Abstract
Introduction:
Chymase is primarily found in mast cells (MCs), fibroblasts, and vascular endothelial cells. MC chymase is released into the extracellular interstitium in response to inflammatory signals, tissue injury, and cellular stress. Among many functions, chymase is a major extravascular source for angiotensin II (Ang II) generation. Several recent pre-clinical and a few clinical studies point to the relatively unrecognized fact that chymase inhibition may have significant therapeutic advantages over other treatments in halting progression of cardiac and vascular disease.
Area covered:
The present review covers patent literature on chymase inhibitors for the treatment of cardiac diseases registered between 2010 and 2018.
Expert opinion:
Increase in cardiac MC number in various cardiac diseases has been found in pathological tissues of human and experimental animals. Meta-analysis data from large clinical trials employing angiotensin converting enzyme (ACE) inhibitors show a relatively small risk reduction of clinical cardiovascular endpoints. The disconnect between the expected benefit associated with Ang II blockade of synthesis or activity underscore a greater participation of chymase compared to ACE in forming Ang II in humans. Emerging literature and a reconsideration of previous studies provide lucid arguments to reconsider chymase as a primary Ang II forming enzyme in human heart and vasculature.
Keywords: mast cells, serine protease, chymase, chymase inhibitors, angiotensin-converting enzyme, angiotensin II, angiotensin I, angiotensin-(1-12), renin, metabolism, renin-angiotensin system, angiotensinogen
1. Introduction
Cardiovascular diseases (CVD) remain the leading causes of death globally. CVD include conditions that changes the structure and function of the heart, such as hypertension, coronary artery disease (CAD, narrowing of the arteries), heart muscles disease (cardiomyopathy), vascular disease (blood vessel disease), congenital heart disease, abnormal heart rhythms (arrhythmias), pericardial disease, heart attack and stroke. Hypertension, high cholesterol, smoking, obesity, and diabetes are major risk factors for the development of CVD. Health conditions such as lifestyle, age, and family history can also increase the risk of heart disease. While age, sex, genetic, and family history are unmodifiable risks for CVD predisposition, its burden can be reduced by approaches entailing implementing a diet low in fats and sodium, maintaining physical activity, and avoiding weight gain. Diet can affect most modifiable risk factors for CVD [1], while medicines blocking the pathological consequences of exacerbated sympathetic nervous and renin angiotensin systems (RAS) can slow down the progression of diseases of the heart and the blood vessels.
Although several risk factors have been identified in the progression of CVD, the precise cellular and molecular mechanism(s) for the development of cardiac diseases remain largely unknown [2-4]. Accumulating evidence documents mast cell (MC) chymase as one of the key factors contributing to tissue remodeling and CVD progression [5]. MCs are best known for their role in allergic reactions but are now also recognized for their important contributions to a number of inflammatory conditions through the release of proteoglycans, lysosomal enzymes, chemokines, cytokines, renin, peroxidase and a number of mast cell-specific proteases (such as chymase, cathepsin G, tryptase, carboxypeptidase A) [6]. MCs directly and/or indirectly contribute to the generation of vasoactive and pro-inflammatory products which may be responsible for heart tissue fibrosis and remodeling processes. MCs are derived from multipotent hematopoietic bone marrow precursor cells that circulate in the blood and differentiate into mature immunologic cells until reaching the tissue or organ in which they reside. MCs have been found to synthesize transforming growth factor-β1 (TGF-β1) and fibroblast growth factors in cardiac tissues [7].
The heart is one of the organs rich in MCs. Accumulation of MCs is observed not only in heart failure (HF) animal models (including experimentally induced hypertension, myocardial infarction, and chronic volume overload secondary to aortocaval fistula and mitral regurgitation) but also in heart tissues of diseased patients [8-13]. Studies from acute myocardial infarcted rats show a massive accumulation of MCs in the infarcted region of the heart [14]. Increased MC density has been implicated in human cardiomyopathy [15] and left ventricular fibrosis in hypertensive rat hearts [16]. There is increasing evidence that cardiac MCs participate in the development of atherosclerosis, coronary inflammation, cardiac ischemia [17], as well as the metabolic syndrome [18]. More importantly, immunological and biochemical studies show that human heart MCs differ from other connective tissue MCs, such as skin MCs because the human heart MC did not respond to morphine and substance P induced release of histamine from MC [19].
In human, MCs are classified into two types on the basis of the expression of proteases in their granules; MCs containing tryptase only (MCT) and MCs containing both proteases; tryptase and chymase (MCTC) [5,20-23]. In humans, cardiac MCs expressed both proteases (MCTC) [24]. In rodents, MCs can be divided into two types based on their tissue distribution; named as connective tissue MCs (CTMC) and mucosal MCs (MMC) [21]. In terms of tissue localization, human MCT corresponds to rodent MMC, predominantly located in mucosal tissues (such as intestine and respiratory tract), whereas human MCTC corresponds to rodent CTMC and are mainly found in connective tissues, such as the skin and peritoneal cavity [26,26]. During altered pathophysiological conditions, cellular stress and tissue injury, chymase is released from MCs into the extracellular environment. Chymase is synthesized as an inactive precursor (pro-chymase) in MCs. Activation of pro-chymase in MC granules occurs by the removal of the dipeptide residue (Gly-Glu) from the N-terminus by dipeptidyl peptidase (DPPI) [27]. Although chymase is stored in MC granules as a fully active form, it has no functional effects. The potent protease activity is liberated after MCs degranulate in response to the presence of inflammatory cytokines or tissue injury.
A growing number of studies suggest a significant role of chymase in accounting for Ang II formation in human cardiovascular tissue and the consequent pathological remodeling associated with increased RAS activity [5,28-30]. Serine proteases and their inhibitors are being extensively studied in various diseased conditions like inflammation, cancer, skin diseases, atherosclerosis, immunological disorders and other pathologies [31,32]. In this review, we will document in detail the specific role of MC serine protease (chymase) including the new concept of RAS pathways in the development and progression of various cardiac diseases. Several chymase inhibitors have been developed and tested in pre-clinical studies using chymase-mediated diseased animal models to prevent CVD. Although pre-clinical results are promising, only a few of them were further tested clinically. An overview of current patents (from 2010 until present) on chymase inhibitors completes this article’s content.
