Abstract
Cognitive enhancements following a single bout of exercise are frequently attributed to increases in cerebral blood flow, however to date we have little understanding of the extent to which such bouts of exercise actually even influence cerebral blood flow following the cessation of exercise. To gain such insight, both regional and global changes in cerebral blood flow were assessed using 3D pseudo-continuous arterial spinlabeled magnetic resonance imaging in a sample of 41 preadolescent children. Using a within-participants randomized crossover design, cerebral blood flow as assessed prior to and following 20-minutes of either aerobic exercise or an active-control condition during two separate, counterbalanced sessions. The aerobic exercise condition consisted of walking/jogging on a motor driven treadmill at an intensity of approximately 70% of age-predicted maximum heart rate (HR = 136.1 ± 11.1 bpm). The active control condition consisted of walking on the treadmill at the lowest possible intensity (0.5 mph and 0% grade; HR = 92.0 ± 12.2 bpm). Findings revealed no differences in cerebral blood flow following the cessation of exercise relative to the active control condition. These findings demonstrate that cerebral blood flow may not be altered in preadolescent children following the termination of the exercise stimulus during the period when cognitive enhancements have previously been observed.
Keywords: Acute exercise, Arterial spin-labeling (ASL), functional magnetic resonance imaging (fMRI)
Over the past decade, there has been a growing focus on examining the effects of single bouts of exercise on cognition, with evidence generally observing enhancements in cognition following the cessation of the bout of exercise (Chang, Labban, Gapin, & Etnier, 2012; Lambourne & Tomporowski, 2010; Verburgh, Kӧnigs, Scherder, & Oosterlaan, 2014). In particular, cognitive processes generally clustered within the construct referred to as cognitive control appear to be disproportionately influenced following these single bouts of exercise. During the period 5 to 48 minutes following a 20–30 minute moderate intensity bout of aerobic exercise, enhancements in cognitive control operations have exhibited moderate-to-large effect sizes (0.4 to 0.9) in both preadolescent and young-adult populations (Drollette, Shishido, Pontifex, & Hillman, 2012; Pontifex, Hillman, Fernhall, Thompson, & Valentini, 2009; Sandroff, Hillman, Benedict, & Motl, 2016; Tomporowski et al., 2005; Weng, Pierce, Darling, & Voss, 2015). Similarly, acute bouts of exercise have been observed to alter neural responses to reward (Crabtree, Chambers, Hardwick, & Blannin, 2014; Masterson et al., 2018) and affect (Petruzzello & Tate, 1997). However, while the evidence base demonstrating the beneficial after effects of these single bouts of exercise continues to grow, we still have limited understanding of the neurobiological mechanisms that underlie this relationship.
One mechanism that has been attributed to underlie these post-exercise induced enhancements in cognition is increased cerebral blood flow (Pontifex et al., 2009). Given that cerebral blood flow at rest is regulated through a cascade of reflexive responses sensitive to neurogenic activity, metabolic processes, arterial blood gas concentration, as well as cardiac output (K. J. Smith & Ainslie, 2017), the supposition is that exercise might elicit — potentially through increased cardiac output and changes in the partial pressure of blood gasses —an increase in cerebral blood flow independent of the brain actually requiring additional blood flow. Thus, such exercise-induced increases in cerebral blood flow would thereby result in greater metabolic resource availability and waste clearing, which in turn might induce facilitations in cognitive processing and alter neural responses (Delp et al., 2001; Pereira et al., 2007; Vingerhoets & Stroobant, 1999). Indeed, a growing body of evidence using techniques such as Doppler ultrasonography and near infrared spectroscopy has demonstrated that exercise results in a curvilinear increase in cerebral blood flow in an intensity dependent fashion (Ogoh & Ainslie, 2009; Rooks, Thom, McCully, & Dishman, 2010; K. J. Smith & Ainslie, 2017). In adult populations, the greatest enhancements in cerebral blood volume and cerebral oxygenation occur during moderate to vigorous exercise intensities occurring around 60% of maximal oxygen uptake (Rooks et al., 2010; K. J. Smith & Ainslie, 2017), however children appear to exhibit a more muted response with similar increases in cerebral blood volume being elicited across a wider range of exercise intensities (Ellis et al., 2017). However, while the vast majority of work has focused on changes in cerebral blood flow during exercise, relatively little work has investigated the extent to which these increases in cerebral blood flow persist following the bout of exercise during the period associated with cognitive enhancements.
Although the measurement of cerebral blood flow during exercise has largely relied upon the use of measurement techniques such as Doppler ultrasonography and near infrared spectroscopy, the “gold standard” measurement technique for non-invasive assessments of whole-brain cerebral blood flow is arterial spin-labeled (ASL) MRI (Buckley, Parthasarathy, Grant, Yodh, & Franceschini, 2014; Goff, Buckley, Durduran, Wang, & Licht, 2010). Arterial spin-labeling bypasses the need for intravascular contrast agents or radioactive labeled tracers by using electromagnetically tagged arterial blood flowing towards the brain as an endogenous contrast agent to determine where blood perfuses in the brain (Goff et al., 2010). This approach therefore enables measurement of both global and regional modulations in cerebral blood flow, and has been found to be particularly effective in pediatric populations which exhibit greater cerebral blood flow than adult populations (Wang et al., 2003). In an initial proof of concept investigation, Smith and colleagues (J. C. Smith, Paulson, Cook, Verber, & Tian, 2010) examined the extent to which cerebral blood flow was modulated following a single bout of exercise in a sample of 5 college-aged adults. Following a 30 minute bout of moderately-intense aerobic exercise, resting-state cerebral blood flow was increased globally relative to pre-exercise. Yet regional analysis specifically focusing on the motor cortex revealed no exercise-induced modulations (J. C. Smith et al., 2010). Utilizing a larger sample size, MacIntosh and colleagues (2014) failed to observe any global changes in cerebral blood flow following a 20 minute bout of moderately-intense aerobic exercise in a sample of 16 college-aged adults. However, planned contrasts observed reductions in cerebral blood flow in gray matter 10 minutes following exercise relative to pretest (MacIntosh et al., 2014).
Some caution is warranted in interpreting the results of these previous investigations as neither study utilized a control condition or group to ensure that the effects were the result of exercise rather than the result of repeated exposure to the assessment. Although much of the acute-exercise and cognition literature has utilized passive control experimental conditions such as seated rest or reading (Drollette et al., 2012; Hillman, Snook, & Jerome, 2003; Pontifex et al., 2009; Pontifex, Parks, Henning, & Kamijo, 2015; Pontifex, Saliba, Raine, Picchietti, & Hillman, 2013), even postural changes have been demonstrated to impact cerebral blood flow (Olufsen, Tran, & Ottesen, 2004). Accordingly, the present investigation utilized a within-subjects repeated measures crossover design to examine resting-state cerebral blood flow prior to and following moderate intensity aerobic exercise relative to an active-control condition during two separate, counterbalanced sessions using an arterial spin labeled MRI approach.
