Abstract
Structural birth defects of the cerebellum, or cerebellar malformations, in humans, have long been recognized. However, until recently there has been little progress in elucidating their developmental pathogenesis. Innovations in brain imaging and human genetic technologies over the last 2 decades have led to better classifications of these disorders and identification of several causative genes. In contrast, cerebellar malformations in model organisms, particularly mice, have been the focus of intense study for more than 70 years. As a result, many of the molecular, genetic and cellular programs that drive formation of the cerebellum have been delineated in mice. In this review, we overview the basic epochs and key molecular regulators of the developmental programs that build the structure of the mouse cerebellum. This mouse-centric approach has been a useful to interpret the developmental pathogenesis of human cerebellar malformations. However, it is becoming apparent that we actually know very little regarding the specifics of human cerebellar development beyond what is inferred from mice. A better understanding of human cerebellar development will not only facilitate improved diagnosis of human cerebellar malformations, but also lead to the development of treatment paradigms for these important neurodevelopmental disorders.
Keywords: Cerebellar malformation, neurogenetics, development, model organism, mouse, pathogenesis, human
Introduction
In the last two decades tremendous advances have been made in defining the molecular and cellular programs that orchestrate cerebellar development. This recent progress has been almost exclusively based on genetic analysis of both human and mouse cerebellar malformations; where atypical development has resulted in cerebellar structure that is macroscopically abnormal in size, shape, and/or position.
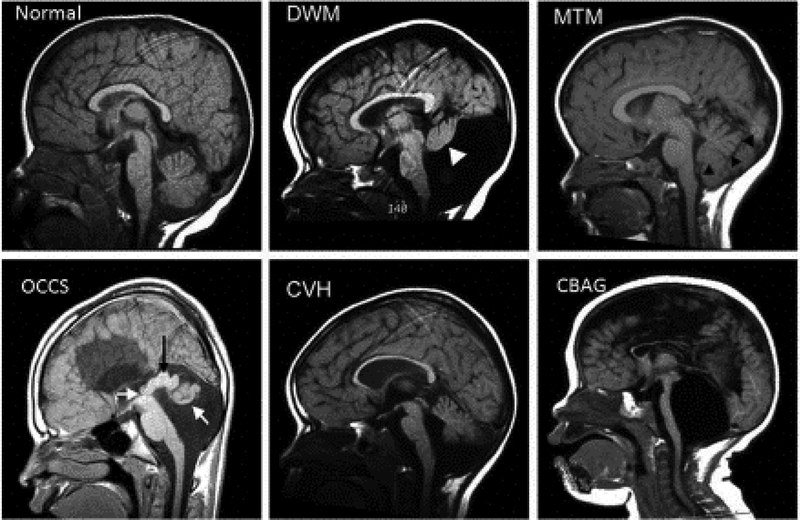
Malformations of the human cerebellum have long been recognized with initial human case reports relying solely on neuropathology (Fusari, 1891; Rossi, 1891; Rossi, 1892). However the advent of routine CT imaging, followed by MRI imaging, dramatically increased diagnosis of human brain malformations. From these studies, it is evident that cerebellar malformations collectively, are not rare, yet population-based prevalence data is difficult to ascertain since imaging studies are required for diagnosis and are variably performed based on clinical circumstances and available resources. It is also clear that cerebellar malformations are extremely heterogeneous and a multitude of malformations exist: from malformations that predominantly affect the cerebellum to those that affect the cerebellum and brainstem and malformations that also encompass multiple brain regions. Any or all of these cerebellar malformations can also include multiple other birth defects outside of the brain. However, with a focus on the cerebellum, specific definitions and classification systems have been devised (Barkovich et al., 2009). Cerebellar hypoplasia and Dandy-Walker malformation are likely the best recognized (FIGURE 1). Recent focus on genetic analyses of these human disorders aided by the rapid evolution of genetic technologies, has resulted in identification of numerous causative genes (Aldinger and Doherty, 2016).
Figure 1: Examples of Human cerebellar malformations.
Mid sagittal MRI views of human cerebellar malformations with an unaffected individual shown for comparison. Dandy-Walker malformation (DWM) with white arrowhead highlighting the small cerebellar vermis rotated away from the brainstem in an enlarged posterior fossa encompassing a very large fourth ventricle. Molar Tooth Malformation (MTM) with black arrowheads marking the edge of the small vermis with cerebellar hemispheres occupying the residual space in a normally sized posterior fossa. Occulocerebrocutaneous syndrome (OCCS) with left white arrow indicating the third ventricle and black arrow highlighting a massively enlarged tectum; right white arrow points to rudimentary cerebellum. Also note lack of corpus callosum. Cerebellar vermis hypoplasia (CVH). Cerebellar agenesis (CBAG); note reduced size of pontine nucleus of the small brain stem.
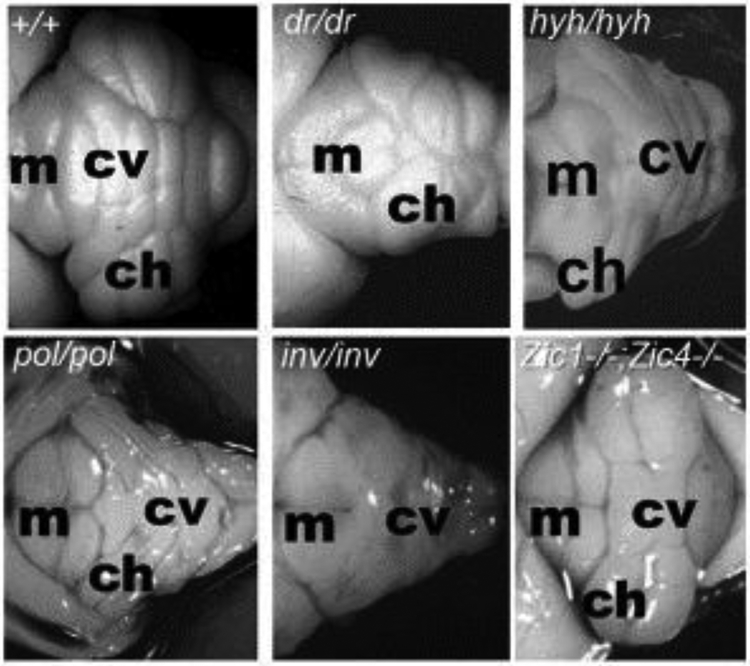
In parallel, phenotypic and genetic analysis of cerebellar malformations in mice (FIGURE 2), supplemented by classical embryological studies in avian and fish models laid the foundation upon which we are only now beginning to truly define the developmental pathogenesis of human cerebellar malformations. Historically, spontaneous mutations in mice have been valuable models of human disorders (Artzt, 2012). An early catalog established the first database of spontaneous neurological mutations of which at least four mutations had dramatic effect on cerebellar structural development (Sidman et al., 1966). The names of these mutant strains (reeler, weaver, staggerer and leaner) clearly reflect the obvious motor phenotypes also associated with the mutant alleles and point to the ease with which spontaneous mouse mutations affecting cerebellar function, including the subset that affect cerebellar structure can be identified in large breeding populations. As a result, a plethora of spontaneous cerebellar mutants have been documented. Studies of these spontaneous mutant mice revealed the first insights into cerebellar neurogenesis, migration and other developmental processes required to build the cerebellum. Later, cloning of the causative genes was facilitated by the availability of the mouse genome sequence, revealing the first molecular insights into the genetic programs driving these events. The advent and rapid evolution of genome engineering technologies (Singh et al., 2015; Joyner, 2016) has since enabled the generation of a multitude of new mouse models to specifically assess the function of any gene expressed during cerebellar development. Further, mouse mutants directly modelling human cerebellar disease mutations are readily constructed. Current mouse databases now list more than 800 spontaneous and targeted mutant alleles encompassing more than 450 genes (www.informatics.jax.org) with cerebellar developmental defects.
Figure 2. Examples of mouse cerebellar malformations.
Dorsal whole mount views of cerebellar malformations in 4 spontaneous mutants and 1 engineered mouse strain. Wild-type (+/+) cerebellum with cerebellar vermis (cv) and cerebellar hemispheres (ch) indicated showing stereotypical foliation pattern. Disruption of this patterning is obvious in many mouse mutant strains. For example, in dreher (dr) homygous mutants, a reduced cerebellar vermis causes juxtaposition of the cerebellar hemispheres. In hydrocephalus with hop gait (hyh) homozygous mutants, the vermis is more prominent than the hemispheres. Although these mice are not models for any specific human malformation, investigation of the underlying pathogenesis has provided insights into the role of the roof plate in cerebellar development and vermis formation. Polaris (pol) and inversus (inv) homozygous mutants have severely disrupted cerebellar morphology and are models for cilia related MTM human cerebellar malformations. Zic1/4 double homozygous mouse mutants model human DWM and display simplified vermis foliation. Anterior is to the left, indicated by the presence of midbrain colliculi (m). Photos are optimized to show pattering differences and are not all at the same magnification.
In this review, we present an overview of the basic epochs and key molecular regulators of the developmental programs that build the structure of the cerebellum based primarily on analysis in mice. We will then outline our current understanding of the developmental pathogenesis of several key human cerebellar malformations based on this model framework since there is considerable evolutionary conservation of cerebellar form and molecular programs across species. We will also highlight examples where significant species-specific differences in cerebellar phenotypes of comparable genotypes have emphasized that we still have much to learn about human cerebellar development. Indeed, some human cerebellar malformations remain unexplained by current mouse-centric developmental models. Finally, we will end our review discussing some known human-specific developmental features that predispose human cerebellar development to genetic and environmental insult underlining the importance of ongoing human fetal research.