2. Tissue renin-angiotensin pathways
Ang II is an effector molecule mainly responsible for blood pressure regulation and water and electrolyte balance through its actions on multiple target receptors, at the cell’s surface nuclear membranes of the vascular wall, kidney tubulo-glomerular components and the adrenal gland. The RAS is both an endocrine and tissue paracrine/intracrine hormonal system in which the production of Ang II in the circulation may be primarily dependent upon the conversion of angiotensin I (Ang I) into Ang II by ACE (Figure 1). Research in Dr. Ferrario’s laboratories demonstrated the existence of an alternative processing of Ang I into the vasodilator and anti-growth heptapeptide angiotensin-(1-7) [33-36], which is generated through the action of tissue endopeptidases such as neprilysin (EC 3.4.24.11), prolyl oligopeptidase (EC 3.4.21.26), and thimet oligopeptidase (EC 3.4.24.15) (Figure 1) [30,36-38]. Further research demonstrated the existence of an exopeptidase - angiotensin converting enzyme 2 (ACE2) - which catalyzes the metabolism of Ang II into Ang-(1-7) [39]. Additional recent work identified the existence of a third enzymatic pathway which is upstream from Ang I and depends upon the conversion of the angiotensinogen (Aogen) protein into the intermediate peptides -angiotensin-(1-25) [Ang-(1-25)] and angiotensin-(1-12) [Ang-(1-12)] [40,41]. As documented in Figure 1, these newly identified components of the processing cascade leading to the functional generation of the biologically active hormones Ang II and Ang-(1-7) is highly relevant to this paper’s theme as the enzyme chymase (EC 3.4.21.39) appears to have a specific avidity for the catalysis of these substrates [42].
Figure 1.
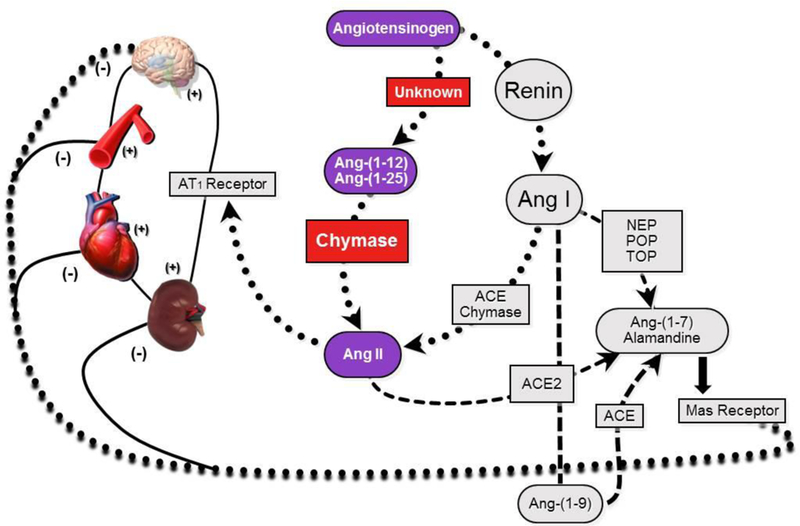
Schematic description of the main biochemical pathways involved in the formation of biologically active angiotensins. Neprilysin, NEP; Prolyl oligopeptidase, POP; Thimet oligopeptidase, TOP; Other abbreviations as in text.
As reviewed elsewhere [5], chymases are members of a family of serine proteases with broad peptidolytic activity and expressed primarily in MCs, fibroblasts, vascular endothelial cells, and granulocytes. Early studies by Cleveland Clinic investigators first demonstrated the role of chymase as an Ang II forming enzyme [43-45]. During the following decades multiple lines of research confirmed and extended the importance of chymase as a legitimate enzymatic pathway for Ang II production [5,21,30,46-49]. Recent studies of Ang-(1-12) processing in human and rodent hearts revealed a primacy of chymase as the Ang II forming enzyme from either Ang I or directly from Ang-(1-12) [28,29,50,51]. Expression of Ang-(1-12) is significantly higher in neonatal cardiac myocytes isolated from spontaneously hypertensive rats (SHR) pups compared to normotensive Wistar-Kyoto (WKY) pups [50]. Ang-(1-12) is highly expressed in human cardiac tissues obtained from patients undergoing cardiac interventions for correction of resistant atrial fibrillation and normal human ventricular tissues [28,51]. As shown in Figure 1, in vitro studies suggest that the rate-limiting step in cardiac tissue Ang II generation is mediated by chymase rather than ACE [29,42].
3. Cardiac mast cell chymases
MCs, derived from hematopoietic progenitor cells of the bone marrow, circulate in peripheral blood or penetrate to connective or mucosal tissue where they proliferate and differentiate into morphologically mature MCs [52]. Substantial research supports a role of MCs in cardiac remodeling and heart failure [53]. MCs secrete mainly three types of proteases [tryptases, chymases and carboxypeptidase A (CPA)]. Tryptases are tetrameric enzymes having trypsin-like substrate specificity and preferentially cleaving after Lys-Arg peptide bonds. In contrast to tryptases, chymases are monomeric serine proteases having chymotrypsin-like specificity cleaving preferentially after aromatic amino acid residues. The preferred cleavage site of chymase enzymes on angiotensin substrates to generate Ang II is the -Phe8-His9- bond. In rodents, MCs have a variety of combinations of at least ten types of protease populations but only five of them are considered to display chymase activity. The five MC chymases are designated in mouse as mouse mast cell proteases-1 to 5 (MMCP-1 to MMCP-5) and in rat as rat mast cell proteases-1 to 5 (RMCP-1 to RMCP-5). Out of these five proteases, only few of them have been purified and characterized in both models, the mouse (MMCP-1 and MMCP-4) and the rat (RMCP-1 and RMCP-2) [21,54,55,56-60].
Based on the structure and substrate specificity, mammalian chymases are classified into two subgroups (α- and β-chymase) [21,47]. In the human only the α-chymase is expressed, whereas in rodents several β-chymases are expressed in addition to the α-chymase. Details of the chymase isoforms and preferred cleavage sites on angiotensin peptides of rat and human are described in Table 1. Both sub-groups of chymases (α- and β-chymase) are shown to generate Ang II from Ang I. But in the rat one of the β-chymases (RMCP-1) is known as an Ang II degrading enzyme as it cleaves the -Tyr4-Ile5- bond in addition to -Phe8-His9- to generate inactive fragments [61,62]. The precise role and impact of these isoforms in CVD and the rationale behind the existence of these isoforms in rodents remain unclear. Because chymase plays a crucial role in the direct formation of Ang II peptide from Aogen precursor peptides [Ang-(1-12) and Ang I] in human heart tissues, an inhibitor may have potential use as a treatment for chymase-mediated CVD (such as vascular wall injury, atherosclerosis and cardiac hypertrophy).