Beyond the assessment of global or regional changes in cerebral blood flow on a structure-by-structure basis; the present investigation additionally sought to determine to what extent acute exercise might induce modulations in cerebral blood flow within functionally connected neural structures or networks proposed to be involved in cognitive control such as the left frontoparietal network, right fronto-parietal network, and executive control network. Given the dynamic state of these neural networks, it may be that the conflicting modulations in cerebral blood flow observed within the present literature are the result of summating cerebral blood flow across neural networks that are differentially modulated in response to exercise. By directly evaluating modulations in cerebral blood flow within empirically derived neural networks, and in response to both exercise and control conditions, a more precise understanding of the after-effects of single bouts of exercise on cerebral blood flow may be obtained.
Given the preliminary nature of the present investigation, the potential to detect the after-effects of single bouts of exercise on cerebral blood flow was maximized by utilizing a preadolescent population who demonstrate less intensity-related variation in their exercise induced cerebral blood flow response (Ellis et al., 2017) and in whom the arterial spin-labeled MRI technique has been demonstrated to be particularly robust (Wang et al., 2003), relative to adult populations. Given the acute exercise and cognition literature, it was hypothesized that cerebral blood flow would be increased following a 20 minute bout of moderate-intensity aerobic exercise within neural networks underling aspects of high-level cognitive operations, with no such changes observed for neural networks involved in motor control given the use of the active-control condition involving walking at the slowest intensity possible on a motor-driven treadmill.
Method
Participants
Forty-one typically developing preadolescent children (18 female; 10.2 ± 1.0 years) from the greaterLansing, Michigan region participated in this investigation. An original sample of 49 participants were assessed for eligibility, with 4 participants not meeting the inclusionary criteria (i.e., presence of ADHD, braces, or uncomfortable in small spaces) and 4 participants declining to participate in the MRI portion of the experiment. All participants provided written assent and their legal guardians provided written informed consent in accordance with the Institutional Review Board at Michigan State University. Further, all participants were reported as being free of any neurological disorder, psychological condition, previous history of head trauma, cardiovascular disease, physical disabilities, and indicated normal or corrected to normal vision. Demographic data is provided in Table 1.
Table 1.
Mean (SD) values for participant demographics
| Measure | All Participants |
|---|---|
| N | 41 (18 females) |
| Age (years) | 10.2 ± 1.0 |
| Tanner scale | 1.5 ± 0.6 |
| IQ | 112.6 ± 13.1 |
| Percent identifying as a race other than white | 19.5% |
| Percent identifying as American Indian or Alaska Native | 0% |
| Percent identifying as Asian | 2.4% |
| Percent identifying as Black or African American | 7.3% |
| Percent identifying as Native Hawaiian or Other Pacific Islander | 0% |
| Percent identifying as White or Caucasian | 75.6% |
| Percent identifying as More than One Race | 9.8% |
| Socioeconomic status | 0.8 ± 0.2 |
| Percent participating in free or reduced-price lunch | 14.6% |
| Household income as a percent of the federal poverty level (%) | 364.6 ± 130.8 |
| Age predicted heart rate max (bpm) | 198.8 ± 0.7 |
| Lowest observed heart rate (bpm) | 58.5 ± 10.5 |
Note: Socioeconomic status (ranging from 0 [lowest] to 1 [highest]) was computed as the mean across financial capital, human capital, and social capital domains based upon parental employment, participation in free or reduced price lunch, parental education, and family structure (Ensminger et al., 2000). Federal poverty level was computed using the U.S. Department of Health and Human Services 2016 poverty guidelines.
Imaging Data Acquisition
The MRI data acquisition was conducted using a GE 3T Signa® HDx MR scanner (GE Healthcare, Waukesha, WI) with an 8-channel head coil. During each scan session, higher-order shimming procedures were first carried out to improve magnetic field homogeneity. Then, a commercial 3D pseudo-continuous arterial spin-labeled (3D PCASL) (Alsop et al., 2015) pulse sequence implemented as a product in the GE scanner was used to quantify regional cerebral blood flow. This pulse sequence and the recommended parameters have been demonstrated to be reliable in both patients and healthy volunteers and are optimized to the GE scanner (Huang et al., 2013; Zeng et al., 2017). The sequence parameters were: fast spiral acquisition, 30 4-mm axial slices, time of echo (TE) = 9.83 ms, time of repetition (TR) = 4.52 s, 8 spiral arms with 512 sampling points per arm, effective resolution = 3.22 mm × 3.22 mm, reconstructed matrix size = 128 × 128, number of excitation (number of label-control pairs) = 3, field of view (FOV) = 22 cm × 22 cm, receiver bandwidth = ± 62.5 kHz, labeling duration = 1.45 s, saturation time = 2 s, and post labeling delay time = 1.525 s. A set of 180 T1weighted 1-mm3 isotropic volumetric inversion recovery fast spoiled gradient-recalled sagittal images (10 minute scan time), with cerebrospinal fluid (CSF) suppressed, was obtained to cover the whole brain with the following parameters: TE = 3.8 ms, TR of acquisition = 8.6 ms, time of inversion (TI) = 831 ms, TR of inversion = 2332 ms, flip angle = 8°, FOV = 25.6 cm × 25.6 cm, matrix size = 256 × 256, slice thickness = 1 mm, and receiver bandwidth = ± 20.8 kHz.
Imaging Data Processing
Cerebral blood flow maps were generated in the unit of ml of blood/100 g of tissue/min based on the equation recommended by Alsop and colleagues (Alsop et al., 2015) with the necessary scaling corrections recommended by the manufacturer (GE Healthcare) on the partial saturation of reference images, inversion efficiency, background suppression efficiency and the number of excitation. First, to account for any slight motion during the scan, the control-label perfusion weighted difference images were aligned linearly to the proton-density weighted images via the “3dvolreg” software in AFNI (Cox, 1996). The proton-density weighted images were then co-registered to the subject- and session specific T1-weighted images using asl_reg in FSL’s BASIL toolset, which is optimized for co-registration of CBF images to structural images with more anatomical detail (Chappell, Groves, Whitcher, & Woolrich, 2009). The resulting transformation was used to co-register the CBF maps to the T1-weighted image for each subject- and session using asl_reg. The global gray matter mask was in standard MNI152 space provided through the Harvard-Oxford atlas available within FSL. The network masks were also provided in standard MNI152 space through NITRC (www.nitrc.org/projects/genr/). Multiplication of the masks and each cerebral blood flow image then allowed for the computation of mean cerebral blood flow within the global gray matter and within each network mask for each session and condition. For whole-brain voxelwise analyses, asl_reg was used to warp each cerebral blood flow image into standard MNI152 space, and resulting images were masked using the MNI brain mask to remove non-brain tissues. Spatial smoothing of FWHM 5mm was then applied to cerebral blood flow images using FSL’s SUSAN (Smith & Brady, 1997) replicating the methods of MacIntosh and colleagues (2014). Smoothing was done to improve the signal to noise ratio of the spatially transformed images for between-subject analyses that assume each voxel was aligned across participants.
