the Injured Spinal Cord, Front Pharmacol, 8 (2017) 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Thomas AM, Palma JL, Shea LD, Sponge-mediated lentivirus delivery to acute and chronic spinal cord injuries, J Control Release, 204 (2015) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, Boulis NM, Federici T, Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery, Hum Gene Ther, 22 (2011) 1129–1135. [DOI] [PubMed] [Google Scholar]
- [50].Lima R, Monteiro S, Lopes JP, Barradas P, Vasconcelos NL, Gomes ED, Assuncao-Silva RC, Teixeira FG, Morais M, Sousa N, Salgado AJ, Silva NA, Systemic Interleukin-4 Administration after Spinal Cord Injury Modulates Inflammation and Promotes Neuroprotection, Pharmaceuticals (Basel), 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Takami T, Oudega M, Bethea JR, Wood PM, Kleitman N, Bunge MB, Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome, J Neurotrauma, 19 (2002) 653–666. [DOI] [PubMed] [Google Scholar]
- [52].Thomas AM, Seidlits SK, Goodman AG, Kukushliev TV, Hassani DM, Cummings BJ, Anderson AJ, Shea LD, Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury, Integr Biol (Camb), 6 (2014) 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO, Christiani DC, Mostoslavsky G, Diamond DV, Duane EG, Byers K, Winters T, Gelfand JA, Fujimoto G, Hudson TW, Vyas JM, Risks Associated With Lentiviral Vector Exposures and Prevention Strategies, J Occup Environ Med, 58 (2016) 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Milone MC, O'Doherty U, Clinical use of lentiviral vectors, Leukemia, 32 (2018) 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hanada T, Yoshimura A, Regulation of cytokine signaling and inflammation, Cytokine Growth Factor Rev, 13 (2002) 413–421. [DOI] [PubMed] [Google Scholar]
- [56].Wirjatijasa F, Dehghani F, Blaheta RA, Korf HW, Hailer NP, Interleukin-4, interleukin-10, and interleukin-1-receptor antagonist but not transforming growth factor-beta induce ramification and reduce adhesion molecule expression of rat microglial cells, J Neurosci Res, 68 (2002) 579–587. [DOI] [PubMed] [Google Scholar]
- [57].Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A, Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury, Mediators Inflamm, 2016 (2016) 9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gensel JC, Zhang B, Macrophage activation and its role in repair and pathology after spinal cord injury, Brain Res, 1619 (2015) 1–11. [DOI] [PubMed] [Google Scholar]
- [59].Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG, Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord, J Neurosci, 29 (2009) 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR, Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord, J Neurotrauma, 18 (2001) 1107–1119. [DOI] [PubMed] [Google Scholar]
- [61].Gordon S, Alternative activation of macrophages, Nat Rev Immunol, 3 (2003) 23–35. [DOI] [PubMed] [Google Scholar]
- [62].Roszer T, Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms, Mediat Inflamm, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT, Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro, Neuron, 35 (2002) 711–719. [DOI] [PubMed] [Google Scholar]
- [64].Pannell M, Labuz D, Celik MO, Keye J, Batra A, Siegmund B, Machelska H, Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides, J Neuroinflammation, 13 (2016) 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Devesa I, Planells-Cases R, Fernandez-Ballester G, Gonzalez-Ros JM, Ferrer-Montiel A, Fernandez-Carvajal A, Role of the transient receptor potential vanilloid 1 in inflammation and sepsis, J Inflamm Res, 4 (2011) 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen Z, Park J, Butler B, Acosta G, Vega-Alvarez S, Zheng LX, Tang J, McCain R, Zhang WP, Ouyang Z, Cao P, Shi RY, Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury, Journal of Neurochemistry, 138 (2016) 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kochukov MY, McNearney TA, Yin H, Zhang L, Ma F, Ponomareva L, Abshire S, Westlund KN, Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes, Mol Pain, 5 (2009) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ma W, Quirion R, Partial sciatic nerve ligation induces increase in the phosphorylation of extra cellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus, Pain, 99 (2002) 175–184. [DOI] [PubMed] [Google Scholar]
- [69].Ji JD, Park-Min KH, Ivashkiv LB, Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages, Hum Immunol, 70 (2009) 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Suzuki K, Kumanogoh A, Kikutani H, Semaphorins and their receptors in immune cell interactions, Nature Immunology, 9 (2008) 17–23. [DOI] [PubMed] [Google Scholar]
- [71].Yang Y, De Laporte L, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, Shea LD, Multiple channel bridges for spinal cord injury: cellular characterization of host response, Tissue Eng Part A, 15 (2009) 3283–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pawar K, Cummings BJ, Thomas A, Shea LD, Levine A, Pfaff S, Anderson AJ, Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function, Biomaterials, 65 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schnell L, Schwab ME, Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord, Eur J Neurosci, 5 (1993) 1156–1171. [DOI] [PubMed] [Google Scholar]
- [74].Helgren ME, Goldberger ME, The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways, Exp Neurol, 123 (1993) 17–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.