Table 1:
Mast Cell Serine Proteases in Human and Rat
| Species and Chymase Name |
α/β- Form |
Ang Substrate Specificity |
NC- IUBMB Number |
aGene Name (NCBI Accession#) |
bPeptidase (UniPort#) |
Remarks [reference] |
|---|---|---|---|---|---|---|
| Human chymase (CMA1) | α-form | Ang I → Ang II | EC 3.4.21.39 (BRENDA) | CMA1 (NM_001836) | S01.140 (P23946) | Ang II-forming enzyme in human (α-chymase) [21] |
| Rat mast cell protease-1 (RMCP-1) | β-form | Ang I → Ang II and Ang II → Ang-(1-4) | EC 3.4.21.39 (BRENDA) | Mcpt1 (NM_017145) | S01.149 (P09650) | Abundant in CTMCs, Ang II degrading chymase [56,61,62] |
| Rat mast cell protease-2 (RMCP-2) | β-form | Ang I → Ang II | Not yet included in NC-IUBMB | Mcpt2 (NM_172044) | S01.141 (P00770) | Abundant in MMCs [56,61,62] |
| Rat mast cell protease-3 (RMCP-3) | β-form | Ang substrate not determined | Not yet included in NC-IUBMB | Mcpt3 (NM_001170466) | S01.012 (Q9Z1D3) | Widely expressed in both types of MCs (predominantly in CTMC) [57] |
| Rat mast cell protease-4 (RMCP-4) | β-form | Ang substrate not determined | Not yet included in NC-IUBMB | Mcpt4 (NM_019321) | S01.005 (P97592) | Abundant in MMCs, not characterized at protein level [58] |
| Rat mast cell protease-5 (RMCP-5) | α-form | Ang substrate not determined | EC 3.4.21.B5 (BRENDA) | Cma1 & Mcpt5 (NM_013092) | S01.150 (P50339) | Initially designated as RMCP-3 [57] |
| Rat vascular chymase (RVCH) | β-form | Ang I → Ang II | Not yet included in NC-IUBMB | VCH (AF063851) | S01.095 (O70500) | Expressed by vascular smooth muscles cells in spontaneously hypertensive rat [59,60] |
NCBI GeneBank accession number
MEROPE database of peptidase S1 family (serine endopeptidases); Ang (Angiotensin); RMCP-1 is involved in both Ang II production and degradation; RMCP-5 initially designated as RMPC-3, CTMCs, connective tissue mast cells; MMCs, mucosal mast cells; VCH, vascular chymase; NC-IUBMB, Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. [Adapted from MEROPS and BRENDA enzyme data bases]
Chymase is also involved in diverse local pathophysiological functions, including the generation and activation of profibrotic factors, matrix metalloproteases (MMPs), and transforming TGF-β1 [5]. The participation of chymase as a major enzyme responsible for Ang II formation (80-90%) in cardiovascular tissues or cells obtained from human subjects and animal models has been extensively documented [28,51,63-65]. Human cardiac chymase has been shown to generate Ang II from both Ang I and Ang-(1-12) substrates at significantly higher rates compared to cardiac ACE [28].
Chymase has been shown to be closely associated with tissue damage although it does not appear to directly alter the elevated blood pressure in hypertensive rat models [66-68]. Metabolism of Ang peptides in the circulation seems to be predominantly ACE-mediated because ACE is present in the plasma and it is located in endothelial cell membranes with its catalytic site exposed to the luminal surface. In contrast, chymase is located intracellularly or in the interstitial spaces of the heart. Studies reported either no chymase in the plasma [69] or its catalytic activity inhibited by various protease inhibitors present in the serum (such as α2-macroglobulin and α1-antichymotrypsin) [70]. An early and sustained increase in chymase activity has been reported in the hamster’s infarcted left ventricle to be present ahead of any changes in ACE activity [71]. MC numbers are increased in patients with gastrointestinal tract (GI) disorders (such as inflammatory bowel disease, IBD) [72]. Preclinical studies suggest that inhibition of chymase in IBD could be beneficial by down regulating profibrotic mediators TGF-β1 and matrix metalloprotease-9 (MMP-9) [73].
4. Chymase inhibitors (therapeutic approach in cardiac disease prevention)
A growing number of studies suggest that chymase plays an important role in the progression and development of cardiac diseases. Chymase enzymatic activity is significantly higher compared to ACE activity in human heart tissues [28,29,51,64,74,75]. Cardiac tissue Ang II primarily generated by chymase may be responsible for cardiac remodeling and disease progression. Multifunctional actions of chymase include up-regulation of degradation of extracellular matrix proteins (direct breakdown of fibronectin, autophagic digestion of procollagen and vitronectin) and degradation of apolipoproteins, activation of MMPs, TGF-β1, Interleukin-1β (IL-1β), big endothelin-1 formation and participation in lipid metabolism [5]. These extracellular matrix proteins as well as other molecules are important in cell adhesion, survival, functional and structural integrity of the cardiac cells. Chymase breaks down these factors and disrupts the normal function and structural integrity of cardiac cells. In heart tissues, 75-80% of Ang II is generated by chymase rather than ACE. Cardiac membranes isolated from diseased human left atrial appendages exhibited a 25-fold higher chymase activity compared to ACE activity [28]. Earlier studies with coronary artery homogenates from human hearts demonstrated that chymostatin but not captopril had the capacity to reduce Ang II formation [76]. Since MCs are a primary source of chymase, MC stabilizers (tranilast) rather than chymase inhibitors were first used to assess the cardiovascular effects of this approach. Tranilast proved effective in animal models of atherosclerosis but failed in two human trials (TREAT-2 and PRESTO) to provide any benefit in preventing neointimal formation following coronary angioplasty [76-78]. The primary reason for failure of the MC stabilizer in both clinical trials is that the tranilast was administered to the patients after percutaneous coronary intervention, whereas in animal models of cardiovascular injury tranilast was given prior to or at the time of the injury and was effective. Another drawback of tranilast is its side effects (liver and kidney dysfunction), which occurred mainly within one month after administration of the drug.
Several orally active chymase inhibitors (Table-2) [SUN-C8257, BCEAB, Suc-Val-Pro-PheP (OPh)2, TY-51469, NK3201 and TEI-E548] have been developed and validated in pre-clinical models of cardiovascular disease [67,71,79-92]. Additional studies evaluated the effects of chymase inhibition in an experimental transgenic mouse model of scleroderma expressing the human chymase gene (Tsk mice) [80]. Since hamsters and dogs have α-chymase in common with human, cardiac diseased models in these species are probably more relevant than in rats and mice, which contain several β-chymases in addition to α-chymase. Moreover, one of the rat’s form β-chymase (RMCP-1), further degrades the biologically active Ang II peptide to an inactive fragment. The chymase inhibitor (SUN-C8257) was initially tested in dog models. This inhibitor has been found to prevent cardiac fibrosis and improve diastolic dysfunction in a dog model with tachycardia induced heart failure [93]. During grafting and balloon injury, the neointima proliferative response associated with vascular endothelial injury or denudation is associated with increased recruitment of MCs and concomitant chymase activation. In a canine model of vascular injury induced by balloon injury model, the chymase inhibitor NK3201 has been found to prevent vascular proliferation [94]. NK3201 selectively decreased chymase activity while having no effect on ACE activity [94]. NK3201 has demonstrated beneficial effects post-myocardial infarction in hamsters where the increased chymase activity was reduced after treatment [71]. In another study, NK3201 treatment significantly reduced the number of MCs recruited in grafted veins at 28 days after the operation [95]. NK3201 toxicity after oral administration is reported to be low at doses under 100 mg/kg (for 2 weeks). Furthermore, very promising results have been found with BCEAB, NK3201 and TEI-E548 (selective chymase inhibitors) in dog and hamster models with myocardial infarction, cardiomyopathy and tachycardia-induced heart failure [67,71,76,80-82,88,91,94,95]. All these studies clearly suggest that chymase inhibition is a novel therapeutic target to prevent cardiac diseases. As mentioned above, studies using chymase inhibitors have been extended to address the role of chymase blockade in collagenous diseases such as scleroderma [80] and atopic dermatitis [84]. Positive outcomes have been obtained in these models. Based on these pre-clinical studies in mice, Asubio Pharmaceuticals Inc. (Asubio Pharma Kabushiki Kaisha) entered SUN13834 for phase II clinical trials for the treatment of atopic dermatitis, a common inflammatory skin disease regulated by genetic and environmental factors [96]. Oral administration of SUN13834 improved dermatitis in NC/Nga mice when dosed between 15 mg/kg (bid) and 30 mg/kg (qd) [85]. The trial was discontinued in 2012 due to adverse side effects.