Procedure
Using a within-participants design, participants visited the laboratory on three separate days. The first day consisted of a paperwork and screening session where, following provision of informed assent/consent, participants were administered the two subtest Wechsler Abbreviated Scale of Intelligence, Second Edition (Wechsler, 2011) to estimate IQ and completed the Edinburgh Handedness Inventory (Oldfield, 1971) to determine hand dominance. Participants, in collaboration with their legal guardian, then completed the Tanner Staging System questionnaire (Taylor et al., 2001), a magnetic resonance screening form, and a health history and demographics questionnaire. Following completion of all paperwork, participants were given a 15-minute exposure in a high-fidelity mock-MRI, mimicking the bore size and sounds occurring during scanning.
Participants were then randomly counterbalanced into two different session orders (day 2: control, day 3: exercise or day 2: exercise, day 3: control; see Figure 1) to ensure that any observed effects were unrelated to the specific order in which participants received the exercise and control conditions. The exercise and control sessions occurred approximately 16.3 ± 17.8 days apart and at approximately the same time of day (1.8 ± 1.9 hours different between sessions) with all tests occurring between 9am and 5pm given evidence suggesting that differences in neural activation can occur as a function of time of day (Hasler, Forbes, & Franzen, 2014; Masterson, Kirwan, Davidson, & LeCheminant, 2016). Each session occurred on a day in which the participant had not participated in physical education or other structured physical activity. Of the 41 participants that were randomized, three participants discontinued participation after the second session (2 following the control condition, 1 following the exercise condition; see Figure 2). Consistent with previous investigations demonstrating exercise induced enhancements in cognition in this population (Hillman et al., 2009; Pontifex et al., 2013), the exercise experimental condition consisted of 20 minutes on a motor-driven treadmill at an aerobic exercise intensity of approximately 70% of age-predicted (205.8 – (0.685*Age)) (Robergs & Landwehr, 2002) maximum heart rate (HR = 136.1 ± 11.1 bpm). This intensity was equivalent to a fast walk or slow jog for the majority of participants. Given that cerebral blood flow is impacted by postural changes (Bode, 1991; Olufsen et al., 2004) and to maximize internal validity, an active-control experimental condition was used to reduce confounds related to body position, demand characteristics/expectancy, and locomotion patterns. This activecontrol condition consisted of 20 minutes on a motor-driven treadmill at the lowest possible intensity (0.5 mph and 0% grade; HR = 92.0 ± 12.2 bpm; see Table 2). A polar HR monitor (Model H7, Polar Electro, Finland) was used to measure HR throughout the test alongside OMNI ratings of perceived exertion (Robertson et al., 2000). To ensure that any observed effects were unrelated to experimenter interaction or non-exercise related stimuli, participants watched an emotionally neutral video (minutes 65 – 85 and 85 – 105 from Wonders of the Universe) (Wonders of the Universe, 2011) during the entire 20 minute experimental period for both the exercise and active-control conditions. Regional cerebral blood flow was assessed prior to each experimental condition (pre-test) and again as soon as possible following termination of the experimental condition and completion of all MRI localizer procedures (post-test; approximately 25 minutes following each experimental condition; see Table 2), with structural MRI scans conducted at the very end of post-test scanning. Prior to entry into the MRI, participants’ blood pressure was assessed in a seated position using an electronic blood pressure cuff (Omron, Kyoto, Japan). Heart rate and respiratory rate were assessed in a supine position at the start of each cerebral blood flow assessment using the peripheral gating and respiratory straps of the GE 3T Signa® HDx MR scanner (GE Healthcare, Waukesha, WI).
Figure 1.
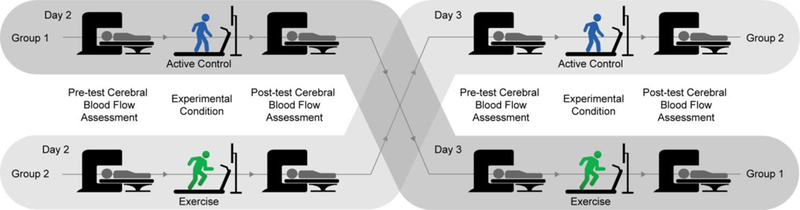
Diagram of the experimental procedure
Figure 2.
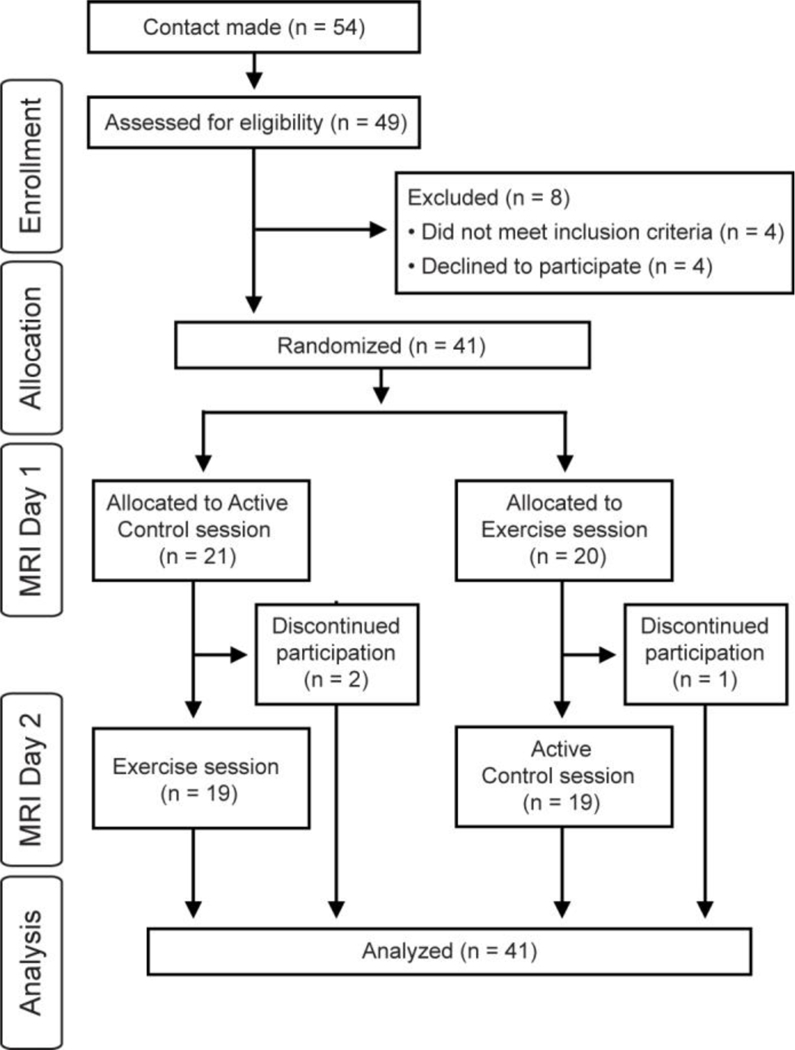
Consort flowchart
Table 2.