Overview of cerebellar development from model organism studies
The cerebellum is a derivative of the anterior-most dorsal hindbrain, or dorsal rhombomere 1 (Martinez and Alvarado-Mallart, 1989b; Martinez and Alvarado-Mallart, 1989a; Hallonet and Le Douarin, 1993; Wingate and Hatten, 1999)(FIGURE 3). The boundary defining the anterior hindbrain from the more anterior midbrain is the first segmental division of the developing neural plate and forms due to activation of a gene cascade at neural plate stages culminating in juxtaposed expression of two key transcription factors; OTX2 (Orthodenticle Homeobox 2) and GBX2 (Gastrulation Brain Homeobox 2). Otx2 is expressed in the forebrain and midbrain, with its posterior limit at the presumptive mid/hindbrain boundary. Concurrently, Gbx2 is expressed in the posterior CNS, with an anterior boundary at the presumptive mid/hindbrain boundary. Loss of Otx2 shifts the mid/hindbrain boundary anteriorly, enlarging the cerebellar anlage at the expense of posterior midbrain tissue and loss of Gbx2 shifts the mid/hindbrain boundary posteriorly causing an expansion of the midbrain at the expense of cerebellar tissue (Hidalgo-Sanchez et al., 2005). The establishment of juxtaposed Otx2 and Gbx2 results in formation of a transient signaling center called the Isthmic Organizer (IsO) straddling the mid/hindbrain boundary. The IsO secretes Fibroblast Growth Family 8 (FGF8) and WNT1 which are required for cell survival, and pattern the adjacent tissue from e8–11.5 in mice (Sato and Joyner, 2009; Harada et al., 2016). Specifically, loss of Fgf8 or Wnt1 causes a deletion of tissue adjacent to mid/hindbrain junction, while modulating Fgf8 and Wnt1 activity levels influences cerebellar patterning. The posterior limit of the cerebellum is defined by Hoxa2 which is expressed in the caudal CNS with its anterior boundary at the rhombomere 1/2 boundary. Loss of Hoxa2 results in a caudal enlargement of the cerebellum at the expense of more posterior hindbrain structures (Gavalas et al., 1997). Ectopic Hoxa2 expression in rhombomere 1 suppresses the specification of cerebellar neurons (Mason et al., 2000; Eddison et al., 2004). Hoxa2 expression is normally excluded from rhombomere 1 via repression by FGF8 from the IsO (Irving and Mason, 2000; Mason et al., 2000).
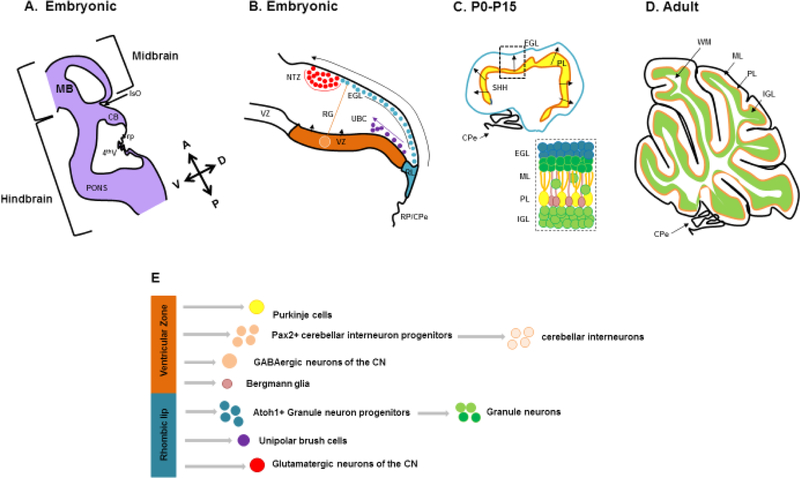
Figure 3 -.
(A) Schematic representation of an embryonic mouse cerebellum between e12.5-e18.5 (CB) sectioned along the sagittal plane. The cerebellum is derived from the dorsal region of rhombomere 1 (rh1) under the influence of signaling factors from the Isthmic organizer (IsO) and roof plate (RP). (B) A composite of embryonic developmental processes during embryogenesis. The developing cerebellum has two zones of neurogenesis, the ventricular zone (VZ) and the rhombic lip (RL). The cerebellar ventricular zone consists of a lining of radial glia (RG) and gives rise to all cerebellar GABAergic neurons and interneurons. GABAergic cerebellar nuclei neurons are produced first, followed by Purkinje cells and PAX2-expressing cerebellar interneuron progenitors. Bergmann Glia are also derived from the cerebellar ventricular zone. The rhombic lip on the other hand gives rise to the three major glutamatergic neuronal subtypes that populate the cerebellum. Firstly, cerebellar nuclei projection neurons migrate from the rhombic lip into the Nuclear Transitory Zone (NTZ) over the anlage as the rostral migratory stream. As embryonic development proceeds, granule neuron progenitors (GNPs) next migrate out of the rhombic lip between embryonic day 12.5 and 16. These cell progenitors migrate tangentially under the pial surface to establish the EGL of the developing cerebellum in an anterior to posterior manner. The RL also gives rise to unipolar brush cells (UBC) later in development, that migrate into the cerebellar anlage, (C) The EGL is a secondary germinal zone, or transit amplifying center. The EGL is composed of 2 sublayers – a proliferating external zone and an inner differentiating zone. Proliferation of GNPs takes place during postnatal days P0–P14. This proliferation is largely driven by the mitogen sonic hedgehog (SHH) secreted from Purkinje cells which have formed the Purkinje layer (PL) under the EGL. (D) Proliferation of GNPs in the EGL is responsible for the dramatic size increase of the post-natal mouse cerebellum. As granule neurons exit the cell cycle, they migrate tangentially within the inner EGL and then exit the EGL migrating radially inward to settle below the developing Purkinje cell layer to form the internal granule layer (IGL), resulting in the final laminar arrangement of the mature cerebellum. (E) Schematic representation of the multiple cell types that arise in the cerebellar ventricular zone and rhombic lip. Reference : Haldipur P., Dang D. and Millen K.J., (In press) Embryology. In: M. Manto and T.A.G.M. Huisman (Eds.) The cerebellum in children and adults. Elsevier, Amsterdam.
Much less is known regarding the mechanisms which define the dorsal coordinates of the cerebellum. However, the dorsal roof plate clearly plays a role. The roof plate is the single layer thick roof of the dorsal midline of the early neural tube which acts as another transient signaling center, expressing BMP and WNT secreted factors. The roof plate will eventually differentiate into the choroid plexus epithelium of the fourth ventricle. In rhombomere 1, roof plate derived Wnt expression is required to drive early cerebellar anlage ventricular zone proliferation, while secreted Bone morphogenetic protein (BMP) gene expression is required to induce the cerebellar rhombic lip and correctly pattern expression of Pancreatic transcription factor (Ptf1a) in the ventricular zone of the nascent cerebellar anlage (Mishima et al., 2009; Millen et al., 2014; Yamada et al., 2014). Loss of Ptf1a leads to transformation of cerebellar ventricular zone fates into more ventral brain stem fates (Millen et al., 2014). To date, Ptf1a is the sole known gene defining the ventral boundary of the cerebellar territory of rhombomere 1, although the molecular cascades that precisely regulate this ventral limit of Ptf1a cerebellar expression remain unknown.
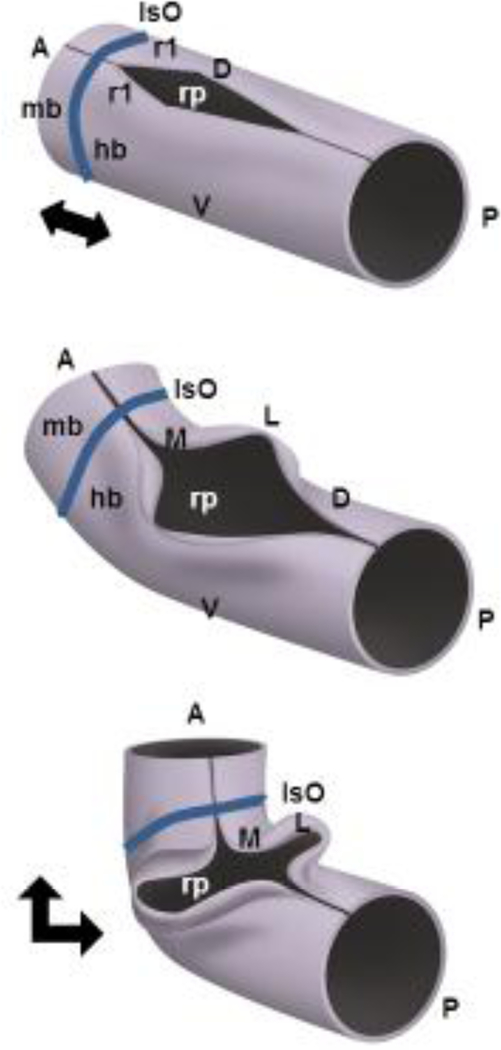
Once established, the developing cerebellar anlage undergoes a series of morphogenetic events between mouse embryonic (e) days 9 to e12.5 that rotate its anterior posterior axis by 90 degrees and convert it to the medio-lateral axis of the bilateral cerebellar wings (Sgaier et al., 2005) (FIGURE 4). In large part, this is driven by the pontine flexure of the developing neural tube. Prolific cell division of the neurepithelium and cerebellar ventricular zone, and subsequent fusion of the medial edges of the previously independent wings results in a contiguous structure that will become the medial cerebellar vermis and lateral hemispheres.
Figure 4 -.