Table 2.
Pre-clinical and Clinical Studies on Chymase specific Inhibitors
| Chymase inhibitors [reference] |
Substrate Specificity |
Species | Disease Models | Remarks |
|---|---|---|---|---|
| TY-51469 [67,79] | Chymase | Pig and Rat | Acute myocardial ischemia/reperfusion in pig and stroke-prone SHR | In pig attenuates fibrosis induced by activated chymase after myocardial ischemia/ reperfusion, reduce inflammatory markers (MMP-9, eNOS) and in rat useful for preventing vascular remodeling and prolonging survival. |
| SUN-C8257 [80] | Chymase | Mice | Genetic mouse model for human scleroderma [tight-skin (Tsk) mice having widespread disorder of connective tissues] | Reduced chymase activity and MMCP-4 mRNA level, also significantly decreased the thickness of the subcutaneous fibrous layer of Tsk mice. |
| BCEAB [81,82] | Chymase | Hamster | Cardiac disease and peritoneal adhesion formation | Suppresses heart chymase, cardiac fibrosis and peritoneal adhesions. |
| BAY 1142524 [83] | Chymase | Human | Heart failure, clinically stable patients with left-ventricular dysfunction after myocardial infarction | Phase 2 trial, no change in blood pressure, drug is safe and well tolerated up to single oral dose of 200 mg. |
| SUN13834 [84,85] | Chymase | Mice and Human | Atopic dermatitis | Phase 2 trial discontinued due to adverse side effects. |
| Suc-Val-Pro-PheP (OPh)2 [86] | Chymase | Hamster | Cardiac adhesions formation | Suppress cardiac chymase activity and TGF-1β level in postoperative cardiac adhesion. |
| NK3201 [67,81,87] | Chymase | Dog and Hamster | Balloon injury, vascular intimal hyperplasia and myocardial infarction | Reduce chymase activity in injured arteries, prevented intimal thickening and vascular proliferation. |
| TEI-E548 [88] | Chymase | Hamster | Chymase-induced microvascular leakage and coronary artery ligation | Improves survival and cardiac hypertrophy of the post-myocardial infarction. |
| RO5066852 [89] | Chymase | Mice | Atherosclerosis ApoE(−/−) mice | Therapeutic modality for atherosclerotic plaque stabilization, normalized the increased frequency and size of intraplaque hemorrhages observed in ApoE(−/−) mice. |
| Y-40613 [90] | Chymase | Mice | Atopic dermatitis | Suppresses the production of IgE and pruritus, ameliorate symptoms of atopic dermatitis. |
As we described in Table-2, several chymase inhibitors have been tested in various cardiac diseases as well as other diseased models in different species. Although some of the chymase specific inhibitors works well in animal models, these drugs failed when tested in early clinical testing. Also, some trials were discontinued due to adverse side effects. This could be due to significant species variability in the expression of chymases genes. As discussed above, humans have a single α-chymase gene, whereas mice and rats possess several β-chymase genes in addition to a single α-chymase gene [97]. The species variability might contribute to the controversy over the possible pathophysiological roles for chymase in rodents and human subjects [98]. Species differences in Ang II generation and degradation by MC chymases were also reported [99]. This study demonstrates that in terms of Ang II generating activity, the chymases ranked as follows: dog > human > hamster > mouse > rat, and that in terms of Ang II degrading activity, the order was hamster > rat > mouse > dog. Human chymase does not degrade Ang II at all. The MC subtypes of human and dog appear to be similar. Therefore, the functions of the dog α-chymase may closely reflect the function of the human α-chymase. The dog may serve as good models for studies of human MC functions and MC-related diseases.
A series of chymase inhibitors have been developed and published. The peptidyl human heart chymase inhibitor 12h is selective against bovine α-chymotrypsin (chymotrypsin Ki = > 100 μM) [100,101]. Chloromethyl ketone derivatives (Compound 21) is reported to be a potent human chymase inhibitor with no inhibitory activity against human leukocyte cathepsin G [102,103]. X-ray crystallographic structures guided the elaboration/linking of an oxindole fragment that was >100-fold selective over cathepsin G [104]. These efforts are augmented in the filing of several patent applications from 2010-2018 (Table 3). The development of these small molecules/substances take advantage of known reactivity of the selected chemical scaffolds, the structural properties of the chymase protein and substrate recognition sites, and combinatory chemistry. Recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters’ tongues is used as the enzyme source. The substrate used for chymase activity measures is Abz-HPFHL-Lys(Dnp)-NH2. Most of the inhibitors show good pharmacokinetic profiles to inhibit human specific chymase enzyme (CMA1) and demonstrated quite good efficiencies with IC50 in the nanomolar (nM) to micromolar (μM) range. In 2011, Janssen Pharmaceutical NV (United States Patent: 7,872,044) developed orally active phosphonic acid and phosphinic acid compounds (small molecules) that can inhibit the tissue specific serine protease involved in inflammatory or serine protease mediated disorders [105,106]. These novel compositions may be administered orally or by injection. Several small molecular inhibitors were patented by Boehringer Ingelheim International GmbH (Ingelheim am Rhein, DE) in the years 2013 and 2015. These compounds are Quinazolinedione (US Patent 8,377,949), Aza-quinazolinedione (US Patent 8,501,749), Benzimidazole (US Patent 9,150,556) and Aza-benzimidazolone (US Patent 9,062,056) [104,107-113]. The preferred modes of administration for these small orally active inhibitors are oral and intravenous routes. The compounds described herein may be administered alone or in combination with adjuvants that enhance stability of the inhibitors. Combinations with other therapeutics include but are not limited to: Ang II receptor blockers, ACE inhibitors, renin inhibitors, β-blockers, calcium channel blockers, diuretics, fibrates, and vasopeptidase inhibitors. The IC50 of these small molecules are in the range of 7-360 nM.