Mean (SD) values for the experimental sessions
| Measure | Active Control | Exercise | t | p |
|---|---|---|---|---|
| Pre-test Cerebral Blood Flow assessment | ||||
| Systolic blood pressure (mmHg) | 105.0 ± 19.2 | 106.7 ± 17.5 | 0.4 | 0.68 |
| Diastolic blood pressure (mmHg) | 71.5 ± 12.2 | 70.9 ± 17.9 | 0.2 | 0.85 |
| Time prior to experimental condition (min) | 42.1 ± 3.8 | 41.7 ± 5.9 | 0.3 | 0.77 |
| Heart rate (bpm) | 77.1 ± 11.4 | 76.2 ± 11.4 | 0.3 | 0.74 |
| Respiratory frequency (breaths perminute) | 20.5 ± 4.3 | 20.0 ± 4.3 | 0.5 | 0.60 |
| Experimental condition | ||||
| Heart rate (bpm) | 92.0 ± 12.2 | 136.1 ± 11.1 | 16.7 | < 0.001 |
| Heart rate reserve (%) | 23.2 ± 8.1 | 54.5 ± 8.0 | 16.7 | < 0.001 |
| Heart rate percent of max (%) | 45.7 ± 5.6 | 68.2 ± 5.5 | 17.4 | < 0.001 |
| Borg rating of perceived exertion | 1.3 ± 1.7 | 4.0 ± 2.4 | 5.8 | < 0.001 |
| Treadmill speed (mph) | 0.5 | 3.5 ± 0.4 | 47.0 | < 0.001 |
| Treadmill grade (%) | 0 | 1.7 ± 1.1 | 9.9 | < 0.001 |
| Post-test Cerebral Blood Flow assessment | ||||
| Systolic blood pressure (mmHg) | 104.8 ± 18.8 | 101.2 ± 16.3 | 0.9 | 0.39 |
| Diastolic blood pressure (mmHg) | 73.6 ± 17.1 | 71.0 ± 13.0 | 0.7 | 0.46 |
| Time following experimental condition (min) | 25.0 ± 3.6 | 25.3 ± 4.2 | 0.4 | 0.68 |
| Heart rate (bpm) | 75.2 ± 9.4 | 76.2 ± 9.8 | 0.5 | 0.63 |
| Respiratory frequency (breaths per minute) | 19.5 ± 3.0 | 19.9 ± 3.7 | 0.4 | 0.68 |
Note: t-tests reflect the difference between active control and exercise at each time point for each measure of interest. No statistical difference in blood pressure, heart rate, or respiratory frequency were observed between pre-test and post-test for either the active control or exercise conditions, t’s (37) ≤ 1.2, p’s ≥ 0.2, drm’s ≤ 0.16 [95% CI: −0.29 to 0.49]. Similarly, no interactions of Mode × Time were observed, F’s (1, 37) ≤ 1.5, p’s ≥ 0.2, f 2’s ≤ 0.46 [95% CI: 0.0 to 1.06]. Heart rate during both the active control and exercise conditions (measured in an upright position) was statistically different from heart rate at pre-test and post-test (measured in a supine position), t’s (73) ≥ 4.1, p’s < 0.001, drm’s ≥ 0.81 [95% CI: 0.39 to 5.23].
Statistical Analysis
As estimation of cerebral blood flow in white matter (i.e., myelinated axon tracts) has been shown to be unreliable (van Gelderen, de Zwart, & Duyn, 2008), all analysis were restricted to gray matter (i.e., neuronal cell bodies). Primary statistical analysis of cerebral blood flow was conducted on global gray matter as well as within previously identified pediatric resting-state fMRI networks (Muetzel et al., 2016). Specifically, analysis focused on networks (left frontoparietal network, right fronto-parietal network, and executive control network) underlying aspects of cognition previously observed to be enhanced following a single bout of exercise, with the motor network included in analysis as a control network. Analyses were conducted for global gray matter and in each of these specific regions with α = .05 and Benjamini-Hochberg false discovery rate control (d = 0.05) for post-hoc decompositions. Each analysis was conducted separately using a 2 (Mode: control, exercise) × 2 (Time: pre-test, post-test) univariate multi-level model including the random effects of Participant, Participant × Mode interactions, and Participant × Time interactions with Kenward-Roger degrees of freedom approximations. Analysis were performed using the lme4 (Bates, Mächler, Bolker, & Walker, 2015), lmerTest (Kuznetsova, Brockhoff, & Christensen, 2017), and emmeans (Lenth, Love, & Herve, 2017) packages in R version 3.4.0. This approach maximized experimental power by allowing participants with missing data (either as a result of withdrawal or motion artifact) to be retained within the analysis (final N = 41; control pre-test: missing 6 cases; control post-test: missing 7 cases; exercise pre-test: missing 5 cases; exercise post-test: missing 6 cases). Statistical findings using this approach were consistent with analysis only including participants with complete data across all time points. Preliminary analysis were conducted to examine the Pearson product moment correlation between the change in cerebral blood flow and age, sex, pubertal status, IQ, change in heart rate or blood pressure from pre- to post-test for either the exercise or active control conditions. As no associations were observed (p’s ≥ 0.09); these factors were not included as covariates within the analysis. For each inferential finding, Cohen’s d with 95% confidence intervals were computed as a standardized measure of effect size, using appropriate variance corrections for repeated-measures comparisons (drm; Lakens, 2013). Given a sample size of 41 participants and beta of .20 (i.e., 80% power), the present research design theoretically had sufficient sensitivity to detect t-test differences exceeding d = 0.395 (with a two-sided alpha) as computed using G*Power 3.1.2 (Faul, Erdfelder, Lang, & Buchner, 2007).
Additionally, secondary voxel-wise analyses of cerebral blood flow across the whole brain gray matter were conducted using a series of non-parametric t-tests with FSL’s randomise tool (Winkler, Ridgway, Webster, Smith, & Nichols, 2014). Given the moderate sample size, variance images used to estimate the tstatistics were smoothed at 5 mm FWHM using the –v option in randomise. All models were run with 5,000 permutations, and threshold-free cluster enhancement was used for voxel-based thresholding, with family-wise error corrected p-values of p < 0.01 considered statistically significant. As such models assume balanced designs, only participants without missing data (either as a result of withdrawal or motion artifact) could be included (N = 25). First, paired-samples non-parametric t-tests of the difference between Mode (control, exercise) in the change from pre- to post-test were conducted to test for a potential Mode × Time interaction. This was then followed by simple exploratory comparisons of time within each Mode, by running 1-sample t-tests to estimate where the change from pre- to post-test was greater than zero.