The cerebellum is a derivative of the anterior-most dorsal hindbrain, or dorsal (D) rhombomere 1 (r1). The establishment mid-hindbrain (mb-hb) boundary results in formation of a transient signaling center called the Isthmic Organizer (IsO), which secretes Fibroblast Growth Family 8 (FGF8) and WNT1 which are required for cell survival and pattern the adjacent tissue from e8–11.5 in mice. The developing cerebellar anlage undergoes a series of morphogenetic events between mouse embryonic (e) days 9 to e12.5 that rotate its anterior posterior (AP) axis by 90 degrees and convert it to the medio-lateral axis (ML) of the bilateral cerebellar wings. This reorientation is in large part driven by pontine flexure which converts the horizontal alignment of mid/hindbrain to nearly right angles (indicated by black double headed arrows). The roof plate (rp) is the single layer thick roof of the dorsal midline of the early neural tube which acts as another transient signaling center, expressing BMP and WNT secreted factors. The roof plate will eventually differentiate into the choroid plexus epithelium of the fourth ventricle. In rhombomere 1, roof plate derived Wnt expression is required to drive early cerebellar anlage ventricular zone proliferation, while secreted Bone morphogenetic protein (BMP) gene expression is required to induce the cerebellar rhombic lip and correctly pattern expression of Pancreatic transcription factor (Ptf1a) in the ventricular zone of the nascent cerebellar anlage.
In overlapping waves of neurogenesis in the e10.5–e14.5 mouse, progenitors in the Ptf1a+ cerebellar ventricular zone give rise to all GABAergic neurons of the cerebellum (FIGURE 3E). The GABAergic Cerebellar Nuclei (CN) neurons are born initially, followed by Purkinje neurons which migrate radially outwards from the ventricular zone to the developing cerebellar cortex along radial glial fibers extending from the progenitors in the ventricular zone to the pial surface. Finally cerebellar interneuron progenitors exit the ventricular zone (Sudarov et al., 2011). Once the last neurons exit the ventricular zone, the remaining radial glial cells lose their apical attachment and differentiate into Bergmann Glial cells retaining and elaborating their pial endfoot attachments. The molecular control of these cell fate switches remains largely unknown. Expression of oligodendrocyte-specific bHLH transcription factor (Olig2) defines Purkinje cell progenitors within the Ptf1a+ ventricular zone, while interneuron progenitors are derived from a Ptf1a+ ventricular subzone expressing homeodomain-containing transcription factor GSX1. At e12.5 in the developing mouse embryo, Olig2+ Purkinje cell progenitors comprise a predominant portion of the PTF1a+ ventricular zone. By e14.5, Gsx1 expression has swept across the ventricular zone and interneuronal progenitors become the predominant output of the ventricular zone. Gsx1 inhibits Olig2 expression and acts as a brake for temporal identity transition. There is some evidence that Olig gene expression is also required for the Purkinje cell progenitor identity (Seto et al., 2014). However, the molecular regulatory details of these dramatic changes in gene expression and subsequent changes in ventricular zone output remain to be determined.
By e10.5 another germinal zone, the cerebellar rhombic lip, is established between the cerebellar ventricular zone and dorsal roof plate (FIGURE 3). This stem cell zone gives rise to all the glutamatergic neurons of the cerebellum. Initially glutamatergic Cerebellar Nuclei neurons emerge from this zone and migrate tangentially over the top of the anlage to form the Nuclear Transitory Zone (NTZ), which is a staging zone for Cerebellar Nuclei assembly. By e11.5, large numbers of granule neuron progenitors (GNPs) emerge from the rhombic lip to migrate over the anlage to form the external granule layer (EGL) on the pial surface of the developing anlage, but under the developing meninges. Within the EGL, GNPs divide extensively, driven by the mitogen Sonic Hedgehog (SHH), secreted by the underlying differentiating Purkinje cells (Dahmane and Ruiz i Altaba, 1999). In mice, peak EGL proliferation occurs around post-natal day (P)7 and is complete by P15. Exponential GNP proliferation in the EGL drives cerebellar growth and foliation (Sudarov and Joyner, 2007). Since the size of the posterior fossa does not concomitantly increase, increased cerebellar size is accommodated by folding along the anterior/posterior axis. In mice, the circumference of the cerebellar medial anterior/posterior axis increases 17.6-fold between e17.5 and P14 compared with only a 2.2-fold increase in the mediolateral axis (Legue et al., 2015). GNP differentiation occurs continually from P0–P14. As granule neurons exit the cell cycle, they migrate tangentially within the inner EGL and then exit the EGL, migrating radially inwardly along Bergmann Glial fibers, trailing a long t-shaped axon behind. The parallel fibers of the granule neurons comprise the cerebellar molecular layer where they interact with the flat, elaborate dendrites of Purkinje cells. Migrating granule cells settle below the developing Purkinje cell layer to form the internal granule layer (IGL), achieving the final laminar arrangement of the mature cerebellum (Millen and Gleeson, 2008; Butts et al., 2014; Marzban et al., 2014; Leto et al., 2016).
The differentiation programs of each cerebellar cell type are all interdependent. For example, the generation of the flat and elaborate stereotypical Purkinje dendritic tree occurs from P5–P15 with its planarity and branching pattern heavily influenced by signaling from differentiating granule neurons (Baptista et al., 1994; Hirai and Launey, 2000; Ohashi et al., 2014). Likewise, expansion of cerebellar interneuron progenitors within the developing cerebellar white matter occurs postnatally, influenced by Purkinje cell-derived SHH. However, their final fates are determined by signals from their eventual locations within the molecular and internal granule layers (De Luca et al., 2015; Fleming and Chiang, 2015). We have specifically focused on structural cerebellar malformations, and a full discussion on the mechanisms that pattern and establish the cerebellar circuitry, including afferents and efferent is beyond the scope of this review.
Human cerebellar development
Most available data describing human cerebellar development has come from limited fetal and neonatal pathology studies, with in utero brain imaging studies now providing complementary data. Importantly, cerebellar foliation, lamination, neuronal morphology and circuitry are conserved from mice to humans indicating significant conservation of developmental programs. There are however, some important species specific differences.
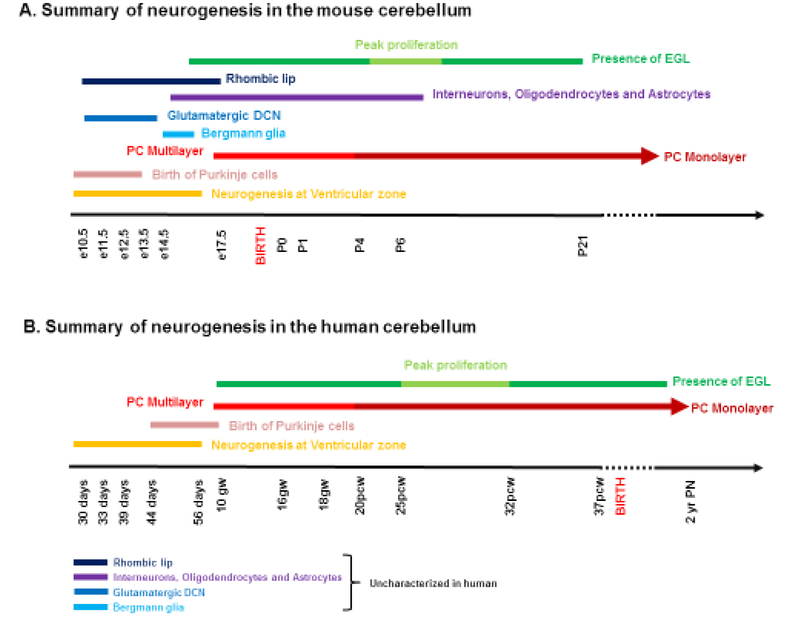
Human cerebellar development is highly protracted compared to mice (Figure 5). In mice, the cerebellum develops over a period of 30–35 days with peak EGL expansion, foliation, IGL formation and Purkinje cell maturation occurring during the first two postnatal weeks (Marzban et al., 2014; Leto et al., 2016). In striking contrast, human cerebellar development extends from the early first trimester to final circuit maturity which is achieved by the end of the second postnatal year. A significant portion of human cerebellar development however, occurs in utero, including peak proliferation of GNPs and folia formation during the last trimester (Raaf, 1944; Rakic and Sidman, 1970). The final cerebellar structures also have distinct features. For example, human cerebellar hemispheres are greatly expanded relative to mice, with a concomitant increase in the size of the dentate nucleus. The human cerebellum is also much more foliated than the mouse cerebellum, yet the basic pattern of cardinal fissures is identical to mice. Lamination is also conserved, yet cellular ratios are different across species. For example, in mice there are 200 GNPs per Purkinje cell. In humans there are 3000 GNPs per Purkinje cell (Lange, 1975). The mechanisms contributing to these differences in timing and scaling remain unknown.
Figure 5 -.
Summary of neurogenesis in the developing (A) mouse and (B) human cerebellum. Human cerebellar development is highly protracted compared to mice. In mice, the cerebellum develops over a period of 30–35 days with peak EGL expansion, foliation and IGL formation and Purkinje cell maturation occurring during the first two postnatal weeks. In striking contrast, human cerebellar development extends from the early first trimester to final circuit maturity which is achieved by the end of the second postnatal year. Also, a significant portion of human cerebellar development occurs in utero, including peak proliferation of GNPs and folia formation during the last trimester. Reference: Haldipur P., Dang D., and Millen K.J., (In press) Embryology. In: M. Manto and T.A.G.M. Huisman (Eds.) The cerebellum in children and adults. Elsevier, Amsterdam
The most comprehensive histological studies of human cerebellar development were conducted in the 1940s and 1970s, (Larsell, 1947; Larsell and Stotler, 1947; Rakic and Sidman, 1970), prior to the inclusion of extensive photographic plates, modern molecular markers or the many insights derived from model organism studies. The published data include widely spaced developmental stages, missing many critical important epochs of cerebellar development known from mice. Recently detailed fetal MRI atlases have been compiled from extensive in utero imaging studies. While these provide valuable volumetric and growth parameters useful for some aspects of clinical prenatal diagnoses, they only cover clinically relevant gestational weeks 20–24, long after most major genetic programs have established and patterned the developing cerebellum. Further, important histological features are not resolvable by MRI (Habas et al., 2010a; Habas et al., 2010b).