Table 3:
Patents Granted on Chymase Inhibitors (2010-2018)
| Patent [reference] |
Year | Institution | Title | Applications |
|---|---|---|---|---|
| US 7,872,044 B2 [106] | 2011 | Janssen Pharmaceutical NV | Inhibitors of chymase | To treat inflammatory or serine protease mediator’s disorders. |
| US 8,377,949 B2 [109] | 2013 | Boehringer Ingelheim International GmbH | Quinazolinedione chymase inhibitors | Useful in treating various diseases and conditions involving chymase |
| US 8,501,749 B2 [110] | 2013 | Boehringer Ingelheim International GmbH | Aza-quinazolinedione chymase inhibitors | Small molecule inhibitors of the formula (I), which are useful in treating various diseases and conditions involving chymase. |
| US 8,969,348 B2 [111] | 2015 | Boehringer Ingelheim International GmbH | Chymase inhibitors | Chronic heart failure, atherosclerosis, restenosis and myocardial infarction. |
| US 9,150,556 B2 [112] | 2015 | Boehringer Ingelheim International GmbH | Benzimidazolone chymase inhibitors | Small molecule inhibitors useful in treating various diseases and conditions involving chymase |
| US 9,062,056 B2 [113] | 2015 | Boehringer Ingelheim International GmbH | Aza-benzimidazolone chymase inhibitors | Small molecule inhibitors of the formula (I): and the pharmaceutical compositions thereof and processes of making the same. The compounds are useful in treating various diseases and conditions involving chymase. |
| US 9,695,131 B2 [114] | 2017 | Bayer Pharma Aktiengesellschaft (Berlin, DE) | Substituted uracils as chymase inhibitors | Use alone or in combinations for the treatment of prophylaxis of diseases |
In 2017, Bayer Pharma (Berlin, Germany) developed novel substituted uracil derivative substances, which act as inhibitors of chymase and are suitable for treatment and/or prophylaxis of cardiovascular, inflammatory, allergic and/or fibrotic disorders in humans and animals (US Patent: 9,695,131) [114]. These compounds are also suitable for treatment and/or prophylaxis of kidney disorders, particularly acute and chronic renal insufficiency and acute and chronic renal failure. The novel substituted uracil derivatives inhibit the degradation and alteration of the extracellular matrix MMPs, particularly MMP-1, MMP-3, MMP-8, MMP-9, MMP-10, MMP-11 and MMP-13. Activation of some of these MMPs are related to heart remodeling. Compounds in the context of the invention, which were tested in this assay, inhibited chymase activity with an IC50 of less than 10 μM. A total of 63 novel substituted uracil derivatives were tested for hamster chymase inhibition and the IC50 range was 1.8 nM to 1590 nM.
Very recently (2018), an orally active chymase inhibitor “Fulacimstat” (alternative name BAY 1142524) is currently being developed by Bayer Healthcare for the treatment of left ventricular dysfunction after myocardial infarction [83]. Preliminary reports demonstrated the ability of this compound to improve hamster’s cardiac function post-myocardial infarction and cardiac remodeling in dogs [114,115]. After very promising results from 3 randomized phase I studies in healthy male volunteers which examined the safety, tolerability, and pharmacokinetics of this specific chymase inhibitor [83], this drug has entered into phase II clinical trials. The results published from this phase I study shows that BAY 1142524 could suppress the abnormal cardiac tissue remodeling after myocardial infarction and improves cardiac function. On-going clinical trials include an evaluation of safety and efficacy of the chymase inhibitor BAY 1142524 at a dose of 25 mg BID in comparison to placebo using a 6 month treatment period in type II diabetic patients with a clinical diagnosis of diabetic kidney disease (NCT: 03412006) and a double-blind study to investigate efficacy, safety and tolerability of BAY 1142524 in patients after acute myocardial infarction with left-ventricular dysfunction (CHIARA MIA 2) (NCT: 02976467).
5. Concluding remarks
MCs are best known to produce immune defence mediators associated with allergic reactions. However, MCs are also capable of producing several inflammatory and pro-inflammatory mediators (such as chymase, tryptase and CPA), which are stored in secretory granules in their fully active form. After degranulation from MCs, the pre-stored proteases are released into the extracellular environment. MC recruitment increases in response to tissue injury contributing to induction of collagen deposition and organ remodeling. The biological actions of chymase released by MCs critically influence the adaptation of the heart to injury due to ischemia or changes in cardiac pre- and afterload [5]. Since cardiac MC derived proteases directly and/or indirectly play a significant role in the development and progression of heart disease, several other novel therapeutic strategies could also be used (such as dual targeting of neutrophil- and MC derived proteases, and inhibition of the MC chymase maturation process using DPPI inhibition) to reduce post ischemia reperfusion and improve cardiac remodeling [27,116]. In keeping with this interpretation, chymase inhibitors have demonstrated their capacity to reverse adverse cardiac remodeling in experimental models of disease. Recent studies with orally active chymase inhibitors should pave a way to their inclusion in the medical armamentarium.
6. Expert opinion
Although a significant number of studies underscore a role of MCs as tissue participants of the inflammatory mechanisms involved in the development of cardiac and vascular disease, a paucity in the availability of specific chymase inhibitors militates against progress. In addition, consensus regarding the clinical effectiveness of therapies that focus on suppressing the formation or activity of Ang II is another factor accounting for the limited appreciation of the role of chymase as a primary component of the biotransformation processes that lead to Ang II pathological actions. Authoritative statements [4] negating chymase’s role as a major Ang II-forming enzyme based on pre-selected studies are accepted without any critical evaluation of the merits of these opinions. In accepting these biased opinions, scientists and clinicians ignore data documenting the limited efficacy of RAS inhibitors in reducing the risk of well-defined cardiovascular events in landmark clinical trials [30,117,118].
The introduction of novel, orally active chymase inhibitors provides now the opportunity to document the critical role of chymase as an Ang II-forming enzyme in human tissue. On-going trials using BAY 1142524 could affirm these concepts.
Article highlights.
We review the role of mast cell chymases in the progression of cardiovascular disease in humans
We underscore how species differences in chymase genes and hydrolytic activity of chymase isoforms influences the actions of MC proteases.
We document what US patents have been filed for the use of orally active chymase inhibitors between 2010 to-date;
Acknowledgments
Funding
This work was supported by grant from the National Heart, Lung and Blood Institute of the NIH (P01 HL-051952).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.De Caterina R, Zampolli A, Del Turco S, et al. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr 2006;83(2):421S–26S. [DOI] [PubMed] [Google Scholar]
- 2.Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 2006;7(1):3–14. [DOI] [PubMed] [Google Scholar]
- 3.Oparil S, Acelajado MC, Bakris GL, et al. Hypertension. Nat Rev Dis Primers 2018;4:18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 2015;116(6):960–75. [DOI] [PubMed] [Google Scholar]
- 5.Dell’Italia LJ, Collawn JF, Ferrario CM. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ Res 2018;122(2):319–36.**Excellent review which illustrates the action of chymase in the development of CVD.