Results
Global Gray Matter Analysis
For analysis of global gray matter, a main effect of Time was observed, F(1, 37) = 33.2, p < 0.001, drm = 0.39 [95% CI: 0.23 to 0.55], with greater cerebral perfusion at pre-test (65.9 ± 6.8 ml/100g/min) relative to posttest (63.5 ± 6.4 ml/100g/min). No main effects of Mode or interactions of Mode × Time were observed, F’s (1, 34) ≤ 0.1, p’s ≥ 0.7, f 2’s < 0.01 [95% CI: 0.0 to 0.04] (see Table 3).
Table 3.
Statistical summary of post-hoc comparisons of cerebral blood flow (ml/100g/min) at pre-test relative to post-test for each region and mode.
| Pre-test | Post-test | t | p | drm [95% CI] | |
|---|---|---|---|---|---|
| Global Gray Matter | |||||
| Active Control | 65.7 ± 6.8 | 63.4 ± 6.9 | 4.0 | < 0.001 | 0.31 [0.15 to 0.47] |
| Exercise | 66.0 ± 6.8 | 63.6 ± 6.2 | 4.1 | < 0.001 | 0.39 [0.19 to 0.58] |
| Left Frontoparietal Network | |||||
| Active Control | 71.1 ± 7.6 | 68.3 ± 7.7 | 4.5 | < 0.001 | 0.33 [0.17 to 0.48] |
| Exercise | 71.3 ± 7.9 | 69.2 ± 7.6 | 3.3 | 0.001 | 0.29 [0.11 to 0.46] |
| Right Frontoparietal Network | |||||
| Active Control | 64.3 ± 7.3 | 61.9 ± 7.8 | 3.7 | < 0.001 | 0.31 [0.14 to 0.48] |
| Exercise | 64.8 ± 7.4 | 62.0 ± 7.3 | 4.3 | < 0.001 | 0.38 [0.20 to 0.56] |
| Executive Control Network | |||||
| Active Control | 64.2 ± 7.3 | 62.4 ± 7.1 | 3.0 | 0.004 | 0.26 [0.08 to 0.43] |
| Exercise | 64.2 ± 7.3 | 61.7 ± 7.5 | 4.3 | < 0.001 | 0.33 [0.17 to 0.48] |
| Motor Network | |||||
| Active Control | 53.2 ± 5.4 | 52.3 ± 5.7 | 1.5 | 0.1 | 0.16 [−0.06 to 0.38] |
| Exercise | 53.7 ± 5.8 | 52.2 ± 5.0 | 2.4 | 0.02* | 0.25 [0.04 to 0.46] |
Note: t-tests reflect the difference in cerebral blood flow between pre-test and post-test for each experimental condition for each neural region. The change in cerebral blood flow in the motor network in response to exercise did not remain significant following false discovery rate control (Benjamini-Hochberg critical alpha = 0.02). No statistical difference in cerebral blood flow was observed between the active control and exercise conditions for either pre-test or post-test, t’s (34) ≤ 0.6, p’s ≥ 0.5, drm’s ≤ 0.08 [95% CI: −0.21 to 0.32]. No interactions of Mode × Time were observed, F’s (1, 37) ≤ 0.9, p’s ≥ 0.3, f 2’s ≤ 0.03 [95% CI: 0.0 to 0.14].
Network Analysis
Across each of the networks examined (left frontoparietal network, right fronto-parietal network, executive control network, and motor network), analysis revealed a main effect of Time, F’s (1, 37) ≥ 7.3, p’s ≤ 0.01, drm’s = 0.25 [95% CI: 0.06 to 0.53], with greater cerebral perfusion at pre-test relative to post-test. However, for the motor network that main effect did not remain significant following false discovery rate control (Benjamini-Hochberg critical alpha = 0.01). No main effects of Mode or interactions of Mode × Time were observed, F’s (1, 34) ≤ 0.9, p’s ≥ 0.3, f 2’s ≤ 0.03 [95% CI: 0.0 to 0.14] (see Table 3).
Voxel-wise Analysis
Voxel-wise analysis of the whole brain gray matter observed no differences between control and exercise in the change in cerebral blood flow from pre- to post-test, p’s ≥ 0.1. Follow-up exploratory analysis of the change in cerebral blood from pre- to post-test following the control condition revealed six broad clusters demonstrating reductions in cerebral blood flow, with the strongest and most dispersed effects observed in the left inferior frontal gyrus, extending across lateral prefrontal cortex, superior temporal gyrus, planum temporale, insular cortex, angular gyrus, and inferior temporal cortex, and additional clusters in the anterior cingulate cortex and bilateral insular cortices, t’s ≥ 3.03, p’s ≤ 0.01 (see Table 4 and Figure 3). Follow-up exploratory analysis of the change in cerebral blood from pre- to post-test following the exercise condition revealed nine broad clusters demonstrating reductions in cerebral blood flow, with the strongest and most dispersed effects observed in the right temporal pole extending into planum temporale, superior temporal gyrus, angular gyrus, insular cortex, and right lateral frontal cortex, with effects also observed in the anterior cingulate cortex and bilateral anterior insular cortices, t’s ≥ 2.99, p’s ≤ 0.01 (see Table 4 and Figure 3).
Table 4.
Statistical summary of whole-brain voxel-wise comparisons of cerebral blood flow (ml/100g/min) at pre-test relative to post-test for each region and mode. Regions summarized below showed reduced cerebral blood flow from pre-to-post test.
| Number of Voxels | MNI coordinates (x,y,z) for peak |
t-statistic cluster mean |
Anatomical description of regions within cluster |
|---|---|---|---|
| Active Control | |||
| 6345 | −56,26,20 | 3.11 | Left inferior frontal gyrus, extending across lateral prefrontal cortex, superior temporal gyrus, planum temporale, insular cortex, angular gyrus, and inferior temporal cortex |
| 2821 | 68,−30,24 | 3.52 | Right supramarginal gyrus, extending into middle and superior temporal gyrus and temporal pole |
| 310 | 46,−8,6 | 3.03 | Right posterior insular cortex |
| 199 | 44,28,−2 | 3.52 | Right frontal orbital cortex extending into anterior insula |
| 197 | 8,46,2 | 3.66 | Bilateral medial prefrontal cortex, paracingulate gyrus |
| 40 | −48,−46,48 | 3.05 | Left supramarginal gyrus |
| Exercise | |||
| 8011 | 42,24,−24 | 3.04 | Right temporal pole extending into planum temporale, superior temporal gyrus, angular gyrus, insular cortex, and right lateral frontal cortex |
| 378 | 0,38,6 | 2.99 | Bilateral anterior cingulate cortex |
| 223 | −60,6,−2 | 3.4 | Left temporal pole extending into left insular cortex |
| 223 | −68,−26,2 | 3.54 | Left superior temporal gyrus extending into planum temporale |
| 199 | 10,−76,32 | 3.36 | Right precuneus cortex extending into intracalcarine cortex |
| 160 | 10,52,12 | 3.04 | Right medial prefrontal cortex, paracingulate gyrus |
| 77 | 44,−72,6 | 3.64 | Right lateral occipital cortex |
| 39 | −34,18,2 | 3.4 | Left anterior insula |
| 33 | −46,−12,4 | 3.46 | Left posterior insula |
Figure 3.