The earliest available histological data is from 8 gestational weeks (Larsell, 1947; Rakic and Sidman, 1970; Zecevic and Rakic, 1976). At this early stage, both zones of neurogenesis, the rhombic lip and ventricular zone are visible. By 10–11 gestational weeks, a stream of cells can be seen present along the pial surface connecting to the rhombic lip, which presumably is the EGL, but cannot be confirmed due to lack of immunohistochemical and transcriptome data (Rakic and Sidman, 1970). The ventricular zone is thinner than that of the mouse at e11.5, indicating that Purkinje cells and interneuron progenitors were likely born prior to this stage. This is supported by the fact that a broad multilayered Purkinje cell layer extending from the ventricular zone to the nascent molecular layer is evident between 10 and 13 gestational weeks, with a Purkinje cell monolayer achieved by 20–24 gestational weeks. In the third trimester, Purkinje cells initiate development of their characteristic extensive and flattened dendritic arbor and long axon (Rakic and Sidman, 1970; Zecevic and Rakic, 1976; Haldipur et al., 2011;Haldipur et al., 2012). This final maturation period is 6-fold longer in humans vs mice (Zecevic and Rakic, 1976).
As in mice, the maturation of the human cerebellar Purkinje cell monolayer coincides with the phase during peak EGL proliferation between the 20th and 32nd gestational week (Rakic and Sidman, 1970). Indeed the human cerebellum increases in size 5 fold from gestational weeks 24–40 (Limperopoulos et al., 2005b; Volpe, 2009) due to extensive EGL proliferation. Foliation also correlates with EGL proliferation. The primary fissure first appears around the 11th gestational week while the secondary fissure, seen around the 16th gestational weeks becomes more prominent with age. Foliation between the 20th and 32nd gestational week increases dramatically as the cerebellum rapidly increases in size and volume.
Differentiation and maturation of the human granule cells progresses as in mice, however once again there are several species-specific features. The developing human fetal cerebellar cortex has a unique transient layer - the lamina dissecans. The lamina dissecans is a cell sparse region that is present between the maturing Purkinje cells and the nascent IGL between the 20th and 32nd gestational week. The lamina dissecans disappears after the 32nd gestational week. No function has been attributed to this layer, however, electron microscopic studies indicate that the lamina dissecans shows the presence of many cellular processes which may indicate a site of incipient synapse formation (Rakic and Sidman, 1970). At birth, the human cerebellar cortex still has a prominent EGL. By 12–24 months, the EGL gradually decreases in thickness as a result of decreased proliferation and migration of granule neurons into the IGL. By the end of the second postnatal year the EGL is depleted while concurrently, the thickness of the molecular layer and length of the Purkinje layer increase with increased cerebellar volume (Raaf, 1944; Rakic and Sidman, 1970; Haldipur et al., 2011; Haldipur et al., 2012).
Overview of structural malformations of the human cerebellum
The study of human cerebellar malformations has lagged behind study of human cerebral cortex malformations (Barkovich et al., 2012). However, recognition that abnormal cerebellar development can result in significant cognitive and behavioural deficits (Stoodley and Limperopoulos, 2016) coupled with advances in brain imaging and human genetics has led to increased focus on human cerebellar malformations. Human cerebellar malformations can be classified as predominantly involving the cerebellum or involving both the cerebellum and brainstem. Here we focus on structural malformations that arise during embryonic, fetal and early post-natal development as the cerebellum achieves its stereotypical structure. We will not address medulloblastoma, a developmental cerebellar tumor, nor discuss spinocerebellar ataxias which are primarily degenerative disorders of the mature cerebellum.
Human cerebellar malformations can occur in isolation or as part of broader syndromes involving other brain regions and/or multiple systems (Bolduc et al., 2011; Brossard-Racine et al., 2015). Collectively human cerebellar malformations are relatively common, although the true incidence is difficult to ascertain due to several factors including the fact that imaging is required for diagnosis and clinical outcomes are extremely variable (Aldinger and Doherty, 2016). Unfortunately, depending on the clinical circumstances and resources available, imaging studies are not routinely performed in all cases of developmental disability. Further, cerebellar malformations are often under recognized even if imaging studies are available. Despite these obstacles, there has been considerable progress in defining cerebellar malformations and their causes over the last 2 decades through interdisciplinary, collaborative work by pediatric neurologists, pathologists, radiologists, geneticists and developmental biologists.
Clinical dysmorphologists classically define malformations as non progressive congenital morphological anomaly due to alteration of a primary developmental program. Alterations may be genetic in nature or involve a teratogen such as retinoic acid which can derail normal development (Piersma et al., 2017). Malformations are classically distinguished from disruptions, which are defined by a breakdown of structure which, until the event, had normal developmental potential (Poretti et al., 2016; Brossard-Racine et al., 2017). Examples of disruptions include viral infections such as CMV (Teissier et al., 2014) and Zika (Cugola et al., 2016; Mlakar et al., 2016) or destructive vascular events which also can be associated with genetic risk factors. In practice, malformations and disruptions are often difficult to distinguish. Further, there is accumulating evidence that disruptions such as fetal hemorrhage can entirely mimic some genetic forms of cerebellar malformation.
Notably, histological and clinical imaging studies represent snapshots in time for any one individual. In mice, developmental time course studies can be conducted on multiple mice of identical genotypes. However, human patients with multiple imaging studies across time are rare and these series are usually only available when atrophy is suspected based on clinical deterioration. Hence, even when human cerebellar malformations are recognized during fetal stages, it is evident that cerebellar development was derailed at earlier developmental stages when no data is available for analysis. Thus, the human cerebellar malformation literature has historically been rife with conjecture about the underlying causes of cerebellar malformations.
Fortunately, modern genetic and genomic technologies have revolutionized the diagnosis of cerebellar malformations and a multitude of genes have been identified (Aldinger and Doherty, 2016). Further, considerable advances in basic model vertebrate cerebellar developmental biology have contextualized the molecular diagnoses and revealed remarkable new insights into the pathogenesis of human structural cerebellar malformations. A full discussion of all cerebellar malformations and disease causative genes is well beyond the scope of this review and has recently been reviewed elsewhere (Aldinger and Doherty, 2016). Instead, we have chosen to highlight some of the prominent findings and themes of the review. Additionally, we have emphasized new insights into cerebellar development that have been gained specifically because of the study on human cerebellar malformations.
So what have human cerebellar malformations taught us about development?
1). Cerebellar malformations are rarely exclusively cerebellar.
Perhaps most importantly, the genetic study of human cerebellar malformations have emphasized that many cerebellar malformations are not actually simply cerebellar malformations. Many cerebellar malformations in both human and mice involve other brain regions. This isn’t surprising, since many genes that regulate cerebellar development are essential for development throughout the CNS.
Example 1:
In mice, many spontaneous neurological mutants were historically readily identified because of cerebellar-related gait abnormalities. The relative simplicity of cerebellar anatomy made cerebellar structural malformations more readily recognizable than malformations of many other CNS regions. For example, the classical spontaneous mouse cerebellar reeler mutant was first identified because of its striking cerebellar–related reeling phenotype and severe hypoplasia of the cerebellum (Falconer, 1951). Yet these mice have a multitude of other CNS phenotypes. These include striking cerebral cortical lamination deficits (Hamburgh, 1963). Indeed, Reelin (RELN), the protein encoded by the reeler gene is expressed in many regions of the developing CNS where it coordinates cell positioning during neurodevelopment.
Similar to mice, human RELN mutations cause cerebellar hypoplasia due to Purkinje and granule neuron migration abnormalities. As in mice, homozygous human RELN mutations are also associated with cerebral cortical lamination abnormalities. In humans however, the normally folded or gyrencephalic cortex is smooth – or lissencephalic when RELN function is lost (Hong et al., 2000). This is a very striking phenotype on MRI imaging studies and hence patients were initially ascertained as having a primary cerebral cortex phenotype. Intriguingly a number of studies have shown altered expression of RELN in epilepsy, psychiatric and aging disorders (Folsom and Fatemi, 2013; Dazzo et al., 2015; Ishii et al., 2016).
RELN mutations are not the only example of “cerebellar” genes with widespread, fundamental cellular and developmental function. Severe cerebellar hypoplasia and or dysmorphology is common in dystroglycanopathies or muscle-eye-brain disorders caused by underglycosylation of α dystroglycan with O-linked carbohydrates (Martin, 2005). Loss of various α and β tubulins are a major cause of brain malformations, including lissencephaly, pachygyria and microgyria, all with varying degrees of cerebellar hypoplasia and dysmorphology (Kato, 2015) reflecting the fundamental role of tubulin in neuronal migration.
Example 2:
Early gene targeting studies in mice demonstrated that complete loss of Wnt1 cause deletions of the anterior cerebellum and other mid/hindbrain defects. This is due to its role in the function of the IsO and thus, establishment of the cerebellar territory during very early neural development. Given the importance of Wnt1 gene function in hindbrain development and specifically in development of brain stem nuclei regulating critical autonomic functions, complete loss of Wnt1 is incompatable with neonatal life. Hypomorphic Wnt1 function seen in the spontaneous swaying mouse and some targeted alleles cause less severe, nevertheless prominent cerebellar mispatterning and hypoplasia (Thomas et al., 1991). Yet, despite its iconic role in cerebellar development from mouse studies, surprisingly, human WNT1 mutations were initially identified in patients with a range of mild-to-severe recessively inherited Osteogenesis imperfecta, a genetic disorder characterized by bone fragility coupled with low bone mass (Ogawa, 1987; Keupp et al., 2013; Pyott et al., 2013). These human disease gene variants are not null mutations as human homozygous null mutations are presumably incompatible with viability. However, human studies prompted a reexamination of post-natal phenotypes in mouse hypomorphic Wnt1 mutants, revealing previously overlooked bone deficits. In parallel, further examination of human patients with osteogenesis imperfecta caused by recessive hypomorphic WNT1 mutations revealed brainstem and cerebellar hypoplasia consistent with disrupted IsO function.