- 6.Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci 2011;68(6):965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiota N, Rysa J, Kovanen PT, et al. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertension 2003;21(10):1935–44. [DOI] [PubMed] [Google Scholar]
- 8.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 2007;217:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levick SP, Melendez GC, Plante E, et al. Cardiac mast cells: the centerpiece in adverse myocardial remodeling. Cardiovascular Res 2011;89(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JA, Wei CC, Brower GL, et al. Cardiac mast cell- and chymase-mediated mast cell number and matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 2003;35(3):311–19. [DOI] [PubMed] [Google Scholar]
- 11.Brower GL, Janicki JS, Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail 2005;11(7):548–56. [DOI] [PubMed] [Google Scholar]
- 12.Fu L, Wei CC, Powell PC, et al. Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol 2016;92:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YW, Pat B, Gladden JD, et al. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol 2011;300(6):H2251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels W, Reiters PH, Daemen MJ, et al. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol 1995;177(4):423–29. [DOI] [PubMed] [Google Scholar]
- 15.Patella V, de Crescenzo G, Lamparter-Schummert B, et al. Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res 1997;46 (Suppl 1):S31–32. [PubMed] [Google Scholar]
- 16.Levick SP, McLarty JL, Murray DB, et al. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension 2009;53(6):1041–47. [DOI] [PubMed] [Google Scholar]
- 17.Kovanen PT. Role of mast cells in atherosclerosis. Chem Immunol 1995;62:132–70. [PubMed] [Google Scholar]
- 18.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochem Biophys Acta 2012;1822(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patella V, de Crescenzo G, Ciccarelli A, et al. Human heart mast cells: a definitive case of mast cell heterogenecity. Int Arch Allergy Immunol 1995;106(4):386–93. [DOI] [PubMed] [Google Scholar]
- 20.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 2007;217:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta 2000;1480(1–2):245–57. [DOI] [PubMed] [Google Scholar]
- 22.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol 2007;95:167–255. [DOI] [PubMed] [Google Scholar]
- 23.Pejler G, Knight SD, Henningsson F, Wernersson S. Novel insights into the biological function of mast cell carboxypeptidase A. Trends Immunol 2009;30(8):401–8. [DOI] [PubMed] [Google Scholar]
- 24.Sperr WR, Banki HC, Mundigler G, et al. The human cardiac mast cell: Localization, isolation, phenotype, and functional characterization. Blood 1994;84(11):3876–84. [PubMed] [Google Scholar]
- 25.Ekoff M, Strasser A, Nilsson G. FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells. J Immunol 2007;178(7):4177–83. [DOI] [PubMed] [Google Scholar]
- 26.Enerback L Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand 1966;66(3):289–302. [DOI] [PubMed] [Google Scholar]
- 27.Wolters PJ, Pham CTN, Muilenburg DJ, et al. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Bio Chem 2001;276(21):18551–56. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad S, Simmons T, Varagic J, et al. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One 2011;6(12):e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S, Varagic J, VonCannon JL, et al. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun 2016;478(2):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrario CM, Ahmad S, Nagata S, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014;126(7):461–9.*Useful review on Angiotensin-II-forming pathway differences in rodents and human.
- 31.Soualmia F, Amri CE. Serine proteases inhibitors to treat inflammation: a patent review (2011–2016). Exp Opin Ther Patents 2018;28(2):93–110. [DOI] [PubMed] [Google Scholar]
- 32.Rachel KV, Sirisha GVD. Serine proteases and their inhibitors in human health and disease Proteases in Human Diseases (Springer, Singapore: ) 2017;195–226. [Google Scholar]
- 33.Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1–7). Peptides 1993;14(4):679–84. [DOI] [PubMed] [Google Scholar]
- 34.Campagnole-Santos MJ, Diz DI, Santos RA, et al. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. Am J Physiol 1989;257 (1 Pt 2):H324–9. [DOI] [PubMed] [Google Scholar]
- 35.Schiavone MT, Khosla MC, Ferrario CM. Angiotensin-[1–7]: evidence for novel actions in the brain. J Cardiovasc Pharmacol 1990;16 (Suppl 4):S19–S24. [PubMed] [Google Scholar]
- 36.Schiavone MT, Santos RA, Brosnihan KB, et al. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci U S A 1988;85(11):4095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension 2010;55(2):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci 1993;52(18):1461–80. [DOI] [PubMed] [Google Scholar]
- 39.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417(6891):822–8.**Landmark report on ACE2 role in cardiac function.
- 40.Nagata S, Hatakeyama K, Asami M, et al. Big angiotensin-25: a novel glycosylated angiotensin-related peptide isolated from human urine. Biochem Biophys Res Commun 2013;441(4):757–62. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S, Kato J, Sasaki K, et al. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 2006;350(4):1026–31. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad S, Varagic J, Groban L, et al. Angiotensin-(1–12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep 2014;16(5):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urata H, Healy B, Stewart RW, et al. Angiotensin II receptors in normal and failing human hearts. J Clin Endocrinol Metab 1989;69(1):54–66. [DOI] [PubMed] [Google Scholar]
- 44.Urata H, Healy B, Stewart RW, et al. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res 1990;66(4):883–90. [DOI] [PubMed] [Google Scholar]
- 45.Urata H, Kinoshita A, Misono KS, et al. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem 1990;265(36):22348–57.*Very important report on ACE-independent pathway of Ang II generation in human heart.