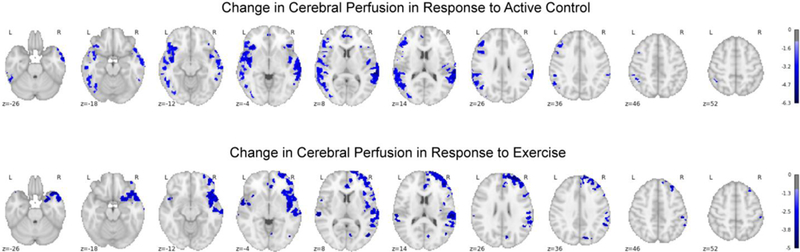
Voxel-wise plot of the family-wise error corrected t-statistics for the difference in cerebral blood flow at post-test relative to pre-test overlayed onto the MNI152 template, in response to the active control and exercise conditions.
Discussion
The aim of the present investigation was to provide greater insight into the extent to which a single bout of exercise might induce regional modulations in cerebral blood flow following the cessation of exercise — during the period that has been previously associated with cognitive enhancements in preadolescent children. In contrast to our a priori hypothesis that cerebral blood flow would be enhanced following the cessation of exercise in neural networks underlying aspects of high-level cognitive operations (left frontoparietal network, right fronto-parietal network, and executive control network); findings revealed that a single 20-minute bout of moderate intensity aerobic exercise was not associated with any differences in cerebral blood flow following exercise relative to following an active control condition. Rather, cerebral blood flow was found to decrease from pre-test to post-test across each of the neural networks assessed — as well as for global gray matter — for both exercise and control conditions. Similarly, exploratory whole-brain voxel-wise analysis of changes in cerebral blood flow from pre- to post-test revealed reductions in cerebral blood flow within the insular cortex, superior temporal gyrus, angular gyrus, anterior cingulate cortex, and the bilateral insular cortices. Although the pattern of change in cerebral blood flow observed in the exploratory whole-brain voxel-wise analysis were not identical between the moderate intensity aerobic exercise and active control conditions, no statistical differences between these two conditions were observed.
Findings from the present investigation are in agreement with the reductions in gray matter cerebral blood flow 10 minutes following a bout of moderate intensity aerobic exercise observed by MacIntosh and colleagues (2014). However, highlighting the importance of utilizing a control condition, findings from the present investigation demonstrate that such reductions in cerebral blood flow may not be the result of moderate intensity aerobic exercise in preadolescent children. It is important to note that the use of an active control experimental condition precludes ruling out ‘physical activity’ per se and/or locomotion as potentially inducing the reductions in cerebral blood flow observed within the present investigation. Nevertheless, as an active control condition, walking on the treadmill at the lowest-possible speed (0.5 mph) and grade (0%) satisfies two critical criteria: 1) it ensures that any differences were not the result of differences in body position between exercise and control conditions (Bode, 1991; Olufsen et al., 2004), and 2) it minimizes the potential influence of expectancy/demand characteristics whereby the outcome of the experiment may have been subconsciously altered by the participant to fit their hypothesized outcome given that they were recruited to take part in an exercise related study (Weber & Cook, 1972).
Although the physiological demands imposed by the active control condition (heart rate = 92 bpm [95% CI: 88.1 to 95.9]; heart rate reserve = 23.2 % [95% CI: 20.5 to 25.8]) technically fall within the tail end of the light physical activity classification (20 to < 40% of heart rate reserve) (American College of Sports Medicine, 2014), it is important to highlight that in this investigation resting heart rate was recorded in a supine rather than sitting position which may have artificially elevated the heart rate reserve calculations given the effect of body position on autonomic regulation (Watanabe, Reece, & Polus, 2007). Accordingly, the physiological demands of the active control condition based upon the mean heart rate are more consistent with activities of daily living such as domestic or academic tasks than with what is typically construed as ‘exercise’ (Norton, Norton, & Sadgrove, 2010). Although previous research in both children and adults has demonstrated that increases in cerebral blood flow during exercise exhibit a curvilinear relationship with the greatest increases occurring during moderate intensity exercise (Ellis et al., 2017; Rooks et al., 2010; K. J. Smith & Ainslie, 2017), it is important to note that children exhibit a more generalized increase in cerebral blood flow across a wide range of exercise intensity (Ellis et al., 2017). Thus, future research is necessary to examine the extent to which modulations in cerebral blood flow may persist following the cessation of exercise when examined relative to other suitable control conditions as well as in response to a sedentary condition. Nevertheless, the finding that gray matter cerebral blood flow was reduced following both the active control and exercise conditions warrants further examination. That is, at present it is unclear if such findings are the result of the motor-control demands involved with physical activity engagement — regardless of the intensity of the physical activity — or are the result of repeated assessments/acclimation to the MRI during each session. However, such an effect may be important to consider for investigations utilizing blood-oxygen-level dependent contrast imaging given that reductions in cerebral blood flow may alter background levels of activation.
Despite the methodological strength of the present investigation, there are a number of limitations that warrant further discussion. First and foremost is the timing of the cerebral blood flow assessment following exercise. Within the present investigation, cerebral blood flow was assessed during the period approximately 23 minutes following each experimental condition. Although this timing largely overlaps with previous investigations that have observed exercise-induced enhancements in cognition during this period (Drollette et al., 2012; Pontifex et al., 2009; Sandroff et al., 2016; Tomporowski et al., 2005; Weng et al., 2015), further research is necessary to determine how rapidly modulations in cerebral blood flow incurred during moderate intensity exercise return to baseline following the cessation of the exercise stimulus. It is important to point out, however, that the present investigation did not assess cerebral blood flow during exercise. Thus, while it is inferred that cerebral blood flow was enhanced during moderate-intensity exercise given this well-established finding (Rooks et al., 2010; K. J. Smith & Ainslie, 2017); further research is necessary to determine to what extent the magnitude of the cerebral blood flow enhancement during exercise as well as the intensity of the exercise bout impact upon how long after the cessation of exercise such modulations in cerebral blood flow persist.