From these and numerous other examples we have learned several important lessons, likely applicable to many studies of human genetic disease. 1) Our perceptions of primary phenotypes in both humans and mice, are clearly heavily influenced by ascertainment bias. Further, the cerebellum does not develop in isolation of the rest of the embryo, hence consideration of other phenotypes is essential to gain a full understanding of the cellular and molecular developmental pathologies of any particular malformation and the differential diagnosis of the disorder. 2) Although human developmental biology is similar to mice, the study of human disorders can provide significant new insights regarding gene function beyond the cerebellum. For example, psychiatric and cognitive phenotypes are difficult to assess in mice yet may be prominent features of human disease. 3) Human populations carry many allelic variants of any gene including hypomorphic alleles and non-coding regulatory variants. This is in striking contrast to the few alleles that have either spontaneously arisen or have been constructed in mice, which are often null alleles. Thus, although mouse studies are important to dissect the mechanisms of gene function, the full phenotypic spectrum of altered gene function is best revealed in human versus mouse studies.
2). Cerebellar hypoplasia is common and has a multitude of genetic and non-genetic causes
Cerebellar hypoplasia (CH) refers to a small and often underdeveloped cerebellum. It is perhaps the most common and most non-specific cerebellar malformation given that it is observed in many different disorders and is often a feature of genomic imbalances (Aldinger and Doherty, 2016). Cerebellar development is a complex orchestration of genetic programs controlling neurogenesis, migration, histogenesis and connectivity. There are many opportunities for cerebellar development to be derailed. This is reflected in the large number of genes and disorders that include CH, including RELN and WNT1 as already discussed. CH is frequently associated with other brain malformations including malformations of the cerebral cortex and brain stem and often has a relatively poor clinical prognosis. This is not perhaps surprising, given that brain imaging studies are most often indicated in children with neurodevelopmental delays. Hence, once again, ascertainment bias is an important consideration regarding cerebellar malformations.
The association of CH with brain stem malformations is of particular note, particularly the association of CH with hypoplasia of the Pons. Neurons comprising the pontine nucleus originate from the dorsal rhombic lip and undergo extensive migration to the ventral brain stem (Kratochwil et al., 2017). Since the rhombic lip also gives rise to all cerebellar granule neuron progenitors, it makes sense that developmental disruption of the rhombic lip lineage has both cerebellar and extracerebellar repercussions. Several progressive, neurodegenerative disorders of the developing cerebellum have been identified and specifically termed Pontocerebellar hypoplasia (PCH). 8 distinct PCH types are recognized based on distinguishing clinical features and causative genes (Aldinger and Doherty, 2016). It is important to note many non-degenerative cerebellar hypoplasia cases are associated with pontine hypoplasia reflecting the common developmental origins of these readily identified CNS structures. RELN mutation represents an excellent example of this phenomenon.
Although often occurring with other brain malformations, CH can be diagnosed as an isolated malformation. For example, patients harboring OPHN1 mutations have CH as the most predominant feature in imaging studies (Tentler et al., 1999; Philip et al., 2003). Strikingly, OPHN1 encodes a Rho-GTPase-activating protein expressed throughout the entire CNS. Given that loss of OPHN is also associated with intellectual disability and seizures, cerebral cortical involvement is certain. However, on imaging studies, the almost crystalline, regular structure of the cerebellum makes it easier for structural abnormalities in this brain region to be compared to the more amorphous structure of the cerebral cortex. Hence CH is the earliest abnormality to diagnose.
One of the most striking findings in recent years is that genetic abnormalities do not readily explain many cases of CH (Sajan et al., 2013). Indeed there is growing awareness that prematurity and perinatal cerebellar injury are significant causes of cerebellar hypoplasia. During the third trimester, many critical cerebellar developmental events occur including foliation, peak proliferation and migration of granule neurons, interneuron differentiation, late stages of Purkinje cell differentiation and synapse formation all take place in utero (Rakic and Sidman, 1970; Zecevic and Rakic, 1976). Premature birth is a major risk factor for cerebellar injury and is associated with deficits in motor coordination, and cognition and is also associated with reduced cerebellar volume (Limperopoulos et al., 2005b; Limperopoulos et al., 2007; Peralta-Carcelen et al., 2017). Preterm infants are also prone to cerebellar hemorrhage which often leads to cerebellar atrophy (Limperopoulos et al., 2005a). Additionally, prenatal or neonatal exposure to glucocorticoids (Heine et al., 2011), hypoxia (Darnall et al., 2017), and hyperoxia (Scheuer et al., 2017) can also dramatically alter GNP proliferation and migration, contributing to cerebellar injury.
3). Analysis of Joubert Syndrome and Dandy-Walker malformation have revealed new cerebellar developmental principles not previously appreciated in model organisms.
There are several examples where human cerebellar malformations have revealed new insights not previously appreciated in the study of model organisms.
One example is Joubert Syndrome (FIGURE 6), a syndromic malformation of the brainstem involving a distinctive elongation of the cerebellar peduncles in addition to cerebellar vermis hypoplasia. To date, more than 30 genes have been shown to be causative for this recessive syndrome, all notable for their association with the primary cilia and basal body organelles; crucial cellular structures required for multiple signaling pathways including sonic hedgehog, WNT and platelet derived growth factor (Parisi and Glass, 1993; Aldinger and Doherty, 2016). The importance of primary cilia during cerebellar development was unknown when the first Joubert genes were identified (Parisi et al., 2004; Castori et al., 2005; Sayer et al., 2006; Valente et al., 2006a; Valente et al., 2006b). These human genetic studies prompted analysis of mice lacking cerebellar cilia which demonstrated that severe cerebellar hypoplasia and foliation abnormalities in these disorders are likely primarily attributable to a failure of expansion of the neonatal granule cell progenitor population due to the loss of the ability to receive the SHH mitotic signal from Purkinje cells during EGL expansion (Chizhikov et al., 2007; Spassky et al., 2008). Later studies also revealed an earlier role for cilia in medial cerebellar fusion (Doherty and Millen, 2011; Lancaster et al., 2011).
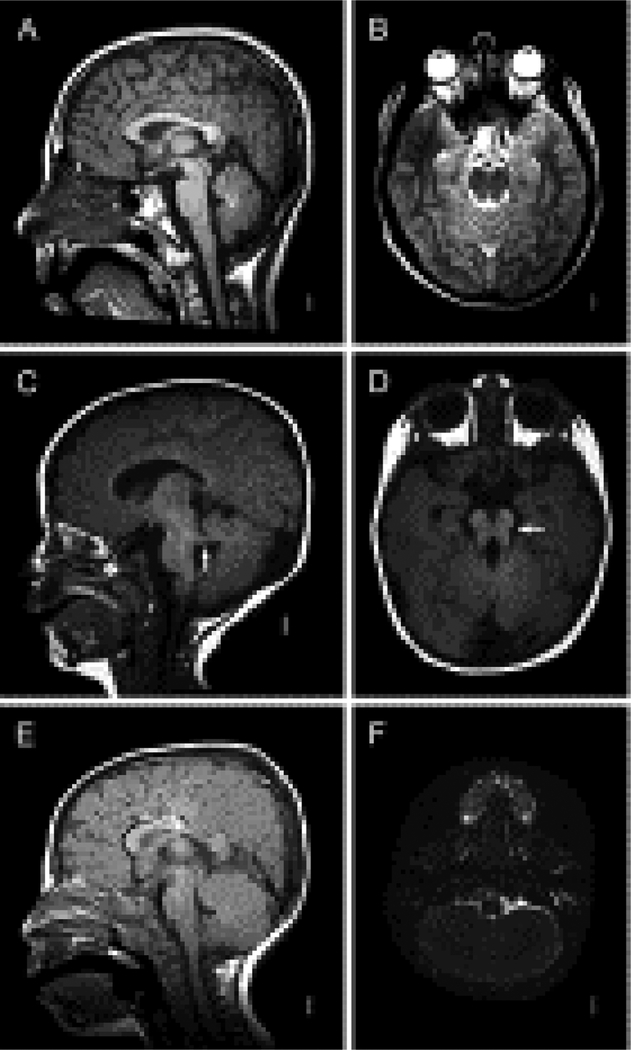
Figure 6 :
MRI views of human Joubert syndrome, a syndromic malformation of the brainstem involving a distinctive elongation of the cerebellar peduncles in addition to cerebellar vermis hypoplasia (C,D) and rhombencephalosynapsis (E, F) with an unaffected individual (A,B) shown for comparison. A,C and E are midsagittal views while B,D and F are along the horizontal plane. JS is characterized by distinct molar tooth sign (D, arrow), while RCS is characterized by missing cerebellar vermis with apparent fusion of the cerebellar hemispheres (F).
Additional new insights into cerebellar development have come from analysis of Dandy-Walker malformation (DWM). DWM is perhaps the most recognizable and well known human cerebellar malformation (Figure 1). Case reports exist from as early as the late 19th century (Fusari, 1891; Rossi, 1891; Rossi, 1892). The core features of DWM are a small cerebellar vermis, rotated up and away from the brainstem and concomitant large fourth ventricle, all contained within a large posterior fossa. Although early cases were all initially recognized due to accompanying hydrocephalus (Dandy, 1921; Taggart and Walker, 1942; Benda, 1954; Dandy and Blackfan, 1964), hydrocephalus is not a consistent feature. Other variable features include brain stem hypoplasia, agenesis of corpus callosum, yet it most often occurs as an isolated malformation.