- 46.Balcells E, Meng QC, Hageman GR, et al. Angiotensin II formation in dog heart is mediated by different pathways in vivo and in vitro. Am J Physiol 1996;271 (2 Pt 2):H417–21. [DOI] [PubMed] [Google Scholar]
- 47.Chandrasekharan UM, Sanker S, Glynias MJ, et al. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science 1996;271(5248):502–5. [DOI] [PubMed] [Google Scholar]
- 48.Dell’Italia LJ, Meng QC, Balcells E, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest 1997;100(2):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zisman LS, Abraham WT, Meixell GE, et al. Angiotensin II formation in the intact human heart. Predominance of the angiotensin-converting enzyme pathway. J Clin Invest 1995;96(3):1490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad S, Varagic J, Westwood BM, et al. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One 2011;6(1):e15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad S, Wei CC, Tallaj J, et al. Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens 2013;7(2):128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitamura Y, Oboki K, Ito A. Development of mast cells. Proc Jpn Acad Ser B Phys Biol Sci 2007;83(6):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levick SP, Melendez GC, Plante E, et al. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res 2011;89(1):12–9.*Important review on regulation and role of cardiac mast cells
- 54.Newlands GF, Gibson S, Knox DP, et al. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology 1987;62(4):629–34. [PMC free article] [PubMed] [Google Scholar]
- 55.Woodbury RG, Everitt MT, Neurath H. Mast cell proteases. Methods Enzymol 1981;80 Pt C:588–609. [DOI] [PubMed] [Google Scholar]
- 56.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77(4):1033–79. [DOI] [PubMed] [Google Scholar]
- 57.Ide H, Itoh H, Tomita M, et al. Cloning of the cDNA encoding a novel rat mast-cell proteinase, rMCP-3, and its expression in comparison with other rat mast-cell proteinases. Biochem J 1995;311 ( Pt 2): 675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karlson U, Pejler G, Froman G, Hellman L. Rat mast cell protease 4 is a beta-chymase with unusually stringent substrate recognition profile. J Biol Chem 2002;277(21):18579–85. [DOI] [PubMed] [Google Scholar]
- 59.Gallwitz M, Enoksson M, Hellman L. Expression profile of novel members of the rat mast cell protease (rMCP)-2 and (rMCP)-8 families, and functional analyses of mouse mast cell protease (mMCP)-8. Immunogenetics 2007;59(5):391–405. [DOI] [PubMed] [Google Scholar]
- 60.Guo C, Ju H, Leung D, et al. A novel vascular smooth muscle chymase is upregulated in hypertensive rats. J Clin Invest 2001;107(6):703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wintroub BU, Schechter NB, Lazarus GS, et al. Angiotensin I conversion by human and rat chymotryptic proteinases. J Invest Dermatol 1984;83(5):336–9. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto D, Shiota N, Takai S, et al. Three-dimensional molecular modeling explains why catalytic function for angiotensin-I is different between human and rat chymases. Biochem Biophys Res Commun 1998;242(1):158–63. [DOI] [PubMed] [Google Scholar]
- 63.Balcells E, Meng QC, Johnson WH, Jr., et al. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol 1997;273(4 Pt 2):H1769–74. [DOI] [PubMed] [Google Scholar]
- 64.Butts B, Goeddel LA, George DJ, et al. Increased Inflammation in Pericardial Fluid Persists 48 Hours After Cardiac Surgery. Circulation 2017;136(23):2284–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chymase Urata H. and matrix metalloproteinase. Hypertens Res 2007;30(1):3–4. [DOI] [PubMed] [Google Scholar]
- 66.Kirimura K, Takai S, Jin D, et al. Role of chymase-dependent angiotensin II formation in regulating blood pressure in spontaneously hypertensive rats. Hypertens Res 2005;28(5):457–64. [DOI] [PubMed] [Google Scholar]
- 67.Takai S, Jin D, Chen H, et al. Chymase inhibition improves vascular dysfunction and survival in stroke-prone spontaneously hypertensive rats. J Hypertens 2014;32(8):1637–49. [DOI] [PubMed] [Google Scholar]
- 68.Roszkowska-Chojecka MM, Walkowska A, Gawrys O et al. Effects of chymostatin, a chymase inhibitor, on blood pressure, plasma and tissue angiotensin II, renal haemodynamics and renal excretion in two models of hypertension in the rat. Exp Physiol 2015;100(9):1093–105. [DOI] [PubMed] [Google Scholar]
- 69.Urata H, Kinoshita A, Misono KS, et al. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem 1990;265(36):22348–57. [PubMed] [Google Scholar]
- 70.Lindstedt L, Lee M, Castro GR, et al. Chymase in exocytosed rat mast cell granules effectively proteolyzes apolipoprotein AI-containing lipoproteins, so reducing the cholesterol efflux-inducing ability of serum and aortic intimal fluid. J Clin Invest 1996;97(10):2174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin D, Takai S, Yamada M, et al. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol 2001;86(2):203–14. [DOI] [PubMed] [Google Scholar]
- 72.Heuston S, Hyland NP. Chymase inhibition as a pharmacological target: a role in inflammatory and functional gastrointestinal disorders? Br J Pharmacol 2012;167(4):732–40.**Outstanding review detailing the association between chymase and inflammatory diseases.
- 73.Takai S, Jin D, Miyazaki M. New approaches to blockade of the renin-angiotensin-aldosterone system: chymase as an important target to prevent organ damage. J Pharmacol Sci 2010;113(4):301–09. [DOI] [PubMed] [Google Scholar]
- 74.Froogh G, Pinto JT, Le Y, et al. Chymase-dependent production of angiotensin II: an old enzyme in old hearts. Am J Physiol Heart Circ Physiol 2017;312(2):H223–H31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinoshita A, Urata H, Bumpus FM, Husain A. Multiple determinants for the high substrate specificity of an angiotensin II-forming chymase from the human heart. J Biol Chem 1991;266(29):19192–7. [PubMed] [Google Scholar]
- 76.Doggrell SA, Wanstall JC. Vascular chymase: pathophysiological role and therapeutic potential of inhibition. Cardiovasc Res 2004;61(4):653–62. [DOI] [PubMed] [Google Scholar]
- 77.Tamai H, Katoh K, Yamaguchi T, et al. The impact of tranilast on restenosis after coronary angioplasty: The second tranilast restenosis following angioplasty trial (TREAT-2). Am Heart J 2002;143:506–13. [DOI] [PubMed] [Google Scholar]
- 78.Holmes DR, Savage M, LaBlanche JM, et al. Results of preventionof restenosis with tranilast and its outcomes (PRESTO) trial. Circulation 2002;106(10):1243–50. [DOI] [PubMed] [Google Scholar]
- 79.Oyamada S, Bianchi C, Takai S, et al. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther 2011;339(1):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shiota N, Kakizoe E, Shimoura K, et al. Effect of mast cell chymase inhibitor on the development of scleroderma in tight-skin mice. Br J Pharmacol 2005;145(4):424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takai S, Jin D, Sakaguchi M, et al. A novel chymase inhibitor, 4-[1-([bis-(4-methyl-phenyl)-methyl]-carbamoyl)3-(2-ethoxy-benzyl)-4-oxo-azetidin e-2-yloxy]-benzoic acid (BCEAB), suppressed cardiac fibrosis in cardiomyopathic hamsters. J Pharmacol Exp Ther 2003;305(1):17–23. [DOI] [PubMed] [Google Scholar]
- 82.Takai S, Jin D, Sakaguchi M, et al. An orally active chymase inhibitor, BCEAB, suppresses heart chymase activity in the hamster. Jpn J Pharmacol 2001;86(1):124–6. [DOI] [PubMed] [Google Scholar]
- 83.Kanefendt F, Thuss U, Becka M, et al. Pharmacokinetics, Safety, and Tolerability of the Novel Chymase Inhibitor BAY 1142524 in Healthy Male Volunteers. Clin Pharmacol Drug Dev 2018. June 7. **First orally active chymase inhibitor (BAY 1142524) entered into phase II clinical trials for the treatment of left ventricular dysfunction after myocardial infarction.