Further research is also necessary in order to provide a greater understanding of the extent to which modulations in cerebral blood flow, both during and following exercise, are moderated by individual differences such as aerobic fitness. That is, a growing body of evidence in older adults has demonstrated that aerobic fitness is associated with greater resting-state cerebral blood flow (Ainslie et al., 2008; Brown et al., 2010). While such findings have not yet been demonstrated in preadolescent populations, such a higher baseline cerebral blood flow may influence the effects of a single bout of exercise. Although the research design of the present investigation largely mitigates the potential for other systematic bias to be introduced, further research should investigate the extent to which cerebral blood flow modulates in response to other factors such as sleep, caffeine consumption, and hydration status. Additionally, although the present investigation set the target exercise intensity based upon the percent of age-predicted maximal heart rate — consistent with methods employed previously in the acute exercise and cognition literature; further research is necessary to determine the extent to which modulations in cerebral blood flow may be observed by prescribing exercise intensity in relation to the percent of ventilatory threshold (Hall, Ekkekakis, & Petruzzello, 2010; Heck et al., 1985; Kashihara & Nakahara, 2005; McMorris, 2016). Such an approach would thus provide greater insight into the potential contribution of aerobic and anaerobic metabolism as they relate to modulations in cerebral blood flow.
Collectively, the present finding that cerebral blood flow may not be differentially modulated following the termination of the exercise stimulus exercise reduces the likelihood that cerebral blood flow may underlie postexercise induced enhancements in cognition and alterations in neural activation. That is, speculatively, it may be that cognitive enhancements/neural alterations following exercise are more strongly related to modulations in cerebral blood flow that occur during the bout of exercise. Similarly, although the focus of the present investigation was on cerebral blood flow, modulations in cognition/neural activity following exercise could also relate to a cascade of other cerebral vascular responses (Ogoh & Ainslie, 2009). Clearly then, further research in this area is necessary to better understand those cerebral vascular factors that are influenced by acute bouts of exercise and to what extent such modulations relate to the cognitive enhancements and neural alterations following the cessation of the exercise bout.
Highlights.
Examination of persistent modulations in cerebral blood flow following exercise.
Rigorous randomized within-subjects repeated-measures cross-over design.
Cerebral blood flow was not differentially modulated following exercise.
Acknowledgments
Support for the preparation of this manuscript was provided by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to Matthew Pontifex (R21 HD078566).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, … Atkinson G (2008). Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. The Journal of Physiology, 586, 4005–4010. 10.1113/jphysiol.2008.158279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, … Zaharchuk G (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73, 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2014). ACSM’s guidelines for exercise testing and prescription (9th ed.). New York: Lippincott Williams & Wilkins. [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [Google Scholar]
- Bode H (1991). Cerebral blood flow velocities during orthostasis and physical exercise. European Journal of Pediatrics, 150, 738–743. 10.1007/BF01958769 [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, & Poulin MJ (2010). Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging, 31, 2047–2057. 10.1016/j.neurobiolaging.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Buckley EM, Parthasarathy AB, Grant PE, Yodh AG, & Franceschini MA (2014). Diffuse correlation spectroscopy for measurement of cerebral blood flow: future prospects. Neurophotonics, 1 10.1117/1.NPh.1.1.011009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YK, Labban JD, Gapin JI, & Etnier JL (2012). The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research, 1453, 87–101. 10.1016/j.brainres.2012.02.068 [DOI] [PubMed] [Google Scholar]
- Chappell MA, Groves AR, Whitcher B, & Woolrich MW (2009). Variational bayesian inference for a nonlinear forward model. IEEE Transactions on Signal Processing, 57, 223–236. 10.1109/TSP.2008.2005752 [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Crabtree DR, Chambers ES, Hardwick RM, & Blannin AK (2014). The effects of high-intensity exercise on neural responses to images of food. The American Journal of Clinical Nutrition, 99, 258–267. 10.3945/ajcn.113.071381 [DOI] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, & Wilkerson MK (2001). Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. The Journal of Physiology, 533, 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drollette ES, Shishido T, Pontifex MB, & Hillman CH (2012). Maintenance of cognitive control during and after walking in preadolescent children. Medicine and Science in Sports and Exercise, 44, 2017–2024. 10.1249/MSS.0b013e318258bcd5 [DOI] [PubMed] [Google Scholar]
- Ellis LA, Ainslie PN, Armstrong VA, Morris LE, Simair RG, Sletten NR, … McManus AM (2017). Anterior cerebral blood velocity and end-tidal CO2 responses to exercise differ in children and adults. American Journal of Physiology-Heart and Circulatory Physiology, 312, H1195–H1202. 10.1152/ajpheart.00034.2017 [DOI] [PubMed] [Google Scholar]
- Ensminger ME, Forrest CB, Riley AW, Kang M, Green BF, Starfield B, & Ryan SA (2000). The validity of measures of socioeconomic status of adolescents. Journal of Adolescent Research, 15, 392–419. 10.1177/0743558400153005 [Google Scholar]
- Goff DA, Buckley EM, Durduran T, Wang J, & Licht DJ (2010). Noninvasive cerebral perfusion imaging in high-risk neonates. Seminars in Perinatology, 34, 46–56. 10.1053/j.semperi.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EE, Ekkekakis P, & Petruzzello SJ (2010). Predicting affective responses to exercise using resting EEG frontal asymmetry: Does intensity matter? Biological Psychology, 83, 201–206. 10.1016/j.biopsycho.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Hasler BP, Forbes EE, & Franzen PL (2014). Time-of-day differences and short-term stability of the neural response to monetary reward: A pilot study. Psychiatry Research: Neuroimaging, 224, 22–27. 10.1016/j.pscychresns.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck H, Mader A, Hess G, Mücke S, Müller R, & Hollman W (1985). Justification of the 4-mmol/l lactate threshold. International Journal of Sports Medicine, 6, 117–130. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, & Kramer AF (2009). The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience, 159, 1044–1054. 10.1016/j.neuroscience.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Snook EM, & Jerome GJ (2003). Acute cardiovascular exercise and executive control function. International Journal of Psychophysiology, 48, 307–314. 10.1016/S0167-8760(03)00080-1 [DOI] [PubMed] [Google Scholar]
- Huang D, Wu B, Shi K, Ma L, Cai Y, & Lou X (2013). Reliability of three-dimensional PseudoContinuous Arterial Spin Labeling MR imaging for measuring visual cortex perfusion on two 3T scanners. PLOS ONE, 8, e79471 10.1371/journal.pone.0079471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara K, & Nakahara Y (2005). Short-term effect of physical exercise at lactate threshold on choice reaction time. Perceptual and Motor Skills, 100, 275–291. 10.2466/pms.100.2.275-291 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26. 10.18637/jss.v082.i13 [Google Scholar]