The genetic causes of DWM remain largely unknown. The recurrence risk is low (1–5%) with very few familial cases reported. This suggests de novo, somatic mosaic or complex causes. Indeed, best characterized are genomic imbalances; heterozygous deletions of chromosome (chr) 3q24 involving the zinc finger in cerebellum (ZIC)1 and ZIC4 genes (Grinberg and Millen, 2005; Blank et al., 2011). Additionally heterozygous deletions of chr 6p25 encompassing the Forkhead box 1 (FOXC1) gene are also associated with DWM (Aldinger et al., 2009; Delahaye et al., 2012; Haldipur et al., 2014; Haldipur et al., 2017).
Analysis of the cerebellar phenotype of Foxc1 mutant mice which model human del chr 6p25 DWM has been essential in revealing the extent of mesenchymal control of cerebellar development. Until human genetic studies identified FOXC1 as a transcription factor relevant to cerebellar development, mouse biologists had largely focused on the role of this gene in cardiovascular development (Kume, 2009).
Foxc1 is a transcription factor that is not expressed in the developing cerebellum. Rather, its expression is limited to the head mesenchyme surrounding the early cerebellar anlage. Head mesenchyme is a mixture of head neural crest and mesodermally derived cells that will give rise to the meninges, bone, and musculature of the head. Foxc1 likely directly regulates posterior fossa size through its cell-autonomous regulation of meningeal and osteoblast development (Vivatbutsiri et al., 2008). Importantly however, complete loss of Foxc1 non-autonomously induces a rapid and devastating decrease in embryonic cerebellar ventricular zone radial glial proliferation and a concurrent increase in cerebellar neuronal differentiation. Subsequent migration of cerebellar neurons is disrupted, associated with disordered radial glial morphology. In vitro, SDF1α, a direct Foxc1 target also expressed in the head mesenchyme, acts as a cerebellar radial glial mitogen and a chemoattractant for nascent Purkinje cells. Its receptor, Cxcr4, is expressed in cerebellar radial glial cells and conditional Cxcr4 ablation in these radial glial cells mimics the Foxc1−/− cerebellar phenotype. SDF1α also rescues the Foxc1−/− phenotype. Together, these studies demonstrate that Foxc1-dependent Sdf1a-Cxcr4 signaling from the mesenchyme to the developing anlage regulates a multitude of cerebellar developmental programs (Haldipur et al., 2014).
Although homozygous null Foxc1 mouse mutants have revealed the importance of mesenchymal control of many aspects of cerebellar development, the mouse cerebellar phenotypes in homozygous null animals are much more severe than those associated with human DWM. Indeed, deletion 6p25 DWM patients do not have complete loss of FOXC1. Rather, one normal chromosome is retained. Mice carrying a homozygous hypomorphic allele of Foxc1 mutant have phenotypes more ready comparable to human DWM. Particularly striking is the presence of a partially formed posterior lobule which echoes the posterior vermis DW ‘tail sign’ observed in human imaging studies (Bernardo et al., 2015). Lineage tracing experiments in Foxc1 mutant mouse cerebella indicated that aberrant migration of granule cell progenitors destined to form the posterior-most lobule causes this unique phenotype. Analyses of rare human del chr 6p25 fetal cerebella demonstrate extensive phenotypic overlap with our Foxc1 mutant mouse models, validating our DWM models and pointing to disruption of mesenchymal signaling during cerebellar rhombic lip development as likely central to the developmental pathogenesis of human DWM (Haldipur et al., 2017).
4). Not all human cerebellar malformations are readily explained by current model organism-derived developmental paradigms
Rhombencephalosynapsis (FIGURE 6) is human cerebellar malformation which cannot be explained by current models of cerebellar development. Rhombencephalosynapsis is very rare midline brain malformation characterized by missing cerebellar vermis with apparent fusion of the cerebellar hemispheres. Rhombencephalosynapsis can be seen in isolation or together with other central nervous system and extra-central nervous system malformations (Ishak et al., 2012). To date, no genes have been associated with this malformation and current mouse models cannot explain the origin of this malformation.
As illustrated in Figure 4, in mice, the cerebellar vermis is a derivative of the anterior-most portion of dorsal rhombomere 1. As pontine flexure displaces posterior rhomobomere 1 laterally, the vermis forms from extensive proliferation of the anterior/medial cerebellar ventricular zone with subsequent fusion of the growing bilateral wings of the cerebellar anlage.
Several mouse mutants exist that lack a cerebellar vermis. Specifically, these mutants all lack IsO function. Loss of IsO in Wnt1 or Engrailed 1 homozygous mutants cause a large deletions of mid and hindbrain tissue during early neural tube stages when the cerebellar territory is defined. In these mice, no cerebellar vermis forms but residual cerebellar hemispheres remain unfused across the dorsal midline (McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Wurst et al., 1994). In mouse mutants with hypomorphic IsO function, a vermis forms, albeit mispatterned (Basson et al., 2008). There have never been any mutant mice described with fusion of cerebellar hemispheres in the absence of a vermis. Until genes causative for Rhombencephalosynapsis are identified, the developmental pathogenesis of this malformation will remain a mystery.
Summary
In this review, we have described key developmental mechanisms that drive formation of the cerebellum. As is evident, almost all of this information has been obtained from genetic analysis of cerebellar malformations, largely in model organisms, mostly in mice. Although human cerebellar malformations have long been recognized, their genetic basis has only recently begun to be elucidated. These studies have revealed several new insights into cerebellar development that were previously unappreciated in model organisms, including significant extra-cerebellar influences on cerebellar development — both genetic and environmental. However, in truth, we actually know very little about the specifics of human cerebellar development. Normal embryonic and fetal human cerebellar development has never been fully described. Recent studies on the development of the human cerebral cortex have identified many striking human-specific features during fetal neurogenesis including the organization of germinal zones (Nowakowski et al., 2016; Nowakowski et al., 2017). These features have been shown to be relevant in the context of cortical malformations such as lissencephaly (Bershteyn et al., 2017). It is certain that human-specific cerebellar developmental programs exist. A better appreciation of these will not only facilitate improved diagnosis of human cerebellar malformations, but also lead to the development of treatment paradigms for these important neurodevelopmental disorders.
Highlights.
Cerebellar malformations are rarely exclusively cerebellar.
Cerebellar hypoplasia is common and has a multitude of genetic and non-genetic causes.
Analyses of Joubert Syndrome and Dandy-Walker malformation have revealed new cerebellar developmental principles not previously appreciated in model organisms.
Not all human cerebellar malformations are readily explained by current model organism-derived developmental paradigms.
Acknowledgements:
MRI images used in Figures 1 and 7 were graciously provided by Dr. William B. Dobyns. Figure 4 was expertly illustrated by Benedict Rossi. This work was supported by NIH grants R01NS080390 and R01NS095733 to KJM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldinger KA and Doherty D (2016) ‘The genetics of cerebellar malformations’, Seminars in fetal & neonatal medicine 21(5): 321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB and Millen KJ (2009) ‘FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation’, Nature genetics 41(9): 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzt K (2012) ‘Mammalian developmental genetics in the twentieth century’, Genetics 192(4): 1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista CA, Hatten ME, Blazeski R and Mason CA (1994) ‘Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro’, Neuron 12(2): 243–60. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD and Dobyns WB (2012) ‘A developmental and genetic classification for malformations of cortical development: update 2012’, Brain : a journal of neurology 135(Pt 5): 1348–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Millen KJ and Dobyns WB (2009) ‘A developmental and genetic classification for midbrain-hindbrain malformations’, Brain : a journal of neurology 132(Pt 12): 3199–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson MA, Echevarria D, Ahn CP, Sudarov A, Joyner AL, Mason IJ, Martinez S and Martin GR (2008) ‘Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development’, Development 135(5): 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda CE (1954) ‘The Dandy-Walker Syndrome or the so called Stresia of the Foramen of Magendie’, Journal of neuropathology and experimental neurology 13: 14–29. [DOI] [PubMed] [Google Scholar]
- Bernardo S, Vinci V, Saldari M, Servadei F, Silvestri E, Giancotti A, Aliberti C, Porpora MG, Triulzi F, Rizzo G et al. (2015) ‘Dandy-Walker Malformation: is the ‘tail sign’ the key sign?’, Prenatal diagnosis 35(13): 1358–64. [DOI] [PubMed] [Google Scholar]
- Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A and Kriegstein AR (2017) ‘Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia’, Cell stem cell 20(4): 435–449 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MC, Grinberg I, Aryee E, Laliberte C, Chizhikov VV, Henkelman RM and Millen KJ (2011) ‘Multiple developmental programs are altered by loss of Zic1 and Zic4 to cause Dandy-Walker malformation cerebellar pathogenesis’, Development 138(6): 1207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc ME, Du Plessis AJ, Sullivan N, Khwaja OS, Zhang X, Barnes K, Robertson RL and Limperopoulos C (2011) ‘Spectrum of neurodevelopmental disabilities in children with cerebellar malformations’, Dev Med Child Neurol 53(5): 409–16. [DOI] [PubMed] [Google Scholar]