- 84.Terakawa M, Fujieda Y, Tomimori Y, et al. Oral chymase inhibitor SUN13834 ameliorates skin inflammation as well as pruritus in mouse model for atopic dermatitis. Eur J Pharmacol 2008;601(1–3):186–91. [DOI] [PubMed] [Google Scholar]
- 85.Ogata A, Fujieda Y, Terakawa M, et al. Pharmacokinetic/pharmacodynamic analyses of chymase inhibitor SUN13834 in NC/Nga mice and prediction of effective dosage for atopic dermatitis patients. Int Immunopharmacol 2011;11(10):1628–32. [DOI] [PubMed] [Google Scholar]
- 86.Soga Y, Takai S, Koyama T, et al. Attenuation of adhesion formation after cardiac surgery with a chymase inhibitor in a hamster model. J Thorac Cardiovasc Surg 2004;127(1):72–8. [DOI] [PubMed] [Google Scholar]
- 87.Doggrell SA, Wanstall JC. Will chymase inhibitors be the next major development for the treatment of cardiovascular disorders? Expert Opin Investig Drugs 2003;12(8):1429–32.*Classical reference in the field of chymase inhibitors.
- 88.Hoshino F, Urata H, Inoue Y, et al. Chymase inhibitor improves survival in hamsters with myocardial infarction. J Cardiovasc Pharmacol 2003;41(Suppl 1):S11–8. [PubMed] [Google Scholar]
- 89.Bot I, Bot M, van Heiningen SH, et al. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/− mice. Cardiovasc Res 2011;89(1):244–52. [DOI] [PubMed] [Google Scholar]
- 90.Imada T, Komorita N, Kobayashi F, et al. Therapeutic potential of a specific chymase inhibitor in atopic dermatitis. Jpn J Pharmacol 2002;90(3):214–7. [DOI] [PubMed] [Google Scholar]
- 91.Doggrell SA, Wanstall JC. Cardiac chymase: pathophysiological role and therapeutic potential of chymase inhibitors. Can J Physiol Pharmacol 2005;83(2):123–30. [DOI] [PubMed] [Google Scholar]
- 92.Roszkowska-Chojecka MM, Walkowska A, Gawrys O, et al. Effects of chymostatin, a chymase inhibitor, on blood pressure, plasma and tissue angiotensin II, renal haemodynamics and renal excretion in two models of hypertension in the rat. Exp Physiol 2015;100(9):1093–105. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto T, Wada A, Tsutamoto T, et al. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation 2003;107(20):2555–8. [DOI] [PubMed] [Google Scholar]
- 94.Takai S, Miyazaki M. Application of a chymase inhibitor, NK3201, for prevention of vascular proliferation. Cardiovasc Drug Rev 2003;21(3):185–98. [DOI] [PubMed] [Google Scholar]
- 95.Takai S, Jin D, Nishimoto M, et al. Oral administration of a specific chymase inhibitor, NK3201, inhibits vascular proliferation in grafted vein. Life Sci 2001;69(15):1725–32. [DOI] [PubMed] [Google Scholar]
- 96.Guttman-Yassky E, Dhingra N, Leung DY. New era of biologic therapeutics in atopic dermatitis. Expert Opin Biol Ther 2013;13(4):549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallwitz M, Hellman L. Rapid lineage-specific diversification of the mast cell chymase locus during mammalian evolution. Immunogenetics 2006;58(8):641–54. [DOI] [PubMed] [Google Scholar]
- 98.Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther 2006;112(3):668–76. [DOI] [PubMed] [Google Scholar]
- 99.Kunori Y, Muroga Y, Iidaka M, el al. Specises differences in angiotensin II generation and degradation by mast cell chymases. J Recept Signal Transduct Res 2005;25(1):35–44. [DOI] [PubMed] [Google Scholar]
- 100.Eda M, Ashimori A, Akahoshi F, et al. Peptidyl human heart chymase inhibitors. 2. Discovery of highly selective difluoromethylene ketone derivatives with Glu at P3 site. Bioorg Med Chem Lett 1998;8(8):919–24. [DOI] [PubMed] [Google Scholar]
- 101.Eda M, Ashimori A, Akahoshi F, et al. Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P’ binding subsites. Bioorg Med Chem Lett 1998;8(8):913–8. [DOI] [PubMed] [Google Scholar]
- 102.Hayashi Y, Iijima K, Katada J, Kiso Y. Structure-activity relationship studies of chloromethyl ketone derivatives for selective human chymase inhibitors. Bioorg Med Chem Lett 2000;10(3):199–201. [DOI] [PubMed] [Google Scholar]
- 103.Iijima K, Katada J, Hayashi Y. Symmetrical anhydride-type serine protease inhibitors: structure-activity relationship studies of human chymase inhibitors. Bioorg Med Chem Lett 1999;9(3):413–8. [DOI] [PubMed] [Google Scholar]
- 104.Taylor SJ, Padyana AK, Abeywardane A, et al. Discovery of potent, selective chymase inhibitors via fragment linking strategies. J Med Chem 2013;56(11):4465–81. [DOI] [PubMed] [Google Scholar]
- 105.Greco MN, Hawkins MJ, Powell ET, et al. Discovery of potent, selective, orally active, nonpeptide inhibitors of human mast cell chymase. J Med Chem 2007;50(8):1727–30. [DOI] [PubMed] [Google Scholar]
- 106.Janssen Pharmaceutical NV. Inhibitors of chymase. US7872044 (2011). [Google Scholar]
- 107.Aoyama Y Non-peptidic chymase inhibitors. Expert Opinion on Therapeutic Patents 2001;11(9):1423–28. [Google Scholar]
- 108.Aoyama Y, Konoike T, Kanda A, et al. Total synthesis of human chymase inhibitor methyllinderone and structure--activity relationships of its derivatives. Bioorg Med Chem Lett 2001;11(13):1695–7. [DOI] [PubMed] [Google Scholar]
- 109.Boehringer Ingelheim International GmbH. Quinazolinedione chymase inhibitors. US8377949 (2013).
- 110.Boehringer Ingelheim International GmbH. Azaquinazolinedione chymase inhibitors. US8501749 (2013)
- 111.Boehringer Ingelheim International GmbH. Chymase inhibitors. US8969348 (2015)
- 112.Boehringer Ingelheim International GmbH. Benzimidazolone chymase inhibitors. US9150556 (2015)
- 113.Boehringer Ingelheim International GmbH. Aza-benzimidazolone chymase inhibitors. US9062056 (2015)
- 114.Bayer Pharma Aktiengesellschaft. Substituted uracils as chymase inhibitors. US9695131 (2017).
- 115.Tinel H, Zubov D, Zimmermann K, et al. A novel chymase inhibitor BAY 1142524 reduces fibrosis and improves cardiac function after myocardial infarction in hamster. Circulation 2017;136 (suppl 1):A13624. [Google Scholar]
- 116.Hooshdaran B, Kolpakov MA, Guo X, et al. Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res Cardiol 2017;112(6):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res 2017;125(Pt A): 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reyes S, Varagic J, Ahmad S, et al. Novel Cardiac Intracrine Mechanisms Based on Ang-(1–12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease. Curr Hypertens Rep 2017;19(2):16.*Very useful review on an intracrine renin-angiotensin system pathway.