- Lambourne K, & Tomporowski P (2010). The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Research, 1341, 12–24. 10.1016/j.brainres.2010.03.091 [DOI] [PubMed] [Google Scholar]
- Lenth R, Love J, & Herve M (2017). emmeans: Estimated marginal means, aka least-squares means Retrieved from https://github.com/rvlenth/emmeans
- MacIntosh BJ, Crane DE, Sage MD, Rajab AS, Donahue MJ, McIlroy WE, & Middleton LE (2014). Impact of a single bout of aerobic exercise on regional brain perfusion and activation responses in healthy young adults. PLoS ONE, 9 10.1371/journal.pone.0085163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson TD, Kirwan CB, Davidson LE, Larson MJ, Keller KL, Fearnbach SN, … LeCheminant JD (2018). Brain reactivity to visual food stimuli after moderate-intensity exercise in children. Brain Imaging and Behavior, 12, 1032–1041. 10.1007/s11682-017-9766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson TD, Kirwan CB, Davidson LE, & LeCheminant JD (2016). Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging and Behavior, 10, 68–78. 10.1007/s11682-015-9366-8 [DOI] [PubMed] [Google Scholar]
- McMorris T (2016). History of research into the acute exercise-cognition interaction: A cognitive psychology approach. In McMorris T (Ed.), Exercise-cognition interaction: Neuroscience Perspectives (pp. 1–22). London, UK: Academic Press. [Google Scholar]
- Muetzel RL, Blanken LME, Thijssen S, van der Lugt A, Jaddoe VWV, Verhulst FC, … White T (2016). Resting-state networks in 6-to-10 year old children. Human Brain Mapping, 37, 4286–4300. 10.1002/hbm.23309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton K, Norton L, & Sadgrove D (2010). Position statement on physical activity and exercise intensity terminology. Journal of Science and Medicine in Sport, 13, 496–502. 10.1016/j.jsams.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Ogoh S, & Ainslie PN (2009). Cerebral blood flow during exercise: mechanisms of regulation. Journal of Applied Physiology, 107, 1370–1380. 10.1152/japplphysiol.00573.2009 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Olufsen M, Tran H, & Ottesen J (2004). Modeling cerebral blood flow control during posture change from sitting to standing. Cardiovascular Engineering: An International Journal, 4, 47–58. 10.1023/B:CARE.0000025122.46013.1a [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, … Small SA (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences, 104, 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzello SJ, & Tate AK (1997). Brain activation, affect, and aerobic exercise: an examination of both state-independent and state-dependent relationships. Psychophysiology, 34, 527–533. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH, Fernhall B, Thompson KM, & Valentini TA (2009). The effect of acute aerobic and resistance exercise on working memory. Medicine & Science in Sport & Exercise, 41, 927–934. 10.1249/MSS.0b013e3181907d69 [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Parks AC, Henning DA, & Kamijo K (2015). Single bouts of exercise selectively sustain attentional processes. Psychophysiology, 52, 618–625. 10.1111/psyp.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, & Hillman CH (2013). Exercise improves behavioral, neurocognitive, and scholastic performance in children with ADHD. The Journal of Pediatrics, 162, 543–551. 10.1016/j.jpeds.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robergs RA, & Landwehr R (2002). The surprising history of the “HRmax=220-age” equation. Journal of Exercise Physiology Online, 5, 1–10. [Google Scholar]
- Robertson RJ, Goss FL, Boer NF, Peoples JA, Foreman AJ, Dabayebeh IM, … Thompkins T (2000). Children’s Omni scale of perceived exertion: Mixed gender and race validation. Medicine and Science in Sports and Exercise, 32, 452–458. [DOI] [PubMed] [Google Scholar]
- Rooks CR, Thom NJ, McCully KK, & Dishman RK (2010). Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: A systematic review. Progress in Neurobiology, 92, 134–150. 10.1016/j.pneurobio.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Sandroff BM, Hillman CH, Benedict RHB, & Motl RW (2016). Acute effects of varying intensities of treadmill walking exercise on inhibitory control in persons with multiple sclerosis: A pilot investigation. Physiology & Behavior, 154, 20–27. 10.1016/j.physbeh.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Smith JC, Paulson ES, Cook DB, Verber MD, & Tian Q (2010). Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: Implications for fMRI. Journal of Neuroscience Methods, 191, 258–262. 10.1016/j.jneumeth.2010.06.028 [DOI] [PubMed] [Google Scholar]
- Smith KJ, & Ainslie PN (2017). Regulation of cerebral blood flow and metabolism during exercise. Experimental Physiology, 102, 1356–1371. 10.1113/EP086249 [DOI] [PubMed] [Google Scholar]
- Smith SM, & Brady JM (1997). SUSAN—A New Approach to Low Level Image Processing. International Journal of Computer Vision, 23, 45–78. 10.1023/A:1007963824710 [Google Scholar]
- Taylor SJC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, & Cook DG (2001). Performance of a new pubertal self-assessment questionnaire: A preliminary study. Paediatric and Perinatal Epidemiology, 15, 88–94. 10.1046/j.1365-3016.2001.00317.x [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Cureton K, Armstrong LE, Kane GM, Sparling PB, & Millard-Stafford M (2005). Short-term effects of aerobic exercise on executive processes and emotional reactivity. International Journal of Sport and Exercise Psychology, 3, 131–146. 10.1080/1612197X.2005.9671763 [Google Scholar]
- van Gelderen P, de Zwart J. a., & Duyn J. h. (2008). Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine, 59, 788–795. 10.1002/mrm.21515 [DOI] [PubMed] [Google Scholar]
- Verburgh L, Kӧnigs M, Scherder EJA, & Oosterlaan J (2014). Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. British Journal of Sports Medicine, 48, 973–979. 10.1136/bjsports-2012-091441 [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, & Stroobant N (1999). Lateralization of cerebral blood flow velocity changes during cognitive tasks: A simultaneous bilateral transcranial doppler study. Stroke, 30, 2152–2158. 10.1161/01.STR.30.10.2152 [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Jahng G-H, Liu C-S, Rubin JT, Haselgrove J, … Detre JA (2003). Pediatric perfusion imaging using pulsed arterial spin labeling. Journal of Magnetic Resonance Imaging, 18, 404–413. 10.1002/jmri.10372 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Reece J, & Polus BI (2007). Effects of body position on autonomic regulation of cardiovascular function in young, healthy adults. Chiropractic & Osteopathy, 15, 19 10.1186/17461340-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SJ, & Cook TD (1972). Subject effects in laboratory research: An examination of subject roles, demand characteristics, and valid inference. Psychological Bulletin, 77, 273–295. 10.1037/h0032351 [Google Scholar]
- Wechsler D (2011). Wechsler abbreviated scale of intelligence (2nd ed.). San Antonio, TX: Pearson Assessments. [Google Scholar]
- Weng TB, Pierce GL, Darling WG, & Voss MW (2015). Differential effects of acute exercise on distinct aspects of executive function. Medicine and Science in Sports and Exercise, 47, 1460–1469. 10.1249/MSS.0000000000000542 [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, & Nichols TE (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders of the Universe (2011). BBC America. [Google Scholar]
- Zeng Q, Jiang B, Shi F, Ling C, Dong F, & Zhang J (2017). 3D Pseudocontinuous Arterial SpinLabeling MR imaging in the preoperative evaluation of gliomas. American Journal of Neuroradiology, 38, 1876–1883. 10.3174/ajnr.A5299 [DOI] [PMC free article] [PubMed] [Google Scholar]