- Brossard-Racine M, du Plessis AJ and Limperopoulos C (2015) ‘Developmental Cerebellar Cognitive Affective Syndrome in Ex-preterm Survivors Following Cerebellar Injury’, Cerebellum 14(2): 151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard-Racine M, Poretti A, Murnick J, Bouyssi-Kobar M, McCarter R, du Plessis AJ and Limperopoulos C (2017) ‘Cerebellar Microstructural Organization is Altered by Complications of Premature Birth: A Case-Control Study’, The Journal of pediatrics 182: 28–33 e1. [DOI] [PubMed] [Google Scholar]
- Butts T, Green MJ and Wingate RJ (2014) ‘Development of the cerebellum: simple steps to make a ‘little brain’’, Development 141(21): 4031–41. [DOI] [PubMed] [Google Scholar]
- Castori M, Valente EM, Donati MA, Salvi S, Fazzi E, Procopio E, Galluccio T, Emma F, Dallapiccola B and Bertini E (2005) ‘NPHP1 gene deletion is a rare cause of Joubert syndrome related disorders’, Journal of medical genetics 42(2): e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK and Millen KJ (2007) ‘Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool’, The Journal of neuroscience : the official journal of the Society for Neuroscience 27(36): 9780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S et al. (2016) ‘The Brazilian Zika virus strain causes birth defects in experimental models’, Nature 534(7606): 267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N and Ruiz i Altaba A (1999) ‘Sonic hedgehog regulates the growth and patterning of the cerebellum’, Development 126(14): 3089–100. [DOI] [PubMed] [Google Scholar]
- Dandy WE (1921) ‘Diagnosis and Treatment of hydrocephalus due to occlusions of the foramina of Luschka and Magendie’, Surgery, Gynecology and Obstetrics 32: 112–124. [Google Scholar]
- Dandy WE and Blackfan K (1964) ‘Internal Hydrocephalus: An Experimental Clinical and Pathological Study’, Journal of neurosurgery 21(7): 585–635. [Google Scholar]
- Darnall RA, Chen X, Nemani KV, Sirieix CM, Gimi B, Knoblach S, McEntire BL and Hunt CE (2017) ‘Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism’, Pediatric research 82(1): 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo E, Fanciulli M, Serioli E, Minervini G, Pulitano P, Binelli S, Di Bonaventura C, Luisi C, Pasini E, Striano S et al. (2015) ‘Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy’, American journal of human genetics 96(6): 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Parmigiani E, Tosatto G, Martire S, Hoshino M, Buffo A, Leto K and Rossi F (2015) ‘Exogenous Sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development’, Cerebellum 14(2): 72–85. [DOI] [PubMed] [Google Scholar]
- Delahaye A, Khung-Savatovsky S, Aboura A, Guimiot F, Drunat S, Alessandri JL, Gerard M, Bitoun P, Boumendil J, Robin S et al. (2012) ‘Pre- and postnatal phenotype of 6p25 deletions involving the FOXC1 gene’, American journal of medical genetics. Part A 158A(10): 2430–8. [DOI] [PubMed] [Google Scholar]
- Doherty D and Millen KJ (2011) ‘Wormless without wingless’, Nature medicine 17(6): 663–5. [DOI] [PubMed] [Google Scholar]
- Eddison M, Toole L, Bell E and Wingate RJ (2004) ‘Segmental identity and cerebellar granule cell induction in rhombomere 1’, BMC Biol 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS (1951) ‘Two new mutants, ‘trembler’ and ‘reeler’, with neurological actions in the house mouse (Mus musculus L.)’, Journal of genetics 50(2): 192–201. [DOI] [PubMed] [Google Scholar]
- Fleming J and Chiang C (2015) ‘The Purkinje neuron: A central orchestrator of cerebellar neurogenesis’, Neurogenesis (Austin) 2(1): e1025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom TD and Fatemi SH (2013) ‘The involvement of Reelin in neurodevelopmental disorders’, Neuropharmacology 68: 122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusari R (1891) ‘Caso di mancanza quasi totale del cerveiletto’, Memorie della R. accademia delle scienze dell’Istituto di Bologna 2(5): 643–658. [Google Scholar]
- Gavalas A, Davenne M, Lumsden A, Chambon P and Rijli FM (1997) ‘Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning’, Development 124(19): 3693–702. [DOI] [PubMed] [Google Scholar]
- Grinberg I and Millen KJ (2005) ‘The ZIC gene family in development and disease’, Clinical genetics 67(4): 290–6. [DOI] [PubMed] [Google Scholar]
- Habas PA, Kim K, Corbett-Detig JM, Rousseau F, Glenn OA, Barkovich AJ and Studholme C (2010a) ‘A spatiotemporal atlas of MR intensity, tissue probability and shape of the fetal brain with application to segmentation’, NeuroImage 53(2): 460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas PA, Kim K, Rousseau F, Glenn OA, Barkovich AJ and Studholme C (2010b) ‘Atlas-based segmentation of developing tissues in the human brain with quantitative validation in young fetuses’, Human brain mapping 31(9): 1348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Bharti U, Alberti C, Sarkar C, Gulati G, Iyengar S, Gressens P and Mani S (2011) ‘Preterm delivery disrupts the developmental program of the cerebellum’, PloS one 6(8): e23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Bharti U, Govindan S, Sarkar C, Iyengar S, Gressens P and Mani S (2012) ‘Expression of Sonic hedgehog during cell proliferation in the human cerebellum’, Stem cells and development 21(7): 1059–68. [DOI] [PubMed] [Google Scholar]
- Haldipur P, Dang D, Aldinger KA, Janson OK, Guimiot F, Adle-Biasette H, Dobyns WB, Siebert JR, Russo R and Millen KJ (2017) ‘Phenotypic outcomes in Mouse and Human Foxc1 dependent Dandy-Walker cerebellar malformation suggest shared mechanisms’, Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Gillies GS, Janson OK, Chizhikov VV, Mithal DS, Miller RJ and Millen KJ (2014) ‘Foxc1 dependent mesenchymal signalling drives embryonic cerebellar growth’, Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallonet ME and Le Douarin NM (1993) ‘Tracing neuroepithelial cells of the mesencephalic and metencephalic alar plates during cerebellar ontogeny in quail-chick chimaeras’, Eur J Neurosci 5(9): 1145–55. [DOI] [PubMed] [Google Scholar]
- Hamburgh M (1963) ‘Analysis of the Postnatal Developmental Effects of “Reeler,” a Neurological Mutation in Mice. A Study in Developmental Genetics’, Developmental biology 8: 165–85. [DOI] [PubMed] [Google Scholar]
- Harada H, Sato T and Nakamura H (2016) ‘Fgf8 signaling for development of the midbrain and hindbrain’, Development, growth & differentiation 58(5): 437–45. [DOI] [PubMed] [Google Scholar]
- Heine VM, Griveau A, Chapin C, Ballard PL, Chen JK and Rowitch DH (2011) ‘A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury’, Science translational medicine 3(105): 105ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Millet S, Bloch-Gallego E and Alvarado-Mallart RM (2005) ‘Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary’, Brain research. Brain research reviews 49(2): 134–49. [DOI] [PubMed] [Google Scholar]
- Hirai H and Launey T (2000) ‘The regulatory connection between the activity of granule cell NMDA receptors and dendritic differentiation of cerebellar Purkinje cells’, The Journal of neuroscience : the official journal of the Society for Neuroscience 20(14): 5217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND and Walsh CA (2000) ‘Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations’, Nature genetics 26(1): 93–6. [DOI] [PubMed] [Google Scholar]
- Irving C and Mason I (2000) ‘Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression’, Development 127(1): 177–86. [DOI] [PubMed] [Google Scholar]
- Ishak GE, Dempsey JC, Shaw DW, Tully H, Adam MP, Sanchez-Lara PA, Glass I, Rue TC, Millen KJ, Dobyns WB et al. (2012) ‘Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity’, Brain : a journal of neurology 135(Pt 5): 1370–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kubo KI and Nakajima K (2016) ‘Reelin and Neuropsychiatric Disorders’, Frontiers in cellular neuroscience 10: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL (2016) ‘From Cloning Neural Development Genes to Functional Studies in Mice, 30 Years of Advancements’, Current topics in developmental biology 116: 501–15. [DOI] [PubMed] [Google Scholar]
- Kato M (2015) ‘Genotype-phenotype correlation in neuronal migration disorders and cortical dysplasias’, Frontiers in neuroscience 9: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keupp K, Beleggia F, Kayserili H, Barnes AM, Steiner M, Semler O, Fischer B, Yigit G, Janda CY, Becker J et al. (2013) ‘Mutations in WNT1 cause different forms of bone fragility’, American journal of human genetics 92(4): 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil CF, Maheshwari U and Rijli FM (2017) ‘The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry’, Frontiers in neural circuits 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T (2009) ‘The cooperative roles of Foxc1 and Foxc2 in cardiovascular development’, Advances in experimental medicine and biology 665: 63–77. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM, Thacker BE, Williams Y, Zaki MS and Gleeson JG (2011) ‘Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome’, Nature medicine 17(6): 726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W (1975) ‘Cell number and cell density in the cerebellar cortex of man and some other mammals’, Cell Tissue Res 157(1): 115–24. [DOI] [PubMed] [Google Scholar]
- Larsell O (1947) ‘The development of the cerebellum in man in relation to its comparative anatomy’, The Journal of comparative neurology 87(2): 85–129. [DOI] [PubMed] [Google Scholar]
- Larsell O and Stotler WA (1947) ‘Some morphological features of the human cerebellum’, The Anatomical record 97(3): 352. [PubMed] [Google Scholar]
- Legue E, Riedel E and Joyner AL (2015) ‘Clonal analysis reveals granule cell behaviors and compartmentalization that determine the folded morphology of the cerebellum’, Development 142(9): 1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Arancillo M, Becker EB, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME et al. (2016) ‘Consensus Paper: Cerebellar Development’, Cerebellum 15(6): 789–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr., Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA et al. (2007) ‘Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors?’, Pediatrics 120(3): 584–93. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, Ringer SA, Volpe JJ and du Plessis AJ (2005a) ‘Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors’, Pediatrics 116(3): 717–24. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ and du Plessis AJ (2005b) ‘Late gestation cerebellar growth is rapid and impeded by premature birth’, Pediatrics 115(3): 688–95. [DOI] [PubMed] [Google Scholar]
- Martin PT (2005) ‘The dystroglycanopathies: the new disorders of O-linked glycosylation’, Seminars in pediatric neurology 12(3): 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S and Alvarado-Mallart RM (1989a) ‘Rostral Cerebellum Originates from the Caudal Portion of the So-Called ‘Mesencephalic’ Vesicle: A Study Using Chick/Quail Chimeras’, Eur J Neurosci 1(6): 549–560. [DOI] [PubMed] [Google Scholar]
- Martinez S and Alvarado-Mallart RM (1989b) ‘Transplanted mesencephalic quail cells colonize selectively all primary visual nuclei of chick diencephalon: a study using heterotopic transplants’, Brain Res Dev Brain Res 47(2): 263–74. [DOI] [PubMed] [Google Scholar]
- Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM and Rastegar M (2014) ‘Cellular commitment in the developing cerebellum’, Frontiers in cellular neuroscience 8: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I, Chambers D, Shamim H, Walshe J and Irving C (2000) ‘Regulation and function of FGF8 in patterning of midbrain and anterior hindbrain’, Biochem Cell Biol 78(5): 577–84. [PubMed] [Google Scholar]
- McMahon AP and Bradley A (1990) ‘The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain’, Cell 62(6): 1073–85. [DOI] [PubMed] [Google Scholar]
- Millen KJ and Gleeson JG (2008) ‘Cerebellar development and disease’, Current opinion in neurobiology 18(1): 12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen KJ, Steshina EY, Iskusnykh IY and Chizhikov VV (2014) ‘Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function’, Proceedings of the National Academy of Sciences of the United States of America 111(17): E1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Lindgren AG, Chizhikov VV, Johnson RL and Millen KJ (2009) ‘Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth’, The Journal of neuroscience : the official journal of the Society for Neuroscience 29(36): 11377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V et al. (2016) ‘Zika Virus Associated with Microcephaly’, The New England journal of medicine 374(10): 951–8. [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D et al. (2017) ‘Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex’, Science 358(6368): 1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Sandoval-Espinosa C and Kriegstein AR (2016) ‘Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development’, Neuron 91(6): 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T (1987) ‘[The electroencephalography (EEG), somatosensory evoked potential (SEP), and multiple unit activity (MUA) during etomidate and althesin anesthesia in cats]’, Masui. The Japanese journal of anesthesiology 36(5): 757–70. [PubMed] [Google Scholar]
- Ohashi R, Sakata S, Naito A, Hirashima N and Tanaka M (2014) ‘Dendritic differentiation of cerebellar Purkinje cells is promoted by ryanodine receptors expressed by Purkinje and granule cells’, Developmental neurobiology 74(4): 467–80. [DOI] [PubMed] [Google Scholar]
- Parisi M and Glass I (1993) Joubert Syndrome in Adam MP Ardinger HH Pagon RA Wallace SE Bean LJH Mefford HC Stephens K Amemiya A and Ledbetter N (eds.) GeneReviews((R)). Seattle (WA). [Google Scholar]
- Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF and Glass IA (2004) ‘The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome’, American journal of human genetics 75(1): 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Carcelen M, Carlo WA, Pappas A, Vaucher YE, Yeates KO, Phillips VA, Gustafson KE, Payne AH, Duncan AF, Newman JE et al. (2017) ‘Behavioral Problems and Socioemotional Competence at 18 to 22 Months of Extremely Premature Children’, Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Chabrol B, Lossi AM, Cardoso C, Guerrini R, Dobyns WB, Raybaud C and Villard L (2003) ‘Mutations in the oligophrenin-1 gene (OPHN1) cause × linked congenital cerebellar hypoplasia’, Journal of medical genetics 40(6): 441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma AH, Hessel EV and Staal YC (2017) ‘Retinoic acid in developmental toxicology: Teratogen, morphogen and biomarker’, Reproductive toxicology 72: 53–61. [DOI] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E and Huisman TA (2016) ‘Prenatal Cerebellar Disruptions: Neuroimaging Spectrum of Findings in Correlation with Likely Mechanisms and Etiologies of Injury’, Neuroimaging clinics of North America 26(3): 359–72. [DOI] [PubMed] [Google Scholar]
- Pyott SM, Tran TT, Leistritz DF, Pepin MG, Mendelsohn NJ, Temme RT, Fernandez BA, Elsayed SM, Elsobky E, Verma I et al. (2013) ‘WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta’, American journal of human genetics 92(4): 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaf J a. K., J. W. (1944) ‘A study of the external granular layer in the cerebellum. The disappearance of the external granular layer and the growth of the molecular and internal granular layers in the cerebellum’, Am. J. Anat 75: 151–172. [Google Scholar]
- Rakic P and Sidman RL (1970) ‘Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans’, The Journal of comparative neurology 139(4): 473–500. [DOI] [PubMed] [Google Scholar]
- Rossi V (1891) ‘Un caso di mancanza del lobo mediano del cerveilletto con presenza della fossetta occipitale media ‘, Sperimentale 45: 418–526. [Google Scholar]
- Rossi V (1892) ‘Nuova ossevazione di mancanza del verme cerebellare’, Sperimentale 46: 310–313. [Google Scholar]
- Sajan SA, Fernandez L, Nieh SE, Rider E, Bukshpun P, Wakahiro M, Christian SL, Riviere JB, Sullivan CT, Sudi J et al. (2013) ‘Both rare and de novo copy number variants are prevalent in agenesis of the corpus callosum but not in cerebellar hypoplasia or polymicrogyria’, PLoS genetics 9(10): e1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T and Joyner AL (2009) ‘The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures’, Development 136(21): 3617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV et al. (2006) ‘The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4’, Nature genetics 38(6): 674–81. [DOI] [PubMed] [Google Scholar]
- Scheuer T, Sharkovska Y, Tarabykin V, Marggraf K, Brockmoller V, Buhrer C, Endesfelder S and Schmitz T (2017) ‘Neonatal Hyperoxia Perturbs Neuronal Development in the Cerebellum’, Molecular neurobiology. [DOI] [PubMed] [Google Scholar]
- Seto Y, Nakatani T, Masuyama N, Taya S, Kumai M, Minaki Y, Hamaguchi A, Inoue YU, Inoue T, Miyashita S et al. (2014) ‘Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum’, Nat Commun 5: 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaier SK, Millet S, Villanueva MP, Berenshteyn F, Song C and Joyner AL (2005) ‘Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping’, Neuron 45(1): 27–40. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Green MC and Appel SH (1966) Catalog of the Neurological Mutants of the Mouse: Harvard University Press.
- Singh P, Schimenti JC and Bolcun-Filas E (2015) ‘A mouse geneticist’s practical guide to CRISPR applications’, Genetics 199(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM and Alvarez-Buylla A (2008) ‘Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool’, Developmental biology 317(1): 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ and Limperopoulos C (2016) ‘Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders’, Seminars in fetal & neonatal medicine 21(5): 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov A and Joyner AL (2007) ‘Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers’, Neural Dev 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov A, Turnbull RK, Kim EJ, Lebel-Potter M, Guillemot F and Joyner AL (2011) ‘Ascl1 genetics reveals insights into cerebellum local circuit assembly’, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(30): 11055–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart JK Jr and Walker AE (1942) ‘Congenital Atresia of the Foramens of Luschka and ‘, Arch Neurol Psychiatry 48: 583–612. [Google Scholar]
- Teissier N, Fallet-Bianco C, Delezoide AL, Laquerriere A, Marcorelles P, Khung-Savatovsky S, Nardelli J, Cipriani S, Csaba Z, Picone O et al. (2014) ‘Cytomegalovirus-induced brain malformations in fetuses’, Journal of neuropathology and experimental neurology 73(2): 143–58. [DOI] [PubMed] [Google Scholar]
- Tentler D, Gustavsson P, Leisti J, Schueler M, Chelly J, Timonen E, Anneren G, Willard HF and Dahl N (1999) ‘Deletion including the oligophrenin-1 gene associated with enlarged cerebral ventricles, cerebellar hypoplasia, seizures and ataxia’, European journal of human genetics : EJHG 7(5): 541–8. [DOI] [PubMed] [Google Scholar]
- Thomas KR and Capecchi MR (1990) ‘Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development’, Nature 346(6287): 847–50. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Musci TS, Neumann PE and Capecchi MR (1991) ‘Swaying is a mutant allele of the proto-oncogene Wnt-1’, Cell 67(5): 969–76. [DOI] [PubMed] [Google Scholar]
- Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, Barrano G, Bertini E, Boltshauser E, Zaki MS, Abdel-Aleem A et al. (2006a) ‘AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders’, Annals of neurology 59(3): 527–34. [DOI] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L et al. (2006b) ‘Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome’, Nature genetics 38(6): 623–5. [DOI] [PubMed] [Google Scholar]
- Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K and Iseki S (2008) ‘Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant’, Journal of anatomy 212(5): 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ (2009) ‘Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important’, Journal of child neurology 24(9): 1085–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate RJ and Hatten ME (1999) ‘The role of the rhombic lip in avian cerebellum development’, Development 126(20): 4395–404. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB and Joyner AL (1994) ‘Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum’, Development 120(7): 2065–75. [DOI] [PubMed] [Google Scholar]
- Yamada M, Seto Y, Taya S, Owa T, Inoue YU, Inoue T, Kawaguchi Y, Nabeshima Y and Hoshino M (2014) ‘Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons’, The Journal of neuroscience : the official journal of the Society for Neuroscience 34(14): 4786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N and Rakic P (1976) ‘Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man’, The Journal of comparative neurology 167(1): 27–47. [DOI] [PubMed] [Google Scholar]